Abstract
Background:
Calligonum polygonoides L. subsp. comosum (L’Hér.) Sosk. is a plant species belonging to family Polygonaceae. Susceptibility to threaten, presence of various chemical constituents, and many medicinal effects reported for this plant in addition to rareness of in vitro culture studies have fuelled the need for its micropropagation and phytochemical investigations of the produced cultures.
Objectives:
To employ in vitro culture technique for ex situ conservation of C. polygonoides, using the fruit as an explant; establish callus and cell suspension cultures from in vitro germinated plantlets; investigate the production of phenolics through callus, redifferentiated shoot, and cell suspension cultures; attempt to enhance cell capacity to accumulate phenolics using salicylic acid and yeast extract and provide a brief demonstration of biosynthetic pathway leading to phenolic production.
Materials and Methods:
Modified Murashige and Skoog media supplemented with growth hormones such as kinetin, 1-naphthaleneacetic acid, 6-benzylaminopurine, and indole-3-acetic acid were used to establish callus, redifferentiated shoots, and cell suspension cultures. Elicitation of cell suspension culture was performed using salicylic acid and yeast extracts. A reversed phase-high performance liquid chromatography method for determination of phenolic content in the aforementioned cultures was developed.
Results:
The unorganized callus and cell suspension cultures contained fewer amounts of phenolic compounds than redifferentiated shoots. Elicitation produced massive quantitative reprogramming of phenolic content.
Conclusion:
The present study offers an alternative and renewable source for this valuable natural plant, provide a chance to improve secondary metabolite yield and serve as a useful tool for studying the biosynthesis of these compounds and its regulation in plant cells.
SUMMARY
In vitro culture techniques provided a strategy for ex situ conservation of the endangered C. polygonoides.
Unorganized callus and cell suspension cultures accumulated less phenolic content than re-differentiated shoots.
Elicitation produced massive quantitative reprogramming of phenolic content.
Phenolic biosynthesis was discussed briefly.
Abbreviation used: H2O2: Hydrogen peroxide, Kin: Kinetin, NAA: Naphthaleneacetic acid, BAP: 6-benzylaminopurine, IAA: Indole-3-acetic acid, HPLC: High-performance liquid chromatography.
Keywords: Calligonum polygonoides, cell cultures, elicitation, ex-situ conservation, micropropagation, phenolics
INTRODUCTION
Calligonum polygonoides L. subsp. Comosum (L’Hér.) Sosk. (Polygonaceae) is a small leafless shrub, which has a reputation in folklore medicine as a stimulant and astringent, under the local names “ghardaq,” “rusah,” “arta,” or “Wargat Al-shamas.”[1,2] Leaves and stems are chewed to wash teeth and to treat gummosis while young shoots infusion is used as tonic.[3] It was reported that C. polygonoides possesses hypoglycemic,[4] cytotoxic, antioxidant,[1] antimicrobial,[5] antiulcer, anti-inflammatory,[6] antifungal,[7] and mosquitocidal activities.[8] Chemical analysis from previous studies revealed the presence of (+)-catechin, dehydrodicatechin A, kaempferol-3-O-rhamnopyranoside, quercitrin, β-sitosterol-3-O-glucoside, isoquercitrin, kaempferol-3-O-glucuronide, and mequilianin.[1] Campesterol, stigmasterol, (3 β, 5 α, 24 S)-stigmastan-3-ol, and stigmast-4-en-3-one were isolated from the roots of the plant,[9] whereas β-sitosterol, kaempferol, quercetin, taxfolin, gallic acid, and astragalin were isolated from leaves.[10]
C. polygonoides has been quoted in Red Data Book of International Union for Conservation of Nature and Natural Resources as an endangered plant species.[11] Endangered, threatened, and rare species should be grown and conserved by in vitro culture because of the efficient multiplication and small demands on a number of initial plants and space.[12] Another advantage of this technology is that cell suspension culture systems could be used for large-scale culturing of plant cells from which secondary metabolites could be extracted so; it can ultimately provide a continuous, reliable source of natural products.[13] Furthermore, elicitation is one of the most important approaches to enhance the yield of secondary metabolites produced by in vitro cultures.[14] Previous attempts for regeneration of C. polygonoides were fruitful only using green shoots as explants[15] but were unsuccessful when the seeds are chosen. Therefore, mature seeds did not germinate in the medium while isolated embryos provided 58.3% germination but failed to produce callus or organs and ultimately died.[16] Subsequently, achieving a protocol for in vitro establishment and multiplication from the fruit explants will definitely assist ex-situ conservation of such valuable plant species. Furthermore, it will introduce the produced aseptic tissues for investigations of their phytoconstituents. The accumulation of phenolic constituents by callus, redifferentiated shoot, and cell suspension cultures has not been studied before. Thus, the objectives of the present study were to employ in vitro culture technique for ex situ conservation of one of the medicinally valuable and environmentally threatened plant species, C. polygonoides, using the fruit as an explant; establish callus and cell suspension cultures from in vitro germinated plantlets; investigate the production of phenolics through callus, redifferentiated shoot, and cell suspension cultures; attempt to enhance cell capacity to accumulate phenolics using elicitors such as salicylic acid and yeast extract and provide a brief demonstration of biosynthetic pathway leading to phenolics production. The current results are reported for the first time.
