In psoriasis, IFN-α–stimulated mast cells release exosomes containing cytoplasmic PLA2 that are transferred to CD1a-expressing cells and generate neolipid antigens which induce the production of IL-22 and IL-17A by CD1a-reactive T cells.
Abstract
Psoriasis is a chronic inflammatory skin disease associated with a T helper 17 response. Yet, it has proved challenging to identify relevant peptide-based T cell antigens. Antigen-presenting Langerhans cells show a differential migration phenotype in psoriatic lesions and express constitutively high levels of CD1a, which presents lipid antigens to T cells. In addition, phospholipase A2 (PLA2) is highly expressed in psoriatic lesions and is known to generate neolipid skin antigens for recognition by CD1a-reactive T cells. In this study, we observed expression of a cytoplasmic PLA2 (PLA2G4D) in psoriatic mast cells but, unexpectedly, also found PLA2G4D activity to be extracellular. This was explained by IFN-α–induced mast cell release of exosomes, which transferred cytoplasmic PLA2 activity to neighboring CD1a-expressing cells. This led to the generation of neolipid antigens and subsequent recognition by lipid-specific CD1a-reactive T cells inducing production of IL-22 and IL-17A. Circulating and skin-derived T cells from patients with psoriasis showed elevated PLA2G4D responsiveness compared with healthy controls. Overall, these data present an alternative model of psoriasis pathogenesis in which lipid-specific CD1a-reactive T cells contribute to psoriatic inflammation. The findings suggest that PLA2 inhibition or CD1a blockade may have therapeutic potential for psoriasis.
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease affecting up to 2–3% of the population worldwide (Gelfand et al., 2005). Psoriasis immunopathology is characterized by an infiltration of CD4+ and CD8+ T cells, neutrophils, NK cells, NKT cells, mast cells, macrophages, and innate lymphoid cells (Valdimarsson et al., 1995; Vissers et al., 2004; Griffiths and Barker, 2007; Lin et al., 2011; Dyring-Andersen et al., 2014; Keijsers et al., 2014; Schön, 2014; Teunissen et al., 2014; Villanova et al., 2014). Initially, psoriasis was regarded as being dominated by a T helper 1 (Th1) response because of highly expressed Th1 cytokines including IFN-γ, IL-1, and IL-12 in psoriatic lesions (Austin et al., 1999). This was consistent with relatively lower expression of Th2 cytokines such as IL-4 (Henseler and Christophers, 1995; Landgren et al., 2006). However, the discovery of increased numbers of IL-17–secreting T cells and elevated levels of the Th17-polarizing cytokine IL-23 in psoriatic lesions suggested a central role for the Th17 response in psoriasis pathogenesis (Lowes et al., 2008; Kagami et al., 2010; Res et al., 2010). This has significant therapeutic implications as anti–IL-23p19, anti–IL-17A, and anti–IL-17RA showed significant clinical efficacy and therefore support the role of the Th17 response (Papp et al., 2008, 2012, 2015; Hueber et al., 2010; Kimball et al., 2013; Vitiello et al., 2013; Gottlieb et al., 2015; Lebwohl et al., 2015). However, despite important and extensive investigations suggesting reactivity to bacterial, keratin, LL37, and melanocyte peptide antigens (Kobayashi et al., 2002; Johnston et al., 2004; Lande et al., 2014; Arakawa et al., 2015), the identity of peptide-based antigens for psoriatic T cells has proved elusive in multiple cohorts, raising the possibility of a role for nonpeptide antigens. In addition, activation and degranulation of mast cells is thought to contribute to the pathology of psoriasis skin lesions (Brody, 1984; Schubert and Christophers, 1985), and production of proinflammatory cytokines from mast cells is thought to be involved in the development of the disease (Balato et al., 2012; Shefler et al., 2014). IFN-α produced by plasmacytoid DCs is also involved in the early development of psoriasis, as expression of IFN-α and infiltration of plasmacytoid DCs have been observed in psoriasis skin lesions, and blocking of the IFN-α signaling pathway was shown to inhibit the development of disease in a psoriasis model (Nestle et al., 2005).
The CD1 family of proteins presents lipid antigens to T cells (Mori and De Libero, 2008). Sharing structural similarities with MHC class I molecules, they possess hydrophobic antigen-binding pockets and noncovalently associate with β2 microglobulin. However, contrary to MHC, CD1 molecules have limited polymorphism and are encoded outside the MHC gene cluster (Gumperz, 2006). CD1a molecules have been reported to present a range of lipid antigens to T cells, including the self-lipid sulfatide and foreign lipids such as the mycobacterial lipopeptide dideoxymycobactin (Zajonc et al., 2003, 2005). Recent studies have shown that CD1a can also present headless lipid antigens such as fatty acids, wax esters, and squalene (de Jong et al., 2010, 2014), with the TCR binding to CD1a without direct contact with the lipid cargo (Birkinshaw et al., 2015).
CD1a is expressed by thymocytes and subsets of DCs including some dermal DCs and specialized DCs at mucosal sites. Importantly, CD1a is also constitutively expressed at high levels by Langerhans cells (LCs) of the epidermis (Dougan et al., 2007; Yakimchuk et al., 2011). Interestingly LCs show impaired migration in patients with psoriasis, consistent with a role in disease pathogenesis (Cumberbatch et al., 2006; Eaton et al., 2014; Shaw et al., 2014).
Recently phospholipase A2 (PLA2) activity has been linked to lipid-specific T cell inflammatory skin responses. It has been shown that exogenous PLA2 from bee venom and house dust mite generates neolipid fatty acid and lysophospholipid antigens for CD1a presentation to T cells (Bourgeois et al., 2015; Jarrett et al., 2016), and elevated CD1a-reactive T cell responses were described in bee and wasp venom allergic individuals (Subramaniam et al., 2016). Furthermore, elevated levels of PLA2 products, including prostaglandin E2 (PGE2), PGF2α, and 12-HETE (12-hydroxyeicosatetraenoic acid), were found in the epidermis of psoriatic lesions (Hammarström et al., 1975; Ryborg et al., 1995). Several studies also revealed raised PLA2 activity in epidermal samples from psoriasis patients (Forster et al., 1983a,b, 1985; Verhagen et al., 1984). Endogenous human PLA2 can be broadly classified into secretory and cytosolic subsets. The latter are further subdivided into tissue-specific forms with substrate and condition preferences. In particular, expression of a novel cytosolic PLA2, namely cPLA2δ or PLA2G4D, was observed in psoriatic lesions, yet was absent in healthy normal skin (Chiba et al., 2004), which was further supported by a recent study showing psoriasis-specific gene expression of PLA2 (Quaranta et al., 2014). Cytosolic PLA2 hydrolyzes membrane phospholipids at the sn-2 position and produces fatty acids such as arachidonic acid (Leslie, 2004; Leslie and Gelb, 2004). Different subtypes of cytosolic PLA2 have enzymatic properties, substrate preferences, tissue expression patterns, and subcellular localization particular to their specialized functions (Ohto et al., 2005; Ghosh et al., 2006). Elevated levels of systemic PLA2 activity in patients with psoriasis have been described and proposed to contribute to the associated metabolic syndrome (Izaki et al., 1996). Despite all these studies, the role of PLA2 in the pathogenesis of psoriasis has not been fully elucidated.
Given the colocalization of CD1a-expressing LCs and T cells as well as elevated PLA2 in psoriasis, we hypothesized that CD1a-reactive T cells may be relevant to the pathogenesis of this disease.
RESULTS
CD1a-autoreactive responses in healthy and psoriasis cohorts
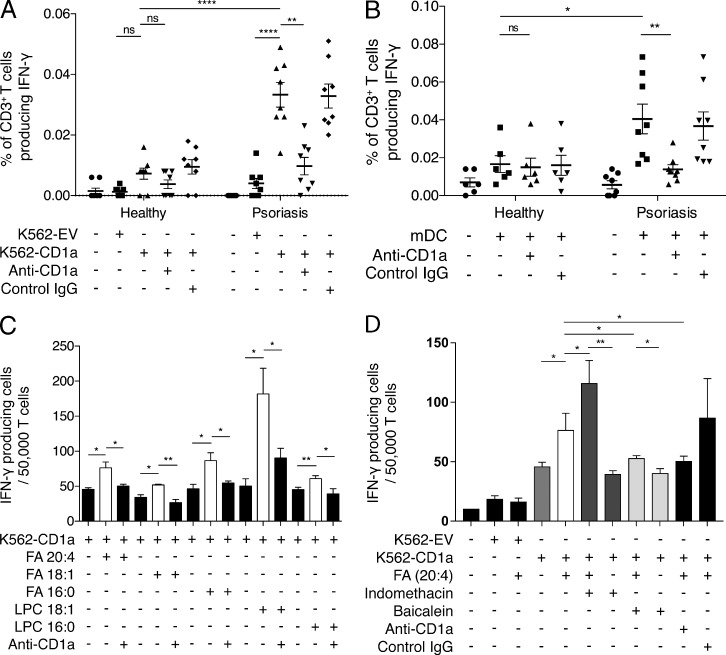
To investigate the potential role of CD1a in psoriasis, we first isolated CD3+ polyclonal T cells from the blood of individuals with or without psoriasis and incubated the T cells ex vivo with K562 cells transfected with CD1a (K562-CD1a) or empty vector (K562-EV). HLAlow K562 cells expressing CD1a have the advantage of being a universal lipid antigen–presenting population to compare responses between unrelated individuals (de Jong et al., 2010, 2014; Bourgeois et al., 2015). We observed that T cells from healthy individuals responded to K562-CD1a cells detected through secretion of IFN-γ (Fig. 1 A), compatible with our previous findings and others’ findings (de Jong et al., 2010, 2014; Bourgeois et al., 2015). Furthermore, a significantly higher frequency of T cells from psoriasis patients responded to K562-CD1a cells but not to mock-transfected K562 cells. The CD1a autoreactivity could be blocked by anti-CD1a antibody but not by control IgG antibody, confirming CD1a dependence (Fig. 1 A). To confirm whether CD1a-expressing DCs could also present antigen, we generated autologous monocyte-derived DCs (mDCs) and observed a greater CD1a-autoreactive T cell response in psoriatic patients than in healthy individuals (Fig. 1 B). These data are compatible with the presence of elevated frequencies of circulating lipid-specific CD1a-reactive T cells in individuals with psoriasis. Moreover, polyclonal T cells from psoriatic donors that were expanded with autologous mDCs responded to a hierarchy of lipids in a CD1a-dependent manner, including fatty acids and lysophospholipids, all of which are enzymatic products of PLA2 activity (Fig. 1 C) and are consistent with previous findings which show broad classes of permissive CD1a ligands (de Jong et al., 2014; Birkinshaw et al., 2015; Bourgeois et al., 2015). Furthermore, it is of interest that the polyclonal T cells responding to arachidonic acid or its derivatives were potentiated by indomethacin, a cyclooxygenase inhibitor that diverts PLA2 products down the lipoxygenase pathway (Fig. 1 D), and responses were reduced by baicalein, a lipoxygenase inhibitor (Fig. 1 D). It is well known that nonsteroidal antiinflammatory drugs such as indomethacin can exacerbate psoriasis.
