ABSTRACT
Nitrate-reducing Fe(II)-oxidizing microorganisms were described for the first time ca. 20 years ago. Most pure cultures of nitrate-reducing Fe(II) oxidizers can oxidize Fe(II) only under mixotrophic conditions, i.e., when an organic cosubstrate is provided. A small number of nitrate-reducing Fe(II)-oxidizing cultures have been proposed to grow autotrophically, but unambiguous evidence for autotrophy has not always been provided. Thus, it is still unclear whether or to what extent Fe(II) oxidation coupled to nitrate reduction is an enzymatically catalyzed and energy-yielding autotrophic process or whether Fe(II) is abiotically oxidized by nitrite from heterotrophic nitrate reduction. The aim of the present study was to find evidence for the existence of autotrophic nitrate-reducing Fe(II) oxidizers in coastal marine sediments. Microcosm incubations showed that with increasing incubation times, the stoichiometric ratio of reduced nitrate/oxidized Fe(II) [NO3−reduced/Fe(II)oxidized] decreased, indicating a decreasing contribution of heterotrophic denitrification and/or an increasing contribution of autotrophic nitrate-reducing Fe(II) oxidation over time. After incubations of sediment slurries for >10 weeks, nitrate-reducing activity ceased, although nitrate was still present. This suggests that heterotrophic nitrate reduction had ceased due to the depletion of readily available organic carbon. However, after the addition of Fe(II) to these batch incubation mixtures, the nitrate-reducing activity resumed, and Fe(II) was oxidized, indicating the activity of autotrophic nitrate-reducing Fe(II) oxidizers. The concurrent reduction of 14C-labeled bicarbonate concentrations unambiguously proved that autotrophic C fixation occurred during Fe(II) oxidation and nitrate reduction. Our results clearly demonstrated that autotrophic nitrate-reducing Fe(II)-oxidizing bacteria were present in the investigated coastal marine sediments.
IMPORTANCE Twenty years after the discovery of nitrate-reducing Fe(II) oxidizers, it is still controversially discussed whether autotrophic nitrate-reducing Fe(II)-oxidizing microorganisms exist and to what extent Fe(II) oxidation in this reduction/oxidation process is enzymatically catalyzed or which role abiotic side reactions of Fe(II) with reactive N species play. Most pure cultures of nitrate-reducing Fe(II) oxidizers are mixotrophic; i.e., they need an organic cosubstrate to maintain their activity over several cultural transfers. For the few existing autotrophic isolates and enrichment cultures, either the mechanism of nitrate-reducing Fe(II) oxidation is not known or evidence for their autotrophic lifestyle is controversial. In the present study, we provide evidence for the existence of autotrophic nitrate-reducing Fe(II) oxidizers in coastal marine sediments. The evidence is based on stoichiometries of nitrate reduction and Fe(II) oxidation determined in microcosm incubations and the incorporation of carbon from CO2 under conditions that favor the activity of nitrate-reducing Fe(II) oxidizers.
INTRODUCTION
Iron is an abundant redox-active element that makes up about 5% of the earth's crust (1). In the environment, the two most important redox states of iron are Fe(II) (ferrous iron) and Fe(III) (ferric iron). In the biogeochemical Fe cycle, different biotic and abiotic reactions are involved in the oxidation of Fe(II) to Fe(III) or the reduction of Fe(III) to Fe(II). At circumneutral pH, the most important abiotic reactions include the oxidation of Fe(II) by O2, reactive N species, or Mn(IV) minerals as well as the reduction of Fe(III) by reduced sulfur species, humic substances, or light-induced reactions (2). Microbial Fe-converting metabolic processes include the oxidation of Fe(II) by microaerophilic (3), anoxygenic phototrophic (4), and nitrate-reducing (5) Fe(II) oxidizers as well as the reduction of Fe by Fe(III)-reducing microorganisms that either live heterotrophically and use organic carbon as an electron donor or live autotrophically and use hydrogen as an electron donor (6, 7).
Iron is an important element, not only because it can be used as an electron acceptor or electron donor by many different microbes that gain energy from that reaction but also because the Fe cycle is connected to many other elementary cycles, such as the C and N cycles (3–5, 8–10). Furthermore, the speciation of Fe and the properties of the Fe minerals that are formed, transformed, or dissolved by microbial activity or by abiotic reactions can influence the fate of nutrients, pollutants, and trace metals (11–16). Unfortunately, there are knowledge gaps that hamper our understanding of the biogeochemical Fe cycle, mainly regarding the mechanisms of Fe(II) oxidation in general and particularly nitrate-reducing Fe(II) oxidation. The energetic yield of the reaction would allow autotrophic growth (equation 1) (17), but now, 20 years after the first discovery of organisms that concurrently reduce nitrate and oxidize Fe(II) (5), it is still unclear whether, or to which extent, this process is enzymatically catalyzed and if bacteria can gain energy from this thermodynamically favorable reaction:
| (1) |
Most known cultures of nitrate-reducing Fe(II) oxidizers cannot be cultivated autotrophically over a long period or over several transfers with nitrate and Fe(II) alone but need an organic cosubstrate (18–22). Particularly for these mixotrophic cultures, it is unknown whether Fe(II) oxidation is enzymatically catalyzed and coupled to cell metabolism or whether Fe(II) oxidation is merely an abiotic side reaction caused by reactive N intermediates of heterotrophic nitrate reduction (23–26). An indication for an unintended abiotic side reaction is that most known mixotrophic nitrate-reducing Fe(II)-oxidizing cells become encrusted in Fe(III) minerals during Fe(II) oxidation (26), a process that is potentially harmful for cells (27). In contrast, microaerophilic or phototrophic autotrophic Fe(II) oxidizers that enzymatically oxidize Fe(II) have developed strategies to avoid the encrustation of cells (27–30), and it is remarkable that no such strategies have been demonstrated to be present in nitrate-reducing Fe(II) oxidizers. Furthermore, it appears that the ability for Fe(II) oxidation is common among all nitrate-reducing bacteria, when an organic substrate is provided (24, 26), even for Escherichia coli (31). Nevertheless, there are a few strains with the proposed ability to live autotrophically with only nitrate and Fe(II): Pseudogulbenkiania sp. strain 2002 (32), Ferroglobus placidus (33), Paracoccus ferrooxidans BDN-1 (34), Thiobacillus denitrificans (5), Geobacter metallireducens (35), Desulfitobacterium frappieri strain G2 (36), Citrobacter freundii strain PXL1 (37), and Mycobacterium sp. strain W5 (38). In addition to these pure cultures, there is the nitrate-reducing Fe(II)-oxidizing enrichment culture KS (5, 39), which is a coculture of several microbial strains (5, 39) that grows autotrophically and can be transferred continuously with only Fe(II) and nitrate. However, the mechanism of Fe(II) oxidation, and also which strain in the coculture is oxidizing Fe(II), is still unknown (39). For most of the above-mentioned “autotrophic” cultures, except for the KS enrichment culture, unambiguous evidence for their ability to oxidize Fe(II) autotrophically with nitrate [such as data for repeatedly transferred cultures and data for CO2 fixation coupled to Fe(II) oxidation and nitrate reduction, etc.] is still missing, and no enzymes involved in Fe(II) oxidation by nitrate-reducing Fe(II) oxidizers have been identified.
Additionally, no evidence for the actual occurrence of autotrophic nitrate-reducing Fe(II) oxidation in the environment has been presented so far. Nitrate-reducing Fe(II)-oxidizing bacteria were found in many different environments, including soils, freshwater sediments, marine sediments, hypersaline sediments, and hydrothermal vents (5, 33, 40–44). Furthermore, the potential activity of nitrate-reducing Fe(II) oxidizers has been investigated in enrichment cultures from wetland sediment or natural sediment from river floodplains, lake sediment, marine sediment, or soil (40, 42, 45–49). However, the nitrate-dependent Fe(II) oxidation observed in most of these setups was probably mixotrophic.
The aim of the present study, therefore, was to find evidence for the presence of autotrophic nitrate-reducing Fe(II) oxidizers in the environment. This was achieved by quantification of Fe(II) oxidation and nitrate reduction rates in microcosm incubations with coastal marine sediments from Norsminde Fjord and Kalø Vig, Denmark, before and after readily available organic carbon had been depleted. Additionally, we quantified the incorporation of 14C-labeled bicarbonate in microcosms with sediment from Norsminde Fjord under conditions that favor the activity of autotrophic nitrate-reducing Fe(II)-oxidizing bacteria. For the 14C incorporation study, we chose the sediment from the Norsminde Fjord field site, because we previously characterized this field site and showed active microbial Fe cycling in this sediment (49).
MATERIALS AND METHODS
Study site.
In June 2015, sediment was sampled at Norsminde Fjord (Denmark), a shallow brackish estuary with freshwater inflow mainly through the Odder River in the western part and a narrow opening to the Baltic Sea in the eastern part. Samples from Norsminde Fjord were taken near the narrow entrance to the Baltic Sea in the southeastern part of the fjord (56°01.171′N, 010°15.390′E). The material collected from Norsminde Fjord was organic-rich mud. In February 2015, sediment was sampled at a beach at Kalø Vig, Denmark (56°16.811′N, 010°28.056′E), at a 0.5- to 1-m water depth. At Kalø Vig, the collected material was an organic-poor silty-sand sediment.
Sediment sampling.
Sediment for microcosm incubations was sampled in core liners with a 2.5-cm inner diameter (50-ml syringes cut off at the front ends). The uppermost 3 cm of several sediment cores was pooled and homogenized. Any visible stones, animals, or other organic-rich particles were removed from the sediment by using tweezers.
Geochemical measurements.
