Sunwoo et al. show that the aryl hydrocarbon receptor is a critical regulator of liver natural killer cell homeostasis.
Abstract
A tissue-resident population of natural killer cells (NK cells) in the liver has recently been described to have the unique capacity to confer immunological memory in the form of hapten-specific contact hypersensitivity independent of T and B cells. Factors regulating the development and maintenance of these liver-resident NK cells are poorly understood. The aryl hydrocarbon receptor (AhR) is a transcription factor modulated by exogenous and endogenous ligands that is important in the homeostasis of immune cells at barrier sites, such as the skin and gut. In this study, we show that liver-resident NK (NK1.1+CD3−) cells, defined as CD49a+TRAIL+CXCR6+DX5− cells in the mouse liver, constitutively express AhR. In AhR−/− mice, there is a significant reduction in the proportion and absolute number of these cells, which results from a cell-intrinsic dependence on AhR. This deficiency in liver-resident NK cells appears to be the result of higher turnover and increased susceptibility to cytokine-induced cell death. Finally, we show that this deficiency has functional implications in vivo. Upon hapten exposure, AhR−/− mice are not able to mount an NK cell memory response to hapten rechallenge. Together, these data demonstrate the requirement of AhR for the maintenance of CD49a+TRAIL+CXCR6+DX5− liver-resident NK cells and their hapten memory function.
INTRODUCTION
NK cells comprise a subset of lymphocytes typically considered to be a component of the innate immune system and initially described by their ability to recognize and kill cells, infected by virus or undergoing malignant transformation, without prior sensitization. More recently, the broad category of NK cells has been reclassified into subsets distinguished by surface marker expression and function and termed innate lymphoid cells (ILCs; Spits et al., 2013). Although felt to have mainly features of innate immunity, NK cells have also been found to be able to confer adaptive immunological memory to viruses and in the form of contact hypersensitivity (CHS) to haptens that is distinct and independent of T cell memory (O’Leary et al., 2006; Sun et al., 2009; Paust et al., 2010; Peng et al., 2013).
CHS is a delayed-typed hypersensitivity reaction, in which the application of small molecule chemicals, or haptens, to an epithelial surface, such as the skin, sensitizes the host and leads to long-term immunological memory. Upon rechallenge with the same molecule even many weeks later, a rapid hapten-specific lymphocyte-dependent inflammatory response is generated. This has traditionally been considered to be a T cell–mediated phenomenon; however, recent studies have definitively demonstrated that, in mice, CHS memory responses can be generated by NK cells independent of T or B cells (O’Leary et al., 2006). Interestingly, not all NK cells are able to confer a CHS response. More precisely, liver-resident NK cells comprise the population that is able to transfer hapten-specific memory in Rag recombinase–deficient mice. This population expresses CXCR6, a chemokine receptor that has been shown to be critical for liver-resident NK cells’ function in CHS (Paust et al., 2010). More recently, this subset has been further defined by the expression of CD49a (α1 integrin) and the lack of expression of the classical NK cell marker α2 integrin (also known as CD49b and more commonly called DX5, after the antibody clone that recognizes this molecule; Peng et al., 2013). Similarly, a human liver-resident memory NK cell population expressing CD49a has also been described (Hydes et al., 2015; Marquardt et al., 2015). These human CD49a+ NK cells produce robust amounts of IFN-γ, TNF, and GM-CSF in response to stimulation, similar to the hepatic CD49a+DX5− liver-resident NK cells in mice (Demehri et al., 2014; Sojka et al., 2014). Thus, liver-resident NK cells comprise a distinct subset of lymphocytes that have an important role in memory NK cell responses.
The development and maintenance of the DX5− liver-resident memory NK cells is poorly understood. This population appears to arise from the fetal liver and persists as a lineage of NK cells distinct from conventional DX5+ NK cells, which develop from BM progenitors (Daussy et al., 2014). Furthermore, DX5− liver NK cells are dependent on the transcription factor T-bet but are eomesodermin (eomes)-negative, unlike conventional mature NK cells (Gordon et al., 2012; Daussy et al., 2014). Besides T-bet, very little is known about the transcriptional requirements of DX5− liver-resident NK cells. Recent gene expression analysis of this population in mice demonstrated that the aryl hydrocarbon receptor (AhR) is more highly expressed in DX5− NK cells in the liver (Peng et al., 2013). AhR is a cytoplasmic receptor that also serves as a transcription factor upon binding to exogenous and endogenous ligands. A member of the Per-Arnt-Sim superfamily, it contains a basic helix-loop-helix domain and two Per-Arnt-Sim domains. These facilitate binding of AhR to its ligands and chaperone proteins, as well as binding partners for transcriptional activity. In its inactive state, AhR exists within the cytosol bound to chaperone proteins. Upon ligand binding, AhR translocates to the nucleus and regulates specific gene transcription. Many AhR ligands are environmentally derived from sources like the diet. The liver is an important site where immune cells are exposed to and can interact with such molecules via blood that is delivered directly from the gut to the liver by the portal venous system. Because AhR mRNA transcripts have been observed to be more highly expressed in the DX5− liver NK cells and because AhR is regulated by several exogenous ligands that would be encountered in the liver, we hypothesized that AhR may have a role in the development and/or maintenance of liver-resident NK cells. Here, we assessed this possibility by examining liver-resident NK cells in the context of AhR loss of function.