Figure 1.
Circulating CD1a-autoreative T cells are enriched in psoriasis patients. (A and B) T cells from healthy (n = 8) or psoriatic (n = 8) donors were isolated by CD3 magnetic-activated cell-sorting separation from peripheral blood and incubated with K562-CD1a or mock-transfected K562 cells (K562-EV; A) or mDCs (B) overnight, and IFN-γ production was measured by ELISPOT in the presence or absence of anti-CD1a antibody. Data are mean ± SEM and were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. (C) T cells from psoriatic donors were expanded for 10–14 d with autologous mDCs that were pulsed with 10 µg/ml of the following antigens separately: fatty acid (FA) 20:4 (arachidonic acid), FA 18:1 (oleic acid), FA 16:0 (palmitic acid), lysophosphatidylcholine (LPC) 18:1 (oleoyl-LPC), and LPC 16:0 (palmitoyl-LPC). Next, the corresponding lipids and T cells were co-incubated with K562-CD1a cells in the presence or absence of anti-CD1a antibody. IFN-γ production was measured by ELISPOT. Results represent one donor and are a typical representation from at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. (D) T cells from psoriatic donors were expanded for 10–14 d with autologous mDCs that were pulsed with 10 µg/ml of FA 20:4 (arachidonic acid). The corresponding lipids and T cells were next co-incubated with K562-CD1a cells in the presence or absence of 10 µM indomethacin, 10 µM baicalein, or anti-CD1a antibody. IFN-γ production was measured by ELISPOT. Results represent one donor and are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
PLA2G4D in psoriatic lesions activates CD1a-restricted T cells
Based on the prior knowledge that the increased expression of PLA2G4D, a cytosolic PLA2, was associated with psoriasis (Chiba et al., 2004; Quaranta et al., 2014), a possible role for PLA2G4D in the generation of CD1a ligands was investigated. From the Gene Expression Omnibus (GEO), we collected the results of three gene expression studies between nonlesional and lesional skin from psoriasis patients (Fig. S1 A). Expression levels of relevant genes were analyzed and compared across the groups using GEO2R. High expression levels of PLA2G4D were detected in psoriatic lesional skin (Fig. S1 B).
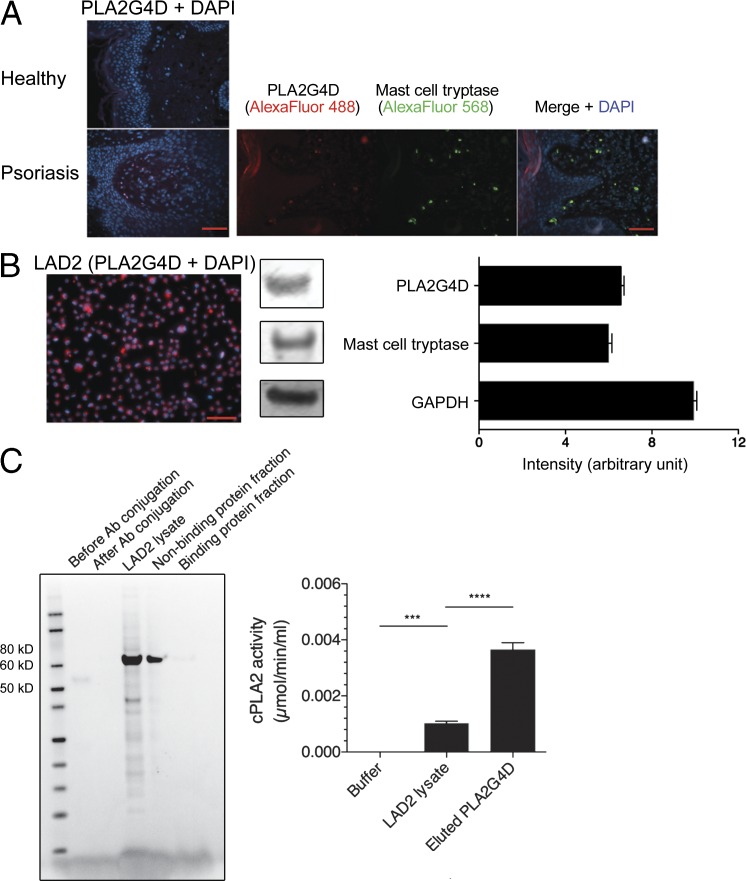
Based on these findings, we then investigated the source of PLA2G4D protein in psoriatic lesional skin. Although no detectable PLA2G4D protein expression was found in normal healthy skin using immunofluorescence, expression of PLA2G4D was unexpectedly observed in the dermis and to a lesser extent in keratinocytes of psoriatic lesional skin (Fig. 2 A). Intriguingly, PLA2G4D expression in the dermis colocalized with the expression of tryptase, which is a specific marker for mast cells, hence indicating that mast cells are a source of PLA2G4D in psoriatic lesions (Fig. 2 A). To confirm this and to proceed to functional investigations, we went on to study the expression of PLA2G4D in the LAD2 human mast cell–like line. LAD2 cells were found to express PLA2G4D as well as mast cell tryptase (Fig. 2 B). We isolated PLA2G4D protein from LAD2 cell lysate by column purification using PLA2G4D-capturing antibody. Both lysate- and binding protein fraction–derived PLA2G4D showed cytosolic PLA2 activity (Fig. 2 C).
Figure 2.
Cytosolic PLA2G4D protein expression in mast cells in psoriatic lesions and in the LAD2 mast cell–like line. (A) PLA2G4D (red) and mast cell tryptase (green) expression in healthy control and psoriatic lesional skin was determined by immunofluorescence. Results represent one donor of each group and are a typical representation of at least three individual experiments. Bars, 60 µm. (B) Immunofluorescence staining of LAD2 mast cells for expression of PLA2G4D (red), with concomitant Western blot staining intensity, was evaluated by ImageJ (National Institutes of Health). Mast cell tryptase and GAPDH were used as positive control proteins. Results represent responses of one sample and are a typical representation of at least three individual experiments. Data are mean ± SEM. Bar, 100 µm. (C) PLA2G4D protein was prepared from cell lysate and eluent of the LAD2 mast cell–like line using immunoprecipitation and Coomassie blue–stained SDS-PAGE. The PLA2 was collected in the binding protein fraction, whereas the nonbinding protein fraction contained any proteins that were not captured in immunoprecipitation. The cytosolic PLA2 activity was measured using detection of free thiol release from arachidonoyl thio-PC, where elute refers to the protein-binding material. Results represent one sample and are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. ***, P < 0.001; ****, P < 0.0001. Ab, antibody.
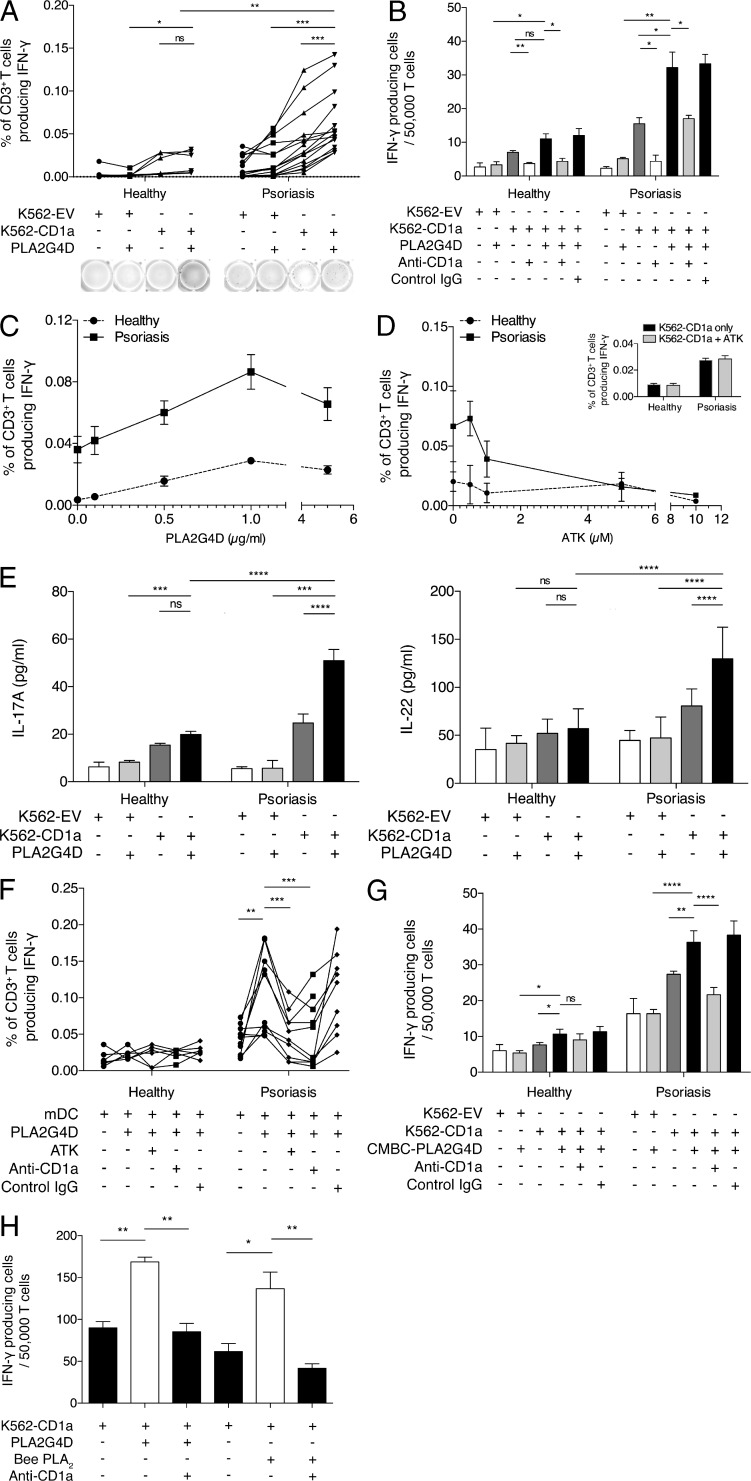
After the protein preparation, we investigated the ability of PLA2G4D to activate a CD1a-reactive T cell response. Based on our previous data (Bourgeois et al., 2015), we hypothesized that PLA2G4D will generate CD1a ligands by cleaving phospholipids in self-cellular plasma membranes and producing neolipid antigens for presentation to CD1a-reactive T cells. Polyclonal T cells from either healthy or psoriatic individuals were isolated from blood ex vivo. In the experiments, both mock-transfected K562 cells and K562-CD1a cells were pulsed with PLA2G4D protein overnight. The T cells were co-incubated with K562/K562-CD1a cells, and IFN-γ secretion was determined. The ELISPOT results using PLA2G4D-pulsed K562-CD1a cells, compared with unpulsed K562-CD1a cells, revealed increased IFN-γ responsiveness of T cells from psoriasis patients (P < 0.001; Fig. 3 A). There was also a significant increase in IFN-γ secretion compared with pulsed mock-transfected K562 cells (P < 0.001). In contrast, T cells from healthy individuals demonstrated a significantly lower CD1a-reactive PLA2G4D response. Low IFN-γ secretion levels were found in the absence of PLA2G4D and/or CD1a (Fig. 3 A). The CD1a reactivity was blocked by anti-CD1a antibody but not control IgG antibody, confirming CD1a dependence (Fig. 3 B). The reactivity of T cells from both healthy and psoriatic individuals varied with the amount of PLA2G4D, confirming a dose dependence (Fig. 3 C). To confirm specificity, we also tested the inhibition of PLA2G4D-responsive CD1a reactivity by arachidonoyl trifluoromethyl ketone (ATK), a cytosolic PLA2 inhibitor. Although the reactivity of T cells from healthy donors remained low throughout, reactivity of T cells from psoriasis patients declined with increasing concentrations of ATK (Fig. 3 D). Because no reduction of CD1a-autoreactive responses to T cells from healthy donors and psoriatics was observed, nonspecific toxicity of ATK on K562 cells was ruled out (Fig. 3 D). In addition to IFN-γ secretion, the T cells from psoriasis patients also produced large amounts of IL-17A and IL-22, consistent with their contribution toward elevated IL-17A and IL-22 found in psoriatic lesions (Fig. 3 E).