Sediment, pore water, and surface water characteristics were determined by a combination of field measurements with a field multimeter (WTW Multi 3430 for measurement of oxygen saturation, pH, temperature, and salinity of the water column) and microelectrode measurements (glass electrodes with a 100-μm tip diameter [Unisense, Denmark] for measurement of oxygen and sulfide concentrations as well as pH and redox potential over sediment depth in the first few millimeters of the sediment). Microelectrode measurements were performed on freshly sampled sediment cores directly at the field site. We determined sediment water and total organic carbon (TOC) contents and pore water concentrations of dissolved organic carbon (DOC), nitrate, nitrite, Fe(II), and Fe(III) as previously described by Laufer et al. (44).
MPN enumeration of nitrate-reducing Fe(II)-oxidizing and Fe(III)-reducing bacteria.
Enumeration of Fe-metabolizing microorganisms was done with the original sediments used for the preparation of the microcosms (homogenized sediment from a 0- to 3-cm sediment depth). Numbers of viable cells of mixotrophic nitrate-reducing Fe(II) oxidizers and Fe(III)-reducing bacteria were determined according to the most probable number (MPN) method with artificial seawater (ASW) medium as described previously by Laufer et al. (44). It was not possible to use the MPN method to determine viable cell numbers of autotrophic nitrate-reducing Fe(II)-oxidizing bacteria due to the high organic carbon content of the sediments used for inoculation of the MPNs, which favored mixotrophic nitrate-reducing Fe(II) oxidizers and prevented us from separately quantifying autotrophs in these sediments.
Microcosm incubations with Norsminde Fjord sediment. (i) Preincubation and depletion of bioavailable organic carbon.
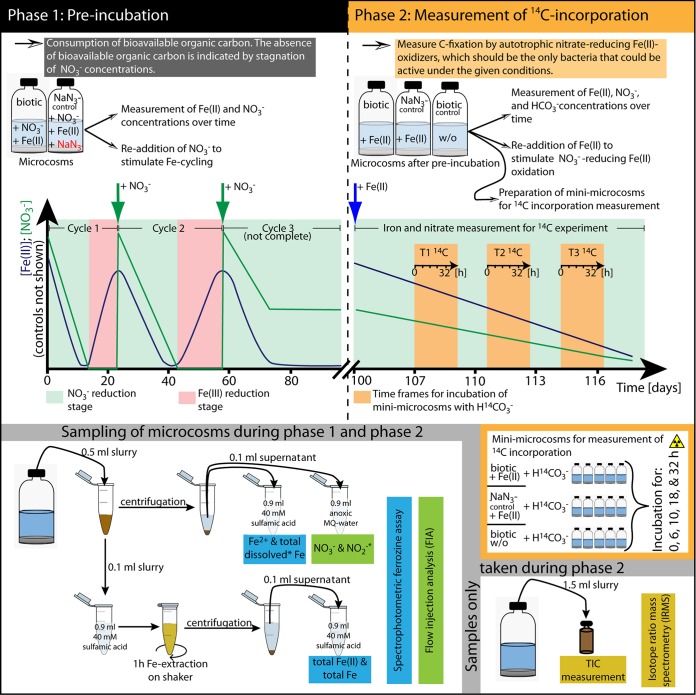
The experimental setup for the microcosm incubations is shown in Fig. 1. In the first phase, the sediment was preincubated in the presence NO3− and Fe(III)/Fe(II) in order to starve the microcosms of readily available organic carbon. Preincubation (phase 1) was composed of three iron redox cycles. In the first redox cycle, in the Fe(II) oxidation stage, NO3− was reduced by heterotrophs and autotrophs, while Fe(II) was oxidized until NO3− was depleted. The following Fe(III) reduction stage was initiated by the depletion of NO3−. Now, the heterotrophic activity shifted to Fe(III) reduction until all reactive iron was converted to Fe(II). The second and third redox cycles were then initiated by the readdition of NO3− (Fig. 1). Cyclic preincubation was continued until NO3− was no longer depleted, indicating that all readily available organic carbon was depleted.
FIG 1.
Schematic representation of the experimental setup. (Top) The microcosm experiment was divided into two phases. The purpose of the first phase was to deplete the microcosms from bioavailable organic carbon to be able to quantify the incorporation of 14CO2 in the second phase by autotrophic nitrate-reducing Fe(II) oxidizers. (Bottom) Sampling procedure. Asterisks indicate parameters that were measured only during the first phase or that were measured by a different method in the second phase. MQ, Milli-Q water.
(ii) 14C incorporation experiments.
The main incubation (phase 2) was started after 103 days by the readdition of Fe(II) to the microcosms, after which NO3− reduction resumed. As NO3− was the only available electron acceptor and Fe(II) was the only available electron donor in this carbon-starved system, the process of nitrate-reducing Fe(II) oxidation was expected to happen autotrophically. Autotrophic CO2 fixation during the main incubation was quantified via 14C incorporation in separate subsamples (mini-microcosms) (Fig. 1).
(iii) Preparation, incubation, and sampling of microcosms.
All preparation steps for the microcosms were performed in an anoxic glove box (100% N2 atmosphere). Microcosm incubations were set up with 100-ml serum vials that were wrapped twice with aluminum foil to keep them dark. Five grams of the homogenized sediment and 50 ml seawater medium were used for each microcosm. Seawater that was sampled at the field site was made anoxic by flushing with N2 for 1 h per liter water, filtering the seawater through a 0.22-μm filter (polyethersulfone [PES]) (Steritop; EMD Millipore), replacing the headspace with N2-CO2 (90:10), and adding 20 mM MOPS [3-(N-morpholino)propanesulfonic acid] as a buffer. One milliliter of vitamin solution (50), 1 ml trace-element solution (51), and 1 ml selenite-tungstate solution (52) were added for each liter of seawater medium. An anoxic and sterile Na2MoO4 solution was added to a final concentration of 20 mM to inhibit the activity of sulfate-reducing bacteria (53, 54). The pH of the medium was adjusted to 7.1 and regularly checked during incubation. The following additives (all sterile and anoxic) were added to the microcosms in the beginning of the first phase: FeCl2 (2 mM) and NaNO3 (4 mM). In total, 32 replicates of this treatment were prepared. Nine inhibited controls were prepared with the same addition of FeCl2 and NaNO3, supplemented with 160 mM NaN3. The efficiency of NaN3 inhibition was evaluated with several enrichment cultures of nitrate-reducing Fe(II) oxidizers and Fe(III) reducers at different concentrations of NaN3 (1 to 160 mM), and no Fe(II) oxidation or Fe(III) reduction was detected in these tests (see Table S1 in the supplemental material).
All microcosms were incubated at 20°C in the dark in sets of 3 replicates. In the preincubation phase of the experiment, we monitored Fe(II) and Fe(III) concentrations in the solid fraction of the slurry (extraction with 40 mM sulfamic acid, which extracts poorly crystalline Fe phases [23]) and Fe(II)/Fe(III) plus NO3− and NO2− concentrations in the dissolved phase. After 23 and 57 days, 4 mM NO3− was readded to all microcosms to initiate the second and third Fe redox cycles, respectively (Fig. 1, top left).
After 103 days of incubation, the second phase of the experiment was started. Before that, the Fe(II) concentration remained stable at ca. 0.6 mM after day 71, and no further NO3− reduction was observed after day 71. To start the second phase, 2 mM FeCl2 was added to the following preincubated microcosms from the first phase: (i) six microcosms that were preincubated with Fe(II) and NO3− and (ii) six control microcosms that were preincubated with Fe(II), NO3−, and NaN3 (inhibited control). As a control for background C fixation without an involvement of Fe(II) oxidation, six preincubated microcosms from phase 1 were incubated without any amendments (background C fixation control). In the second phase, the Fe(II) and Fe(III) concentrations in the slurry and concentrations of NO3− in the dissolved phase were monitored. Additional samples were taken for the quantification of total inorganic carbon (TIC) concentrations. At three time points during the second phase of the experiment, mini-microcosms for the quantification of 14C incorporation were prepared from the original microcosms (see below).
(iv) Preparation, incubation, and sampling of mini-microcosms.
At three time points (after 105, 109, and 113 days of incubation) during the second phase of the experiment, mini-microcosms were prepared from subsamples of the original microcosms. For the mini-microcosms, 10-ml glass vials were sterilized and closed with a butyl stopper and an aluminum crimp cap, and the headspace was flushed with N2-CO2 gas. The vials were wrapped twice with aluminum foil to exclude light. Five milliliters of the slurry was then sampled anoxically from the respective microcosm with a syringe and a thick hypodermic needle (1.2 by 40 mm) and injected into anoxic, sterile glass vials. Afterwards, the mini-microcosms were incubated overnight before the radioactive 14C tracer was added. At each of the three time points, seven mini-microcosms were prepared from (i) microcosms preincubated with Fe(II) and NO3−, to which no additives were added at the beginning of the second phase (to measure background C fixation); (ii) microcosms that were preincubated with Fe(II) and NO3−, to which Fe(II) was added at the beginning of the second phase; and (iii) control microcosms that were preincubated with Fe(II), NO3−, and NaN3, to which Fe(II) was added at the beginning of the second phase (inhibited control). To each mini-microcosm, 25 kBq of a carrier-free H14CO3− tracer was added. The mini-microcosms were incubated with the 14C tracer for different time periods ranging from 0 to 32 h (0, 6, 10, 18, and 32 h). The incubations were stopped by adding 0.25 ml of a 0.5 M Na2CO3 solution (to bring the pH above 9 and avoid the loss of nonfixed 14CO2 to the atmosphere when the vials are opened later) and freezing the mixtures at −20°C. For the microcosms that were incubated for 0 h, incubation was stopped within 1 to 2 min after the addition of the tracer. One mini-microcosm was sampled for each treatment at each time interval except after 18 h, when triplicate mini-microcosms were sampled.
Microcosm experiment with Kalø Vig sediment.
