Abstract
Drug-addiction may trigger early onset of age-related disease, due to drug-induced multi-system toxicity and perilous lifestyle, which remains mostly undetected and untreated. We present the literature on pathophysiological processes that may hasten aging and its relevance to addiction, including: oxidative stress and cellular aging, inflammation in periphery and brain, decline in brain volume and function, and early onset of cardiac, cerebrovascular, kidney, and liver disease. Timely detection of accelerated aging in addiction is crucial for the prevention of premature morbidity and mortality.
What is biological aging?
Aging is a progressive process spanning from optimal performance at maturity to gradual and advanced diminishing function involving the entire organism [1], impacting physiologic systems, functional characteristics and clinical features. Chronological age conveys a rough approximation of a status, whereas biological aging results from the interaction of genetic, environmental and behavioral factors, as well as disease. Accelerated aging occurs when biological age outpaces chronological age and the emergence of typical aging phenotypes at an earlier age than commonly observed [1, 2]. While operational definitions of aging phenotypes are inconclusive [1, 3] and various age-related biomarkers are used in the literature [3, 4], determining whether and to what extent a disease or risk factors contribute to premature aging is crucial in a chronic disease as addiction. Our review centers on the major pathways and interacting pathophysiological processes that may contribute to accelerated aging in drug addiction (see Figure and Table).
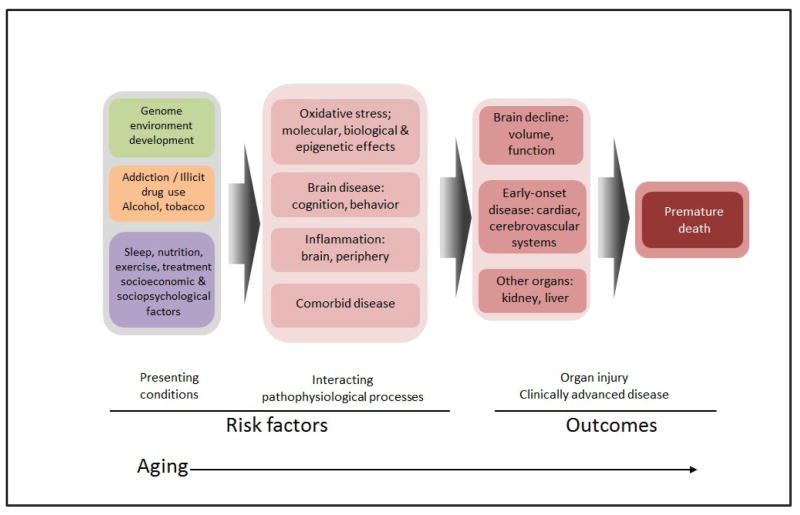
Figure. Conceptual model for aging in addiction.
This figure illustrates the progression of disease among drug addicted individuals, from presenting phenotypic conditions constituting risk factors, through interacting with pathophysiological processes to cumulative organ system injury, and outcome of advanced clinical disease and eventually death.
Table.
Factors by which substance use disorders contribute to accelerated aging in drug addicted individuals including alcoholics and cigarette smokers
| Toxic Effects of Drugs in Brain |
| Acceleration of aging in dopamine systems |
| Cerebrovascular pathology |
| Neuroinflammation |
| Enhanced sensitivity to stressors |
| Physiological Drug Effects |
| Cardio vascular |
| Pulmonary |
| Metabolic |
| Immune |
| Circadian |
| Behavioral/Social Drug Effects |
| Unhealthy life styles (poor nutrition, poor sleep patterns, lack of physical activity) |
| Social isolation (stigmatization, impaired access to healthcare and family and community support, poverty) |
| Infectious diseases (HIV, hepatitis, other sexually transmitted diseases) |
| Involvement with Criminal Justice System |
Why study aging in addiction?
Drug-addiction involves premature mortality and early onset of age-related disease. For example, due to arterial, cardiac and cerebrovascular toxicity, cocaine is involved in 40.3% of emergency admissions related to illicit drug use with the highest rates occurring in men aged 35–44 [5]. Similarly the median life of smokers is reduced by at least 10 years not just from cancer, which is a hallmark disorder of aging [6], but also from damage to vascular and pulmonary systems and the brain. Vast efforts are geared towards psychosocial and occupational rehabilitation of individuals with substance use disorders. Yet, the early-onset of diseases triggered (e.g., cirrhosis of the liver by alcohol) or exacerbated by drugs of abuse (e.g., pulmonary hypertension by methamphetamine [7]) remains mostly undetected, resulting in silent disease progression, amounting to a vast personal, social and economic burden [8]. We propose that substance use promotes accelerated aging, and that its early detection is crucial for the prevention of premature morbidity and mortality.
