Abstract
Guidelines for the diagnosis and monitoring of hypertension were historically based on in-office blood pressure measurements. However, the United States Preventive Services Task Force recently expanded their recommendations on screening for hypertension to include out-of-office blood pressure measurements to confirm the diagnosis of hypertension. Out-of-office blood pressure monitoring, including ambulatory blood pressure monitoring and home blood pressure monitoring, are important tools in distinguishing between normotension, masked hypertension, white-coat hypertension, and sustained (including uncontrolled or drug-resistant) hypertension. Compared to in-office readings, out-of-office blood pressures are a greater predictor of renal and cardiac morbidity and mortality. There are multiple barriers to the implementation of out-of-office blood pressure monitoring which need to be overcome in order to promote more widespread use of these modalities.
Keywords: white-coat hypertension, masked hypertension, hypertension screening, ambulatory blood pressure monitoring, home blood pressure monitoring
Introduction
Hypertension is the most common diagnosis made in adults by primary care practitioners, occurring in twenty to thirty percent of the United States population (1). Hypertension is widely considered to be a principal risk factor in the development of multiple seriously morbidities including stroke, cardiovascular disease, congestive heart failure, and chronic kidney disease (2–5). High quality randomized controlled trials consistently demonstrate a marked reduction in adverse renal and cardiovascular outcomes in hypertensive patients who undergo treatment for their hypertension (2–4). Furthermore, there is minimal measurable harm associated with screening for hypertension (6–11). As a result, since 2003, the United States Preventive Services Task Force (USPSTF) has strongly recommended screening for hypertension for in adults age 18 and older (Grade A recommendation) (6,7).
In the United States, screening and monitoring of hypertension typically occurs in the healthcare setting based on preexisting recommendations (6,7). However, in October 2015, the USPSTF released its first addendum to the original recommendations on screening for hypertension in adults (12). In the updated recommendations, the USPSTF indicated that blood pressure measurements should be obtained outside of the clinical setting in order to confirm the diagnosis of hypertension and before starting treatment (12).
While out-of-office blood pressure monitoring has been widely endorsed in European guidelines (13,14), the new USPSTF recommendations represent the first time that out-of-office blood pressure monitoring has been endorsed in the United States. Previous screening recommendations and practice guidelines for hypertension diagnosis and management were largely developed using office-based blood pressure measurements (6,7,15,16). This is likely due to a combination of the existing high-quality study data, economic considerations, and relative ease of execution. Nonetheless, repeated measures of blood pressure, whether in or out of the office, provide much greater predictive value for the diagnosis of hypertension than any single measurement (17). Out-of-office blood pressure measurement modalities, including home blood pressure monitoring and ambulatory blood pressure monitoring, are superior to office-based measurements for prognostication of renal and cardiovascular outcomes (18–21).
There are a number of barriers to the wide implementation of home blood pressure monitoring and ambulatory blood pressure monitoring, including limited access, the need for patient-level quality assurance, and insufficient reimbursement. However, these out-of-office modalities exist as an important complement to in-office measurements, and can provide more accurate assessment of blood pressures for the initial diagnosis of hypertension in addition to ongoing monitoring of therapeutic effectiveness.
In-Office vs. Out-of-Office Measurements
The majority of guidelines regarding the initiation of treatment for and ongoing management of hypertension are based on studies using in-office blood pressure readings (15,16). However, in real world practice, restricting screening for hypertension to in-office blood pressure measurement poses the risk of under- or over-diagnosing hypertension, and missing isolated nocturnal hypertension. Repeated measures of blood pressure have significantly greater positive predictive value for diagnosing hypertension than any single measurement (17). Nonetheless, even repeated in-office blood pressure measurements are often weakly correlated with out-of-office blood pressures (22). Furthermore, in-office blood pressures are an inferior predictor of long-term cardiovascular outcomes compared to out-of-office measurements (18,19,23). Masked hypertension, white-coat effect, and isolated nocturnal hypertension are major contributing factors to the discrepancies between in-office and out-of-office blood pressures as well as their prognostic values.
