Abstract
The components of the adult extracellular matrix in the central nervous system form a lattice-like structure that is deposited as perineuronal nets, around axon initial segments and as synapse-associated matrix. An abundant component of this matrix is the lecticans, chondroitin sulfate-bearing proteoglycans that are the major substrate for several members of the ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) family. Since lecticans are key regulators of neural plasticity, ADAMTS cleavage of lecticans would likely also contribute to neuroplasticity. Indeed, many studies have examined the neuroplastic contribution of the ADAMTSs to damage and recovery after injury and in central nervous system disease. Much of this data supports a role for the ADAMTSs in recovery and repair following spinal cord injury by stimulating axonal outgrowth after degradation of a glial scar and improving synaptic plasticity following seizure-induced neural damage in the brain. The action of the ADAMTSs in chronic diseases of the central nervous system appears to be more complex and less well-defined. Increasing evidence indicates that lecticans participate in synaptic plasticity in neurodegenerative disease states. It will be interesting to examine how ADAMTS expression and action would affect the progression of these diseases.
Keywords: Brevican, Chondroitin sulfate, Spinal cord injury, Seizure, Neuroplasticity, Glial scar, Proteolytic fragment
Extracellular matrix in the developing and adult CNS
The extracellular matrix (ECM) in the central nervous system (CNS) shares similarities to other tissues, although there are several aspects of nervous system matrix that make it unique [1]. In the CNS there is little collagen or other fibril forming molecules. The extracellular space in the parenchyma is limited to 20% of the total CNS volume [2] and is filled with a lattice-forming structure composed of hyaluronan, glycoproteins including tenascin C/R, and a small family of chondroitin sulfate (CS)-bearing proteoglycans (PGs) termed lecticans [3–5]. The lecticans include CNS-specific brevican and neurocan [6], aggrecan (also highly abundant in cartilage), and versican (found in nearly all tissues including blood vessels and basement membranes) [4,7]. The amino terminus of lecticans binds hyaluronan and the carboxy terminus binds tenascin.
The make-up of the matrix changes significantly during CNS development [8,9] and is quite varied in different regions of the adult CNS. During development, the ECM directly influences neuronal and glial migration and differentiation, neurogenesis, and axonal outgrowth and guidance that are vital for synaptogenesis [10–12]. Compared to the ECM during early development and later developmental “critical periods”, adul tECM is located just adjacent to the active zone of many synapses and directly affects synaptic plasticity and stability [3,6]. Additionally in the adult CNS, the ECM forms perineuronal nets (PNNs) around selected neuronal soma where it can influence synapses on the membrane of the cell body [13]. After injury to the CNS, glial scars form that contains robust amounts of ECM molecules, especially CS-bearing PGs, secreted mainly by activated glia. Although these scars are thought to assist in the isolation of the injured region of the brain, the scar inhibits repair mechanisms [11]. In fact, be it synaptic or neurite plasticity, the role of CS-bearing PGs appears to be negative in every case, but little is known about how the presence and turnover of matrix is regulated. However, it is known that extracellular proteases act selectively on lecticans, other CS-bearing PGs, other matrix proteins, and a small number of non-matrix protein substrates. These proteinase families are called a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) and matrix metalloproteinases (MMPs) [5,14]; tissue plasminogen activator is also capable of cleaving lecticans. Changes in deposits of matrix protein in the CNS involve the action of these proteases. The remainder of this chapter will describe what is known about the expression and activity of ADAMTS glutamyl-endopeptidases (proteases that cleave on the carboxy-end of glutamate or aspartate residues), in the CNS, how they influence the functional plasticity of brain ECM in CNS injury and disease, and the prospects for future discovery.
Detection of changes in ADAMTS activity
One consistent and evident feature of Western blots conducted for identification of the lectican proteins, brevican, aggrecan, versican and neurocan, is the presence of low molecular weight fragments detected by antibodies originally raised to bind epitopes on the full length proteins. These fragments were initially observed for aggrecan and led to the notion that specific proteinases could cleave the full length protein [15]. The cleavage sites for each lectican in the intraglobular domain are conserved across species and are located near the junction between the N-terminal G1 domain and the central, CS-bearing domain. MMPs and ADAMTSs cleave specific but different sites in this region. Originally, a protein termed glial hyaluronan binding protein was thought to be an independent protein, but was found to be an ADAMTS-derived N-terminal fragment of versican V2 [16].
