Abstract
Background:
α-glucosidase inhibitors controls postprandial hyperglycemia (PPHG) by lowering sharp rise in blood glucose levels after ingestion of carbohydrate rich meal in type 2 diabetic (T2D) individuals. Acalypha indica commonly known as Indian copper leaf is used in traditional medicinal system to treat various diseases. In our previous in-vitro investigation, methanolic extract of A. indica stems (AIS) proved to be an effective a-glucosidase inhibitor, antioxidant, and well tolerated in acute and subchronic toxicity studies in albino wistar rats
Objective:
In this perspective, this study was designed to evaluate postprandial antihyperglycemic potential of AIS in maltose, sucrose, and glucose loaded streptozotocin (STZ)-induced normal and diabetic rats. As, the acute hyperglycemia at postprandial period has more triggering effect on oxidative stress, study was also aimed to evaluate the antioxidant potential of AIS on STZ-induced Albino–Wistar rats.
Materials and Methods:
Rats were treated with AIS (300–600 mg/kg b.w.) to investigate effect of AIS in controling PPHG after carbohydrate loading. Hepatoprotective activity of AIS is evaluated in diabetic rats by treating them at the dosages 300–600 mg/kg b.w.
Results:
Studies revealed 69.10 and 80.35% blood glucose-lowering effect of AIS in maltose and sucrose loaded diabetic rats in comparison with the diabetic control group. AIS recovered the liver damage caused by streptozotocin
Conclusion:
The present study confirmed high potential of AIS in controling PPHG by inhibiting a-glucosidase enzyme in maltose and sucrose loaded diabetic rats. AIS also exhibited hepatoprotective activity in STZ-induced diabetic rats. Thus, AIS could be used as a nutraceutical supplement to treat T2D effectively.
SUMMARY
AIS extract is effective in suppressing maltose and sucrose-induced postprandial hyperglycemic spikes in rats
AIS treat ment showed a 69.10 and80.35% blood glucose-lowering effect in maltose and sucrose loaded diabetic rats in comparison with the diabetic control group.
AIS also improved the antioxidant status in diabetic rats and also has recovered the liver damage caused by streptozotocin.
The α-glucosidase inhibitor isolated from AIS is a good supplement to control postprandial blood glucose level in the management of type 2 diabetes.
Abbreviations used: AIS: Acalypha indica Stems, ALP: Alkaline Phosphatase, b/w: Body Weight, PPHG: Postprandial hyperglycemia, SE: Standard Error, SGOT: Serum glutamate oxaloacetate transaminase, SGPT: Serum glutamate pyruvate transaminase, SOD: Superoxide dismutase, STZ: Streptozotocin, TB: Total Bilirubin, T2D: Type 2 diabetes
Keywords: Albino–Wistar rats, Acalypha indica, antioxidant, a-glucosidase inhibitor, postprandial hyperglycemia
INTRODUCTION
Diabetes mellitus is a condition in which there is an impaired function in carbohydrates, protein, and lipid metabolism due to the deficiency in insulin secretion or ineffectiveness of insulin by the pancreas leading to increased levels of fasting and postprandial blood glucose levels. The prolonged condition of these imbalanced metabolic processes within the body results initially in hyperglycemia, which in turn periodically develops into a syndrome known as diabetes mellitus.[1] Diabetes is classified into two main classes on the basis of the origin. Type 1 diabetes or insulin-dependent diabetes mellitus (IDDM) and Type 2 diabetes or noninsulin-dependent diabetes mellitus. Type 1 diabetes (T1D) is a catabolic disorder most commonly seen in the children and young adults in developing countries. T1D results when the circulating insulin is absent, plasma glucagon is elevated, and beta cells of the pancreas fail to respond to insulinogenic stimuli. People suffering from T1D are about 5–10% all over the world. Type 2 diabetes (T2D) occurs when the insulin receptors within the body cells are incapable of the uptake of insulin in insulin signaling pathway and as a result, glucose is unable to enter into the cells leading to high concentration of glucose in the blood stream. It is the most common form of diabetes and 90% of people are with T2D all over the world.[2] India has the highest number of diabetes cases in the world. There are 62 million people with diabetes in India and that may reach to 79.4 million at the end of 2030.[3,4] Postprandial hyperglycemia plays a crucial role in the progress of T2D and its associated microvascular and macrovascular complications.[5] Mammalian a-glucosidase is a key enzyme present in the brush border of the small intestine that helps in the breakdown of dietary carbohydrates such as maltose and sucrose by the hydrolysis of terminal glyosidic bonds at the nonreducing end of the saccharide polymers to release glucose.[6] The hindering of postprandial hyperglycemia could be an important strategy in the type 2 diabetic therapies. Inhibition of a-glucosidase enzyme by the action of a-glucosidase inhibitor delays the postprandial glucose elevation and consequently can be a major therapeutic approach for the type 2 diabetes treatment. Acarbose, voglibose, and miglitol are the synthetic a-glucosidase inhibitors that are available commercially in the market, which acts effectively in controling sharp rise in blood glucose level that occur immediately after the intake of meals. However, prolonged use of these synthetic medicines causes hepatotoxicity, flatulence, diarrhea, abdominal discomfort, and hypoglycemia.[7] At present, there is a renewed interest in the practice of traditional system of medicine in developing countries to treat diseases that comprise economic problems such as diabetes because drugs of herbal origin are inexpensive, effective, and with no side effects. Subsequently, medicinal plants gained scientific acceptability for efficacious treatment of diabetes mellitus.[8]
Medicinal plants play an important role in disease hindrance and control because of the antioxidant property of the chemical constituents within them that are broadly termed as polyphenolic compounds. The antioxidant nature of the polyphenolic compounds is because of their perfect structural chemistry and high reactivity that allow them to act as free radical scavengers, hydrogen donors, reducing agents, metal chelators, and quenchers of singlet oxygen.[9] Polyphenols present in plants have been reported to reduce oxidative stress and also contribute to the carbohydrate hydrolyzing enzyme inhibitor activities.[10,11] A good approach in the management of diabetes as a whole is with plants that exhibit enzyme inhibitory and antioxidant activities.[12]
A. indica is commonly known as Indian copper leaf belongs to the family Euphorbiaceae is an erect annual weed plant growing to a height of 60 cm. It grows extensively in most of the regions of Asian continent, including India, Pakistan, Yemen, the southern districts of China, and South Africa.[13] Stems are longitudinally ribbed and pubescent. Leaves are ovate with toothed margins except near the base. Flowers are unisexual with axillary spikes. Fruits are tuberculate, three lobed, and pubescent. A. indica as a whole or its parts were used widely as phytomedicine in tropical and subtropical countries. The whole plant of A. indica is used in traditional medicinal system to treat various diseases. In Ayurveda, A. indica used as a laxative, antihelmintic, emetic, expectorant, and also used to treat scabies, earache, syphilitic ulcers, and snake bites.[14] In Siddha medicine, A. indica is used to treat diseases associated with teeth and gums, stomach ache, irritations, burns, wheezing, and respiratory diseases.[15] This plant has been studied for antidiabetic, wound healing, postcoital infertility, antivenom, antiulcer, antimalarial, antiinflammatory, antimicrobial, alpha amylase inhibitor, neuroprotective, and antioxidant activities.[16,17,18,19,20,21,22,23,24,25,26,27] However, there are no scientific reports on the postprandial antihyperglycemic and antioxidant activities of A. indica stems (AIS). Hence, this study focused on postprandial antihyperglycemic and antioxidant activities of methanolic stem extract of A. indica Linn.
