Significance
Septic shock is one of the major causes of morbidity and mortality throughout the world. Despite extensive efforts, septic shock remains a major medical challenge. In this study, using Arid5a-deficient mice, we demonstrated a critical role of this protein in lipopolysaccharide (LPS)-induced shock through regulation of IFN-γ expression in T cells by preventing the mRNA degradation of its major transcription factor T-bet. Further, we identified the conserved stem loop structure at 3′UTR of T-bet mRNA required for binding Arid5a during its action. Our study indicates the therapeutic potential of anti-agonistic agents for Arid5a in the prevention of pathological conditions of septic shock.
Keywords: septic shock, Arid5a, IFN-γ, T-bet, IL-6
Abstract
Adenine-thymine (AT)-rich interactive domain containing protein 5a (Arid5a) is an RNA-binding protein that has been shown to play an important immune regulatory function via the stabilization of IL-6 and STAT3 mRNA. However, the role of Arid5a in the overwhelming and uncontrolled immune response that leads to septic shock is unknown. Here, we report that Arid5a-deficient mice are highly resistant to lipopolysaccharide (LPS)-induced endotoxic shock and secrete lower levels of major proinflammatory cytokines, including IFN-γ, IL-6, and TNF-α, than WT mice in response to LPS. Arid5a deficiency resulted in decreased levels of IFN-γ under Th1 cell conditions, in which T-box expressed in T cells (T-bet) mRNA expression was inhibited. Arid5a bound to the conserved stem loop structure of the 3′UTR of T-bet and stabilized its mRNA. Arid5a-deficient mice were also resistant to Propionibacterium acnes-primed LPS injection, which is considered to be a T-cell–mediated IFN-γ dependent endotoxic shock mouse model. Thus, regulation of IFN-γ by Arid5a via the stabilization of T-bet mRNA in Th1 cells contributes to the development of septic shock in mice. In addition, our previous study suggests that Arid5a control the IL-6 level in vivo in response to LPS by stabilization of IL-6 mRNA. We also observed that neutralization of IFN-γ and IL-6 significantly recovered the mice from endotoxic shock. Taken together, we conclude that Arid5a regulates the augmentation of IL-6 and IFN-γ in response to LPS, which possibly works synergistically for amplification of various other cytokines that ultimately cause the development of septic shock in mice.
Sepsis is a frequent and severe systemic inflammatory response to infection associated with the excessive production of cytokines. The Gram-negative bacterial wall constituent endotoxin (LPS) is the major active agent in the pathogenesis of septic shock (1). IFN-γ plays an essential role in the development of septic shock. IFN-γ is produced by activated T lymphocytes and natural killer (NK) cells and mediates its action by binding a unique cell surface receptor (2). Treatment of mice with IFN-γ increases susceptibility to endotoxic shock (3), and IFN-γ receptor knockout mice are resistant to endotoxic shock (4), clearly indicating its importance in the pathogenesis of septic shock.
T cells play an important role in sepsis by secreting IFN-γ. Mice depleted of T cells are resistant to polymicrobial sepsis and Escherichia coli infection (5, 6). T-box expressed in T cells (T-bet) is a transcription factor that plays a major role in regulating IFN-γ production in T lymphocytes (7, 8). The expression of T-bet in primary T cells or developing Th2 cells results in the activation of IFN-γ production (7), whereas mice lacking T-bet fail to develop Th1 cells and display a dramatic reduction of IFN-γ production (8). T-bet dysregulation has been associated with several Th1-driven immunopathological disorders, suggesting that regulation of T-bet is essential to control the innate immunity (9, 10).
AT-rich interactive domain containing protein 5a (Arid5a) is a member of the Arid protein family that functions as a unique RNA-binding protein (11, 12). It is expressed highly in macrophage in response to LPS and controls posttranscriptional regulation of IL-6 by stabilizing IL-6 mRNA through binding to its 3′UTR (11). Arid5a deficiency was shown to inhibit the elevation of IL-6 serum levels in LPS-treated mice (11). Our recent study also showed an intrinsic role for Arid5a in T cells, where Arid5a was found to direct the differentiation of naive CD4+ T cells into inflammatory CD4+ T cells by the stabilization of STAT3 mRNA (12). Overall, these findings suggest Arid5a has an important immune regulatory function. However, the role of Arid5a in the overwhelming and uncontrolled immune response that leads to endotoxic shock is unknown.
In this study, we identified an essential role for Arid5a in LPS-induced endotoxic shock. We report here that Arid5a augments IFN-γ secretion in Th1 cells via stabilization of the mRNA of its transcription factor, T-bet, which in turn contributes to IFN-γ–mediated endotoxic shock in mice.
Results
Arid5a-Deficient Mice Are Highly Resistant to Endotoxic Shock.
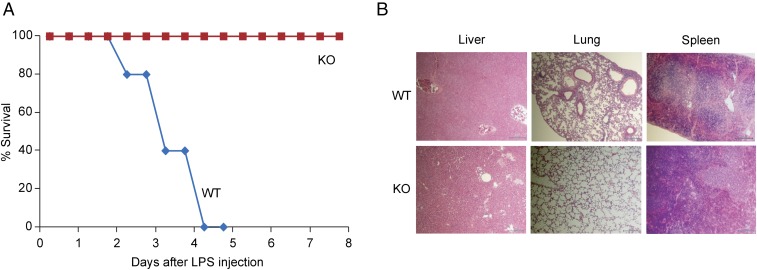
To determine the role of Arid5a in the innate immune response, we first investigated the role of Arid5a in a murine model of endotoxic shock, using Arid5a-deficient mice. Arid5a-deficient and WT control mice were injected intraperitoneally (i.p.) with LPS, and survival was monitored. As shown in Fig. 1A, all the WT mice died within 96 h of LPS administration, whereas 100% of the Arid5a-deficient mice survived under similar conditions. As endotoxemia in septic shock is associated with multiple organ damage, which leads to death, we next evaluated histopathology of the liver, lung, and spleen of the WT and Arid5a-deficient mice, using H&E staining (Fig. 1B). The WT mice exhibited widespread hemorrhaging in the liver, severe fibrosis in the lung, and tissue disorganization in the spleen, whereas the Arid5a-deficient mice were protected from tissue damage after endotoxic shock (Fig. 1B). Thus, our data suggest Arid5a plays an essential role in the progression of endotoxic shock.