MATERIALS AND METHODS
Plant materials
C. polygonoides fruits were collected from western desert, Egypt, on April 2012, during flowering season and kindly authenticated by Dr. Abdelhalim Mohamed (Plant Taxonomy Department, Agricultural Research Center, Cairo, Egypt) for whom the authors are thankful. Voucher specimen was deposited at Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University under the registration number BUPD-40.
Fruit surface sterilization and germination
Several trials were performed on the fruits before in vitro germination:
Clorox treatment: Fruits were surface sterilized by dipping in an aqueous ethanol solution (70%) for 5, 10, 15, and 30 min, then in a 5% solution of commercial detergent; Clorox (5.25% sodium hypochlorite) for 5, 10, 20, and 30 min and finally rinsed exhaustively with sterilized distilled water three times before in vitro culture
Boiling water treatment: Fruits were dipped in boiling water for 5, 10, and 20 min then in a solution of commercial Clorox (5%) for 5, 10, 20, and 30 min and finally rinsed with sterilized distilled water
Abrasion of fruit wall: Fruits were surface-scratched manually using a knife, dipped in a solution of commercial Clorox (5%) for 5, 10, and 20 min and finally rinsed thoroughly with sterilized distilled water
Flame treatment: Fruits were exposed to direct flame for 1 s. just to remove their dense hair surface, dipped in a solution of commercial Clorox (5%) for 5, 10, and 20 min and finally rinsed exhaustively with sterilized distilled water
Sulfuric acid immersion: Fruits were dipped in sulfuric acid 10, 15, 25, 50, and 100% for 5, 10, 15, 20, and 30 min and rinsed exhaustively with sterilized distilled water
Cold water maceration: Fruits were soaked in distilled water overnight, dipped in a solution of commercial Clorox (5%) for 5, 10, and 20 min and finally rinsed exhaustively with sterilized distilled water
-
Hydrogen peroxide treatments:
- Fruits were dipped in a solution of commercial Clorox (5%) for 5 min followed by hydrogen peroxide (H2O2) for 15 min
- Fruits were surface-scratched, dipped in a solution of commercial Clorox (5%) for 5 min followed by H2O2 for 15 min
- Fruits were exposed to direct flame for 1 s, dipped in a solution of commercial Clorox (5%) for 5 min followed by H2O2 for 15 min.
The sterilized fruits in each case were germinated aseptically on quarter-strength Murashige and Skoog (MS) basal liquid medium,[17] supplemented with 30 g/L sucrose and 9 g/L agar. The pH of the medium was adjusted to 5.2. The fruits were kept at 27 ± 2°C in the dark. Germination was expressed as percentage of viable germinated fruits.
Callus formation
The in vitro germinated plantlets were used as explants to produce calli. Hypocotyls segments (1 cm long) obtained from 14-days-old young plants were cultured on modified full-strength MS solid medium containing 30 g/L sucrose, 1 mg/L nicotinic acid, 1 mg/L pyridoxine HCl, and 10 mg/L thiamine HCl. The following plant growth regulators have been added, and the two media were tested:
1 mg/L 6-benzylaminopurine (BAP) and 0.5 mg/L naphthaleneacetic acid (NAA)
2 mg/L kinetin (Kin) and 1 mg/L NAA.
The pH of the medium was adjusted to 5.8. The media were solidified with 9 g/L agar and incubated at 27 ± 2°C in the dark. Callus was multiplied on the same medium under the same conditions every 3 weeks for few subcultures.