Figure 3.
Cytosolic PLA2G4D from the LAD2 mast cell–like line activates CD1a-restricted T cells in blood of psoriasis patients. (A) K562-EV/K562-CD1a cells were incubated with 1 µg/ml PLA2G4D and then incubated with T cells from psoriasis patients (n = 15) or controls (n = 6). IFN-γ production was measured by ELISPOT, showing cumulative and example ELISPOT data. Data were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. (B) K562/K562-CD1a cells were incubated with 1 µg/ml PLA2G4D and then incubated with T cells from one psoriatic patient and one healthy donor in the presence or absence of anti-CD1a antibody or isotype control. Results represent one donor of each group and are representative of 15 patients with psoriasis and 6 controls. Data are mean ± SEM and were analyzed using two-way ANOVA. (C) K562-EV/K562-CD1a cells were incubated with increasing concentrations of PLA2G4D and then incubated with T cells from psoriasis patients (n = 5) or controls (n = 3). IFN-γ production was measured by ELISPOT. Data are mean ± SEM. (D) K562-EV/K562-CD1a cells were incubated with 1 µg/ml PLA2G4D and then incubated with T cells from psoriatic patients (n = 3) or controls (n = 3) in the presence or absence of ATK, a specific cytosolic PLA2 inhibitor. IFN-γ production was measured by ELISPOT. No toxicity or nonspecific inhibition of IFN-γ production of ATK on K562-CD1a cells was observed. Data are mean ± SEM. (E) K562-EV/K562-CD1a cells were incubated with PLA2G4D and then incubated with T cells from psoriatic patients (n = 9) or controls (n = 6). IL-17A and IL-22 production was measured using ELISA. Data are mean ± SEM and were analyzed using two-way ANOVA. (F) Autologous mDCs were incubated with PLA2G4D and then incubated with T cells from psoriatic patients (n = 10) or controls (n = 6) with anti–HLA-ABC (W6/32)– and anti–HLA-DR–blocking antibodies (L243) and in the presence or absence of anti-CD1a antibody, isotype control, or ATK. Data were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. (G) K562-EV/K562-CD1a cells were incubated with 1 µg/ml PLA2G4D isolated from mast cells derived from healthy human cord blood (CBMC-PLA2G4D) and then incubated with T cells from a psoriatic patient and a healthy donor in the presence or absence of anti-CD1a antibody or isotype control. Results represent one donor of each group and are representative of four patients with psoriasis and five controls. Data are mean ± SEM and were analyzed using two-way ANOVA. (H) T cells from psoriatic donors were expanded for 10–14 d before the assay using autologous mDCs that were pulsed with 1 µg/ml PLA2G4D or bee PLA2 protein. Next, the corresponding PLA and T cells were co-incubated with K562-CD1a cells in the presence or absence of anti-CD1a antibody. IFN-γ production was measured by ELISPOT. Results represent one donor and are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
We further examined the PLA2G4D-responsive CD1a reactivity of T cells using autologous mDCs as APCs. Here, we detected a significantly greater PLA2G4D-dependent CD1a-reactive T cell response from psoriasis patients compared with healthy donors (Fig. 3 F). Similarly, the responses were blocked by anti-CD1a antibody but not IgG isotype control antibody. As expected, the CD1a reactivity was reduced in the presence of ATK (Fig. 3 F). We also observed the CD1a reactivity of T cells from psoriatic patients using PLA2G4D, which was isolated from mast cells differentiated from cord blood from healthy donors, as an antigen source (Fig. 3 G). Moreover, T cells from psoriasis donors expanded in the presence of autologous mDCs not only could recognize PLA2G4D-derived lipids, but also showed cross-reactivity with bee venom PLA2 suggesting shared substrate specificities and products (P < 0.01 and P < 0.05, respectively; Fig. 3 H). Overall, these data demonstrated that the generation of neolipid antigens by PLA2G4D contributes to the CD1a-reactive response in T cells from psoriasis patients.
Mast cell exosomes are the source of cytosolic PLA2 contributing to a CD1a-reactive T cell response in psoriasis patients
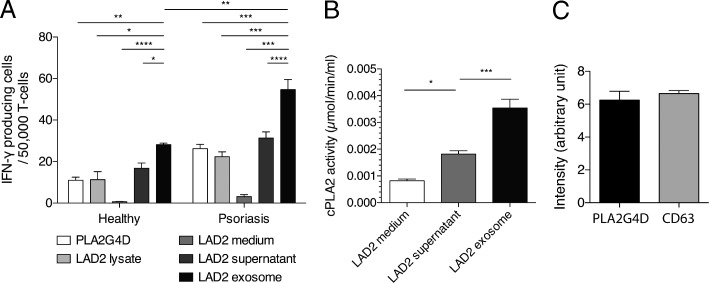
Having observed PLA2G4D in dermal mast cells within psoriatic lesional skin, we sought to determine how PLA2G4D as a cytosolic PLA2 generates neolipid antigens for CD1a lipid presentation to T cells by neighboring APC. Using K562-CD1a as presenting cells, we screened a panel of various antigen sources, namely purified PLA2G4D, LAD2 cell lysate, LAD2 medium control, and LAD2 cell supernatant, by ELISPOT (Fig. 4 A). We observed CD1a reactivity to the LAD2 cell lysate, which was expected because the lysate contained cytosolic PLA2 as well as relevant lipids that might also be CD1a ligands. However, unexpectedly, we observed that the LAD2 supernatant also generated a T cell response (Fig. 4 A). This counterintuitive result was explained by showing the reactivity associated with LAD2-derived exosomes (Fig. 4 A) and further supported by the biochemical activity and protein expression of cytosolic PLA2 found in the exosome fraction of LAD2 cells (Fig. 4, B and C).
Figure 4.
PLA2G4D protein expression in exosomes of the LAD mast cell–like line. (A) K562-CD1a cells were pulsed with PLA2G4D (1 µg/ml), LAD2 lysate (10 µg/ml protein content), LAD2 culture medium control (1 ml per 106 cells), LAD2 supernatant (1 ml per 106 cells), or LAD2 exosome (10 µg/ml protein content). Then, the cells were incubated with T cells from patients with psoriasis (n = 3) and controls (n = 3). IFN-γ production was measured by ELISPOT. Data are mean ± SEM and were analyzed using two-way ANOVA. (B) Cytosolic PLA2 activity was measured in exosome fraction, culture supernatant, and culture medium of the LAD2 mast cell–like line using biochemical activity assay. Results represent one sample and are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. (A and B) *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (C) Protein expression of PLAG4D by LAD2 exosomes was detected using Western blotting, and intensity was evaluated by ImageJ and compared with CD63, a known exosomal marker. Results represent one sample and are a typical representation of at least three individual experiments. Data are mean ± SEM.