Sampling and preparation of microcosms with Kalø Vig sediment were done similarly to those of the microcosms with sediment from Norsminde Fjord. Microcosms were prepared in 100-ml serum vials, to which 5 g of homogenized sediment and 50 ml seawater medium (anoxic and sterile filtered) that was buffered with 20 mM MOPS buffer were added. The same vitamins and trace-element solutions as those used in the Norsminde Fjord medium were added. FeCl2 and NO3− were both added at a starting concentration of 2 mM. Control incubation mixtures were treated with 160 mM NaN3 (inhibited control). Similarly to the Norsminde Fjord microcosms, the concentrations of Fe(II) and total Fe were analyzed over time in the solid phase (40 mM sulfamic acid extraction) and the dissolved phase. Also, the concentrations of NO3− and NO2− were monitored over time by using the same analytical techniques as those used on the Norsminde Fjord microcosms. After 32 days, 2 mM FeCl2 was readded to all microcosms.
Analytical techniques and calculations. (i) Quantification of Fe(II), Fe(III), NO3−, NO2−, and TIC concentrations in microcosms.
Quantification of concentrations of Fe(II) and total Fe in the dissolved phase and of sulfamic acid-extractable Fe was performed by using a ferrozine assay (55). Sampling was performed as shown in Fig. 1. A flow injection analysis (FIA) instrument equipped with a dialysis membrane for removal of Fe to prevent side reactions during measurement (Seal Analytical, Germany) was applied for quantification of NO3− and NO2− concentrations. Fe(II) and total Fe concentrations in the dissolved phase were quantified only in the first phase of the experiment. In the second phase, only Fe(II) and total Fe concentrations in the sulfamic acid-extractable Fe fraction and NO3− concentrations in the dissolved phase were quantified. Concentrations of NO2− in the second phase of the experiment were evaluated with indicator sticks (MQuant nitrite test, with a detection limit of 2 mg NO2− liter−1, equal to 40 μM; Merck). In the second phase, additional samples for quantification of TIC concentrations were taken. Therefore, 1.5 ml of the slurry was sampled with a syringe and needle and transferred into a small-headspace vial that was completely filled and closed immediately. Samples were stored at 4°C until measurement. For quantification of TIC concentrations, 0.1 ml of the sample was transferred to a sealed Exetainer (Labco Limited, UK) and acidified with 0.05 ml of phosphoric acid (85%). After 24 h of equilibration, the evolved CO2 was measured in the headspace of the Exetainer by isotope ratio mass spectrometry (IRMS) (Delta V Plus; Thermo).
(ii) Calculation of rates of Fe(II) oxidation, Fe(III) reduction, and NO3− reduction and stoichiometries of NO3−reduced versus Fe(II)oxidized.
Rates of Fe(II) oxidation, Fe(III) reduction, and NO3− reduction were calculated by linear regression of the measured concentrations of the respective compounds over time at the highest rate of increasing or decreasing concentrations. At least 3 time points were used for each rate calculation. The stoichiometries of reduced NO3− (NO3−reduced) versus oxidized Fe(II) [Fe(II)oxidized] during different time intervals were calculated by dividing the amount of nitrate that was reduced during that time interval (calculated from the total decrease in nitrate concentrations, including nonlinear segments) by the amount of Fe(II) that was oxidized during that same time interval.
Incubations with H14CO3− and measurement of 14C incorporation into biomass. (i) Determination of 14C incorporation into biomass via wet fumigation.
Fixation of H14CO3− into biomass was quantified via wet fumigation according to a method described previously by Ji et al. (56), with modifications to make it suitable for sediment samples and an inorganic 14C tracer. First, the slurry from the mini-microcosm was transferred into a Falcon tube (15 ml) and centrifuged (5 min at 5,000 × g). For quantification of the amount of unreacted 14C tracer (counts per minute of dissolved inorganic carbon [cpmDIC]), 100 μl of the supernatant was added to a scintillation vial containing 900 μl CarboSorbE (PerkinElmer) and 19 ml Permafluor (PerkinElmer) scintillation cocktail. Radioactivity (in counts per minute) was determined with a liquid scintillation counter (Tri-Carb 2900TR; Packard Instruments). The sediment pellet was transferred into one side vial of a custom-made H-shaped glass vessel, of which both openings were closed with butyl rubber stoppers and a screw cap (see Fig. S1 in the supplemental material). A total of 0.5 ml of H2SO3 was then added to the sediment to decrease the pH to ca. 2, and the headspace of the vessel was flushed for 12 h with N2 while stirring the sediment-acid mixture with a magnetic stirrer to remove residual carbonate. The emerging CO2 was trapped in CarboSorbE to test if the tracer was lost to crystalline carbonate during incubation. For measurement of the amount of 14CO2 released after acidification, 100 μl of CarboSorbE was transferred into a scintillation vial containing 900 μl CarboSorbE and 19 ml Permafluor scintillation cocktail, and radioactivity was measured by liquid scintillation counting. To oxidize all organic carbon in the sediment-acid mixture left after acidification, 300 mg of K2Cr2O7 and 5 ml of K2Cr2O7 in an H2SO4 solution (12 g/100 ml) were added to the sediment and mixed well with the sediment by stirring with a magnetic stirrer. Afterwards, the tubes were autoclaved for 2 h at 121°C and then cooled to room temperature. This treatment with a strong oxidant and heat converts all organic carbon into CO2. To trap the organic-derived CO2 present in the headspace of the H tube, 1 ml of CarboSorbE was added to the second side tube of the vessel, and the mixtures in both vessels of the H-shaped glass vessel were stirred for 24 h. Finally, CarboSorbE was transferred into a scintillation vial containing 19 ml of Permafluor scintillation cocktail for quantification of the fraction of 14C incorporated into organic matter during incubation (cpmfix). In addition, the remaining waste from the wet fumigation (mixture of sediment pellet-acid-K2Cr2O7) was prepared for scintillation counting by adding 100 μl of the waste to a scintillation vial containing 900 μl CarboSorbE and 19 ml Permafluor, and radioactivity was determined by liquid scintillation counting.
(ii) Calculation of 14C incorporation rates.
The rates of CO2 incorporation per milliliter of slurry per hour at each time point were calculated according to the following equation:
| (2) |
where cpmfix is the counts from scintillation counting for 14C that was fixed into biomass (corrected for the counts per minute that were already found in the time zero control); cpmDIC is the counts from the supernatants of the respective mini-microcosms by liquid scintillation counting (back-calculated to the total amount of radioactivity in the full volume of the mini-microcosm); 1.05 is a conversion factor, as bacteria preferentially take up the lighter isotope (e.g., see reference 57); DIC is the concentration of inorganic carbon in the microcosm (millimoles per milliliter); and t is the time that the mini-microcosms was incubated with the 14C tracer.
RESULTS
Characterization of the sediment and pore water.
Visual observation of the sediment from Norsminde Fjord showed that it was oxidized in the upper ca. 0.5 cm, indicated by a light brown color. The deeper part of the sediment was black, indicating reducing conditions and the presence of FeS. Oxygen penetrated only 2 mm into the sediment. The DOC concentration in the water column was 4.9 mg liter−1, and the TOC content in the sediment was 2.9 wt% [i.e., 2.9% (wt/wt)]. The sediment from Kalø Vig had a light brown color in the uppermost centimeter and was slightly grayish below that. Oxygen penetrated the sediment to about 5 mm. The DOC concentration in the water column was 4.4 mg liter−1, and the TOC content in the sediment was 0.45 wt%. Detailed results of the geochemical measurements are shown in Table 1.
TABLE 1.
Geochemical parameters and MPN of Fe(III) reducers and nitrate-reducing Fe(II) oxidizers in sediment from Norsminde Fjord and Kalø Viga
| Parameter | Value for sediment |
|
|---|---|---|
| Norsminde Fjord | Kalø Vig | |
| % O2 saturation of water column | 100 | 100 |
| O2 penetration depth in sediment (mm) | 2 | 5 |
| Salinity | 22.1 | 23.3 |
| pH of water column | 8.2 | 7.8 |
| pH in anoxic part of sediment (sediment depth [mm]) | 7.4 (2) | 7.2 (7) |
| Redox potential in water column (mV) | +380 | +435 |
| Redox potential in sediment (mV) (sediment depth [mm]) | −98 (5) | +263 (15) |
| Mean Fe(II) concn in pore water (μmol liter−1) ± SD | 92 ± 60 | 23 ± 53 |
| Mean Fe(II) concn in sediment (1 M HCl extraction) (μmol g−1 [dry wt]) ± SD | 35 ± 21 | 0.4 ± 0.1 |
| NO3− concn in water column (μmol liter−1) | 64 | ND |
| NO2− concn in water column (μmol liter−1) | BDL | ND |
| Mean NO3− concn in pore water (μmol liter−1) ± SD | BDL | 11.66 ± 2.9 |
| NO2− concn in pore water (μmol liter−1) | BDL | BDL |
| Mean TOC concn in sediment (%) ± SD | 2.9 (per wt) | 0.45 ± 0.32 |
| Mean DOC concn in water column (mg liter−1) ± SD | 4.9 | 4.4 ± 0.1 |
| Mean MPN NO3−-reducing Fe(II) oxidizersb (cells g−1 [dry wt]) (range) | 6.9 × 103 (3.2 × 103–8.3 × 103) | 5.7 × 103 (2.4 × 103–7.9 × 103) |
| Mean MPN Fe(III) reducers (cells g−1 [dry wt]) (range) | 4.7 × 103 (9.8 × 102–6.2 × 103) | 2.9 × 102 (1.2 × 102–6.7 × 102) |
BDL, below the detection limit; ND, not determined.
MPN were determined only for mixotrophic nitrate-reducing Fe(II) oxidizers, as due to the high TOC concentration in the sediment, it was not possible to determine MPN for autotrophic nitrate-reducing Fe(II) oxidizers.
MPN enumeration of nitrate-reducing Fe(II) oxidizers and Fe(III) reducers.