MATERIALS AND METHODS
Plant material
Stems of A. indica were collected from Seshachalam forest area (40 35’02’N7943’19’N), Chittoor district, Andhra Pradesh, India. The collected plant material was brought to the molecular microbiology laboratory, VIT University, Vellore and their identification was confirmed by Dr. P. Jayaraman, Director, Institute of herbal botany, Plant Anatomy Research Centre, Chennai, India with herbarium no: PARe/2013/2146.
Sequential extraction of the collected sample
The collected plant material was rinsed under tap water and then by distilled water. The plant material was shade dried, homogenized into a fine powder using a mechanical grinder and extracted serially with the solvents in ascending order of their polarity with hexane, chloroform, methanol, and water using a Soxhlet apparatus. The extract obtained was then concentrated in rotary evaporator and stored in airtight container at 4°C in a refrigerator.
Animals used for the study
Adult male albino–Wistar rats of 12-weeks old, weighing 150–200 g were accommodated in Animal house Centre for Biomedical Research, VIT University, Vellore, Tamil Nadu, India. Animals were housed in polycarbonate cages and were maintained in a room at 20 ± 2°C with 12 h light to dark cycle. The animals were fed with standard rodent diet obtained from Hindustan Lever Ltd (Mumbai, India) and had free access to water ad libitum. The experimental protocols were approved by the ethical committee in accordance with institutional Animal Ethical Committee, 1333/C/10/CPCSEA.
Induction of diabetes
Rats previously fasted for 16 h received a single intraperitoneal injection of Streptozotocin (STZ) at a dosage of 45 mg/kg body weight (b.w.) dissolved in 0.1 M citrate buffer (pH 4.5). After three days of STZ induction, fasting blood glucose levels was checked and the rats with fasting blood glucose level over 250 mg/dl were considered as diabetic and, thus, used as experimental models.
Administration of test material
Rats were deprived of food (16 h) but had free access to water before the experimentation. Overnight fasted rats received test material orally by intragastric route at 300–600 mg/kg b.w. in a volume of 1 ml of distilled water.
Experimental design
The postprandial antihyperglycemic effect of the potent extract was evaluated against carbohydrates challenged Albino–Wistar rats in normal and STZ-induced diabetic rats.[28] In-vivo antioxidant activity of the plant extract was evaluated against STZ-induced hepatotoxicity in Albino–Wistar rat model.[29]
Maltose and sucrose loading in normal rats
Rats with normal blood glucose levels were segregated into four groups of six animals in each group. After 16 h of fasting, Group I was administered with maltose (2 g/kg b.w.) and served as a normal control. Group 2 was coadministered with maltose (2 g/kg b.w.) and AIS (300 mg/kg b.w.) served as a treated group. Group 3 was coadministered with maltose (2 g/kg b.w.) and AIS (600 mg/kg b.w.), which was also served as treated group. Group 4 received maltose (2 g/kg b.w.) and Acarbose (5 mg/kg b.w.) was served as standard control group. Selected dosages were reported to be well tolerated as per the previous studies on whole plant ethanolic extracts of A. indica.[30] The levels of blood glucose were determined before and after 30, 60, and 120 min of maltose loading using a Glucometer (One Touch HorizonTM). The variation of blood glucose levels from the basal level were analyzed and represented as delta blood glucose. The same experimental design was followed for sucrose loaded normal rat model.
Maltose and sucrose loading in diabetic rats
Rats with fasting blood glucose levels over 250 mg/dL were segregated into four groups of six animals in each group. After 16 h of fasting, Group 1 was administered with maltose (2 g/kg b.w.) and served as a diabetic control. Group 2 was coadministered with maltose (2 g/kg b.w.) and AIS (300 mg/kg b.w.) served as a treated group. Group 3 was coadministered with maltose (2 g/kg b.w.) and AIS (600 mg/kg b.w.), which was also served as treated group. Group 4 received maltose (2 g/kg b.w.) and Acarbose (5 mg/kg b.w.) was served as standard control group. Blood glucose levels were measured before and after 30, 60, and 120 min of maltose loading by using Glucometer (One Touch HorizonTM). The alteration in blood glucose levels from the basal level was analyzed and represented as delta blood glucose. The same experimental design was followed for sucrose loaded diabetic rat model.
