Significance
Specialized secretion systems transport proteins across the double-membrane cell envelope of Gram-negative bacteria. Gram-positive bacteria possess a single membrane and lack many of these secretion systems. During endospore formation in Gram-positive bacteria such as Bacillus subtilis, a double-membrane envelope surrounds the developing spore. A transenvelope complex with similarities to Gram-negative specialized secretion systems spans the two membranes separating mother cell and endospore. This complex is essential for development and has been hypothesized to serve as a channel for molecular transport between the two cells. Here we show that it contains an oligomeric ring with architecture and dimensions similar to those found in type III secretion systems, providing direct evidence for a conduit connecting mother cell and developing spore.
Keywords: sporulation, SpoIIIAG, type III secretion system, EscJ/PrgK/FliF, SigG
Abstract
During spore formation in Bacillus subtilis a transenvelope complex is assembled across the double membrane that separates the mother cell and forespore. This complex (called the “A–Q complex”) is required to maintain forespore development and is composed of proteins with remote homology to components of type II, III, and IV secretion systems found in Gram-negative bacteria. Here, we show that one of these proteins, SpoIIIAG, which has remote homology to ring-forming proteins found in type III secretion systems, assembles into an oligomeric ring in the periplasmic-like space between the two membranes. Three-dimensional reconstruction of images generated by cryo-electron microscopy indicates that the SpoIIIAG ring has a cup-and-saucer architecture with a 6-nm central pore. Structural modeling of SpoIIIAG generated a 24-member ring with dimensions similar to those of the EM-derived saucer. Point mutations in the predicted oligomeric interface disrupted ring formation in vitro and impaired forespore gene expression and efficient spore formation in vivo. Taken together, our data provide strong support for the model in which the A–Q transenvelope complex contains a conduit that connects the mother cell and forespore. We propose that a set of stacked rings spans the intermembrane space, as has been found for type III secretion systems.
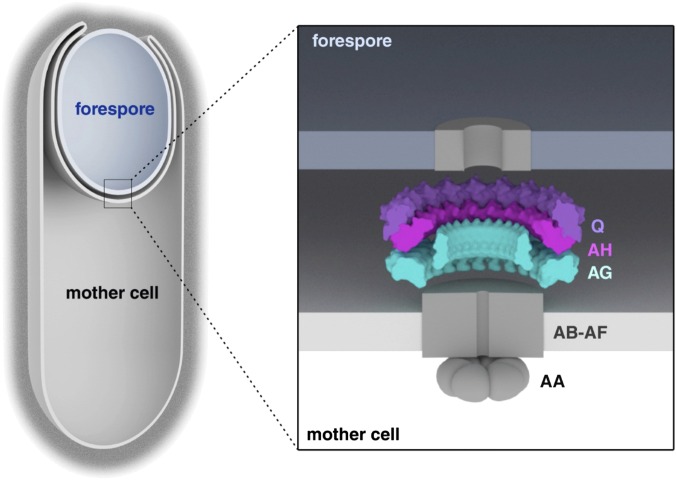
Transport of proteins across the outer membrane of Gram-negative bacteria requires specialized secretion systems (1, 2). These transenvelope complexes span the inner and outer membranes and use ATP hydrolysis in the cytoplasm to power secretion across the outer membrane. Gram-positive bacteria lack an outer member and in most cases lack these specialized secretion systems. Endospore formation in bacteria such as Bacillus subtilis provides an unusual and noteworthy example of a Gram-positive, double-membrane envelope. As a result of the phagocytic-like process of engulfment, the developing endospore (called the “forespore”) is released into its sister cell (referred to as the “mother cell”), surrounded by an inner membrane derived from the forespore and an outer membrane derived from the mother cell (Fig. 1B) (3). Intriguingly, the mother cell and forespore assemble a multimeric complex spanning these two membranes that bears similarity to specialized secretion systems and is required to maintain forespore development (4–9). It has been proposed that these proteins constitute a hybrid specialized secretion system with a channel connecting mother cell and forespore. Here we provide direct evidence that this complex contains a ring-like conduit in the space between the two membranes.
Fig. 1.
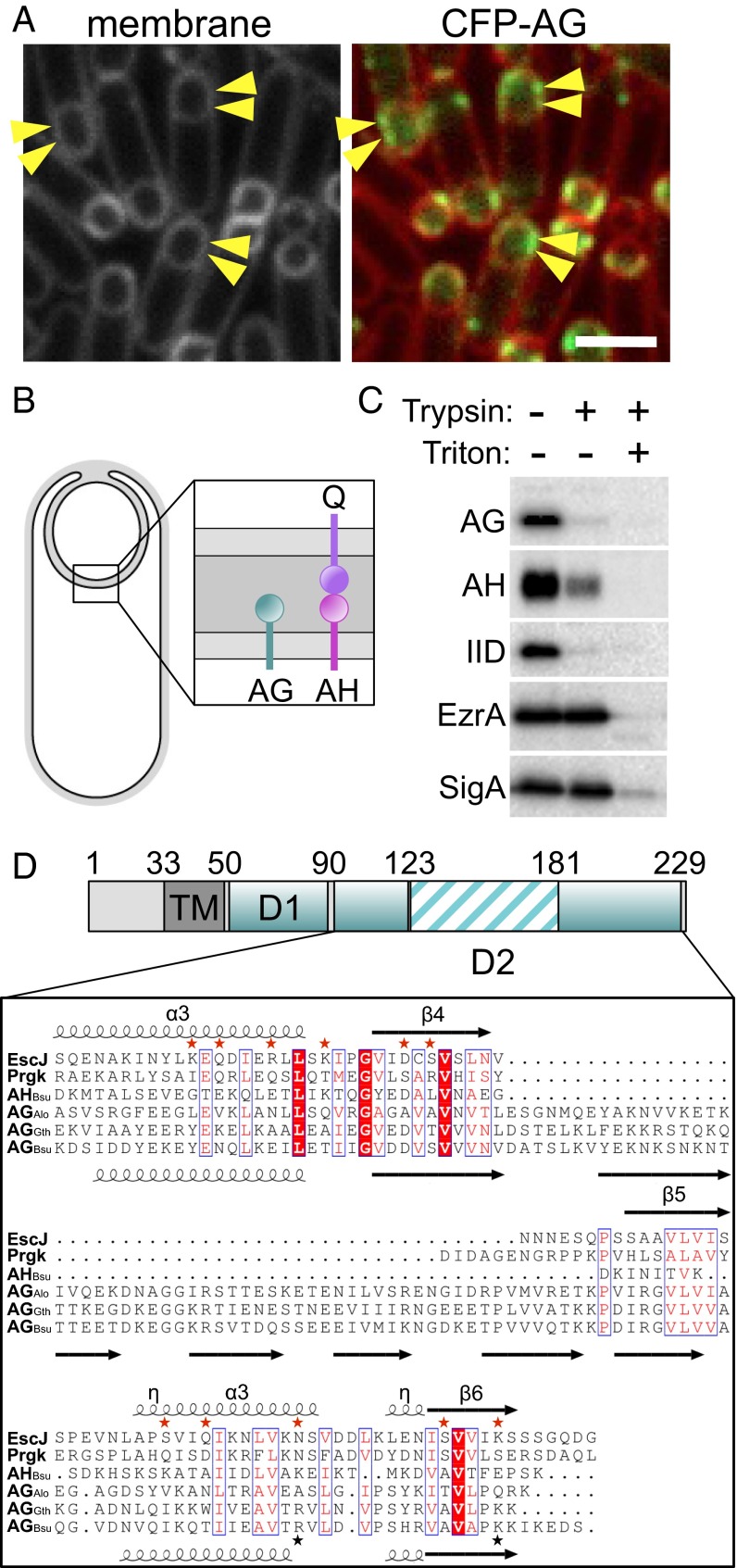
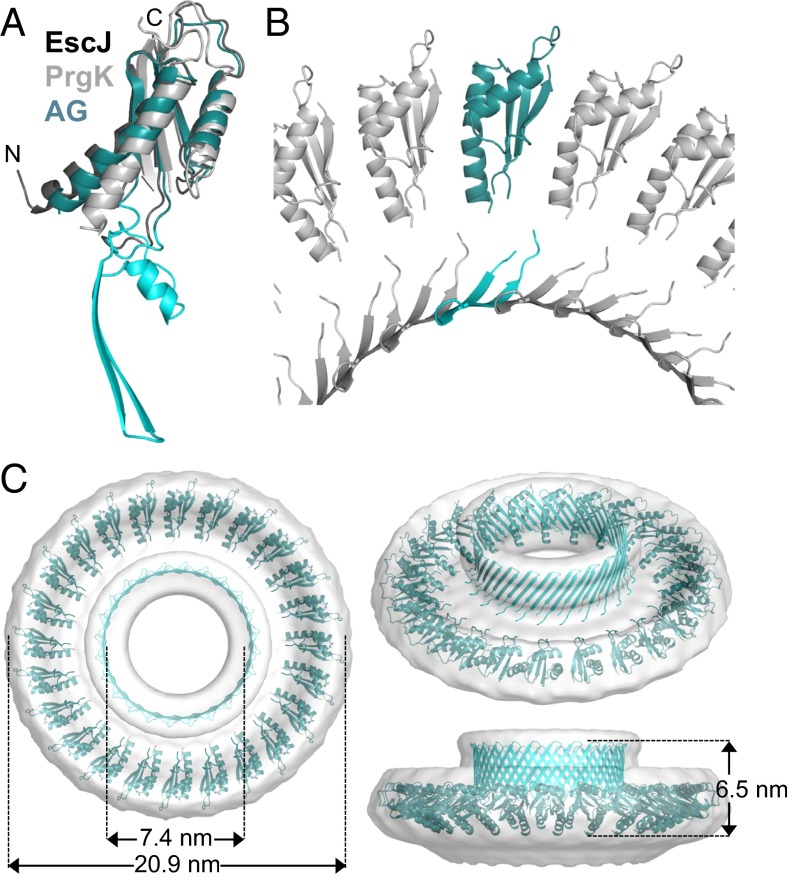
The extracellular domain of AG has remote homology to PrgK/EscJ proteins in type III secretion systems. (A) CFP-SpoIIIAG (CFP-AG, false-colored green) localizes as discrete foci (arrowheads) in the mother cell membranes that surround the forespore. CFP-AG displays weak localization at the second potential polar division site, but the relevance of this localization remains unclear. CFP-AG and membranes (stained with the fluorescent probe TMA-DPH) were visualized by fluorescence microscopy at hour 2 of sporulation. (Scale bar, 2 μm.) (B) Schematic diagram showing the localization and topology of AG, SpoIIIAH (AH), and SpoIIQ (Q) during sporulation. (C) The extracellular domains of AG, AH, and SpoIID (IID) are extracytoplasmic. Immunoblot of a protease susceptibility assay in which sporulating cells were protoplasted and treated with trypsin with or without Triton X-100. A soluble transcription factor (SigA) and a membrane-anchored cytoplasmic protein (EzrA) were inaccessible to trypsin. (D) Domain structure of AG showing the transmembrane segment (TM) and the extracytoplasmic D1 and D2 domains. Numbering refers to the B. subtilis AG sequence. The sequences of AG from B. subtilis (AGBsu), G. thermodenitrificans NG-80 (AGGth), and A. longum (AGAlo) were aligned with S. typhimurium SPI‐1 and PrgK and E. coli EPEC LEE EscJ. Conserved residues are in red boxes; similar residues are shown by red letters boxed in blue. The secondary structures of EscJ and the ones predicted for AGBsu are indicated above and below the sequence alignment, respectively. Arrows indicate β-strands; α, α-helix; η, 310-helices. Residues at the oligomeric interface of EscJ protomers are indicated by red stars. Predicted AGBsu interface residues important for ring formation and sporulation are indicated by with black stars.