Presenting conditions
Contribution of addiction-related phenomenology to premature aging
Addiction is considered a chronically relapsing brain disorder associated with abnormal brain morphology [9] and function [10]. It has been conceptualized as a syndrome of impaired response inhibition and salience attribution (“iRISA”) encompassing deficits in higher-order cognitive control and motivational functions, functions of the prefrontal cortex including the orbitofrontal cortex and anterior cingulate cortex, and neuroanatomically connected subcortical and limbic reward structures. Frontal cortical areas are activated in addicted individuals during intoxication, craving, and bingeing, and they are deactivated during withdrawal, and their functioning vis-à-vis the subcortical reward pathways are suggested to contribute to long-term self-control and motivational deficits [11], leading to perilous lifestyle.
Genetic factors that contribute to variability in response to psychoactive agents can enhance hemodynamic reactivity, incidence of coronary vasoconstriction, vascular damage and cardiac and pulmonary pathology. Additionally, synergetic effects between multiple environmental, psychosocial and behavioral factors comprising the addiction phenomenology could enhance potential age-related disease.
The life-course and complexity of addiction is comprised of years (often decades) of chronic stress and comorbid use of drugs (including illicit drugs with alcohol and/or tobacco) that potentiate cellular harm and systemic toxicity [12]. Drug addiction is associated with risky health behaviors including unprotected sex or intravenous drug use that increase exposure to infections, which in turn activate the immune and inflammatory systems and potentially hasten vasculature-aging and neuronal toxicity. The prevailing low socio-economic status, limited use of health and follow-up care, lack of sleep, insufficient exercise, and poor nutrition, could further promote age-related disease [13]. Such lifestyle factors may mediate aging-related mechanisms rather than being directly causally inducing accelerated aging. For example, cocaine and alcohol addiction are associated with robust alterations in sleep architecture, including disturbances in slow-wave sleep and rapid eye movement (REM), which has implications for several age-related diseases, since sleep contributes to the homeostatic regulation of the neuroendocrine and immune systems [14] [e.g., the amount of REM sleep correlates with the production of the proinflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α [15]].
Pathophysiological processes
Oxidative stress and cellular aging
Oxidative stress occurs when levels of reactive oxygen/nitrogen species overwhelm the cellular antioxidant capacities [16]. While lower and moderate levels of mitochondrial reactive oxygen species (mROS) have positive function-promoting functions, elevated mROS directly damage cells [16]. When severe, extensive modifications or damage to macromolecules including nucleic acids (DNA, RNA), lipids and proteins [17] can eventually induce cell death and tissue injury [18, 19]. Such damage has been linked to multiple age-dependent pathologies, including diabetes, cancer and neurodegenerative diseases [18] (e.g., neurodegeneration is associated with overproduction of mROS that induces cellular damage and subsequent neuronal deficits [19]). Addictive drugs slow cell growth and potentiate apoptosis [20]. Their use (cannabis [21], alcohol, amphetamine or its derivatives [22]; cocaine [23]; opiates [24]; inhalants, synthetic drugs [25]), or withdrawal from their acute effects, induce oxidative stress contributing to their cytotoxicity in brain, heart, liver, and kidney [26]. Cannabis increases the uncoupling of oxidative phosphorylation increasing the free radical flux from mitochondria [21]. Beyond oxidative metabolism of drugs, monoamine oxidation (by monoamine oxidases or by auto-oxidation), mitochondrial dysfunction, excitotoxicity, microglial activation, inflammation, are present [26]. The interaction of multiple drugs can lead to further oxidative damage [26]. For example, the combination of cocaine and opioids (“speedball”) accentuates mitochondrial dysfunction [27], and co-abuse of cocaine and ethanol (produces an active metabolite, cocaethylene) resulted in increased ROS generation [28].