RESULTS AND DISCUSSION
AhR is constitutively expressed in CD49a+TRAIL+DX5− liver-resident NK cells
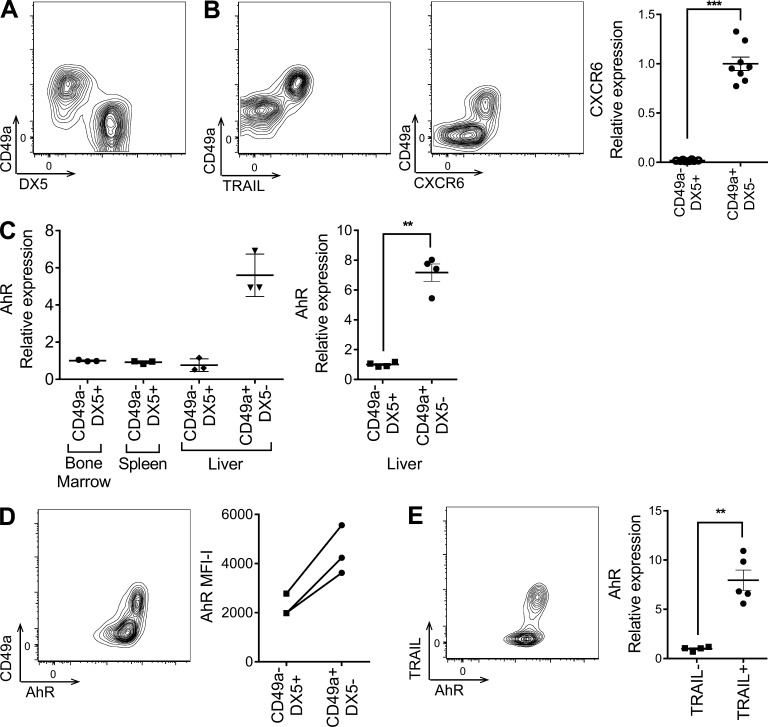
We first confirmed the presence of a previously described distinct liver-resident (NK1.1+CD3−) NK cell subpopulation in mice defined by its expression of CD49a and lack of expression of the conventional NK cell marker DX5 (Fig. 1 A; Peng et al., 2013; Sojka et al., 2014). Liver NK cells have also been described to express TNF-related apoptosis-inducing ligand (TRAIL) and CXCR6 (Takeda et al., 2005; Paust et al., 2010), and therefore, we examined more closely the coexpression of these markers on the CD49a+ liver-resident NK cell subset. By flow cytometry, we observed exclusive expression of both TRAIL and CXCR6 on the CD49a+ NK cell subset in the liver (Fig. 1 B). In addition, mRNA transcript assessment of sorted CD49a+DX5− and CD49a−DX5+ cells by quantitative RT-PCR confirmed that CXCR6 was expressed exclusively in the CD49a+ subset (Fig. 1 B). Thus, CD49a+TRAIL+CXCR6+DX5− NK cells are a distinct subset within the mouse liver.
Figure 1.
AhR is constitutively expressed in CD49a+TRAIL+DX5− liver-resident NK cells. (A) Representative flow cytometry profile of CD49a and DX5 expression on liver NK (NK1.1+CD3−) cells. (B) Expression of TRAIL (left) and CXCR6 (middle) on CD49a+ liver NK cells. (Right) CXCR6 mRNA expression in FACS-sorted CD49a−DX5+ and CD49a+DX5− liver NK cells, normalized to HPRT and shown relative to CD49a+DX5− liver NK cells. (C) CD49a−DX5+ or CD49a+DX5− NK cells were sorted from the BM, spleen, and liver of WT mice. AhR mRNA expression was assessed by quantitative RT-PCR, normalized to HPRT, and shown relative to BM NK cells (left) or CD49a−DX5+ liver NK cells (right). (D, left) Representative flow cytometry profile of CD49a and AhR expression in liver NK cells. (Right) Mean fluorescent intensity (MFI)–isotype control of AhR staining in CD49a−DX5+ versus CD49a+DX5− liver NK cells. (E, left) Representative flow cytometry profile of TRAIL and AhR expression in liver NK cells. (Right) AhR mRNA expression in FACS-sorted TRAIL− and TRAIL+ liver NK cells, normalized to HPRT and shown relative to relative to TRAIL− liver NK cells. Dot plots are from at least two independent experiments, and n = 3–8 mice per group. All contour plots are representative of at least three independent experiments. Data are shown as mean ± SEM. **, P < 0.01; ***, P < 0.001 (paired Student’s t test).
Because AhR has been previously observed to be expressed more highly in DX5− NK cells compared with DX5+ NK cells in a gene expression microarray study (Peng et al., 2013), we further characterized the expression of AhR in the CD49a+DX5− liver-resident subset. Compared with BM, spleen, and CD49a−DX5+ liver NK cells isolated from naive C57BL/6 (B6) mice, CD49a+DX5− liver-resident NK cells had a sevenfold greater constitutive expression of AhR at the mRNA level (Fig. 1 C). AhR protein was also selectively expressed in the CD49a+DX5− NK cell population (Fig. 1 D). Similarly, both AhR mRNA and protein were primarily expressed in TRAIL+ NK cells compared with TRAIL− NK cells in the liver, consistent with TRAIL being selectively expressed on the CD49a+DX5− liver-resident subset (Fig. 1 E). Thus, these data demonstrate that CD49a+TRAIL+DX5− liver-resident NK cells in the mouse constitutively express AhR.
