Abstract
Background
Recently, the radiation application research center for the atomic energy organization of Yazd (Iran) has developed a hydrogel dressing which was evaluated for quality and safety in 2008. Its efficacy for assisting in the wound healing process was approved for animal use, and its use has proven to be more effective than a related Syrian material.
Objectives
We have already confirmed the safety and efficacy of Irgel use on mice (1, 2), so this study was conducted in order to further evaluate its effectiveness on human burn wounds, and to compare its efficacy with MaxGel, another hydrogel. A randomized clinical trial study was conducted to compare the efficacy of hydrogel produced by the radiation application research center (Yazd Branch) with MaxGel and routine dressing on burn repair in the Yazd Burn hospital.
Materials and Methods
In this study, 90 patients with second-degree burn injuries who were admitted to the Yazd Burn hospital were randomly divided into three equal groups. In the negative control group, the wounds were covered with sterile vaseline gauze followed by double sterile dry gauze and ultimately bandaged. In the test group, the wounds were covered by an Iranian hydrogel sheet (Irgel) instead of vaseline gauze, while in the positive control group, the wounds were covered by MaxGel instead of Irgel. At each visit (every other day), each dressing was renewed by its respective method and the wound area, pain score, and body temperature were recorded. At the beginning and at the end of the first and second week, five milliliters of venous blood were taken from all patients to evaluate hematologic parameters such as peripheral blood cell count, liver function, blood urea nitrogen, and creatinine.
Results
Before the intervention, the extent of the burns and pain sensations were quite similar among the different groups, but at the second week, the burn areas and pain scores for the Irgel group were significantly less than those of the normal control and the positive control groups (P < 0.05).
Conclusions
Based on our findings, both gels assist in the process of burn wound healing and pain reduction more effectively as compared with routine dressing. However, Irgel had better effects on wound healing and pain relief than MaxGel, which indicates a better quality of Irgel for this particular kind of treatment.
Keywords: Hydrogel, Burn, Iranian Gel, MaxGel
1. Background
Depending on their depth, ulcers (or wounds that fail to heal) are classified into four stages, from one to four depending on the level of depth (one being the lowest). Most ulcers are in stage one or two, and in these cases, the bulk of the tissue in the dermis can be repaired and reconstruction is the task of the fibroblast cells, which replace the normal tissue with new scar tissue (3).
Wound healing is a complex but regular process associated with cell migration, proliferation, adhesion, and phenotype differentiation (4). In events such as burning, wound healing is of particular importance in order to avoid the development of more complex ulcers, and in certain conditions such as diabetes, tissue repair may be delayed, thus leading to infection (5). Extensive research on the role of accelerating wound healing, including factors such as angiotensin (6), growth (7), antioxidants, and vitamins (8, 9), have been conducted on wounds of various origins. In addition, the use of infrared (10), electromagnetic (11), or electrical stimulation (12, 13), in addition to lasers (12) and a variety of dressings, plasters, and ointments on wound healing have been considered (14). The main issue in wound care involves appropriate management in order to assure that the wound can heal in the shortest possible time and with minimal side effects.
In such cases, there are two important issues that must be addressed. First, it is important to keep the wound clean and prevent the entry of potentially harmful environmental materials, and second, it is necessary to preserve the remaining healthy tissue and prevent newly formed tissue damage (15). Many substances which are used for cleaning wounds, such as Betadine, hydrogen peroxide, and alcohol, usually cause damage to the skin cells and interfere with the activity of the fibroblast cells, which leads to a delay in wound healing. For instance, Lineaweaver et al. compared four different methods of wound irrigation, including Betadine, hydrogen peroxide, acetic acid, and sodium hydrochloride, and declared their toxic effect on fibroblasts. They also revealed that Betadine causes a delay of four to eight days in the wound healing of rats, as well as a decrease in the tensile strength of the repaired tissue (16). Rodeheaver et al. also showed that Betadine in comparison to saline cannot reduce the chance of wound infection (17). Hydrogen peroxide is known to release oxygen via activating the catalase, and it can therefore reduce the level of bacteria in wounds, but Tatnall et al. demonstrated that this substance is toxic to keratinocytes and produces microscopic gaps within the tissues, ultimately leading to a delay in wound healing (18). Furthermore, the lethal effect of hydrogen peroxide from oxygen embolism in high-pressure wound dressings has been reported (19, 20).