Exposure to oxidation, inflammation and stress hormones may lead to telomere (nucleotides at the ends of chromosomes) attrition thereby expediting aging [2] (e.g., leukocyte telomere length (LTL) generally decreases progressively over the lifespan, with estimates of average attrition rates ranging between 32.2 and 45.5 base pairs per year [29]). The effect of inflammation on LTL is potentially caused by LTL’s association with increased immune cell replication, as well as by pathways leading from inflammation to oxidation. Oxidative stress is associated with memory and learning impairments [26], that are common in drug addiction [30]. The combination of ethanol and methylenedioxymethamphetamine increases hyperthermic and hepatotoxic effects [31]. Also, drug use (especially of opioids) reduced LTLs, as associated with relapse and route of administration (sniffing related to longer LTLs compared to other methods) [32]. In addition, stem cells (pluripotential progenitor cells from which a whole class of cells differentiate) and their health are a special focus of aging medicine and associated deficits [33]. The decline in stem cells circulating in the peripheral blood appears to be three to four times faster in addicted individuals than in the general population.
Inflammation in periphery and brain
Immune parameters in periphery and brain change with age (suppression or hyperstimulation) and are considered biological markers of aging [34]. As a result of telomere shortening in lymphocytes that hypersecrete peripheral pro-inflammatory cytokines, senescent immune cells can lead to a vicious cycle of added inflammation, oxidative stress and subsequent telomere shortening [35]. Furthermore, inflammation coupled with increased oxidation may be especially damaging and likely to foster accelerated cell aging [36]. In the brain, whether activated microglia, involved in both addiction-related [37] and other age-related disorders [38] have a role in neuroinflammation is inconclusive [39]. Yet, it has been proposed that a collection of inflammatory changes in the hypothalamus, a critical nucleus for aging development and lifespan control, underlies advanced aging [40].
Peripheral pro-inflammatory cytokine concentrations are inversely correlated with LTL in major depressive disorder and in individuals with history of early life stress [41], comorbidities highly associated with drug abuse. Inflammation and atherosclerosis (deposition of plaques of fatty material on inner walls of arteries) are potential lethal effects of drug use that have acute and chronic systemic impact [42]. Opiate addiction is characterized by a chronic immune stimulatory profile with elevated lymphocytes and monocytes, cytokines and globulins immune activity as well as suppression. Similarly, cocaine creates an hyperactive immune-mediated inflammatory state with increased pro-inflammatory cytokines (e.g., TNFa, IL-1β) [42, 43] and decreased basal anti-inflammatory markers (e.g., IL-10) [42, 43], all contributing to vascular disease (e.g., endocarditis). Also, high-sensitivity C-reactive protein (a protein produced by the liver following injury, infection, or inflammation) values were higher in addiction [44]. In the brain, most stimulants dysregulate the blood-brain barrier through alterations in tight junction complexes or through inflammation and oxidative stress. Neuroinflammation plays a particularly important role as it contributes to a feed-forward process leading to vulnerability to infiltration of inflammation markers from the periphery [45].
Similarly to oxidative stress, inflammatory processes are further accelerated by comorbid substance abuse especially with cigarette smoking, which in addition to having negative effects on the vascular and pulmonary systems, inhibits monoamine oxidases, which are necessary for proper body detoxification [46].
Organ system injury and advanced clinical disease
Brain decline: volume, function
Aging causes changes in brain structure, neurochemistry, and function including damage to its vasculature as well as the deposition of substances that affects cognition. Brain weight declines on average by 5% per decade after age 40, where the frontal lobes undergo the greatest shrinkage in men whereas the parietal lobes show more atrophy in women [47]. Brain volume loss encompasses the loss of neurons, synapses, neurotransmitters and receptors, especially in the neocortex, basal forebrain nuclei and brainstem monoaminergic systems. Both brain small vessels [48] and carotid arteries [49] show decrease in lumen diameter with age. Other microscopic changes with senescence include the accumulation of lipofuscin (a pigmented lipid composed of lysosomal digestion), and the proteins hyperphosphorilated tau and beta-amyloid in the form of neurofibrillary tangles and senile plaques [50]. The presence of similar changes in cognitively impaired patients may indicate that a disease state (e.g. dementia) is an exaggerated example of normal aging [47] and therefore the “pace of aging” may be crucial for transition into disease.
