Abstract
We present a recently introduced three tier pattern-based histopathologic system to stratify endocervical adenocarcinoma (EAC) that better correlates with lymph node (LN) metastases than FIGO staging alone, and has the advantage of safely predicting node-negative disease in a large proportion of EAC patients. The system consists of stratifying EAC into one of three patterns: pattern A tumors characterized by well-demarcated glands frequently forming clusters or groups with relative lobular architecture and lacking destructive stromal invasion or lymphvascular invasion (LVI), pattern B tumors demonstrating localized destructive invasion (small clusters or individual tumor cells within desmoplastic stroma often arising from pattern A glands), and pattern C tumors with diffusely infiltrative glands and associated desmoplastic response. Three hundred and fifty-two cases were included; mean follow-up 52.8 months. Seventy-three patients (21%) had pattern A tumors; all were stage I and there were no LN metastases or recurrences. Pattern B was seen in 90 tumors (26%); all were stage I and LVI was seen in 24 cases (26.6%). Nodal disease was found in only 4 (4.4%) pattern B tumors (one IA2, two IB1, one IB not further specified (NOS)), each of which showed LVI. Pattern C was found in 189 cases (54%), 117 had LVI (61.9%) and 17% were stage II or greater. Forty-five (23.8%) patients showed LN metastases (one IA1, 14 IB1, 5 IB2, 5 IB NOS, 11 II, 5 III and 4 IV) and recurrences were recorded in 41 (21.7%) patients. This new risk stratification system identifies a subset of stage I patients with essentially no risk of nodal disease, suggesting that patients with pattern A tumors can be spared lymphadenectomy. Patients with pattern B tumors rarely present with LN metastases, and sentinel LN examination could potentially identify these patients. Surgical treatment with nodal resection is justified in patients with pattern C tumors.
Key words of phrases: endocervical adenocarcinoma, invasive carcinoma, pattern-based, risk stratification, classification system, lymph node metastasis
Introduction
We recently reported on a new classification system that stratifies endocervical adenocarcinoma (EAC) by the morphologic pattern of invasion into three categories. This new system better predicts compared to FIGO stage, for the presence of lymph node (LN) metastasis as well as clinical behavior in patients with EAC [1, 2]. A subsequent study from an independent group indicated the system’s good reproducibility [3].
Staging cervical cancer is based on a combination of clinical and pathologic evaluation when using the International Federation of Gynecology and Obstetrics (FIGO) staging system [4]. Same criteria apply to both squamous and glandular lesions; however, these represent different tumor types; cervical cancer is not just one disease [5]. When an organ confined tumor is visible or palpable on examination, it is staged IB. When a tumor is not clinically visible, pathologists use the depth of invasion (DOI) of the tumor to determine its stage [4, 6]. The accurate pathologic measurement of DOI in some tumors can be quite challenging [7,8]. By definition, DOI is calculated from the basement membrane of the epithelium from which the invasive tumor arose [6]. This is easier for squamous carcinomas, since the overlying squamous epithelium is flat but endocervical glands normally extend into the superficial cervical stroma, vary in size and shape as well as normal extension and location in the underlying stroma [7,8]. The lack of a specific point of reference could determine a difference of several mm when calculating the depth of invasion; polypoid or ulcerated tumors could also affect DOI. Given the architectural complexity of the endocervical glands that are formed by a deeply invaginated epithelium with secondary branching and tunnel formation, the accurate measurement of depth of invasion is often problematic in early EAC [7,8].
While pathologists may struggle to determine an accurate DOI, this measurement has significant implications since it is the basis to stage and treat non-visible lesions; according to current NCCN guidelines only patients with EAC Stage IA1 (DOI 3 mm or less), without LVI can be spared pelvic LN dissection [9]. In addition, recently established NCCN guidelines following the Society of Gynecologic Oncology guidelines, recommend that tumors with less than 3 mm DOI but with LVI should undergo the same procedures as higher stage tumors including radical hysterectomy (radical trachelectomy in fertility sparing procedures) in addition to pelvic LN dissection and possible paraaortic LN sampling [9–12]. However, the literature reports few patients with early stage tumors and evidence of LN metastasis; less than 1% of patients with stage IA1 tumors had LN metastasis; while stage IA2 tumors revealed LN metastasis in about 2% of the cases [13–23]. This low yield is troubling since there can be significant morbidity after LN dissection [23–25].
Our objective is to present this recently introduced histopathologic pattern-based risk stratification system, highlighting that it better predicts nodal status and outcome than FIGO staging, and would allow for personalized selection of patients who can safely undergo conservative treatment.
Materials and methods
After Institutional Review Board approvals were obtained, cases diagnosed and treated as invasive endocervical adenocarcinoma of usual type were retrieved and studied from 12 national and international institutions.
Selection criteria included: 1) tumors diagnosed as invasive endocervical adenocarcinoma, usual type (as defined by most recent World Health Organization classification [26]); 2) tumor resected by cone/LEEP procedure, trachelectomy and/or hysterectomy with tumor slides available for microscopic examination; and 3) lymphadenectomy with more than one lymph node or clinical/radiological evidence of metastatic nodal disease.