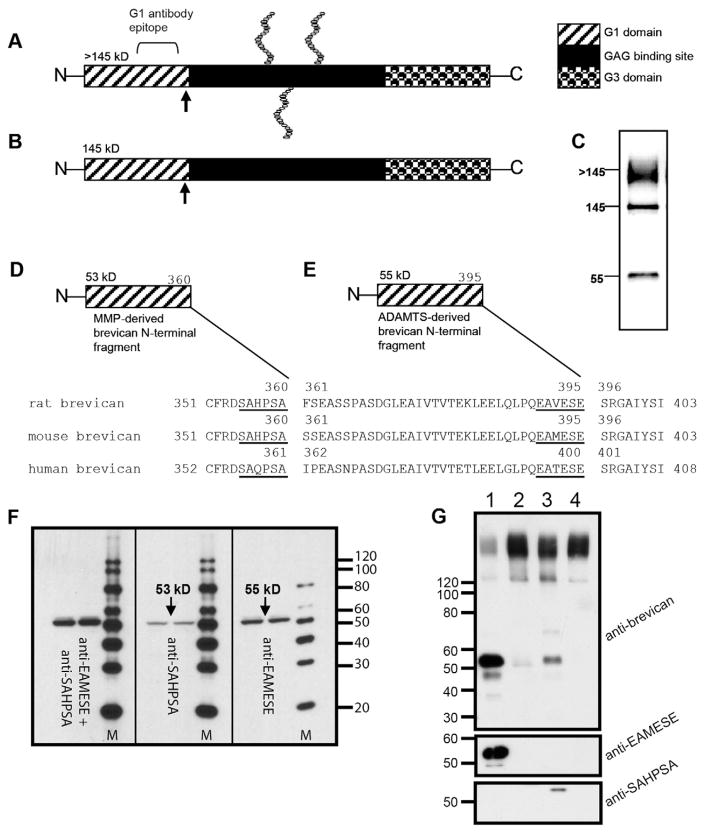
The C-terminal peptide sequences of the N-terminal fragments produced by lectican proteolysis were used to raise “neoepitope” antibodies that specifically recognize only the cleaved fragment, and not the intact proteoglycan [17,18]. These neoepitope antibodies may be used in immunohistochemistry and Western blots to determine when there is an increase in the lectican fragment (and potentially a decrease in full length lectican) that would signify increased activity of the ADAMTSs or MMPs in a particular region of the brain after injury, in a brain disorder, or after treatment with an agent that may affect the activity of these proteinases. For example, a Western blot using an antibody against the brevican N-terminal region shows a typical ~55 kDa fragment (Fig. 1c). Brevican may be cleaved by either ADAMTSs or MMPs at sites that are proximate to one another. The N-terminal fragments produced by ADAMTS or MMP cleavage of brevican are 55 kDa and 53 kDa, respectively (Fig. 1D, E), and thus an increase in either the 55 kDa or 53 kDa fragment may signify increased ADAMTS or MMP activity, respectively. Since ADAMTSs require proteolytic activation to generate a functional protease, or may be inhibited by endogenous TIMP3, detecting an altered quantity of ADAMTSs alone does not signify a change in protease activity. However, changes in the levels of the ADAMTS-specific lectican fragment indicate that the activity of the proteinase has changed. The major ADAMTSs expressed in the brain which have the ability to cleave lecticans are ADAMT1, ADAMTS4, ADAMTS5, ADAMTS8, and ADAMTS9. It should also be noted that the lecticans are not the only substrates for these ADAMTSs, as they can cleave phosphacan [19] and reelin [20,21].
Fig. 1.