Fig. 1.
Arid5a-deficient mice are resistant to LPS-induced endotoxic shock. (A) 8-wk-old WT (n = 15) and Arid5a-deficient (n = 10) mice were injected with E. coli LPS (15 mg/kg weight), and survival was monitored for 7 d. (B) H&E staining of the liver, lung, and spleen sections of WT and Arid5a-deficient mice 48 h after LPS challenge.
Arid5a-Deficient Mice Produce Significantly Decreased Levels of Proinflammatory Cytokines in Response to Endotoxic Shock.
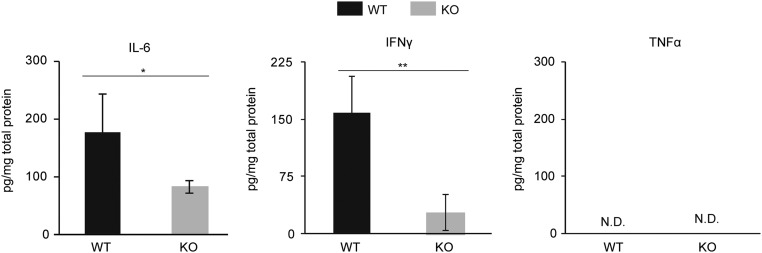
The toxic effects of LPS are mainly mediated by proinflammatory cytokines such as TNF-α, IL-6, and IFN-γ (13). Therefore, to determine the influence of Arid5a on proinflammatory cytokines during LPS-induced shock, WT and Arid5a-deficient mice were injected i.p. with LPS, and serum cytokine levels were determined by ELISA at various times after LPS injection. As shown in Fig. 2 A–C, the production of major proinflammatory cytokines, including IFN-γ, IL-6, and TNF-α, was significantly decreased in Arid5a-deficient mice compared with WT mice after in vivo injection of LPS. Consistent with these data, splenocytes collected from LPS-challenged Arid5a-deficient mice expressed significantly decreased levels of IL-6 and IFN-γ compared with control mice, whereas TNF-α was not detected in either WT or Arid5a-deficient splenocytes 24 h after LPS challenge (Fig. S1). Taken together, these results suggest that the resistance of Arid5a null mice to endotoxic shock is the result of a diminished response to proinflammatory cytokines.
Fig. 2.
Serum proinflammatory cytokines were significantly decreased in Arid5a-deficient mice after LPS challenge. (A–C) The serum concentrations of IFN-γ (A), IL-6 (B), and TNF-α (C) in Arid5a-deficient mice (n = 5) were measured at the indicated points after LPS (5 mg/kg) injection. Error bars show the means ± SEM *P < 0.05; **P < 0.01 (Student’s t test).
Fig. S1.
Expression of the proinflammatory cytokines IL-6 and IFN-γ was significantly decreased in Arid5a-deficient splenocytes after LPS challenge. Twenty-four hours after LPS injection (5 mg/kg; n = 3), spleens were collected from WT and deficient mice, homogenates were prepared, and IL-6, TNF-α and IFN-γ levels were measured by ELISA. Error bars show the means ± SD. *P < 0.05; **P < 0.01; (Student’s t test); N.D., not detected.
Neutralization of IFN-γ and IL-6 Recovers Mice from Endotoxic Shock.
Our results suggest that Arid5a-deficient mice are defective at producing IFN-γ, IL-6, and TNF-α in response to LPS. Next, we asked which cytokine among these three proinflammatory cytokines has the most profound effect on endotoxemia. To answer this question, we compared the effects of neutralizing antibodies against IFN-γ, IL-6, and TNF-α 4 h after LPS treatment. As shown in Fig. 3, anti–IFN-γ treatment rescued all the mice from LPS-mediated septic shock, whereas anti–IL-6 receptor antibody moderately rescued (78%) and anti–TNF-α treatment partially rescued (30%) the mice. Collectively, these results demonstrate that IFN-γ and IL-6 play an important role in endotoxic shock.
Fig. 3.
Anti–IFN-γ and Anti–IL-6R treatment recover the mice from endotoxic shock. Age-matched WT B6 mice were administered E. coli LPS (25 mg/kg). Four hours later, the mice were treated with PBS (n = 10), anti–IL-6R (n = 13), anti–IFN-γ (n = 10), or anti–TNF-α (n = 5) antibodies, and survival was monitored for 7 d.
IFN-γ and T-bet Expression Is Significantly Decreased in Arid5a-Deficient Activated T Cells.