B. subtilis differentiates into a stress-resistant spore in response to nutrient limitation (3). The first morphological event in this process is the formation of an asymmetrically placed septum generating a small forespore and larger mother cell. Shortly afterwards, the mother cell membranes migrate around the forespore, generating a cell within a cell. Eight mother cell proteins encoded in the spoIIIA operon (AA, AB, AC, AD, AE, AF, AG, and AH) and one forespore protein, SpoIIQ (Q), are required during and/or shortly after engulfment to maintain forespore development. Cells lacking any of these nine proteins have the same phenotype: The engulfed forespores fail to grow to their full size and frequently develop membrane invaginations and appear to collapse (7, 10). In addition, these forespores are unable to maintain transcriptional potential including gene expression under the late-acting forespore transcription factor SigG (5, 7).
Previous work indicates that most of the A–Q proteins reside in a multimeric complex that spans the two membranes surrounding the forespore; for simplicity, we refer to this complex as a “transenvelope complex,” although whether it spans the nascent spore coat remains unclear (7). Q produced in the forespore localizes to the inner forespore membrane and is required for the localization of AH in the outer forespore membrane (11, 12). This localization is mediated by direct protein–protein interaction between the extracellular domains of AH and Q in the intermembrane space (Fig. 1B) (11, 12). Furthermore, coimmunoprecipitation experiments have shown that AB, AD, AE, AF, and AG reside in a multimeric membrane complex (7). Finally, AG has been found to localize in the membranes surrounding the forespore (Fig. 1A), and this localization depends on AH and Q (7).
The role of this complex in maintaining forespore development has been informed by the remote homologies (13) of many of the proteins in this complex (4, 6, 7). AA resembles secretion ATPases found in type IV secretion systems, whereas AB and AE both have domains with remote homology to GspF that helps tether the secretion ATPase to the membrane complex in type II secretion systems. Finally, AF, AG, and AH have remote homology to the EscJ/PrgK family of ring-forming proteins found in type III secretion systems. Importantly, cocrystal structures of a heterodimeric complex consisting of the extracellular domains of AH and Q revealed structural similarity between AH and EscJ/PrgK family members (14, 15). Furthermore, using this structure, the extracellular domains of AH and Q could be modeled into oligomeric rings. Based on the remote sequence homologies and this structural similarity, it has been proposed that AH and Q form a channel in the intermembrane space and that the A–Q complex functions as a specialized secretion system or a feeding tube, allowing the mother cell to nurture the forespore and maintain forespore development (4–7, 14, 15). The structure of this transenvelope complex and whether it functions to transport molecules between the two cells remain important outstanding questions.
Here, we show that the extracellular domains of AG from three endospore formers, B. subtilis, Geobacillus thermodenitrificans, and Acetonema longum, assemble into large oligomeric rings in vitro. 3D reconstruction of images generated by cryo-EM indicates that the B. subtilis AG ring has a “cup-and-saucer” architecture, similar to that of the EscJ/PrgK family member FliF, which is part of the flagellar basal body (16). Structural modeling of AG generated a ring with dimensions similar to those of the saucer, and point mutants in the predicted oligomeric interface disrupted ring formation in vitro and impaired SigG activity and spore formation in vivo. These data indicate that the A–Q complex contains a conduit that connects the mother cell and the forespore and support a model in which stacked rings similar to those found in type III secretion systems span the intermembrane space.
Results
The Extracellular Domain of AG Has Remote Homology to the EscJ/PrgK Family.
The AG protein has a single N-terminal transmembrane segment and a large soluble domain (Fig. 1D). A sequence alignment of more than 20 AG orthologs using ClustalW (17) indicates that the soluble domain displays low sequence conservation over the first ∼40 amino acids (designated “D1”) and higher sequence conservation over the remaining ∼140 C-terminal residues (called “D2”) (Fig. 1D). The D2 domain of AG has been reported previously to have remote homology to the EscJ/PrgK family of ring-forming proteins in type III secretion systems (6, 7). Alignment of the D2 domain from AG orthologs with EscJ from Escherichia coli EPEC (18) and PrgK from Salmonella typhimurium SPI‐1 (19, 20) revealed that AG proteins contain two regions (residues 90–123 and 181–229) that share weak sequence and secondary structure similarities with these ring-forming proteins. However, AG orthologs contain an insertion between these regions (residues 124–180) with no homology to known structures (Fig. 1D). This extended D2 domain was recently reported by Bergeron (21). To investigate whether the soluble portion of AG (D1+D2) resides in the cytoplasm or, like AH, in the space between the mother cell and forespore membranes (Fig. 1B), we analyzed its localization by protease susceptibility. Sporulating cells were treated with lysozyme in hypertonic buffer to generate protoplasts. The protoplasts then were treated with trypsin followed by immunoblot analysis using anti-AG antibodies. Consistent with the idea that the D1 and D2 domains of AG are extracellular, these domains and the extracellular domain of AH and the sporulation protein SpoIID were susceptible to protease digestion (Fig. 1C). By contrast, an integral membrane protein with a large cytoplasmic domain (EzrA) and a soluble transcription factor (SigA) were both fully protected.
The Extracellular Domain of AG Forms Large Oligomeric Rings.