We found significant numbers of mixotrophic nitrate-reducing Fe(II) oxidizers and Fe(III) reducers in the original sediment from Norsminde Fjord of 6.9 × 103 cells g−1 (dry weight) (range, 3.2 × 103 to 8.3 × 103 cells g−1 [dry weight]) and 4.7 × 103 cells g−1 (dry weight) (range, 9.9 × 102 to 6.2 × 103 cells g−1 [dry weight]), respectively (Table 1). In the sediment from Kalø Vig, numbers of viable cells of nitrate-reducing Fe(II) oxidizers and Fe(III) reducers were 5.7 × 103 cells g−1 (dry weight) (range, 2.4 × 103 to 7.9 × 103 cells g−1 [dry weight]) and 2.9 × 102 cells g−1 (dry weight) (range, 1.2 × 103 to 6.8 × 102 cells g−1 [dry weight]), respectively (Table 1).
Fe(II) oxidation and Fe(III) reduction in microcosms over time.
The microcosm experiment with sediment from Norsminde Fjord was divided into two phases (Fig. 1). The first phase consisted of three oxidation-reduction cycles, the third of which was not complete and consisted of Fe(II) oxidation only. This preincubation was intended to deplete the sediment of organic carbon readily available for heterotrophic processes. The second phase of the experiment consisted of only Fe(II) oxidation and was set up to reveal potential autotrophic nitrate-reducing Fe(II) oxidation. In Fig. 2 and 3, total Fe(II) concentrations are shown for both phases of the microcosm experiment. Table 2 provides an overview of the concentrations of Fe(II) and Fe(III) as well as the rates of Fe(II) oxidation and Fe(III) reduction. The extents of Fe(II) oxidation and Fe(III) reduction vary in different cycles. The rates of Fe(III) reduction were comparable (0.16 and 0.18 mM day−1) in redox cycles 1 and 2. Rates of Fe(II) oxidation decreased from cycle 1 (0.26 mM day−1) to cycle 2 (0.15 mM day−1) and increased slightly again in cycle 3 (0.19 mM day−1) (Table 2).
FIG 2.
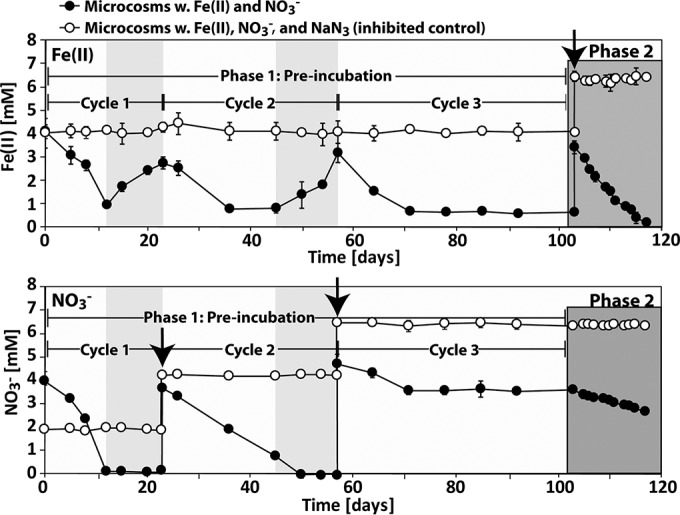
Temporal development of Fe(II) (top) and NO3− (bottom) concentrations in the first and second phases of the microcosm experiment with sediment from Norsminde Fjord. Shown are the data for total Fe(II) (extraction with 40 mM sulfamic acid) and NO3− in the dissolved phase. In the top panel, the black arrow indicates the time point at which Fe(II) was readded to the microcosms. In the bottom panel, the black arrows indicate the time points at which NO3− was readded to the microcosms. Phase 1 is the first phase of the experiment, in which nitrate was added, organic carbon was depleted, and Fe was cycled. The light gray boxes in phase 1 indicate when Fe(III) reduction was occurring. Error bars show standard deviations of data from three replicates.
FIG 3.
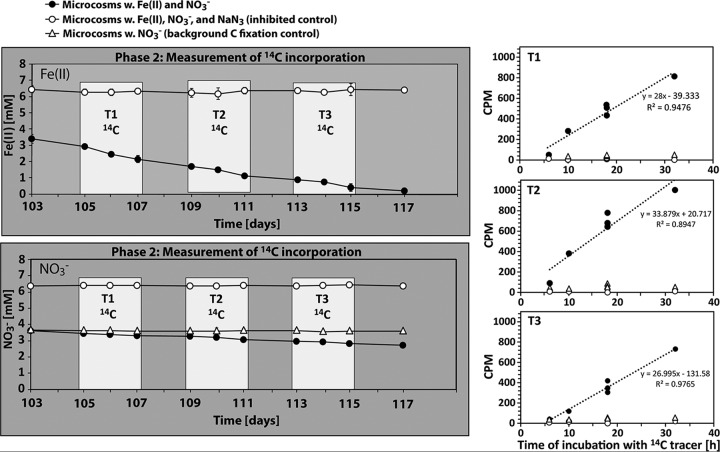
(Left) Temporal development of Fe(II) (top) and NO3− (bottom) concentrations in the second phase of the microcosm experiment with sediment from Norsminde Fjord. Shown are the data for total Fe(II) (extraction with 40 mM sulfamic acid) and NO3− in the dissolved phase. The incorporation of an inorganic 14C tracer (14C-labeled bicarbonate) into biomass was quantified in mini-microcosms at three time intervals (T1, T2, and T3) (indicated by the white boxes). Error bars show standard deviations of data from three replicates (in some cases, they are smaller than the symbol). (Right) Results of liquid scintillation counting of 14C incorporated into biomass from incubations in mini-microcosms during the three different time intervals.
TABLE 2.
Decreases of Fe(II) and NO3− concentrations and rates and stoichiometries of Fe(II) oxidation, Fe(III) reduction, and NO3− reduction in the two phases of the microcosm experiment with sediment from Norsminde Fjord
| Parameter | Value for expt |
|||
|---|---|---|---|---|
| Phase 1 |
Phase 2 | |||
| Cycle 1 | Cycle 2 | Cycle 3 | ||
| Decrease of Fe(II) concn (mM) | 3.15 | 1.98 | 2.59 | 3.20 |
| Decrease of Fe(III) concn (mM) | 1.79 | 2.41 | —a | —a |
| Decrease of NO3− concn (mM) | 3.87 | 3.69 (−1.74b) | 1.16 | 0.90 |
| Rate of Fe(II) oxidation (mM day−1) | 0.26 | 0.15 | 0.19 | 0.23 |
| Rate of Fe(III) reduction (mM day−1) | 0.16 | 0.18 | —a | —a |
| Rate of NO3− reduction (mM day−1) | 0.31 | 0.14 | 0.083 | 0.064 |
| Stoichiometry of NO3−reduced/Fe(II)oxidized | 1.23 | 0.89 | 0.44 | 0.28 |
No Fe(III) reduction was measured.
Amount of nitrate that was reduced while Fe(II) was oxidized.
The second phase of the experiment was initiated by the readdition of 2 mM Fe(II) after 103 days of incubation, when readily available organic carbon was depleted and the nitrate concentration remained constant at ca. 3.6 mM. The extent and the rate of Fe(II) oxidation in phase 2 were comparable to those for the first cycle in phase 1 (Table 2 and Fig. 2 and 3). No changes in Fe(II) concentrations were measured in NaN3-inhibited controls.
In the microcosm incubations of sediment from Kalø Vig, the Fe(II) concentration in the 40 mM sulfamic acid extract decreased continuously over the first 25 days and remained constant at ca. 0.2 mM (Fig. 4) until day 32. At that point, 2 mM FeCl2 was readded to the microcosms, and Fe(II) oxidation continued until day 88. The amounts of Fe(II) oxidized and the calculated Fe(II) oxidation rates are shown in Table 3. No changes in Fe(II) concentrations were observed in the NaN3-inhibited controls.
FIG 4.
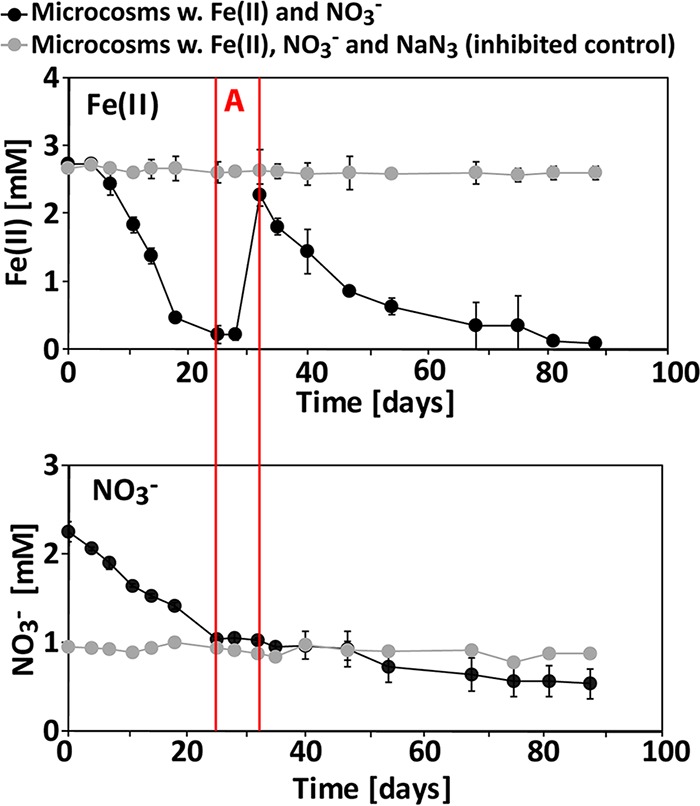
Development of Fe(II) and NO3− concentrations in a microcosm experiment with sediment from Kalø Vig. Shown are data for total Fe(II) (extraction with 40 mM sulfamic acid) and NO3− in the dissolved phase. The time interval “A,” marked by red lines in the graphs, shows that nitrate reduction had ceased because oxidizable Fe(II) was depleted, but nitrate reduction started again at day 32 when fresh Fe(II) was added. Error bars show standard deviations of data from triplicates (in some cases, they are smaller than the symbol).
TABLE 3.