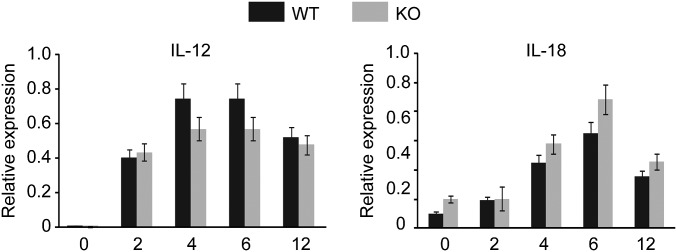
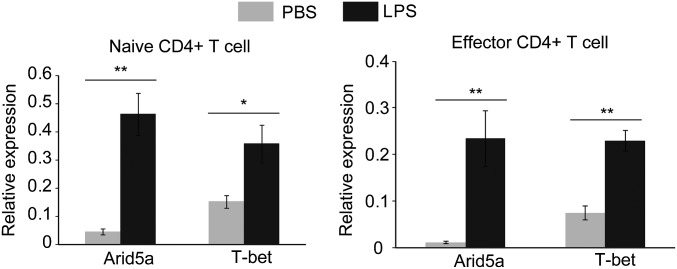
Having confirmed that IFN-γ and IL-6 play a profound role in endotoxemia, we next focused on the mechanism by which Arid5a augments these proinflammatory cytokines in the serum in response to LPS. Our previous study already clarified that Arid5a controls the IL-6 level in vivo by preventing the decay of IL-6 mRNA (11). Therefore, in this study we emphasized the mechanism by which Arid5a regulates the IFN-γ during endotoxic shock. IFN-γ is not expressed directly in response to LPS. The interaction of LPS with Toll-like receptor 4 (TLR4) on macrophages and monocytes first triggers the production of various initial proinflammatory cytokines, such as IL-12 and IL-18, which in turn activate T cells or NK cells to release IFN-γ (14). Initially, we evaluated the expression of IL-12 and IL-18 in peritoneal macrophages in WT and Arid5a-deficient mice. These cytokines are directly affected by LPS and trigger the release of IFN-γ in other immunological cells, such as activated T cells. However, their expression levels were found to be comparable between WT and Arid5a-deficient mice (Fig. S2), suggesting Arid5a may not directly affect the expression of IL-12 and IL-18 for IFN-γ induction. T cells have been demonstrated to play an important role in acute sepsis via the increased production of IFN-γ (5, 6, 15). Moreover, injection of mice with LPS significantly increases Arid5a expression in both naive and effector CD4+ T cells (Fig. S3). Thus, to examine the possible role of Arid5a in controlling IFN-γ, we next examined the intrinsic role of Arid5a in T cells. When naive CD4+ T cells collected from WT and Arid5a-deficient spleens were stimulated under Th1 differentiation conditions, Arid5a-deficient Th1 cells were found to express significantly lower levels of IFN-γ (Fig. 4A) compared with WT Th1 cells. To determine whether Arid5a-deficient CD4+ T cells also expressed low levels of IFN-γ under endotoxic shock conditions, we isolated CD4+ T cells from WT and Arid5a-deficient splenocytes after in vivo injection of LPS and measured the IFN-γ expression by quantitative PCR. Consistent with the in vitro data, we also observed that Arid5a-deficient T cells expressed severely reduced levels of IFN-γ (Fig. 4C) after endotoxic shock.
Fig. S2.
IL-12 and IL-18 expression were comparable between WT and Arid5a-deficient mice (n = 3) in peritoneal macrophages after LPS administration. Peritoneal macrophages were treated with LPS (500 ng/mL) at the indicated points. Total RNA was isolated, and equal amounts of RNA were used for the quantitative RT-PCR assay. Error bars show the means ± SD.
Fig. S3.
Expression of Arid5a and T-bet in CD4+ T cells isolated from LPS-treated mice. Four hours after LPS injection, spleens were collected, and naive and effector CD4+ T cells were isolated using MACS microbeads. Total RNA was isolated, and equal amounts of RNA were used for the quantitative RT-PCR assay. Error bars show the means ± SD. *P < 0.05; **P < 0.01 (Student’s t test).
Fig. 4.
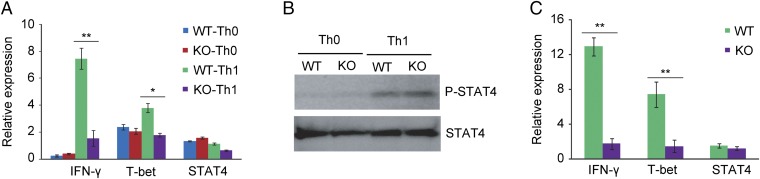
Arid5a-deficient Th1 cells expressed decreased levels of IFN-γ and T-bet in vitro and in vivo. (A) Naive CD4+ T cells from WT and Arid5a-deficient mice were stimulated under Th0 conditions (stimulated with CD3ε and CD28) or Th1 conditions (stimulated with CD3ε, CD28, and IL-12). Forty-eight hours after differentiation, IFN-γ, T-bet, and STAT4 mRNA expression was measured by RT-PCR. (B) Immunoblot analysis of phosphorylated STAT4 and total STAT4 in WT or Arid5a-deficient CD4+ T cells was stimulated for 12 h under Th0 and Th1 cell conditions. (C) Quantitative real-time PCR analysis of IFN-γ, T-bet, and STAT4 mRNA in CD4+ T cells isolated from the spleens of LPS (5 μg/kg)-challenged WT or Arid5a-deficient mice. The data are representative of three independent experiments (A and C). Error bars show the means ± SD (A–D). *P < 0.05; **P < 0.01 (Student’s t test).
