Abstract
Background
Graft reperfusion poses a critical challenge during liver transplantation and can be associated with hemodynamic instability/postreperfusion syndrome. This is sequel to ischemia-reperfusion injury and normothermic machine preservation (NMP) may affect hemodynamic changes. Herein, we characterize postreperfusion hemodynamics in liver grafts after NMP and traditional cold preservation.
Materials and methods
Intraoperative records of patients receiving grafts after NMP (n = 6; NMP group) and cold storage (CS) (n = 12; CS group) were compared. The mean arterial pressure (MAP) was defined as the average pressure in the radial artery during 1 cardiac cycle by invasive monitoring. Postreperfusion syndrome was defined as MAP drop greater than 30% of baseline, lasting for 1 minute or longer within the first 5 minutes from graft reperfusion.
Results
Donor, recipient, demographics, and surgical parameters were evenly matched. Normothermic machine preservation grafts were perfused for 525 minutes (395-605 minutes) after initial cold ischemic time of 91 minutes (73-117 minutes), whereas in CS group cold ischemic time was 456 minutes (347-685 minutes) (P = 0.001). None developed postreperfusion syndrome in the NMP group against n = 2 (16.7%) in CS group (P = 0.529). Normothermic machine preservation group had better intraoperative MAP at 90 minutes postreperfusion (P = 0.029), achieved with a significantly less vasopressor requirement (P = <0.05) and less transfusion of blood products (P = 0.030) compared with CS group.
Conclusions
Normothermic machine perfusion is associated with a stable intraoperative hemodynamic profile postreperfusion, requiring significantly less vasopressor infusions and blood product transfusion after graft reperfusion and may have benefit to alleviate ischemia-reperfusion injury in liver transplantation.
Graft reperfusion is a critical point during liver transplantation (LT), because it is correlated with the greatest intraoperative hemodynamic and metabolic stresses. During reperfusion, the occurrence of severe hemodynamic instability is commonly referred to as “postreperfusion syndrome” (PRS) and represents an important risk factor for graft injury, recipient mortality and morbidity.1 Postreperfusion syndrome is defined as a decrease in mean arterial pressure (MAP) of more than 30% from the value recorded at anhepatic phase lasting for more than 1 minute, during the first 5 minutes after reperfusion of the graft.2 Several studies report that the incidence of PRS varies between 25% and 50%.3,4 In a recent observational study, PRS was associated with significantly more frequent postoperative renal failure and with more frequent early postoperative death.4 A few studies have attempted to determine the clinical predictors, which could potentially impact on PRS occurrence.3,5 These variables include: (a) donor/graft related factors such as donor type (donors after brain death [DBD]/donor after cardiac death [DCD]), donor age, donor risk index (DRI), graft steatosis; (b) recipient factors including elevated model for end-stage liver disease (MELD) score, left ventricular diastolic dysfunction; and (c) intraoperative factors, such as prolonged cold ischemic time (CIT), prolonged warm ischemic time (WIT), intraoperative blood loss, and absence of a portocaval shunt.6-8 However, the influence of those clinical factors on PRS occurrence remains still controversial.
The pathophysiology of PRS after graft reperfusion is a complex mechanism not clearly understood. Postreperfusion syndrome is influenced by the liver ischemia-reperfusion injury (IRI), which represents an event with “global” consequences that influence the function of many organs including the myocardium, kidney, lung, intestine, pancreas, and adrenal glands.9 Among the multiple mechanisms implicated in IRI pathophysiology, the activation of the oxidative pathway and the excessive systemic inflammatory response, characterized by high circulating levels of proinflammatory cytokines and transcriptional factors (including IL-6 and TNF-α) after reperfusion, have been clearly recognized as having a key role in this phenomenon.9,10 Consequently, the establishment of metabolic acidosis, hypothermia, hypocalcemia, hyperkalemia, and the release of vascular components determine the multiple organ dysfunction and hemodynamic changes, which impact on postoperative morbidity and mortality.11
In recent years, the transplant community has explored many strategies to prevent IRI after transplantation. Novel ex vivo techniques, such as hypothermic, normothermic, and subnormothermic machine perfusion (NMP), have been shown to have a potentially useful role in reducing ischemia-reperfusion liver damage and in “resuscitating” marginal organs.12-14 In particular, several recent studies have reported the feasibility of graft preservation with NMP,15 which continuously perfuses the graft at physiological pressures and oxygenated blood providing cell nutrition, and might have a better outcome than preservation via cold storage (CS). This new technique may improve hemodynamics and attenuate ischemic injury also in marginal livers, but few data of its application in humans are available. The first report (OrganOx trial) on the safety and the feasibility of NMP on transplanted human liver allografts has recently been published with significant contribution from the author's own institution.16 The aim of this substudy is to characterize the hemodynamic changes related to PRS in the cohort of patients transplanted with NMP liver grafts, in comparison to a control group of patients receiving grafts from CS.
