ABSTRACT
In a recent article in Cell Reports, we described a novel mechanism for acquired resistance against new small-molecule antiangiogenic tyrosine-kinase inhibitors (TKIs). Vascular normalization-inducing TKIs block glycolysis and trigger a nutritional stress response in the tumor compartment that induces a (targetable) switch to mitochondrial metabolism. We discuss the implications for clinical/translational studies and suggest areas for future research.
KEYWORDS: Antiangiogenics, resistance, breast cancer, ME-344, metabolic synthetic lethality, nintedanib, phenformin
Abbreviations
- MoAb
monoclonal antibody
- TKI
multi-tyrosine kinase inhibitor
- FMISO
18F-fluoromisonidazole
- WoO
window-of-opportunity
Acquired resistance against antiangiogenic therapy is a major problem in cancer treatment.1 Antiangiogenic agents, which include both monoclonal antibodies (MoAbs) and multitargeted tyrosine kinase inhibitors (multi-TKIs), have been approved for the treatment of malignancies such as kidney, liver, lung, ovarian, colorectal, and breast cancer. Thus, antiangiogenics are currently the biological agents in widest clinical use. However, almost 100% of patients being treated with these agents develop disease progression.1 Several types of “resistance” have been proposed and reviewed elsewhere.2 In our recently published study,3 we aimed to address the most common type, acquired resistance.1,2
Several key design features were incorporated in our study: first, most preclinical investigations on antiangiogenics rely on xenograft mouse models of immunodeficiency, which ignore the role of the immune system in the regulation of angiogenesis, assume aberrant growth kinetics, and introduce the artifact of cross-species interaction. Instead, we used immunocompetent spontaneous tumor models.4 Second, we adjusted the effects according to the type of agent (several multikinase inhibitors and monoclonal antibodies were studied in parallel), since the results from one agent might not be extrapolated to another agent. Finally, the findings were validated in several tumor types.
The key finding of our study was that small-molecule multi-TKIs lead to correction of hypoxia coupled with a reduced rate of glycolysis.3 A high rate of glycolysis is known to be a key factor in tumor progression (especially in MAPK- and/or Pi3K–AKT-activated tumors, which represent the majority of cases), linked not only to rapid energy production but also to increased uptake of carbon skeletons.5 When glycolysis was pharmacologically inhibited, the tumors continued growing at a normal rate because other energy sources were available. In particular, we observed a switch to mitochondrial metabolism in the tumor compartment that was mediated by hyperactivation of AMPK, PKA, and PPARα, a mechanism similar to that of the stress response of healthy tissue to nutritional deprivation.6 The use of phenformin or the mitochondrial inhibitor ME-344 to block mitochondrial metabolism in the absence of a TKI was not therapeutic, but a TKI combined with those blocking agents led to an impressive tumor response. The effects were particularly striking when the tumors were “forced” to rely on mitochondrial metabolism by initial priming with TKI monotherapy; subsequent addition of a mitochondrial blocker led to tumor eradication in many cases, an effect that we called “metabolic synthetic lethality.”
These findings have immediate clinical applicability. Specifically, we have already launched a phase I trial for patients with early breast cancer that combines antiangiogenic treatment with weekly doses of ME-344; the latter agent is added 1 week after the first dose of antiangigogenic agent in order to induce the phenomenon of metabolic synthetic lethality. A second trial investigating the combination of phenformin with an antiangiogenic agent for patients with lung or colon cancer will start at the end of the year.
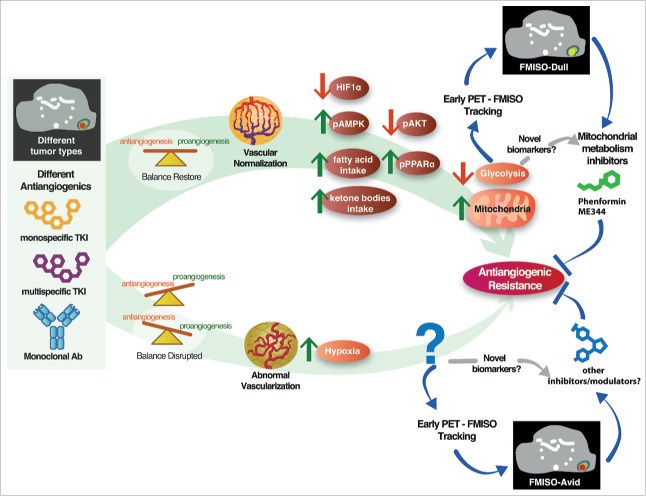
Several points with additional clinical implications should be considered in future research. First, metformin and phenformin have been used in preclinical investigations as “equivalent” inhibitors of mitochondria. However, in our study metformin did not show activity. This result can be explained by 2 observations: metformin seems to be one order of magnitude less potent than phenformin7; and metformin, but not phenformin, requires a membrane transporter to enter cancer cells.8 These factors probably explain the failure of metformin to improve survival in a recent clinical trial.9 We encourage clinical researchers who are considering investigations that incorporate mitochondrial inhibitors to use phenformin or ME-344 instead of metformin. Second, and more importantly, we have observed that the same tumor model can respond to one antiangiogenic agent by correcting interstitial hypoxia (“vessel normalization”), while responding to a different antiangiogenic agent by increasing hypoxia. In a separate study, we showed that the same agent applied to 2 different tumor models induced vessel normalization in one model and hypoxia enhancement in the other.10 We also performed a clinical trial with a window-of-opportunity design (WoO), where the patients in the experimental arm received 2 weeks of single-agent nintedanib (antiangiogenic TKI) prior to the standard treatment. We used 18F-fluoromisonidazole (FMISO)-PET (18F-FMISO is a positron-emitting probe that binds to hypoxic tissues [< 1% O2]) before and after the WoO. We observed that 20% of the patients had increased hypoxia in their tumors, whereas 25% and 55% of the patients had decreased or unchanged hypoxia, respectively (unpublished results). These results highlight the importance of assessing the response of the individual patient to antiangiogenic therapy by 18F-FMISO-PET or other techniques instead of concentrating on general conclusions about whether or not antiangiogenics induce vascular normalization or tumor “choking.” Finally, the mechanisms involved in the development of acquired resistance to antiangiogenic agents when it is mediated by worsening hypoxia instead of normalization remain to be elucidated. These points are summarized in Fig. 1.