Similarly, use of drugs accentuates age-related changes in the brain that may underlie some of the abnormalities found in addiction models. Compared to healthy controls, cocaine-dependent individuals show twice the rate of brain volume reduction a year, especially of the prefrontal and temporal regions [9]; in methamphetamine users the rate of decline in grey matter is 6.4–8.5% a year in the frontal, temporal, insula and occipital cortices [51]. Cannabis is associated with reduced hippocampus volume [52], Cocaine [53], methamphetamine [54], and heroin [55] addicted-individuals show intra- and extra-cranial artery dysfunction. Pathologies commonly observed in the brain of aged individuals (e.g., primary age-related taupathy) indeed appear earlier in drug addicted individuals as compared to non-addicted individuals. Importantly, cognitive decline in addiction [56] may be attributed to hyperphosphorylated tau and p62-positive inclusions (neurodegeneration-related proteins) as reported with heroin use [57] or the accumulation of amyloid as reported in cocaine [58] and methamphetamine [59] users.
Early onset of age-related illness: cardiac, cerebrovascular, kidney, and liver disease
Drug addiction causes irreversible structural and functional changes not only in the brain but also in the heart, lung and other organs such as the liver and kidney. Cocaine-induced injury to the cardiovascular and cerebrovascular systems is well known [60]. Specifically, cocaine use is linked with hypertension, tachycardia, ventricular arrhythmias [61], myocardial infarction [62], and stroke [63]. Furthermore, cocaine is associated with muscle damage, electrolyte disturbances and elevated liver enzymes leading to renal and liver failure resulting in severe functional impairments or sudden mortality [64]. Likewise, amphetamines produce cardiac-rhythm disturbances and infarction, stroke, nephropathy (kidney damage) and generate toxic metabolites that may be the cause of hepatic injury. Similarly, heroin has been a known cause of chronic brain small vessel disease, heart disease, and as a leading cause of death among young individuals. Moreover, in combination with alcohol, heroin triggers liver dysfunction and cirrhosis and nephropathy [65]. Endocrine pathology casts a wide net of deleterious effects. For example, the suppression of sex steroids by many addictions is far from benign, and a focus of the aging literature [66]. Also the link between opioid addiction and diabetes is often unrecognized and overlooked [67].
Conclusion
Addiction and substance abuse, prevalent conditions worldwide, contribute to accelerate aging and age-related diseases. Although the mechanisms by which these drugs accelerate the aging process remain to be established, evidence points to multiple events (e.g. oxidative stress, excitotoxicity, and mitochondrial dysfunction) which ultimately lead to degeneration and neuronal apoptosis. Drug addicted individuals exhibit unique aging-related biomarkers that are not routinely identified, and therefore, they require additional attention. Further research is needed to adapt screening tools, risk scores and prognostic models for early identification of at-risk individuals in order to reduce premature morbidity and mortality in this population.
Highlights.
Accelerated aging happens when biological age outpaces chronological age
In addiction the early-onset of comorbid diseases remains mostly undetected
oxidation, inflammation, stress hormones may lead to telomere attrition expediting aging
cognitive decline in addiction may be attributed to premature accumulation of amyloid
Acknowledgments
Funding
This work was supported by: the National Institute on Drug Abuse: [KB: T32-DA007135-31; NDV: Intramural funding; RZG: 1U01DA041174, R01DA041528]; the National Institutes of Health [NAK: R01MH090134]; Department of Preventive Medicine, and Social Work Services support, Icahn School of Medicine at Mount Sinai [KB]; Grant from Alfonso Martin Escudero Foundation [SS].