T-bet and STAT4 are the major transcription factors that regulate IFN-γ expression in Th1 cells (10, 16). To determine whether defective production of IFN-γ in Arid5a-deficient Th1 cells is a result of the abnormal production of T-bet and STAT4, we measured their expression under Th1-promoting conditions. Interestingly, we found that T-bet expression, but not STAT4, was significantly impaired in Arid5a-deficient Th1 cells (Fig. 4A) compared with WT cells. As Arid5a and T-bet expression were significantly increased in CD4+ T cells after LPS injection (Fig. S3), we next determined whether the depletion of Arid5a in CD4+ T cells affected T-bet expression under endotoxic shock conditions. Similar to the in vitro data, we observed that T-bet expression, but not STAT4, was severely decreased in Arid5a-deficient CD4+ T cells under endotoxic shock conditions (Fig. 4C). STAT4 is known to be activated via phosphorylation under Th1-promoting conditions, contributing to IFN-γ production (17). To determine whether Arid5a contributes to IFN-γ production in Th1 cells through STAT4 activation, we compared the phosphorylation of STAT4 in WT and Arid5a-deficient mice under Th1-promoting conditions by immunoblotting (Fig. 4B). However, no significant differences were observed in STAT4 phosphorylation or in total STAT4 levels between WT and Arid5a-deficient Th1 cells (Fig. 4B), which suggests Arid5a is not involved in STAT4 activation. Thus, our in vitro and in vivo results indicate that Arid5a plays an important role in activated T cells by regulating the expression of T-bet and IFN-γ.
Arid5a Binds to and Stabilizes the 3′UTR Region of T-bet mRNA.
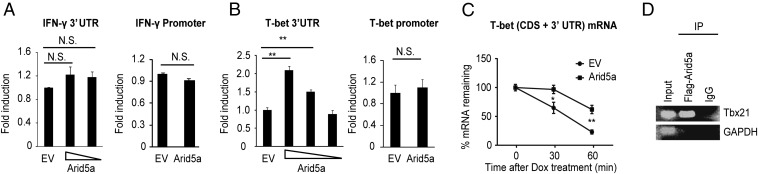
Having confirmed that Arid5a regulates T-bet and IFN-γ expression in Th1 cells, we next asked how Arid5a controls their expression. Arid family proteins are believed to have DNA-binding properties (18), but our group demonstrated that Arid5a can function as an RNA-binding protein (11, 12). Thus, there is a possibility that Arid5a could regulate T-bet and IFN-γ transcriptionally, by activating their promoters, or posttranscriptionally, by activating their UTRs. To evaluate both possibilities, we constructed luciferase vectors encoding the promoters or 3′UTR regions of T-bet and IFN-γ and performed luciferase reporter assays by overexpressing Arid5a or empty vector in HEK293T cells (Fig. 5 A and B). Overexpression of Arid5a affected neither the promoter nor the UTR activity of IFN-γ (Fig. 5A). However, as shown in Fig. 5B, Arid5a overexpression significantly augmented the luciferase activity of the pGL3 vector encoding the T-bet 3′UTR compared with that of the control vector. No effect of Arid5a was observed on the T-bet promoter region (Fig. 5B), suggesting Arid5a is essential for T-bet mRNA stabilization, but not for IFN-γ. This result was further confirmed by measurement of the T-bet mRNA half-life after overexpressing Arid5a in HEK Tet-off cells stably expressing the enhancer of a tetracycline-responsive promoter (TRE). Overexpression of Arid5a was shown to prevent the T-bet mRNA degradation after stimulation with tetracycline derivative doxycycline compared with the control cells transfected with empty vector (Fig. 5C). To evaluate the association of T-bet mRNA with Arid5a protein, we next performed an RNA immunoprecipitation assay after overexpression of the T-bet (CDS + 3′UTR) plasmid together with Flag-tagged Arid5a or control vector in HEK293T cells. Arid5a was immunoprecipitated with T-bet mRNA, but not GAPDH (Fig. 5D). These results indicate the physical association of Arid5a with T-bet 3′UTR mRNA. Taken together, our results demonstrate that Arid5a stabilizes T-bet mRNA by associating with its 3′UTR region.
Fig. 5.
Arid5a stabilizes T-bet, but not IFN-γ, mRNA. (A and B) HEK 293T cells were transfected with a luciferase vector encoding the full-length 3′UTR and promoter regions of IFN-γ (A) or T-bet (B) together with an empty vector (EV) or an Arid5a expression vector. Luciferase activity was measured 48 h after transfection. The values were normalized to those obtained with transfection with EV. (C) HEK293 Tet-off cells were transfected with pTREtight-T-bet-CDS + 3′UTR together with an Arid5a expression vector or an empty vector. The cells were uniformly divided 3 h after transfection and incubated overnight. Total RNA was prepared after doxycycline (1 μg/mL) treatment, and T-bet mRNA levels were determined by RT-PCR. (D) Association of Arid5a with T-bet mRNA by RIP assay. HEK293T cells were transfected with the T-bet (CDS + 3′UTR) and a Flag-tagged Arid5a expression vector. The cell lysates were immunoprecipitated with Flag or IgG antibody, and immunoprecipitates were analyzed using primers specific to T-bet and GAPDH mRNA. The data are representative of three independent experiments (A–D). Error bars show the means ± SD (A–D). *P < 0.05; **P < 0.01; N.S., not significant (Student’s t test).
The Conserved Stem Loop Region of the T-bet 3′UTR (616-641) Is Required for Arid5a Binding.
After confirming that Arid5a controls T-bet 3′UTR mRNA stability via the 3′UTR, we next attempted to identify which portions of the T-bet 3′UTR are critical for the stabilization of T-bet mRNA by Arid5a. Many RNA-binding proteins have been shown to associate with adenine-uridine-rich element (ARE) regions (19). Mouse T-bet mRNA contains one ARE in its 3′UTR. To investigate regions of the T-bet 3′UTR that are critical for conferring Arid5a responsiveness, we prepared constructs of the pGL3 luciferase vector encoding the non-ARE-containing fraction of the T-bet 3′UTR (1–320) or the ARE-containing fraction of the T-bet 3′UTR (321–701), as shown in Fig. 6A. Overexpression of Arid5a enhanced the luciferase activity of the pGL3 vector encoding the ARE-containing region (321–701) of the T-bet 3′UTR (Fig. 6B) more than twofold compared with the control, whereas Arid5a was less responsive to the non-ARE region (1–301) (Fig. 6C). This result suggests that Arid5a stabilizes T-bet mRNA by binding to the ARE-containing fraction of the 3′UTR of T-bet.