Detection of brevican isoforms and proteolytic degradation by endogenous proteases at specific cleavage sites. Brevican is secreted as a >145 kDa protein bearing 1–3 CS chains (A). Brevican is also secreted as the holoprotein without CS chains at 145 kDa (B). When probed on Western blot with an N-terminal antibody (BD Biosciences, San Jose, CA) three immunoreactive bands appear: a >145 kDa smear (glycosylated brevican), the 145 kDa core protein, and a ~55 kDa proteolytic fragment. Arrows in (A) and (B) indicate proteolytic cleavage sites. Fragments of brevican are generated by endogenous proteases, the MMPs (D) and ADAMTSs (E). Each has a distinct, specific cleavage site sequence on the brevican protein. Shown here are the specific cleavage sequences for the MMPs and ADAMTSs in mouse, rat and human brevican (based on data from [51]). The MMP cleavage-site is 35 amino acid residues upstream from the ADAMTS-specific site (D and E). Distinct “neoepitope” antibodies recognize the MMP- and ADAMTS-derived cleavage fragments of brevican in mouse brain extracts subjected to Western blot on 4–20% gradient SDS-PAGE gels (F). Anti-SAHPSA recognizes the 53 kDa, MMP-derived fragment of brevican (F; middle panel) whereas anti-EAMESE detects the 55 kDa, ADAMTS-derived form (F; right panel). Mixing the two antibodies detects a “thicker” band in this region (F; left panel). “M” indicates molecular weight markers in (F). Antibodies recognize distinct products after proteolytic cleavage with hrADAMTS4 or hrMMP-2 (G). Proteoglycan purified from the mouse brain was incubated with 50 nM hrADAMTS-4 (G, lane 1), 50 nM hrADAMTS4 + 5 mM EDTA (G, lane 2), 50 nM hrMMP-2 (G, lane 3) or 50 nM hrMMP-2 + 5 mM EDTA (G, lane 4) and immunoblotted for brevican. Note that the ADAMTS-derived brevican fragment was selectively recognized by anti-EAMESE and the brevican MMP product was recognized by anti-SAHPSA (Figure used with permission from Journal of Neurochemistry [52]).
ADAMTS in the CNS: regional and cellular expression in the CNS
ADAMTSs are expressed throughout the CNS, including the spinal cord, brain stem, hippocampus, striatum and cortex, as detected by direct immunohistochemistry, Western blot, RT-PCR, and by using ADAMTS-specific neoepitope antibodies to detect changes in fragments of various lectican substrates [21–29]. ADAMTS4 appears to be the most highly expressed ADAMTS in the brain of the adult under basal conditions [23], although ADAMTS1 was found to be expressed in the mouse and rat brain during development [30,31], in the mouse motor neurons after hypoglossal nerve injury [32] and in the frontal cortex of human Down’s syndrome, Alzheimer’s and Pick’s disease tissue [25]. In the frontal cortex of mice, transcripts for the ADAMTS proteoglycanases were compared at postnatal day 8, day 28 and in the adult. ADAMTS1 and ADAMTS4 had higher transcript levels at all developmental periods compared to ADAMTS5, ADAMTS9 and ADAMTS15. Most interestingly, ADAMTS4 levels were quite low at P8, increased markedly at P28 and decreased to between these two levels in the adult [29]. ADAMTS4 is found in astrocytes and microglia in spinal cord in vivo and it increased in experimental autoimmune encephalomyelitis [27]. Neuronal expression of ADAMTS4 mRNA was observed in normal rat hippocampus—-dentate gyrus granule cells and CA1 and CA3 neurons—and cortical neurons; its expression markedly increased in these regions following kainic acid-induced seizures [23]. In addition, ADAMTS1 mRNA markedly increased in the dentate gyrus and CA1 and CA3 regions of the hippocampus following kainic acid-induced seizures. This expression was closely associated with an increase in the ADAMTS-specific N-terminal cleavage fragment of brevican [23]. In vivo data supports that most ADAMTSs are produced by astrocytes, especially after injury, although ADAMTSs are also produced by neurons and microglia [33]. In vitro, many of the proteoglycan-cleaving ADAMTSs are produced by astrocytes and neurons [33]. In cultured astrocytes ADAMTS4 is expressed basally and inhibited in response to treatment with transforming growth factor β [34] and its production is stimulated by β-amyloid [35]. Further, tumor necrosis factor α induced the production of ADAMTS1 and ADAMTS4 in astrocytes [36]. Overall, most data indicates that ADAMTSs increase their expression in response to CNS disease, CNS disorders or after CNS injury. In these studies, there is either speculation about how the ADAMTSs might influence CNS repair or some studies produced data to support the functional role of the ADAMTSs.