The main function of dressings is to provide the proper environment for wound healing and, according to Lawrence, a good dressing should be sterile, strong, absorbent, protective, non-adhesive, nontoxic, and cost-effective (21). Dressings are of several types, each with its advantages and disadvantages. One of the most popular dressings is the traditional approach, or wet-to-dry. In this method, after washing, the wound is covered with wet gauze which is then dried spontaneously. The dried gauze dressing is removed and is replaced with a new one. The most important disadvantage of this method is that the dressing changes lead to the removal and destruction of the superficial parts of the repaired tissue and, as a result, healing is delayed (22). An alternative method is film dressing. Usually these films are made of polyurethane and they allow for the wound and its contents to be partially visible. The disadvantage here is that this type of dressing is not absorbent, it sticks to scar tissue, and it damages the epithelialized tissues which lead to the loss of the skin barrier system (22).
The use of hydrogel dressing is one of the newest methods. These types of dressings are made of polyethylene oxide or polyvinylpyrrolidone, and over 90% of the dressing is water (22). Hydrogel dressings generally have good features and provide some cold contact in the acute phase of wound healing, thus reducing pain and inflammation particularly in the dressing of burns (23, 24). They also prevent drying of the newly formed tissue in the wound, since the material does not stick to the dressing and removal and destruction of newly formed tissues can be avoided, which allows for faster (even daily) dressing changes. The main disadvantage of these dressings is due to their hydrophobic property which does not absorb water and is less useful for secretory wound dressing (25). Hydrocolloid dressing has recently been made of a gel containing hydrophilic materials such as pectin and gelatin, which absorb wound exudate and its secretory contents so that the dressing gradually becomes bulky and spongy; upon maximum absorption, its color changes, which is a reminder of the need to change the dressing (26).
Several companies have produced and marketed hydrogel dressings and, according to producers’ comments, they can accelerate wound healing in different ways (14). Recently, the radiation application research center for the atomic energy organization of Yazd (Iran) has produced a hydrogel dressing (Irgel) that was evaluated for quality and safety by Ajji et al. in 2008 (27). In support of this determination, we have also observed that there were not any pathological or hematological adverse effects of Irgel in our own animal studies (1, 2).
In 2009, a study was designed and implemented by Noorbala et al. to evaluate and compare the effectiveness of Irgel and saline on wound healing in rats. The results of this study showed that the hydrogel constructed by the Atomic Energy Organization of Yazd had positive effects on wound healing and is as effective as normal saline; furthermore, it did not cause any measurable histological and/or clinical defects in the rats’ vital organs (1). However, in 2011, the efficacy of this hydrogel on wound healing in rats was compared with a Syrian hydrogel, the former of which was demonstrated to be significantly more effective (2).
2. Objectives
Following these experimental studies, this clinical trial study was conducted to compare the efficacy of the hydrogel produced by the radiation application research center (Yazd branch) with MaxGel and routine dressing on human burn repair in the Yazd Burn hospital.
3. Materials and Methods
3.1. Study Design and Population
In this randomized clinical trial, based on the variables of similar studies at a confidence level of 95% and a test power of 80%, the appropriate sample size was determined to be 60. However, for elevating the confidence and accuracy of the study, 90 patients with second-degree burns who were admitted to Yazd Burn hospital were randomly divided into three equal groups according to the table of random numbers. The following inclusion/exclusion criteria were considered in the subject selection process:
1. The patient provided written consent after becoming familiar with the potential study results and its possible complications.
2. No more than 24 hours had passed since the burn.
3. Burns had not been tampered with or treated by local or nonscientific methods.
4. The patient was between seven and 60 years old.
5. No more than ten percent of the body’s surface area was burned.
6. The burn location had not been on the head, armpits, groin, or perineal area.
7. The patient did not suffer from underlying chronic inflammatory diseases and/or disorders of the immune system.
Furthermore, during treatment, patients who developed hypersensitive reactions, bacterial infections, or any other conditions that required special drugs, as well as those patients who did not wish to continue working on the project were excluded, but after departure, their information and data were still used in the analysis.