Shoot induction and multiplication
Shoot induction was established from 3-month-old callus grown on modified full-strength MS solid medium involved 30 g/L sucrose, 1 mg/L BAP, and 0.5 mg/L indole-3-acetic acid (IAA) then subcultured regularly every 3 weeks for proliferation on a medium supplemented with 5 mg/L BAP. All media were incubated at 27 ± 2°C in the light (intensity 2000 lux).
Establishment of cell suspension culture
Cell suspension culture was established from 3-month-old callus. About 1 g callus tissues were added to 100 ml Erlenmeyer flasks containing 30 ml full-strength liquid MS medium and incubated at 27 ± 2°C in the dark on a rotary shaker at 100 rpm. Cells were subcultured on the same medium under the same condition every 3 weeks.
Growth time course for cell suspension culture
Growth ratio of the cultured cells was assessed by harvesting cells at specified times (7, 14, 21, and 28 days) after inoculation. Final fresh weight of the harvested cells was determined, and the growth ratio was calculated according to the following equation:
Growth ratio = final fresh weight/initial fresh weight.
Treatment of cell suspension cultures with elicitors
Two grams fresh weight cells were inoculated into 100 ml Erlenmeyer flasks containing 30 ml liquid MS medium. Seven-day-old cultured cells were separately treated for 48 h with filter sterilized aqueous solution of crude yeast extract (50, 100 μg/ml) or salicylic acid (100, 200 μg/ml). Control received equivalent volumes of solvent.
Phenolic extraction and high-performance liquid chromatography analysis
Callus, redifferentiated shoot, and cell suspension cultures (control, 50, 100 μg/ml yeast extract-treated cells, 100 and 200 μg/ml salicylic acid-treated cells) were separately harvested. The cells of suspension cultures were separated from the medium by filtration. Callus and redifferentiated shoot were washed with distilled water to discard the adherent agar. All cultures were separately extracted with 75% aqueous methanol (3 × 10 ml) at room temperature and filtered. Each filtrate was separately pooled and evaporated to dryness. One gram of each dried extract was separately dissolved in 1 ml methanol (high-performance liquid chromatography [HPLC] grade, Sigma-Aldrich), clarified using Millipore filters (0.22 μm) and subjected to HPLC analysis to quantify their phenolic content. The used HPLC (Agilent 1260 Infinity) instrument was equipped with an Agilent 1260 Infinity preparative pump (G1361A), Agilent 1260 Diode array detector VL (G1315D), Agilent 1260 infinity thermostatted column compartment (G1361A), and Agilent 1260 Infinity autosampler (G2260A). Separation and quantitation were performed on a ZORBAX Eclipse plus C8 analytical column (250 × 4.6 mm i.d, 5 μm particle size). An aliquot of 50 μL was injected. Phenolics were quantified at 275 nm using peak area by comparison to a calibration curve derived from commercially available standards (gallic acid, catechin, taxifolin, isoquercitrin, kaempferol-3-O-glucuronide, astragalin, quercetin, and kaempferol). Ambient temperature was used. Elution was carried out at a flow rate of 1 ml/min using water:formic acid (99.97:0.03, v/v) as solvent A and methanol:formic acid (99.97:0.03, v/v) as solvent B in a gradient mode as follows: 0–5 min with 80% A, 5–20 min with 80–50% A, 20–25 min with 50–20% A, 25–30 min with 20–0% A.
RESULTS
Fruit surface sterilization and germination
The highest germination (100% viable germinated fruits) was achieved in all cases of H2O2 treatment (a, b, c) as shown in Figure 1a and 1b. On the other hand, the sulfuric acid treatment produced 20% viable germinated fruits when 100% concentrated solution was used for 30 min. All other treatments gave 0% viable germinated fruits. In vitro germinated plantlets were successfully transferred to soil to complete their subsequent growth as shown in Figure 1c.
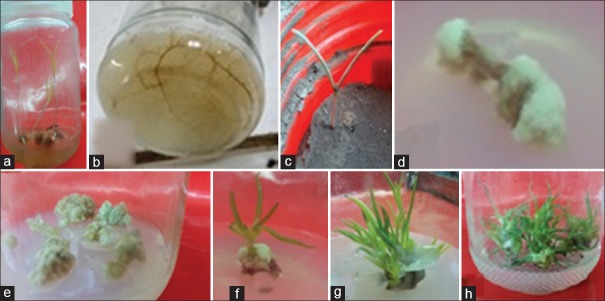
Figure 1.