The functional role of ADAMTSs in the CNS
The specific role(s) ADAMTSs play in physiological and pathological nervous system functions remains under investigation. Studies have predominantly focused on ADAMTS family members that can cleave lecticans. There is substantive data that demonstrates that ADAMTS cleavage of brevican may facilitate the invasiveness of the glial tumors. ADAMTS4 mRNA is expressed by glioma cell lines with invasive properties and early data indicated that cleavage of brevican by ADAMTS4 is closely associated with invasiveness of these brain tumors [22,37,38]. Interestingly, only ADAMTS5 mRNA was upregulated in human glioma tissue. Although increased transcript alone does not indicate increased proteolytic activity, ADAMTS5 may also be involved in glioma invasiveness [39]. Compounds that could inhibit ADAMTS cleavage of brevican could potentially serve to limit glioma migration and aid in surgical removal. However, in other pathological conditions, increasing ADAMTS activity could prove beneficial.
Several studies indicate that the ADAMTSs are involved in recovery from spinal cord injury (SCI). SCI leads to a glial scar that is rich in growth inhibitory molecules, including lecticans, which diminishes functional recovery. Following SCI, ADAMTS1, 5, and 9 (but not ADAMTS4) mRNA was upregulated and this change was accompanied by increased cleavage of aggrecan, brevican, and versican [40]. Although ADAMTS4 mRNA was not altered in this report, another study demonstrated both increased ADAMTS4 protein and aggrecan cleavage following SCI [19]. Additionally, ADAMTS13 mRNA, a family member that cleaves von Willebrand Factor (vWF) but not lecticans, was increased in astrocytes and microglia following SCI. While vWF is systemically important for regulating blood coagulation, upregulation at the injury site was hypothesized to regulate secondary inflammation that would result from blood brain barrier breakdown. Given the prominent lectican component of the glial scar, recent studies examined the ability of ADAMTS4 to aid in recovery from SCI. When ADAMTS4 was infused into the rat spinal cord following SCI, it promoted functional recovery comparable to that observed with chondroitinase ABC, a bacterial enzyme that cleaves chondroitin sulfate from CS-bearing PGs [19]. Recently, tPA was shown to cleave pro-A-DAMTS4 into its active form. Infusion of either tPA or active ADAMTS4 at the lesion after SCI promoted axonal sprouting and functional recovery [33]. Overall, ADAMTS4 may be a potentially promising therapeutic candidate that could increase plasticity and functional recovery after SCI, especially when delivered soon after the injury.
In studies both within and outside the CNS, ADAMTSs have been identified as having inflammatory and anti-angiogenic actions [33], including their necessity for macrophage invasion in vitro [41] and monocyte/macrophage differentiation and activation [42]. In addition, because several ADAMTSs can cleave versican deposited in the vasculature, it is possible that the ADAMTSs, especially ADAMTS4, may contribute to the opening of the blood brain barrier after spinal cord injury or ischemic stroke. Although ADAMTS1 was the first ADAMTS to be cloned and identified with both inflammatory and anti-angiogenic properties [43], other ADAMTSs also show these actions [33]. Both the inflammatory and anti-angiogenic properties of the ADAMTSs have the potential to contribute to repair following ischemic stroke, other brain injury and certainly in a number of different neurodegenerative disease states. In fact, the ADAMTSs have a great deal of speculative potential toward their role in neural and synaptic plasticity, and potential for repair mechanisms that involves turnover of the ECM proteoglycans [5].