Fig. 6.
Arid5a associates with the stem loop-like structure located near the ARE region of T-bet mRNA. (A) Schematic diagram of the luciferase vectors encoding T-bet 3′UTR region (1–701), T-bet 3′UTR region (1–302), or T-bet 3′UTR region (321–701). The black circle shows AU-rich elements (AREs). (B) Luciferase activity of the pGL3 vectors encoding T-bet 3′UTR region (1–701), T-bet 3′UTR region (1–302), or T-bet 3′UTR region (321–701) cotransfected for 48 h with an Arid5a expression vector or empty vector. The data are representative of three independent experiments. Error bars show the means ± SD (B). *P < 0.05; **P < 0.01; N.S., not significant (Student’s t test). (C) Schematic diagram shows the conserved stem loop-like element (616–641) between humans and mice near the ARE region (closed circle) of T-bet 3′UTR mRNA. (D) Diagram of the stem loop structure (616–641) of the T-bet 3′UTR. (E) EMSA evaluating the interaction of recombinant Arid5a protein with 3′ biotinylated nucleotides, as in D.
Having confirmed that Arid5a controls T-bet mRNA stability via binding the ARE-containing region of the 3′UTR, we next attempted to identify which elements in the ARE fraction of the T-bet 3′UTR are critical for the stabilization of T-bet mRNA by Arid5a. Our group recently reported that Arid5a can recognize stem loop structure of the 3′UTR region of STAT3 mRNA (12). Thus, we looked for the stem loop-like structure in the ARE-containing fraction of the 3′UTR of T-bet (321–701). Interestingly, amino acids 616–641 of the T-bet 3′UTR were found to form a stem loop-like structure (Fig. 6D) and were highly conserved between humans and mice (Fig. 6C). To confirm whether this conserved stem loop-like element (616-GCUAUUUAUUGUAGAGAGUGGU-641) in the T-bet 3′UTR is physically associated with Arid5a, we performed an electrophoretic mobility shift assay (EMSA), using the biotinylated RNA sequence of the conserved element (616–641) and recombinant Arid5a protein. As shown in Fig. 5D, Arid5a tightly bound with the stem loop region (616–641) of the T-bet 3′UTR. Overall, our data suggest that Arid5a physically associates with the conserved stem loop region near the ARE site of the T-bet 3′UTR mRNA.
Arid5a-Deficient Mice Are Resistant to Propionibacterium acnes-Primed Endotoxic Shock.
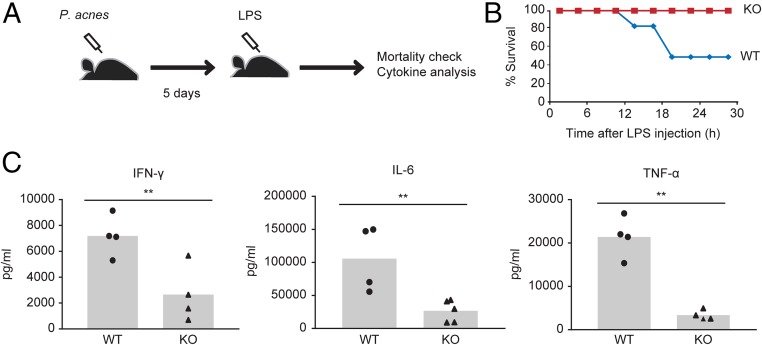
Our results demonstrated that Arid5a controls IFN-γ by stabilizing its master regulator, T-bet, in Th1 cells. To confirm whether this phenomenon is related to endotoxic shock, we evaluated the sensitivity of Arid5a-deficient mice in a P. acnes-primed endotoxic shock mouse model. Mice deficient in T cells (nu/nu), Th1 cells (IL-12p40−/−), or IFN-γ are highly resistant to P. acnes-primed, LPS-induced septic shock (15). Thus, it is believed that the P. acnes-primed endotoxic shock mouse model is T-cell–mediated and IFN-γ–dependent. Previously, it was proposed that priming mice with heat-killed P. acnes results in the induction of IL-12 by macrophages, which causes increased IFN-γ production by Th1 cells and primes the macrophages to be susceptible to LPS (15). Thus, to evaluate whether the regulation of IFN-γ expression in Th1 cells by Arid5a affects endotoxic shock in mice, we challenged WT and Arid5a-deficient mice with P. acnes for 5 d, followed by a sublethal dose of LPS (Fig. 7A). As shown in Fig. 7B, 24 h after LPS administration, 50% of the WT mice died, whereas all of the Arid5a-deficient mice survived under similar conditions. The serum proinflammatory cytokine levels were measured 4 h after LPS treatment. The serum IFN-γ, IL-6, and TNF-α levels were significantly decreased in Arid5a-deficient mice compared with WT mice (Fig. 7C) after LPS administration. Because IFN-γ is known to play a key role in the pathogenesis of disease in this mouse model, this is likely to be mediated by Arid5a regulation of IFN-γ expression.
Fig. 7.
Arid5a-deficient mice are protected from T-cell–mediated, IFN-γ–dependent endotoxic shock. (A) Schematic diagram shows the experimental design. Mice were administered heat-killed P. acnes (1 mg in 200 μL PBS) i.p. On day 5, the mice were treated with low-dose LPS (5 μg/mouse). Serum cytokines and the mortality of the mice were analyzed after LPS treatment. (B) Survival analysis of WT (n = 7) and Arid5a-deficient mice (n = 7) after P. acnes-primed LPS treatment (C) Serum was collected 4 h after LPS treatment from WT (n = 4) and Arid5a-deficient mice (n = 4), and the proinflammatory cytokines IFN-γ, IL-6, and TNF-α were measured by ELISA. **P < 0.01, (Student’s t test).