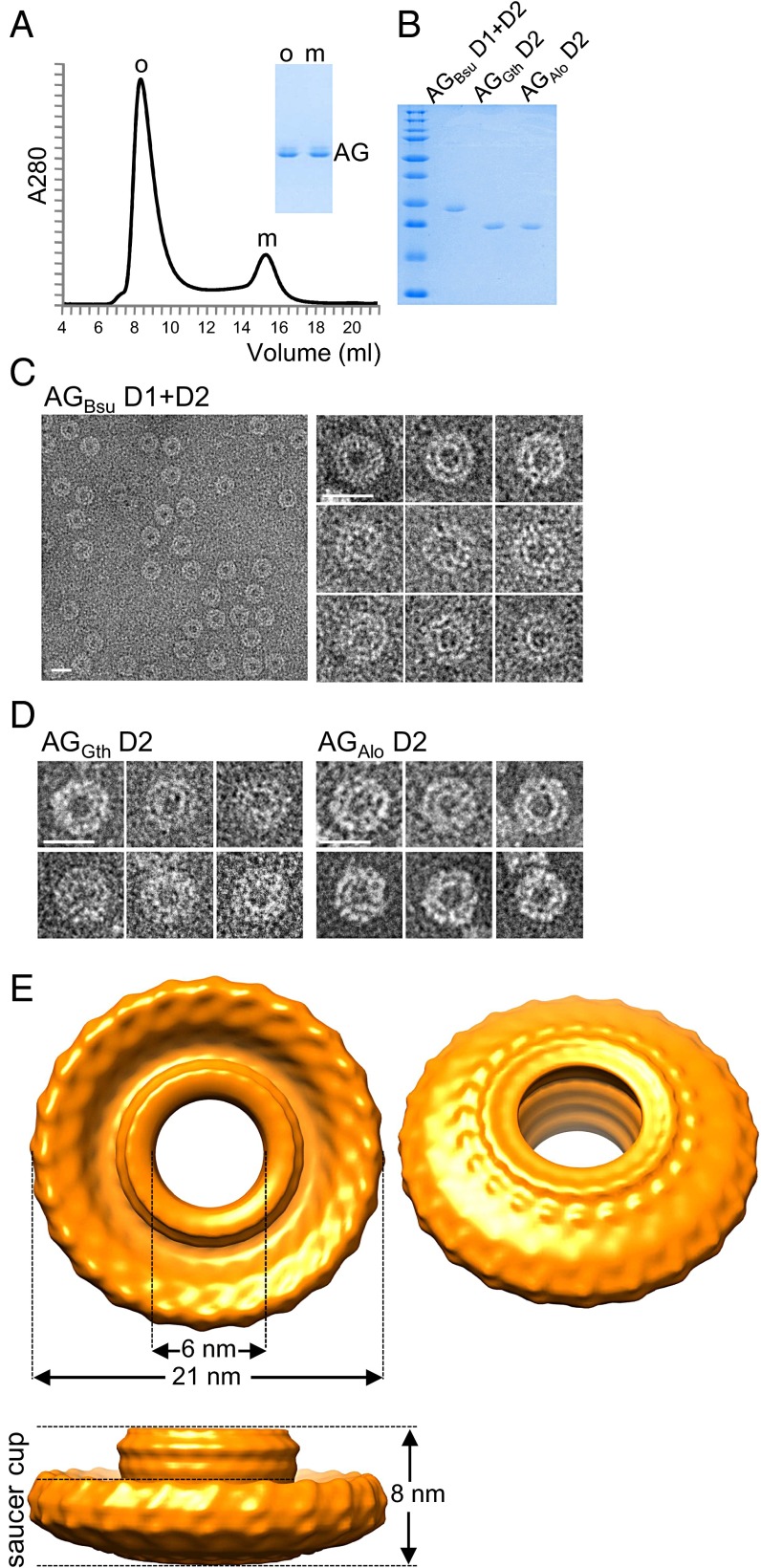
As a first step toward structural determination, we expressed the extracellular domain (D1+D2) of AG from B. subtilis (AGBsu) in E. coli and purified it to homogeneity (Fig. 2A). Size-exclusion chromatography resolved the purified protein into two distinct species, a small one eluting at ∼15 mL and a larger one eluting at ∼8.5 mL, indicative of an oligomeric complex (Fig. 2A). Calibration using protein standards indicate apparent molecular masses of 22.5 and 550 kDa for the small and large species, respectively. Negative-stain EM of the large molecular weight species revealed homogeneous rings with a large central cavity (Fig. 2C). The majority of the particles had the same orientation and dimensions, with a diameter of ∼20 nm and an apparent central pore of ∼7 nm. To investigate whether these structures were unique to B. subtilis AG, we expressed and purified the D2 domains from two other AG orthologs (Fig. 2B). One was from G. thermodenitrificans (AGGth, 46% identity to AGBsu), and the other was from the Gram-negative bacterium A. longum (AGAlo, 25% identity to AGBsu), which differentiates into an endospore. Negative-stain EM revealed ring-shaped structures similar to those of AGBsu (Fig. 2D). Thus, the ability to assemble into oligomeric rings in vitro is a property shared among distantly related AG family members, suggesting that these rings are a physiologically relevant structure.
Fig. 2.
The extracellular domain of AG forms large oligomeric rings in vitro. (A) Size-exclusion chromatography of the purified AGBsu D1+D2 protein. The elution volume (from a Superdex 200 column) is plotted on the x axis, and the 280-nm absorbance is plotted on the y axis. The void volume was 7.5 mL. Coomassie-stained gel of the oligomeric (o) and the monomeric (m) fractions is shown. (B) Coomassie-stained gel of the purified D1+D2 protein from B. subtilis and the D2 domain from G. thermodenitrificans and A. longum. (C and D) Negative-stained EM images of AG oligomers purified by size exclusion. (Scale bars, 20 nm.) (E) 3D cryo-EM reconstruction of AG D1+D2 from B. subtilis. The models are visualized at 2.0σ.
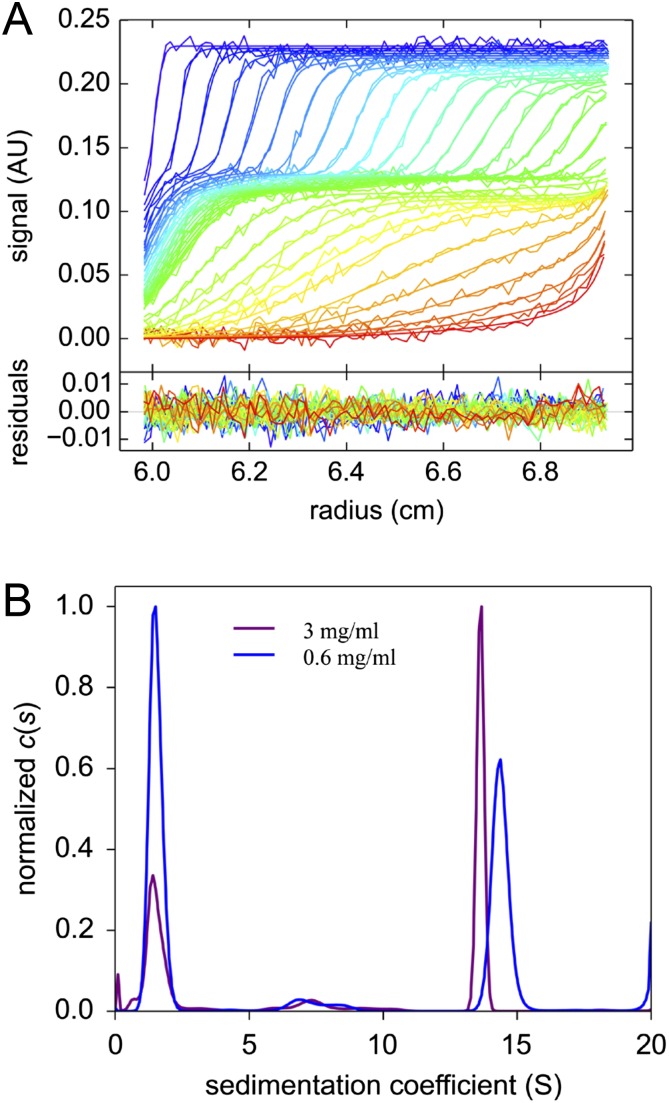
To characterize the AGBsu rings further, we performed analytical ultracentrifugation. Differential sedimentation coefficient distributions [c(s)] (Fig. S1) defined a species at a sedimentation coefficient (S) of 1.5 consistent with an elongated AGBsu monomer of 19.7 kDa. A second species was determined at 14.5 S, compatible with a 24-mer with a slightly anisotropic shape (frictional ratio f/fmin = 1.5) and a hydrodynamic radius (RH) of 7.6 nm. Because analytical ultracentrifugation is accurate to approximately ±10%, we tentatively conclude that the AGBsu ring is composed of 24 protomers. This order of rotational symmetry is similar to that of the EscJ, PrgK, and FliF rings (24 for EscJ/PrgK and 24–26 for FliF) (16, 18, 20), further supporting the idea that members of the SpoIIIA complex resemble the periplasmic rings found in type III secretion systems.
Fig. S1.
Biochemical characterization of AGBsu. (A) Analytical ultracentrifugation analysis of AGBSu D1+D2 (at 3 mg/mL). The superposition of experimental and fitted sedimentation velocity profiles (Upper) and their differences (Lower) are shown. (B) Sedimentation coefficient distributions obtained for AGBSu D1+D2 at 3 and 0.6 mg/mL.
Cryo-EM 3D Reconstruction of the AGBsu Ring Reveals a Cup-and-Saucer Architecture.
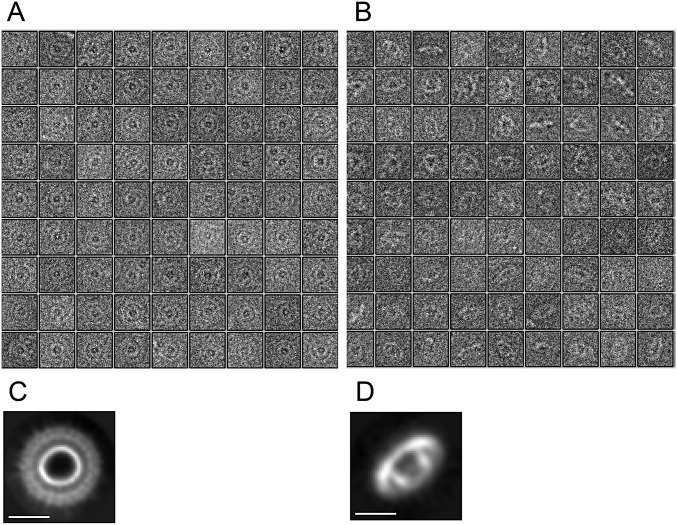
More than 15,000 particle images were collected from vitrified samples, and ∼6,000 were analyzed by projection matching (Fig. S2; see SI Materials and Methods for details). To obtain a 3D reconstruction, 24-fold rotational symmetry was imposed based on the results from analytical ultracentrifugation. Unfortunately, very few (<200) side views of the AGBsu ring were collected (Fig. S2 B and D). Accordingly, the resolution of the resulting map (Fig. 2E) was estimated to be ∼35 Å. Nevertheless, the reconstruction of the AGBsu ring revealed a cup-and-saucer architecture with a central pore (Fig. 2E). The outer diameter of the saucer was 21 nm, and the outer and inner diameters of the cup were 11 nm and 6 nm, respectively. The height of the cup and saucer together was 8 nm. The AG saucer had dimensions similar to those of the rings formed by PrgK, EscJ, and FliF (outer diameters of 18.7, 18, and 24 nm, respectively) (16, 18, 20). Interestingly, the AG cup is reminiscent of domain R in the FliF ring (16), which forms a cup-like structure that is thought to contact the cylindrical rod of the flagellum that traverses the outer membrane (Discussion).