(a) In vitro germinated Calligonum polygonoides L. fruits treated with H2O2 and the produced distinct plantlet (a) shoot (b) root; (c) in vitro plantlet shifted successfully to soil bed; (d and e) callus induced from the in vitro shoot segments; and (f-h) shoot differentiation and subsequent proliferation
Callus induction
The highest C. polygonoides callus induction response of 100% was found in media (b) containing 2 mg/L Kin and 1 mg/L NAA after 4 weeks of hypocotyl segments culture when compared to media (a) containing 1 mg/L BAP and 0.5 mg/L NAA, which produced callus from 80% of cultured initial explants. Shoot callus or callus produced from shoot segments was friable, white to off-white in color as shown in Figure 1d and 1e.
Shoot differentiation and multiplication
Differentiated callus with green small shoots was observed in the MS basal medium containing 1 mg/L BAP and 0.5 mg/L IAA after 6–8 weeks [Figure 1f]. The addition of BAP and IAA to MS medium was effective in increasing the number of induced-shoots per callus (90%). The shoot regeneration capacity was improved when the BAP concentration increased in the medium into 5 mg/L for further subcultures [Figure 1g and 1h].
Growth time course for cell suspension culture
Growth parameters of cell cultures of C. polygonoides in liquid MS medium are shown in Figure 2. It showed high growth ratios with an exponential phase between 6th and 14th day of culture. The growth ratios were almost constant after 3 weeks of inoculation.
Figure 2.
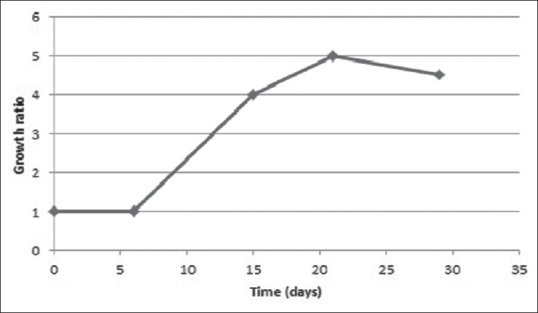
Time growth course of Calligonum polygonoides L. cultured cells cultivated in Murashige and Skoog liquid media. Mean values ± standard error of the mean
Regeneration and production of phenolics
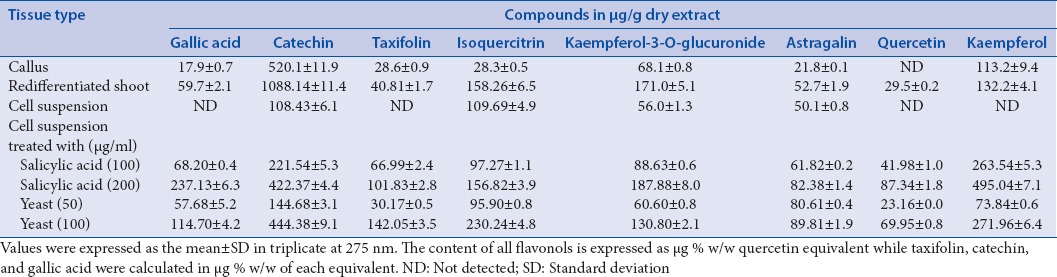
The capacity of callus, redifferentiated shoot, and cell suspension cultures of C. polygonoides to produce secondary metabolites was investigated by determining their phenolic content using HPLC. Levels and profiles of phenolics varied significantly with respect to differentiation state of the cultures [Table 1]. The unorganized callus and cell suspension cultures contained fewer amounts of all phenolic compounds than redifferentiated shoots. Catechin and kaempferol-3-O-glucuronide were the major compounds in regenerated multiple shoots, whereas catechin and kaempferol were the majors in case of callus. On the other hand, isoquercitrin and catechin were prominent in cell suspension culture which did not produce gallic acid, taxifolin, quercetin, and kaempferol.
Table 1.
High-performance liquid chromatography determination of phenolic content in different in vitro cultures of Calligonum polygonoides L.
