ABSTRACT
Amplification of cyclin D1 is a frequent alteration in many cancers of different type and origin. We recently described a novel regulatory axis involving cyclin D1 in the regulation of tumor invasion and metastasis. Membrane-associated cyclin D1-CDK4 complexes promote activation of the small GTPase RAC1 through phosphorylation of the regulatory protein paxillin.
KEYWORDS: Cdk4, cell invasion, cyclin D1, metastasis, paxillin, Rac1
D-type cyclins are the regulatory subunits of the cyclin-dependent kinases 4/6 (CDK4/6). These complexes trigger cell cycle entry by phosphorylation and inactivation of the retinoblastoma protein (RB1, hereafter pRB) and, consequently, by releasing E2F-dependent transcription of S-phase genes. Among D-type cyclins, cyclin D1 (CCND1) is one of the most-studied oncogenes, and the presence of abundant amounts of this cyclin in cells and tissues is a mark of proliferation and tumor growth (for a review see ref. 11). Indeed, many tumors of different type and origin show amplification of Ccnd1. Besides the pRB-dependent role of Ccnd1 in the regulation of proliferation, this cyclin also regulates other cellular processes such as cell adhesion and migration. Ccnd1−/− macrophages and fibroblasts show increased capacity for attachment to the extracellular matrix and reduced potential for cell migration and invasion.2 We and others have previously shown that the Ccnd1–Cdk4 complex impinges on the activity of different small GTPases such as RHOA and RALA/B. Ccnd1–Cdk4 negatively regulates RhoA activity through stabilization of the cyclin-dependent kinase inhibitor p27 (CDKN1B),3 and the same complex seems to positively regulate Ral A and B activities by phosphorylation of the GTPase exchange factor (GEF) RGL2.4 The spatial and temporal adjustment of all these small GTPases is essential for efficient cell migration and invasion. In a recent study we identified another member of this repertoire, the GTPase RAC1, and showed that Ccnd1 regulates cell adhesion and migration via activation of this small GTPase.5 The Ccnd1–Cdk4 complex reduces cell adhesion and increases cell migration/invasion through the phosphorylation of paxillin (PXN) at serines 83 and 178. Although paxillin is a scaffold protein that is mainly localized at focal adhesions we have shown that a subpopulation of paxillin and Ccnd1 exclusively co-localizes at the cell membrane, and that Ccnd1–Cdk4 triggers the accumulation of Rac1–GTP in a manner that depends on its ability to phosphorylate paxillin. Hence, we have described a new regulatory axis composed of Ccnd1–Cdk4/Paxillin–Rac1 that controls cell adhesion and migration.
Nuclear Ccnd1–Cdk4 exerts its function as a modulator of transcription acting on different targets.1 In agreement with this, Ccnd1 is visualized by fluorescence microscopy and immunohistochemistry in the nucleus of cells in culture and in tissues, respectively. However, Ccnd1 can be localized in the cytoplasm and the cell membrane under certain conditions (see Fig. 1). Diehl and co-workers demonstrated that Ccnd1 is re-localized and degraded in the cytoplasm of fibroblasts during S phase after phosphorylation by GSK3β (GSK3B).6 Also, Balda and collaborators revealed that tight junction proteins sequester Ccnd1–Cdk4 in the membrane of epithelial cells to reduce cell proliferation as a mechanism of proliferative arrest during cell-to-cell contact inhibition.7 In both cases, Ccnd1 does not seem to play an active role in the cytoplasm and its re-localization is viewed as part of a mechanism to keep Ccnd1 out of the nucleus. Despite this, there are some previous indications pointing to an active role of Ccnd1–Cdk4 in the cytoplasm and in the cell membrane. For instance, Ccnd1 has been shown to interact with filamin A (FLNA) and to co-localize with this protein in membrane ruffles.8 Our recent contribution confirms that a subpopulation of the Ccnd1 protein is localized in the membrane,5 together with paxillin and active Rac1, and strongly suggests that this localization is functionally relevant in the regulation of cell adhesion and invasion. Hence, we provide new and compelling evidence that cytoplasmic Ccnd1 has an important role in regulating different pathways to induce migration and invasion.
Figure 1.
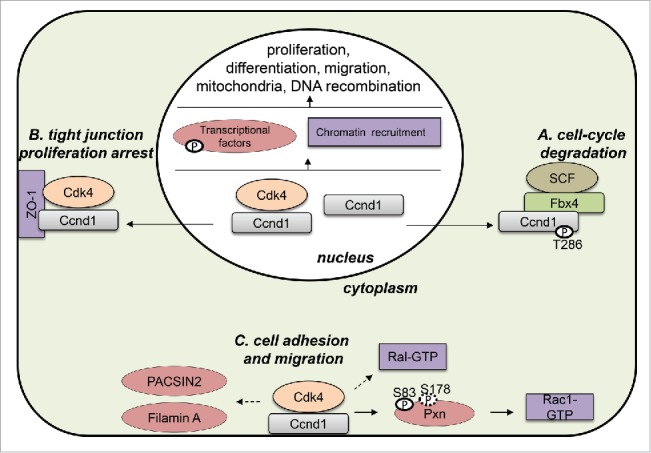
Subcellular localization of cyclin D1 (CCND1). In the nucleus, Ccnd1 is important for the regulation of many different processes by Cdk4-dependent and -independent mechanisms. During S phase, Ccnd1 is phosphorylated at threonine 286 by GSK3β (GSK3B) and exported to the cytoplasm to be ubiquitinated and degraded via the ubiquitin-ligase complex SCF-Fbx4 (A). In epithelial cells, Ccnd1–Cdk4 is sequestered to the membrane by the tight junction protein ZO-1 (TJP1) to arrest cell proliferation (B). Ccnd1–Cdk4 is also associated with the cell membrane, interacting with different targets such as paxillin (PXN), filamin A (FLNA), PACSIN2, RALA/B, and RAC1. The activity of the complex in this location is important for regulation of cell adhesion and migration (C).
