ABSTRACT
Accumulating data support the bimodal action of several key cellular factors in cancer. The dogma of p21WAF1/Cip1 as a tumor suppressor has been recently challenged since new data support its tumor promoting features depending on the tumor environment. Here we discuss the Janus face of p21WAF1/Cip1.
KEYWORDS: Genomic instability, p21WAF1/Cip1, p53, PCNA, replication stress, tumor heterogeneity
Accumulating evidence indicates that a number of proteins involved in key cellular processes display a bimodal behavior in cancer, acting as either tumor suppressors or oncoproteins (1- Supplementary Table 1). This duality is commonly attributed to the cellular or environmental context in which the tumors develop. The mechanistic basis underlying such context-dependent phenomena is vague in most cases, and its elucidation is essential for both understanding cell biology and the rational design of cancer therapy.*
For quite a long period, more than 20 years, p21WAF1/Cip1 expression was considered a strong indicator of wild-type (wt) tumor protein p53 (TP53, best known as p53) activity and thus a marker of good prognosis.2 However, sparse evidence in the literature pointed out that this was not always the case.3 For example, double knockout (Trp53−/−Cdkn1a−/−) * mice display a reduced incidence of spontaneous and radiation-induced lymphomas, contrary to expectations.3
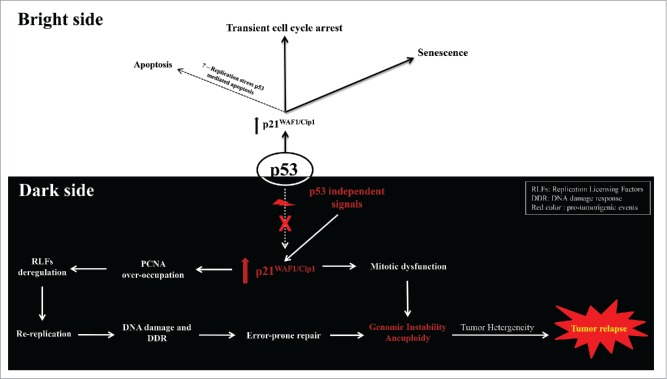
Within this framework we recently demonstrated that protracted p21WAF1/Cip1 expression in wt p53-deficient tumors stimulated genomic instability by deregulating the replication licensing machinery and triggering re-replication, a form of replication stress. These biochemical events took place during an initial senescence-like phase and represented a selective process that led to the emergence of a subpopulation of p21WAF1/Cip1 aggressive and chemoresistant cells (Fig. 1). We found that a RAD52-mediated error-prone double-strand break repair pathway was implicated in forming the genetic landscape of the cells that escaped senescence (termed “escape cells”).1 At the mechanistic level, 2 key features dictate the above outcome. The first is the interaction of p21WAF1/Cip1 with proliferating cell nuclear antigen (PCNA), a sliding DNA clamp involved in essential cell cycle functions such as DNA replication and repair.4 Among all PCNA-interacting proteins, p21WAF1/Cip1 possesses the highest binding affinity (KD ∼2.5 nM).1 Thus, under conditions of “chronic” expression p21WAF1/Cip1 could displace all other PCNA competitors, altering vital PCNA-dependent functions. In agreement with this perception, we revealed that protracted p21WAF1/Cip1 expression abrogated the ability of PCNA to control turnover of the replication licensing factors Cdt1 and Cdc6,1 eliciting re-replication.1 The second feature is loss of wt p53 activity. When wt p53 is intact the cells undergoing re-replication are eliminated via p53-mediated apoptosis, possibly as a result of the increasing amount of DNA damage accumulated from the collapsed re-replicated forks.1 Consequently, in an environment of loss of wt p53 function this antitumor barrier is erased, paving the way for re-replication–driven genomic instability.
Figure 1.
The Janus face of p21. On the bright side, p53 activation leads to p21WAF1/Cip1 expression that triggers transient cell cycle arrest or senescence (acute phase), depending on the magnitude of the damage. These networks are turned off provided toxic events are neutralized by the cell. The relationship of p21WAF1/Cip1 with apoptosis is not completely clear and most reports suggest that it suppresses apoptotic pathways. However, in the presence of p53 the cells undergoing p21WAF1/Cip1-driven re-replication are eliminated via apoptosis. On the dark side, in p53-deficient tumors, p21WAF1/Cip1 can be induced by p53-independent protumorigenic signals, causing deregulation of the replication licensing machinery that triggers replication stress in the form of re-replication. Subsequently, DNA damage is produced and repaired by error-prone procedures thus altering the landscape of the genome and fueling genomic instability. The latter might be enhanced by p21WAF1/Cip1-mediated blockage of mitosis and S/M phase dissociation triggered via re-replication, promoting overall aneuploidy, tumor heterogeneity, and aggressiveness. DDR, DNA damage response; PCNA, proliferating cell nuclear antigen; RLF, replication licensing factor. Red color indicates protumorigenic events.