MATERIALS AND METHODS
Study Design
Patients receiving liver grafts perfused with NMP that contributed to the first OrganOx trial at the authors' institution were compared with a matched control group of patients (1:2 control) receiving liver grafts from CS. The control group was chosen from contemporary transplants performed at the same institution and enrolled in the original OrganOx trial according to following criteria as 1:2 match (donor age within 5 years, MELD ±2, etiology, recipient age within 10 years, and the graft type).16 The anesthetic records and perioperative data were collected. The following definitions were used: mean arterial pressure was defined as “the average pressure in the radial artery during 1 cardiac cycle by invasive arterial monitoring.” Postreperfusion syndrome was defined as “decrease of more than 30% in MAP immediately after the anhepatic stage for at least 1 minute within the first 5 minutes after reperfusion” with other surrogate hemodynamic changes recognized previously.2 The anhepatic phase was defined as “the duration of time from the removal of the recipient's liver to the graft reperfusion” and the implantation time referred to “the duration of time from the start of the caval anastomosis to the portal reperfusion.” Cold ischemic time was defined as “the time between the cold perfusion of the liver is commenced at the cross-clamping and the time the organ is taken out from the CS for implantation” in the control group, meanwhile in the NMP group this constituted a much shorter time period until the liver graft is connected to the NMP device after the organ procurement. Donor WIT was defined as “time elapsed since the onset of hypotension (when systolic blood pressure falls <50 mm Hg) or hypoxemia (desaturation with SpO2 < 80% measured by pulse oximetry)—whichever comes first—until the cold arterial flush is started in the donor.”
To compare the graft quality in the 2 groups and to assess the impact of NMP on DRI, we calculated the DRI17 in the following way. In the NMP group, the DRI was calculated as an actual score and a projected score. Actual DRI score was calculated taking into account only the brief period of CIT during the bench preparation of graft before it was connected to OrganOx machine. The projected DRI was calculated assuming that there was no normothermic component, and the liver grafts in this group were also preserved in traditional CS and assuming the transplant operation was planned in the same way. Finally, the actual and the projected score of the NMP group were compared with the DRI of the control group.
Anaesthesia Protocol
Anaesthesia was induced with intravenous Propofol (1.5-2.0 mg/kg) and Fentanyl (1-1.5 μg/kg) and maintained with volatile anaesthetics (isoflurane or desflurane), opiate (alfentanil or remifentanil) and atracurium infusions. Mechanical ventilation was characterized by a tidal volume of 6 to 10 mL/kg and a respiratory rate appropriate to achieve an end-tidal CO2 of 4 to 5.5 kPa. Invasive arterial and central venous monitoring was used. The MAP was measured by invasive BP traces during the entire operation. Cardiac output and right ventricular function were monitored using either a pulmonary artery catheter with thermodilution or transesophageal echocardiography. A continuous infusion of norepinephrine (NE) was started in all recipients with the aim of maintaining a MAP greater than 60 to 65 mm Hg. Continuous venovenous hemofiltration was used in cases with significantly impaired renal function. At reperfusion, the rate of NE infusion was increased to maintain the MAP above 65 mm Hg, and boluses of epinephrine of 10 to 30 μg was used if the hypotension was severe, arterial pressure did not recover promptly, or if there was evidence of reduced myocardial contractility on transesophageal echocardiography. During surgery patients received intravenous fluids (such as crystalloids and colloids) for volume replacement, and packed RBCs to maintain a blood hemoglobin level above 70 g/L. Fresh-frozen plasma and platelet administration was guided by thromboelastography parameters.
Surgical Techniques
Liver allografts from both DBD and DCD were included. All organs were retrieved by senior surgeons and were perfused with cold University of Wisconsin solution.
NMP Group
Immediately after the liver was retrieved from the donor, the back table preparation of the graft was performed in the donor's hospital at 4°C. Then, the liver was connected to NMP device (OrganOx), and any bleeding from the graft was fixed before the transport to the recipient's hospital, as described in the original trial (16). During the perfusion, the OrganOx device provided automated pumping, oxygen/air delivery, and heat exchange to maintain the perfusate at normal temperature (37°C), with physiological range for pO2 (12 kPa), pCO2 (5 kPa), pH (7.35), at physiological pressures in the vascular inflows and outflows of the liver (hepatic artery pressure 60 to 75 mm Hg and inferior vena cava pressure −1 to 2 mm Hg) and at stable portal flow. Arterial and portal perfusions were performed with a combined oxygenated solution of 3 units of RBCs, 1 L of colloids, bile salts, insulin, prostacyclin, and heparin. Pressures, arterial and portal flows, temperature, blood gases, pH, and bile production were monitored continuously during the entire time that the livers were on the machine and evaluated for the preimplantation viability of the graft. When ready for the transplant, organs were flushed with 2 to 3 L of cold perfusion solution (University of Wisconsin solution/histidine-tryptphan-ketoglutarate solution) through the portal vein and then implanted.
CS Group
A standard cold preservation by storage of the liver at 4°C was performed.
The piggyback technique, with a side-to-side caval anastomosis, was used for all transplants. All grafts were flushed in situ with 1 L of cold dextrose 5% or 0.9% physiological saline (according to the surgeon preference) before the reperfusion. A temporary portocaval shunt was performed only selectively. Initial revascularization of the graft was performed with the portal vein in all cases.
Data Collection
Demographic characteristics of recipients (age, sex, body mass index [BMI], primary liver disease, MELD score, United Kingdom MELD, preoperative echocardiographic examination) and donor criteria (age, BMI, DRI score, CIT) were collected. Intraoperative variables, included duration of surgery and other operative variables, transfusion requirements, the inotropic requirements were recorded and compared between the 2 groups at the initiation of surgery during hepatic dissection and during the anhepatic phase at 5, 30, 60, 90 minutes after graft reperfusion. Liver biopsy performed after the disconnection of the graft from the NMP (postperfusion) and after 1 hour from reperfusion were analyzed. Postoperative variables included intensive care unit and hospital stays, intraoperative death, early death, and morbidity occurring during the first 15 days. All patients included in this study signed an informed consent before the transplant to be enrolled in the original OrganOx trial. This retrospective study was approved by the clinical audit and research management system (CARMS) of the institution (registration number: CARMS-11423).