Indeed, increasing evidence suggests that ADAMTSs may play a role in neuroplasticity. After kainic acid-induced seizure, there was an association between increased ADAMTS-derived proteolytic brevican fragment and a loss of synaptic density (as detected by reduced synaptophysin immunoreactivity) in dentate gyrus outer molecular layer. ADAMTS cleavage of brevican occurred early with elevated brevican fragment by 8 h of post-seizure. This finding suggested that the disrupted brevican–tenascin–hyaluronan complex reduced the stability of synapses and may have led to synapse loss [23]. Localized inhibition of ADAMTS activity using a small molecule inhibitor [44] or localized treatment with siRNA that would block ADAMTS expression to determine if these treatments would reverse the loss of synaptic density might directly verify and support this finding. On the other hand, when ADAMTS activity was examined for longer periods after localized entorhinal cortex lesion, data indicated that ADAMTSs may exert a positive action in stimulating neuritic sprouting, synaptic remodeling and synaptogenesis in the outer molecular layer of the dentate gyrus; this role would make ADAMTSs crucial for recovery and repair after neuronal and/or synaptic loss [24]. Additional evidence for the potential role of ADAMTSs in neuroplasticity comes from in vitro work. When compared to several MMPs that cleave CS-bearing PGs, ADAMTS4 not only increased neurite outgrowth on an inhibitory CS-bearing PG or astrocyte-derived matrix, but also did not process laminin or induce neurotoxicity [45]. Cortical neurons transfected with either ADAMTS4 cDNA or a mutant ADAMTS4 cDNA that produced an inactive proteinase or direct treatment with recombinant ADAMTS4 all exhibited increased neurite outgrowth. The recombinant form of ADAMTS4 stimulated neurite extension in a dose–dependent manner. Thus, it was speculated that the thrombospondin repeats in ADAMTS4 may have induced neurite extension since expressing the inactive form of the protease was just as effective as the active form in stimulating neurite outgrowth [46]. In this study, ADAMTS-induced neuritic growth was shown to be dependent on extracellular signal-regulated kinase 1/2 (ERK-1/2) signaling [46]. This data suggests that ADAMTSs may increase plasticity even in the absence of lectican cleavage [46].
ADAMTS1, although not widely studied in the CNS, appears to play a role in developmental synaptogenesis. In one month old and adult ADAMTS1 knockout mice, several synaptic markers, including PSD-95, synaptophysin and SNAP-25 showed reduced levels in the frontal cortex extracts compared to wild type mice [29,47] and this was associated with an increase in neurocan during early postnatal development. Interestingly, this effect was sexually dimorphic as only female ADAMTS1 knockout mice exhibited these changes. It is unclear, however, whether these changes in synaptic density in female ADAMTS1 knockouts were related to the changes in matrix proteins since the alterations in the neurocan were observed about a week after birth [29]. ADAMTS1 is located on chromosome 21, and thus in Down syndrome or trisomy 21, it would be expected to be upregulated. In the Ts65Dn mouse model of Down syndrome, there was altered synaptic density in the stratum oriens of the hippocampus that was unexpectedly associated with increased deposition of versican V2 in the same region. It is possible that the overexpression of versican was associated with altered inhibitory neurotransmission in this region [48]. Taken together, the majority of data suggests that the ADAMTSs are involved in the activation of plasticity mechanisms, possibly on neurites and synapses, a role that is not surprising since the CS-bearing PGs can inhibit both neurite outgrowth and synaptic plasticity.
Future investigation into the role of ADAMTSs in nervous system disorders may involve synaptic plasticity. There is abundant evidence that lecticans and other CS-bearing proteoglycans have a marked influence on a host of nervous system disorders and diseases [13]. For example, recent data demonstrated a defect in ocular dominance plasticity in two different amyloid precursor protein overexpressing Alzheimer disease model mice at one month of age that was associated with increased ECM deposition [49]. Also, elevated ECM proteins, including brevican, were found at an early age in Alzheimer model mice associated with a loss of contextual fear memory and an LTP defect. Treatment with chondroitinase ABC restored contextual memory as well as LTP [50]. In such models it would be quite interesting to determine whether increased expression of ADAMTSs can produce the same effects as treatment with chondroitinase ABC. This prospect would be especially important since the ADAMTSs are naturally produced proteins where chondroitinase ABC is a bacterial enzyme. This has been performed in spinal cord injury and overexpression of the ADAMTSs was as effective as chondroitinase ABC in producing behavioral recovery [19].