Members of the participating institutions convened in three consensus meetings at Cedar-Sinai Medical center in Los Angeles, California. A presentation of the histopathologic pattern-based risk stratification system started the initial meeting and available slides were then reviewed by the group utilizing a multiheaded microscope. Cases were classified by consensus according to the newly developed system (Silva system of endocervical adenocarcinoma) based on “pattern of invasion” as A, B, or C (Table 1; Figure 1).
Table 1.
New risk stratification system for invasive endocervical adenocarcinomas based on pattern of invasion (Silva system).
| Silva system | |
|---|---|
| Pattern A |
|
| Pattern B |
|
| Pattern C |
|
Adapted from Roma AA, et al. Am J Surg Pathol. 2015 May;39(5):667–72.
Copyright: Wolters Kluwer Health, Inc.
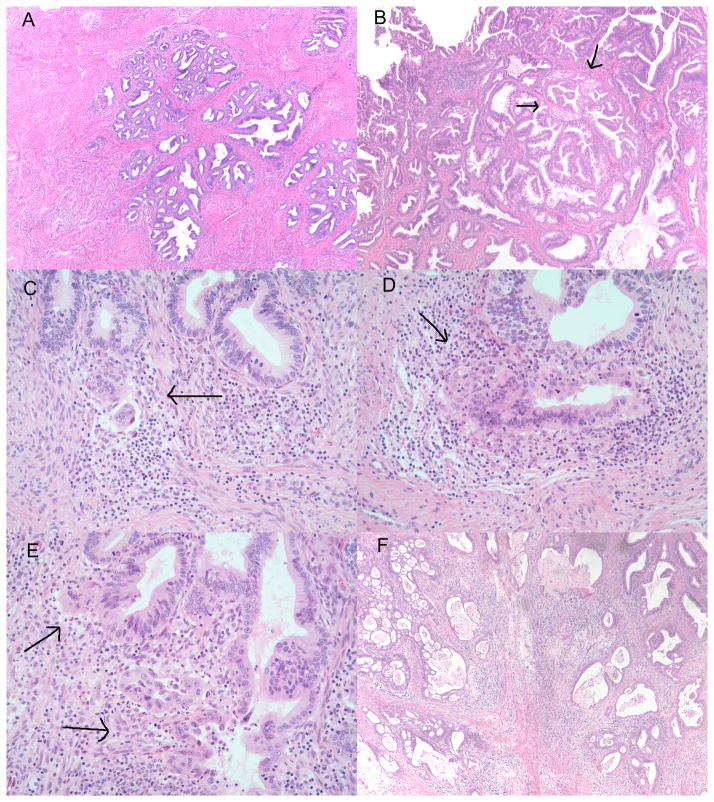
Figure 1.
Figure 1A: Deeply invasive well differentiated endocervical adenocarcinoma corresponding to pattern A tumor. H&E 40X. B: Low power examination of exophytic and invasive endocervical adenocarcinoma also corresponding to pattern A tumor. Higher examination is recommended to rule out questionable pattern B areas (arrows). H&E 40X. C–E: High power examination of invasive endocervical adenocarcinoma composed of glands with irregular contours (arrows) in a focally desmoplastic stroma arising from pattern A type glands. H&E 200X. F: Pattern C composed of diffuse destructive invasion, irregular and incomplete glands, some with cribriform architecture in a diffuse desmoplastic stroma. H&E40X.
EAC with pattern A is characterized by well-demarcated glands with rounded contours, frequently forming clusters or groups and sometimes showing relatively well preserved lobular architecture. Tumor glands demonstrate a pushing or expansile pattern of invasion. Most pattern A cases extend below the level of benign endocervical glands, with the neoplastic glands often adjacent to thick walled blood vessels, an established criteria for invasion [27]. Complex intraglandular pattern including cribriform morphology or papillary intraglandular growth can also be seen in gland profiles exceeding the size of normal glands. The presence of LVI excludes a tumor from pattern A.
Pattern B tumors show early or limited, localized destructive invasion, defined as individual or small clusters of tumor cells or fragments of glands set in a desmoplastic, edematous, or inflamed stroma adjacent to an intact gland. The typical appearance is that of limited destructive invasion arising from glands with a pattern A appearance. LVI may be seen in pattern B tumors.
Pattern C tumors have diffusely infiltrative glands, with associated extensive, diffuse desmoplastic response; the glands show a destructive (or tentacular) pattern with angulated and often incomplete glands open to the stroma. Additional criteria for pattern C tumors include confluent growth of cribriform or papillary structures within stroma, filling a low power microscopic field (4x field; 5 mm), and/or extensive mucin lakes with tumor cells. Solid and/or poorly differentiated component (architecturally high grade) is also included in pattern C. Cases with mixed patterns are classified based on the worst tumor pattern areas.
Data analyzed included: tumor size, horizontal spread, DOI, LVI, and LN metastasis. Data collected were summarized using descriptive statistics with Microsoft Excel (e.g., averages, frequencies, percentages). Data is normally distributed. All statistical analyses performed in IBM -SPSS 22.0 using Levene statistics of homogeneity of variance and Q-Q plots of each variable. Normality rechecked with Kurtosis measurement in IBM-SPSS 23.0.