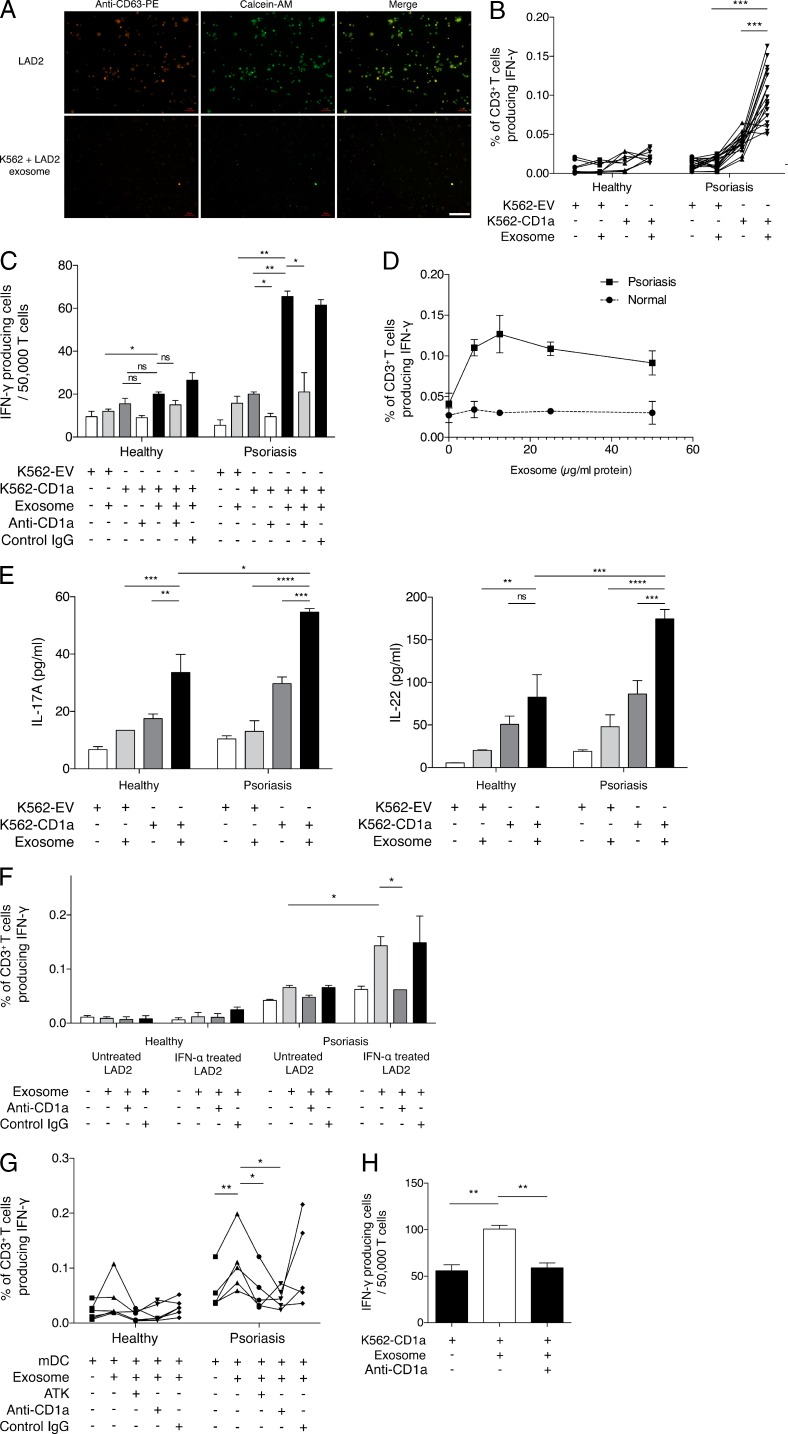
We next sought to understand how exosomes deliver their content to the APC. First, we stained LAD2 cells with anti-CD63–PE antibody and calcein-acetoxymethyl (calcein-AM; Fig. 5 A). The CD63 antibody binds to surface of exosomes, while calcein-AM enters the cells and remains in the cytosol and can thus also enter exosomes. After 24-h incubation of the cells with anti-CD63–PE and calcein-AM, exosomes were isolated from LAD2 supernatant and transferred to K562 cells. After 4 h, we observed detectable fluorescence of K562 cells indicating uptake of exosomes (Fig. 5 A). This experiment demonstrated the docking, internalization, and loading of the exosomal content into K562 cells.
Figure 5.
CD1a-reactivity of T cells from psoriasis patients in response to PLA2G4D-containing exosomes from mast cells. (A) The LAD2 mast cell–like line was stained with anti-CD63–PE (representing membrane) and calcein-AM (cytosolic) for 30 min and then washed and cultured overnight to allow the production of exosomes, which were stained with both fluorochromes. The next day, culture supernatants were collected, and the exosome fraction was obtained using total exosome extraction reagent. K562 cells were incubated with the double-stained exosomes for 4 h. Fluorescence from both fluorochromes was detected using fluorescence microscopy. Results represent one sample and are a typical representative of at least three individual experiments. Bar, 100 µm. (B) K562-EV/K562-CD1a cells were incubated with 10 µg/ml LAD2 exosomes and then incubated with T cells from psoriatic patients (n = 18) and healthy donors (n = 12). IFN-γ was measured by ELISPOT. Data were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. (C) K562-EV/K562-CD1a cells were incubated with 10 µg/ml LAD2 exosomes and then incubated with T cells from one healthy donor and one psoriatic patient in the presence or absence of anti-CD1a antibody or isotype control. Results are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. (D) K562-EV/K562-CD1a cells were incubated with varying concentrations of LAD2 exosomes and then incubated with T cells from psoriatic patients (n = 3) or controls (n = 3). IFN-γ production was measured by ELISPOT. Data are mean ± SEM. (E) K562-EV/K562-CD1a cells were incubated with 10 µg/ml LAD2 exosomes and then incubated with T cells from one healthy donor and one psoriatic patient. IL-17A and IL-22 production were measured by ELISA. Results are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. (F) K562-CD1a cells were either incubated with exosomes from untreated LAD2 cells or exosomes from IFN-α (4 U/ml)–treated LAD2 cells and then incubated with T cells from one healthy donor and one psoriatic patient in the presence or absence of anti-CD1a antibody or isotype control. Results are representative of four patients with psoriasis and five controls. Data are mean ± SEM and were analyzed using two-way ANOVA. (G) mDCs were incubated with 10 µg/ml LAD2 exosomes and then incubated with T cells from psoriasis patients (n = 5) and healthy donors (n = 6) with anti–HLA-ABC (W6/32)– and anti–HLA-DR–blocking antibodies (L243) and in the presence or absence of anti-CD1a antibody or isotype control. Data were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. (H) T cells from psoriatic donors were expanded for 10–14 d before the assay using autologous mDCs that were pulsed with LAD2 exosomes (10 µg/ml of protein content). Next, the exosomes and T cells were co-incubated with K562-CD1a cells in the presence or absence of anti-CD1a antibody. IFN-γ production was measured by ELISPOT. Results represent one donor and are a typical representation of at least three individual experiments. Data are mean ± SEM and were analyzed using two-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To determine the antigenic potential of mast cell–derived exosomes in patients with psoriasis, we performed IFN-γ ELISPOT experiments, stimulating T cells with LAD2-derived exosomes. Although T cells from healthy individuals had low exosome-responsive CD1a reactivity, T cells from psoriasis patients showed increased CD1a responses in the presence of LAD2-derived exosomes (P < 0.001; Fig. 5 B), which could be blocked by anti-CD1a antibody but not control IgG antibody (Fig. 5 C). Different concentrations of LAD2 exosome determined the level of CD1a reactivity (Fig. 5 D). In contrast, the reactivity of T cells from healthy donors remained low regardless of the amount of LAD2-derived exosome added (Fig. 5 D). The exosome-responsive CD1a-reactive T cells also produced large amounts of IL-17A and IL-22 (Fig. 5 E). Notably, the CD1a reactivity in T cells from psoriasis patients was potentiated by IFN-α. When K562-CD1a cells pulsed with LAD2-derived exosomes were pretreated with IFN-α overnight, the T cell IFN-γ response was significantly enhanced (Fig. 5 F).
A similar set of IFN-γ ELISPOT experiments was performed using mDCs as APCs. Again, T cells from psoriasis patients, but not from healthy donors, demonstrated strong LAD2 exosome–responsive CD1a reactivity that could be blocked by ATK or anti-CD1a antibody but not control IgG antibody (Fig. 5 G). Furthermore, the exosome-responsive CD1a reactivity of T cells that were expanded with autologous mDCs was also demonstrated (Fig. 5 H). These data show that psoriatic patients have elevated frequencies of LAD2-derived exosome-responsive CD1a-reactive T cells compared with healthy individuals and that this is inhibited by cPLA2 blockade.
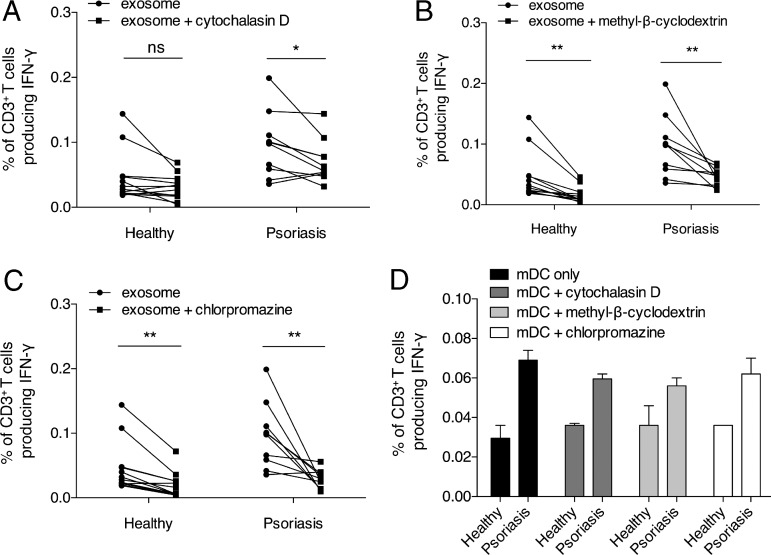
Inhibition of endocytic pathway reduces exosome-responsive CD1a reactivity
We further investigated the mechanism of exosome influence on the CD1a reactivity of T cells. As we have shown that exosomes can be taken up and internalized by K562 cells, to study the endocytic mechanism, we investigated the effects of inhibitors of endocytosis on exosome-responsive CD1a reactivity. We observed that a 30-min preincubation of autologous mDCs with 5 µg/ml cytochalasin D, which inhibits actin polymerization, significantly reduced the CD1a reactivity of T cells from psoriasis patients (P < 0.05). However, there was no inhibition of responses of T cells derived from healthy donors, ruling out nonspecific toxicity of the cytochalasin D (Fig. 6 A). This was supported by the significant reduction of CD1a reactivity by methyl-β-cyclodextrin (2%), an inhibitor of endocytosis that depletes membrane cholesterol (P < 0.01; Fig. 6 B). Inhibition of clathrin-mediated endocytosis by 10 µg/ml chlorpromazine also decreased the CD1a reactivity (P < 0.01; Fig. 6 C), indicating clathrin dependency for exosome entry to mDCs. CD1a autoreactivity of mDCs was not reduced by the inhibition of the endocytic pathway (Fig. 6 D).