Effect of elicitors on the production of phenolics
Cell suspension cultures of C. polygonoides undergo massive quantitative reprogramming in response to elicitation with salicylic acid (100, 200 μg/ml), and yeast extracts (50, 100 μg/ml), added to 7-day-old cultures for 48 h [Table 1]. Salicylic acid and yeast extracts induced the accumulation of gallic acid, taxifolin, quercetin, and kaempferol, which was not detected primarily in the untreated control. Incubation of the cells with salicylic acid, at a concentration of 100 μg/ml produced an increase in catechin, kaempferol-3-O-glucuronide, and astragalin levels by approximately 2, 1.5, and 1.2-fold, respectively, whereas an increase of approximately 3.8, 3.3, and 1.6-fold, respectively, was observed when 200 μg/ml salicylic acid was used, in comparison to control cells.
On the other hand, incubation of the cells with crude yeast extract, at a concentration of 50 μg/ml produced an increase in catechin, kaempferol-3-O-glucuronide, and astragalin levels by approximately 1.3, 1, and 1.6-fold, respectively, whereas an increase of approximately 4, 2.3, and 1.7-fold, respectively, was observed when 100 μg/ml crude yeast extract was used, in comparison to control cells. A little decrease in isoquercitrin level was observed when both salicylic acid (100 μg/ml) and yeast extract (50 μg/ml) were used but an increase in its level by approximately 1.6, 2.4-fold was noticed when higher concentrations of salicylic acid and yeast were applied, respectively. Extensive browning and considerable loss of viability were detected in all yeast extract cultures after 48 h. This browning of cells as well as the culture medium is a common feature observed after treating cell suspension cultures with fungal elicitors.[18]
DISCUSSION AND CONCLUSION
The present study provides a strategy for ex-situ conservation of the endangered medicinal plant species, C. polygonoides, through the application of in vitro culture techniques using the fruits as initial explants. Sterilization and germination of these fruits were so difficult due to the structure nature of the fruit. The seed wall adheres with fruit wall which is characterized by dense long hairy and extra rigid solid wooden wall. The difficulty of raising seedlings from seeds may be due to its hard impermeable testa which prevents imbibitions of water and germination.[19] In the current study, several trials were performed on the fruits to overcome these problems and only H2O2 and sulfuric acid treatments succeed. The obtained results conflict with Derbel and Chaieb, 2007, who reported that integumental inhibition was easily eliminated by Clorox. About 45% germination was observed when they used Clorox followed by 30% and 5% for abrasion and boiling water treatments, respectively.[19]
Callus was successfully established from in vitro germinated plantlets. The kind and concentration of plant growth regulators in the medium were very important for shoot induction and differentiation. It is well known that plant regeneration ability from callus is usually enhanced by the synergistic action of auxins and cytokinins. BAP is considered as the most effective plant growth regulator for shoot proliferation and breaking dormancy in several medicinal plant species.[15] It worth to mention that the above-mentioned protocol for micropropagation from the fruit explants into ex vitro plants will strengthen the efforts to conserve such plant and may be other similar species ex-situ.
Callus when cultivated in MS liquid medium produced cell suspension culture which showed stable growth and accumulated phenolic constituents. This phenolic content was higher when the cells were organized into shoots. Our results were in agreement with previous studies where the production of secondary metabolites was higher in differentiated structures than in nondifferentiated cells.[20,21] The expression of secondary metabolic pathways in redifferentiated cultures is actually not surprising because it mimics exactly what the plant does. However, the inconvenience of manipulating plants or parts of them, and relatively slow growth remains the main disadvantage of this plant tissue culture system.[22] To overcome these problems, a great effort has been directed toward increasing secondary metabolite production from cell culture using various techniques such as elicitation.
Elicitation is an effective strategy to enhance the production of secondary metabolites such as alkaloids, terpenoids, flavonoids, and phenolic compounds.[23] Cell suspension cultures are preferred due to its rapid growth cycles. They have been used for generating large amounts of cells for quantitative or qualitative analysis of growth responses and metabolism of novel chemicals.[24] It can ultimately provide a continuous, reliable source of natural products.[13] This was in agreement with our results where the capacity of cell suspension culture to accumulate phenolics was enhanced after application of salicylic acid and yeast extract thus provide a chance to improve yield.