Statistical Methods
Data were recruited from a prospectively collected consecutive database (Microsoft Access 2.0; Microsoft Corporation, Redmond, WA). Univariate data were analyzed using the Mann-Whitney test and Fisher exact test. Repeated-measures analysis of variance models were then produced to analyze the NE and MAP levels. The NE values were log10-transformed before the analysis to reduce the level of skew in the distribution that meets the assumption of normality. The model included the patient group and measurement time as factors, as well as an interaction term. Results are reported as medians and ranges for demographic and surgical factors, geometric means with 95% confidence intervals for NE, and as arithmetic means with 95% confidence intervals for MAP. All analyses were performed using IBM SPSS Statistics 22 (IBM Corp. Armonk, NY), with P less than 0.05 deemed to be indicative of statistical significance.
RESULTS
Patient Population, Donor Variables, and Survival
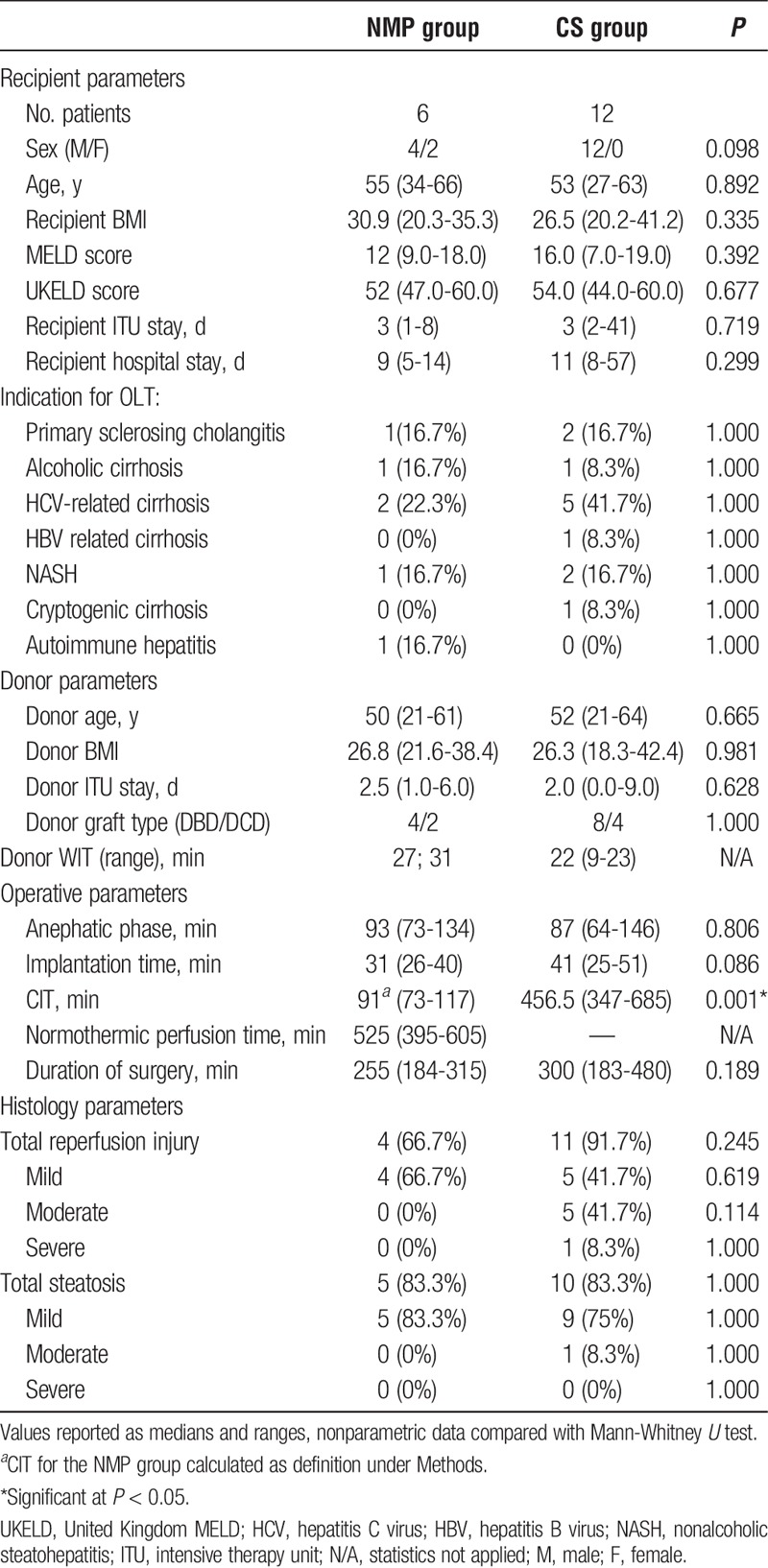
A total number of 18 patients (male, n = 16; median age [range], 58 [33-63] years) were retrospectively reviewed. This included 6 recipients (male, n = 4; median age [range], 55 [34-66] years) who received grafts preserved with NMP (NMP group) matched with 12 controls (male, n = 12; median age [range], 53 [27-63] years) who underwent LT after conventional CS (CS group). The 2 groups were comparable in terms of recipient demographic variables, such as age, sex, BMI, MELD, and United Kingdom MELD score, and etiology of liver disease (Table 1).