Results
Clinical data previously reported is summarized in Tables 2 [1, 2]. Overall, 352 patients diagnosed with invasive EAC were included in this study.
Table 2.
Outcome data comparing the standard method of tumor evaluation (depth of invasion) versus the newly proposed pattern-based system.
| Patients | Patients with metastatic LN | # metastatic LNs | Stage I | Stage II–IV | |
|---|---|---|---|---|---|
| Standard | 352 | 49 (14%) | 83 (1%) | 320 (91%) | 32 (9%) |
| Pattern A | 73 (20.7%) | 0 | 0 | 73 (100%) | 0 |
| Pattern B | 90 (25.6%) | 4 (4.4%) | 5 (0.2%) | 90 (100%) | 0 |
| Pattern C | 189 (53.7%) | 45 (24%) | 76 (1.7%) | 157 (83%) | 32 (17%) |
LNs: lymph nodes
Seventy-three cases (20.7%) contained morphologic features that corresponded to pattern A. All tumors were stage I, including 14 IA1, 8 IA2, 46 IB1, 1 IB2 and 4 IB not further specified. Tumor size ranged from 1.8 mm to 42 mm (mean 13.4 mm). DOI ranged from 0.1 mm to 10 mm (mean 3.8 mm). None of the cases had LVI. LNs were resected in all cases (total 1333 LNs; range 2 to 78 resected LNs per patient; mean 20.5 LNs); all were negative for metastatic carcinoma. Follow-up was available in 71 patients (range 6 to 252 months; mean 50.1 months; median 44 months). Recurrences were not observed and all patients were alive and well at last follow-up.
Ninety patients (25.6%) had tumors with morphologic features that corresponded to pattern B. All patients had FIGO stage I tumors, including 7 IA1, 8 IA2, 51 IB1, 4 IB2 and 20 IB not further specified. Tumor size ranged from 2 mm to 65 mm (mean 17 mm). DOI ranged from 0.2 to 27 mm (mean 9.1 mm). Twenty-four cases (26.6%) demonstrated LVI. LNs resected totaled 1750 (range 4 to 60 resected LNs per patient, mean 22.1). Only 4 (4.4%) patients demonstrated metastatic adenocarcinoma in pelvic LNs, and all 4 of the primary tumors had LVI. There were 5 metastatic LNs in total. Follow-up was available in 83 patients and ranged from 6 month to 392.5 months (mean, 56.3; median 42 months). All four patients with positive LNs had no recurrence at last follow-up; two patients received chemotherapy and radiation treatment and had no evidence of disease (NED) 22 and 54 months after surgery, respectively. The other two patients did not receive adjuvant therapy, but also were NED at 56 and 60 months, respectively. Only 1 (1.2%) patient suffered recurrence in the vagina, 8 months after hysterectomy; this patient had 60 resected negative LNs, no evidence of LVI in the tumor, and was alive and well 49 months after resection of the recurrence. No patient with pattern B tumors died of disease (DOD).
The remaining 189 patients (53.7%) had tumors with morphologic features of pattern C. Most of these patients, 157 (83%), had stage I tumor (4 IA1, 10 IA2, 107 IB1, 12 IB2 and 24 IB not further specified), while 32 (16.9%) had stage II or higher stage tumors (21 II, 6 III and 5 IV). Tumor size ranged from 1.5 mm to 70 mm (mean 26.7 mm). DOI ranged from 0.3 to 20 mm (mean 9.1 mm). LVI was present in 117 (61.9%) cases. LN metastases were recorded in 45 (23.8%) patients; 43 with metastatic carcinoma documented in surgically resected LNs, and 2 patients with clinically positive LNs. At least 73 LNs had metastatic carcinoma out of 3423 resected LNs, with only a single positive node in 21 patients. Follow-up was available in 179 patients and ranged from 3 to 258 months (mean 56 months, median 39 months). Recurrences were recorded in 41 (21.5%) patients; 10 (5.2%) with vaginal or vulvar recurrence and 31 (16.4%) with pelvic or distant recurrence. At last follow-up, 18 patients were DOD at 9 to 107 months (mean 45.1 months, medium 39 months). Table 3 summarizes data on pattern C tumors in patients with metastatic lymph nodes at presentation and status at last follow-up including tumors with recurrence; while Table 4 shows recurrences by stage and pattern and Table 5 shows patients with metastatic LN by stage and pattern.
Table 3.