Fig. S2.
AGBsu forms a large oligomeric complex. (A and B) Selection of 81 cryo-EM images of the AGBSu D1+D2 ring particles seen in top (A) or side (B) orientations. (C and D) Class averages of AGBsu D1+D2 ring particles in top (C) and side (D) orientations obtained by ab initio classification using RELION. (Scale bars, 10 nm.)
In Silico Modeling of the AGBsu Monomer and Ring.
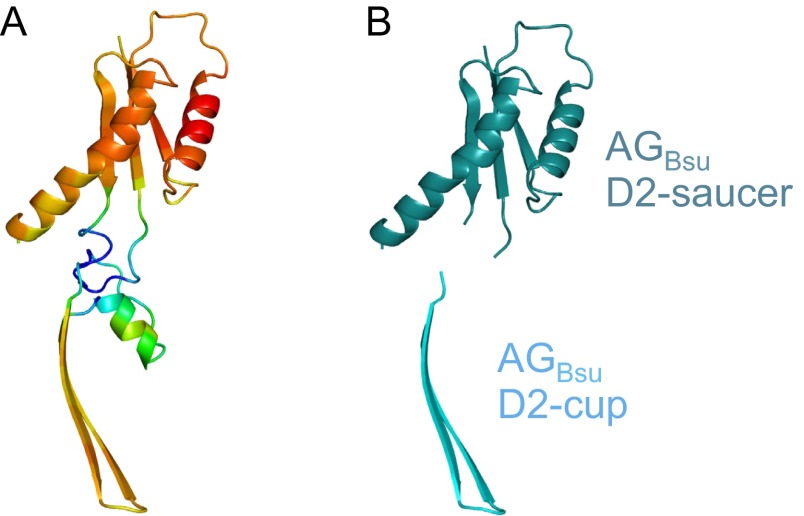
Despite intensive efforts to provide atomic resolution of the AG ring, we were unable to obtain crystals that diffracted to high resolution from either the D2 or the D1+D2 domains from AGBsu, AGGth, or AGAlo. Accordingly, we performed in silico modeling on the D2 domain of AGBsu and fitted the structural model into our EM density map. Using the Swiss Model server (22), a model based on the structure of PrgK was generated (Fig. 3A). In the two regions of AGBsu homologous to the EscJ/PrgK family (residues 90–123 and 181–229), the model contained high-confidence factors (mean value ± SD, 0.6 ± 0.2). Modeling of the insertion between these regions (residues 124–180) was more problematic: Confidence factors were particularly low in the regions encompassing residues 124–128 and 155–180 (0.13 ± 0.1) (Fig. S3). However, the region encompassing residues 129–154 was predicted to form a two-stranded antiparallel β-sheet with a mean confidence factor of 0.54 ± 0.2 (Fig. 3A). This latter prediction is supported by in silico secondary structure predictions and the high proportion of β-structures in the AGBsu rings detected by solid-state NMR (Fig. S4).
Fig. 3.
3D modeling of the AG ring. (A) Ribbon representation of the raw AGBsu D2 model (AG) superimposed on the D2 structures of EscJ (dark gray) and PrgK (light gray). The AGBsu regions homologous to EscJ/PrgK are in teal, and the insertion region is in cyan. (B) Close-up view of the modeled AGBsu D2 ring. (C) Ribbon representation of the AGBsu D2 ring model in the EM map density (SI Materials and Methods).
Fig. S3.
3D modeling of the AGBsu ring. (A) Ribbon representation of the raw AGBsu D2 model colored by model quality value. Red corresponds to regions with high-quality values (∼0.8) and are built on X-ray structures. Blue regions have low-quality values (∼0.1) and are built from loop databases without template contribution. (B) Ribbon representation of the domains in the AGBsu D2 model that were used for modeling the AGBsu D2 ring in the cup (AGBsu D2-cup, in cyan) and saucer (AGBsu D2-saucer, in teal).
Fig. S4.
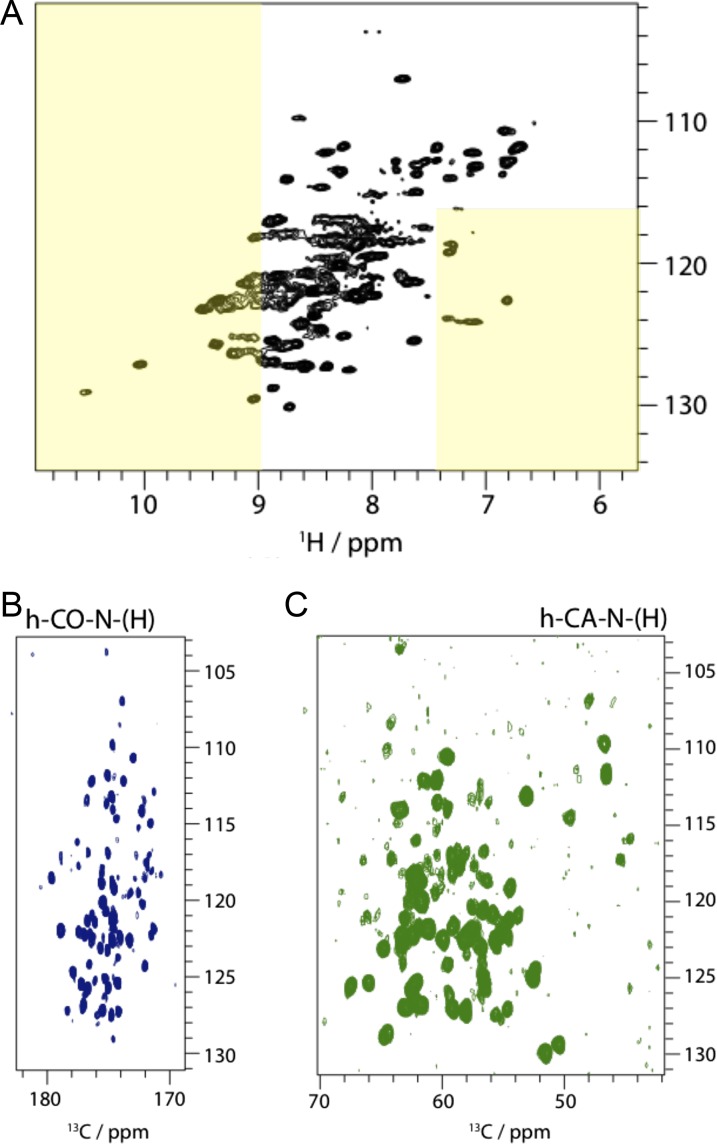
Solid-state NMR studies of AGBsu D1+D2. NMR spectra obtained on AGBsu D1+D2 (∼1.5 mg), using proton-detected solid-state magic-angle-spinning (MAS) NMR, obtained at 55-kHz MAS frequency at a magnetic field strength of 14.1 T at an effective sample temperature of 22 ± 2 °C. (A) 2D H–N correlation spectrum. Narrow peak widths indicate a high degree of sample homogeneity and symmetry among the rings. The dispersion of amide signals, over more than 3 ppm in the 1H dimension strongly suggests that the rings contain β-strand structures. Yellow rectangles show the region of the spectrum where β-sheet signals are typically found. Amide signals in β-sheets do not necessarily lie in these regions, but signals in these regions are most often caused by β-sheets. The two signals shifted down-field at >10 ppm are backbone amide signals, and their strong down-field shift strongly suggests that these residues have a β-sheet conformation of these residues. AGBsu D1+D2 does not contain Trp residues, excluding the possibility that these signals arise from Trp side chains. (B and C) 3D CO-N-H (B) and CA-N-H (C) correlation experiments. Both experiments are 2D projections along the 1H dimension. Experiments were performed with cross-polarization schemes, which focus exclusively on rigid proteins, i.e., all the observed signals arise from residues in rigid structures. Taken together, the solid-state NMR data suggest that AGBSu D1+D2 rings form rigid structures containing a high proportion of β-sheet conformations.