Figure 1.
Mechanisms of acquired resistance against antiagiogenic agents. Antiangiogenic agents are diverse in their mechanisms of action; thus, it is not surprising that some agents can tip the imbalance of pro- and antiangiogenic factors in the tumor microenvironment back to normal (upper side) whereas other agents can lead to increased vascular abnormality (lower side). When vascular abnormality and hypoxia are corrected, glycolysis decreases and the tumor switches to mitochondrial metabolism. In this situation, the tumor is highly sensitive to mitochondrial inhibition by phenformin or another agent. However, a tumor can become and remain normoxic after exposure to an antiangiogenic agent. What mediates the acquisition of resistance, and how those mediators can be targeted, is currently unknown. Whether a given antiangiogenic agent (monoclonal antibody or multitargeted tyrosine kinase inhibitor) will tip the balance toward increased or corrected hypoxia cannot be currently predicted; however, hypoxia is easily evaluated by 18F-fluoromisonidazole-PET. Other biomarkers are currently under development, including protein hydroxylation.
Our findings, together with those of other investigators, highlight the therapeutic importance of manipulating the metabolism of cancer tissues, and prove that tumors can exist in different metabolic states. More importantly, our findings point toward a model for acquired resistance that, because it does not involve a single mechanism, will require assessment of individual patients using hypoxia/normoxia biomarkers. We envision a treatment protocol in the near future that will allow personalized decisions after the patient receives the first course of antiangiogenic therapy. Subsequent treatment will be based on the assessment of tumor hypoxia. The results of biomarker testing will indicate whether a patient should receive a mitochondrial inhibitor or another agent that blocks the hypoxia-driven adaptive response.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 2012; 12:699-709; PMID:23001349; http://dx.doi.org/ 10.1038/nrc3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mastri M, Rosario S, Tracz A, Frink RE, Brekken RA, Ebos JM. The challenges of modeling drug resistance to antiangiogenic therapy. Curr Drug Targets 2015; PMID:26648063; http://dx.doi.org/ 10.2174/1389450117666151209123544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarro P, Bueno MJ, Zagorac I, Mondejar T, Sanchez J, Mouron S, Munoz J, Gomez-Lopez G, Jimenez-Renard V, Mulero F, et al.. Targeting Tumor Mitochondrial Metabolism Overcomes Resistance to Antiangiogenics. Cell Rep 2016; 15:2705-18; PMID:27292634; http://dx.doi.org/ 10.1016/j.celrep.2016.05.052 [DOI] [PubMed] [Google Scholar]
- 4.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov 2006; 5:741-54; PMID:16915232; http://dx.doi.org/ 10.1038/nrd2110 [DOI] [PubMed] [Google Scholar]
- 5.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011; 27:441-64; PMID:21985671; http://dx.doi.org/ 10.1146/annurev-cellbio-092910-154237 [DOI] [PubMed] [Google Scholar]
- 6.Soeters MR, Soeters PB, Schooneman MG, Houten SM, Romijn JA. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am J Physiol Endocrinol Metab 2012; 303:E1397-407; PMID:23074240; http://dx.doi.org/ 10.1152/ajpendo.00397.2012 [DOI] [PubMed] [Google Scholar]
- 7.Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, Bosenberg MW, McMahon M, Cantley LC, Zheng B. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A 2013; 110:18226-31; PMID:24145418; http://dx.doi.org/ 10.1073/pnas.1317577110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal ED, Yasmeen A, Beauchamp MC, Rosenblatt J, Pollak M, Gotlieb WH. Relevance of the OCT1 transporter to the antineoplastic effect of biguanides. Biochem Biophys Res Commun 2011; 414:694-9; PMID:21986525; http://dx.doi.org/ 10.1016/j.bbrc.2011.09.134 [DOI] [PubMed] [Google Scholar]
- 9.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2015; 16(7):839-47; PMID:26067687; 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Agudo E, Mondejar T, Soto-Montenegro ML, Megias D, Mouron S, Sanchez J, Hidalgo M, Lopez-Casas PP, Mulero F, Desco M, et al.. Monitoring vascular normalization induced by antiangiogenic treatment with (18)F-fluoromisonidazole-PET. Mol Oncol 2016; 10:704-18; PMID:26778791; http://dx.doi.org/ 10.1016/j.molonc.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]