TABLE 1.
Recipient and donor and operative characteristics
Preoperative cardiac examinations, in terms of ejection fraction, were comparable in patients of both groups (>60%), except 1 in each group with left ventricular diastolic dysfunction.
Similarly, the donor profile was also comparable in the 2 groups (BMI, days of intensive therapy unit stay, DBD/DCD, donor WIT) (Table 1). Grafts preserved with the NMP were perfused on the machine for a median time of 525 (395-605) minutes before transplantation. All grafts preserved with NMP maintained consistent hepatic arterial (0.1-0.2 L/min) and portal venous (1-1.2 L/min) flows during the perfusion, and pH between 7.2 and 7.4 without pharmacological correction. Also, decreasing of lactate levels as bile productions was observed after the first hour and maintained throughout NMP.
Intraoperative times including duration of surgery, duration of the anhepatic phase, and implantation times were similar in both groups (Table 1). The median CIT, as defined in the methods, was significantly shorter in the NMP group compared with the CS group (91 minutes [73-117] vs 456 minutes [347-685], respectively) (P < 0.001). All organs were reperfused by portal vein and temporary portocaval shunt was performed in 4 patients (66.7%) of the NMP group and in 5 patients (41.7%) of the CS group (P = 0.612).
In the NMP group, liver biopsies performed when the graft was disconnected from the OrganOx machine and after 1 hour from reperfusion showed similar findings. When these were compared with the 1-hour postreperfusion graft biopsy of the CS group there were no significant differences reported in terms of postreperfusion injury and steatosis (Table 1).
Influence of NMP on DRI Score
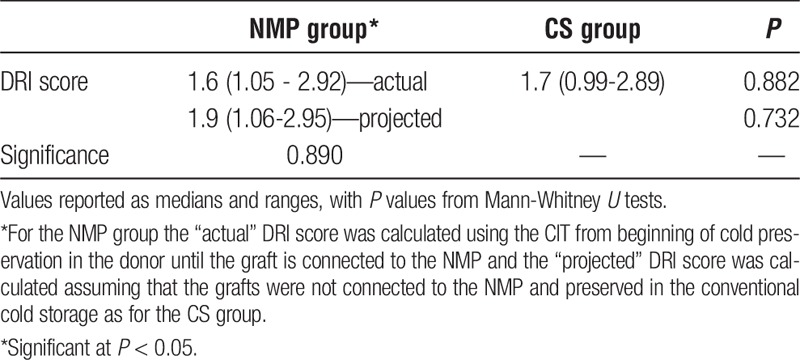
As described under the methods DRI was calculated on the grafts used in both NMP and CS groups. The median actual DRI of the NMP group was 1.6 (1.05-2.92) compared with CS group (1.7 [0.99-2.89]; P = 0.882) (Table 2). The projected DRI (assuming that there was no normothermic component) in NMP group was 1.9 (1.06-2.95), and this did not significantly differ with the actual DRI in the NMP group (P = 0.890) or that of CS group (P = 0.732).
TABLE 2.
Actual and projected donor risk index score for the 2 groups
Intraoperative Hemodynamic Outcomes
Mean Arterial Pressure
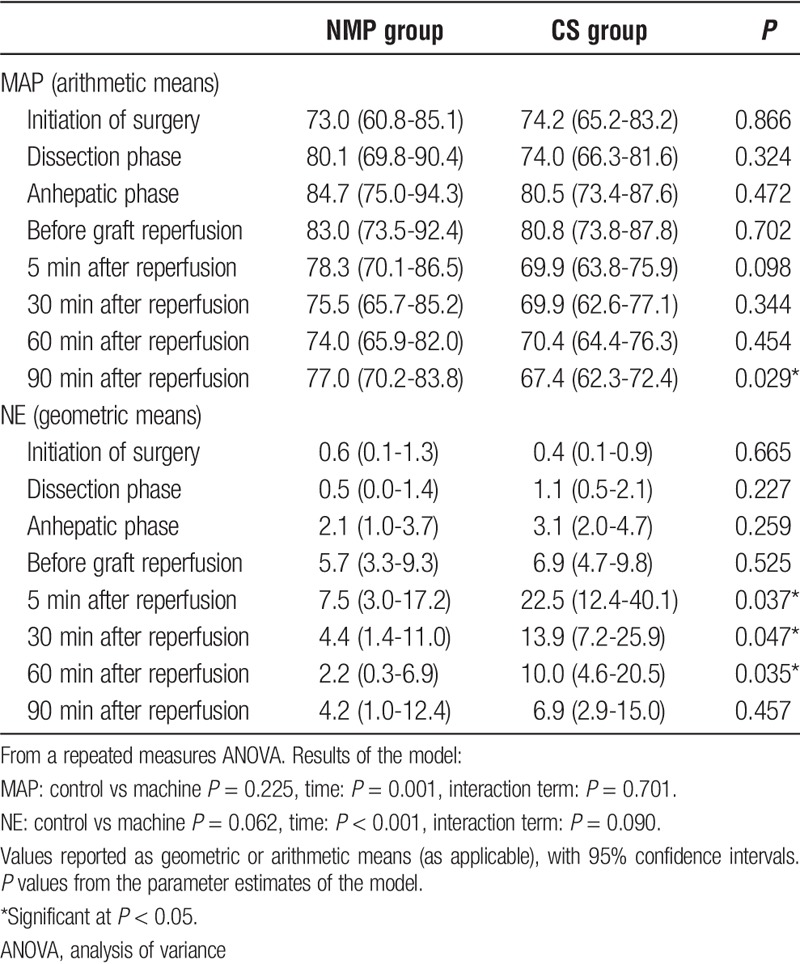
The intraoperative MAP was similar in both groups at all times during surgery, except for MAP at 90 minutes after graft reperfusion (67.4 [62.3-72.4] mm Hg for the CS group vs 77.0 [70.2-83.8] mm Hg for the NMP group [P = 0.029]), despite appropriate dose titration of the vasopressor (Table 3). Compared with the prereperfusion value within each group, the MAP of CS group significantly reduced 5 minutes after reperfusion (P = 0.020) and slowly increased to the prereperfusion values after 30 minutes (P = 0.050), whereas MAP remained unchanged compared with the prereperfusion values in the NMP group (P = 0.280). This change of the MAP in the NMP group was evident without the need for increased requirement of vasopressors to support adequate perfusion pressures as reported in detail below.