Discussion
The pathology of endotoxemia is believed to be jointly mediated by multiple cytokines, including TNF-α, IL-6, and IFN-γ (13). However, to the best of our knowledge, their effects have not yet been compared under similar experimental conditions by using neutralizing antibodies after the development of LPS-induced shock. In this study, we showed that anti–IFN-γ and anti–IL-6 receptor antibody treatment at 4 h after LPS treatment had the most pronounced effect in recovering mice from endotoxic shock compared with anti–TNF-α treatment (Fig. 3), suggesting IFN-γ and IL-6 play the most significant role at late points after endotoxic shock in mice.
Arid5a is an RNA-binding protein that was shown to regulate the IL-6 level in vivo by stabilization of its mRNA (11). In this study, we demonstrated a new and critical role of this protein in LPS-induced shock through regulation of IFN-γ expression in Th1 cells. The interaction of LPS with TLR4 on macrophages and monocytes first triggers the production of various initial proinflammatory cytokines such as IL-12 and IL-18 that activate the Th1 cells to release the IFN-γ (14), which in turn trigger the release of additional immunomodulatory factors that together contribute to the development of endotoxic shock (20). Our studies showed that Arid5a did not inhibit LPS-dependent production of IL-12 or IL-18 in peritoneal macrophage (Fig. S2). However, IL-12–induced activated Arid5a-deficient Th1 cells expressed a significantly low level of IFN-γ compared with WT (Fig. 3A). Arid5a-deficient T cells isolated from the LPS-injected mice also showed a significantly low expression of IFN-γ in comparison with WT (Fig. 3C), suggesting that activation of T cells after LPS injection was defective in Arid5a-deficient mice, which possibly contributes to low expression of IFN-γ.
The transcription factor T-bet is a master regulator of the generation of IFN-γ–producing Th1 cells (7, 8). IFN-γ secretion was reported to be impaired in T-bet-deficient mice (9). Recent studies have strongly indicated that regulation of T-bet in Th1 cells is required for combating bacterial and viral infection (21, 22). In this report, we showed that the expression of T-bet is impaired in Arid5a-deficient Th1 cells (Fig. 3A). T-bet expression was also significantly decreased in Arid5a-deficient T cells isolated from endotoxic shock mice compared with WT T cells (Fig. 3C). We reasoned that Arid5a-deficient Th1 cells produce decreased levels of IFN-γ because of the defective expression of T-bet, which contributes to the decreased serum levels of IFN-γ in Arid5a-deficient mice after LPS injection.
The combination of signals from antigens, costimulation, and proinflammatory cytokines such as IL-12 promotes the activation of the TCR, IL-12–STAT4, and IFN-γ–STAT1 signaling pathways in T cells, which induces the expression of T-bet and results in the differentiation of naive T cells into Th1 cells (10). T-bet also positively regulates its own expression through an autoregulator loop involving Hlx, a homeobox gene (10). Although transcriptional regulation of T-bet has already been well studied, the posttranscriptional regulation of T-bet is not well understood. In this study, we show that Arid5a regulates T-bet expression by protecting the degradation of its mRNA via binding to the stem loop structure at 3′UTR region (Figs. 5 and 6). This mechanism of Arid5a is consistent with our previous observations, where Arid5a was shown to work as a stabilizer of IL-6 and STAT3 mRNA (11, 12).
Although the posttranscriptional degradation mechanism of T-bet mRNA is not clearly known, previous study showed that RNA-binding protein Regnase-1 is highly expressed in T cells, and overexpression of Regnase-1 degraded the 3′UTR region of T-bet (Tbx21) mRNA (23). Moreover, transcriptome analysis also showed that Tbx21 mRNA is highly upregulated in Regnase-1-deficient CD4+ T-cells (23), suggesting Regnase-1 is the potential candidate for posttranscriptional destabilization of T-bet mRNA. In addition, our previous study suggests that Arid5a counteract the destabilizing effect of Regnase-1 by preventing its binding to the stem loop structure of 3′UTR mRNA of IL-6 and STAT3 (11, 12). On the basis of those observations, we speculate that Arid5a stabilizes the T-bet mRNA, possibly by counteracting with Regnase-1.
In this report, we did not focus on the role of Arid5a in NK cells, which have also been shown to play important roles in endotoxemia by secreting IFN-γ (24). We cannot ignore the possibility that Arid5a might also have a similar role in NK cells, as well as in the regulation of IFN-γ. In fact, T-bet is also expressed in NK cells (10). Further studies are required by generating cell-specific Arid5a-deficient mice to reveal the complete mechanism of Arid5a in endotoxic shock.
In summary, we conclude that Arid5a regulates the augmentation of IFN-γ via stabilization of T-bet mRNA in Th1 cell, which in turn contributes to development of LPS-induced septic shock in mice. However, the role of Arid5a in the development of septic shock is not solely dependent on its regulation of IFN-γ. The previously described role of Arid5a in regulation of IL-6 (11) also possibly participates in the establishment of septic shock, as Arid5a-deficient mice secreted less IL-6 in response to LPS (Fig. 2) and anti–IL-6 receptor antibody treatment showed a significant effect in recovering the mice from septic shock (Fig. 3). So, it is reasonable to speculate that Arid5a regulates the augmentation of both IL-6 and IFN-γ in response to LPS, which possibly works synergistically for the amplification of various other cytokines that ultimately cause the development of septic shock in mice. Thus, our study indicates the therapeutic potential of anti-agonistic agents for Arid5a in the prevention of pathological conditions of sepsis.