AhR−/− mice have a deficiency in CD49a+TRAIL+DX5− liver-resident NK cells
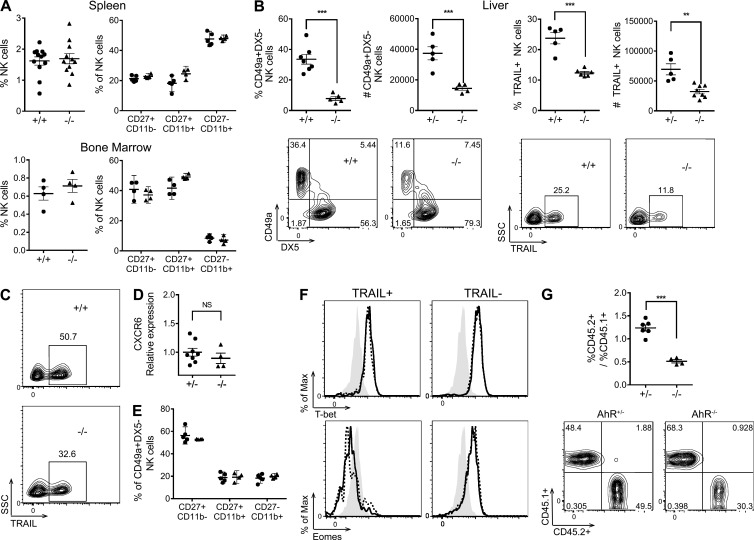
The selective constitutive expression of AhR in the CD49a+TRAIL+DX5− NK cells prompted us to examine the consequences of AhR loss of function on this population in the naive mouse. In both the spleen and BM, there was no apparent deficiency in the proportion or maturation (measured by CD27 and CD11b expression) of NK1.1+CD3− NK cells in AhR−/− mice (Fig. 2 A), as observed previously (Shin et al., 2013). However, in the liver, we observed a striking reduction in both the proportion and the absolute number of liver-resident NK cells measured as either CD49a+DX5− NK cells or TRAIL+ NK cells in AhR−/− mice (Fig. 2 B). This defect was also seen in young mice (Fig. 2 C). Although younger mice have greater proportions of TRAIL+DX5− NK cells compared with adult mice (Takeda et al., 2005), young AhR−/− mice still have a significant reduction in this population compared with age-matched AhR+/+ mice. No differences were observed between the liver-resident NK cells in AhR+/+ and AhR+/− mice. Of the liver-resident NK cells that did develop in AhR−/− mice, there was no apparent defect in the expression of CXCR6 (Fig. 2 D), indicating that AhR does not regulate the expression of this critical chemokine receptor. There was also no difference in the expression of CD69, KLRG1, or Ly49 receptors (not depicted). Furthermore, analysis of the maturation markers CD27 and CD11b on CD49a+DX5− liver-resident NK cells revealed no differences between AhR+/+ and AhR−/− mice, suggesting that the defect is not developmental in nature (Fig. 2 E). This is supported by the intracellular staining of T-bet and eomes in conventional CD49a−DX5+ and liver-resident CD49a+DX5− NK cells, which did not reveal any differences in the expression of these transcription factors between AhR+/+ and AhR−/− mice (Fig. 2 F), indicating that the reduction in the CD49a+DX5− NK cells that we observed (Fig. 2 B) was not caused by T-bet or eomes and that AhR was acting through a different mechanism.
Figure 2.
AhR−/− mice have a deficiency in CD49a+TRAIL+DX5− liver-resident NK cells. (A) NK (NK1.1+CD3−) cell percentages (left) and expression of CD27 and CD11b on NK cells (right) from the spleen (top) and BM (bottom) of AhR+/+ or AhR−/− mice. (B) Percentage, cell number, and representative plots of CD49a+DX5− (left) and TRAIL+ (right) liver NK cells from AhR+/+ or AhR+/− versus AhR−/− mice. SSC, side scatter. (C) Representative plots of TRAIL+ CD3−NK1.1+ NK cells from the liver of AhR+/+ and AHR−/− mice at 4 wk. (D) CD49a+DX5− NK cells, sorted from the liver of AhR+/− and AhR−/− mice, were assessed for CXCR6 mRNA expression by quantitative RT-PCR, normalized to HPRT, and shown relative to AhR+/− cells. (E) Expression of CD27 and CD11b on CD49a+DX5− liver NK cells from AhR+/+ (circles) and AhR−/− (triangles) mice. (F) Representative histograms of eomes (bottom) and T-bet (top) staining in TRAIL+ (left) and TRAIL− (right) liver NK cells of AhR+/+ (continuous line) and AhR−/− mice (dashed line), as well as an isotype control (shaded). (G) Sublethally irradiated CD45.1+ recipient host mice were reconstituted i.v. with an equal mixture of CD45.1+ WT and CD45.2+ AhR+/− or AhR−/− fetal liver cells. (Top) At 2 mo, mice received three i.v. injections of 3 µg FICZ over the course of 1 wk before analysis of liver NK cells. The ratio of CD45.2+ to CD45.1+ cells in TRAIL+ liver NK cells from recipients of AhR+/− versus AhR−/− fetal liver cells is shown. (Bottom) Representative plots of CD45.2 and CD45.1 staining on TRAIL+ liver NK cells. Scatter plots are from at least two independent experiments, and n = 4–11 mice per group. All contour plots are representative of at least two independent experiments. Data are shown as mean ± SEM. **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
To determine whether the deficiency in liver-resident NK cells observed in AhR−/− mice was cell intrinsic, hematopoietic progenitor mixed chimera transplant experiments were performed (Fig. 2 G). Fetal liver cells from embryos at gestational age E18 were harvested from CD45.1 WT and CD45.2 AhR−/− mice. These were mixed at equal ratio and transplanted i.v. into sublethally irradiated CD45.1 WT recipients. Likewise, fetal liver cells from CD45.1 WT and CD45.2 AhR+/− control mice were also harvested, mixed, and transplanted into sublethally irradiated CD45.1 WT recipients. After 8 wk, liver NK cells in the recipient hosts were examined (Fig. 2 G). We observed a significant deficiency in the proportion of TRAIL+ liver-resident NK cells derived from AhR−/− (CD45.2+) progenitors, compared with TRAIL+ liver-resident NK cells derived from AhR+/+ (CD45.1+) progenitors. Unlike the TRAIL+ liver-resident NK cells, there was normal development of DX5+ NK cells derived from AhR−/− progenitors (Fig. S1). The deficiency in the proportion of TRAIL+ liver-resident NK cells derived from the AhR−/− progenitors was not observed in the control mixed chimera recipients where the CD45.2+ cells were derived from AhR+/− fetal liver progenitors. Interestingly, very few liver-resident NK cells developed if the donor cells were derived from BM progenitors (not depicted), consistent with previous observations indicating that liver-resident NK cells arise from fetal liver hematopoietic progenitors distinct from the BM progenitors of conventional NK cells (Jiang et al., 2013; Peng et al., 2013; Daussy et al., 2014). Of note, the full reconstitution of liver-resident NK cells in the recipients of transferred AhR+/− or WT fetal liver hematopoietic progenitors depended on administration of the exogenous AhR agonist 6-formylindolo[3,2-b]carbazole (FICZ; Fig. S1), further indicating that AhR plays an active role in the development of these cells. Thus, these data demonstrate that AhR loss of function impacts liver-resident NK cells in a cell-intrinsic manner. Although AhR has been shown to be involved in the development of ILC3 cells and human NK cell maturation (Lee et al., 2011; Qiu et al., 2012; Hughes et al., 2014; Montaldo et al., 2014), to our knowledge, the requirement of AhR in a memory NK cell subset has not been previously described.