Masked Hypertension
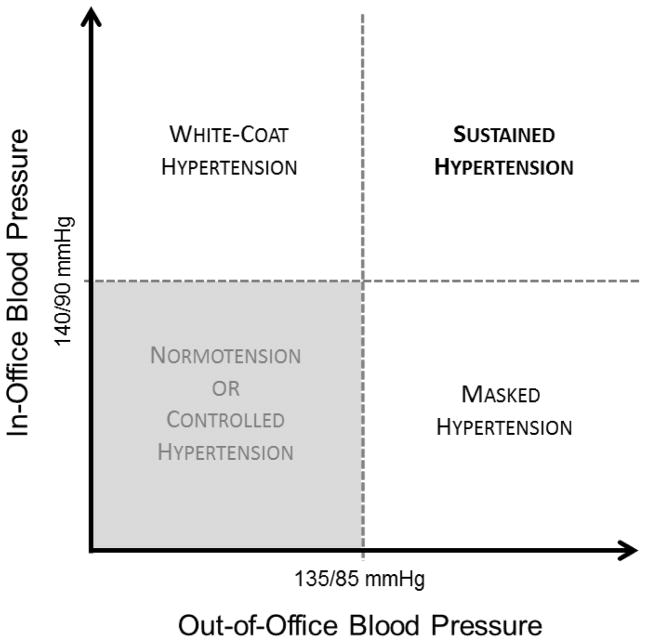
Masked hypertension is defined as normal in-office blood pressure in the setting of elevated ambulatory or home blood pressure (Figure 1) (24). In population-based studies, masked hypertension occurs in up to 30% of individuals with normal in-office blood pressure readings (25). Previous meta-analyses have demonstrated up to a 2-fold increased hazards of cardiovascular events and mortality in patients with masked hypertension (confirmed by either ambulatory blood pressure monitoring or home blood pressures) compared to patients who are truly normotensive (26,27). More recent studies corroborate these findings. A 2014 retrospective analysis of 6458 patients across five countries using data from the International Database of Home Blood Pressure demonstrated a significantly increased hazards of adverse cardiovascular events (defined as cardiovascular mortality, nonfatal myocardial infarction, surgical and percutaneous coronary revascularization, heart failure, pacemaker implantation, and stroke) in patients with untreated masked hypertension compared to untreated patients who were normotensive (adjusted hazard ratio [HR] 1.55; 95% confidence interval [CI] 1.12–2.14) (28). The study also demonstrated a significantly increased hazards of adverse cardiovascular events in patients with masked hypertension who were on antihypertensive treatment compared to treated patients with normal achieved in-office and out-of-office blood pressure (adjusted HR 1.76; 95% CI 1.23–2.53) (28).
Figure 1.
Hypertension diagnostic classifications based on in-office versus out-of-office measurements.
In a 2015 analysis of 972 patients enrolled in the Jackson Heart Study, a population-based cohort of African American patients living in the southern United States, 34.4% of patients with normal in-office blood pressures had an elevated 24-hour ambulatory blood pressure value consistent with masked hypertension (29). Patients with masked hypertension had more severe signs of target organ damage than patients with who were normotensive, as demonstrated by higher common carotid artery intima-media thickness, left ventricular mass index, and urinary albumin-to-creatinine ratio. The findings of more severe target organ damage were similar between patients with masked hypertension and patients with uncontrolled hypertension (i.e. sustained elevated blood pressure both in and out of the office) (29).
Similarly, in a 2015 study of 3027 patients from the Dallas Heart Study, a multiethnic, probability-based cohort, 17.8% of patients had masked hypertension (30). Compared to normotensive patients, patients with masked hypertension had significantly increased signs of target organ damage, including increased aortic pulse wave velocity, cystatin C, and urinary albumin-to-creatinine ratio. Patients with masked hypertension also had a significantly higher hazard for cardiovascular events (adjusted HR 2.03, 95% CI 1.36- 3.03) (30).
Of note, masked hypertension is significantly associated with increased risk for development and progression of chronic kidney disease (31,32). In a study of 1492 patients enrolled in the Chronic Renal Insufficiency Cohort, an ongoing multicenter observational study of patients with an estimated glomerular filtration rate between 20 and 70 mL/min/1.73m2, 27.8% of patients had masked hypertension based on 24-hour ambulatory blood pressure monitoring. Among these patients, masked hypertension was independently associated with more severe renal and cardiovascular target organ damage, including lower estimated glomerular filtration rate, higher degree of proteinuria, greater left ventricular mass, and worsening arterial stiffness (33).
White-Coat Effect
White-coat hypertension is defined as elevated in-office blood pressure in the setting of normal out-of-office blood pressure in patients not yet undergoing treatment for hypertension. White-coat effect occurs when blood pressure is elevated in the healthcare setting in patients who are already undergoing treatment for hypertension whereas ambulatory or home readings are not elevated (Figure 1) (24). The reported prevalence of white-coat hypertension varies widely and ranges from 5% to 65% in various studies of untreated patients (11). The results of existing literature are mixed with regard to long-term cardiovascular risk associated with white-coat hypertension, particularly among patients with untreated white-coat hypertension.