Glucose loading in normal rats
Rats with normal blood glucose level were allocated into two groups of six animals in each group. Group 1 received glucose (2 g/kg b.w.) and served as a normal control wherein as Group 2 was coadministered with glucose (2 g/kg b.w.) and AIS (300 mg/kg b.w.), which served as treated group. Blood glucose concentration was measured before and after 30, 60, and 120 min of glucose loading by commercially available Glucometer (One Touch HorizonTM). The change in blood glucose concentrations from the basal level were analyzed and represented as delta blood glucose.
Glucose loading in diabetic rats
Rats with a blood glucose level of 250 mg/dl and above were segregated into two groups of six animals in each group. Group 1 received glucose (2 g/kg b.w.) and served as a diabetic control wherein as Group 2 was coadministered with glucose (2 g/kg b.w.) and AIS (300 mg/kg b.w.), which served as treated group. The levels of blood glucose were determined before and after 30, 60, and 120 min of glucose loading by using Glucometer (One Touch HorizonTM). The change in blood glucose concentrations from the basal level were analyzed and represented as delta blood glucose.
In-vivo antioxidant studies
Rats were segregated into four groups of six animals in each group. Group 1 received saline and served as normal control. Group 2 to Group 4 were given a single toxic dosage of STZ (45 mg/kg b.w.) by intraperitoneal injection. Group 2 served as an experimental control. Group 3 received AIS (300 mg/kg b.w.) and served as a treated group. Group 4 received Metformin (10 mg/kg b.w.) and served as standard drug treated group. Treatment was started after three days of STZ induction and was carried out for seven days. On the last day of the experimental period, rats were euthanized with diethyether, sacrificed and the blood samples were collected by cardiac puncture. The collected blood samples were transferred into a sterile, nonheparinized centrifuge tube. The Liver of the animals were dissected, washed with ice cold phosphate buffer saline (pH 7.4) and processed for further experimentation.
Estimation of enzymatic antioxidant levels in the liver
A 10% w/v liver homogenates were prepared using phosphate buffer saline. Thus, obtained liver homogenates were used to estimate the levels of enzymatic antioxidants by performing catalase, superoxide dismutase (SOD) and glutathione peroxidase activities. Catalase activity was performed following the method of Sinha.[31] The method of Marklund and Marklund[32] was followed for evaluating superoxide dismutase activity and Glutathione peroxidise assay was carried out as per the protocol of Rotruk et al.[33]
Assessment of liver marker enzymes
To evaluate the levels of liver marker enzymes, rat blood was collected by cardiac puncture. The collected blood was then allowed for clotting. Once the blood was clotted, centrifugation was carried out at 2500 rpm for 5 min. The supernatant containing serum was collected and was analyzed for liver marker enzymes such as serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), and total bilirubin (TB). All liver marker enzymes in serum were assayed using biochemistry semiautomatic Analyzer (3000 Evolution, Tulip Group, India).
Statistical analysis
All the results were expressed as mean ± SE (n = 6) for in-vivo studies. Statistical analysis of the data was done with Microsoft Excel 2007 (Roselle, IL, USA) and Graphpad prism software. In the Graphpad prism software, results were analyzed using t-test or one-way analysis of variance followed by Dunnett's Multiple Comparison Test. P values of less than 0.05 were considered to be statistically significant.
RESULTS AND DISCUSSION
The diabetes treatment is aimed not only to control fasting blood glucose levels, but also to lower postprandial blood glucose levels into normalcy. Only most of the previous works on herbal medicine were reported to possess control over fasting blood glucose levels and causes very less effect in controling postprandial blood sugar levels.[34] Consequently, studies have made on carbohydrate hydrolyzing enzyme inhibitors and found that inhibition of a-glucosidase enzyme delays the carbohydrate digestion, thus, reducing postprandial blood glucose levels.[35] There is a renewed interest in the usage of medicinal plants as a-glucosidase inhibitors because of the side effects associated with chronic consumption of synthetic a-glucosidase inhibitors.[36] Hence, this study aimed to evaluate the potential of AIS to control postprandial blood glucose levels after maltose, sucrose, and glucose load in normal and streptozotocin-induced diabetic rats.
Maltose loading in normal rats
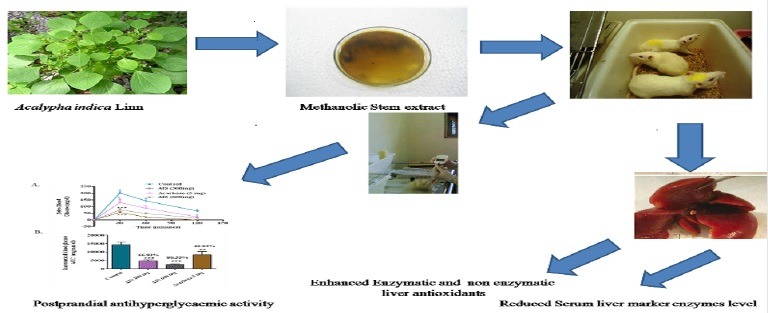
In the control group, delta blood glucose level increased to an average of 55 mg/dl at 30 min after the maltose load. In the group that received an AIS extract along with maltose, the 30 min postload glucose level increased only marginally by 15.5 mg/dl on an average. This indicates the potency of the AIS extract to significantly suppress high maltose diet associated postprandial glucose elevation. Compared with the control group, the area under the curve (AUC) of AIS treated rats has been significantly reduced by 70.41% shown in the Figure 1.
Figure 1.
a-glucosidase enzyme inhibitor potential of A. indica stems on blood glucose levels challenged with maltose load in normal rats (a). The glycemic response curve after maltose challenge; (b). The incremental AUC0-120 min in normal rats after maltose administration; data expressed as mean ± SE, n = 6.