TABLE 3.
Mean arterial pressure and norepinephrine infusion during the surgery
Intraoperative data showed that PRS did not occur in the NMP group, whereas it occurred in 2 patients (16.7%) in the CS group (P = 0.529; Fisher exact test). The MAP dropped more than 30% that of prereperfusion recordings at any time of point after reperfusion in 4 recipients (33.3%) in the CS group, but no such variations were reported in the NMP group (P = 0.245).
Inotropic Support
Intravenous NE requirement was significantly higher in the CS group at 5 (P = 0.937), 30 (P = 0.047) and 60 (P = 0.035) minutes after liver reperfusion (Table 3). In the CS group, 3 recipients (25.0%) required boluses of epinephrine after graft revascularization until hemodynamic parameters were stabilized, whereas no patients required epinephrine boluses in the NMP group. The intraoperative mean NE infusion levels and MAP profiles are reported in Figure 1. No other inotropes were used. One patient in the CS group, without preoperative cardiovascular disease (left ventricular ejection fraction of 60-65% at the preoperative echocardiographic examination) and normokalemia before reperfusion, died from intraoperative cardiac arrest due to ventricular fibrillation, which occurred 30 minutes after portal reperfusion and 10 minutes after artery reperfusion.
FIGURE 1.
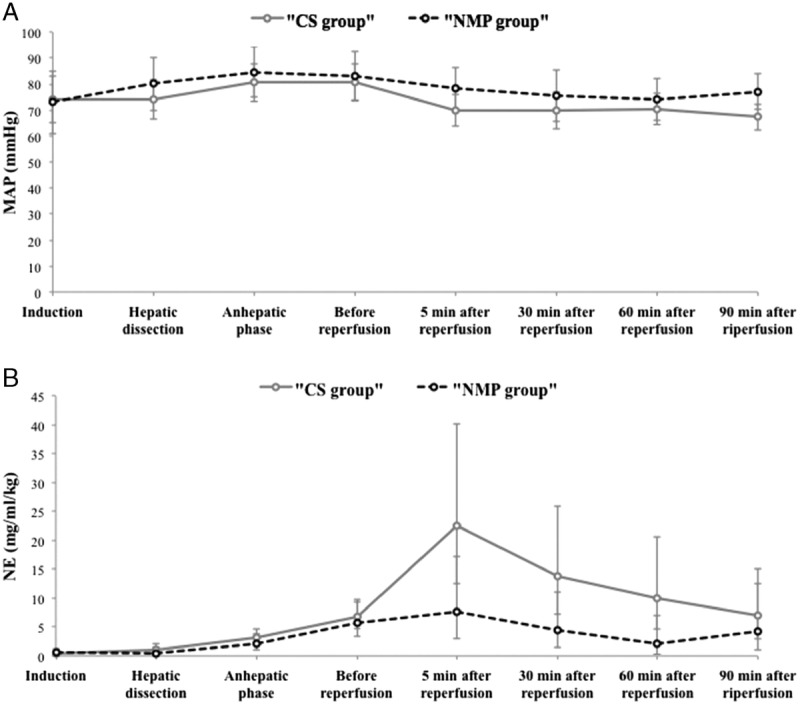
Course of norepinephrine infusion and mean arterial pressure over time during the liver transplantation. Arithmetic mean MAP (A) and geometric mean NE (B) over the course of the surgery. Bars represent 95% confidence intervals.