In the 2015 analysis of 3027 patients from the Dallas Heart Study, described above, 3.3% of patients had white-coat hypertension. Compared to normotensive patients, patients with white-coat hypertension had a significantly increased rate of target organ damage (i.e. aortic pulse wave velocity, cystatin C, and urinary albumin-to-creatinine ratio) as well as a significantly increased hazard of cardiovascular events (adjusted HR 2.09, 95% CI 1.05–4.15) (30). However, in 2012, analyses from the Finn-Home Study, a population-based cohort of 2046 patients in Finland, demonstrated no significant increase in cardiovascular events (unadjusted HR 1.18, p=0.5) and mortality (unadjusted HR 1.23, p=0.5) among subjects with white-coat hypertension compared to normotensive subjects (23).
The 2014 analysis of 6458 patients from the International Database of Home Blood Pressure, described above, demonstrated a significantly increased hazard of adverse cardiovascular events and mortality among untreated patients with white-coat hypertension compared to untreated normotensive patients (adjusted HR 1.42, 95% CI 1.06–1.91) (28). However, there was no increased hazard of adverse cardiovascular events or mortality among patients on antihypertensive medication who had white-coat effect compared to treated patients with controlled hypertension (adjusted HR 1.16, 95% CI 0.79–1.72) (28). In 2012, a meta-analysis of data from 7295 elderly patients with systolic hypertension in the same cohort demonstrated no significant difference in hazard of fatal and nonfatal cardiovascular events in untreated patients with white-coat hypertension compared to untreated normotensive patients (adjusted HR 1.17, 95% CI 0.87–1.57). Similar to the subsequent findings in this cohort, patients with treated white-coat hypertension also had no increased hazard of fatal and nonfatal cardiovascular events compared to patients with treated, controlled hypertension (adjusted HR 1.10, 95% CI: 0.79–1.53).
As a result of these conflicting results, there is no existing consensus about whether white-coat hypertension is a benign finding or a marker of future cardiovascular risk. While the effects of untreated white-coat hypertension are unclear, patients with treated hypertension who have white-coat effect seem to be at similar cardiovascular risk to patients with treated, controlled hypertension.
Ambulatory Blood Pressure Monitoring
Ambulatory blood pressure measurement is more highly prognostic of chronic kidney disease, adverse cardiovascular events, and mortality than in-office blood pressure measurement (Table 1) (20,23,34). Ambulatory blood pressure monitoring provides automated blood pressure measurements in regular intervals over a 24 hour period, comprising a complete cycle of wakefulness and sleep. A normal daytime mean ambulatory blood pressure is considered to be <135/85 mmHg (35,36).
Table 1.
Advantages of ambulatory blood pressure monitoring and home blood pressure monitoring over in-office blood pressure measurement
| Ambulatory Blood Pressure Monitoring | Home Blood Pressure Monitoring |
|---|---|
| Practical Advantages | Practical Advantages |
| Provides repeated measures of blood pressures over 24-hours | Provides repeated measures of daytime blood pressures longitudinally |
| Captures the pattern of circadian blood pressure over a 24-hour period | Captures changes in blood pressures associated with changes in therapy over time |
| Diagnostic Advantages | Diagnostic Advantages |
| Identifies patients with white-coat effect, potentially preventing over-treatment | Identifies patients with white-coat effect, potentially preventing over-treatment |
| Identifies patients with masked hypertension, potentially preventing under-treatment | Identifies patients with masked hypertension, potentially preventing under-treatment |
| Prognostic Advantages | Prognostic Advantages |
| Better prognostication (than office BP) for chronic kidney disease, cardiovascular disease, and mortality | Better prognostication (than office BP) for chronic kidney disease, cardiovascular disease, and mortality |
| Circadian variations (non-dipping, reverse dipping, and augmented morning surge) are independently associated with increased risk of development and progression of chronic kidney disease, cardiovascular disease, stroke, and mortality | Day-to-day variability may be associated with increased risk of cardiovascular disease and mortality |
In performing ambulatory blood pressure monitoring, patients are instructed to place the cuff on their non-dominant arm, engage in their usual daily activities, and avoid strenuous exercise (37). Blood pressure is typically measured over a 24-hour period every 15–30 minutes while awake and every 30–60 minutes during sleep (38). Patients are also instructed to keep a diary documenting time to bed, time waking up, medications taken, periods of stress, and any other events of significance. Although there is no existing gold standard for adequacy of a 24-hour ambulatory blood pressure assessment, testing is typically considered adequate if there are ≥14 daytime and ≥6 nighttime readings (39).