Follow-up and adjuvant treatment modalities in patients with pattern C tumors and initial metastatic lymph nodes
| Adjuvant treatment in patients with recurrence | ||||
|---|---|---|---|---|
| Chemotherapy (4 cases) | Radiation (2 cases) | Both (8 cases) | None (3 cases) | |
| Site of recurrence; stage; status at last follow-up | Abdomen; stage IIB; NED | Ovary; stage IIIB; AWD | Lung; stage IIA1; AWD | Vagina; stage IB1, lost follow-up |
| Para-aortic LN; stage IIB; DOD | Para-aortic LN; stage IB1; DOD | Lung and pelvis; stage IV; AWD | Ovary, pelvis; stage IV; DOD | |
| Lung; IB1; AWD | Pleural fluid; stage IIB; DOD | Ovary, bladder wall; stage IIIB; AWD | ||
| Lung and liver; stage IB1; DOD | Pelvis; stage IV; AWD | |||
| Abdominal wall; stage IB1; DOD | ||||
| Retroperitoneum; stage IB2; AWD | ||||
| Ovary, liver, retroperitoneum; stage IV; AWD | ||||
| Vagina; stage IIA1; AWD | ||||
| Adjuvant treatment in patients with no recurrence at last follow-up | ||||
| Chemotherapy (4 cases) | Radiation (7 cases) | Both (13 cases) | None (6 cases) | |
| Stage | IB1 | IB1 (5 cases) | IB1 (8 cases) | IA1 |
| IIA | IB2 | IB2 (2 cases) | IA2 | |
| IIIB (2 cases) | IIA | IIA (2 cases) | IB1 | |
| IIIB | IB2 | |||
| IIA | ||||
| IIB | ||||
NED: no evidence of disease; AWD: alive with disease; DOD: died of disease
Table 4.
Comparison of FIGO stage vs. tumor pattern and recurrence.
| Stage | IA1 | IA2 | IB1 | IB2 | IB NOS | II | III | IV | Total |
|---|---|---|---|---|---|---|---|---|---|
| Recurrence/Pattern A | 0/14 | 0/8 | 0/46 | 0/1 | 0/4 | 0/0 | 0/0 | 0/0 | 0/73 |
| Recurrence/Pattern B | 0/7 | 0/8 | 1/51 (2%) | 0/4 | 0/20 | 0/0 | 0/0 | 0/0 | 1/90 (1.1%) |
| Recurrence/Pattern C | 0/4 | 0/10 | 14/107 (13%) | 4/12 (33.3%) | 6/24 (25%) | 9/21 (43%) | 3/6 (50%) | 5/5 (100%) | 41/189 (21.7%) |
FIGO: International Federation of Gynecology and Obstetrics; NOS: not otherwise specified
Table 5.
Comparison of FIGO stage vs. tumor pattern and lymph node metastasis.
| Stage | IA1 | IA2 | IB1 | IB2 | IB NOS | II | III | IV | Total |
|---|---|---|---|---|---|---|---|---|---|
| LN mets/Pattern A | 0/14 | 0/8 | 0/46 | 0/1 | 0/4 | 0/0 | 0/0 | 0/0 | 0/73 |
| LN mets/Pattern B | 0/7 | 1/8 (12.5%) | 2/51 (3.9%) | 0/4 | 1/20 (5%) | 0/0 | 0/0 | 0/0 | 4/90 (4.4%) |
| LN mets/Pattern C | 1/4 (25%) | 0/10 | 14/107 (13%) | 5/12 (41.7%) | 5/24 (20.8%) | 11/21 (52.4%) | 5/6 (83.3%) | 4/5 (80%) | 45/189 (23.8%) |
| LN mets by stage | 1/25 (4%) | 1/26 (3.8%) | 16/204 (7.8%) | 5/17 (29.4%) | 6/48 (12.5%) | 11/21 (52.4%) | 5/6 (83.3%) | 4/5 (80%) | 49/352 (13.9%) |
FIGO: International Federation of Gynecology and Obstetrics; NOS: not otherwise specified
Statistical analysis revealed that pattern B tumors had statistically more extensive horizontal spread (p<0.002) than pattern A tumors. Pattern C tumors were larger (p<0.0001), had deeper invasion (tumor thickness p<0.0001), and demonstrated a higher frequency of LN metastasis (p<0.0001) than pattern A tumors. Pattern C tumors were larger (p<0.0001), had more extensive horizontal spread (p<0.0001), had deeper invasion (tumor thickness p<0.0001 or DOI p<0.0001), and demonstrated LVI (p<0.0001) and LN metastases (p<0.001) more frequently than tumors with pattern B.
Discussion
This new histopathologic pattern-based risk stratification system separates EAC into pattern A tumors with no risk of LN metastasis; therefore, avoiding LN dissection should be considered. In addition, there were no recurrences seen in pattern A tumors, and this may indicate that adjuvant therapy following surgery would not be indicated even for bulky tumors, using Sedlis criteria [28]. Patients with pattern B tumors rarely show nodal metastasis, and only if there is LVI; recurrences are very rare and all these patients had stage I tumors. In addition to conservative surgery, limited LN dissection or sentinel LN sampling in these patents might be beneficial and should be investigated. Patients with pattern C tumors require radical treatment with nodal resection, since nearly one-quarter of these tumors showed LN metastases and over 20% suffered recurrences.
It is important to note that all study cases were diagnosed as invasive EAC, including all pattern A tumors. A small minority of pattern A tumors may be interpreted by some pathologists as endocervical adenocarcinoma in situ (AIS); however, many of the cases in this study classified as pattern A had large grossly visible and deep tumors that could not have been considered as in situ lesions. While studies indicate that a clear distinction of invasive EAC from adenocarcinoma in situ is not possible in up to 20% of cases [29–30], this new system makes that distinction in difficult cases irrelevant as nodal dissection is unnecessary in both AIS and pattern A tumors, as patients would have an excellent prognosis.