Decreases of Fe(II) and NO3− concentrations and rates and stoichiometries of Fe(II) oxidation and NO3− reduction at different time intervals in the microcosm experiment with sediment from Kalø Vig
| Time of incubation (days) | Decrease of Fe(II) concn (mM) [rate of Fe(II) oxidation (mM day−1)] | Decrease of NO3− concn (mM) (rate of NO3− oxidation [mM day−1]) | Stoichiometry of NO3−reduced/Fe(II)oxidized |
|---|---|---|---|
| 0–25 | 2.5 (0.10) | 1.25 (0.05) | 0.49 |
| 32–88 | 2.17 (0.039) | 0.47 (0.008) | 0.22 |
Nitrate and nitrite concentrations in microcosms over time.
The temporal development of the NO3− concentrations in microcosms from Norsminde Fjord is shown in Fig. 2 and 3. In the first phase of the experiment, NO3− was completely consumed in the first and second redox cycles after day 12 and day 50 (Fig. 2). In the third redox cycle, however, only 1.16 mM NO3− was reduced until day 71, and from that time point on, the NO3− concentration remained constant at 3.6 ± 0.05 mM until day 103, when Fe(II) was replenished. The rates of NO3− reduction decreased throughout the duration of the experiment (Table 2). Nitrite accumulated only during the first Fe redox cycle in phase 1, where it reached a maximum concentration of 0.62 ± 0.23 mM after 12 days. During the following cycles, maximum nitrite concentrations were <50 μM (see Fig. S2 in the supplemental material). In the NaN3-inhibited controls, NO3− concentrations remained constant over time, and no accumulation of NO2− was observed (Fig. 2; see also Fig. S2 in the supplemental material).
In the microcosms with sediment from Kalø Vig, NO3− concentrations decreased except between days 25 and 32, when concentrations remained constant (Fig. 4). The rates of NO3− reduction were higher at the beginning of the incubation than toward the end (Table 3). NO2− was found to accumulate only between days 4 and 35, with a maximum concentration of 0.27 mM after 11 and 14 days (see Fig. S3 in the supplemental material). In the NaN3-inhibited controls, NO3− concentrations remained constant over time, and no accumulation of NO2− was observed (Fig. 4; see also Fig. S3 in the supplemental material).
Stoichiometries of NO3−reduced versus Fe(II)oxidized.
The ratio of reduced NO3− to oxidized Fe(II) changed significantly during the microcosm incubation experiment. In microcosms with sediment from Norsminde Fjord, 1.23 mol of NO3− was initially reduced for every mole of Fe(II) oxidized (Table 2). The ratio decreased throughout the experiment, and during the second phase of the experiment, only 0.28 mol of NO3− was reduced per mol of Fe(II) oxidized. Similarly, in microcosms with sediment from Kalø Vig, the ratio of reduced NO3− to oxidized Fe(II) changed from initially 0.49 to 0.22 over time (Table 3).
14C incorporation into biomass during nitrate-reducing Fe(II) oxidation.
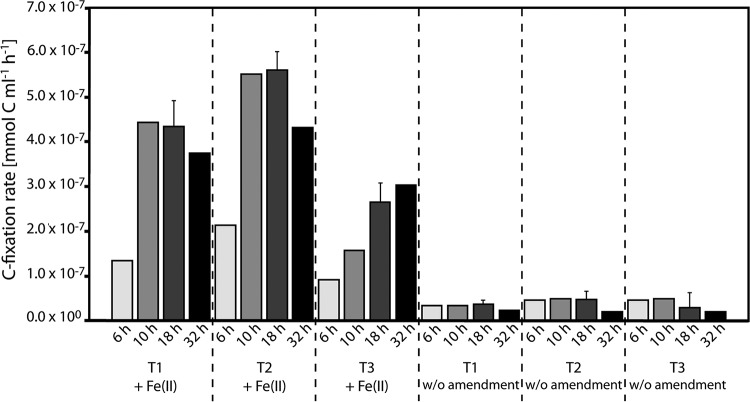
To demonstrate the activity of autotrophic nitrate-reducing Fe(II)-oxidizing bacteria, we quantified the incorporation of 14C-labeled bicarbonate into biomass during the second phase of the experiment, i.e., when we saw Fe(II) oxidation coupled to nitrate reduction after organic carbon depletion. We found that background 14C incorporation in the control microcosms that contained NO3− but no Fe(II) amendment was about 5 times higher (3.8 × 10−8 ± 1.1 × 10−8 mmol of C ml−1 h−1) than that in the NaN3-inhibited controls (7.2 × 10−9 ± 1.1 × 10−8 mmol of C ml−1 h−1) (Fig. 3). The addition of Fe(II) to the microcosms increased the rate of 14C incorporation to 3.8 × 10−7 ± 1.6 × 10−7 mmol of C ml−1 h−1 (Fig. 5), i.e., ∼10-fold higher than that of the Fe-free controls and 50-fold higher than that of the NaN3-inhibited controls (Fig. 3).
FIG 5.
C fixation rates measured in mini-microcosms during three time intervals in phase 2 of the experiment. Rates from incubations where Fe(II) was added (NO3− was still present after preincubation) are shown on the left. Rates from incubations where no Fe(II) was added and also no consumption of NO3− was observed over time (NO3− was still present after preincubation) are shown on the right. Error bars show standard deviations of data for three samples (available for the 18-h samples only).
Stoichiometries of Fe(II)oxidized per Cfixed.
To determine how many moles of Fe(II) were oxidized by autotrophic nitrate-reducing Fe(II) oxidizers to fix 1 mol of CO2, we plotted the amount of Fe(II) oxidation against the amount of CO2 fixation during the second phase of the experiment and applied linear regression. The linear regression showed that 26.5 mol of Fe(II) was oxidized per mol of fixed CO2 (R2 = 0.85) (see Fig. S4 in the supplemental material).
DISCUSSION
Transition from heterotrophic denitrification and chemodenitrification to autotrophic Fe(II) oxidation coupled to nitrate reduction.
This study shows evidence of the presence of autotrophic nitrate-reducing Fe(II) oxidizers in sediment from Norsminde Fjord and Kalø Vig. We quantified the contributions of heterotrophic nitrate reduction versus autotrophic nitrate-reducing Fe(II) oxidation based on stoichiometries of NO3−reduced versus Fe(II)oxidized. In microcosm experiments with both sediments, we found a decreasing activity of heterotrophic nitrate reduction over time due to the gradual depletion of readily available organic carbon. This was concluded based on the continuous decrease of the ratio of NO3−reduced to Fe(II)oxidized throughout the experiment. In microcosms from Norsminde Fjord and Kalø Vig, the ratios decreased from 1.23 and 0.49 at the beginning of the experiment to 0.28 and 0.22 at the end, respectively (Tables 2 and 3).
As can be seen from equation 1, a molar stoichiometry of 0.2 for reduced nitrate per Fe(II)oxidized would be indicative of nitrate-reducing Fe(II) oxidation with N2 as the end product. However, some of the electrons from Fe(II) oxidation must be used for the reduction of CO2 (for biomass production) rather than the reduction of nitrate (for energy generation). In our microcosm experiments with sediment from Norsminde Fjord, 26.5 mol of Fe(II) was oxidized to fix 1 mol of carbon (Table 3). Based on this value, and the theoretical value of 4 mol of electrons that is needed to fix 1 mol of carbon, the stoichiometry of reduced nitrate per Fe(II)oxidized for autotrophic nitrate-reducing Fe(II) oxidation should be 0.17.
We found initially high ratios of NO3−reduced to Fe(II)oxidized in the Norsminde Fjord microcosms. This implies that early nitrate reduction in the sediment microcosms was due primarily to heterotrophic nitrate reduction or mixotrophic nitrate-reducing Fe(II) oxidation. In the first phase of the experiment, even if all measured Fe(II) oxidation was microbially catalyzed, only 16, 23, and 45% of the measured nitrate reductions could be attributed to microbial nitrate-reducing Fe(II) oxidation in the first, second, and third redox cycles, respectively. However, the occurrence of chemodenitrification (58, 59) or Fe(III) reduction [which would replenish some Fe(II)] could lead to an over- or underestimation of the contribution of nitrate-reducing Fe(II) oxidizers, especially in the beginning of the microcosm incubations. Nitrite was formed only at the beginning of the incubation (accumulation of maximums of 0.62 mM after 12 days and 0.27 mM after 14 days in microcosms from Norsminde Fjord and Kalø Vig, respectively). After day 20 and day 25 for Norsminde Fjord and Kalø Vig microcosms, respectively, nitrite accumulation of >0.04 mM was no longer detected. As this shift in nitrite production is correlated with the expected shift of the relative contribution from mostly heterotrophic processes to an increasing importance of autotrophic processes, we assume that nitrite production in the presence of Fe(II) is linked to heterotrophic nitrate reduction, as outlined previously by Klueglein et al. (26).
Bioavailability of Fe(II) and Fe extraction with sulfamic acid.
One of the main differences between the redox processes in the second phase and those in the first phase of the experiment was the concentration of nonoxidized Fe(II) in the 40 mM sulfamic acid extract. In the first phase, 0.9, 0.7, and 0.6 mM Fe(II) remained nonoxidized in redox cycles 1, 2, and 3, respectively. At these time points, when the total Fe(II) concentrations were not decreasing further, dissolved Fe(II) was no longer present (data not shown). In the second cycle, sufficient NO3− (ca. 2 mM) was present (and also, NO3− reduction continued), while no changes in Fe(II) concentrations (remaining at ca. 0.7 mM) in the 40 mM sulfamic acid extract were measured. This indicated that not all of the 40 mM sulfamic acid-extractable Fe(II) was available for microbial oxidation. In the second phase of the experiment, however, only 0.2 mM Fe(II) remained nonoxidized. The lower value of nonoxidized Fe(II) in phase 2 than that in phase 1 could be due to a higher crystallinity of Fe minerals in the microcosms in phase 2, leading to a lower extraction efficiency of 40 mM sulfamic acid. Alternatively, it could be due to increased Fe(II) bioavailability and Fe(II) oxidation caused by a change in Fe(II) mineralogy or a change/adaptation of the Fe(II)-oxidizing bacterial community during the transition from phase 1 to phase 2 caused by repeated Fe cycling.