AhR regulates the homeostasis of NK cells
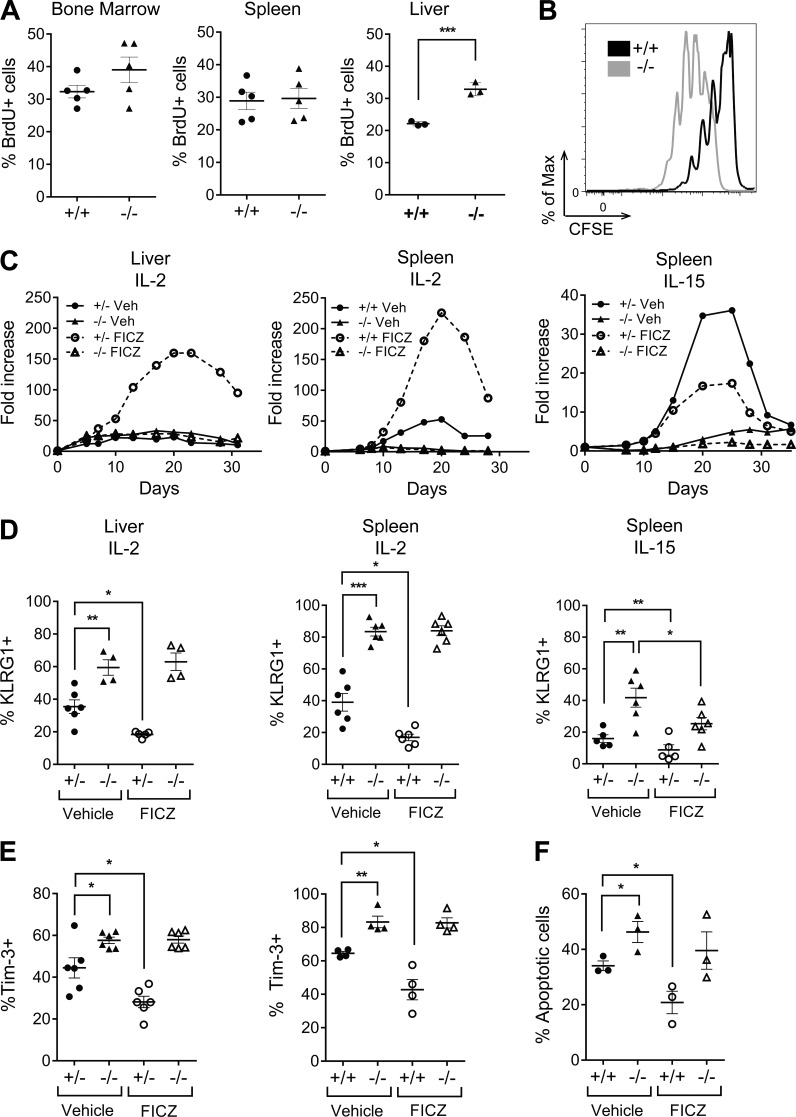
To understand further what may be affecting the differences in liver-resident NK cell proportions in AhR−/− mice, in vivo BrdU labeling of naive AhR+/+ and AhR−/− mice was performed. There was no significant difference of BrdU labeling of NK cells isolated from the BM or spleens of AhR+/+ and AhR−/− mice; however, interestingly, there was an ∼50% increase in the BrdU labeling of liver NK cells in AhR−/− mice (Fig. 3 A). Because unstimulated NK cells from the spleen and BM do not constitutively express AhR like NK cells from the liver (Fig. 1 C) and because AhR expression can be induced in NK cells by stimulation with cytokines, such as IL-2 (Shin et al., 2013), we examined the effects of IL-2 stimulation on AhR+/+ and AhR−/− splenic NK cells. We observed a significantly more robust early proliferative response to IL-2 stimulation by AhR−/− NK cells compared with AhR+/+ NK cells, as measured by CFSE dilution (Fig. 3 B). This was consistent with the greater BrdU incorporation that we observed in AhR−/− liver NK cells, which normally express AhR constitutively in vivo in the naive context (Fig. 1 C). Given the deficiency in the liver-resident NK cell population in the AhR−/− mice (Fig. 2 B), this increased proliferative capacity was unexpected.
Figure 3.