3.2. Ethical Considerations
Although, the efficacy and lack of toxicity of Irgel in laboratory animals has been supported by previous studies (1, 2), this study was registered on the “Iranian Registry of Clinical Trials” website and assigned the number IRCT138902123856N1. Furthermore, the university’s research ethics committee approval was obtained (94895 - 01/11/2009) and, as mentioned above, informed consent was provided by patients following the provision of sufficient and appropriate information.
3.3. Experimental Procedure
Patients enrolled in the study after providing written consent were physically examined according to the established medical plan. The demographic information of each patient, the extent and location of the burn, its etiology, and previous medical records were obtained and recorded in each patient’s medical file. Then, the same basic procedure involving injecting the analgesics (if necessary) and washing the wound with normal saline was performed on each patient. Based on a random number table, each patient was assigned to either the negative control, test, or positive control group. In the negative control group undergoing the traditional method of dressing, the wounds were covered with sterile vaseline gauze followed by double sterile dry gauze and ultimately bandaged. In the test group, the wounds were covered by an Iranian hydrogel sheet (Irgel) instead of vaseline gauze, while in the positive control group, the wounds were covered by MaxGel (Maxford Medical Technology Co., Hong Kong) instead of Irgel.
All patients were discharged with recommendations to continue treatment and change the dressing every other day in the hospital. At each visit, after removing the previous dressing, a new dressing was applied by the same method and the wound area, pain score, and body temperature were recorded. The pain intensity felt by the patient during dressing was recorded using the visual analog scale method in which a score of zero indicated absolutely no pain and a score of ten was reserved for the most intense pain that had ever been felt. The level of wound healing was calculated in terms of the primary wound area minus the area of the secondary wound divided by the initial area and multiplied by a hundred. At the beginning and at the end of the first and second week, five milliliters of venous blood were taken from all patients to evaluate hematologic parameters such as the peripheral blood cell count, liver function, blood urea nitrogen, and creatinine. All data were collected by expert nurses who were unaware of the group that each patient belonged to.
3.4. Statistical Analysis
Data were considered as mean ± SD of the burn area and the pain score in the studied groups and it was statistically analyzed by the Kruskal-Wallis test via SPSS software. P < 0.05 was considered as the level of significance. Where a significant difference between groups was identified, a binary comparison test (Mann-Whitney test) was used to determine the specific differences. The trends of the variables for the different time courses was analyzed by a repeated measures test.
4. Results
Over the course of more than two years, 82 patients completed the study. The burn area for all patients at any stage of visiting and during dressing change was measured and recorded. The mean ± SD of the burn areas for each of the studied groups are presented in Table 1.
Table 1. Comparison of Mean Burn Area in the Studied Groups During the Dressing Stagesa,b.
| Groups Time Course | MaxGel (N = 28) (M ± SD) | Conventional (N = 27) (M ± SD) | Iranian Gel (Irgel) (N = 27) (M ± SD) | P Value |
|---|---|---|---|---|
| 1st Day | 112.25 ± 95.39 | 116.84 ± 78.41 | 132.46 ± 138.52 | 0.792 |
| 3rd Day | 118.42 ± 96.43 | 133.37 ± 79.13 | 143.66 ± 128.49 | 0.820 |
| 5th Day | 105.50 ± 87.30 | 119.85 ± 67.65 | 121.55 ± 103.69 | 0.690 |
| 7th Day | 91.60 ± 83.31 | 103.40 ± 61.25 | 92.92 ± 76.74 | 0.500 |
| 9th Day | 75.32 ± 71.39 | 87.22 ± 57.62 | 65.22 ± 56.80 | 0.340 |
| 11th Day | 60.46 ± 63.84 | 69.70 ± 51.55 | 41.14 ± 44.08 | 0.070 |
| 13th Day | 45.78 ± 51.28 | 53.66 ± 43.59 | 21.51 ± 29.75A,B | 0.001 |
aP value is determined by comparing the three groups using the Kruskal-Wallis test.
bA significant difference as compared with the use of MaxGel (P = 0.046) and conventional dressing (P = 0.000), respectively, using the Mann-Whitney test.