Nitrate consumption and potential formation of the N intermediates N2O and NO.
In the second phase of the Norsminde Fjord microcosm incubation, the NO3−reduced/Fe(II)oxidized stoichiometry was 0.28. The calculated stoichiometry for autotrophic nitrate-reducing Fe(II) oxidation is 0.17 (see above). Taking this stoichiometry and the measured decrease in the Fe(II) concentration of 3.2 mM, we assign 0.55 mM the consumed NO3− to Fe(II) oxidation. Since we measured a decrease in the NO3− concentration of 0.9 mM, this yields 0.35 mM NO3− disappearing that cannot be attributed to autotrophic nitrate-reducing Fe(II) oxidation. However, we analyzed only nitrate and nitrite concentrations in solution and no other N products or N intermediates of nitrate reduction. Several other processes could lead to a higher decrease in nitrate concentrations than expected; e.g., (i) nitrate reduction by heterotrophic or mixotrophic bacteria (despite the depletion of carbon during the first phase), (ii) storage of nitrate inside microbial cells, or (iii) incomplete reduction of nitrate to intermediate products such as NO or N2O could lead to an increased value for NO3−reduced versus Fe(II)oxidized. In the control incubation, where no Fe(II) was added in the beginning of the second phase, no measurable decrease in the NO3− concentration was observed. Therefore, it is unlikely that heterotrophic nitrate reduction occurred to any substantial degree in the microcosms to which Fe(II) was added. Also, even assuming the highest reported microbial storage capacities for nitrate (60, 61), with the low numbers of cells of nitrate-reducing Fe(II) oxidizers present, a substantial contribution of nitrate removal by intracellular storage is also unlikely. Therefore, the most likely explanation is that not all of the nitrate was reduced to N2, but rather, it was reduced only to N intermediates, such as N2O or NO, which is common for nitrate reducers (62, 63).
Autotrophic nitrate-reducing Fe(II) oxidation depending on carbon availability.
The sediment from Norsminde Fjord contained around 3 wt% TOC. To detect the activity of autotrophic nitrate-reducing Fe(II) oxidizers, it was necessary to preincubate the sediment (phase 1) for >3 months to deplete the bioavailable organic carbon. The presence of bioavailable organic carbon and the resulting activity of heterotrophic nitrate-reducing bacteria, as well as possible abiotic side reactions, would have complicated 14C incorporation measurements in phase 1 for several reasons. First, it is likely that as long as there is still readily available organic carbon present, bacteria with the potential for autotrophic nitrate-reducing Fe(II) oxidation would instead grow heterotrophically or mixotrophically. Second, due to the activity of heterotrophic nitrate reducers in the first phase of the experiment, a significant fraction of Fe(II) oxidation in the beginning of the experiment could have happened via chemodenitrification by reaction with reactive nitrogen species produced by heterotrophic bacteria during nitrate reduction (58, 59). These abiotic reactions would have made it impossible to calculate the ratio of oxidized Fe(II) to fixed carbon. Third, carbon fixation by heterotrophic bacteria in anaplerotic reactions typically accounts for 5 to 10% of the total biomass produced by heterotrophic bacteria (64–66). This substantial amount of C fixation could have masked the expected low carbon fixation rates by autotrophic nitrate-reducing Fe(II) oxidizers at the early stages of the experiment.
However, after the long preincubation, we were able to detect and quantify autotrophic 14C fixation by nitrate-reducing Fe(II) oxidizers, although it is unclear whether these bacteria perform this process in situ in this organic-rich sediment. Our study rather suggests that a habitat with relatively high nitrate and Fe(II) concentrations combined with a low organic carbon content would be most suitable for studying the activity of autotrophic nitrate-reducing Fe(II) oxidizers. Under such conditions, they would not have to compete with heterotrophic nitrate reducers for nitrate, and because of organic carbon limitation, they would have a competitive advantage when living autotrophically. However, the organic carbon content should also not be too low. Otherwise, heterotrophic Fe(III) reduction would be limiting, and the nitrate-reducing Fe(II) oxidizers would have a smaller supply of Fe(II). Consequently, we expect that autotrophic nitrate-reducing Fe(II)-oxidizing bacteria play only a minor role in Fe(II) oxidation in the TOC-rich sediments of Norsminde Fjord. Indeed, the microcosm experiments with the low-TOC sediment from Kalø Vig (TOC concentration of 0.45 wt%) already showed evidence of autotrophic nitrate-reducing Fe(II)-oxidizing activity after a much shorter time than in experiments with the higher-TOC sediments from Norsminde Fjord. We therefore suggest that the Kalø Vig field site is suitable for further investigations of autotrophic nitrate-reducing Fe(II) oxidation. However, based on the low cell numbers for nitrate-reducing Fe(II) oxidizers that we determined by MPN analyses, it remains to be determined how relevant microbial nitrate-reducing Fe(II) oxidation is in the environment. A recent study by Jewell et al. (67) highlighted the importance of chemolithoautotrophic nitrate-reducing microorganisms in an oligotrophic groundwater environment. Based on metatranscriptomic analyses, those authors showed that after injection of nitrate, the abundances of Fe(II)-oxidizing Gallionellaceae and S-oxidizing Sulfurimonas spp. were increasing. Based on the limited knowledge about these microorganisms, it is difficult to predict or evaluate how important sulfide-oxidizing nitrate reducers are at the field sites that were investigated in the present study compared to Fe(II)-oxidizing nitrate reducers. At least in our microcosm incubations where sulfate reduction was inhibited, no sulfide was produced, and therefore, S-oxidizing nitrate reducers should not have been active.
Fe(II) oxidation required for CO2 fixation and for energy generation.
We calculated that ca. 26 mol of Fe(II) was oxidized by nitrate-reducing Fe(II) oxidizers to fix 1 mol of carbon. To calculate how much energy is needed by nitrate-reducing Fe(II) oxidizers to fix 1 mol of carbon, we use a value of 1 mol of carbon fixation from CO2 that requires 4 mol of electrons (in the form of reducing equivalents, such as, e.g., NADH) and energy in the form of ATP. Consequently, if nitrate-reducing Fe(II) oxidizers oxidize 26.5 mol Fe(II) in order to fix 1 mol of carbon, and they need only 4 mol electrons/reducing equivalents for the fixation of 1 mol of carbon, they can use the other 22.5 mol of Fe(II) for energy generation.
From the estimated energy gain of −96.23 kJ/mol Fe(II)oxidized (equation 1), we calculate that nitrate-reducing Fe(II) oxidizers need −2,165 kJ per mol CO2 fixed. This value is within the range reported previously for other chemolithoautotrophs such as aerobic sulfide oxidizers (68). Furthermore, we calculate that ∼15% of the electrons from Fe(II) are used for carbon fixation. This is also in good agreement with what was reported previously for other chemolithoautotrophs (usually 10 to 20% [69–72]). The molar ratios of the oxidized substrate to fixed carbon for other chemolithoautotrophs are, e.g., 2:1 to 5:1 for aerobic sulfur oxidizers (depending on the sulfur species and the electron acceptor [66, 72–74]), 10:1 for ammonium oxidizers (75, 76), and 25:1 to 80:1 for nitrite oxidizers (77). Calculating how many electrons these microbes need to transfer to fix 1 mol of CO2, this translates to a range of 16 to 40 for sulfur oxidizers, ca. 80 for ammonium oxidizers, and 50 to 160 for nitrite oxidizers. Thus, the amount of electrons transferred by nitrate-reducing Fe(II) oxidizers to fix 1 mol of C (ca. 26) is in the lower range and is most similar to what is known for aerobic sulfide oxidizers. The energy gain of sulfur oxidation per mole of electrons transferred is −99.25 kJ/mol (oxidation of sulfide to sulfate with oxygen as the electron acceptor [78]), which is similar to the energy yield of nitrate-reducing Fe(II) oxidizers (equation 1).
The values for oxidized Fe(II) per fixed C that we found for nitrate-reducing Fe(II) oxidizers are significantly lower than what was estimated previously for microaerophilic Fe(II) oxidizers, for which values of 43 to 70 mol oxidized Fe(II) per mol of fixed C were suggested (79, 80). However, these values probably contain large uncertainties due to the difficulties that are related to the accurate quantification of microbial microaerophilic Fe(II) oxidation rates, which compete with abiotic Fe(II) oxidation rates.
Biomass production during autotrophic nitrate-reducing Fe(II) oxidation.
The rates of C fixation from all measurements in mini-microcosms that contained nitrate and Fe(II) were on average 2.9 × 10−7 ± 1.6 × 10−7 mmol C ml−1 h−1. Based on these C fixation rates, within the 14 days of the second phase of the experiment, there was ∼9.7 × 10−4 mmol C fixed per ml sediment slurry. This approximately equals the amount of C that is present in 5 × 106 cells that could have been produced per ml of slurry (assuming 0.2 × 10−12 g per cell [dry weight] and a carbon content of 50% [dry weight]). However, this is only a rough estimation, and nitrate-reducing Fe(II)-oxidizing bacteria can produce substantial amounts of extracellular polymeric substances (EPSs) (26). Therefore, an unknown amount of the fixed carbon may not be going into the production of new cells but rather may be going to EPS production.
Conclusions and outlook.
Our microcosm data on the stoichiometry of nitrate reduced per Fe(II) oxidized and 14CO2 incorporated provide evidence for the presence and activity of autotrophic nitrate-reducing Fe(II) oxidation in a marine sediment. Our study suggests that autotrophic nitrate-reducing Fe(II) oxidation is most likely to occur in sediments with low or intermediate organic carbon concentrations.