Maltose loading in diabetic rats
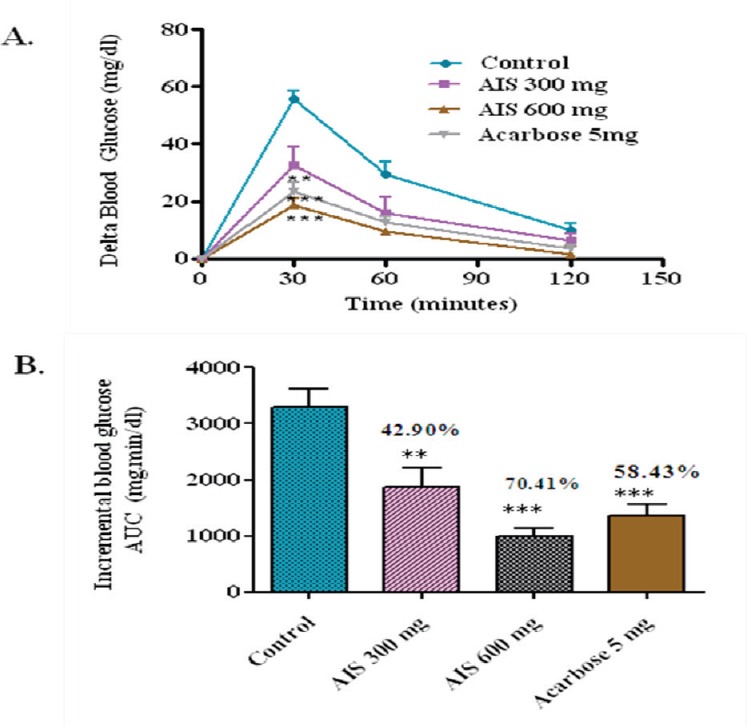
The results clearly showed ameliorated postprandial hyperglycemia upon treatment with AIS in the Figure 2. Compared with controls, the whole glycemic response is reduced significantly by 56.47, 69.10, and 44.87% when treated with 300–600 mg/kg b.w. of AIS and 5 mg/kg b.w. of acarbose, respectively.
Figure 2.
a-glucosidase enzyme inhibitor potential of A. indica stems on blood glucose levels challenged with maltose load in diabetic rats (a). The glycemic response curve after maltose challenge; (b). The incremental AUC0-120 min in diabetic rats after maltose administration; data expressed as mean ± SE, n = 6.
Sucrose loading in normal rats
In the control group, the delta blood glucose level increased to an average of 33.33 mg/dl at 30 min after the sucrose load. In the group that received an AIS extract along with sucrose, the 30 min postload glucose level increased only by 12.5 mg/dl on an average. This indicates the potency of the AIS extract to significantly suppress high sucrose diet associated postprandial glucose elevation. Compared with control, the whole glycemic response is reduced by 52.7% in AIS treatment and 40.09 % of Acarbose treatment (Figure 3).
Figure 3.
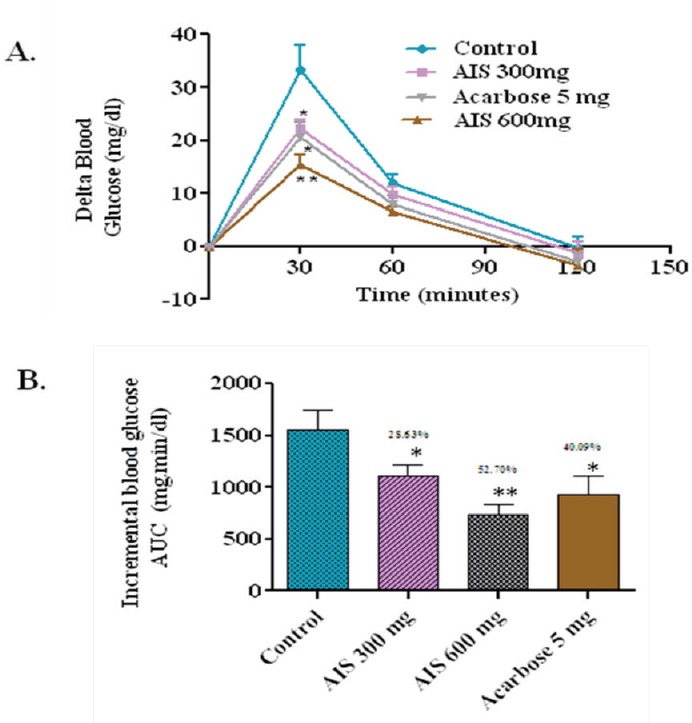
a-glucosidase enzyme inhibitor potential of A. indica stems on blood glucose levels challenged with sucrose load in normal rats (a). The glycemic response curve after sucrose challenge; (b). The incremental AUC0-120 min in normal rats after sucrose administration; data expressed as mean ± SE, n = 6.
Sucrose loading in diabetic rats
The effect of AIS on sucrose loaded diabetic rats was checked. Compared with control, the whole glycemic response is reduced by 66.92, 80.35, and 40.93% when treated with 300, 600 mg/kg b.w. of AIS and 5 mg/kg b.w. of acarbose, respectively [Figure 4].
Figure 4.