AhR regulates the homeostasis of NK cells. (A) NK (NK1.1+CD3−) cells in the BM, spleen, and liver of WT mice were assessed for proliferation by BrdU incorporation. (B) Enriched splenic NK cells from AhR+/+ and AhR−/− mice were labeled with CFSE and cultured in the presence of 1,000 U/ml IL-2. CFSE dilution staining in NK cells is shown at day 3. (C) Representative growth curves for liver mononuclear cells (left) or enriched splenic NK cells (middle) isolated and cultured in 1,000 U/ml IL-2 and 200 nM FICZ or vehicle control. (Right) Representative growth curves of enriched splenic NK cells cultured in 100 ng/ml IL-15 and 200 nM FICZ or vehicle control. (D) Expression of KLRG1 on NK cells in IL-2 or IL-15 assessed by flow cytometry on day 7. (E) Expression of Tim-3 on IL-2–cultured liver (left) and splenic (right) NK cells at day 7. (F) Expression of annexin V on IL-2–cultured splenic NK cells at day 7. Circles, AhR+/+ or AhR+/−; triangles, AhR−/−; closed, vehicle control; open, FICZ. Scatter plots are from at least two independent experiments, and n = 3–8 mice per group. All representative data are from at least three independent experiments. Data are shown as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test for AhR+/+ vs. AhR−/−; paired Student’s t test for vehicle vs. FICZ).
To explore this further, we cultured liver and splenic NK cells from AhR+/+ and AhR−/− mice in the presence of IL-2, with and without FICZ, and assessed fold expansion over 30 d. AhR−/− splenic NK cells displayed an attenuated expansion compared with AhR+/+ splenic NK cells cultured in either IL-2 or IL-15 (Fig. 3 C). Interestingly, the addition of FICZ to the IL-2 culture conditions induced a marked increase in the expansion of AhR+/+ NK cells but not AhR−/− NK cells from both the liver and spleen (Fig. 3 C). Further, AhR−/− NK cells cultured in IL-2 displayed an increased proportion of cells expressing KLRG1 and Tim-3 at day 7, suggestive of early exhaustion, and an increased frequency of apoptotic cells (Fig. 3, D–F). Of note, the addition of FICZ to the culture reduced the proportion of AhR+/+ and AhR+/− NK cells expressing KLRG1 and Tim-3 and also reduced the proportion of apoptotic cells, consistent with the ability of this AhR agonist to increase the fold expansion of these cells in IL-2 culture (Fig. 3, C–E). These effects seen with FICZ in AhR+/+ and AhR+/− NK cells were not observed in AhR−/− NK cells, indicating that FICZ was acting specifically through AhR. Thus, these data indicate that in the absence of AhR, NK cells undergo an increased proliferative burst, early exhaustion, and apoptosis, which could account for the reduction in the numbers of liver-resident NK cells found in AhR−/− mice.
AhR-deficient mice have impaired NK cell–mediated delayed CHS
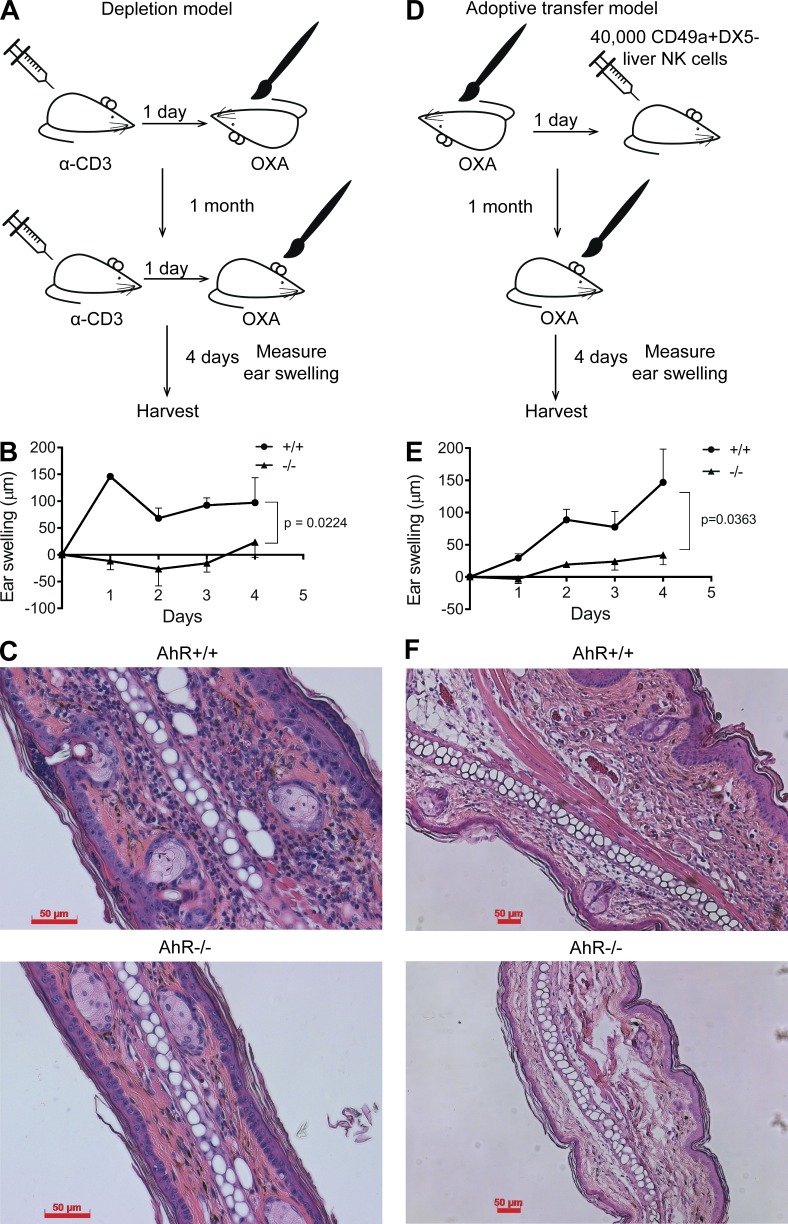
The reduced numbers of CD49a+DX5− liver-resident NK cells in AhR−/− mice led us to address the biological relevance of this observation and examine NK cell–mediated memory responses to haptens in the context of delayed CHS. As mentioned, these liver-resident NK cells have previously been demonstrated to have the intrinsic ability to provide immunological memory of specific topically applied haptens in the absence of T cells (Paust et al., 2010; Peng et al., 2013). Therefore, to assess functional defects of the liver-resident NK cells in AhR−/− mice, we first depleted T cells with systemic administration of anti-CD3 antibody and then applied a well characterized CHS hapten, oxalozone (OXA), topically to the undersurface skin of AhR+/+ or AhR−/− mice. After 30 d, the mice were again administered anti-CD3 antibody to deplete T cells 1 d before challenge with OXA on the skin overlying the external ear. Ear swelling was measured over the next 4 d (Fig. 4 A). In the AhR+/+ mice, immediate ear swelling in response to OXA was observed; however, this was significantly attenuated in the AhR−/− mouse cohort (Fig. 4, B and C), consistent with the deficiency observed in the CD49a+DX5− liver-resident NK cell population in AhR−/− mice (Fig. 2). Although we observed thorough T cell depletion with anti-CD3 antibody administration (not depicted), residual T cells could still be confounding the results. To address this and to determine whether the defect in ear swelling was caused by a reduction in the liver-resident NK cell number or an intrinsic defect, we adoptively transferred 40,000 OXA-sensitized CD49a+DX5− liver-resident NK cells, isolated from AhR+/+ or AhR−/− mice, into immune-deficient (B10;B6 Rag2−/−γc−/− mice) recipient mice before OXA rechallenge (Fig. 4 D). Similar to the depletion model, mice receiving AhR−/− liver-resident NK cells displayed significantly reduced ear swelling upon secondary challenge with OXA (Fig. 4, E and F). Thus, these data indicate that AhR−/− mice have a cell-intrinsic defect in the ability of liver-resident NK cells to confer CHS.