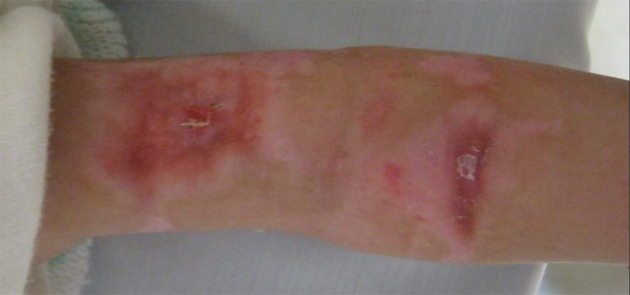
According to our data, before the intervention and during the early stages of repair, the extents of the burns were quite similar among the different groups, and there was no significant difference between the groups (P > 0.05). However, once healing began, the wound area reduced and at the second week (13th day), the burn areas for the Irgel group were significantly less than those of the normal control (P = 0.000) and positive control groups (P < 0.046). Figures 1 -3 show wound remission of the burned areas in the three groups treated with MaxGel, conventional methods, and Irgel, respectively.
Figure 1. Burned Area After Two Weeks In the MaxGel Group.
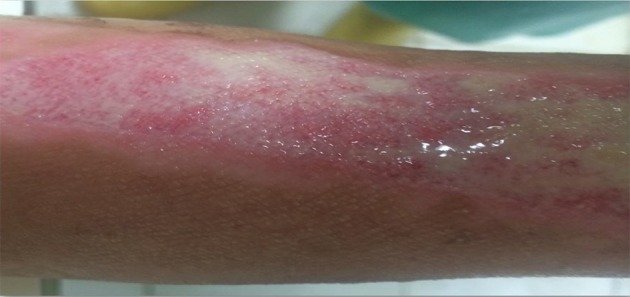
Figure 2. Burned Area After Two Weeks In the Conventional Group.
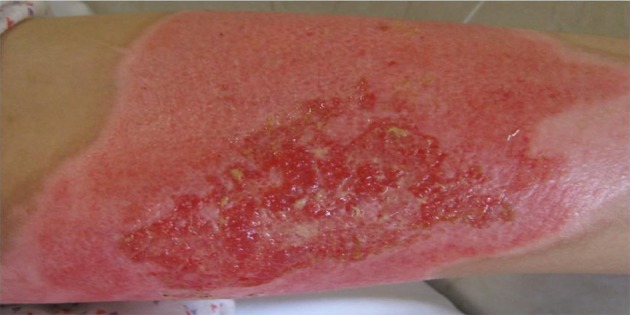
Figure 3. Burned Area After Two Weeks In the Irgel Group.
Before the intervention, the intensity of pain sensation was quite similar among the different groups, and there was no significant difference between them (P > 0.05). However, the pain scores for the Irgel group from the third session of redressing were significantly lower than the scores of the other groups (P < 0.001, Table 2). According to the Mann-Whitney test, the significant level of difference between Irgel and conventional dressing on the 5th and 13th days of intervention was P < 0.001, and between Irgel and MaxGel on the same days was P < 0.014 and P < 0.000, respectively. There was a significant difference between MaxGel and conventional dressing only on the 13th day of redressing (P < 0.002).
Table 2. Comparison of the Mean Pain Intensity In the Studied Groups During the Dressing Sessionsa,b.
| Group | MaxGel (N = 28) (M ± Sd ) | Conventional (N = 27) (M ± Sd ) | Iranian Gel (Irgel) (N = 27) (M ± Sd ) | P Value |
|---|---|---|---|---|
| 1st day | 6.25±1.84 | 6.62 ± 1.64 | 6.53 ± 1.58 | 0.614 |
| 3rd day | 5.50 ±1.79 | 5.66 ± 1.41 | 5.11 ± 1.31 | 0.270 |
| 5th day | 4.25 ± 1.57 | 4.62 ± 1.49 | 3.33 ± 1.20A, B | 0.001 |
| 7th day | 3.17 ± 1.46 | 3.48 ± 1.15 | 1.77 ± 0.80 A, B | < 0.001 |
| 9th day | 2.07 ± 1.21 | 2.55 ± 0.80 | 1.00 ± 0.73 A, B | < 0.001 |
| 11th day | 1.39 ± 1.06 | 1.77 ± 0.69 | 0.33 ± 0.55 A, B | < 0.001 |
| 13th day | 0.67 ± 0.72C | 1.18 ± 0.39 | 0.00 ± 0.00 A, B | < 0.001 |
aP value is determined by comparing the three groups using the Kruskal-Wallis test.
bAccording to the Mann-Whitney Test, A and C are indicative of a significant difference as compared with conventional dressing (P < 0.001) and MaxGel (P < 0.05), respectively, and B is indicative of a significant difference as compared with conventional dressing (P < 0.001).