Most patients demonstrate circadian variations in ambulatory blood pressure readings, with at least a 10% lowering of blood pressure during sleep known as “nocturnal dipping” (34). The absence of nocturnal dipping has been associated with increased risk of stroke and cardiovascular mortality, independent of degree of blood pressure control (40). Patients with chronic kidney disease, obstructive sleep apnea, and obesity have blunted amplitudes of circardian variation, and are significantly more likely to be non-dippers, which may be linked to increased risk of progression to end stage renal disease, stroke, and mortality (40–42). Chronic kidney disease is also associated with an increased likelihood of paradoxical rise in blood pressure during sleep, known as “reverse dipping.” The prevalence of reverse dipping increases as renal function worsens, occurring in up to 35% of patients with advanced chronic kidney disease (43). The presence of reverse dipping is associated with increased risk of stroke among patients with chronic kidney disease (44).
Ambulatory blood pressure monitoring also captures normal circadian increases in blood pressure from nighttime to early morning, known as the morning surge. Augmented morning surge observed on ambulatory blood pressure monitoring may be independently associated with an increased risk of chronic kidney disease, cardiovascular disease, and stroke (45–47). High degree of blood pressure variability across 24-hour ambulatory blood pressure monitoring (measured as the standard deviation of BP during ABPM) may also be associated with increased risk of cardiovascular disease (48).
Although ambulatory blood pressure monitoring provides superior precision and prognostication compared to in-office readings, it is infrequently used in practice due to poor reimbursement and limited access (Table 2). Ambulatory blood pressure monitoring is typically only available in hypertension subspecialty clinics. Insurance companies usually only reimburse for ambulatory blood pressure monitoring for white-coat hypertension, and the reimbursement rates are poorly balanced with the degree of clinician demand and overhead costs (49). Additionally, while it is often well-tolerated, some patients are unable to wear the ambulatory blood pressure monitoring for the full 24-hour period due to sleep disruption.
Table 2.
Barriers associated with out-of-office blood pressure monitoring by modality
| Ambulatory Blood Pressure Monitoring | Home Blood Pressure Monitoring |
|---|---|
| Physician-level barriers | Physician-level barriers |
| Limited reimbursement | No Reimbursement |
| Time-consuming interpretation of results | Time-consuming patient education |
| Formal guidelines on interpretation and implementation not available | |
| Device cost | |
| Device validation | |
| No standard for assessing quality of measurements | |
| Patient-level barriers | Patient-level barriers |
| Limited access | Longitudinally time-consuming |
| Often only available in hypertension clinics | Device cost |
| Poor tolerance | Device validation |
| Temporary intrusion into day-to-day activities | Requires literacy |
| Sleep disturbance | Need for quality assessment and calibration by provider |
Home Blood Pressure Monitoring
Home blood pressure monitoring has become an increasingly acceptable alternative to ambulatory blood pressure monitoring. As opposed to 24-hour ambulatory blood pressure monitoring, home blood pressure monitoring is more widely available, readily interpretable by most providers, and can provide information on resting blood pressure over long periods of time. It gives patients and physicians the opportunity to readily confirm the diagnosis of hypertension and provide ongoing monitoring of adequate therapy. An elevated home blood pressure is considered to be a mean blood pressure value of ≥135/85 across a minimum of 3 days of home blood pressure monitoring (13,35).
Compared to ambulatory blood pressure monitoring, home blood pressure monitoring is limited by its inability to provide data on nocturnal dipping and other variations in circadian blood pressure rhythm. However, as opposed to limiting screening and monitoring to in-office measurements, home blood pressure monitoring allows for accurate distinction of white-coat hypertension and masked hypertension (50). Home blood pressure monitoring provides patients with the opportunity to confirm elevated blood pressure readings with repeated measures of blood pressures, potentially avoiding hypotensive events due to over-treatment. Furthermore, home blood pressure measurement provides notably greater prognostication for chronic kidney disease, cardiovascular disease, and mortality compared to in-office measurements (21,23). Additionally, although detection of circadian variations is limited in home blood pressure monitoring compared to ambulatory blood pressure monitoring, increased variability in daily home blood pressure readings may be associated with increased risk of cardiovascular disease and mortality (51).