Figure 4.
AhR-deficient mice have impaired NK cell–mediated delayed CHS. (A) Schematic of CHS assay after T cell depletion. Mice were injected with 50 µg of anti-CD3–depleting antibody 1 d before sensitization with OXA. At 1 mo, mice were again injected with 50 µg anti-CD3 1 d before challenging with OXA by painting the ears. Ears were measured by micrometer, and ears were harvested 4 d after rechallenge. (B) Ear swelling of AhR+/+ and AhR−/− mice relative to control ears. Data are representative of two independent experimental cohorts, and n = 3–4 mice per group. (C) Representative H&E stains of OXA-challenged ears from AhR+/+ (top) and AhR−/− (bottom) mice at day 4 after rechallenge. (D) Schematic of adoptive transfer CHS assay. AhR+/+ and AhR−/− mice were sensitized with OXA, and 40,000 CD49a+DX5− liver NK cells were adoptively transferred into B10;B6 Rag2−/−γc−/− mice. At 1 mo, mice were challenged with OXA by painting the ears. Ears were measured by micrometer, and ears were harvested 4 d after rechallenge. (E) Ear swelling of mice receiving 40,000 AhR+/+ or AhR−/− CD49a+DX5− liver NK cells. n = 4–5 mice per group. (F) Representative H&E stains of OXA-challenged ears from AhR+/+ and AhR−/− recipient mice at day 4 after rechallenge. (C and F) Bars, 50 µm. Data are shown as mean ± SEM (two-way ANOVA).
Concluding remarks
Our data demonstrate the cell-intrinsic requirement of AhR for the maintenance of liver-resident NK cell proportions and function in the mouse liver. The liver-resident NK cell population in mice are considered to be a subset of group 1 ILCs, along with conventional NK cells, other tissue-resident NK cells, and intraepithelial ILC1s (Cella et al., 2014). Although AhR is required for the maintenance of ILC3s (which, unlike ILC1s, secrete IL-17 and/or IL-22; Kiss et al., 2011; Lee et al., 2011, 2012; Qiu et al., 2012), AhR has not been previously implicated in group 1 ILC subset development, maintenance, or function. Therefore, our observations that liver-resident NK cells require AhR for proper maintenance and function introduce important new information that distinguishes the liver-resident NK cells from ILC1s and conventional NK cells among the group 1 ILC subsets.
There are abundant AhR ligands found in the diet, and several studies have noted the importance of dietary ligands in activating AhR and maintaining the presence of certain gut-associated immune cells (Kiss et al., 2011; Lee et al., 2011; Li et al., 2011). In addition, the microbiota found in the intestines are also a potential source of AhR ligands. Our data demonstrate the importance of AhR in the homeostasis of the CD49a+DX5− liver-resident NK cell population. It is intriguing to consider that environmentally derived ligands may influence systemic immunity by affecting AhR activity in these tissue-resident cells. In future studies, it will be important to investigate the influence of dietary ligands and microbiota on the homeostasis of CD49a+DX5− liver-resident NK cells and CHS, as well as the role of AhR polymorphisms among the population in determining susceptibility to inflammatory conditions.
Longevity is a hallmark of memory cells, and having the ability to survive and persist is vital for liver-resident NK cells to mediate their memory function. Factors regulating the homeostasis of these memory NK cells are still poorly understood. However, our study introduces AhR as an important component of NK cell memory. Because cytokine-induced memory NK cells have also been described (Cooper et al., 2009) and because cytokines, such as IL-2, IL-15, and IL-12, induce AhR expression (Shin et al., 2013), it is possible that AhR is playing an important role in the maintenance of these NK cells as well. Given the role of NK cells in immunological memory to viruses (O’Leary et al., 2006; Sun et al., 2009; Paust et al., 2010; Gillard et al., 2011), it will also be interesting to see whether AhR plays a role in these responses. In the context of immunological memory formation, our data suggest that the activation of AhR may be providing a survival benefit through the prevention of proliferation-induced exhaustion and death.