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
*of special interest
** of outstanding interest
- 1*.Margolick JB, Ferrucci L. Accelerating aging research: how can we measure the rate of biologic aging? Exp Gerontol. 2015;64:78–80. doi: 10.1016/j.exger.2015.02.009. Excellent opinion/review on the four domains of the aging phenotype and how the concept of accelerated aging can be quantified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neuroscience and biobehavioral reviews. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE. Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences. 2015;112(30):E4104–E4110. doi: 10.1073/pnas.1506264112. Longitudinal cohort study providing quantification of the pace of physiological deterioration across multiple organ systems to assess biological aging demonstrating a normal distribution of the later as copared to chronological aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Scott KM, Lim C, Al-Hamzawi A, et al. Association of mental disorders with subsequent chronic physical conditions: World mental health surveys from 17 countries. JAMA Psychiatry. 2016;73(2):150–158. doi: 10.1001/jamapsychiatry.2015.2688. Data from 17 courntries demonstrate that alcohol abuse was associated with early onset of 10 physical conditions including stroke, even after controlling for co-morbidities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. 2013. HHS Publication No. (SMA) 13–4760. [PubMed] [Google Scholar]
- 6.Rosso T, Malvezzi M, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Cancer mortality in Europe, 1970–2009: an age, period, and cohort analysis. European Journal of Cancer Prevention. 2016 doi: 10.1097/CEJ.0000000000000282. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS One. 2010;5(12):e15269. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diercks DB, Fonarow GC, Kirk JD, Jois-Bilowich P, Hollander JE, Weber JE, Wynne J, Mills RM, Yancy C, Peacock WFt. Illicit stimulant use in a United States heart failure population presenting to the emergency department (from the Acute Decompensated Heart Failure National Registry Emergency Module) The American journal of cardiology. 2008;102(9):1216–1219. doi: 10.1016/j.amjcard.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 9**.Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Molecular psychiatry. 2013;18(2):134–135. doi: 10.1038/mp.2012.31. Cocaine dependent individuals had greater age-related prefrontal and temporal gary matter atrophy than non-using controls, indicating accelerated loss in cortical areas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. The New England journal of medicine. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and Telomere Biology: A Lifespan Perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janković S, Stojisavljević D, Janković J, Erić M, Marinković J. Association of socioeconomic status measured by education, and cardiovascular health: a population-based cross-sectional study. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2014-005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Irwin MR, Bjurstrom MF, Olmstead R. Polysomnographic measures of sleep in cocaine dependence and alcohol dependence: Implications for age-related loss of slow wave, stage 3 sleep. Addiction (Abingdon, England) 2016;111(6):1084–1092. doi: 10.1111/add.13300. Study recorded marked disturbance in cocaine and alcohol addicted including increased rapid eye movement and accelerated age-related loss of slow wave, Stage 3 sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18(4):349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Yan L-J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biology. 2014;2:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Annals of the New York Academy of Sciences. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 18.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Molecular cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70(6):1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Sarafian TA, Habib N, Oldham M, Seeram N, Lee RP, Lin L, Tashkin DP, Roth MD. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo. American journal of physiology. Lung cellular and molecular physiology. 2006;290(6):L1202–1209. doi: 10.1152/ajplung.00371.2005. [DOI] [PubMed] [Google Scholar]
- 22.Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pomierny-Chamiolo L, Moniczewski A, Wydra K, Suder A, Filip M. Oxidative stress biomarkers in some rat brain structures and peripheral organs underwent cocaine. Neurotoxicity research. 2013;23(1):92–102. doi: 10.1007/s12640-012-9335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skrabalova J, Drastichova Z, Novotny J. Morphine as a Potential Oxidative Stress-Causing Agent. Mini-Reviews in Organic Chemistry. 2013;10(4):367–372. doi: 10.2174/1570193X113106660031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virmani A, Ali SF, Binienda ZK. Neuroprotective strategies in drug abuse-evoked encephalopathy. Annals of the New York Academy of Sciences. 2010;1199(1):52–68. doi: 10.1111/j.1749-6632.2009.05171.x. [DOI] [PubMed] [Google Scholar]