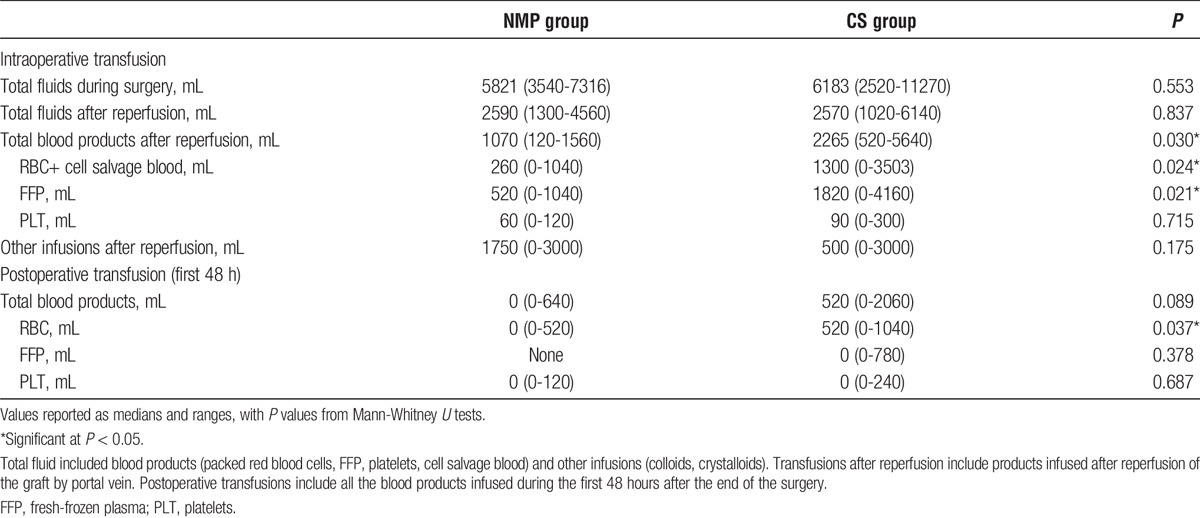
Transfusion Requirements
The total amount of intraoperative fluid infusion (blood products and other fluids) and the quantity infused after organ reperfusions were comparable in both groups (Table 4). However, in the grafts preserved with NMP, the volume of blood products transfused (RBCs, fresh frozen plasma, platelets, and cell salvage blood) after the liver reperfusion was significantly lower compared with that of the organs treated with standard CS (P = 0.030). In the NMP group 2 (33.3%), patients required cell-sever reinfusion, whereas 8 (66.7%) recipients in the control group (P = 0.321). The median intraoperative blood losses were 639 mL (256-1022 mL) and 957 mL (500-2326 mL) in the NMP group and CS group, respectively (P = 0.087). The hemoglobin level at the end of the operation were similar in both group ([8.9 mg/dL in the NMP group vs 8.6 g/dL in the control group [P = 0.504]). During the first 48 hours after the surgery, the total amount of blood products transfused was similar in both groups (P = 0.089); however, the volume of RBCs required in the control group was significantly higher than in the NMP group (P = 0.037) (Table 4).
TABLE 4.
Intraoperative and postoperative transfusion of blood products and fluids
DISCUSSION
Normothermic organ preservation has been investigated in all solid organ transplant models including laboratory, animal, and preclinical setting and currently being incorporated in the clinical transplantation scenery.18-21 The ability to mitigate cold ischemia and the ischemia-reperfusion syndrome, which causes significant graft dysfunction after early posttransplant period is projected as the principle benefit of normothermic preservation. The organ damage during the CS is mainly responsible for ischemic injury; complete alleviation of the cold ischemic component may allow liver grafts to be preserved in normal physiological conditions. In LT, normothermia could theoretically be applied in various stages.22-24
Graft reperfusion represents a critical challenge during LT and it may be associated with severe hemodynamic instability and PRS, which represent an important intraoperative risk of liver injury such as recipient morbidity and mortality.1,4 Mechanism of PRS is complex and many pathways are involved, and the severity is correlated with IRI.1,25 The physiopathology of liver IRI has been extensively investigated and has been divided in 2 principal phases, namely ischemia and reperfusion.7,26,27 Ischemic phase occurs during the CS due to absence of tissue perfusion, and therefore of oxygen and delivery of metabolites. This phase is characterized by depletion of energy stores, failure of Na+/K+ ATPase, anaerobic metabolism, cellular swelling/oedema, and acidosis. In the second phase, the graft reperfusion causes a rebound oxygen reuptake from cells, release of free radicals, endothelial damage, and intrahepatic inflammatory infiltration leading to organ damage.28 The release of proinflammatory cytokines into the circulation, along with perfusion of cooled allograft, bring about a myriad of physiological changes, such as myocardial depression, due to hypothermia (cooled blood returning to the heart), changes in pulmonary vascular resistance, and significant vasodilatation along with an increased cardiac output. These are systemic manifestations of postreperfusion injury. Therefore, the initial insult to the liver has been related with remote mechanisms, which can affect distant organ systems too.9
This study suggests that the systemic effects of IRI, leading to PRS, may be mitigated by NMP. It shows that to maintain the physiological blood pressures during the immediate reperfusion period, those in the CS group required a significantly greater dose of vasopressor, denoting that the peripheral vasodilatory response in this group that was brought about by IRI is greater. Even after hemodynamic and volume optimization of the patient, the mean blood pressures between the 2 groups were significantly different. Our data are in keeping with a similar study, where improved hemodynamics after NMP was shown in an animal reperfusion model.29 What is equally important is that the NMP did not significantly alter the particular risk of a graft, as denoted by the DRI, and despite this, there were significant physiological benefits and stability in the recipient during the post reperfusion phase. In other words, a high-risk graft remained “high risk” even after NMP, but the physiological response after reperfusion was comparatively better.