Current NCCN guidelines determine how patients with EAC at different stages should be treated, with radical surgery and nodal resection in most patients [9]. However, morbidity for radical surgery and lymph node resection are significant including voiding difficulty, urinary tract infection, hemorrhage, port-site hematoma, pain, pyrexia, and nerve injury as early complications. Late complications include lymphedema, lymphocyst, urinary incontinence, voiding difficulty, venous thrombosis and rectocele, in addition to the loss of childbearing capability for these often young patients [24, 25, 31].
Although several studies reported on the potential safety of conservative management of early-stage glandular lesions, there remains controversy regarding the use of less radical treatments [13–23, 32–51]. In the last few years, there have been attempts to better determine patients that can be safely treated with conservative surgery. Reynolds et al, found only one case of nodal metastasis, a micrometastasis, in a set of 66 Stage IA EAC cases [17]. There was no parametrial involvement nor recurrences in their patients. Similar data was confirmed by other studies, supporting consideration for less radical surgery performed in conjunction with pelvic lymphadenectomy for patients with tumor size no greater than 2 cm, no LVI, negative pelvic lymph nodes and DOI less than 10 mm [33–37]. However, most these studies included both squamous carcinoma and adenocarcinomas, with a predominance of the former; pelvic lymph nodes were still resected, but in some series, sentinel lymph node mapping plus or minus completion of pelvic node resection depending on sentinel lymph node status was performed. There is an attempt to predict tumor behavior but it is independent of the morphologic features of the tumor (aside from lymphovascular invasion) and in particular not recognizing EAC as a specific tumor.
We are proposing a different, and we believe more specific, risk stratification system for EAC that better discriminates patients’ LN status and recurrence rate; it decreases the significance of measuring DOI with all its difficulties, instead focusing on tumor morphologic features. Analysis of morphologic tumor pattern enabled stratification of EAC into three distinct groups identified by histologic examination. Table 4 illustrates that when pattern A and B tumors are excluded, recurrence rate increases with stage: 13% in stage IB1, 33.3% in stage IB2, 43% in stage II, 50% in stage III and 100% in stage IV; this risk stratification system improves on the current FIGO staging system. Table 5 shows that early stage tumors can have LN metastasis, which are rare and hard to predict with the current staging system and better predicted with the new risk stratification system.
We are completing a reproducibility study and the preliminary data confirms that pathologists who have not been included in the original reviews of the cases were able to classify most EAC agreeing with our interpretation.
We are also currently moving into the next stage which is working with a group of gynecologic oncologists to explore the possibility of modifying the staging and treatment of this tumor. In our opinion, if these findings are confirmed in additional, prospective studies, consideration may be given to staging based on the tumor pattern with a new three tier stage I: IA for pure pattern A tumors, IB for pattern B tumors with or without pattern A, and IC for pattern C tumors, pure or combined with any of the other patterns. If new studies are successful in stratifying either pattern B or pattern C tumors, substaging could be incorporated to better predict tumor behavior and treatment. Alternatively, the transition from the current system to the new system could require adding tumor pattern (A, B or C) and still assessing DOI, albeit redefining it to measure depth of destructive invasion (DODI) (i.e. measuring destructive invasion from pattern A type glands). The refinement of the definition of DOI would determine that pattern A tumors have no DODI, most Pattern B have DODI < 3 mm and < 7 mm horizontal extent, and most but not all Pattern C have DODI > 3 mm. This change would allow pathologists and clinicians to determine the FIGO stage as it currently stands, but clinicians will have more data to evaluate the need for radical vs. conservative surgery, lymphadenectomy vs. sentinel lymph node or close follow-up with no additional treatment.
Further validation and study of additional cases is necessary; however, this new system has significant clinical implications. We propose the following algorithm to help gynecologic oncologists and other treating physicians better determine patient treatment, along with other criteria. Tumor pattern in the diagnostic cervical biopsy would be reported which might consist of a pure pattern or combination thereof; the worst or highest pattern should be reported. If any pattern C is present in the biopsy, then based on published observations, current treatment modalities would be appropriate. Radical surgery (e.g. radical hysterectomy or trachelectomy) plus pelvic lymph node resection would be the recommended treatment. However, the treating physician could include other known variables or criteria that might modify treatment accordingly (i.e. cervical conization or simple trachelectomy and LN evaluation seems to be an acceptable treatment strategy for selected patients with small-volume (2 cm or less) stage I cervical cancer [40–50].
Conservative management similar to management for AIS would be the recommended treatment approach when pure pattern A tumor is seen in the biopsy. Treatment would proceed with a cervical cone, entirely submitted for microscopic examination. If pattern A tumor persists in the cone and the margins are negative, the patient might be managed with observation. If the tumor involves the surgical margins of the cone, a second cervical conization or wider excision, could be necessary; the tumor should be entirely resected with negative margins but radical surgery or lymph node resection is not necessary. If the subsequent cervical cone reveals pattern C tumor, a hysterectomy or trachelectomy plus pelvic lymph node resection would be appropriate treatment (as we have presented, these are aggressive tumors); the role of pelvic radiation therapy, in particular, in patients with Sedlis criteria might need to be investigated and could decrease recurrence rate [28]. If tumor in the initial biopsy or subsequent cervical conization specimen revealed pattern B, then the treatment would include conservative surgery plus sentinel lymph node sampling. Future studies might determine that only patients with LVI need sentinel lymph node sampling since very few patients had lymph node metastasis (only 4.4% of patients with pattern B tumors). If frozen section evaluation of the sentinel lymph nodes is performed, metastatic EAC should trigger completion of the lymphadenectomy. We recommend conservative surgery to resect pattern B tumors since all were stage I and parametrial involvement was not reported [2].
