Abstract
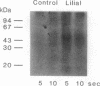
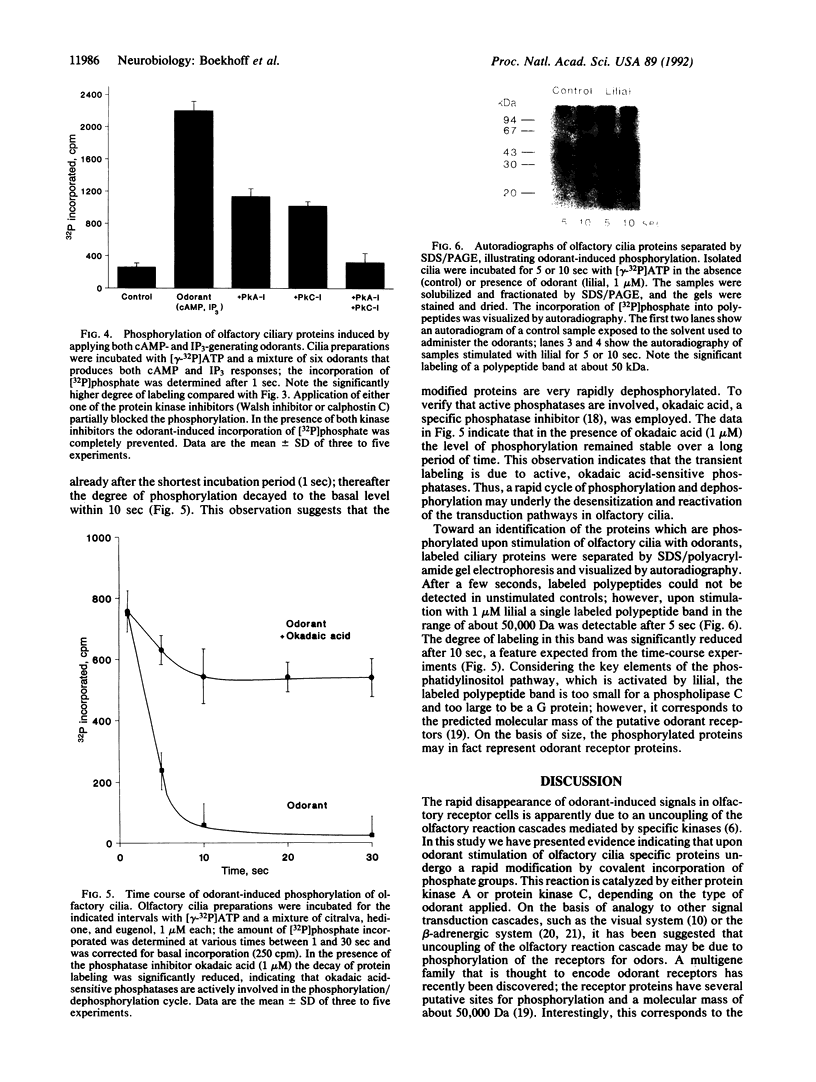
Stimulation of isolated rat olfactory cilia in the presence of [gamma-32P]ATP leads to a significantly enhanced incorporation of [32P]phosphate. Depending on the type of odorants applied, the induced phosphorylation is completely blocked by specific inhibitors of either protein kinase A or protein kinase C. Time-course experiments indicate that the odor-induced modification of ciliary proteins is transient; the intensity of labeling decayed over time (1-10 sec). Separation of ciliary proteins by SDS/polyacrylamide gel electrophoresis followed by autoradiography demonstrated that upon stimulation with lilial, a single polypeptide (50,000 Da) was phosphorylated; the size of the modified protein is in line with the hypothesis that odorant receptors are phosphorylated subsequent to activation by specific odors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anholt R. R., Aebi U., Snyder S. H. A partially purified preparation of isolated chemosensory cilia from the olfactory epithelium of the bullfrog, Rana catesbeiana. J Neurosci. 1986 Jul;6(7):1962–1969. doi: 10.1523/JNEUROSCI.06-07-01962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I., Breer H. Termination of second messenger signaling in olfaction. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):471–474. doi: 10.1073/pnas.89.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I., Tareilus E., Strotmann J., Breer H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990 Aug;9(8):2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H., Boekhoff I., Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990 May 3;345(6270):65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Pace U., Heldman J., Shapira A., Lancet D. Isolated frog olfactory cilia: a preparation of dendritic membranes from chemosensory neurons. J Neurosci. 1986 Aug;6(8):2146–2154. doi: 10.1523/JNEUROSCI.06-08-02146.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Fowles C., Akhtar M., Cohen P. Interplay of phosphorylation and dephosphorylation in vision: protein phosphatases of bovine rod outer segments. Biochemistry. 1989 Nov 28;28(24):9385–9391. doi: 10.1021/bi00450a020. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. J Physiol. 1978 Sep;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Heldman J., Lancet D. Cyclic AMP-dependent protein phosphorylation in chemosensory neurons: identification of cyclic nucleotide-regulated phosphoproteins in olfactory cilia. J Neurochem. 1986 Nov;47(5):1527–1533. doi: 10.1111/j.1471-4159.1986.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Kearney K. A., Gurd J. W. Phosphorylation of synaptic membrane glycoproteins: the effects of Ca2+ and calmodulin. J Neurochem. 1986 Jun;46(6):1683–1691. doi: 10.1111/j.1471-4159.1986.tb08485.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kurahashi T., Shibuya T. Ca2(+)-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990 May 7;515(1-2):261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Parfitt D. J., Hester L. D., Snyder S. H. Odorant-sensitive adenylate cyclase: rapid, potent activation and desensitization in primary olfactory neuronal cultures. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2366–2369. doi: 10.1073/pnas.88.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Tamaoki T., Nakano H. Potent and specific inhibitors of protein kinase C of microbial origin. Biotechnology (N Y) 1990 Aug;8(8):732–735. doi: 10.1038/nbt0890-732. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]