Our study provides the basis for several further investigations. In particular, it will now be possible to identify autotrophic nitrate-reducing bacteria in the Norsminde Fjord and Kalø Vig sediments, e.g., by DNA-SIP (stable isotope probing). Additionally, if it is known which bacteria are oxidizing Fe(II) autotrophically, this will assist in isolation attempts, as it can be evaluated whether these bacteria or closely related bacteria have special demands, e.g., regarding vitamins or growth conditions. Finally, in environments that are likely to support the activity of autotrophic nitrate-reducing Fe(II) oxidizers in situ, such as the Kalø Vig field site, the general environmental relevance of this process can be determined.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ellen Struve, Karina Bomholt Oest, Susanne Nielsen, Anne Stentebjerg, and Trine Bech Søgaard for technical assistance and help with measurements. Furthermore, we thank Bente A. Lomstein for supervision and discussion of the radiotracer experiments.
This project was supported by the European Research Council under European Union Seventh Framework Program (FP/2007-2013)/ERC grant agreements 307320-Microfox and 294200-Microenergy.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01570-16.
REFERENCES
- 1.Rankama K, Sahama TG. 1950. Geochemistry. University of Chicago Press, Chicago, IL. [Google Scholar]
- 2.Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A. 2014. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808. doi: 10.1038/nrmicro3347. [DOI] [PubMed] [Google Scholar]
- 3.Emerson D, Moyer C. 1997. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol 63:4784–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B. 1993. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362:834–836. doi: 10.1038/362834a0. [DOI] [Google Scholar]
- 5.Straub KL, Benz M, Schink B, Widdel F. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62:1458–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovley DR, Phillips EJP. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovley DR, Phillips EJP, Lonergan DJ. 1989. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol 55:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canfield DE. 1989. Reactive iron in marine sediments. Geochim Cosmochim Acta 53:619–632. doi: 10.1016/0016-7037(89)90005-7. [DOI] [PubMed] [Google Scholar]
- 9.Martin JH, Gordon M, Fitzwater SE. 1991. The case for iron. Limnol Oceanogr 36:1793–1802. doi: 10.4319/lo.1991.36.8.1793. [DOI] [Google Scholar]
- 10.Boyd PW, Jickells T, Law C, Blain S, Boyle E, Buesseler K, Coale K, Cullen J, De Baar H, Follows M. 2007. Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science 315:612–617. doi: 10.1126/science.1131669. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Caccavo F, Fendorf S, Rosenzweig RF. 1999. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ Sci Technol 33:723–729. doi: 10.1021/es980541c. [DOI] [Google Scholar]
- 12.Kim S, Picardal FW. 1999. Enhanced anaerobic biotransformation of carbon tetrachloride in the presence of reduced iron oxides. Environ Toxicol Chem 18:2142–2150. doi: 10.1002/etc.5620181005. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DC, Picardal F, Rivera J, Talbot C. 2000. Zinc immobilization and magnetite formation via ferric oxide reduction by Shewanella putrefaciens 200. Environ Sci Technol 34:100–106. doi: 10.1021/es990510x. [DOI] [Google Scholar]
- 14.Zachara JM, Fredrickson JK, Smith SC, Gassman PL. 2001. Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim Cosmochim Acta 65:75–93. doi: 10.1016/S0016-7037(00)00500-7. [DOI] [Google Scholar]
- 15.Borch T, Kretzschmar R, Kappler A, Cappellen PV, Ginder-Vogel M, Voegelin A, Campbell K. 2010. Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23. doi: 10.1021/es9026248. [DOI] [PubMed] [Google Scholar]
- 16.Muehe EM, Kappler A. 2014. Arsenic mobility and toxicity in South and South-East Asia—a review on biogeochemistry, health and socio-economic effects, remediation and risk predictions. Environ Chem 11:483–495. doi: 10.1071/EN13230. [DOI] [Google Scholar]
- 17.Kappler A, Emerson D, Gralnick JA, Roden EE, Muehe EM. 2015. Geomicrobiology of iron, p 343–399. In Ehrlich HL, Newman DK, Kappler A (ed), Ehrlich's geomicrobiology, 6th ed CRC Press, Boca Raton, FL. [Google Scholar]
- 18.Straub KL, Schonhuber WA, Buchholz-Cleven BEE, Schink B. 2004. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol J 21:371–378. doi: 10.1080/01490450490485854. [DOI] [Google Scholar]
- 19.Kappler A, Schink B, Newman DK. 2005. Fe(III) mineral formation and cell encrustation by Fe(III) minerals precipitated by nitrate-dependent Fe(II)-oxidizers the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology 3:235–245. doi: 10.1111/j.1472-4669.2006.00056.x. [DOI] [Google Scholar]
- 20.Muehe EM, Gerhardt S, Schink B, Kappler A. 2009. Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate-reducing bacteria. FEMS Microbiol Ecol 70:335–343. doi: 10.1111/j.1574-6941.2009.00755.x. [DOI] [PubMed] [Google Scholar]
- 21.Byrne-Bailey KG, Weber KA, Bose S, Knox T, Spanbauer TL, Chertkov O, Coates JD. 2010. Completed genome sequence of the anaerobic iron-oxidizing bacterium Acidovorax ebreus strain TPSY. J Bacteriol 192:1475–1476. doi: 10.1128/JB.01449-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty A, Roden EE, Schieber J, Picardal F. 2011. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl Environ Microbiol 77:8548–8556. doi: 10.1128/AEM.06214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klueglein N, Kappler A. 2013. Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1—questioning the existence of enzymatic Fe(II) oxidation. Geobiology 11:180–190. doi: 10.1111/gbi.12019. [DOI] [PubMed] [Google Scholar]
- 24.Carlson HK, Clark IC, Blazewicz SJ, Iavarone AT, Coates JD. 2013. Fe(II) oxidation is an innate capability of nitrate-reducing bacteria that involves abiotic and biotic reactions. J Bacteriol 195:3260–3268. doi: 10.1128/JB.00058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty A, Picardal F. 2013. Induction of nitrate-dependent Fe(II) oxidation by Fe(II) in Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Appl Environ Microbiol 79:748–752. doi: 10.1128/AEM.02709-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klueglein N, Zeitvogel F, Stierhof Y-D, Floetenmeyer M, Konhauser KO, Kappler A, Obst M. 2014. Potential role of nitrite for abiotic Fe(II) oxidation and cell encrustation during nitrate reduction by denitrifying bacteria. Appl Environ Microbiol 80:1051–1061. doi: 10.1128/AEM.03277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schädler S, Burkhardt C, Hegler F, Straub KL, Miot J, Benzerara K, Kappler A. 2009. Formation of cell-iron-mineral aggregates by phototrophic and nitrate-reducing anaerobic Fe(II)-oxidizing bacteria. Geomicrobiol J 26:93–103. doi: 10.1080/01490450802660573. [DOI] [Google Scholar]
- 28.Hegler F, Schmidt C, Schwarz H, Kappler A. 2010. Does a low-pH microenvironment around phototrophic FeII-oxidizing bacteria prevent cell encrustation by Fe(III) minerals? FEMS Microbiol Ecol 74:592–600. doi: 10.1111/j.1574-6941.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- 29.Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ. 2011. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J 5:717–727. doi: 10.1038/ismej.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallbeck L, Pedersen K. 1995. Benefits associated with the stalk of Gallionella ferruginea, evaluated by comparison of a stalk-forming and a non-stalk-forming strain and biofilm studies in situ. Microb Ecol 30:257–268. [DOI] [PubMed] [Google Scholar]
- 31.Brons HJ, Hagen WR, Zehnder AJ. 1991. Ferrous iron dependent nitric oxide production in nitrate reducing cultures of Escherichia coli. Arch Microbiol 155:341–347. [DOI] [PubMed] [Google Scholar]
- 32.Weber KA, Pollock J, Cole KA, O'Connor SM, Achenbach LA, Coates JD. 2006. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl Environ Microbiol 72:686–694. doi: 10.1128/AEM.72.1.686-694.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hafenbradl D, Keller M, Dirmeier R, Rachel R, Rossnagel P, Burggraf S, Huber H, Stetter KO. 1996. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol 166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- 34.Kumaraswamy R, Sjollema K, Kuenen G, van Loosdrecht M, Muyzer G. 2006. Nitrate-dependent [Fe(II)EDTA]-oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor. Syst Appl Microbiol 29:276–286. doi: 10.1016/j.syapm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Finneran KT, Housewright ME, Lovley DR. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ Microbiol 4:510–516. doi: 10.1046/j.1462-2920.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 36.Shelobolina ES, VanPraagh CG, Lovley DR. 2003. Use of ferric and ferrous iron containing minerals for respiration by Desulfitobacterium frappieri. Geomicrobiol J 20:143–156. doi: 10.1080/01490450303884. [DOI] [Google Scholar]
- 37.Li B, Tian C, Zhang D, Pan X. 2013. Anaerobic nitrate-dependent iron(II) oxidation by a novel autotrophic bacterium, Citrobacter freundii strain PXL1. Geomicrobiol J 31:138–144. [Google Scholar]
- 38.Zhou J, Wang H, Yang K, Ji B, Chen D, Zhang H, Sun Y, Tian J. 2016. Autotrophic denitrification by nitrate-dependent Fe(II) oxidation in a continuous up-flow biofilter. Bioprocess Biosyst Eng 39:277–284. doi: 10.1007/s00449-015-1511-7. [DOI] [PubMed] [Google Scholar]