Of the above causes impacting on IRI, cold ischemia is potentially the only variable that could be modified—either through minimizing CIT though logistics of transplant setting up or by other means of organ preservation. Currently, the 2 alternative approaches to organ preservation being researched and incorporated in the clinical practice are normothermic and subnormothermic/hypothermic preservation.12 Both these principles incorporate taking away the ischemic insult to the graft and aim at replenishing the energy status of a graft. By minimizing the “ischemic insult,” subsequent reperfusion injury is also minimized.25,30-34 Recently, the Zurich group suggested that oxygenated hypotermic liver perfusion (HOPE) at the end of cold preservation is beneficial for DCD graft.35 However, NMP (37°), compared with oxygenated hypotermic liver perfusion, may create more physiological conditions to the graft and may represent the best option to improve the viability of suboptimal organs, such as DCD, before transplantation.36-40 Until now, the use of NMP has been reported mainly in animal models29,41-46 or in discarded human donor livers.12,15,24,47 Recently, the first 2 cases of successful transplantation of human livers after NMP preservation have been reported.48,49 The current study is the first report of the use of NMP in adult LT with the aim to evaluate his impact on the intraoperative hemodynamic events and PRS occurrence.
This study has limitations. The small sample size can be considered as a limiting factor leading to lack of statistical power, as is the use of the matched control group within the confines of a phase 1 study. Another major limitation is the lack of proinflammatory marker assays, which could have been helpful to corroborate the clinical pattern observed in the postreperfusion period. These limitations were unavoidable because the current study was a substudy of preplanned study where safety of NMP was assessed in the humans for the first time. Despite these limitations, one of the key findings that emerged from the study was that NMP removes or minimizes the cold ischemia component of preserved allografts, but this does not alter the perceived risk of a particular graft. Therefore, the immediate hemodynamic effects on the patient, having received an equal risk graft, are significantly different after NMP.
In conclusion, patients receiving liver grafts preserved in traditional CS require higher dose of vasopressors to counteract the vasodilatory effects manifested during reperfusion phase and a greater requirement of blood product transfusion. In contrast, NMP preserved liver allografts result in smooth reperfusion and achieve more physiological stability, with lesser need of pressor support and blood product transfusion. In an era where the shortage of donors is a universal issue and compromised grafts are increasingly used, normothermic preservation could be a tool to increase the utility of such grafts by modulating the IRI. Normothermic perfusion could also reduce the usage of blood products (another limited resource) during LT. These benefits and additional aspects need to be carefully investigated in the larger multicenter trials that are underway.50
Footnotes
Published online 5 August 2016.
ORGANOX was the sponsor of the original trial and P.J.F. is a director of the ORGANOX Company, Oxford, United Kingdom. All other authors did not have any financial conflicts or financial interests with ORGANOX Company.
The authors declare no funding.
R.A. and M.T.P.R.P. contributed equally to the work.
R.A. participated in data collection, analysis, and article writing. M.T.P.R.P. conceptualised the study, data analysis, interpretation, and article writing. R.R. participated in machine perfusion and data collection. D.H. participated in machine perfusion and data collection. C.C. is the inventor of normothermic technology device and participated in the interpretation of data and intellectual content. H.M. participated in data collection, analysis and interpretation, and intellectual content. J.R.I. participated in data collection, analysis and interpretation, intellectual content. A.I. participated in data collection, analysis and interpretation, and intellectual content. H.C. participated in data collection, analysis and interpretation, intellectual content. P.M. participated in data collection, analysis and interpretation, and intellectual content. P.J.F. is also the inventor of normothermic technology device, and participated in interpretation of data and intellectual content. D.F.M. conceptualized the study, data interpretation, intellectual content, and is the senior author.
REFERENCES
- 1.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504–508. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Kang Y, Freeman JA, et al. Postreperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8:154–160. [DOI] [PubMed] [Google Scholar]
- 3.Ayanoglu HO, Ulukaya S, Tokat Y. Causes of postreperfusion syndrome in living or cadaveric donor liver transplantations. Transplant Proc. 2003;35:1442–1444. [DOI] [PubMed] [Google Scholar]
- 4.Paugam-Burtz C, Kavafyan J, Merckx P, et al. Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl. 2009;15:522–529. [DOI] [PubMed] [Google Scholar]
- 5.Chung IS, Kim HY, Shin YH, et al. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation. Clin Transplant. 2012;26:539–543. [DOI] [PubMed] [Google Scholar]
- 6.Xu ZD, Xu HT, Yuan HB, et al. Postreperfusion syndrome during orthotopic liver transplantation: a single-center experience. Hepatobiliary Pancreat Dis Int. 2012;11:34–39. [DOI] [PubMed] [Google Scholar]
- 7.Khosravi MB, Sattari H, Ghaffaripour S, et al. Post-reperfusion syndrome and outcome variables after orthotopic liver transplantation. Int J Organ Transplant Med. 2010;1:115–120. [PMC free article] [PubMed] [Google Scholar]
- 8.De Boer MT, Christensen MC, Asmussen M, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106:32–44. [DOI] [PubMed] [Google Scholar]