Importance of Measurement Accuracy
Adequate blood pressure measurement, whether in-office or out-of-office, is critical to the accurate diagnosis of hypertension and monitoring of ongoing therapy. Many practitioners are skeptical of the accuracy of home blood pressure monitoring. In part, this is supported by the fact that patients often do not read the instructions, and may use the monitors incorrectly. It is important to provide clear instructions to patients on selecting an appropriate home monitor, and to have patients bring their home monitors into the clinic in order to assess for adequate technique and calibration of the machine.
Upper arm blood pressure readings are more reliable and better-validated than wrist or finger measurements. Additionally, correct cuff-size is crucial, particularly given the high prevalence of obesity in the population. The bladder of the cuff should encircle at least 80% of the arm circumference, taking into account that standard BP cuffs overestimate BP in obese patients (52). Patients should be instructed to sit a quiet room for at least 5 minutes prior to checking their blood pressure, to ensure that they have an empty bladder, and to place their feet uncrossed, flat on the floor (53). Patients should perform their blood pressure measurements two times in the morning and two times in the evening for five to seven consecutive days. When practitioners are calculating the mean blood pressure, the first day of readings should be disregarded (53).
In-office and home measurements may be performed using either automated or manual monitors, as long as they are appropriately calibrated. Most home blood pressure monitors use oscillometric technology, as opposed to the aneroid devices requiring a stethoscope that are often used in the clinical setting. Oscillometric monitors measure the mean arterial pressure, and use internal proprietary algorithms to estimate the systolic and diastolic blood pressure values (54). Based on variations in these proprietary internal algorithms, not all monitors generate the same blood pressure values in the same patient (55). Furthermore, many monitors are not individually validated, (54) reinforcing the importance of having patients bring their monitors into the office for assessment of both patient technique and device quality. Among those machines that are validated, oscillometric measurements are consistently reproducible and seem to provide an accurate approximation of daytime ambulatory blood pressure values (56). An updated listed of validated blood pressure monitors can be found at http://www.dableducational.org/.
Notably, the 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension introduced a Grade C recommendation advising providers to use validated automated oscillometric blood pressure devices for in-office measurements in favor of aneroid auscultatory measurement (57). The authors note that automated oscillometric devices allow for repeated blood pressure measurements to be performed in the clinic while a patient rests alone in a quiet room, in the absence of any patient-provider interaction. In addition to facilitating repeated measurements of blood pressures within a single office visit, automated devices may reduce the impact of many potential confounders to obtain accurate blood pressure measurements, such as conversation during measurement and white-coat effect (58).
Drug-Resistant Hypertension
Up to 28% of treated hypertensive patients have resistant hypertension, defined as requiring a minimum of three antihypertensive medications including a diuretic in order to achieve normotension (59). Out-of-office blood pressure monitoring can be particularly useful in the verification and management of treatment-resistant hypertension. Patients with treatment-resistant hypertension often have white-coat effect (60). In a 2013 cohort study of 423 patients with elevated blood pressure in the setting of resistant hypertension diagnosed by in-office readings, only 60% of the patients had elevated blood pressures on ambulatory blood pressure monitoring (61). Taking into account the high rate of white-coat effect among resistant hypertensive patients, these patients are often adequately controlled in the out-of-office setting, and escalation in therapy can put them at risk for hypotension and other adverse medication effects. Furthermore, much like the general population of hypertensive patients, ambulatory blood pressure monitoring has greater prognostic value than in-office measurements patients with true resistant hypertension with regard to cardiovascular events and mortality (62,63).
Additionally, poor adherence is a common issue, and may result in misperceived resistance to medication, known as pseudoresistance (59,61). As a result, patients may be prescribed a greater number of medications, at higher doses than indicated, increasing the risk of hypotension and other adverse effects when they do take their medications. Pill counting and monitoring of prescription renewals may provide clues into the occurrence of this effect, but are suboptimal options in the usual treatment setting. Ambulatory blood pressure monitoring and home blood pressure monitoring can be particularly helpful in the identification of these patients (64).
Conclusion
The introduction of out-of-office blood pressure monitoring into Unites States practice is an important complement to preexisting in-office hypertension screening measures. When using out-of-office blood pressure values to guide therapy, practitioners should keep in mind that most existing treatment guidelines were based on studies using in-office blood pressure readings. Nonetheless, with the rapid advancement of health-related mobile technology, the use of home-based monitoring is very likely become increasingly common practice. Masked and white-coat hypertension have a high prevalence in the general population and often result in the under and over-treatment of hypertension, respectively. Masked hypertension, in particular, has the potential to pose grave long-term cardiovascular risk if left untreated. Independent of the presence of masked hypertension or white-coat effect, out-of-office blood pressure monitoring provides superior prognostication of long-term renal and cardiac risk compared to in-office blood pressures. Additionally, ambulatory blood pressure monitoring and home blood pressure monitoring can have an important role in the verification and management of drug-resistant and pseudoresistant hypertensive patients. Improved physician familiarity, patient-centered quality assurance, and fairer insurance reimbursement would help to overcome some of the previous barriers currently barring more widespread use of out-of-office blood pressure monitoring.