To verify the possible adverse effects of the different dressings on the patients, at the beginning and at the end of the first and second week, five milliliters of venous blood were taken from the subjects for evaluating the hematologic parameters. In this case no abnormalities were detected and all parameters were in the normal range. None of the patients developed fever and/or wound infection.
5. Discussion
We have already confirmed the safety and efficacy of Irgel use on mice (1, 2), so this study was conducted in order to further evaluate its effectiveness on human burn wounds. This study showed that the rate of wound healing among the three groups was different, and that the group using hydrogel dressing from the Yazd atomic energy organization healed faster; this difference was significant at the end of the second week. The study also revealed that the mean pain intensity during dressing change declined in the groups using gel dressing from the first week, and was significantly lower than that of traditional dressing.
It should be mentioned that wound healing can occur naturally, but many environmental factors such as infection through foreign contamination and other unsuitable conditions can disrupt and delay recovery, while other factors sometimes cause earlier and faster recovery and healing. Numerous studies have been performed in connection with the effectiveness of hydrogel dressings on wound healing which indicate that there is healing acceleration and pain reduction in patients when it is used (28). Since the majority of manufacturers claim that the gel is most effective for chronic wounds, more evidence comes from studies where hydrogel dressing is used to accelerate healing of chronic wounds such as diabetic ulcers and bedsores.
Kaya et al. compared a hydrogel dressing with conventional gauze and Betadine dressings and showed that despite the fact that healing occurred faster and in a shorter time frame for the hydrogel group, the difference was not statistically significant (29). However, another study was conducted by Martineau and Shek on burn wounds on rats using a hydrogel dressing as compared with a control group that received bandages, and it was reported that in hydrogel group, 85% of the wounds recovered in six days rather than eight days in the control group (30). In Hampton’s 2004 study (31), the intensity of pain and the need for dressing changes on twenty patients using hydrogel dressing was compared with the conventional method. The results showed that pain scores were significantly reduced after using hydrogel dressing (8.65 versus. 3.75), which is quite similar to our results. The mean frequency of dressing change within a week after taking hydrogel was also significantly reduced (2.8 versus 1.3 times per week).
Goldenberg et al. showed that hydro-colloid gel dressing significantly reduces the chance of wound infection (32). They stated that among those who were given dressings with hydrocolloid gel, 2% experienced infected wounds, while the other group whose wounds were rinsed with normal saline and who received regular Betadine and alcohol dressing experienced a 7% infection rate. However, no infection was observed in our study, which may be due to our inclusion criteria, because patients were only included if their burn area was less than 10%, and the lesions were in specific locations that have less of a chance of infection.
Dodd and Chalmers (33) studied the effect of a hydrogel dressing as compared with lanoline ointment on a group of lactating women with nipple wounds. The results of this study were the same as our study, with the investigators reporting that the patients receiving hydrogel dressing had significantly less pain during treatment and recovered earlier. In the lanolin ointment group, there were eight cases of breast infection, but no infection was observed in the group treated with hydrogels.
However, some studies still have conflicting results. For instance, in a study by Thomas et al. 30 patients with pressure ulcers were randomly divided into two groups. Patients in group I were treated with a hydrogel dressing and group II with gauze moistened with normal saline. Complete recovery occurred in 63% of the wounds after ten weeks, and no significant differences were observed between the two groups (34).
Following the earlier laboratory and animal studies (1, 2, 27), the present study reveals the efficacy of Irgel in patients with second-degree burns. However, there were some limitations in this study including the small number of samples, the lack of minor burns being referred to the hospital, and incomplete therapy for some patients. These limitations were the main weak point of this study, while the blind collection of the data was the biggest strength.
According to our findings, both gels affect burn wound healing and pain reduction more positively as compared with routine dressing procedures commonly performed in the Yazd Burn hospital. However, a comparison of Iranian hydrogel with its similar foreign type (MaxGel), which the client company (atomic energy agency) provided us, revealed that Irgel had a better effect on wound healing and pain relief. This indicates that Irgel is of better quality than MaxGel in this respect.