Using the regions of the AGBsu monomer that had structural predictions with confidence factors >0.2 (Fig. S3), we modeled the ring. The domain corresponding to the two regions homologous to EscJ/PrgK (residues 90–123 and 181–224) was placed in the EM map manually, and a 24-fold rotational symmetry was applied. This step was followed by energy minimization using NAMD (23). The resulting 24-member ring could be fit easily into the saucer derived from the EM map with an outer diameter of the ring of 20.9 nm (Fig. 3 B and C). However, density at the bottom of the saucer and the entire cup were missing. Because the cup is present in the rings from the D2 domains of AGGth and AGAlo (Fig. 2D), we suspect that the D1 domain (residues 51–90) from AGBsu, which was not used in the modeling, does not make up the cup but instead is the missing density in the saucer. In support of this idea, the ring model predicts that the N terminus of the D2 domain is oriented toward the bottom of the saucer. To investigate whether the insertion region in the D2 domain that was predicted to form a two-stranded antiparallel β-sheet (residues 129–154) could be the cup, we independently placed it in the EM map, applied a 24-fold rotational symmetry, and performed energy minimization (23). The resulting β-barrel had inner and outer diameters of 7.4 and 10.4 nm, respectively, that fit easily into the EM map of the cup (Fig. 3 B and C). Although this structural prediction is consistent with our EM 3D reconstruction, residues 124–128 and 155–180 of the insertion region that could not be modeled with high-confidence factors were not included in modeling the ring. We therefore tentatively conclude that the D2 domain constitutes both the cup and saucer observed by EM.
In Vivo Test of the AG Ring Model.
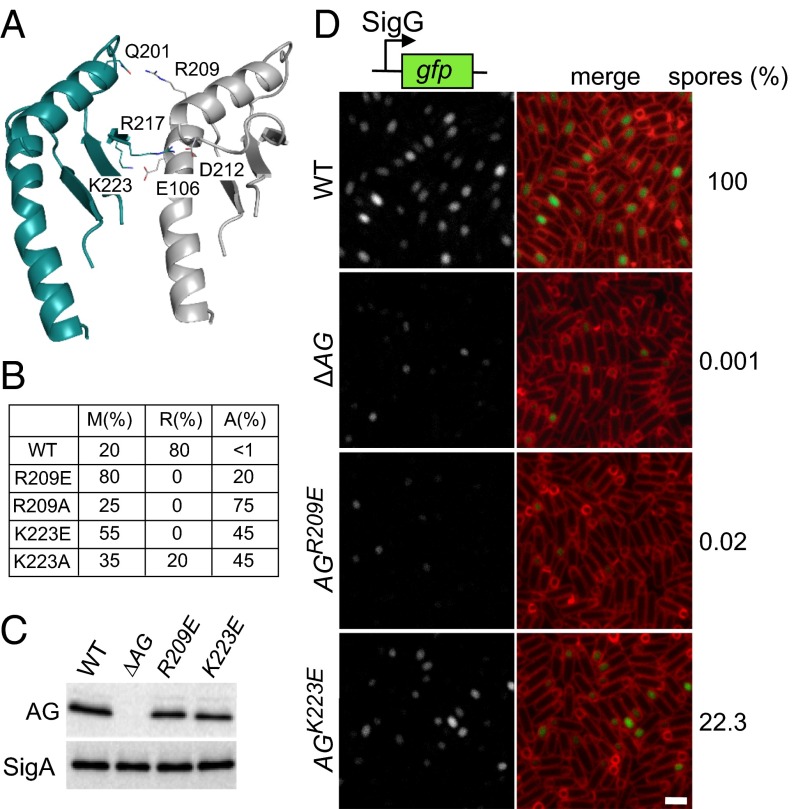
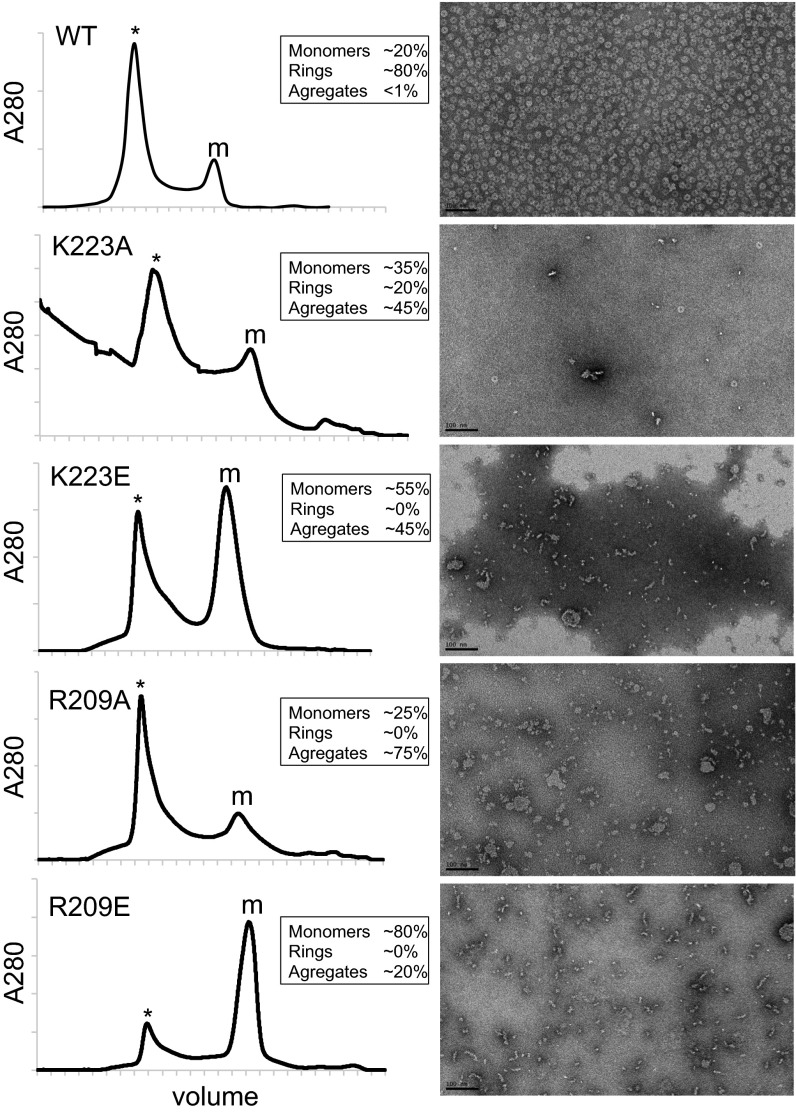
To test the ring model, we focused on the oligomeric interface in the two regions homologous to EscJ/PrgK that make up the saucer. The model predicts hydrophilic interactions between Q201, R217, and K223 on one protomer interface and R209, D212, and E106, respectively, on the other (Fig. 4A). We generated site-directed mutations (R209E, R209A, K223E, and K223A) in the D1+D2 expression construct and purified the four mutant proteins. Size-exclusion chromatography revealed that all four had an increase in monomeric species (Fig. 4B and Fig. S5). Analysis of the larger species by negative-stain EM indicates that most contained aggregates or fibers rather than rings (Fig. S5). These data are consistent with R209 and K223 playing a role in oligomerization. To investigate whether oligomerization is important in vivo, we generated R209E and K223E substitutions in the context of the full-length AG gene and tested them for their ability to support SigG activity and efficient sporulation. These mutants were chosen because they were the most monomeric in vitro and produced the least amount of aggregated protein (Fig. 4B and Fig. S5). Sporulating cells harboring AG(R209E) were impaired in SigG activity, had a small forespore phenotype, and were significantly reduced in sporulation efficiency (Fig. 4D). Cells harboring AG(K223E) were also reduced in sporulation efficiency, although not as strongly (approximately fivefold). Importantly, a subset of forespores was smaller and had impaired SigG activity. Both proteins were produced at levels similar to the wild-type control (Fig. 4C). Collectively, these data are consistent with the idea that AG forms a ring in vivo and that this ring is important for the proper function of the A–Q complex in maintaining forespore physiology, SigG activity, and efficient spore formation.
Fig. 4.
In vitro and in vivo test of the AG ring model. (A) Close-up view of the interface between two adjacent subunits in the saucer region of the AGBsu D2 ring model. Residues predicted to make up the oligomeric interface are labeled. (B) Table showing the proportion of monomers (M), rings (R), and aggregates (A) assessed by gel filtration and negative-stain EM for the AGBsu D1+D2 variants. The primary data can be found in Fig. S5. (C) Immunoblot analysis of whole-cell lysates from sporulating wild-type (BCR1434), ΔAG mutant (BCR776), AG(R209E) (BCR1435), and AG(K223E) (BCR1436) with anti-AG antibodies. SigA levels were monitored to control for loading. (D) SigG activity (Left) and sporulation efficiency (Right) of B. subtilis cells with AG mutants. Wild-type (BCR1438), ΔAG mutant (BCR1437), and cells expressing AG(R209E) (BCR1439) or AG(K223E) (BCR1440) were visualized 4 h after the initiation of sporulation. All strains harbor a SigG-responsive promoter (PsspB) fused to GFP. Images of PsspB-GFP fluorescence (Left) and of PsspB-GFP fluorescence images merged with TMA-DPH–stained membranes (Right) are shown. The images were scaled identically. (Scale bar, 2 μm.)
Fig. S5.