The successful production of phenolic compounds from in vitro cultures may serve as a useful tool for studying the biosynthesis of these compounds and its regulation in plant cells. The flavonoid pathway is part of the larger phenylpropanoid pathway, which produces a range of other secondary metabolites such as phenolic acids, lignins, lignans, and stilbenes. The key flavonoid precursors are phenylalanine, obtained via the shikimate and arogenate pathways, and malonyl-CoA, derived from citrate produced by the TCA cycle.[25] Expression of the phenylpropanoid biosynthetic pathway is precisely regulated in response to developmental signals, nutrient status, and environmental stimuli such as light, heat, and pathogen attack. The induction of phenylpropanoid synthesis under conditions of stress is the result of increased transcription of genes encoding the corresponding biosynthetic enzymes.[26] Elicitors from physical or chemical origin have been widely employed to increase a target natural product formation in plant cell cultures, and this strategy has been effective in stimulating the production of many chemical classes of secondary metabolites including flavonoids. Elicitation has also been employed to dissect plant–microbe interactions and plant defense responses as well as signaling pathways involving elicitors.[27] Salicylic acid can potentiate the elicitation of several defense responses in plants and enhance the accumulation of phenylpropanoid products through elicitation of the phenylpropanoid genes phenylalanine ammonia-lyase and 4-coumarate: CoA ligase.[28] Yeast crude extract can elicit potent defense responses in plant cells through its complex mixture content of chemicals including chitin, N-acetylglucosamine oligomers, β-glucan, glycopeptides, and ergosterol. It can act as a pathogen mimic, induces phenylalanine ammonia-lyase activity and hence, phenolic production.[29] The observed little decrease in isoquercitrin level followed by the increase in its level, when salicylic acid and yeast extract were applied to culture media, might be due to that isoflavonoids occupy a later position than other flavonoids in the biosynthetic pathway leading to their production in C. polygonoides cells.
In conclusion, this experiment with C. polygonoides is another example for the power of tissue culture technique to offers an alternative and renewable source for endangered valuable medicinal plant, provide a good chance to improve secondary metabolite yield and facilitate a better understanding of biosynthetic pathway of these compounds which is mandatory for using biotechnological methods instead of field crops as a basic source of this important pharmaceutical raw material. Our results are reported for the first time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Asmaa I. Owis
Asmaa I. Owis, is a Lecturer at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt. Her research of interest is in the area of pharmacognosy, biotechnology, phytochemistry and natural products research. Additionally, she is the Deputy Director for Quality Assurance Unit, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt.
REFERENCES
- 1.Badria FA, Ameen M, Akl MR. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Z Naturforsch C. 2007;62:656–60. doi: 10.1515/znc-2007-9-1005. [DOI] [PubMed] [Google Scholar]
- 2.Nawash OS, Al-Horani AS. The most important medicinal plants in Wadi Araba desert in South West Jordan: A review article. Adv Environ Biol. 2011;5:418–25. [Google Scholar]
- 3.Shah A, Marwat SK, Gohar F, Khan A, Bhatti KH, Amin M, et al. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal & Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces) Pakistan Am J Plant Sci. 2013;4:98–116. [Google Scholar]
- 4.El-Hawary Z, Kholief T. Biochemical studies on some hypoglycaemic agents (II) effect of Calligonum comosum extract. Arch Pharm Res. 1990;13:113–6. [Google Scholar]
- 5.Zaki D, Abd-El-Aziz M, El-Gengeihy S, Morsi N. Antimicrobial potentialities of some Egyptian desert plants. Herb Hung. 1984;23:73–84. [Google Scholar]
- 6.Liu XM, Zakaria MN, Islam MW, Radhakrishnan R, Ismail A, Chen HB, et al. Anti-inflammatory and anti-ulcer activity of Calligonum comosum in rats. Fitoterapia. 2001;72:487–91. doi: 10.1016/s0367-326x(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 7.Al-Abrahim JS, Mohammed AE, Elobeid MM. Assessment of in vitro anti-fungal potential of ethanolic extract of Calligonum comosum against two fungal postharvest pathogens of fruits and vegetables in Saudi Arabia. IJABPT. 2014;5:90–4. [Google Scholar]