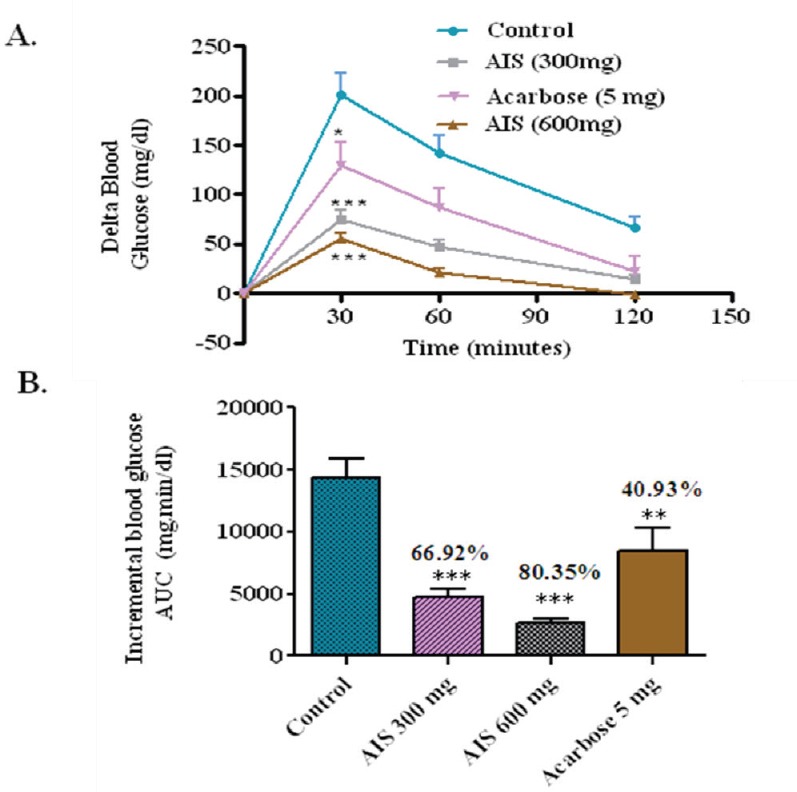
a-glucosidse enzyme inhibitor potential of A. indica stems on blood glucose levels challenged with sucrose load in diabetic rats (a). The glycemic response curve after sucrose challenge; (b). The incremental AUC0-120 min in normal rats after sucrose administration; data expressed as mean ± SE, n = 6.
Glucose loading in normal rats
To affirm that the observed suppression of postprandial glucose is due to the inhibition of a-glucosidase, postprandial blood glucose variation was measured after glucose loading in the normal rats with and without the coadministration of AIS extract. In the control group, the delta blood glucose level increased to an average of 19.1 mg/dl at 30 min after the glucose load. In the group that received an AIS extract along with glucose, the 30 min postload glucose level increased only marginally by 20.6 mg/dl on an average, which shows that the glucose absorption is not significantly affected due to AIS extract given in the Figure 5.
Figure 5.
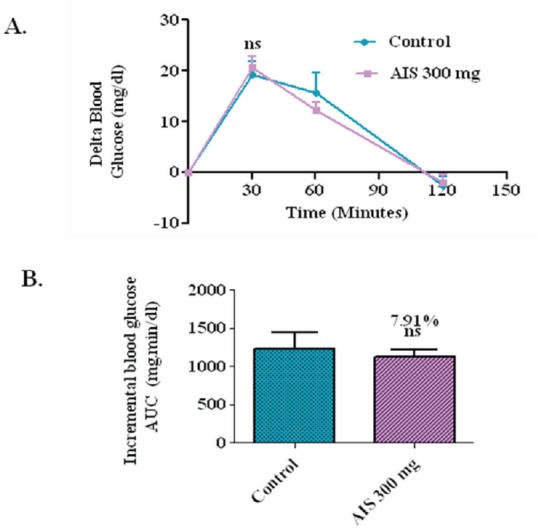
a-glucosidase enzyme inhibitor potential of A. indica stems on blood glucose levels challenged with glucose load in normal rats (a). The glycemic response curve after sucrose challenge; (b). The incremental AUC0-120 min in normal rats after sucrose administration; data expressed as mean ± SE, n = 6.
Glucose loading in diabetic rats
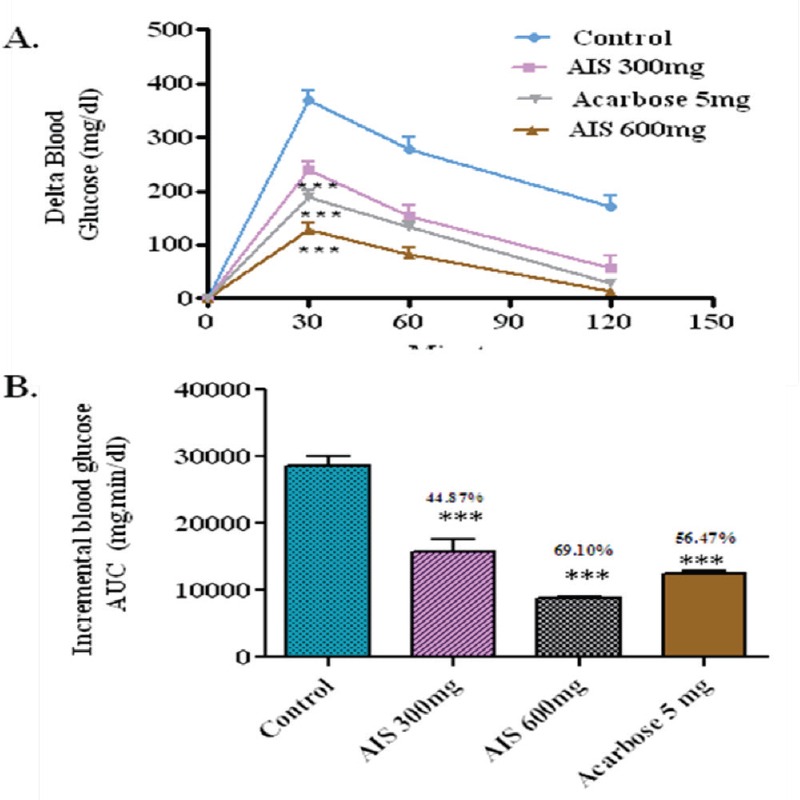
To evaluate the effect of the AIS extract on glucose tolerance in diabetic condition and to elucidate whether the observed postprandial glucose suppression is majorly due to a-glucosidase inhibition, postprandial blood glucose variation was measured after glucose loading in the diabetic rats with and without the coadministration of AIS extract. In the control group, the delta blood glucose level increased to an average of 375.8 mg/dl at 30 min after the glucose load. In the group that received an AIS extract along with glucose, the 30 min postload glucose level increased only marginally by 352 mg/dl on an average, which shows that the glucose absorption is not significantly affected due to AIS extract (Figure 6). As AIS significantly suppressed postprandial hyperglycemia in diabetic rats loaded with maltose, sucrose but not glucose. This indicates the potency of AIS in suppressing the high maltose and sucrose diet associated postprandial glucose elevation. Thus, the present study confirmed the observed suppression of postprandial glucose is due to the inhibition of a-glucosidase in glucose loaded normal and diabetic model. These results ensured the mechanism of AIS as a-glucosidase inhibitor.