In vitro test of the AG ring model. Size-exclusion chromatography of purified wild-type AGBsu D1+D2 and the indicated mutants. (Left) Elution volumes from a Superdex 200 column are plotted on the x axis, and the 280-nm absorbance is plotted on the y axis. The elution peaks containing AGBsu D1+D2 monomers (m) and rings and/or aggregates (*) are indicated. (Right) Negative-stain EM images of the aggregate/oligomer fractions. (Scale bars, 100 nm.) The percentage of monomers, rings, and aggregates based on size exclusion and EM is indicated for each purified protein.
AG Is a Core Component of the A–Q Transenvelope Complex.
Previous work suggested that Q and AH represent the core components of a channel that connects the mother cell and forespore (4). Our data indicating that the extracellular domain of AG resides in the intermembrane space and assembles into an oligomeric ring that is important for forespore development suggest that AG is a central part of an A–Q conduit. Accordingly, we returned to the experiments that defined AH and Q as core elements. In previous work, a small in-frame deletion in pbpG encoding a class A penicillin-binding protein was found to bypass partially the requirement for most of the components of the A–Q complex for SigG activity (4). The mechanism by which this mutant bypassed the need for members of the complex was (and remains) unclear, but it was used as an assay to perform epistasis tests. Only AH and Q mutants could not be bypassed by the in-frame deletion, suggesting that they constitute the core components. The strains used in these studies were specifically engineered to eliminate the contribution of SigG autoregulation and any role of the negative regulator CsfB (also called “Gin”) on SigG activity. Using these same strains and fluorescence microscopy to monitor SigG activity and forespore development in single cells, we found that a deletion of the entire spoIIIA operon (including AH) was partially bypassed by the pbpG mutation (Fig. S6), indicating that the bypass is independent of the A–Q complex. Furthermore, analysis of SigG activity in strains that retain SigG autoregulation and CsfB revealed that the pbpG mutation similarly bypassed AH and AG mutants as well as the operon deletion (Fig. S7). In addition, the small and collapsed forespore phenotypes associated with AH and AG mutants were not suppressed by the pbpG mutation (Fig. S8). These results argue that the partial bypass mediated by the PbpG mutant is not a good predictor of core components and, taken together with the data presented above, suggest that AG and AH both contribute to the conduit that connects the mother cell and the forespore.
Fig. S6.
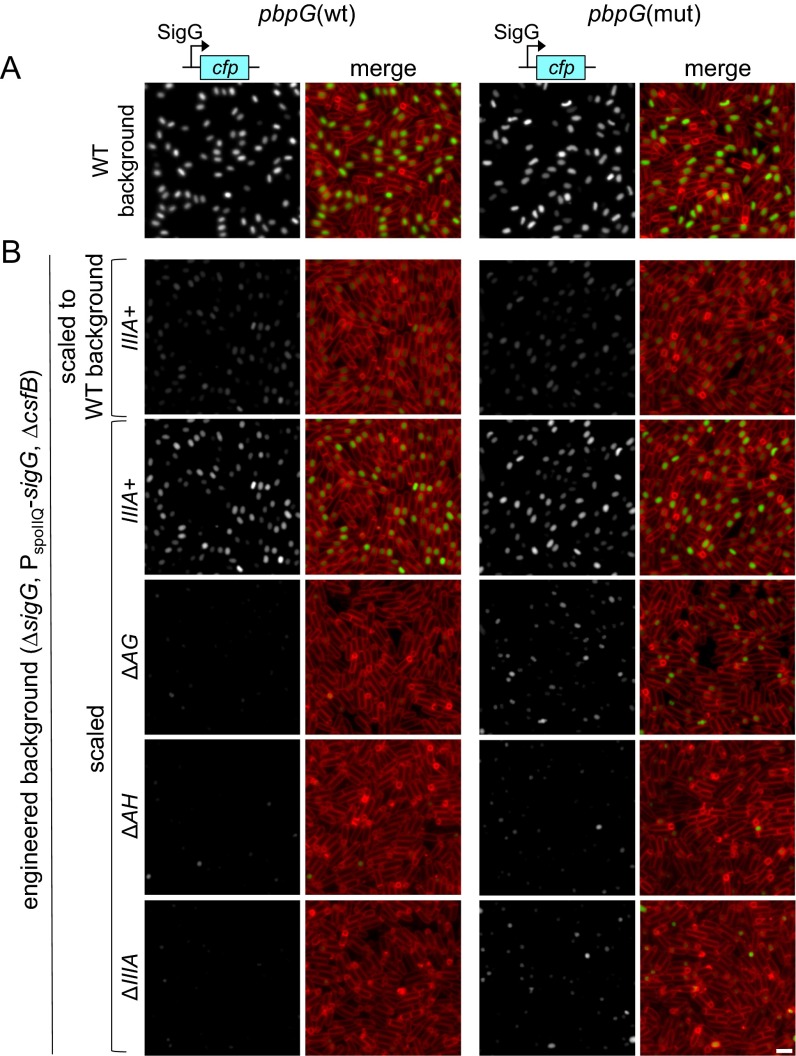
Partial bypass of the spoIIIA operon in sporulating cells with a mutation in pbpG. (A) SigG activity (assessed using PsspB-cfp) in wild-type sporulating cells harboring wild-type pbpG (Left) or a pbpG mutant (pbpG ∆R147-K1480) (Right). Images are from hour 4 of sporulation. Images are PsspB-CFP (Left) and merged with TMA-DPH membrane staining (Right). These strains are shown for comparison with the strains in B. (B) SigG activity (PsspB-cfp) in sporulating cells engineered to bypass SigG autoregulation and lacking the SigG inhibitor CsfB (Gin). Strains harbor the wild-type spoIIIA operon (IIIA+) or lack AG (∆AG), AH (∆AH), or the entire operon (∆IIIA) and contain the wild-type pbpG gene (Left) or a pbpG mutant (pbpG ∆R147-K148) (Right). The first row of panels in B was scaled identically to the panels in A. The remaining panels in B were scaled relative to each other. Strains engineered to bypass autoregulation and lacking CsfB have reduced SigG activity compared with the wild-type strain. The pbpG mutation partially bypasses the requirement of AG or the entire spoIIIA operon (including AH) for SigG activity. The nature of the weaker bypass in the absence of AH in this background is currently unknown. (Scale bar, 2 μm.)
Fig. S7.
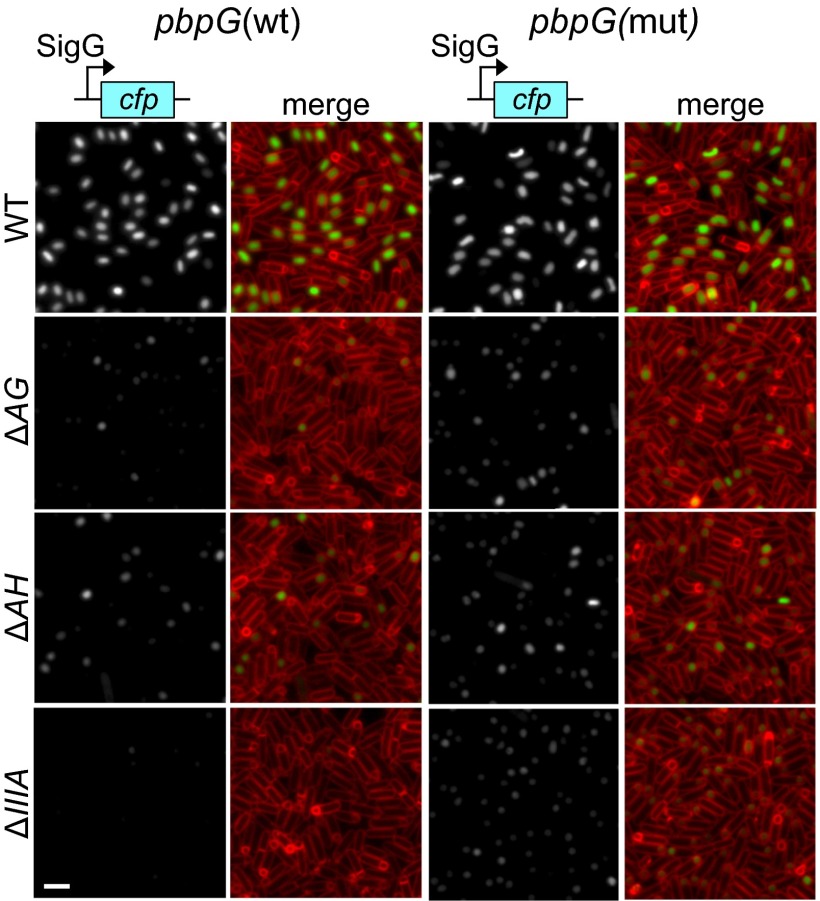
A pbpG mutation partially bypasses the requirement for the spoIIIA operon. Analysis of SigG activity in sporulating cells harboring the indicated mutants in the A–Q complex in the presence of wild-type pbpG (Left) or a pbpG mutant (pbpG ∆R147-K148) (Right). For cells containing wild-type pbpG, the strains used were wild-type (BCR877), ΔAG (BCR878), ΔAH (BCR879), and ΔspoIIIA (∆IIIA) operon-null (BCR990). For the mutant pbpG (mut) allele, the strains used were wild-type (BCR896), ΔAG (BCR959), ΔAH (BCR900), and ΔIIIA (BCR991). Images are PsspB-CFP (Left) and merged with TMA-DPH–stained membranes (Right). The images were scaled identically. (Scale bar, 2 μm.)