Figure 6.
Clathrin-mediated endocytosis is critical for exosome uptake by APCs. (A–C) Autologous mDCs were incubated with 10 µg/ml LAD2 exosomes in the presence of anti–HLA-ABC (W6/32)– and anti–HLA-DR–blocking antibodies (L243), with or without preincubation of inhibitors of the endocytosis pathway (5 µg/ml cytochalasin D, A; 2% methyl-β-cyclodextrin, B; 10 µg/ml chlorpromazine, C) for 30 min, and were then incubated with T cells from psoriatic patients (n = 10) or healthy donors (n = 12). Data were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. *, P < 0.05; **, P < 0.01. (D) Autologous mDCs were preincubated with or without inhibitors (5 µg/ml cytochalasin D, 2% methyl-β-cyclodextrin, or 10 µg/ml chlorpromazine) of the endocytosis pathway for 30 min and were then incubated with T cells from psoriasis patients or healthy donors. Results represent one donor of each group and are representative of 10 patients with psoriasis and 12 controls. Data are mean ± SEM.
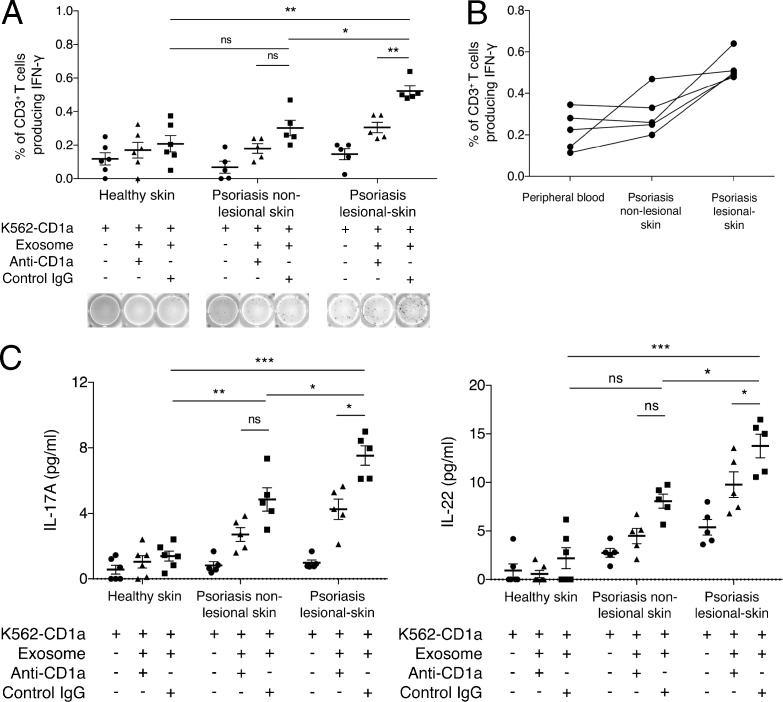
Exosome-responsive CD1a-reactive T cells are enriched in lesional skin of patients with psoriasis
Cutaneous T cells were isolated from lesional and nonlesional skin from psoriasis patients for the study of LAD2 exosome–responsive CD1a reactivity ex vivo. Because of the limited number of T cells obtained from each biopsy, T cells were divided into three experimental conditions. Although T cells from nonlesional psoriatic skin had similar CD1a reactivity to K562 cells compared with those of healthy donors using the LAD2 exosome as an antigen source, lesional psoriatic skin had significantly more IFN-γ–secreting CD1a-restricted T cells (Fig. 7 A). Compared with lesional skin, nonlesional skin had fewer LAD2 exosome–responsive CD1a-reactive T cells (Fig. 7 A). The CD1a reactivity was reduced by anti-CD1a antibody. For each donor of psoriatic skin, peripheral blood T cells were also isolated and tested for comparison. In general, there was a larger proportion of exosome-responsive CD1a-reactive T cells in lesional psoriatic skin than in peripheral blood and nonlesional skin of psoriatics (Fig. 7 B). This was further supported by the results showing that T cells from lesional psoriatic skin secreted significantly higher levels of IL-17A and IL-22 compared with those from healthy skin and nonlesional psoriasis skin (P < 0.0001 and P < 0.05, respectively; Fig. 7 C). To summarize, we show that psoriatic lesional skin has a greater population of resident IFN-γ–, IL-17A–, and IL-22–producing CD1a-restricted T cells that are responsive to exosomes likely derived from mast cells.
Figure 7.
Skin-resident CD1a-autoreactive and exosome-responsive CD1a-reactive T cells in psoriatic lesions. (A) K562-EV/K562-CD1a cells were incubated with LAD2 exosomes and then incubated with T cells from skin biopsies from psoriasis patients (n = 5) or controls (n = 6) in the presence or absence of anti-CD1a or isotype control. IFN-γ production was measured by ELISPOT, showing cumulative and example ELISPOT data. Data are mean ± SEM and were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. (B) K562-EV/K562-CD1a cells were incubated with LAD2 exosomes and then incubated with T cells from blood, nonlesional skin, and lesional skin biopsies from psoriatic patients (n = 5). IFN-γ production was measured by ELISPOT. (C) K562-EV/K562-CD1a cells were incubated with LAD2 exosomes and then incubated with T cells from skin biopsies from psoriatic patients (n = 5) or controls (n = 6) in the presence or absence of anti-CD1a or isotype control. IL-17A and IL-22 production in the supernatant was measured by ELISA. Data are mean ± SEM and were analyzed using a one-tailed Wilcoxon matched-pairs signed rank test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Considerable evidence supports a role for T cells in the pathogenesis of psoriasis, including the presence of T cells within lesions, and the clinical response of patients to therapeutics that target T cell–derived cytokines and function. Many studies have attempted to identify conventional peptide-based T cell antigens that might be relevant to psoriasis, including cross-reactive epitopes between streptococcal and keratinocyte antigens (Kobayashi et al., 2002; Johnston et al., 2004), as well as responses to self-peptide antigens including LL37 and melanocyte antigens (Lande et al., 2014; Arakawa et al., 2015). In the current study, we showed that PLA2-responsive CD1a-reactive T cells are elevated in the blood and skin of patients with psoriasis. We observed that endogenous cytosolic phospholipase PLA2G4D was expressed in mast cells and keratinocytes within psoriatic lesions. However, notably cytoplasmic PLA2 activity was also found in the mast cell supernatant. This was explained by localization of cytoplasmic PLA2 activity within mast cell–derived exosomes, which could be transferred to CD1a-expressing target cells in a clathrin-dependent manner. These findings point to a broader model of psoriatic inflammation, where nonpeptide antigens may also be important activators of lesional T cells, and show that exosomes can transport potential lipid ligands to neighboring APCs.
The role of PLA2 in the pathogenesis of psoriasis was initially suggested 30 yr ago, when elevated levels of products of the PLA2 pathway, such as PGE2 and PGF2α, were observed in the epidermis of psoriatic lesions (Hammarström et al., 1975). Since then, additional studies have demonstrated increased activity of PLA2, which included nonpancreatic PLA2 and cytosolic PLA2, in the skin of psoriasis patients (Forster et al., 1983a,b, 1985; Verhagen et al., 1984; Andersen et al., 1994). In particular, Chiba et al. (2004) reported the expression of PLA2G4D in psoriatic lesional skin but not in healthy skin, in line with the genome expression analysis revealing the up-regulation of PLA2G4D gene expression in psoriatic but not in healthy skin (Quaranta et al., 2014). Interestingly, as well as endogenous phospholipase, proposed microbial triggers of psoriasis such as infection with Streptococcus and Malassezia species, also contain secretory phospholipase activity, which may be relevant to skin inflammation (Leung et al., 1995; Cafarchia and Otranto, 2004; Amaya et al., 2007). Nonsteroidal antiinflammatory drugs are known to have the capacity to exacerbate psoriasis clinically, and so it was of interest that indomethacin could potentiate the CD1a-restricted T cell responses from psoriatics, raising the possibility that a differential modulation of this pathway may lead to therapeutic benefit.
Exogenous PLA2 has recently been shown to generate neolipid antigens for recognition by CD1a-reactive T cells (Bourgeois et al., 2015; Subramaniam et al., 2016). CD1a is expressed at constitutively high levels by LCs of the epidermis, which are known to be functionally altered in patients with psoriasis (Dougan et al., 2007; Yakimchuk et al., 2011). It was unexpected to have found cytosolic PLA2G4D in the supernatant from mast cells, but this was subsequently explained by exosomal transfer. Exosomes are membrane vesicles of 40–100 nm in diameter and secreted by many different cells including B cells, T cells, and tumor cells (Denzer et al., 2000; Keller et al., 2006; Simpson et al., 2009). Their composition depends on their cellular source and can be complex. For instance, exosomes can contain proteins or enzymes including heat shock proteins and tetraspanins (CD63, CD9, and CD81; Stoorvogel et al., 2002; Simons and Raposo, 2009), lipids such as cholesterol and sphingolipids (Krishnamoorthy et al., 2009), and nucleic acids such as micro-RNA (Schorey and Bhatnagar, 2008). Many studies have shown that exosomes are involved in intercellular transfer of proteins and RNA (Katzmann et al., 2001; van Niel et al., 2006; Schorey and Bhatnagar, 2008). Clathrin-mediated endocytosis of exosomes by DCs has been previously described (Morelli et al., 2004). It remains possible that lipid products of PLA2G4D are also being transferred alongside PLA2G4D within the exosomes, but the ability of cytoplasmic PLA2 inhibition to block the T cell recognition of exosome-pulsed CD1a-expressing target cells confirms the requirement for enzymatically active PLA2. The release of exosomes was enhanced in the presence of IFN-α, which is known to be important in the initiation of psoriasis. Package and transfer of PLA2 in exosomes would help ensure their safe transit to relevant CD1-expressing cell types. Mast cells have long been known to be enriched in psoriatic lesions, but the mechanisms underlying their involvement have been unclear. The current study provides a unifying link between mast cells, IFN-α, PLA2, and T cells in the pathogenesis of psoriasis, which points to an alternative model where T cells recognizing lipids may play a role in disease. The data also have therapeutic implications. As well as supporting approaches to inhibit T cell–derived and innate cell–derived cytokines, such as IL-17, the findings would support the development of approaches to inhibit PLA2G4D or CD1a. It is of interest that topical corticosteroids, which are effective in psoriasis, have probable PLA2 inhibitory activity. However PLA2 inhibition by corticosteroids is broad, affecting diverse PLA2 with roles in homeostasis as well as inflammation, and so, it is likely that inhibition of specific PLA2 forms will be required.