Acknowledgments
The authors thank all of the people who have assisted with this study, including the research deputy of Yazd Shahid Sadoughi university, and the head and personnel of Yazd Shahid Sadoughi Burn hospital, with special thanks to Dr. Mahin Felfely.
Footnotes
Authors’ Contribution:Original idea, study concept, design, and supervision: Mohammad Taghi Noorbala; patient visiting, dressing, and data collection: Mohammad Noorbala, Mahdi Noorbala, Roghaye Noorbala, and Bahare Mozafari; drafting of the manuscript: Mohammad Hossein Dashti-Rahmatabadi; critical revision of the manuscript for important intellectual content: Mohammad Taghi Noorbala.
Funding/Support:This research was supported by the Yazd branch of the radiation application research center.
References
- 1.Noorbala MT, DashtiRahmatabadi M, Binesh F, Morshedi A. Efficacy of Iranian hydrogel on wound healing in rat as an animal model. Iranian Red Crescent Medical Journal. 2009;2009(4):387–90. [Google Scholar]
- 2.Dashti-Rahmatabadi MH, Noorbala MT. A Comparison between the Efficacy of Iranian and Syrian Hydrogel Dressings on Wound Healing in Rats. Iran Red Crescent Med J. 2011;13(5):338–41. [PMC free article] [PubMed] [Google Scholar]
- 3.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674–86. doi: 10.1111/j.1524-4725.2005.31612. discussion 686. [DOI] [PubMed] [Google Scholar]
- 4.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8(11):823–31. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 5.Tramontina VA, Machado M, Filho GRN, Kim SH, Vizzioli MR, Toledo S. Effect of bismuth subgallate (local hemostatic agent) on wound healing in rats. Histological and histometric findings. Braz Dent J. 2002;13(1):11–6. [PubMed] [Google Scholar]
- 6.Takeda H, Katagata Y, Hozumi Y, Kondo S. Effects of angiotensin II receptor signaling during skin wound healing. Am J Pathol. 2004;165(5):1653–62. doi: 10.1016/S0002-9440(10)63422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soo C, Hu F, Zhang X, Wang Y, Beanes SR, Lorenz H, et al. Differential Expression of Fibromodulin, a Transforming Growth Factor-β Modulator, in Fetal Skin Development and Scarless Repair. Am J Pathol. 2000;157(2):423–33. doi: 10.1016/s0002-9440(10)64555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson CW. Nutrition and adult wound healing. Nutr Week. 2003;18:1–40. [Google Scholar]
- 9.Musalmah M, Nizrana MY, Fairuz AH, NoorAini AH, Azian AL, Gapor MT, et al. Comparative effects of palm vitamin E and alpha-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids. 2005;40(6):575–80. doi: 10.1007/s11745-005-1418-9. [DOI] [PubMed] [Google Scholar]
- 10.Toyokawa H, Matsui Y, Uhara J, Tsuchiya H, Teshima S, Nakanishi H, et al. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med . 2003;228(6):724–9. doi: 10.1177/153537020322800612. [DOI] [PubMed] [Google Scholar]
- 11.Bouzarjomehri FA, Sharafi AA, Firouzabadi SM, Hajizadeh S. Effects of low-frequency pulsed electromagnetic fields on wound healing in rat skin. AMS. 2000:1–5. [Google Scholar]
- 12.Demir H, Balay H, Kirnap M. A comparative study of the effects of electrical stimulation and laser treatment on experimental wound healing in rats. J Rehabil Res Dev. 2004;41(2):147–54. doi: 10.1682/jrrd.2004.02.0147. [DOI] [PubMed] [Google Scholar]
- 13.Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB J. 2005;19(3):379–86. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert G, Smith DP. Wound Care Product Selection. US Pharmacists. 2006;31(1) [Google Scholar]
- 15.Baxter CR, Rodchcavcr GT. Interventions: hemostasis, cleaning, topical antibiotics, debridement and closure. In: Eaglstein WH, ed Wound Care Manual: New Directions in Wound Healing. Princeton: NJ ConvaTec; 1990. pp. 71–82. [Google Scholar]