In pattern A tumors, it is important that pathologists examine the tumor very carefully to exclude LVI. Deeper sections might be necessary to exclude any areas worrisome for pattern B or LVI.
One of the limitations of the study is that we only included EAC, usual type and did not include special types: clear cell or serous carcinoma, mucinous adenocarcinoma (minimal deviation, intestinal, signet-ring cell or the newly described gastric-type adenocarcinoma) or mesonephric carcinoma; however, we believe most of these special types would represent examples of pattern C tumors [26, 52, 53].
This new risk stratification system combined with modification of the current FIGO stage I and treatment algorithm of patients with EAC better predict patient prognosis and avoids unnecessary lymphadenectomy and its complications in a significant subset of patients. This new personalized approach allows for many of these patients to be treated with conservative modalities, rather than radical hysterectomy and LN dissection for most, hence avoiding morbidity without risking an increase in the rate of recurrence or lymph node metastasis. This is a step in the right direction of personalized medicine that analyzes all features of the tumor, not just size or DOI.
Acknowledgments
This work was sponsored by the International Society of Gynecological Pathologists and Department of Pathology and Laboratory Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Footnotes
Disclosures: The authors declare that there are no conflicts of interest or funding to disclose.
References
- 1.Diaz De Vivar A, Roma AA, Park KJ, Alvarado-Cabrero I, Rasty G, Chanona-Vilchis JG, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol. 2013;32:592–601. doi: 10.1097/PGP.0b013e31829952c6. [DOI] [PubMed] [Google Scholar]
- 2.Roma AA, Diaz De Vivar A, Park KJ, Alvarado-Cabrero I, Rasty G, Chanona-Vilchis JG, et al. Invasive Endocervical Adenocarcinoma: A New Pattern-based Classification System With Important Clinical Significance. Am J Surg Pathol. 2015;39:667–72. doi: 10.1097/PAS.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 3.Paquette C, Jeffus SK, Quick CM, Conaway MR, Stoler MH, Atkins KA. Interobserver variability in the application of a proposed histologic subclassification of endocervical adenocarcinoma. Am J Surg Pathol. 2015;39:93–100. doi: 10.1097/PAS.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 4.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol. 2010;116:140–6. doi: 10.1016/j.ygyno.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–62. [PubMed] [Google Scholar]
- 7.Zaino RJ. Glandular Lesions of the Uterine Cervix. Mod Pathol. 2000;13:261–74. doi: 10.1038/modpathol.3880047. [DOI] [PubMed] [Google Scholar]
- 8.Zaino RJ. Symposium part I: adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 2002;21:314–26. doi: 10.1097/00004347-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. [Accessed 11/16/2014];Clinical Practice Guidelines in Oncology (NCCN Guidelinest) Version 1.2015. Available at: http://www.NCCN.org.
- 10.Tropé C, Kristensen G, Onsrud M, Bosze P. Controversies in cervical cancer staging. CME J Gynecol Oncol. 2001;6:240–5. [Google Scholar]
- 11.Creasman WT, Fetter BF, Clarke-Pearson DL, Kaufmann L, Parker RT. Management of stage IA carcinoma of the cervix. Am I Obstet Gynecol. 1985;153:164–72. doi: 10.1016/0002-9378(85)90105-x. [DOI] [PubMed] [Google Scholar]
- 12.Sevin BU, Nadji M, Averette HE, Hilsenbeck S, Smith D, Lampe B. Microinvasive carcinoma of the cervix. Cancer. 1992;70:2121–8. doi: 10.1002/1097-0142(19921015)70:8<2121::aid-cncr2820700819>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Poynor EA, Marshall D, Sonoda Y, Slomovitz BM, Barakat RR, Soslow RA. Clinicopathologic features of early adenocarcinoma of the cervix initially managed with cervical conization. Gynecol Oncol. 2006;103:960–5. doi: 10.1016/j.ygyno.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85:1547–54. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Kasamatsu T, Okada S, Tsuda H, Shiromizu K, Yamada T, Tsunematsu R, et al. Early invasive adenocarcinoma of the uterine cervix: criteria for nonradical surgical treatment. Gynecol oncol. 2002;85:327–32. doi: 10.1006/gyno.2002.6624. [DOI] [PubMed] [Google Scholar]
- 16.Hirai Y, Takeshima N, Tate S, Akiyama F, Furuta R, Hasumi K. Early invasive cervical adenocarcinoma: its potential for nodal metastasis or recurrence. BJOG. 2003;110:241–6. [PubMed] [Google Scholar]