Materials and Methods
Mice.
C57BL/6J WT mice (8–9 wk) were obtained from CLEA Japan, Inc. Arid5a−/− mice were generated as previously described (11). All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of the Graduate School of Frontier Bioscience (Osaka University, Osaka, Japan).
LPS-Induced Endotoxic Shock.
Arid5a−/− and WT female mice (body weight, 18–20 g) were i.p. injected with LPS (15 mg/kg or 25 mg/kg) from E. coli (Sigma). The survival of the mice was monitored over the next 7 d. The roles of TNF-α, IL-6, and IFN-γ in endotoxic shock were investigated by neutralizing endogenous TNF-α, IL-6, and IFN-γ with their respective monoclonal antibodies. Anti–TNF-α (MP6-XT22 BioLegend, 10 mg/kg), anti–IL-6R (MR16-1, 250 mg/kg), and anti–IFN-γ (R4-6A2 BioLegend, 25 mg/kg) were injected into the mice 4 h after LPS injection, and mortality was monitored over the next 7 d.
Cell Culture and in Vitro Th1 Cell Differentiation.
Cell culture and in vitro Th1 cell differentiation were performed according to the method described previously (11, 12). Details are given in SI Materials and Methods.
Luciferase Assay.
HEK293T cells were transfected with a pGL3 vector encoding the IFN-γ UTR, T-bet 3′UTR region (1–701), T-bet 3′UTR region (1–302), and T-bet 3′UTR region (321–701) or a pLUC-MCS vector encoding the IFN-γ or T-bet promoter, together with an Arid5a expression plasmid or an empty (control) plasmid. Luciferase activity was measured according to the protocol described previously (11). Details are given in SI Materials and Methods.
mRNA Decay Experiments (Tet-off System).
mRNA decay experiment of T-bet mRNA was performed using quantitative RT-PCR (qRT-PCR) Tet-off system in HEK293 Tet-off cells by transfecting with pTREtight-T-bet-CDS + 3′UTR (which has a T-bet coding and noncoding 3′UTR sequence), together with an Arid5a expression plasmid or a control (empty) plasmid. Details are given in SI Materials and Methods.
RIP Assay.
HEK293T cells were transfected with an expression vector encoding the T-bet (CDS + 3′UTR), together with a Flag-tagged Arid5a expression vector or an empty vector (control). Twelve hours after transfection, the cells were collected, and an RNA immunoprecipitation (RIP) assay was performed using the RiboCluster Profiler RIP assay kit (MBL) according to the manufacturer’s instructions, using a Flag monoclonal antibody (Sigma) and control mouse IgG. The eluted RNA was analyzed by RT-PCR.
qRT-PCR, RNA-EMSA, and Immunoblotting.
qRT-PCR analysis, RNA-EMSA, and immunoblotting were performed as previously described (11, 12, 17). Details are given in SI Materials and Methods.
SI Materials and Methods
Histopathological Analysis.
The livers, lungs, and spleens were collected from endotoxic shock mice and fixed with 10% (vol/vol) formalin neutral buffer solution, embedded in paraffin, and cut into 5-μm-thick sections. Histopathological analysis of paraffin-embedded tissue sections was performed by H&E staining.
Cytokine Measurement by ELISA.
The levels of mouse IFN-γ, IL-6, and TNF-α in serum and splenocytes were measured by ELISA according to the manufacturer’s protocol (R&D). Serum was prepared from blood collected from the tail veins at different points after LPS treatment. Spleen homogenates were prepared by disruption and homogenization of LPS-treated spleens using cell extraction buffer (Thermo Fisher Scientific). After centrifugation, the supernatants were used for cytokine measurement.
Cell Culture.
HEK293T and HEK Tet-off cells were cultured in DMEM (Sigma-Aldrich) supplemented with 10% (vol/vol) FBS. The cells were transfected using the Lipofectamine Plus system (Invitrogen).
Peritoneal macrophages were prepared as described previously (11). Thioglycolate-elicited peritoneal macrophages were cultured in RPMI medium 1640 (Sigma-Aldrich) with 10% (vol/vol) FCS, 100 μg/mL streptomycin, and 100 U/mL penicillin G (Nakalai Tesque).
In Vitro Th1 Cell Differentiation.
Naive CD4+ T cells (CD4+ CD44 low CD62L hi CD25−) were isolated from the spleen, using MACS bead-based cell isolation kits (Miltenyi Biotec). Naive CD4+ T cells were stimulated with plate-bound anti-CD3ε (5 μg/mL; 145–2C11; BioLegend) and anti-CD28 (2 μg/mL; 37.51; BioLegend) in the presence of IL-12 (20 ng/mL) and anti–IL-4 (10 μg/mL; 11B11; BioLegend) for the generation of Th1 cells.
Luciferase Assay.
HEK293T cells were transfected with a pGL3 vector encoding the IFN-γ UTR, T-bet 3′UTR region (1–701), T-bet 3′UTR region (1–302), and T-bet 3′UTR region (321–701) or a pLUC-MCS vector encoding the IFN-γ or T-bet promoter together with an Arid5a expression plasmid or an empty (control) plasmid. The Renilla luciferase gene was simultaneously transfected as an internal control. After 48 h of cultivation, these cells were lysed, and the luciferase activities in lysates from the treated samples were determined using the Dual-Luciferase Reporter Assay System (Promega).
mRNA Decay Experiments (Tet-off System).