Figure 6.
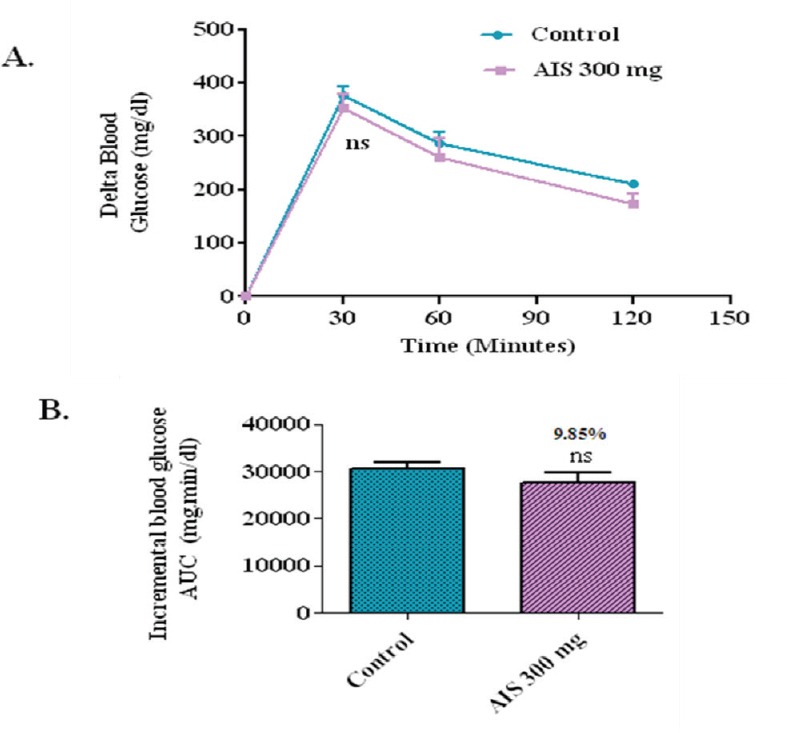
a-glucosidase enzyme inhibitor potential of A. indica stems on blood glucose levels challenged with glucose load in diabetic rats (a). The glycemic response curve aftersucrose challenge; (b). The incremental AUC0-120 min in normal rats after sucrose administration; data expressed as mean ± SE, n = 6.
In-vivo antioxidant studies
ROS-Reactive Oxygen Species are extremely reactive oxidants initiating the onset of various diseases including diabetes mellitus. Studies of oxidative stress and diabetes prevails that ROS is involved in the causation of diabetes and its related complications. Enzymatic and nonenzymatic antioxidants act as a first line of -defense against ROS. The levels of enzymatic antioxidants namely SOD, catalase and reduced glutathione are usually very low in the type 2 diabetic individuals.[37] This condition is mainly due to protein glycation that mediates conformational changes in the function of antioxidant enzymes. Recent report demonstrates that streptozotocin causes diabetes by free radical generation leading to the oxidative damage of various organs like pancreas, liver, and kidney.[38] Therefore, in the present study streptozotocin was used as a diabetogenic agent in Albino–Wistar rats. The liver is selected for the present investigation because of its importance in metabolic functions in the body. Thus, hepatic injury leads to the hindering of normal physiological and metabolic functions in the body. As a result, complications occur within the body that is even fatal to humans. In accordance with this, innumerable herbs have been reported for hepatoprotective activity earlier, wherein these herbs protected the liver from streptozotocin-induced liver damage by improving antioxidant status.[29,39] In the view of these reports, AIS was evaluated for antioxidant property in the liver of streptozotocin-induced Albino–Wistar rats.