- 8.El-Hag E, Harraz F, Zaytoon A, Salama A. Evaluation of some wild herb extracts for control of mosquitoes, (Culicidae, Diptera) J King Saud Univ. 1996;8:135–45. [Google Scholar]
- 9.Samejo MQ, Memon S, Bhanger MI, Khan KM. Isolation and characterization of steroids from Calligonum polygonoides. J Pharm Res. 2013;6:346–9. [Google Scholar]
- 10.Ahmed H, Moawad A, Owis A, AbouZid S, Ahmed O. Flavonoids of Calligonum polygonoides and their cytotoxicity. Pharmaceutical Biology. 2016 doi: 10.3109/13880209.2016.1146778. DOI: 10.3109/13880209.2016.1146778. [DOI] [PubMed] [Google Scholar]
- 11.Vyas GK, Kumar V, Sharma R, Sharma RA, Sharma S, Singh JP, et al. Chemical and genetic diversity among some wild stands of Calligonum polygonoides (Polygonaceae) from the Thar Desert of Rajasthan. Rev Biol Trop. 2012;60:1097–108. doi: 10.15517/rbt.v60i3.1760. [DOI] [PubMed] [Google Scholar]
- 12.Hussain A, Nazir H, Ullah I, Qarshi IA. Plant tissue culture: Current status and opportunities. In: Annarita L, Laura MRR, editors. Recent Advances in Plant in vitro Culture. Croatia: InTech; 2012. pp. 1–28. [Google Scholar]
- 13.Vanisree M, Lee CY, Lo SF, Nalawade SM, Lin CY, Tsay HS. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sin. 2004;45:1–22. [Google Scholar]
- 14.Namdeo A. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn Rev. 2007;1:69–79. [Google Scholar]
- 15.Sadeq MA, Pathak MR, Salih AA, Abido M, Abahussain A. Effect of plant growth regulators on regeneration of the endangered medicinal plant Calligonum comosum L. Henry in the Kingdom of Bahrain. Afr J Biotechnol. 2014;13:2513. [Google Scholar]
- 16.Al-Khalifah NS. Saudi Arabia King, Abdulatziz City: 2004. The Role of Biotechnology in Developing Plant Resources in Deserts Environment. Proceedings of 1st International Conference on Water Resources and Arid Environment (WRAE04); 5-8 December 2004. [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. [Google Scholar]
- 18.Ramawat KG. Biotechnology: Secondary Metabolites. 2nd ed. New York: CRC; 2007. [Google Scholar]
- 19.Derbel S, Chaieb M. Germination behaviour and seedling establishment of two desert shrubs, Calligonum polygonoides (Polygonaceae) and Spartidium saharae (Fabaceae), under experimental conditions. Acta Bot Gallica. 2007;154:533–44. [Google Scholar]
- 20.Lindsey K, Yeoman M. The relationship between growth rate, differentiation and alkaloid accumulation in cell cultures. J Exp Bot. 1983;34:1055–65. [Google Scholar]
- 21.Wink M. Why do lupin cell cultures fail to produce alkaloids in large quantities? Plant Cell Tissue Organ Cult. 1987;8:103–11. [Google Scholar]
- 22.Arnason JT, Mata R, Romeo JT. Phytochemistry of Medicinal Plants. 1st ed. New York: Springer Science & Business Media; 2013. [Google Scholar]
- 23.Ali MB, Yu KW, Hahn EJ, Paek KY. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006;25:613–20. doi: 10.1007/s00299-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 24.Mulabagal V, Tsay HS. Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng. 2004;2:29–48. [Google Scholar]
- 25.Andersen OM, Markham KR. Flavonoids: Chemistry, Biochemistry and Applications. New York: CRC; 2005. [Google Scholar]
- 26.França S, Roberto P, Marins M, Puga R, Rodrigues A, Pereira J. Biosynthesis of secondary metabolites in sugarcane. Genet Mol Biol. 2001;24:243–50. [Google Scholar]
- 27.Sánchez-Sampedro A, Kim HK, Choi YH, Verpoorte R, Corchete P. Metabolomic alterations in elicitor treated Silybum marianum suspension cultures monitored by nuclear magnetic resonance spectroscopy. J Biotechnol. 2007;130:133–42. doi: 10.1016/j.jbiotec.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Thulke O, Conrath U. Salicylic acid has a dual role in the activation of defence-related genes in parsley. Plant J. 1998;14:35–42. doi: 10.1046/j.1365-313X.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari S. Biological elicitors of plant secondary metabolites: Mode of action and use in the production of nutraceutics. In: Giardi M, Rea G, Berra B, editors. Bio-Farms for Nutraceuticals. US: Springer; 2010. pp. 152–66. [DOI] [PubMed] [Google Scholar]