The role of the ADAMTSs in the CNS continues to evolve. More and more evidence indicates that they play an important role in neuroplasticity as well as nervous system dysfunctions. It is hopeful and possible that ADAMTS family members may be utilized to develop therapies for CNS injury and neurodegenerative and neurological disorders.
Acknowledgments
This work was supported by NIH NIA R01 AG022101 (PEG) and funds from the University of Arkansas for Medical Sciences (PEG).
References
- 1.Barros CS, Franco SJ, Muller U. Extracellular matrix: functions in the nervous system. In: Hynes RO, Yamada KM, editors. Extracellular matrix biology. Cold Spring Harbor Laboratory Press; 2012. pp. 333–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell MD, Gottschall PE. Lectican proteoglycans, their cleaving metalloproteinases, and plasticity in the central nervous system extracellular microenvironment. Neuroscience. 2012;217:6–18. doi: 10.1016/j.neuroscience.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–53. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 5.Gottschall PE, Sandy JD, Zimmerman DR. Substrates for metalloendopeptidases in the central nervous system. In: Conant K, Gottschall PE, editors. Matrix metalloproteinases in the central nervous system. Imperial College Press; 2005. pp. 87–118. [Google Scholar]
- 6.Frischknecht R, Seidenbecher CI. Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol. 2012;44:1051–4. doi: 10.1016/j.biocel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Avram S, Shaposhnikov S, Buiu C, Mernea M. Chondroitin sulfate proteoglycans: structure-function relationship with implication in neural development and brain disorders. Biomed Res Int. 2014;2014:642798. doi: 10.1155/2014/642798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–47. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 9.Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, et al. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem Biophys Res Commun. 1998;247:207–12. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- 10.de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, et al. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun. 2013;4:1484. doi: 10.1038/ncomms2491. [DOI] [PubMed] [Google Scholar]
- 11.Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235:5–17. doi: 10.1016/j.expneurol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Pyka M, Busse C, Seidenbecher C, Gundelfinger ED, Faissner A. Astrocytes are crucial for survival and maturation of embryonic hippocampal neurons in a neuronglia cell-insert coculture assay. Synapse. 2011;65:41–53. doi: 10.1002/syn.20816. [DOI] [PubMed] [Google Scholar]
- 13.Soleman S, Filippov MA, Dityatev A, Fawcett JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013;253:194–213. doi: 10.1016/j.neuroscience.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–45. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–6. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, et al. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronan binding protein. Biochem J. 2004;377:787–95. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H, Watanabe K, Shimonaka M, Yamasaki M, Yamaguchi Y. cDNA cloning and the identification of an aggrecanase-like cleavage site in rat brevican. Biochem Biophys Res Commun. 1995;216:957–63. doi: 10.1006/bbrc.1995.2713. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Kelly G, Zerillo C, Jaworski DM, Hockfield S. Expression of a cleaved brain-specific extracellular matrix protein mediates glioma cell invasion in vivo. J Neurosci. 1998;18:2370–6. doi: 10.1523/JNEUROSCI.18-07-02370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauchi R, Imagama S, Natori T, Ohgomori T, Muramoto A, Shinjo R, et al. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation. 2012;9:53. doi: 10.1186/1742-2094-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisanaga A, Morishita S, Suzuki K, Sasaki K, Koie M, Kohno T, et al. A disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) cleaves reelin in an isoform–dependent manner. FEBS Lett. 2012;586:3349–53. doi: 10.1016/j.febslet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Krstic D, Rodriguez M, Knuesel I. Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators. PLoS ONE. 2012;7:e47793. doi: 10.1371/journal.pone.0047793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, et al. Brain-enriched hyaluronan binding (BEHAB)/ brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem. 2000;275:22695–703. doi: 10.1074/jbc.M909764199. [DOI] [PubMed] [Google Scholar]