MATERIALS AND METHODS
Cell culture
K562 cells were maintained in K562 medium, which comprises of RPMI 1640 medium with 10% fetal bovine serum, 100 IU/ml penicillin, 100 µg/ml streptomycin (Sigma-Aldrich), 2 mM l-glutamine, 1× nonessential amino acids (NEAAs), 1 mM sodium pyruvate, 10 mM Hepes, 500 µM 2-mercaptoethanol, and 50 µg/ml G418 antibiotic (Thermo Fisher Scientific). The LAD2 mast cell line was provided by D. Metcalfe and A. Kirshenbaum (National Institutes of Health, Bethesda, MD) and cultured in StemPro-34 serum-free medium (Thermo Fisher Scientific) supplemented with 100 µg/ml human stem cell factor (PeproTech). Human mast cells were cultured and differentiated from CD34+ progenitor cells isolated from human cord blood in the presence of IMDM containing 10% human serum, 100 IU/ml penicillin, 100 µg/ml streptomycin (Sigma-Aldrich), 0.55 µM 2-mercaptoethanol (Thermo Fisher Scientific), 100 ng/ml human recombinant stem cell factor, and 50 ng/ml human rIL-6 (PeproTech) in 5% CO2 at 37°C for 10–12 wk.
Isolation and preparation of human T cells and mDCs from peripheral blood
PBMCs were isolated from healthy adult donors and psoriasis patients with moderate-severe disease but not on systemic therapy. Local ethical approval was given by the Oxford Ethics Committee (09/H0606/71). T cells were purified from PBMCs using Lymphoprep medium (STEMCELL Technologies) and magnetic-activated cell-sorting separation of CD3-positive cells (Miltenyi Biotec) and maintained in T cell culture medium comprising of RPMI 1640 medium supplemented with 5% human serum (Sigma-Aldrich), 100 IU/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine, 1× NEAA, 10 mM Hepes, 500 µM 2-mercaptoethanol, and 2 nM IL-2 (PeproTech). mDCs were prepared by differentiating CD14-positive monocytes after magnetic separation from PBMCs for 3–4 d in complete medium, which comprised of RPMI 1640 medium with 10% fetal bovine serum, 100 IU/ml penicillin, 100 µg/ml streptomycin (Sigma-Aldrich), 2 mM l-glutamine, 1× NEAA, 1 mM sodium pyruvate, 10 mM Hepes, and 500 µM 2-mercaptoethanol, and supplemented with 300 U/ml GM-CSF and 200 U/ml IL-4 (PeproTech). mDCs were harvested at day 4, and CD1a expression was verified by flow cytometry.
Isolation of human T cells from skin
After removing subcutaneous fat, skin sections were cut into 1-mm pieces and incubated in RPMI 1640 medium with 10% fetal bovine serum, glutamine, and penicillin and in the presence of 1 mg/ml collagenase P (Roche) at 37°C overnight at 5% CO2. The skin pieces were then washed with cold PBS with 10 mM EDTA to quench the collagenase digestion, and CD3-positive cells were isolated using Lymphoprep medium (STEMCELL Technologies), followed by magnetic-activated cell-sorting separation (Miltenyi Biotec). CD3-positive T cells were then cultured in T cell medium for further experiments.
Immunofluorescence microscopy
Paraffin-embedded skin sections were dewaxed using Citroclear, rehydrated in a series of descending gradient of ethanol-water solutions, and then boiled in 1× Target Retrieval buffer (Dako) for 15 min for antigen retrieval, followed by cooling down in PBS. Blocking was done with blocking solution (1% BSA in PBS) for 1 h at room temperature. Skin sections were then ready for incubation with primary and secondary antibodies, accordingly. Coverslips were mounted in Fluoroshield mounting medium (Sigma-Aldrich). For LAD2 mast cell imaging, cells were plated on culture slides (BD) pretreated with poly-d-lysine, fixed and permeabilized with acetone for 10 min, and then blocked with blocking solution for 1 h at room temperature, followed by staining with primary and secondary antibodies. Primary antibodies were: rabbit anti-PLA2G4D (1:50; Abcam) and mouse anti–mast cell tryptase (1:1,000; Abcam). Secondary antibodies were: goat anti–rabbit IgG–Alexa Fluor 488 (1:500; Thermo Fisher Scientific) and goat anti–mouse IgG–Alexa Fluor 568 (1:500; Thermo Fisher Scientific). Images were acquired on an Axiovert S100 microscope (ZEISS) coupled with a digital camera (ORCA-ER C4742-80; Hamamatsu Photonics). Laser intensity and amplifier gains were adjusted to avoid pixel saturation. Detection of fluorescence was performed independently and sequentially on each fluorophore. Images were processed by ZEN imaging software (Blue edition; ZEISS).
PLA2G4D extraction
The LAD2 mast cell line or mast cells differentiated from healthy donor cord blood were lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich), the cell lysate was obtained in supernatant after centrifugation and removal of pellet, and PLA2G4D protein was prepared using affinity column protein purification. In brief, the cell lysate was run through a resin column (Thermo Fisher Scientific) precoated covalently with anti-PLA2G4D antibody (Abcam). After extensive washing of the column with PBS, PLA2G4D was eluted with elution buffer (Thermo Fisher Scientific), and the PLA2G4D protein fraction was collected. Protein concentration was measured using the bicinchoninic acid assay method, and cytosolic PLA2 activity was measured by biochemical activity assay.
Exosome preparation
Exosomes of the LAD2 mast cell line were prepared from the culture supernatant using Total Exosome Extraction reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. In brief, two parts of culture supernatant were mixed with one part of exosome extraction reagent, and the mixture was vortexed for 30 s, followed by incubating at 4°C on a roller shaker overnight. Next, the mixture was centrifuged at 10,000 g for 1 h, and exosome fractions were collected as pellets. Pellets of exosomes were dissolved in PBS or other desired buffer for further experiments.
PLA2 biochemical activity assays
PLA2 activities in culture medium, cell lysate, culture supernatant, and exosomes of the LAD2 mast cell line were measured using a cytosolic PLA2 kit (Cayman Chemicals) according to the manufacturer’s protocols. Arachidonoyl thio-PC is a substrate for cPLA2 by virtue of the presence of arachidonic acid at the sn-2 position of the glycerophospholipid. Hydrolysis of the arachidonoyl thioester bond at the sn-2 position by cPLA2 releases a free thiol which can be detected by DTNB (5,5′-dithiobis[2-nitrobenzoic acid]).
ELISPOT experiments
At day 1, ELISPOT plates (EMD Millipore) were pretreated with 35% ethanol, washed six times with water, and then coated with anti–IFN-γ antibody (Mabtech) for overnight at 4°C. K562 cells or mDCs were washed three times with R-Hepes medium (RPMI 1640 medium with 10 mM Hepes), resuspended in resting medium (RPMI 1640 medium, 5% human serum, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine), and pulsed with PLA2G4D protein or exosomes for overnight at 37°C and 5% CO2. After isolation from blood or skin, polyclonal T cells were maintained in T cell medium, as described in the Isolation and preparation of human T cells and mDCs from peripheral blood section, for 2–3 d and further rested for 1 d in medium at 37°C and 5% CO2. On day 2, ELISPOT plates were washed six times with RPMI 1640 medium, blocked with R10 medium (RPMI 1640 medium with 10% FCS, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine) for 30–60 min, and washed twice with RPMI 1640 medium. 50,000 T cells and 25,000 K562 cells/mDCs were added to each well. Wells were set up in duplicates or triplicates. 10 ng/ml PMA and 500 ng/ml ionomycin were used as a positive control, whereas T cells alone served as the negative control. In some experiments, CD1a-transfected or empty vector–transfected K562 cells (a gift from B. Moody, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) were incubated with 10 µg/ml anti-CD1a antibody (eBioscience) or 10 µg/ml mouse control IgG antibody (BD). For the mDC experiments, 10 µg/ml anti–HLA-ABC (W6/32) and anti–HLA-DR blocking antibodies (L243) were added for 2 h before addition of T cells. Inhibitors were 10 µg/ml chlorpromazine, 2% methyl-β-cyclodextrin, 5 µg/ml cytochalasin D, or 10 µM ATK added for 30 min before K562-T cell co-incubation. Cells were then incubated overnight at 37°C and 5% CO2. On day 3, after collecting ELISPOT supernatants for further experiments, plates were first washed five times with 0.05% PBS–Tween 20 and incubated with 1 µg/ml biotin-conjugated anti–IFN-γ monoclonal antibody (Mabtech) for 2–3 h at room temperature, followed by washing six times with PBS–Tween 20. Plates were then incubated with streptavidin–alkaline phosphatase solution (Mabtech) for 1–2 h and washed six times with PBS–Tween 20. Spots were developed and visualized using an alkaline phosphatase conjugate kit (Bio-Rad Laboratories) and detected and analyzed using an ELISPOT plate reader (Autimmun Diagnostika gmbh ELISpot Reader Classic).
ELISA
Supernatants were collected for measurements of IL-17A and IL-22 secretion using ELISA kits (eBioscience) In brief, the supernatants were plated into 96-well ELISA plates (Sigma-Aldrich) precoated with respective coating antibodies (eBioscience) for overnight at 4°C. The next day, plates were washed and developed according to the manufacturer’s protocols, and absorbance at 415 nm was measured on a microplate reader (iMark Microplate Reader; Bio-Rad Laboratories).
Statistics
Cohorts of healthy and psoriasis individuals for CD1a-autoreactive, PLA2-specific, and exosome-specific CD1a-restricted responses were analyzed using one-tailed Wilcoxon matched-pairs signed rank tests. Other T cell responses were analyzed using two-way ANOVA.
Online supplemental material
Fig. S1 shows elevated PLA2G4D gene expression in psoriatic skin in three gene expression studies retrieved from GEO.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the support of the National Institute for Health Research Clinical Research Network, British Association of Dermatologists, British Skin Foundation, and Misses Barrie Charitable Trust. We are grateful to Branch Moody for provision of K562 transfectants and for helpful discussions.
This work was funded by the Medical Research Council (CF7720) and the National Institute for Health Research Biomedical Research Centre (CF7722).