Fig. S8.
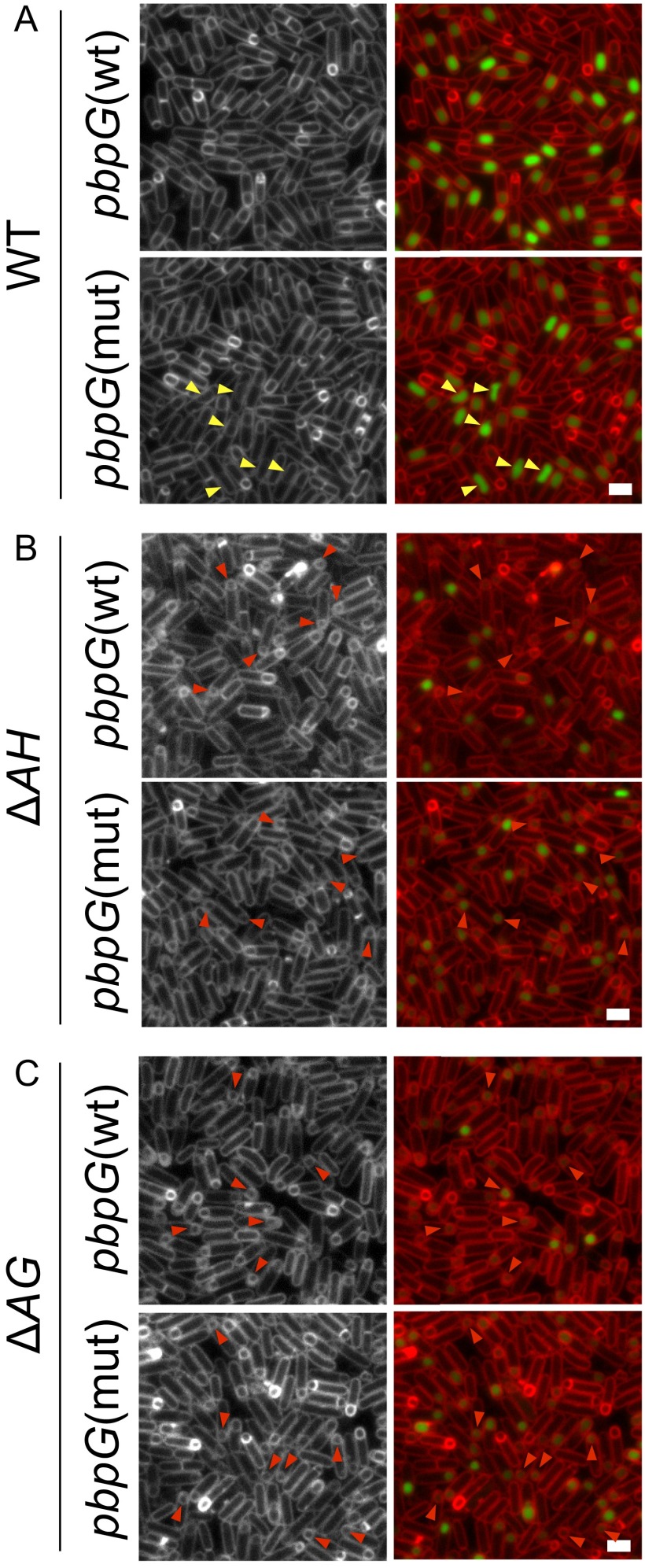
The pbpG mutation does not suppress the forespore morphology defects of the ∆AH and ∆AG mutants. Representative images of SigG activity and forespore morphology assessed with the membrane dye TMA-DPH in strains harboring wild-type pbpG or a pbpG mutant (pbpG ∆R147-K148) in wild-type cells (A), in cells lacking AH (∆AH) (B), and in cells lacking AG (∆AG) (C). Images are from hour 4 of sporulation. We note that sporulating cells harboring the pbpG mutation exhibit forespore morphology defects that are different from the small and collapsed forespores observed in cells lacking members of the A–Q complex. Yellow arrowheads point to forespores with abnormal morphologies in the pbpG mutant, and red arrowheads point to forespores that failed to thrive and/or collapsed. Images show TMA-DPH–stained membrane (Left) and merged with PsspB-CFP (Right). (Scale bars, 2 μm.)
SI Materials and Methods
General Methods.
All B. subtilis strains were derived from the prototrophic strain PY79 (26). Sporulation was induced by resuspension at 37 °C according to the method of Sterlini-Mandelstam (28) or by exhaustion in supplemented Difco Sporulation medium [8 g/L bacto nutrient broth (Difco), 0.1% (wt/vol) KCl, 1 mM MgSO4, 0.5 mM NaOH, 1 mM Ca(NO3)2, 0.01 mM MnCl2, 0.001 mM FeSO4] (29). Sporulation efficiency was determined in 24- to 30-h cultures as the total number of heat-resistant (80 °C for 20 min) cfus compared with wild-type heat-resistant cfus. Deletion mutants were generated by isothermal assembly and direct transformation into B. subtilis.
Fluorescence Microscopy.
Fluorescence microscopy was performed with an Olympus BX61 microscope as previously described (7). Cells were mounted on a 2% agarose pad containing resuspension medium using a gene frame (Bio-Rad). Fluorescent signals were visualized with a phase-contrast objective (UplanF1 100×) and were captured with a monochrome CoolSNAPHQ digital camera (Photometrics) using MetaMorph software version 6.1 (Universal Imaging). The membrane dye 1-(4-(trimethylamino)phenyl)-6-phenylhexa-1,3,5-triene (TMA-DPH) (Molecular Probes) was used at a final concentration of 0.01 mM; exposure times typically were 200 ms. Images were analyzed, adjusted, and cropped using MetaMorph software.
Immunoblot Analysis.
Whole-cell lysates from sporulating cells (induced by resuspension) were prepared as described previously (7). Samples were heated for 10 min at 50 °C before loading. Equivalent loading was based on OD600 at the time of harvest. Proteins were separated by SDS/PAGE on 12.5% polyacrylamide gels, electroblotted onto Immobilon-P membranes (Millipore), and blocked in 5% (wt/vol) nonfat milk in PBS and 0.5% Tween-20. The blocked membranes were probed with anti-SpoIID (1:10,000), anti-SpoIIIAH (1:10,000), anti-SigA (1:10,000), anti-EzrA (1:10,000), and anti-SpoIIIAG (1:10,000), diluted into 3% (wt/vol) BSA in 1× PBS-0.05% Tween-20. Primary antibodies were detected using HRP-conjugated goat, anti-rabbit IgG (1:20,000, Bio-Rad) and the Western Lightning reagent kit as described by the manufacturer (PerkinElmer).
Protease Susceptibility.
Protease susceptibility assays were performed in sporulating cells lacking the SpoIIQ (Q) protein (strain BCR267) to ensure that the membrane proteins present in the inner and outer forespore membranes would not be artificially inaccessible because of protoplast engulfment (30). Twenty-five milliliters of sporulating cells (induced by resuspension) were harvested by centrifugation at hour 2 after the onset of sporulation, washed, and resuspended in 2 mL 1× SMM buffer (0.5 M sucrose, 20 mM MgCl2, 20 mM maleic acid, pH 6.5). The cells were protoplasted by lysozyme (5 mg/mL final concentration) for 10 min with slow agitation. The protoplasts were harvested by centrifugation and resuspended in 1 mL of 1× SMM. Protoplasts (100 μL) were incubated with trypsin (30 μg/mL final concentration) (Worthington), trypsin and Triton X-100 (2% final concentration), or 1× SMM for 15 min. Reactions were terminated by the addition of 100 μL of 2× SDS-sample buffer and incubation for 5 min at 95 °C. Five microliters from each reaction were analyzed by immunoblot.
Protein Expression and Purification.
All recombinant proteins were overexpressed in E. coli Rosetta (DE3) pLysS and purified with a 6×His-SUMO (H-SUMO) tag fused to their N termini (27). Cells were grown in 2 L of Terrific Broth (TB) medium [1.2% (wt/vol) tryptone, 2.4% (wt/vol) yeast extract, 0.4% (vol/vol) glycerol, 0.017 M potassium phosphate dibasic, 0.072 M potassium phosphate monobasic] supplemented with ampicillin (50 μg/mL) at 37 °C to an OD600 of 0.8 and induced by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to 0.5 mM after the cultures were cooled to 25 °C. Growth was continued overnight at 25 °C, and cells were harvested by centrifugation. Cell pellets were resuspended in 1/25th volume of buffer A [50 mM Tris⋅HCl (pH 8.0), 500 mM NaCl, 10% (vol/vol) glycerol] containing 25 mM imidazole and the Complete mixture of protease inhibitor (Roche). Cells were lysed by six passages through a Microfluidizer M110-P (Microfluidics) at 10,000 psi, and cell debris was pelleted by centrifugation at 40,000 × g for 30 min at 4 °C. H-SUMO fusions were purified using 8.0 mL Ni-NTA agarose resin (Qiagen) equilibrated in buffer A with 25 mM imidazole. After loading and extensive washing with buffer A containing 50 mM imidazole, the fusion proteins were eluted with a linear 50–500 mM gradient of imidazole in buffer B [50 mM Tris⋅HCl (pH 8.0), 300 mM NaCl, 10% (vol/vol) glycerol] over 10 column volumes. Peak fractions were pooled and dialyzed overnight at 4 °C in buffer B containing 25 mM imidazole and a 1:500 dilution of a 6×His-tagged Ulp (SUMO) protease (H-SP) preparation (27). Cleavage reactions were passed through Ni-NTA resin to remove free H-SUMO and H-SP, and untagged proteins were collected in the flow through. Flow-through fractions were concentrated with Amicon Ultra Centrifugal filter units with a molecular weight cutoff of 10,000 (Millipore) and were injected onto a 10/300 GL Superdex 200 gel-filtration column (GE Healthcare). Proteins were eluted with buffer C [25 mM Tris⋅HCl (pH 8.0), 150 mM NaCl] and again concentrated with Amicon Ultra Centrifugal filter units. Protein standards used for calibration were ovalbumin (44 kDa), conalbumin (75 kDa), aldolase (158 kDa), and thyroglobulin (669 kDa) (GE Healthcare). Blue Dextran 2000 (GE Healthcare) was used to determine the void volume of the gel-filtration column. Protein concentrations were measured using absorbance at 280 nm.