MATERIALS AND METHODS
Mice
AhR+/− mice lacking exon 2 on a C57BL/6 background were obtained from C. Bradfield (University of Wisconsin-Madison, Madison, WI) and also were purchased from The Jackson Laboratory (which is the provider of the same mice originally donated by C. Bradfield). C57BL/6 and B6 CD45.1 mice were obtained from The Jackson Laboratory. AhR+/+ and AhR−/− mice were bred from AhR+/− mice and maintained as separate colonies. B10;B6 Rag2−/−γ−/− mice were obtained from Taconic. Mice of both sexes were used for experiments at age 8–12 wk. All animal procedures were performed in accordance with protocols approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
Immune cell isolation
BM was harvested by flushing femurs with a 25-gauge needle, and a single-cell suspension obtained. Spleens and livers were mashed in 70-µm cell strainers into a single-cell suspension. Liver mononuclear cells were then isolated through a 30% Percoll (GE Healthcare) gradient spin. All samples had red blood cells lysed with ACK Lysing buffer (Lonza), and the resulting cells were used for analyses.
NK cell culture
NK cells were isolated by negative isolation (STEMCELL Technologies) from the spleen and cultured in 1,000 U/ml IL-2 (NCI BRB Preclinical Repository) or 100 ng/ml IL-15 (PeproTech). For cell proliferation assays, NK cells were loaded with 5 µM CFSE (eBioscience). Cells also received 200 nM FICZ (Enzo Life Sciences) or vehicle control. Cells were maintained at 0.5–1 × 106 cells/ml, and cytokine and FICZ were replenished every 2–3 d.
Flow cytometry
NK cells were stained with antibodies against CD3, NK1.1, CD11b, KLRG1, CD27, TRAIL, Tim-3, c-kit, DX5, CD45.1, CD45.2 (BioLegend), and CD49a (BioLegend and BD). CXCR6 was stained using CXCL16-His and anti-His (R&D Systems). AhR was stained using anti-AhR and goat anti–rabbit antibody (Abcam). For intracellular antibodies, cells were fixed and permeabilized with Cytofix/Cytoperm (BD) before staining. Data were acquired on an LSRFortessa cytometer (BD).
Quantitative RT-PCR
RNA was extracted with the RNeasy mini kit (QIAGEN), and cDNA was made with the Maxima First Strand cDNA kit (Thermo Fisher Scientific). Quantitative gene expression was performed using the Taqman Gene Expression Assay with their recommended primers (Thermo Fisher Scientific). Gene expression was normalized to hypoxanthine phosphoribosyltransferase (HPRT) and then shown relative to an appropriate control.
BrdU labeling
Mice were injected with 300 µg BrdU dissolved in PBS i.p. daily for 3 d. 1 d after the last injection, BM, spleen, and liver were harvested from the mice, and a single-cell suspension was obtained. Cell surface markers were stained as described in the Flow cytometry section, and cells were then fixed and permeabilized with the Cytofix/Cytoperm kit (BD). Then, cells were treated with 300 µg/ml DNase (STEMCELL Technologies) and stained with an anti-BrdU antibody (BD).
CHS
Depletion model
Mice were injected i.p. with 100 µg of CD3-depleting antibody 17A2 (Bio X Cell). On days 1 and 2, the abdomens of mice were shaved and painted with 50 µl of 5% OXA (Sigma-Aldrich). 1 mo later, mice were reinjected i.p. with 100 µg 17A2. After 24 h, the left ear was challenged with 5% OXA, whereas the right was challenged with vehicle control (1:1 mixture of acetone and methanol). Ear thickness was measured by micrometer, and antigen-specific swelling was calculated by (treated ear swelling − control ear swelling) − background swelling of naive mice. At day 4, organs were harvested as described, and ears were fixed overnight in 10% formalin. Hematoxylin & eosin (H&E) stains of paraffin-embedded sections were performed by the Stanford Department of Comparative Medicine’s Histology Lab.
Adoptive transfer model
On days 0 and 1, the abdomen of donor mice were shaved and painted with 50 µl of 5% OXA. On day 2, the mice were euthanized, and the liver mononuclear cells were harvested. Liver-resident NK cells (NK1.1+CD3−CD49a+DX5−) were sorted to high purity, and 40,000 cells were injected i.v. into B10;B6 Rag2−/−γ−/− mice. 1 mo later, mice were rechallenged and analyzed as described in the Depletion model section.
Fetal liver transplant
Fetal livers were harvested from B6 CD45.1+ and AhR+/− or AhR−/− fetuses age embryonic day 18. Red blood cells were lysed using ACK Lysing buffer, and fetal livers were mixed at a 50:50 ratio. Recipient B6 CD45.1+ mice were irradiated with two exposures of 500 rads each at least 4 h apart and placed on an antibiotic feed. Then, recipient mice received the fetal liver cells via a tail vein injection. At 2 mo, mice received three i.v. injections of 3 µg FICZ over the course of 1 wk before isolation and staining of liver NK cells.
Online supplemental material
Fig. S1 shows development of liver NK cells from fetal liver transplant.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Chris Bradfield for the gift of AhR+/− mice, Barbara Storch and Angela Lu for their assistance in this study, and Sungjin Kim for his critical review of and valuable suggestions for the manuscript.