The accumulation of Ccnd1 and Cdk4 in the nucleus promotes growth of the tumors through an increase in the proliferative capabilities of tumor cells.1 It is generally accepted that activation/inactivation of many transcriptional factors by Ccnd1–Cdk4, including the canonical pathway to inactivate pRB, produces this hyperproliferative effect. However, in addition to this phenotype, Ccnd1-Cdk4 also promotes cell invasion and metastasis; for example, the expression of Ccnd1 is associated with metastases from prostate tumors.9 It has been suggested that the metastatic potential of Ccnd1 is related to its ability to promote cell motility and migration.2 Our results suggest that membrane-associated activity of the Ccnd1–Cdk4 complex is responsible at least in part for the induction of invasion and metastasis in tumors overexpressing Ccnd1. As mentioned above, Ccnd1 induces invasion through the phosphorylation of paxillin in the cell membrane.5 In agreement with this, we have observed that a phosphomimetic allele of paxillin rescues the metastatic potential of Ccnd1-deficient cells without affecting their proliferative state. This also agrees with the reported requirement for phospho-Pxn for the induction of metastases in different tumor models. Reinforcing the idea that cytoplasmic Ccnd1 is important in promoting metastases, we have recently demonstrated that expression of a membrane-associated allele of Ccnd1 harboring a C-terminus farnesylation motif increases the invasive and metastatic potential of tumor cells.10
In mammals, the differentiation of many tissues and organs requires cell polarization, migration, and changes in cell–substrate adherence. Polarity may be coordinated with the proliferation machinery to achieve proper organism development and tissue homeostasis. Anomalies in this coordination may be critical in the development of oncogenesis. In view of all the new facts concerning Ccnd1, this cyclin is now a solid candidate for participation in processes involved in the coordination of cell proliferation and migration. Under normal and pathological conditions, changes in the subcellular localization of Ccnd1–Cdk4 activity could be part of a mechanism by which the cell decides whether it proliferates or migrates. Therefore, the presence of abnormal levels of cytoplasmic Ccnd1–Cdk4 in tumor cells may be taken as an indication of high invasion potential and might be useful as a diagnostic marker for high metastatic risk. In addition, the dual role of Ccnd1–Cdk4 in regulating both tumor growth and invasion in many different tumors firmly supports the use of therapies based on Cdk4/6 inhibitors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work has been supported by research grants from Spanish Ministry of Education and Science (BFU2013-42895), Catalan Government (SGR-559), and Fundació Alicia Cuello de Merigó.
References
- 1.Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol 2009; 220:292-6; PMID:19415697; http://dx.doi.org/ 10.1002/jcp.21791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, Dye C, Yang J, Dai M, Ju X, et al.. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol 2006; 26:4240-56; PMID:16705174; http://dx.doi.org/ 10.1128/MCB.02124-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Jiao X, Wang C, Ju X, Lu Y, Yuan L, Lisanti MP, Katiyar S, Pestell RG. Cyclin D1 induction of cellular migration requires p27(KIP1). Cancer Res 2006; 66:9986-94; PMID:17047061; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1596 [DOI] [PubMed] [Google Scholar]
- 4.Fernández RMH, Ruiz-Miró M, Dolcet X, Aldea M, Garí E. Cyclin D1 interacts and collaborates with Ral GTPases enhancing cell detachment and motility. Oncogene 2011; 30:1936-46; PMID:21242975; http://dx.doi.org/17081987 10.1038/onc.2010.577 [DOI] [PubMed] [Google Scholar]
- 5.Fusté NP, Fernández-Hernández R, Cemeli T, Mirantes C, Pedraza N, Rafel M, Torres-Rosell J, Colomina N, Ferrezuelo F, Dolcet X GE. Cytoplasmic cyclin D1 regulates cell invasion and metastasis through the phosphorylation of paxillin. Nat Commun 2016; 7:11581; PMID:27181366; http://dx.doi.org/17081987 10.1038/ncomms11581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin DI, Barbash O, Kumar KGS, Weber JD, Harper JW, Klein-Szanto AP, Rustgi A, Fuchs S, Diehl JA. Phosphorylation-Dependent Ubiquitination of Cyclin D1 by the SCFFBX4-aB Crystallin Complex. Mol Cell 2006; 24:355-66; PMID:17081987; http://dx.doi.org/ 10.1016/j.molcel.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol 2003; 160:423-32; PMID:12566432; http://dx.doi.org/ 10.1083/jcb.200210020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Z, Yeow W-S, Zou C, Wassell R, Wang C, Pestell RG, Quong JN, Quong AA. Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Res 2010; 70:2105-14; PMID:20179208; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-Cardo C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res 2000; 6:1891-5; PMID:10815912 [PubMed] [Google Scholar]
- 10.Fusté NP, Castelblanco E, Felip I, Santacana M, Fernández-Hernández R, Gatius S, Pedraza N, Pallarés J, Cemeli T, Valls J, et al.. Characterization of cytoplasmic cyclin D1 as a marker of invasiveness in cancer. Oncotarget 2016; 7:26979-91; PMID:27105504; http://dx.doi.org/ 10.18632/oncotarget.8876 [DOI] [PMC free article] [PubMed] [Google Scholar]