G. Ogg has served on advisory boards for Eli Lilly, Novartis, Janssen, and UCB Pharma. The authors declare no further competing financial interests.
Footnotes
Abbreviations used:
- ATK
- arachidonoyl trifluoromethyl ketone
- calcein-AM
- calcein-acetoxymethyl
- LC
- Langerhans cell
- mDC
- monocyte-derived DC
- NEAA
- nonessential amino acid
References
- Amaya M., Tajima M., Okubo Y., Sugita T., Nishikawa A., and Tsuboi R.. 2007. Molecular analysis of Malassezia microflora in the lesional skin of psoriasis patients. J. Dermatol. 34:619–624. 10.1111/j.1346-8138.2007.00343.x [DOI] [PubMed] [Google Scholar]
- Andersen S., Sjursen W., Laegreid A., Volden G., and Johansen B.. 1994. Elevated expression of human nonpancreatic phospholipase A2 in psoriatic tissue. Inflammation. 18:1–12. 10.1007/BF01534593 [DOI] [PubMed] [Google Scholar]
- Arakawa A., Siewert K., Stöhr J., Besgen P., Kim S.M., Rühl G., Nickel J., Vollmer S., Thomas P., Krebs S., et al. . 2015. Melanocyte antigen triggers autoimmunity in human psoriasis. J. Exp. Med. 212:2203–2212. 10.1084/jem.20151093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin L.M., Ozawa M., Kikuchi T., Walters I.B., and Krueger J.G.. 1999. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113:752–759. 10.1046/j.1523-1747.1999.00749.x [DOI] [PubMed] [Google Scholar]
- Balato A., Lembo S., Mattii M., Schiattarella M., Marino R., De Paulis A., Balato N., and Ayala F.. 2012. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp. Dermatol. 21:892–894. 10.1111/exd.12027 [DOI] [PubMed] [Google Scholar]
- Birkinshaw R.W., Pellicci D.G., Cheng T.Y., Keller A.N., Sandoval-Romero M., Gras S., de Jong A., Uldrich A.P., Moody D.B., Godfrey D.I., and Rossjohn J.. 2015. αβ T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat. Immunol. 16:258–266. 10.1038/ni.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois E.A., Subramaniam S., Cheng T.Y., De Jong A., Layre E., Ly D., Salimi M., Legaspi A., Modlin R.L., Salio M., et al. . 2015. Bee venom processes human skin lipids for presentation by CD1a. J. Exp. Med. 212:149–163. 10.1084/jem.20141505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody I. 1984. Mast cell degranulation in the evolution of acute eruptive guttate psoriasis vulgaris. J. Invest. Dermatol. 82:460–464. 10.1111/1523-1747.ep12260955 [DOI] [PubMed] [Google Scholar]
- Cafarchia C., and Otranto D.. 2004. Association between phospholipase production by Malassezia pachydermatis and skin lesions. J. Clin. Microbiol. 42:4868–4869. 10.1128/JCM.42.10.4868-4869.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Michibata H., Wakimoto K., Seishima M., Kawasaki S., Okubo K., Mitsui H., Torii H., and Imai Y.. 2004. Cloning of a gene for a novel epithelium-specific cytosolic phospholipase A2, cPLA2δ, induced in psoriatic skin. J. Biol. Chem. 279:12890–12897. 10.1074/jbc.M305801200 [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Singh M., Dearman R.J., Young H.S., Kimber I., and Griffiths C.E.. 2006. Impaired Langerhans cell migration in psoriasis. J. Exp. Med. 203:953–960. 10.1084/jem.20052367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., Peña-Cruz V., Cheng T.Y., Clark R.A., Van Rhijn I., and Moody D.B.. 2010. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat. Immunol. 11:1102–1109. 10.1038/ni.1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., Cheng T.Y., Huang S., Gras S., Birkinshaw R.W., Kasmar A.G., Van Rhijn I., Peña-Cruz V., Ruan D.T., Altman J.D., et al. . 2014. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat. Immunol. 15:177–185. 10.1038/ni.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K., Kleijmeer M.J., Heijnen H.F., Stoorvogel W., and Geuze H.J.. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113:3365–3374. [DOI] [PubMed] [Google Scholar]
- Dougan S.K., Kaser A., and Blumberg R.S.. 2007. CD1 expression on antigen-presenting cells. Curr. Top. Microbiol. Immunol. 314:113–141. [DOI] [PubMed] [Google Scholar]
- Dyring-Andersen B., Geisler C., Agerbeck C., Lauritsen J.P., Gúdjonsdottir S.D., Skov L., and Bonefeld C.M.. 2014. Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. Br. J. Dermatol. 170:609–616. 10.1111/bjd.12658 [DOI] [PubMed] [Google Scholar]
- Eaton L.H., Chularojanamontri L., Ali F.R., Theodorakopoulou E., Dearman R.J., Kimber I., and Griffiths C.E.. 2014. Guttate psoriasis is associated with an intermediate phenotype of impaired Langerhans cell migration. Br. J. Dermatol. 171:409–411. 10.1111/bjd.12960 [DOI] [PubMed] [Google Scholar]
- Forster S., Ilderton E., Summerly R., and Yardley H.J.. 1983a Epidermal phospholipase A2 activity is raised in the uninvolved skin of psoriasis. Br. J. Dermatol. 109:30–35. 10.1111/j.1365-2133.1983.tb06815.x [DOI] [PubMed] [Google Scholar]
- Forster S., Ilderton E., Summerly R., and Yardley H.J.. 1983b The level of phospholipase A2 activity is raised in the uninvolved epidermis of psoriasis. Br. J. Dermatol. 108:103–105. 10.1111/j.1365-2133.1983.tb04585.x [DOI] [PubMed] [Google Scholar]
- Forster S., Ilderton E., Norris J.F., Summerly R., and Yardley H.J.. 1985. Characterization and activity of phospholipase A2 in normal human epidermis and in lesion-free epidermis of patients with psoriasis or eczema. Br. J. Dermatol. 112:135–147. 10.1111/j.1365-2133.1985.tb00077.x [DOI] [PubMed] [Google Scholar]
- Gelfand J.M., Stern R.S., Nijsten T., Feldman S.R., Thomas J., Kist J., Rolstad T., and Margolis D.J.. 2005. The prevalence of psoriasis in African Americans: results from a population-based study. J. Am. Acad. Dermatol. 52:23–26. 10.1016/j.jaad.2004.07.045 [DOI] [PubMed] [Google Scholar]
- Ghosh M., Loper R., Gelb M.H., and Leslie C.C.. 2006. Identification of the expressed form of human cytosolic phospholipase A2β (cPLA2β): cPLA2β3 is a novel variant localized to mitochondria and early endosomes. J. Biol. Chem. 281:16615–16624. 10.1074/jbc.M601770200 [DOI] [PubMed] [Google Scholar]
- Gottlieb A.B., Langley R.G., Philipp S., Sigurgeirsson B., Blauvelt A., Martin R., Papavassilis C., and Mpofu S.. 2015. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: Results from two randomized, phase 3 trials. J. Drugs Dermatol. 14:821–833. [PubMed] [Google Scholar]
- Griffiths C.E., and Barker J.N.. 2007. Pathogenesis and clinical features of psoriasis. Lancet. 370:263–271. 10.1016/S0140-6736(07)61128-3 [DOI] [PubMed] [Google Scholar]
- Gumperz J.E. 2006. The ins and outs of CD1 molecules: bringing lipids under immunological surveillance. Traffic. 7:2–13. 10.1111/j.1600-0854.2005.00364.x [DOI] [PubMed] [Google Scholar]
- Hammarström S., Hamberg M., Samuelsson B., Duell E.A., Stawiski M., and Voorhees J.J.. 1975. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc. Natl. Acad. Sci. USA. 72:5130–5134. 10.1073/pnas.72.12.5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henseler T., and Christophers E.. 1995. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 32:982–986. 10.1016/0190-9622(95)91336-X [DOI] [PubMed] [Google Scholar]
- Hueber W., Patel D.D., Dryja T., Wright A.M., Koroleva I., Bruin G., Antoni C., Draelos Z., Gold M.H., Durez P., et al. Uveitis Study Group . 2010. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2:52ra72 10.1126/scitranslmed.3001107 [DOI] [PubMed] [Google Scholar]
- Izaki S., Yamamoto T., Goto Y., Ishimaru S., Yudate F., Kitamura K., and Matsuzaki M.. 1996. Platelet-activating factor and arachidonic acid metabolites in psoriatic inflammation. Br. J. Dermatol. 134:1060–1064. 10.1111/j.1365-2133.1996.tb07943.x [DOI] [PubMed] [Google Scholar]
- Jarrett R., Salio M., Lloyd-Lavery A., Subramaniam S., Bourgeois E., Archer C., Cheung K.L., Hardman C., Chandler D., Salimi M., et al. . 2016. Filaggrin inhibits generation of CD1a neolipid antigens by house dust mite-derived phospholipase. Sci. Transl. Med. 8:325ra18 10.1126/scitranslmed.aad6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A., Gudjonsson J.E., Sigmundsdottir H., Love T.J., and Valdimarsson H.. 2004. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8+ T cells. Clin. Exp. Immunol. 138:83–93. 10.1111/j.1365-2249.2004.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S., Rizzo H.L., Lee J.J., Koguchi Y., and Blauvelt A.. 2010. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 130:1373–1383. 10.1038/jid.2009.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D.J., Babst M., and Emr S.D.. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 106:145–155. 10.1016/S0092-8674(01)00434-2 [DOI] [PubMed] [Google Scholar]
- Keijsers R.R., Joosten I., van Erp P.E., Koenen H.J., and van de Kerkhof P.C.. 2014. Cellular sources of IL-17 in psoriasis: a paradigm shift? Exp. Dermatol. 23:799–803. 10.1111/exd.12487 [DOI] [PubMed] [Google Scholar]
- Keller S., Sanderson M.P., Stoeck A., and Altevogt P.. 2006. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 107:102–108. 10.1016/j.imlet.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Kimball A.B., Papp K.A., Wasfi Y., Chan D., Bissonnette R., Sofen H., Yeilding N., Li S., Szapary P., and Gordon K.B.. PHOENIX 1 Investigators . 2013. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J. Eur. Acad. Dermatol. Venereol. 27:1535–1545. 10.1111/jdv.12046 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Takahashi M., Takahashi H., Ishida-Yamamoto A., Hashimoto Y., Sato K., Tateno M., and Iizuka H.. 2002. CD4+ T-cells from peripheral blood of a patient with psoriasis recognize keratin 14 peptide but not ‘homologous’ streptococcal M-protein epitope. J. Dermatol. Sci. 30:240–247. 10.1016/S0923-1811(02)00111-1 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy L., Bess J.W. Jr., Preston A.B., Nagashima K., and Mahal L.K.. 2009. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat. Chem. Biol. 5:244–250. 10.1038/nchembio.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R., Botti E., Jandus C., Dojcinovic D., Fanelli G., Conrad C., Chamilos G., Feldmeyer L., Marinari B., Chon S., et al. . 2014. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 5:5621 10.1038/ncomms6621 [DOI] [PubMed] [Google Scholar]