- 9.Nastos C, Kalimeris K, Papoutsidakis N, et al. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965 doi: 10.1155/2014/906965. Epub 2014 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. [DOI] [PubMed] [Google Scholar]
- 11.Nanashima A, Pillay P, Crawford M, et al. Analysis of postrevascularization syndrome after orthotopic liver transplantation: the experience of an Australian liver transplantation centre. J Hepatobiliary Pancreat Surg. 2001;8:557–563. [DOI] [PubMed] [Google Scholar]
- 12.Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol. 2014;61:418–431. [DOI] [PubMed] [Google Scholar]
- 13.Guarrera JV. Assist devices: machine preservation of extended criteria donors. Liver Transpl. 2012;18:S31–S33. [DOI] [PubMed] [Google Scholar]
- 14.Olschewski P, Gass P, Ariyakhagorn V, et al. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology. 2010;60:337–343. [DOI] [PubMed] [Google Scholar]
- 15.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–1335. [DOI] [PubMed] [Google Scholar]
- 16.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–1787. [DOI] [PubMed] [Google Scholar]
- 17.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 18.Vogel T, Brockmann JG, Coussios C, et al. The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplant Rev (Orlando). 2012;26:156–162. [DOI] [PubMed] [Google Scholar]
- 19.Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int. 2015;28:657–664. [DOI] [PubMed] [Google Scholar]
- 20.Erasmus ME, Fernhout MH, Elstrodt JM, et al. Normothermic ex vivo lung perfusion of non-heart-beating donor lungs in pigs: from pretransplant function analysis towards a 6-h machine preservation. Transpl Int. 2006;19:589–593. [DOI] [PubMed] [Google Scholar]
- 21.Tolboom H, Makhro A, Rosser BA, et al. Recovery of donor hearts after circulatory death with normothermic extracorporeal machine perfusion. Eur J Cardiothorac Surg. 2015;47:173–179. [DOI] [PubMed] [Google Scholar]
- 22.Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death—the United Kingdom experience. Am J Transplant. 2014;14:2846–2854. [DOI] [PubMed] [Google Scholar]
- 23.Perera MT, Clutton-Brock T, Muiesan P. One donor, two types of preservation: first description of a donation after circulatory death donor with normothermic abdominal perfusion and simultaneous cold perfusion of lungs. Liver Transpl. 2014;20:1012–1015. [DOI] [PubMed] [Google Scholar]
- 24.Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642 doi: 10.1371/journal.pone.0110642. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bukowicka B, Akar RA, Olszewska A, et al. The occurrence of postreperfusion syndrome in orthotopic liver transplantation and its significance in terms of complications and short-term survival. Ann Transplant. 2011;16:26–30. [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. [DOI] [PubMed] [Google Scholar]
- 27.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury—a fresh look. Exp Mol Pathol. 2003;74:86–93. [DOI] [PubMed] [Google Scholar]
- 28.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. [DOI] [PubMed] [Google Scholar]
- 29.Fondevila C, Hessheimer AJ, Maathuis MH, et al. Superior preservation of DCD livers with continuous normothermic perfusion. Ann Surg. 2011;254:1000–1007. [DOI] [PubMed] [Google Scholar]
- 30.Fukazawa K, Yamada Y, Gologorsky E, et al. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth. 2014;28:994–1002. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchihashi S, Fondevila C, Shaw GD, et al. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol. 2006;176:616–624. [DOI] [PubMed] [Google Scholar]
- 32.Fondevila C, Shen XD, Duarte S, et al. Cytoprotective effects of a cyclic RGD peptide in steatotic liver cold ischemia and reperfusion injury. Am J Transplant. 2009;9:2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fondevila C, Shen XD, Tsuchihashi S, et al. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 2008;14:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl Int. 2015;28:690–699. [DOI] [PubMed] [Google Scholar]
- 35.Dutkowski P, Polak WG, Muiesan P, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg. 2015;262:764–771. [DOI] [PubMed] [Google Scholar]
- 36.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372–381. [DOI] [PubMed] [Google Scholar]
- 37.Hessheimer AJ, Fondevila C, García-Valdecasas JC. Extracorporeal machine liver perfusion: are we warming up? Curr Opin Organ Transplant. 2012;17:143–147. [DOI] [PubMed] [Google Scholar]
- 38.Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73:701–709. [DOI] [PubMed] [Google Scholar]
- 39.Schön MR, Kollmar O, Wolf S, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boehnert MU, Yeung JC, Bazerbachi F, et al. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant. 2013;13:1441–1449. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Berendsen T, Kim K, et al. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J Surg Res. 2012;173:e83–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellomo R, Suzuki S, Marino B, et al. Normothermic extracorporeal perfusion of isolated porcine liver after warm ischaemia: a preliminary report. Crit Care Resusc. 2012;14:173–176. [PubMed] [Google Scholar]
- 45.Tolboom H, Pouw RE, Izamis ML, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 2009;87:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlegel A, Kron P, Graf R, et al. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014;61:1267–1275. [DOI] [PubMed] [Google Scholar]
- 47.Bellomo R, Marino B, Starkey G, et al. Extended normothermic extracorporeal perfusion of isolated human liver after warm ischaemia: a preliminary report. Crit Care Resusc. 2014;16:197–201. [PubMed] [Google Scholar]
- 48.Watson CJ, Kosmoliaptsis V, Randle LV, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant. 2016;16:353–357. [DOI] [PubMed] [Google Scholar]
- 49.Perera T, Mergental H, Stephenson B, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016;22:120–124. [DOI] [PubMed] [Google Scholar]
- 50.COPE Consortium. Available at: www.cope-eu.org/ Accessed on 15 May 2016.