Footnotes
Conflict of Interest
Jordana B. Cohen and Debbie L. Cohen declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Lv J, Neal B, Ehteshami P, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Med. 2012;9(8):e1001293. doi: 10.1371/journal.pmed.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185(11):949–957. doi: 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 2. Effects at different baseline and achieved blood pressure levels--overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2296–2304. doi: 10.1097/HJH.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 5.USRDS. 2014 Annual Data Report. 2014 http://www.usrds.org/2014/download/V2_Ch_01_ESRD_Incidence_Prevalence_14.pdf.
- 6.Sheridan S, Pignone M, Donahue K. Screening for high blood pressure: a review of the evidence for the U.S. Preventive Services Task Force. Am J Prev Med. 2003;25(2):151–158. doi: 10.1016/s0749-3797(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 7.Wolff T, Miller T. Evidence for the reaffirmation of the U. S. preventive services task force recommendation on screening for high blood pressure. Annals of Internal Medicine. 2007;147(11):787–U747. doi: 10.7326/0003-4819-147-11-200712040-00010. [DOI] [PubMed] [Google Scholar]
- 8.Spruill TM, Feltheimer SD, Harlapur M, et al. Are there consequences of labeling patients with prehypertension? An experimental study of effects on blood pressure and quality of life. J Psychosom Res. 2013;74(5):433–438. doi: 10.1016/j.jpsychores.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viera AJ, Lingley K, Esserman D. Effects of labeling patients as prehypertensive. J Am Board Fam Med. 2010;23(5):571–583. doi: 10.3122/jabfm.2010.05.100047. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DW, Haynes RB, Sackett DL, Gibson ES. Longterm follow-up of absenteeism among working men following the detection and treatment of their hypertension. Clin Invest Med. 1981;4(3–4):173–177. [PubMed] [Google Scholar]
- 11.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(3):192–204. doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 12••.Siu AL US Preventitive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. doi: 10.7326/M15-2223. These recommendations, recently released by the US Preventitive Services Task Force, introduce an addendum to the previous recommendations for screening of all adults age 18 years or older. The recommendations now advise obtaining out-of-office blood pressure measurements for the confirmation of hypertension before starting treatment. [DOI] [PubMed] [Google Scholar]
- 13.Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24(12):779–785. doi: 10.1038/jhh.2010.54. [DOI] [PubMed] [Google Scholar]
- 14.Bloch MJ, Basile JN. UK guidelines call for routine 24-hour ambulatory blood pressure monitoring in all patients to make the diagnosis of hypertension--not ready for prime time in the United States. J Clin Hypertens (Greenwich) 2011;13(12):871–872. doi: 10.1111/j.1751-7176.2011.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 17.Handler J, Zhao Y, Egan BM. Impact of the number of blood pressure measurements on blood pressure classification in US adults: NHANES 1999–2008. J Clin Hypertens (Greenwich) 2012;14(11):751–759. doi: 10.1111/jch.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–515. doi: 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 19.Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Jama-Journal of the American Medical Association. 1999;282(6):539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(7):1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol. 2006;26(5):503–510. doi: 10.1159/000097366. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 23.Hanninen MR, Niiranen TJ, Puukka PJ, Johansson J, Jula AM. Prognostic significance of masked and white-coat hypertension in the general population: the Finn-Home Study. J Hypertens. 2012;30(4):705–712. doi: 10.1097/HJH.0b013e328350a69b. [DOI] [PubMed] [Google Scholar]
- 24.Pickering TG, Hall JE, Appel LJ, Falnker BE, Graves JW, Hill MN. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 25.Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28(9):521–528. doi: 10.1038/jhh.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25(11):2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 27.Hansen TW, Kikuya M, Thijs L, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25(8):1554–1564. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 28•.Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63(4):675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741. This large retrospective study of patients across five countries demonstrated a significantly increased hazards of adverse cardiovascular events in patients with untreated masked hypertension compared to untreated patients who were normotensive as well as in patients with treated masked hypertension compared to treated patients with controlled hyperteision. The study also demonstrated a significantly increased hazard of adverse cardiovascular events and mortality among untreated patients with white-coat hypertension compared to untreated normotensive patients, but no increased hazard of adverse cardiovascular events or mortality among patients on antihypertensive medication who had white-coat effect compared to treated patients with controlled hypertension. [DOI] [PubMed] [Google Scholar]