- 17.Reynolds E, Tierney K, Keeney GL, Felix JC, Weaver AL, Roman LD, et al. Analysis of outcomes of microinvasive adenocarcinoma of the uterine cervix by treatment type. Obstet Gynecol. 2010;116:1150–7. doi: 10.1097/AOG.0b013e3181f74062. [DOI] [PubMed] [Google Scholar]
- 18.Elliott P, Coppleson M, Russell P, Liouros P, Carter J, MacLeod C, et al. Early invasive (FIGO stage IA) carcinoma of the cervix: a clinico-pathologic study of 476 cases. Int J Gynecol Cancer. 2000;10:42–52. doi: 10.1046/j.1525-1438.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaku T, Kamura T, Sakai K, Amada S, Kobayashi H, Shigematsu T, et al. Early Adenocarcinoma of the Uterine Cervix. Gynecol Oncol. 1997;285:281–5. doi: 10.1006/gyno.1997.4652. [DOI] [PubMed] [Google Scholar]
- 20.Baalbergen A, Smedts F, Helmerhorst TJM. Conservative Therapy in Microinvasive Adenocarcinoma of the Uterine Cervix Is Justified: An Analysis of 59 Cases and a Review of the Literature. Int J Gynecol Cancer. 2011;21:1640–5. doi: 10.1097/IGC.0b013e3182262059. [DOI] [PubMed] [Google Scholar]
- 21.Bisseling KC, Bekkers RL, Rome RM, Quinn MA. Treatment of microinvasive adenocarcinoma of the uterine cervix: A retrospective study and review of the literature. Gynecol Oncol. 2007;107:424–30. doi: 10.1016/j.ygyno.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 22.Smith HO, Qualls CR, Romero AA, Webb JC, Dorin MH, Padilla LA, et al. Is there a difference in survival for IA1 and IA2 adenocarcinoma of the uterine cervix? Gynecol Oncol. 2002;85:229–41. doi: 10.1006/gyno.2002.6635. [DOI] [PubMed] [Google Scholar]
- 23.Webb JC, Key CR, Qualls CR, Smith HO. Population-Based Study of Microinvasive Adenocarcinoma of the Uterine Cervix. Obstet Gynecol. 2001;97:701–6. doi: 10.1016/s0029-7844(01)01330-8. [DOI] [PubMed] [Google Scholar]
- 24.Zikan M, Fischerova D, Pinkavova I, Slama J, Weinberger V, Dusek L, et al. A prospective study examining the incidence of asymptomatic and symptomatic lymphoceles following lymphadenectomy in patients with gynecological cancer. Gynecol Oncol. 2015;137:291–8. doi: 10.1016/j.ygyno.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Conte M, Panici PB, Guariglia L, Scambia G, Greggi S, Mancuso S. Pelvic lymphocele following radical para-aortic and pelvic lymphadenectomy for cervical carcinoma: incidence rate and percutaneous management. Obstet Gynecol. 1990;76:268–71. [PubMed] [Google Scholar]
- 26.Wilbur DC, Colgan TJ, Ferenczy AS, et al. Tumours of the uterine cervix. Glandular tumours and precursors. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. 4. Geneva, Switzerland: WHO press; 2014. pp. 184–5. [Google Scholar]
- 27.Wheeler DT, Kurman RJ. The relationship of glands to thick-wall blood vessels as a marker of invasion in endocervical adenocarcinoma. Int J Gynecol Pathol. 2005;24:125–30. doi: 10.1097/01.pgp.0000152025.45106.6d. [DOI] [PubMed] [Google Scholar]
- 28.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–83. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 29.Bean SM, Kurtycz DFI, Colgan TJ. Microinvasive and early invasive carcinoma of the uterine cervix. J Low Genit Tract Dis. 2011;15:146–57. doi: 10.1097/LGT.0b013e3181fb425d. [DOI] [PubMed] [Google Scholar]
- 30.Ostör G. Early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 2000;19:29–38. doi: 10.1097/00004347-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Mehra G, Weekes A, Vantrappen P, Visvanathan D, Jeyarajah A. Laparoscopic assisted radical vaginal hysterectomy for cervical carcinoma: morbidity and long-term follow-up. Eur J Surg Oncol. 2010;36:304–8. doi: 10.1016/j.ejso.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Baalbergen A, Ewing-Graham PC, Hop WC, Struijk P, Helmerhorst TJ. Prognostic factors in adenocarcinoma of the uterine cervix. Gynecol Oncol. 2004;92:262–7. doi: 10.1016/j.ygyno.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Covens A, Rosen B, Murphy J, Laframboise S, DePetrillo AD, Lickrish G, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol. 2002;84:145–9. doi: 10.1006/gyno.2001.6493. [DOI] [PubMed] [Google Scholar]
- 34.Steed H, Capstick V, Schepansky A, Honore L, Hiltz M, Faught W. Early cervical cancer and parametrial involvement: is it significant? Gynecol Oncol. 2006;103:53–7. doi: 10.1016/j.ygyno.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Wright JD, Grigsby PW, Brooks R, Powell MA, Gibb RK, Gao F, et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer. 2007;110:1281–6. doi: 10.1002/cncr.22899. [DOI] [PubMed] [Google Scholar]