- Landgren E., Bråbäck L., Hedlin G., Hjern A., and Rasmussen F.. 2006. Psoriasis in Swedish conscripts: time trend and association with T-helper 2-mediated disorders. Br. J. Dermatol. 154:332–336. 10.1111/j.1365-2133.2005.07004.x [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Strober B., Menter A., Gordon K., Weglowska J., Puig L., Papp K., Spelman L., Toth D., Kerdel F., et al. . 2015. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 373:1318–1328. 10.1056/NEJMoa1503824 [DOI] [PubMed] [Google Scholar]
- Leslie C.C. 2004. Regulation of the specific release of arachidonic acid by cytosolic phospholipase A2. Prostaglandins Leukot. Essent. Fatty Acids. 70:373–376. 10.1016/j.plefa.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Leslie C.C., and Gelb M.H.. 2004. Assaying phospholipase A2 activity. Methods Mol. Biol. 284:229–242. [DOI] [PubMed] [Google Scholar]
- Leung D.Y., Travers J.B., Giorno R., Norris D.A., Skinner R., Aelion J., Kazemi L.V., Kim M.H., Trumble A.E., Kotb M., et al. . 1995. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J. Clin. Invest. 96:2106–2112. 10.1172/JCI118263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A.M., Rubin C.J., Khandpur R., Wang J.Y., Riblett M., Yalavarthi S., Villanueva E.C., Shah P., Kaplan M.J., and Bruce A.T.. 2011. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187:490–500. 10.4049/jimmunol.1100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes M.A., Kikuchi T., Fuentes-Duculan J., Cardinale I., Zaba L.C., Haider A.S., Bowman E.P., and Krueger J.G.. 2008. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 128:1207–1211. 10.1038/sj.jid.5701213 [DOI] [PubMed] [Google Scholar]
- Morelli A.E., Larregina A.T., Shufesky W.J., Sullivan M.L., Stolz D.B., Papworth G.D., Zahorchak A.F., Logar A.J., Wang Z., Watkins S.C., et al. . 2004. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 104:3257–3266. 10.1182/blood-2004-03-0824 [DOI] [PubMed] [Google Scholar]
- Mori L., and De Libero G.. 2008. Presentation of lipid antigens to T cells. Immunol. Lett. 117:1–8. 10.1016/j.imlet.2007.11.027 [DOI] [PubMed] [Google Scholar]
- Nestle F.O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y.J., and Gilliet M.. 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202:135–143. 10.1084/jem.20050500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto T., Uozumi N., Hirabayashi T., and Shimizu T.. 2005. Identification of novel cytosolic phospholipase A2s, murine cPLA2δ, ε, and ζ, which form a gene cluster with cPLA2β. J. Biol. Chem. 280:24576–24583. 10.1074/jbc.M413711200 [DOI] [PubMed] [Google Scholar]
- Papp K.A., Langley R.G., Lebwohl M., Krueger G.G., Szapary P., Yeilding N., Guzzo C., Hsu M.C., Wang Y., Li S., et al. PHOENIX 2 study investigators . 2008. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 371:1675–1684. 10.1016/S0140-6736(08)60726-6 [DOI] [PubMed] [Google Scholar]
- Papp K.A., Leonardi C., Menter A., Ortonne J.P., Krueger J.G., Kricorian G., Aras G., Li J., Russell C.B., Thompson E.H., and Baumgartner S.. 2012. Brodalumab, an anti–interleukin-17–receptor antibody for psoriasis. N. Engl. J. Med. 366:1181–1189. 10.1056/NEJMoa1109017 [DOI] [PubMed] [Google Scholar]
- Papp K., Menter A., Strober B., Kricorian G., Thompson E.H., Milmont C.E., Nirula A., and Klekotka P.. 2015. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J. Am. Acad. Dermatol. 72:436–439.e1. 10.1016/j.jaad.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Quaranta M., Knapp B., Garzorz N., Mattii M., Pullabhatla V., Pennino D., Andres C., Traidl-Hoffmann C., Cavani A., Theis F.J., et al. . 2014. Intraindividual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci. Transl. Med. 6:244ra90 10.1126/scitranslmed.3008946 [DOI] [PubMed] [Google Scholar]
- Res P.C., Piskin G., de Boer O.J., van der Loos C.M., Teeling P., Bos J.D., and Teunissen M.B.. 2010. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 5:e14108 10.1371/journal.pone.0014108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryborg A.K., Grøn B., and Kragballe K.. 1995. Increased lysophosphatidylcholine content in lesional psoriatic skin. Br. J. Dermatol. 133:398–402. 10.1111/j.1365-2133.1995.tb02667.x [DOI] [PubMed] [Google Scholar]
- Schön M.P. 2014. The plot thickens while the scope broadens: a holistic view on IL-17 in psoriasis and other inflammatory disorders. Exp. Dermatol. 23:804–806. 10.1111/exd.12541 [DOI] [PubMed] [Google Scholar]
- Schorey J.S., and Bhatnagar S.. 2008. Exosome function: from tumor immunology to pathogen biology. Traffic. 9:871–881. 10.1111/j.1600-0854.2008.00734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C., and Christophers E.. 1985. Mast cells and macrophages in early relapsing psoriasis. Arch. Dermatol. Res. 277:352–358. 10.1007/BF00509232 [DOI] [PubMed] [Google Scholar]
- Shaw F.L., Mellody K.T., Ogden S., Dearman R.J., Kimber I., and Griffiths C.E.. 2014. Treatment-related restoration of Langerhans cell migration in psoriasis. J. Invest. Dermatol. 134:268–271. 10.1038/jid.2013.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefler I., Pasmanik-Chor M., Kidron D., Mekori Y.A., and Hershko A.Y.. 2014. T cell-derived microvesicles induce mast cell production of IL-24: relevance to inflammatory skin diseases. J. Allergy Clin. Immunol. 133:217–224.e3. 10.1016/j.jaci.2013.04.035 [DOI] [PubMed] [Google Scholar]
- Simons M., and Raposo G.. 2009. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21:575–581. 10.1016/j.ceb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Simpson R.J., Lim J.W., Moritz R.L., and Mathivanan S.. 2009. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics. 6:267–283. 10.1586/epr.09.17 [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Kleijmeer M.J., Geuze H.J., and Raposo G.. 2002. The biogenesis and functions of exosomes. Traffic. 3:321–330. 10.1034/j.1600-0854.2002.30502.x [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Aslam A., Misbah S.A., Salio M., Cerundolo V., Moody D.B., and Ogg G.. 2016. Elevated and cross-responsive CD1a-reactive T cells in bee and wasp venom allergic individuals. Eur. J. Immunol. 46:242–252. 10.1002/eji.201545869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen M.B., Munneke J.M., Bernink J.H., Spuls P.I., Res P.C., Te Velde A., Cheuk S., Brouwer M.W., Menting S.P., Eidsmo L., et al. . 2014. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR+ ILC3 in lesional skin and blood of psoriasis patients. J. Invest. Dermatol. 134:2351–2360. 10.1038/jid.2014.146 [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Baker B.S., Jónsdóttir I., Powles A., and Fry L.. 1995. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol. Today. 16:145–149. 10.1016/0167-5699(95)80132-4 [DOI] [PubMed] [Google Scholar]
- van Niel G., Porto-Carreiro I., Simoes S., and Raposo G.. 2006. Exosomes: a common pathway for a specialized function. J. Biochem. 140:13–21. 10.1093/jb/mvj128 [DOI] [PubMed] [Google Scholar]
- Verhagen A., Bergers M., van Erp P.E., Gommans J.M., van de Kerkhof P.C., and Mier P.D.. 1984. Confirmation of raised phospholipase A2 activity in the uninvolved skin of psoriasis. Br. J. Dermatol. 110:731–732. 10.1111/j.1365-2133.1984.tb04712.x [DOI] [PubMed] [Google Scholar]
- Villanova F., Flutter B., Tosi I., Grys K., Sreeneebus H., Perera G.K., Chapman A., Smith C.H., Di Meglio P., and Nestle F.O.. 2014. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Invest. Dermatol. 134:984–991. 10.1038/jid.2013.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers W.H., Muys L., Erp P.E., de Jong E.M., and van de Kerkhof P.C.. 2004. Immunohistochemical differentiation between inflammatory linear verrucous epidermal nevus (ILVEN) and psoriasis. Eur. J. Dermatol. 14:216–220. [PubMed] [Google Scholar]
- Vitiello M., Tosti A., Abuchar A., Zaiac M., and Kerdel F.A.. 2013. Ustekinumab for the treatment of nail psoriasis in heavily treated psoriatic patients. Int. J. Dermatol. 52:358–362. 10.1111/j.1365-4632.2011.05320.x [DOI] [PubMed] [Google Scholar]
- Yakimchuk K., Roura-Mir C., Magalhaes K.G., de Jong A., Kasmar A.G., Granter S.R., Budd R., Steere A., Pena-Cruz V., Kirschning C., et al. . 2011. Borrelia burgdorferi infection regulates CD1 expression in human cells and tissues via IL1-β. Eur. J. Immunol. 41:694–705. 10.1002/eji.201040808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc D.M., Elsliger M.A., Teyton L., and Wilson I.A.. 2003. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat. Immunol. 4:808–815. 10.1038/ni948 [DOI] [PubMed] [Google Scholar]
- Zajonc D.M., Crispin M.D., Bowden T.A., Young D.C., Cheng T.Y., Hu J., Costello C.E., Rudd P.M., Dwek R.A., Miller M.J., et al. . 2005. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 22:209–219. 10.1016/j.immuni.2004.12.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.