- 26.Cunha-Oliveira T, Cristina Rego A, Oliveira RC. Oxidative Stress and Drugs of Abuse: An Update. Mini-Reviews in Organic Chemistry. 2013;10(4):321–334. [Google Scholar]
- 27.Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Neurotoxicity of heroin-cocaine combinations in rat cortical neurons. Toxicology. 2010;276(1):11–17. doi: 10.1016/j.tox.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Bazuaye-Ekwuyasi EA, Ogunbileje JO, Kaphalia BS, Eltorky MA, Okorodudu AO. Comparative effects of cocaine and cocaethylene on alveolar epithelial type II cells. Toxicology Mechanisms and Methods. 2015;25(8):604–613. doi: 10.3109/15376516.2015.1045658. [DOI] [PubMed] [Google Scholar]
- 29.Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing research reviews. 2013;12(2):509–519. doi: 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of Executive and Memory Function Associated with Amphetamine and Opiate Dependence. Neuropsychopharmacology. 2005;31(5):1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pontes H, Sousa C, Silva R, Fernandes E, Carmo H, Remiao F, Carvalho F, Bastos ML. Synergistic toxicity of ethanol and MDMA towards primary cultured rat hepatocytes. Toxicology. 2008;254(1–2):42–50. doi: 10.1016/j.tox.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Ye J, Li C, Zhou D, Shen Q, Wu J, Cao L, Wang T, Cui D, He S, Qi G, He L, Liu Y. Drug addiction is associated with leukocyte telomere length. Scientific reports. 2013;3:1542. doi: 10.1038/srep01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reece AS. Evidence of accelerated ageing in clinical drug addiction from immune, hepatic and metabolic biomarkers. Immunity & ageing : I & A. 2007;4:6–6. doi: 10.1186/1742-4933-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46(2–3):135–140. doi: 10.1016/j.exger.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143–152. doi: 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovács KJ. Microglia and Drug-Induced Plasticity in Reward-Related Neuronal Circuits. Frontiers in Molecular Neuroscience. 2012;5:74. doi: 10.3389/fnmol.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beggs S, Salter MW. SnapShot: Microglia in Disease. Cell. 2016;165(5):1294–1294.e1291. doi: 10.1016/j.cell.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 39.Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. Journal of neuroinflammation. 2016;13:33. doi: 10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497(7448):211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narvaez JC, Magalhaes PV, Fries GR, Colpo GD, Czepielewski LS, Vianna P, Chies JA, Rosa AR, Von Diemen L, Vieta E, Pechansky F, Kapczinski F. Peripheral toxicity in crack cocaine use disorders. Neuroscience letters. 2013;544:80–84. doi: 10.1016/j.neulet.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 43.Fox HC, D'Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R. Immune system inflammation in cocaine dependent individuals: implications for medications development. Human psychopharmacology. 2012;27(2):156–166. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reece AS, Hulse GK. Elevation of central arterial stiffness and vascular ageing in opiate withdrawal: cross-sectional and longitudinal studies. Cardiovascular toxicology. 2013;13(1):55–67. doi: 10.1007/s12012-012-9186-7. [DOI] [PubMed] [Google Scholar]
- 45.Kousik SM, Napier TC, Carvey PM. The Effects of Psychostimulant Drugs on Blood Brain Barrier Function and Neuroinflammation. Frontiers in Pharmacology. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fowler JS, Logan J, Wang G-J, Volkow ND, Telang F, Zhu W, Franceschi D, Pappas N, Ferrieri R, Shea C, Garza V, Xu Y, Schlyer D, Gatley SJ, Ding Y-S, Alexoff D, Warner D, Netusil N, Carter P, Jayne M, King P, Vaska P. Low monoamine oxidase B in peripheral organs in smokers. Proceedings of the National Academy of Sciences. 2003;100(20):11600–11605. doi: 10.1073/pnas.1833106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67(8):1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- 48.Abraham HM, Wolfson L, Moscufo N, Guttmann CR, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: Blood pressure, white matter lesions, and functional decline in older persons. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36(1):132–142. doi: 10.1038/jcbfm.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shenkin SD, Bastin ME, MacGillivray TJ, Eadie E, Deary IJ, Starr JM, Wardlaw JM. Carotid intima-media thickness and cerebrovascular disease in community-dwelling older people without stroke. Stroke; a journal of cerebral circulation. 2010;41(9):2083–2086. doi: 10.1161/STROKEAHA.110.590505. [DOI] [PubMed] [Google Scholar]