- 36.Stegeman M, Louwen M, van der Velden J, ten Kate FJ, den Bakker MA, Burger CW, et al. The incidence of parametrial tumor involvement in select patients with early cervix cancer is too low to justify parametrectomy. Gynecol Oncol. 2007;105:475–80. doi: 10.1016/j.ygyno.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Frumovitz M, Sun CC, Schmeler KM, Deavers MT, Dos Reis R, Levenback CF, et al. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstet Gynecol. 2009;114:93–9. doi: 10.1097/AOG.0b013e3181ab474d. [DOI] [PubMed] [Google Scholar]
- 38.McHale MT, Le TD, Burger RA, Gu M, Rutgers JL, Monk BJ. Fertility sparing treatment for in situ and early invasive adenocarcinoma of the cervix. Obstet Gynecol. 2001;98:726–31. doi: 10.1016/s0029-7844(01)01544-7. [DOI] [PubMed] [Google Scholar]
- 39.Naik R, Cross P, Nayar A, Mayadevi S, Lopes A, Godfrey K, et al. Conservative surgical management of small-volume stage IB1 cervical cancer. BJOG. 2007;114:958–63. doi: 10.1111/j.1471-0528.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 40.Rob L, Charvat M, Robova H, Pluta M, Strnad P, Hrehorcak M, et al. Less radical fertility-sparing surgery than radical trachelectomy in early cervical cancer. Int J Gynecol Cancer. 2007;17:304–10. doi: 10.1111/j.1525-1438.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 41.Rob L, Pluta M, Strnad P, Hrehorcak M, Chmel R, Skapa P, et al. A less radical treatment option to the fertility-sparing radical trachelectomy in patients with stage I cervical cancer. Gynecol Oncol. 2008;111:S116–20. doi: 10.1016/j.ygyno.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Pluta M, Rob L, Charvat M, Chmel R, Halaska M, Jr, Skapa P, et al. Less radical surgery than radical hysterectomy in early stage cervical cancer: a pilot study. Gynecol Oncol. 2009;113:181–4. doi: 10.1016/j.ygyno.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Maneo A, Sideri M, Scambia G, Boveri S, Dell’anna T, Villa M, et al. Simple conization and lymphadenectomy for the conservative treatment of stage IB1 cervical cancer. An Italian experience. Gynecol Oncol. 2011;123:557–60. doi: 10.1016/j.ygyno.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Fagotti A, Gagliardi ML, Moruzzi C, Carone V, Scambia G, Fanfani F. Excisional cone as fertility-sparing treatment in early-stage cervical cancer. Fertil Steril. 2011;95:1109–12. doi: 10.1016/j.fertnstert.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Al-Kalbani M, McVeigh G, Nagar H, McCluggage WG. Do FIGO stage IA and small (≤2 cm) IB1 cervical adenocarcinomas have a good prognosis and warrant less radical surgery? Int J Gynecol Cancer. 2012;22:291–5. doi: 10.1097/IGC.0b013e3182339fff. [DOI] [PubMed] [Google Scholar]
- 46.Palaia I, Musella A, Bellati F, archetti C, Di Donato V, Perniola G, et al. Simple extrafascial trachelectomy and pelvic bilateral lymphadenectomy in early stage cervical cancer. Gynecol Oncol. 2012;126:78–81. doi: 10.1016/j.ygyno.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Raju SK, Papadopoulos AJ, Montalto SA, Coutts M, Culora G, Kodampur M, et al. Fertility-sparing surgery for early cervical cancer-approach to less radical surgery. Int J Gynecol Cancer. 2012;22:311–7. doi: 10.1097/IGC.0b013e3182370f51. [DOI] [PubMed] [Google Scholar]
- 48.Biliatis I, Kucukmetin A, Patel A, Ratnavelu N, Cross P, Chattopadhyay S, et al. Small volume stage 1B1 cervical cancer: is radical surgery still necessary? Gynecol Oncol. 2012;126:73–7. doi: 10.1016/j.ygyno.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 49.Plante M, Gregoire J, Renaud MC, Sebastianelli A, Grondin K, Noel P, et al. Simple vaginal trachelectomy in early-stage low-risk cervical cancer: a pilot study of 16 cases and review of the literature. Int J Gynecol Cancer. 2013;23:916–22. doi: 10.1097/IGC.0b013e3182954ddf. [DOI] [PubMed] [Google Scholar]
- 50.Andikyan V, Khoury-Collado F, Denesopolis J, Park KJ, Hussein YR, Brown CL, et al. Cervical conization and sentinel lymph node mapping in the treatment of stage I cervical cancer: is less enough? Int J Gynecol Cancer. 2014;24:113–7. doi: 10.1097/IGC.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez PT, Pareja R, Rendón GJ, Millan C, Frumovitz M, Schmeler KM. Management of low-risk early-stage cervical cancer: should conization, simple trachelectomy, or simple hysterectomy replace radical surgery as the new standard of care? Gynecol Oncol. 2014;132:254–9. doi: 10.1016/j.ygyno.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Mod Pathol. 2004;17:962–72. doi: 10.1038/modpathol.3800148. [DOI] [PubMed] [Google Scholar]
- 53.Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–72. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]