Luhua Zhang is supported by the National Science Foundation Graduate Research Fellowship Program (DGE-114747) and the National Institutes of Health (5T32AI007290-30). John Sunwoo’s laboratory was supported by the National Institutes of Health (R01CA158516) for this project.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- AhR
- aryl hydrocarbon receptor
- CHS
- contact hypersensitivity
- H&E
- hematoxylin & eosin
- HPRT
- hypoxanthine phosphoribosyltransferase
- ILC
- innate lymphoid cell
- TRAIL
- TNF-related apoptosis-inducing ligand
References
- Cella M., Miller H., and Song C.. 2014. Beyond NK cells: the expanding universe of innate lymphoid cells. Front. Immunol. 5:282 10.3389/fimmu.2014.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M.A., Elliott J.M., Keyel P.A., Yang L., Carrero J.A., and Yokoyama W.M.. 2009. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA. 106:1915–1919. 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daussy C., Faure F., Mayol K., Viel S., Gasteiger G., Charrier E., Bienvenu J., Henry T., Debien E., Hasan U.A., et al. . 2014. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 211:563–577. 10.1084/jem.20131560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S., Cunningham T.J., Hurst E.A., Schaffer A., Sheinbein D.M., and Yokoyama W.M.. 2014. Chronic allergic contact dermatitis promotes skin cancer. J. Clin. Invest. 124:5037–5041. 10.1172/JCI77843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard G.O., Bivas-Benita M., Hovav A.H., Grandpre L.E., Panas M.W., Seaman M.S., Haynes B.F., and Letvin N.L.. 2011. Thy1+ NK cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 7:e1002141 10.1371/journal.ppat.1002141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.M., Chaix J., Rupp L.J., Wu J., Madera S., Sun J.C., Lindsten T., and Reiner S.L.. 2012. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 36:55–67. 10.1016/j.immuni.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T., Briercheck E.L., Freud A.G., Trotta R., McClory S., Scoville S.D., Keller K., Deng Y., Cole J., Harrison N., et al. . 2014. The transcription factor AHR prevents the differentiation of a stage 3 innate lymphoid cell subset to natural killer cells. Cell Reports. 8:150–162. 10.1016/j.celrep.2014.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hydes T., Abuhilal M., Armstrong T., Primrose J., Takhar A., and Khakoo S.. 2015. Natural killer cell maturation markers in the human liver and expansion of an NKG2C+KIR+ population. Lancet. 385:S45 10.1016/S0140-6736(15)60360-9 [DOI] [PubMed] [Google Scholar]
- Jiang X., Chen Y., Peng H., and Tian Z.. 2013. Memory NK cells: why do they reside in the liver? Cell. Mol. Immunol. 10:196–201. 10.1038/cmi.2013.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E.A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., and Diefenbach A.. 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 334:1561–1565. 10.1126/science.1214914 [DOI] [PubMed] [Google Scholar]
- Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M., Mantovani A., Kopan R., Bradfield C.A., Newberry R.D., and Colonna M.. 2011. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13:144–151. 10.1038/ni.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Cella M., and Colonna M.. 2012. AHR and the transcriptional regulation of type-17/22 ILC. Front. Immunol. 3:10 10.3389/fimmu.2012.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Innocentin S., Withers D.R., Roberts N.A., Gallagher A.R., Grigorieva E.F., Wilhelm C., and Veldhoen M.. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 147:629–640. 10.1016/j.cell.2011.09.025 [DOI] [PubMed] [Google Scholar]
- Marquardt N., Béziat V., Nyström S., Hengst J., Ivarsson M.A., Kekäläinen E., Johansson H., Mjösberg J., Westgren M., Lankisch T.O., et al. . 2015. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J. Immunol. 194:2467–2471. 10.4049/jimmunol.1402756 [DOI] [PubMed] [Google Scholar]
- Montaldo E., Teixeira-Alves L.G., Glatzer T., Durek P., Stervbo U., Hamann W., Babic M., Paclik D., Stölzel K., Gröne J., et al. . 2014. Human RORγt+CD34+ cells are lineage-specified progenitors of group 3 RORγt+ innate lymphoid cells. Immunity. 41:988–1000. 10.1016/j.immuni.2014.11.010 [DOI] [PubMed] [Google Scholar]
- O’Leary J.G., Goodarzi M., Drayton D.L., and von Andrian U.H.. 2006. T cell– and B cell–independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7:507–516. 10.1038/ni1332 [DOI] [PubMed] [Google Scholar]
- Paust S., Gill H.S., Wang B.Z., Flynn M.P., Moseman E.A., Senman B., Szczepanik M., Telenti A., Askenase P.W., Compans R.W., and von Andrian U.H.. 2010. Critical role for the chemokine receptor CXCR6 in NK cell–mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 11:1127–1135. 10.1038/ni.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Jiang X., Chen Y., Sojka D.K., Wei H., Gao X., Sun R., Yokoyama W.M., and Tian Z.. 2013. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 123:1444–1456. 10.1172/JCI66381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Heller J.J., Guo X., Chen Z.M., Fish K., Fu Y.X., and Zhou L.. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 36:92–104. 10.1016/j.immuni.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.H., Zhang L., Murillo-Sauca O., Kim J., Kohrt H.E., Bui J.D., and Sunwoo J.B.. 2013. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 110:12391–12396. 10.1073/pnas.1302856110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka D.K., Plougastel-Douglas B., Yang L., Pak-Wittel M.A., Artyomov M.N., Ivanova Y., Zhong C., Chase J.M., Rothman P.B., Yu J., et al. . 2014. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 3:e01659 10.7554/eLife.01659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., et al. . 2013. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13:145–149. 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- Sun J.C., Beilke J.N., and Lanier L.L.. 2009. Adaptive immune features of natural killer cells. Nature. 457:557–561. 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Cretney E., Hayakawa Y., Ota T., Akiba H., Ogasawara K., Yagita H., Kinoshita K., Okumura K., and Smyth M.J.. 2005. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 105:2082–2089. 10.1182/blood-2004-08-3262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.