A question that emerges is whether overexpression of other PCNA interacting proteins could produce an analogous phenomenon. In a recent report, nuclear accumulation of cyclin D1, a well established PCNA-interacting factor, resulted in Cdt1 abundance, re-replication, and genomic instability in a p53-dependent manner.5 The high nuclear levels of cyclin D1 were due to a specific cyclin D1 allele (D1T286A mutation). As this mutation resides outside the cyclin D1 PCNA interacting domains6 it does not prevent PCNA binding and saturation of Cul4-RING protein ligase (CRL4)CDT2, the E3 ligase that targets both Cdt1 and Cdc6.1 Therefore, the model that we propose can function in parallel with the course of action of nuclear cyclin D1, as described previously,5 providing an additional mechanistic explanation.1 Another question, from a different angle, is whether increased expression of certain proteins could lead to accumulation of other substrates that share the same degradation modules. Although such a scenario is theoretically possible, an argument against it is provided by the case of the c-myc oncoprotein, which is frequently overexpressed in lymphomas. While c-myc shares with cyclin-E, c-Jun, and Notch the same E3 ubiquitin ligase, F-box and WD repeat domain containing 7 (FBW7),7 its overexpression is not followed by accumulation of the common substrates. An explanation for this discrepancy is that chronic p21WAFI/Cip1 induction represents a typical case of enzymatic competitive inhibition, whereas myc overexpression in lymphomas is mainly due to translocations [e.g., t(8;14)].8 Most translocated c-myc alleles contain sequence alterations, often point mutations or deletions. The most frequent hotspot is mutation at threonine 58 (T58), which is essential for ubiquitination by FBW7, thus rendering myc unaccessible to FBW79 and leaving FBW7 free to target other available targets.
The model that we propose (Fig. 1) provides an additional mechanistic explanation, distinct from that of mitotic dysfunction,10 for the unclarified relationship between cellular senescence and aneuploidy (chromosomal instability), a well defined hallmark of cancer.1
Lastly, pathologists should cautiously estimate p21WAF1/Cip1 immunopositivity in tumors in everyday practice, always taking into account the p53 status. This parameter is crucial in determining whether p21WAF1/Cip1 overexpression is beneficial for the patient (p53 intact) or harbors deleterious features (p53 mutant) that promote tumor heterogeneity and eventually tumor relapse and aggressiveness.
Footnotes
Cyclin-dependent kinase inhibitor 1 (Cdkn1a) is the gene encoding p21WAF1/Cip1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors' work is funded by the European Union's Seventh Framework Programme (project INsPiRE), the Greek GSRT program of Excellence II (Aristeia II) and NKUA-SARG grants 70/3/12128, 70/3/8916, 70/3/11351.
References
- 1.Galanos P, Vougas K, Walter D, Polyzos A, Maya-Mendoza A, Haagensen EJ, Kokkalis A, Roumelioti FM, Gagos S, Tzetis M, et al.. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat Cell Biol 2016; 18(7):777-89; PMID:27323328; 10.1038/ncb3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall PA, Lane DP. p53 in tumour pathology: can we trust immunohistochemistry? –Revisited! J Pathol 1994; 172(1):1-4; PMID:7931821; http://dx.doi.org/ 10.1002/path.1711720103 [DOI] [PubMed] [Google Scholar]
- 3.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9:400-14; PMID:19440234; http://dx.doi.org/ 10.1038/nrc2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 2003; 116(Pt 15):3051-60; PMID:12829735; http://dx.doi.org/ 10.1242/jcs.00653 [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal P, Lessie MD, Lin DI, Pontano L, Gladden AB, Nuskey B, Goradia A, Wasik MA, Klein-Szanto AJ, Rustgi AK, et al.. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev 2007; 21(22):2908-22; PMID:18006686; http://dx.doi.org/ 10.1101/gad.1586007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuoka S, Yamaguchi M, Matsukage A. D-type cyclin-binding regions of proliferating cell nuclear antigen. J Biol Chem 1994; 269(15):11030-6; PMID:7908906 [PubMed] [Google Scholar]
- 7.Wang L, Ye X, Liu Y, Wei W, Wang Z. Aberrant regulation of FBW7 in cancer. Oncotarget 2014; 5(8):2000-15; PMID:24899581; http://dx.doi.org/ 10.18632/oncotarget.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene 2001; 20(40):5595-610; PMID:11607812; http://dx.doi.org/ 10.1038/sj.onc.1204595 [DOI] [PubMed] [Google Scholar]
- 9.Amati B. Myc degradation: dancing with ubiquitin ligases. Proc Natl Acad Sci USA 2004; 101(24):8843-4; PMID:15187232; http://dx.doi.org/ 10.1073/pnas.0403046101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbert N, Navaratnam N, Augert A, Da Costa M, Martien S, Wang J, Martinez D, Abbadie C, Carling D, de Launoit Y, et al.. Regulation of ploidy and senescence by the AMPK-related kinase NUAK1. EMBO J 2010; 29(2):376-86; PMID:19927127; http://dx.doi.org/ 10.1038/emboj.2009.342 [DOI] [PMC free article] [PubMed] [Google Scholar]