- 50.Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered Proteins in the Aging Brain. Journal of neuropathology and experimental neurology. 2016;75(4):316–325. doi: 10.1093/jnen/nlw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction (Abingdon, England) 2011;106(8):1474–1483. doi: 10.1111/j.1360-0443.2011.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battistella G, Fornari E, Annoni J-M, Chtioui H, Dao K, Fabritius M, Favrat B, Mall J-F, Maeder P, Giroud C. Long-Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology. 2014;39(9):2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massardo T, Quintana JC, Jaimovich R, Saez CG, Cabreras MJ, Pereira-Flores K, Ibanez C, Pallavicini J, Veliz J, Mezzano D, Pereira J. Changes in regional cerebral blood flow are associated with endothelial dysfunction markers in cocaine-dependent patients under recent abstinence. Journal of addiction medicine. 2015;9(2):139–146. doi: 10.1097/ADM.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 54.Ho EL, Josephson SA, Lee HS, Smith WS. Cerebrovascular complications of methamphetamine abuse. Neurocritical care. 2009;10(3):295–305. doi: 10.1007/s12028-008-9177-5. [DOI] [PubMed] [Google Scholar]
- 55.Benoilid A, Collongues N, de Seze J, Blanc F. Heroin inhalation-induced unilateral complete hippocampal stroke. Neurocase. 2013;19(4):313–315. doi: 10.1080/13554794.2012.667125. [DOI] [PubMed] [Google Scholar]
- 56.Sanvicente-Vieira B, Kommers-Molina J, De Nardi T, Francke I, Grassi-Oliveira R. Crack-cocaine dependence and aging: effects on working memory. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999) 2016;38(1):58–60. doi: 10.1590/1516-4446-2015-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Kovacs GG, Horvath MC, Majtenyi K, Lutz MI, Hurd YL, Keller E. Heroin abuse exaggerates age-related deposition of hyperphosphorylated tau and p62-positive inclusions. Neurobiology of aging. 2015;36(11):3100–3107. doi: 10.1016/j.neurobiolaging.2015.07.018. They performed systematic mapping of protein deposits in the brain of heroin addicted individuals and found age-related p62-positive neuritic profiles that were independently affected by duration of drug (Heroine) use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shvartsbeyn M, Phillips DG, Markey MA, Morrison A, DeJong JL, Castellani RJ. Cocaine-induced intracerebral hemorrhage in a patient with cerebral amyloid angiopathy. Journal of forensic sciences. 2010;55(5):1389–1392. doi: 10.1111/j.1556-4029.2010.01410.x. [DOI] [PubMed] [Google Scholar]
- 59.Wallace TL, Vorhees CV, Zemlan FP, Gudelsky GA. Methamphetamine enhances the cleavage of the cytoskeletal protein tau in the rat brain. Neuroscience. 2003;116(4):1063–1068. doi: 10.1016/s0306-4522(02)00795-9. [DOI] [PubMed] [Google Scholar]
- 60.Sordo L, Indave BI, Barrio G, Degenhardt L, de la Fuente L, Bravo MJ. Cocaine use and risk of stroke: a systematic review. Drug and alcohol dependence. 2014;142:1–13. doi: 10.1016/j.drugalcdep.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 61.Afonso L, Mohammad T, Thatai D. Crack Whips the Heart: A Review of the Cardiovascular Toxicity of Cocaine. The American journal of cardiology. 2007;100(6):1040–1043. doi: 10.1016/j.amjcard.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 62.Radunski UK, Fuger U, Reimer J, Lund G, Adam G, Blankenberg S, Muellerleile K. Increased extracellular volume in asymptomatic cocaine abusers detected by cardiovascular magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance (BioMed Central) 2013;15(Suppl 1):1–2. [Google Scholar]
- 63.Ren H, Du C, Yuan Z, Park K, Volkow ND, Pan Y. Cocaine-induced cortical microischemia in the rodent brain: Clinical implications. Molecular psychiatry. 2012;17(10):1017–1025. doi: 10.1038/mp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guollo F, Narciso-Schiavon JL, Barotto AM, Zannin M, Schiavon LL. Significance of alanine aminotransferase levels in patients admitted for cocaine intoxication. Journal of clinical gastroenterology. 2015;49(3):250–255. doi: 10.1097/MCG.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 65.Gasiorowski J, Marchewka Z, Lapinski L, Szymanska B, Glowacka K, Knysz B, Dlugosz A, Wiela-Hojenska A. The investigation of specific biochemical markers in monitoring kidney function of drug addicts. Postepy higieny i medycyny doswiadczalnej (Online) 2013;67:1214–1221. doi: 10.5604/17322693.1078854. [DOI] [PubMed] [Google Scholar]
- 66.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120(4):461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Mysels DJ, Sullivan MA. The relationship between opioid and sugar intake: Review of evidence and clinical applications. Journal of Opioid Management. 2010;6(6):445–452. doi: 10.5055/jom.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]