- 23.Yuan W, Matthews RT, Sandy JD, Gottschall PE. Association between protease-specific proteolytic cleavage of brevican and synaptic loss in the dentate gyrus of kainate-treated rats. Neuroscience. 2002;114:1091–101. doi: 10.1016/s0306-4522(02)00347-0. [DOI] [PubMed] [Google Scholar]
- 24.Mayer J, Hamel MG, Gottschall PE. Evidence for proteolytic cleavage of brevican by the ADAMTSs in the dentate gyrus after excitotoxic lesion of the mouse entorhinal cortex. BMC Neurosci. 2005;6:52. doi: 10.1186/1471-2202-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miguel RF, Pollak A, Lubec G. Metalloproteinase ADAMTS-1 but not ADAMTS-5 is manifold overexpressed in neurodegenerative disorders as Down syndrome, Alzheimer’s and Pick’s disease. Brain Res Mol Brain Res. 2005;133:1–5. doi: 10.1016/j.molbrainres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Jungers KA, Le Goff C, Somerville RP, Apte SS. Adamts9 is widely expressed during mouse embryo development. Gene Expr Patterns. 2005;5:609–17. doi: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Cross AK, Haddock G, Surr J, Plumb J, Bunning RA, Buttle DJ, et al. Differential expression of ADAMTS-1, -4, -5 and TIMP-3 in rat spinal cord at different stages of acute experimental autoimmune encephalomyelitis. J Autoimmun. 2006;26:16–23. doi: 10.1016/j.jaut.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Ajmo JM, Eakin AK, Hamel MG, Gottschall PE. Discordant localization of WFA reactivity and brevican/ADAMTS-derived fragment in rodent brain. BMC Neurosci. 2008;9:14. doi: 10.1186/1471-2202-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howell MD, Torres-Collado AX, Iruela-Arispe ML, Gottschall PE. Selective decline of synaptic protein levels in the frontal cortex of female mice deficient in the extracellular metalloproteinase ADAMTS1. PLoS ONE. 2012;7:e47226. doi: 10.1371/journal.pone.0047226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thai SN, Iruela-Arispe ML. Expression of ADAMTS1 during murine development. Mech Dev. 2002;115:181–5. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- 31.Gunther W, Skaftnesmo KO, Arnold H, Bjerkvig R, Terzis AJ. Distribution patterns of the anti-angiogenic protein ADAMTS-1 during rat development. Acta Histochem. 2005;107:121–31. doi: 10.1016/j.acthis.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki M, Seo-Kiryu S, Kato R, Kita S, Kiyama H. A disintegrin and metalloprotease with thrombospondin type1 motifs (ADAMTS-1) and IL-1 receptor type 1 mRNAs are simultaneously induced in nerve injured motor neurons. Brain Res Mol Brain Res. 2001;89:158–63. doi: 10.1016/s0169-328x(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 33.Lemarchant S, Pruvost M, Montaner J, Emery E, Vivien D, Kanninen K, et al. ADAMTS proteoglycanases in the physiological and pathological central nervous system. J Neuroinflammation. 2013;10:133. doi: 10.1186/1742-2094-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamel MG, Mayer J, Gottschall PE. Altered production and proteolytic processing of brevican by transforming growth factor beta in cultured astrocytes. J Neurochem. 2005;93:1533–41. doi: 10.1111/j.1471-4159.2005.03144.x. [DOI] [PubMed] [Google Scholar]
- 35.Satoh K, Suzuki N, Yokota H. ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) is transcriptionally induced in beta-amyloid treated rat astrocytes. Neurosci Lett. 2000;289:177–80. doi: 10.1016/s0304-3940(00)01285-4. [DOI] [PubMed] [Google Scholar]