- 29••.Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA. Prevalence, Determinants, and Clinical Significance of Masked Hypertension in a Population-Based Sample of African Americans: The Jackson Heart Study. Am J Hypertens. 2015;28(7):900–908. doi: 10.1093/ajh/hpu241. This ipopulation-based study of African American patients living in the southern United States demonstrated a prevelance of masked hypertension of 34.4% among study participants. Individuals with masked hypertension had significantly more advanced signs of target organ (i.e. renal and cardiovascular) damage than patients with sustained normotension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Tientcheu D, Ayers C, Das SR, et al. Target Organ Complications and Cardiovascular Events Associated With Masked Hypertension and White-Coat Hypertension: Analysis From the Dallas Heart Study. J Am Coll Cardiol. 2015;66(20):2159–2169. doi: 10.1016/j.jacc.2015.09.007. This multiethnic, probability-based cohort of patients being followed for development of cardiovascular disease demonstrated a prevalence of 17.8% of masked hypertension and of 3.3% of white-coat hypertension among study participants. Patients with either white-coat hypertension or masked hypretension had a significantly higher rate of target organ damage as well as a greater hazard for cardiovascular events compared to normotensive patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanno A, Metoki H, Kikuya M, et al. Usefulness of assessing masked and white-coat hypertension by ambulatory blood pressure monitoring for determining prevalent risk of chronic kidney disease: the Ohasama study. Hypertens Res. 2010;33(11):1192–1198. doi: 10.1038/hr.2010.139. [DOI] [PubMed] [Google Scholar]
- 32.Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20–27. doi: 10.1161/HYPERTENSIONAHA.108.115154. [DOI] [PubMed] [Google Scholar]
- 33••.Drawz PE, Alper AB, Anderson AH, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol. 2016;11(4):642–652. doi: 10.2215/CJN.08530815. This analysis of patients with chronic kidney disease enrolled in the Chronic Renal Insufficiency Cohort demonstrated a 27.8% prevalence of masked hypertension among study participants. Patients with masked hypertension had significantly more severe renal and cardiovascular target organ damage compared to normotensive subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering TG, Harshfield GA, Kleinert HD, Blank S, Laragh JH. Blood pressure during normal daily activities, sleep, and exercise. Comparison of values in normal and hypertensive subjects. JAMA. 1982;247(7):992–996. [PubMed] [Google Scholar]
- 35.Pickering TG, White WB Group ASoHW. ASH Position Paper: Home and ambulatory blood pressure monitoring. When and how to use self (home) and ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich) 2008;10(11):850–855. doi: 10.1111/j.1751-7176.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parati G, Stergiou G, O’Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32(7):1359–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 37.De la Sierra A. Advantages of Ambulatory Blood Pressure Monitoring in Assessing the Efficacy of Antihypertensive Therapy. Cardiol Ther. 2015;4(Suppl 1):5–17. doi: 10.1007/s40119-015-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1–9. doi: 10.1161/HYPERTENSIONAHA.107.189011. [DOI] [PubMed] [Google Scholar]
- 39.Krause T, Lovibond K, Caulfield M, McCormack T, Williams B Guideline Development G. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891. [DOI] [PubMed] [Google Scholar]
- 40.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997;12(11):2301–2307. doi: 10.1093/ndt/12.11.2301. [DOI] [PubMed] [Google Scholar]
- 42.Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension. 2005;45(4):602–607. doi: 10.1161/01.HYP.0000158261.86674.8e. [DOI] [PubMed] [Google Scholar]
- 43.Mojon A, Ayala DE, Pineiro L, et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30(1–2):145–158. doi: 10.3109/07420528.2012.703083. [DOI] [PubMed] [Google Scholar]