- 39.He S, Tominski C, Kappler A, Behrens S, Roden EE. 2016. Metagenomic analyses of the autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture KS. Appl Environ Microbiol 82:2656–2668. doi: 10.1128/AEM.03493-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melton ED, Schmidt C, Kappler A. 2012. Microbial iron(II) oxidation in littoral freshwater lake sediment: the potential for competition between phototrophic vs. nitrate-reducing iron(II)-oxidizers. Front Microbiol 3:197. doi: 10.3389/fmicb.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emmerich M, Bhansali A, Losekann-Behrens T, Schroder C, Kappler A, Behrens S. 2012. Abundance, distribution, and activity of Fe(II)-oxidizing and Fe(III)-reducing microorganisms in hypersaline sediments of Lake Kasin, southern Russia. Appl Environ Microbiol 78:4386–4399. doi: 10.1128/AEM.07637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelobolina E, Konishi H, Xu H, Benzine J, Xiong MY, Wu T, Blöthe M, Roden E. 2012. Isolation of phyllosilicate-iron redox cycling microorganisms from an illite-smectite rich hydromorphic soil. Front Microbiol 3:134. doi: 10.3389/fmicb.2012.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe AR, Chellamuthu P, Lam B, Okamoto A, Nealson K. 2015. Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism. Front Microbiol 5:784. doi: 10.3389/fmicb.2014.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laufer K, Nordhoff M, Røy H, Schmidt C, Behrens S, Jørgensen BB, Kappler A. 2016. Coexistence of microaerophilic, nitrate-reducing, and phototrophic Fe(II) oxidizers and Fe(III) reducers in coastal marine sediment. Appl Environ Microbiol 82:1433–1447. doi: 10.1128/AEM.03527-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber KA, Urrutia MM, Churchill PF, Kukkadapu RK, Roden EE. 2006. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ Microbiol 8:100–113. doi: 10.1111/j.1462-2920.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- 46.Coby AJ, Picardal F, Shelobolina E, Xu H, Roden EE. 2011. Repeated anaerobic microbial redox cycling of iron. Appl Environ Microbiol 77:6036–6042. doi: 10.1128/AEM.00276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benzine J, Shelobolina E, Xiong MY, Kennedy DW, McKinley JP, Lin X, Roden EE. 2013. Fe-phyllosilicate redox cycling organisms from a redox transition zone in Hanford 300 area sediments. Front Microbiol 4:388. doi: 10.3389/fmicb.2013.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson EK, Roberts KL, Burdorf LD, Cook P, Thamdrup B. 2016. Dissimilatory nitrate reduction to ammonium coupled to Fe(II) oxidation in sediments of a periodically hypoxic estuary. Limnol Oceanogr 61:365–381. doi: 10.1002/lno.10220. [DOI] [Google Scholar]
- 49.Laufer K, Byrne JM, Glombitza C, Schmidt C, Jørgensen BB, Kappler A. 27 May 2016. Anaerobic microbial Fe(II) oxidation and Fe(III) reduction in coastal marine sediments controlled by organic carbon content. Environ Microbiol doi: 10.1111/1462-2920.13387. [DOI] [PubMed] [Google Scholar]
- 50.Widdel F, Pfennig N. 1981. Studies on dissimilatory sulfate-reducing bacteria that compose fatty acids. I. Isolation of a new sulfate-reducer enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov. sp. nov. Arch Microbiol 129:395–400. [DOI] [PubMed] [Google Scholar]
- 51.Tschech A, Pfennig N. 1984. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol 137:163–167. doi: 10.1007/BF00414460. [DOI] [Google Scholar]
- 52.Widdel F. 1980. Anaerober Abbau von Fettsäuren und Benzoesäure durch neu isolierte Arten Sufat reduzierender Bakterien. PhD thesis Universität Göttingen, Göttingen, Germany. [Google Scholar]
- 53.Sørensen J. 1982. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol 43:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parkes R, Gibson G, Mueller-Harvey I, Buckingham W, Herbert R. 1989. Determination of the substrates for sulphate-reducing bacteria within marine and estuarine sediments with different rates of sulphate reduction. J Gen Microbiol 135:175–187. [Google Scholar]
- 55.Stookey LL. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779–781. doi: 10.1021/ac60289a016. [DOI] [Google Scholar]
- 56.Ji R, Kappler A, Brune A. 2000. Transformation and mineralization of synthetic 14C-labeled humic model compounds by soil-feeding termites. Soil Biol Biochem 32:1281–1291. doi: 10.1016/S0038-0717(00)00046-8. [DOI] [Google Scholar]
- 57.Cibic T, Blasutto O, Burba N, Fonda Umani S. 2008. Microphytobenthic primary production as 14C uptake in sublittoral sediments of the Gulf of Trieste (northern Adriatic Sea): methodological aspects and data analyses. Estuar Coast Shelf Sci 77:113–122. doi: 10.1016/j.ecss.2007.09.005. [DOI] [Google Scholar]
- 58.Wullstein LH, Gilmour CM. 1966. Non-enzymatic formation of nitrogen gas. Nature 210:1150–1151. doi: 10.1038/2101150a0. [DOI] [Google Scholar]
- 59.Picardal F. 2012. Abiotic and microbial interactions during anaerobic transformations of Fe(II) and NOx. Front Microbiol 3:112. doi: 10.3389/fmicb.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fossing H, Gallardo VA, Jorgensen BB, Huttel M, Nielsen LP, Schulz H, Canfield DE, Forster S, Glud RN, Gundersen JK, Kuver J, Ramsing NB, Teske A, Thamdrup B, Ulloa O. 1995. Concentration and transport of nitrate by the mat-forming sulphur-bacterium Thioploca. Nature 374:713–715. doi: 10.1038/374713a0. [DOI] [Google Scholar]
- 61.Jørgensen BB, Gallardo VA. 1999. Thioploca spp.: filamentous sulfur bacteria with nitrate vacuoles. FEMS Microbiol Ecol 28:301–313. doi: 10.1111/j.1574-6941.1999.tb00585.x. [DOI] [Google Scholar]
- 62.Betlach MR, Tiedje JM. 1981. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol 42:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Firestone MK, Davidson EA. 1989. Microbiological basis of NO and N2O production and consumption in soil, p 7–21. In Andreae MO, Schimel DS (ed), Exchange of trace gases between terrestrial ecosystems and the atmosphere. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 64.Owen OE, Kalhan SC, Hanson RW. 2002. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 65.Molari M, Manini E, Dell'Anno A. 2013. Dark inorganic carbon fixation sustains the functioning of benthic deep-sea ecosystems. Global Biogeochem Cycles 27:212–221. doi: 10.1002/gbc.20030. [DOI] [Google Scholar]
- 66.Boschker HTS, Vasquez-Cardenas D, Bolhuis H, Moerdijk-Poortvliet TWC, Moodley L. 2014. Chemoautotrophic carbon fixation rates and active bacterial communities in intertidal marine sediments. PLoS One 9:e101443. doi: 10.1371/journal.pone.0101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jewell TNM, Karaoz U, Brodie EL, Williams KH, Beller HR. 4 March 2016. Metatranscriptomic evidence of pervasive and diverse chemolithoautotrophy relevant to C, S, N and Fe cycling in a shallow alluvial aquifer. ISME J doi: 10.1038/ismej.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heijnen J, Van Dijken J. 1992. In search of a thermodynamic description of biomass yields for the chemotrophic growth of microorganisms. Biotechnol Bioeng 39:833–858. doi: 10.1002/bit.260390806. [DOI] [PubMed] [Google Scholar]
- 69.Kelly DP. 1982. Biochemistry of the chemolithotrophic oxidation of inorganic sulphur. Philos Trans R Soc Lond B Biol Sci 298:499–528. doi: 10.1098/rstb.1982.0094. [DOI] [PubMed] [Google Scholar]
- 70.Timmer-ten Hoor A. 1981. Cell yield and bioenergetics of Thiomicrospira denitrificans compared with Thiobacillus denitrificans. Antonie Van Leeuwenhoek 47:231–243. doi: 10.1007/BF00403394. [DOI] [PubMed] [Google Scholar]
- 71.Nelson DC, Jørgensen BB, Revsbech NP. 1986. Growth pattern and yield of a chemoautotrophic Beggiatoa sp. in oxygen-sulfide microgradients. Appl Environ Microbiol 52:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jørgensen BB, Nelson DC. 2004. Sulfide oxidation in marine sediments: geochemistry meets microbiology. Geol Soc Am Spec Pap 379:63–81. [Google Scholar]
- 73.Howarth RW, Jørgensen BB. 1984. Formation of 35S-labelled elemental sulfur and pyrite in coastal marine sediments (Limfjorden and Kysing Fjord, Denmark) during short-term 35SO42− reduction measurements. Geochim Cosmochim Acta 48:1807–1818. doi: 10.1016/0016-7037(84)90034-6. [DOI] [Google Scholar]
- 74.Thomsen U, Kristensen E. 1997. Dynamics of CO2 in a surficial sandy marine sediment: the role of chemoautotrophy. Aquat Microb Ecol 12:165–176. doi: 10.3354/ame012165. [DOI] [Google Scholar]
- 75.Wuchter C, Abbas B, Coolen MJ, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ. 2006. Archaeal nitrification in the ocean. Proc Natl Acad Sci U S A 103:12317–12322. doi: 10.1073/pnas.0600756103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berg C, Listmann L, Vandieken V, Vogts A, Jürgens K. 2014. Chemoautotrophic growth of ammonia-oxidizing Thaumarchaeota enriched from a pelagic redox gradient in the Baltic Sea. Front Microbiol 5:786. doi: 10.3389/fmicb.2014.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Billen G. 1976. Evaluation of nitrifying activity in sediments by dark 14C-bicarbonate incorporation. Water Res 10:51–57. doi: 10.1016/0043-1354(76)90157-3. [DOI] [Google Scholar]
- 78.Jørgensen BB. 2006. Bacteria and marine biogeochemistry, p 169–206. In Schulz HD, Zabel M (ed), Marine geochemistry. Springer, Berlin, Germany. [Google Scholar]
- 79.Neubauer SC, Emerson D, Megonigal JP. 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl Environ Microbiol 68:3988–3995. doi: 10.1128/AEM.68.8.3988-3995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sobolev D, Roden EE. 2004. Characterization of a neutrophilic, chemolithoautotrophic Fe(II)-oxidizing beta-proteobacterium from freshwater wetland sediments. Geomicrobiol J 21:1–10. doi: 10.1080/01490450490253310. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.