HEK293 Tet-off cells were transfected with pTREtight-T-bet-CDS + 3′UTR (which has a T-bet coding and noncoding 3′UTR sequence), together with an Arid5a expression plasmid or a control (empty) plasmid. After 3 h, the cells were subdivided into three 60-mm dishes and cultured overnight. mRNA transcription from pTREtight vectors was terminated by the addition of doxycycline (1 μg/mL), and total RNA was prepared at the indicated periods. The RNA was subjected to RT-PCR analysis.
RNA EMSA.
EMSA was performed according to a previously described protocol (12). The RNA was synthesized as a single strand and was 3′-end labeled by biotin (Hokkaido System Science). The sequence used was 5′-GCUAUUUAUUGUAGAGAGUGGU-3′. Recombinant mouse Arid5a protein was prepared by Chugai Pharmaceutical Co. Ltd., according to the method described previously (12).
Quantitative Real-Time PCR Analysis.
Total cellular RNA was extracted with RNeasy columns (Qiagen). Reverse transcription was performed using a reverse transcription kit (Qiagen). Real-time PCR was performed using the SybrGreen PCR master mix (Roche) with an ABI PRISM 7900 HT Real Time PCR system (Applied Biosystems). The levels of T-bet, STAT4, IFN-γ, IL-6, TNF-α, IL-12, IL-18, and IL-1β mRNA were normalized to GAPDH mRNA levels. The sequences of the PCR primers are shown in Table S1.
Table S1.
List of primers used for qRT-PCR
| Name | Forward | Reverse | Species |
| Arid5a | CAGCACCTCCGGCCAAA | CTTGAAGCCAAGATGGGGCA | Mouse |
| IFN-γ | CTTCTTCAGCAACAGCAAGG | TGAGCTCATTGAATGCTTGG | Mouse |
| IL-12p40 | GGAAGCACGGCAGCAGAATAA | CTTGAGGGAGAAGTAGGAATG | Mouse |
| IL-18 | ACTGTACAACCGCAGTAATACGC | AGTGAACATTACAGATTTATCCC | Mouse |
| T-bet | CAACAACCCCTTTGCCAAAG | TCCCCCAAGCAGTTGACAGT | Mouse |
| STAT4 | CCTGGGTGGACCAATCTGAA | CTCGCAGGATGTCAGCGAA | Mouse |
| GAPDH | TCCACCACCCTGTTGCTGTA | ACCACAGTCCATGCCATCAC | Mouse |
Immunoblotting.
Whole-cell lysates were prepared using RIPA lysis buffer supplemented with protease and phosphatase inhibitors. Immunoblotting was performed as described previously (17). The following antibodies were used: p-STAT4 (Abcam) and STAT4 (Santa Cruz).
Statistical Analysis.
Student’s t test (two tailed) was used to analyze the data for statistically significant differences. Values of P < 0.05 were regarded as statistically significant.
Acknowledgments
We thank Professor Hiroko Tsutsui (Hyogo College of Medicine, Japan) for providing the heat-killed P. acnes bacteria and Chugai Pharmaceutical Co., Ltd. (Tokyo) for making the recombinant Arid5a protein. This work was funded by the Kishimoto Foundation and WPI Immunology Frontier Research Center.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613307113/-/DCSupplemental.
References
- 1.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6(1):19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11(1):571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 3.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145(9):2920–2924. [PubMed] [Google Scholar]
- 4.Car BD, et al. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179(5):1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enoh VT, et al. Mice depleted of alphabeta but not gammadelta T cells are resistant to mortality caused by cecal ligation and puncture. Shock. 2007;27(5):507–519. doi: 10.1097/SHK.0b013e31802b5d9f. [DOI] [PubMed] [Google Scholar]
- 6.van Schaik SM, Abbas AK. Role of T cells in a murine model of Escherichia coli sepsis. Eur J Immunol. 2007;37(11):3101–3110. doi: 10.1002/eji.200737295. [DOI] [PubMed] [Google Scholar]
- 7.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 8.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 9.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12(7):597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarevic V, Glimcher LH, Lord GM. T-bet: A bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13(11):777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda K, et al. Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci USA. 2013;110(23):9409–9414. doi: 10.1073/pnas.1307419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda K, et al. Arid5a regulates naive CD4+ T cell fate through selective stabilization of Stat3 mRNA. J Exp Med. 2016;213(4):605–619. doi: 10.1084/jem.20151289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93(3):329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7(3):167–202. [PubMed] [Google Scholar]
- 15.Kawa K, et al. IFN-gamma is a master regulator of endotoxin shock syndrome in mice primed with heat-killed Propionibacterium acnes. Int Immunol. 2010;22(3):157–166. doi: 10.1093/intimm/dxp122. [DOI] [PubMed] [Google Scholar]
- 16.Thierfelder WE, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382(6587):171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 17.Bacon CM, et al. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci USA. 1995;92(16):7307–7311. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patsialou A, Wilsker D, Moran E. DNA-binding properties of ARID family proteins. Nucleic Acids Res. 2005;33(1):66–80. doi: 10.1093/nar/gki145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9(4):353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 20.Mattner F, et al. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65(11):4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui M, Moriya O, Yoshimoto T, Akatsuka T. T-bet is required for protection against vaccinia virus infection. J Virol. 2005;79(20):12798–12806. doi: 10.1128/JVI.79.20.12798-12806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol. 2005;175(7):4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 23.Uehata T, et al. Malt1-induced cleavage of regnase-1 in CD4(+) helper T cells regulates immune activation. Cell. 2013;153(5):1036–1049. doi: 10.1016/j.cell.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 24.Barkhausen T, et al. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response. Shock. 2008;30(4):401–410. doi: 10.1097/SHK.0b013e31816e2cda. [DOI] [PubMed] [Google Scholar]