- 44.Yano Y, Bakris GL, Matsushita K, Hoshide S, Shimoda K, Kario K. Both Chronic Kidney Disease and Nocturnal Blood Pressure Associate with Strokes in the Elderly. American Journal of Nephrology. 2013;38(3):195–203. doi: 10.1159/000354232. [DOI] [PubMed] [Google Scholar]
- 45•.Turak O, Afsar B, Siriopol D, et al. Morning Blood Pressure Surge as a Predictor of Development of Chronic Kidney Disease. J Clin Hypertens (Greenwich) 2016;18(5):444–448. doi: 10.1111/jch.12707. This prospective study demonstrated that augmented morning blood pressure surge was associated with a significantly increased rate of incident chronic kidney disease among patients with essential hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierdomenico SD, Pierdomenico AM, Di Tommaso R, et al. Morning Blood Pressure Surge, Dipping, and Risk of Coronary Events in Elderly Treated Hypertensive Patients. Am J Hypertens. 2016;29(1):39–45. doi: 10.1093/ajh/hpv074. [DOI] [PubMed] [Google Scholar]
- 47.Pierdomenico SD, Pierdomenico AM, Cuccurullo F. Morning blood pressure surge, dipping, and risk of ischemic stroke in elderly patients treated for hypertension. Am J Hypertens. 2014;27(4):564–570. doi: 10.1093/ajh/hpt170. [DOI] [PubMed] [Google Scholar]
- 48.Hansen TW, Thijs L, Li Y, et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55(4):1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 49.Kent ST, Shimbo D, Huang L, et al. Rates, amounts, and determinants of ambulatory blood pressure monitoring claim reimbursements among Medicare beneficiaries. J Am Soc Hypertens. 2014;8(12):898–908. doi: 10.1016/j.jash.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen MJ, Khawandi W, Agarwal R. Home blood pressure monitoring in CKD. Am J Kidney Dis. 2005;45(6):994–1001. doi: 10.1053/j.ajkd.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Stergiou GS, Ntineri A, Kollias A, Ohkubo T, Imai Y, Parati G. Blood pressure variability assessed by home measurements: a systematic review. Hypertens Res. 2014;37(6):565–572. doi: 10.1038/hr.2014.2. [DOI] [PubMed] [Google Scholar]
- 52.Fonseca-Reyes S, de Alba-Garcia JG, Parra-Carrillo JZ, Paczka-Zapata JA. Effect of standard cuff on blood pressure readings in patients with obese arms. How frequent are arms of a ‘large circumference’? Blood Press Monit. 2003;8(3):101–106. doi: 10.1097/00126097-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691–700. doi: 10.7326/M15-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alpert BS, Quinn D, Gallick D. Oscillometric blood pressure: a review for clinicians. J Am Soc Hypertens. 2014;8(12):930–938. doi: 10.1016/j.jash.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Kallem RR, Meyers KE, Sawinski DL, Townsend RR. A comparison of two ambulatory blood pressure monitors worn at the same time. J Clin Hypertens (Greenwich) 2013;15(5):321–325. doi: 10.1111/jch.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. British Medical Journal. 2011:342. doi: 10.1136/bmj.d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32(5):569–588. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 58.Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. Journal of Hypertension. 2009;27(2):280–286. doi: 10.1097/HJH.0b013e32831b9e6b. [DOI] [PubMed] [Google Scholar]
- 59.Lazaridis AA, Sarafidis PA, Ruilope LM. Ambulatory Blood Pressure Monitoring in the Diagnosis, Prognosis, and Management of Resistant Hypertension: Still a Matter of our Resistance? Curr Hypertens Rep. 2015;17(10):78. doi: 10.1007/s11906-015-0590-9. [DOI] [PubMed] [Google Scholar]
- 60.Rios MT, Dominguez-Sardina M, Ayala DE, et al. Prevalence and clinical characteristics of isolated-office and true resistant hypertension determined by ambulatory blood pressure monitoring. Chronobiol Int. 2013;30(1–2):207–220. doi: 10.3109/07420528.2012.701135. [DOI] [PubMed] [Google Scholar]
- 61.Brambilla G, Bombelli M, Seravalle G, et al. Prevalence and clinical characteristics of patients with true resistant hypertension in central and Eastern Europe: data from the BP-CARE study. J Hypertens. 2013;31(10):2018–2024. doi: 10.1097/HJH.0b013e328363823f. [DOI] [PubMed] [Google Scholar]
- 62.de la Sierra A, Banegas JR, Segura J, Gorostidi M, Ruilope LM Investigators CE. Ambulatory blood pressure monitoring and development of cardiovascular events in high-risk patients included in the Spanish ABPM registry: the CARDIORISC Event study. J Hypertens. 2012;30(4):713–719. doi: 10.1097/HJH.0b013e328350bb40. [DOI] [PubMed] [Google Scholar]
- 63.Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18(11):1422–1428. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 64.Burnier M, Wuerzner G. Ambulatory blood pressure and adherence monitoring: diagnosing pseudoresistant hypertension. Semin Nephrol. 2014;34(5):498–505. doi: 10.1016/j.semnephrol.2014.08.003. [DOI] [PubMed] [Google Scholar]