Clinical findings
During the clinical observation, no sign of toxicity or any disorder was observed in the rats included in normal control group (Group 1). The rats included in diabetic control group (Group 2) exhibited weakness, inactiveness, and unresponsive to external stimuli after 12 h of treatment. A significant decrease in the body weight was observed, however, all the rats survived during the experimental period. The rats included in the treatment groups (Group 3 and 4) exhibited weakness, inactiveness, and unresponsive to external stimuli after 12 h of treatment, however, recovered to normal in consecutive days. A similar pattern was observed in case of body weight
Biochemical parameters
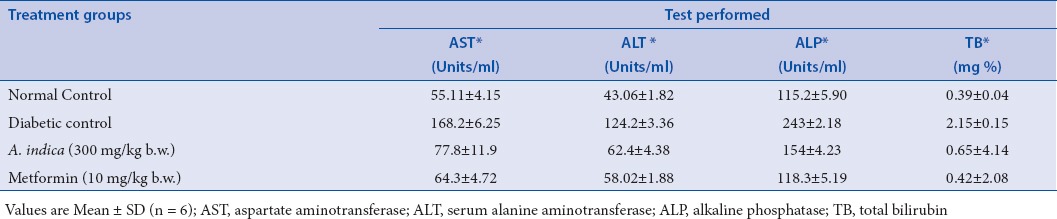
In diabetic control group, there was an absolute lowering of enzymatic and nonenzymatic antioxidant levels in comparison with the normal control group. Whereas in the diabetic rats treated with AIS (300 mg/kg b.w.) extract, there was a significant enhancement of antioxidant status and reached closer to the levels of normal control group. The efficacy of the AIS extract was compared with the standard drug Metformin (Tables 1 and 2). Raised levels of liver marker enzymes, namely, SGOT, SGPT, ALP, and TB were observed in serum collected from an experimental control group of rats (Group 2). The raised level of marker enzymes reflected the pathological alteration in biliary flow. These enzymes are generally present in the cytoplasm of the hepatic cells, streptozotocin-induced damage of hepatocytes resulted in the excessive release of liver marker enzymes in systemic circulation. Extract treatment resulted in substantial hepatoprotective activity in the treated rat groups in a dose-dependent manner. Treatment of rats with AIS significantly (P < 0.05) reduced the levels of liver marker enzymes in the serum (Group 3). These observations suggest that the extract treatment protected the membrane integrity of the liver cells against streptozotocin-induced leakage of liver marker enzymes into the blood circulation. The results were given in the Table 3. Hence, in the present study also proved the efficacy of AIS in recovering the lowered antioxidant enzymatic status in diabetic rats to normal status by abolishing streptozotocin, the initiator of oxidative stress. These findings are in agreement with the earlier research on the antidiabetic and antioxidant activity of Bassilla rubra and Salscia oblonga.[29,40]
Table 1.
Effect of methanolic stem extract of A. indica on liver superoxide dismutase, Catalase, glutathione peroxidase activities of the control and treated groups
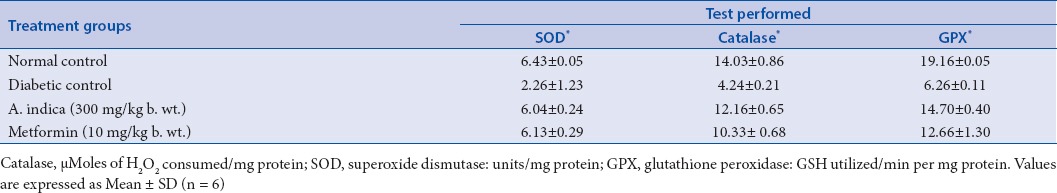
Table 2.
Effect of methanolic stem extract of A. indica on Plasma vitamin C, vitamin E, and reduced glutathione levels of control and treated groups
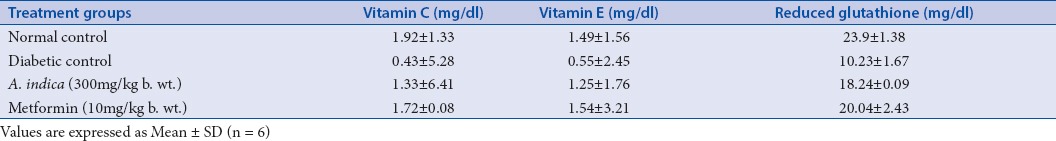
Table 3.
Effect of methanolic stem extract of A. indica on serum aspartate aminotransferase, serum alanine aminotransferase, alkaline phosphatase and total bilirubin activities of the control and treated groups
CONCLUSION
The results of the present study revealed that AIS is effective in suppressing maltose and sucrose-induced postprandial hyperglycemic spikes in rats, as well as improved the antioxidant status in diabetic rats and also has recovered the liver damage caused by streptozotocin. Thus, a-glucosidase inhibitor could be isolated from AIS and used as a good supplement to control postprandial blood glucose level in the management of type 2 diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgmemt
The authors wish to thank the Management and Staff of VIT University, Vellore, TN, India for providing necessary facilities to carry out this study.
REFERENCES
- 1.Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of status phytochemicals: Present and future prospects. Curr Sci. 2002;83:30–38. [Google Scholar]
- 2.Center for Disease Control. National Diabetes Factsheet. 2007. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf .
- 3.Kumar A, Goel MK, Jain RB, Khanna P, Chaudhary V. India towards diabetes control: key issues. Australas Med J. 2013;6:4–31. doi: 10.4066/AMJ.2013.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiting DR, Guariguata L, Weil C, Shawj IDF. Diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:4–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Park MH, Han JS. Hypoglycemic effect of Padina arborescens extract in streptozotocin-induced Diabetic Mice. Prev Nutr Food Sci. 2012;17:4–44. doi: 10.3746/pnf.2012.17.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee A, Sengupta S. Indian medicinal plants known to contain intestinal glucosidase inhibitors also inhibit pancreatic lipase activity-An ideal situation for obesity control by drugs. Indian J. Biotech. 2013;12:32–39. [Google Scholar]
- 7.Laar FA, Lucassen PL, Akkermans RP, Lisdonk EH, Rutten GE, Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–63. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 8.Balunas MJ, Kinghor AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–41. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Demiray S, Pintado ME. Castro PML, Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad Sci Eng Tech. 2009;54:312–17. [Google Scholar]
- 10.Kumar S, Narwal S, Prakash O. a- glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn Rev. 2011;5:19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adefegha SA, Oboh G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. and Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pac J Trop Biomed. 2012;2:774–81. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulati V, Harding IH. Palombo EA, Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complem Altern Med. 2012;12:1–9. doi: 10.1186/1472-6882-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran J. The First 3D Book on Herbs. 1st edition. Chennai, India: Murugan Pathipagam publishers; 2008. Herbs of Siddha Medicine. [Google Scholar]