- 16.Lineaweaver W, Howard R, Soucy D, McMorris S, Freeman J, Crain C, et al. Topical antimicrobial toxicity. Arch Surg. 1985;120(3):267–70. doi: 10.1001/archsurg.1985.01390270007001. [DOI] [PubMed] [Google Scholar]
- 17.Rodeheaver G, Bellamy W, Kody M, Spatafora G, Fitton L, Leyden K, et al. Bactericidal activity and toxicity of iodine-containing solutions in wounds. Arch Surg. 1982;117(2):181–6. doi: 10.1001/archsurg.1982.01380260051009. [DOI] [PubMed] [Google Scholar]
- 18.Tatnall FM, Leigh IM, Gibson JR. Assay of antiseptic agents in cell culture: conditions affecting cytotoxicity. J Hosp Infect. 1991;17(4):287–96. doi: 10.1016/0195-6701(91)90273-b. [DOI] [PubMed] [Google Scholar]
- 19.Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448–50. doi: 10.1136/pgmj.58.681.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider DL, Hebert LJ. Subcutaneous gas from hydrogen peroxide administration under pressure. Am J Dis Child. 1987;141(1):10–1. doi: 10.1001/archpedi.1987.04460010010002. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence JC. Dressings and wound infection. Am J Surg. 1994;167(1A):21S–4S. doi: 10.1016/0002-9610(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 22.Mertz PM ". Prineclon NC. Intervention: dressing effect on wound healing. In: Eaglstein WH, ed. Wound Care Manual:New directions in Wound Healing. Prineclon: ConvaTec; 1990. pp. 83–96. [Google Scholar]
- 23.Davies JW. Prompt cooling of burned areas: a review of benefits and the effector mechanisms. Burns Incl Therm Inj. 1982;9(1):1–6. doi: 10.1016/0305-4179(82)90127-9. [DOI] [PubMed] [Google Scholar]
- 24.Yates DW, Hadfield JM. Clinical experience with a new hydrogel wound dressing. Injury. 1984;16(1):23–4. doi: 10.1016/0020-1383(84)90109-8. [DOI] [PubMed] [Google Scholar]
- 25.Eisenbud D, Hunter H, Kessler L, Zulkowski K. Hydrogel wound dressings: where do we stand in 2003? Ostomy Wound Manage. 2003;49(10):52–7. [PubMed] [Google Scholar]
- 26.Sprung P, Hou Z, Ladin DA. Hydrogels and hydrocolloids: an objective product comparison. Ostomy Wound Manage. 1998;44(1):44. [PubMed] [Google Scholar]
- 27.Ajji Z, Mirjalili G, Alkhatab A, Dada H. Use of electron beam for the production of hydrogel dressings. Radiation Phys Chem. 2008;77(2):200–2. [Google Scholar]
- 28.Gruber RP, Vistnes L, Pardoe R. The effect of commonly used antiseptics on wound healing. Plast Reconstr Surg. 1975;55(4):472–6. [PubMed] [Google Scholar]
- 29.Kaya AZ, Turani N, Akyuz M. The effectiveness of a hydrogel dressing compared with standard management of pressure ulcers. J Wound Care. 2005;14(1):42–4. doi: 10.12968/jowc.2005.14.1.26726. [DOI] [PubMed] [Google Scholar]
- 30.Martineau L, Shek PN. Evaluation of a bi-layer wound dressing for burn care I. Cooling and wound healing properties. Burns. 2006;32(1):70–6. doi: 10.1016/j.burns.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Hampton S. A small study in healing rates and symptom control using a new sheet hydrogel dressing. J Wound Care. 2004;13(7):297–300. doi: 10.12968/jowc.2004.13.7.26639. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg MS. Wound care management: proper protocol differs from athletic trainers' perceptions. J Athl Train. 1996;31(1):12–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Dodd V, Chalmers C. Comparing the use of hydrogel dressings to lanolin ointment with lactating mothers. J Obstet Gynecol Neonatal Nurs. 2003;32(4):486–94. doi: 10.1177/0884217503255098. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DR, Goode PS, LaMaster K, Tennyson T. Acemannan hydrogel dressing versus saline dressing for pressure ulcers. A randomized, controlled trial. Adv Wound Care. 1998;11(6):273–6. [PubMed] [Google Scholar]