- 36.Cross AK, Haddock G, Stock CJ, Allan S, Surr J, Bunning RA, et al. ADAMTS-1 and -4 are up-regulated following transient middle cerebral artery occlusion in the rat and their expression is modulated by TNF in cultured astrocytes. Brain Res. 2006;1088:19–30. doi: 10.1016/j.brainres.2006.02.136. [DOI] [PubMed] [Google Scholar]
- 37.Held-Feindt J, Paredes EB, Blomer U, Seidenbecher C, Stark AM, Mehdorn HM, et al. Matrix-degrading proteases ADAMTS4 and ADAMTS5 (disintegrins and metalloproteinases with thrombospondin motifs 4 and 5) are expressed in human glioblastomas. Int J Cancer. 2006;118:55–61. doi: 10.1002/ijc.21258. [DOI] [PubMed] [Google Scholar]
- 38.Viapiano MS, Hockfield S, Matthews RT. BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. J Neuro-Oncol. 2008;88:261–72. doi: 10.1007/s11060-008-9575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakada M, Miyamori H, Kita D, Takahashi T, Yamashita J, Sato H, et al. Human glioblastomas overexpress ADAMTS-5 that degrades brevican. Acta Neuropathol. 2005;110:239–46. doi: 10.1007/s00401-005-1032-6. [DOI] [PubMed] [Google Scholar]
- 40.Demircan K, Yonezawa T, Takigawa T, Topcu V, Erdogan S, Ucar F, et al. ADAMTS1, ADAMTS5, ADAMTS9 and aggrecanase-generated proteoglycan fragments are induced following spinal cord injury in mouse. Neurosci Lett. 2013;544:25–30. doi: 10.1016/j.neulet.2013.02.064. [DOI] [PubMed] [Google Scholar]
- 41.Ren P, Zhang L, Xu G, Palmero LC, Albini PT, Coselli JS, et al. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2013;95:570–7. doi: 10.1016/j.athoracsur.2012.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagsater D, Bjork H, Zhu C, Bjorkegren J, Valen G, Hamsten A, et al. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514–22. doi: 10.1016/j.atherosclerosis.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–62. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 44.Yao W, Wasserman ZR, Chao M, Reddy G, Shi E, Liu RQ, et al. Design and synthesis of a series of (2R)-N(4)-hydroxy-2-(3-hydroxybenzyl)-N(1)- [(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]butanediamide derivatives as potent, selective, and orally bioavailable aggrecanase inhibitors. J Med Chem. 2001;44:3347–50. doi: 10.1021/jm015533c. [DOI] [PubMed] [Google Scholar]
- 45.Cua RC, Lau LW, Keough MB, Midha R, Apte SS, Yong VW. Overcoming neurite-inhibitory chondroitin sulfate proteoglycans in the astrocyte matrix. Glia. 2013;61:972–84. doi: 10.1002/glia.22489. [DOI] [PubMed] [Google Scholar]
- 46.Hamel MG, Ajmo JM, Leonardo CC, Zuo F, Sandy JD, Gottschall PE. Multimodal signaling by the ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) promotes neurite extension. Exp Neurol. 2008;210:428–40. doi: 10.1016/j.expneurol.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottschall PE, Ajmo JM, Eakin AK, Howell MD, Mehta H, Bailey LA. Panel of synaptic protein ELISAs for evaluating neurological phenotype. Exp Brain Res. 2010;201:885–93. doi: 10.1007/s00221-010-2182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howell MD, Gottschall PE. Altered synaptic marker abundance in the hippocampal stratum oriens of Ts65Dn mice is associated with exuberant expression of versican. ASN Neuro. 2012:4. doi: 10.1042/AN20110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.William CM, Andermann ML, Goldey GJ, Roumis DK, Reid RC, Shatz CJ, et al. Synaptic plasticity defect following visual deprivation in Alzheimer’s disease model transgenic mice. J Neurosci. 2012;32:8004–11. doi: 10.1523/JNEUROSCI.5369-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vegh MJ, Heldring CM, Kamphuis W, Hijazi S, Timmerman AJ, Li KW, et al. Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2014;2:76. doi: 10.1186/s40478-014-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, et al. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–90. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- 52.Ajmo JM, Bailey LA, Howell MD, Cortez LK, Pennypacker KR, Mehta HN, et al. Abnormal post-translational and extracellular processing of brevican in plaque-bearing mice overexpressing APPsw. J Neurochem. 2010;113:784–95. doi: 10.1111/j.1471-4159.2010.06647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]