- 14.Jagatheeswari D, Deepa J, Sheik JAH, Ranganathan P. Acalypha indica L – an important medicinal plant: a review of its traditional uses, and pharmacological properties. Intern J Res in Botany. 2013;3:19–22. [Google Scholar]
- 15.Alagesaboopathi C. Ethnomedicinal plants and their utilization by villagers in Kumaragiri hills of Salem district of Tamilnadu, India. Afr J Tradit Complement Altern Med. 2009;6:222–27. doi: 10.4314/ajtcam.v6i3.57157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begum DT, Kumar KM, Sravanthi P, Prasad KS, Ravindrareddy K. Evaluating phytochemical and antidiabetic activity of ethanolic extract of whole plant of Acalypha indica Linn on alloxan induced diabetic Rats. J Pharm Res. 2011;4:1704–06. [Google Scholar]
- 17.Reddy JS, Rao PR, Reddy MS. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. J Ethnopharmacol. 2002;79:249–51. doi: 10.1016/s0378-8741(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 18.Shivayogi P, Hiremath K, Rudresh Shrishailappa B, Saraswati BP, Somanth RP. Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol. 1999;67:253–58. doi: 10.1016/s0378-8741(98)00213-x. [DOI] [PubMed] [Google Scholar]
- 19.Shirwarikar A, Rajendran K, Bolda R, Kumar CD. Neutralization potential of Viper russelli (Russell’s viper) venom by ethanol leaf extract of Acalypha indica (L.) J Ethnopharmacol. 2004;94:267–73. doi: 10.1016/j.jep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Kalimuthu S, Rajesh P, Rajesh Kannan V, Balamurugan B, Chandrasekar TM. Antiulcer activity of methanolic extract of Acalypha indica Linn.(Euphorbiaceae) by pylorous ligture and swim stress induced ulceration. J Pharm Res. 2010;3:2779–83. [Google Scholar]
- 21.Govindarajan M, Jebanesan A, Pushpanathan T, Samidurai K. Studies on effect of Acalypha indica L.(Euphorbiaceae) leaf extracts on the malarial vector, Anopheles stephensi Liston (Diptera: Culicidae) Parasitol Res. 2008;103:691–95. doi: 10.1007/s00436-008-1032-2. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MA, Sitesh CB, Rahmatullah M. Analgesic and antiinflammatory activity of methanolic extract of Acalypha indica Linn. Pak J Pharm Sci. 2010;23:256–58. [PubMed] [Google Scholar]
- 23.Gopalakrishnan V, Rao KNV, Loganathan V, Shanmuganathan S, Bollu VK, Bhavana ST. Antimicrobial activity of extracts of Acalypha indica Linn. Indian J Pharm Sci. 2000;62:347–50. [Google Scholar]
- 24.Nandhakumar M, Iniyan TG, Senthilkumar M, Kumar DB, Mitra A. In vitro assay of alpha amylase inhibitory activity of Indian medicinal herb Acalypha Indica. J Clin Diagn Res. 2009;3:1475–78. [Google Scholar]
- 25.Purwaningsih EH, Ibrahim N, Zain H. The nerve protection and in vivo therapeutic effect of Acalypha indica extract in frogs. Med J Indones. 2010;19:96–102. [Google Scholar]
- 26.Balakrishnan N, Panda AB, Raj NR, Shrivastava A, Prathani R. The evaluation of nitric oxide scavenging activity of Acalypha Indica Linn root. Asian J Research Chem. 2009;2:148–50. [Google Scholar]
- 27.Priya CL, Rao KVB. Phytochemical composition, antidiabetic and antioxidant activities of Acalypha indica Linn (Euphorbiaceae) stem extract: an in vitro study. Res J Biotechnol. 2014;9:1–9. [Google Scholar]
- 28.Shihabudeen HMS, Priscilla DH, Kavitha T. Cinnamon extract inhibits a-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab. 2011;8:1–1. doi: 10.1186/1743-7075-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nirmala A, Saroja S, Devi GG. Antidiabetic activity of Basella rubra and its relationship: with the antioxidant property. Brit Biotechnol J. 2011;1:1–9. [Google Scholar]
- 30.Sathya M, Kokilavani R, Anantha TKS. Acute and Subacute toxicity studies of ethanolic extract of Acalypha indica Linn in male wistar albino rats. Asian J Pharm Clin Res. 2012;5:97–100. [Google Scholar]
- 31.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 32.Marklund SL. Marklund G, Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 33.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium, biochemical role as a component of glutathione peroxidase purification and assay. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 34.Ratner RE. Controlling postprandial hyperglycemia. Am J Cardiol. 2001;88:26–31. doi: 10.1016/s0002-9149(01)01834-3. [DOI] [PubMed] [Google Scholar]
- 35.Rosa MP, Monica DG. Meliacinolin: a potent a-glucosidase and a-amylase inhibitor isolated from Azadirachta indica leaves and in vivo antidiabetic property in streptozotocinnicotinamide-induced type 2 diabetes in mice. Biol Pharm Bull. 2012;35:1516–24. doi: 10.1248/bpb.b12-00246. [DOI] [PubMed] [Google Scholar]
- 36.Laar FA, Lucassen PL, Akkermans RP, Lisdonk EH, Rutten GE, Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–63. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 37.Desai NK, Bhabhor PH, Domadia D, Bhatt JD. Role of antioxidant therapy in management of type - 2 diabetes mellitus. National J int Res Med. 2013;4:1–6. [Google Scholar]
- 38.Sellamuthu PS, Arulselvan P, Kamalraj S, Fakurazi S, Kandasamy M. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. Pharmacol. 2013:1–10. doi: 10.1155/2013/750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umar IA, Mohammed A, Dawud FA, Kabir AM, Sai JV, Muhammad FS, Okalor ME. The hypolipidemic and antioxidant actions of aqueous extracts of Ocimum basilicum and Ocimum suave in high fat fed Rats. J Med Plants Res. 2012;6:3501–05. [Google Scholar]
- 40.Krishnakumar K, Augusti KT, Vijayammal PL. Hypolipidaemic effect of Salcia oblonga wall root bark in STZ diabetic rats. Med Sci Res. 2000;28:65–67. [Google Scholar]