Analytical Ultracentrifugation Analysis.
Sedimentation velocity experiments were carried out on an analytical ultracentrifuge XLI (Beckman Coulter) with a rotor speed of 130,000 × g, at 20 °C, using an Anti-50 rotor and double-sector cells with an optical path length of 12 or 3 mm equipped with sapphire windows. Acquisitions were made using absorbance at 280 nm. Two samples of AGBsu D1+D2 (0.6 and 3 mg/mL) in buffer C were investigated. Solvent density of 1.004 g/mL and viscosity of 1.054 mPa/s were measured at 20 °C on a DMA 5000 density meter and an AMVn viscosity meter (Anton Paar), respectively, and the partial specific volume was estimated to 0.734 mL/g with the program SEDNTERP. The analysis was carried out in terms of distribution of sedimentation coefficients [c(s)] and noninteracting species with SEDFIT software, version 14.1.
EM.
Samples of AGBsu D1+D2 were diluted to 0.1 mg/mL in buffer C. For negative-stain EM analysis, 3 μL of the sample was loaded on the clean side of carbon on mica (carbon/mica interface), negatively stained with 2% (wt/vol) sodium silico tungstate, and air-dried. Images were collected on a Tecnai 12 microscope (FEI) with a LaB6 electron source operating at 120 kV and equipped with a GATAN camera (Orius 1000). For cryo-EM analysis, 3 μL of the AGBsu D1+D2 sample at 0.4 mg/mL was loaded onto a Quantifoil R2/1 holey grid (Quantifoil Micro Tools GmbH) and then vitrified using a Mark IV Vitrobot (FEI). The frozen grids were transferred onto a Polara electron microscope working at 300 kV. The images were taken under low-dose conditions (<20 e¯/Å2) with a nominal magnification of 59,000×, recorded on Kodak SO-163 films, and developed for 12 min in full-strength Kodak D19 developer. Negatives were digitized on a PhotoScan TD scanner (Z/I Imaging) at a step size of 7 μm, corresponding to 1.19 Å per pixel. The images were corrected for the Contrast Transfer Function using CTFFIND3 (31). Then 6,125 particles were selected semiautomatically from 30 films using the EMAN boxer routine (32); these particles were normalized and filtered between 200 Å and 20 Å (Fig. S2 C and D). SPIDER (33) was used for projection-matching image analysis. The initial model was generated by back-projecting a side view and imposing C24 symmetry (as determined by ultracentrifugation experiments). Several models were generated and averaged to give a 50-Å initial model. The angular step used in the projection-matching procedure was 5°, giving 20 equally spaced reprojections. A lack of side views limited the final resolution to 35 Å as indicated by the standard Fourier-shell correlation (criterion FSC = 0.5) (34). Ab initio classification of the particle images using RELION (35) generated classes that were coherent with the 3D model obtained using SPIDER (Fig. 2E).
3D Homology Modeling of the AGBsu Monomer.
Homology modeling servers, including Phyre2 (www.sbg.bio.ic.ac.uk/phyre2/), Robetta (www.robetta.org/), and Swiss Model (https://swissmodel.expasy.org/) (22), were tested to generate models of AGBsu. All identified EscJ [Protein Databank (PDB) ID code 1YJ7] and PrgK (PDB ID code 3J6D) as the best templates to model the D2 domain of AGBsu. Using the Swiss Model server, we used the structures of EscJ (PDB ID code 1YJ7) and PrgK (PDB ID code 3J6D) separately as target-template alignment files. Both AGBsu D2 models had high-confidence factors in the regions homologous to the EscJ/PrgK family. The model based on EscJ displayed a global QMEAN scoring function of −3.52 and a sequence identity with AGBsu D2 of 24% over 85 residues. The model based on PrgK had a global QMEAN scoring function of −10.44 and a sequence identity of 17% over 85 residues. The insertion region was modeled with lower-confidence values. When analyzed with servers dedicated to secondary structure predictions (PSIPRED, bioinf.cs.ucl.ac.uk/psipred/; JPRED, www.compbio.dundee.ac.uk/jpred/; and PredictProtein, https://www.predictprotein.org/), this region was predicted to be composed mainly of β secondary elements. Because the Swiss model based on EscJ contained only helices and loops in the insertion region, whereas the model based on PrgK contained a large antiparallel β-hairpin that matches the PSIPRED, JPRED, and PredictProtein predictions, we used the Swiss model based on PrgK (Fig. S3) for modeling the AGBsu ring.
EM-Guided Symmetry Modeling of the AGBsu Ring.
To model the ring, we only used the regions of the AGBsu monomer that could be modeled with a confidence factor >0.2 and displayed secondary structures in agreement with PSIPRED, JPRED, and PredictProtein predictions. We split the model of the D2 monomer into two PDB files, one corresponding to the regions homologous to the EscJ/PrgK family (residues 90–123 and 181–224), and the other corresponding to the insertion region (residues 129–154). Each PDB file was placed manually and independently into the EM map density according to its putative localization. Then 24-fold symmetry files were generated using a “pdbsymm” symmetry builder script from the Situs 2.7 package (36). Finally, the position of the protomers in the EM map were refined using the “Fit in map” function in Chimera (37) and were energy minimized using the NAMD program (23).
Protein–Protein Interaction Assay.
Samples of AGBsu rings at 5 mg/mL in buffer C were incubated for 15 min at room temperature with stoichiometric amounts of the purified extracellular domains of B. subtilis AH, Q, or preformed AH/Q complexes. After incubation, samples were injected onto a 10/300 GL Superdex 200 gel-filtration column (GE Healthcare) and eluted with buffer C. In all cases, the elution volume of the oligomeric species was indistinguishable from the elution volume of the AGBsu rings alone (∼8.5 mL), providing no evidence of a higher-order complex. However, the oligomeric species were analyzed by negative-stain EM using the protocol described above. The EM images revealed ring-shaped structures with architecture and dimensions similar to those of AGBsu rings alone, further indicating that, under these experimental conditions, higher-order complexes between AG, AH, and/or Q do not exist.
Plasmid Construction.
pCR094 [His6-SUMO-spoIIIAG (51-229, B. subtilis)] was generated by isothermal assembly of a PCR product containing the relevant DNA segment of spoIIIAG (oligonucleotide primers oCR160 and oCR161 on PY79 genomic DNA) into pTB146 (His6-SUMO) cut with SapI. pTB146 (His6-SUMO) is a protein expression vector provided by T. Bernhardt, Harvard Medical School.
pCR133 [His6-SUMO-spoIIIAG (90-229, B. subtilis)] was generated by isothermal assembly of a PCR product containing the relevant DNA segment of spoIIIAG (oligonucleotide primers oCR161 and oCR302 on PY79 genomic DNA) into pTB146 (His6-SUMO) cut with SapI. pTB146 (His6-SUMO) is a protein expression vector provided by T. Bernhardt, Harvard Medical School.
pCR134 [His6-SUMO-spoIIIAG (66-201, A. longum)] was generated by a two-way ligation of a BamHI-XhoI gBlock (IDT Technologies) containing the relevant DNA segment of spoIIIAGAlo (NCBI protein accession number WP_004095879.1) into pTB146 (His6-SUMO) cut with BamHI-XhoI.
pCR137 [His6-SUMO-spoIIIAG (78-212, G. thermodenitrificans)] was generated by a two-way ligation of a BamHI-XhoI gBlock (IDT Technologies) containing the relevant DNA segment of spoIIIAGGth (accession number WP_008879812.1) into pTB146 (His6-SUMO) cut with BamHI-XhoI.
pCR244 [His6-SUMO-spoIIIAGR209E (51-229, B. subtilis)] was generated by site-directed mutagenesis using oligonucleotide primers oCR515 and oCR516 and pCR094.
pCR245 [His6-SUMO-spoIIIAGK223E (51-229, B. subtilis)] was generated by site-directed mutagenesis using oligonucleotide primers oCR517 and oCR518 and pCR094.
pCR247 [His6-SUMO-spoIIIAGR209A (51-229, B. subtilis)] was generated by site-directed mutagenesis using oligonucleotide primers oCR521 and oCR522 and pCR094.
pCR248 [His6-SUMO-spoIIIAGK223A (51-229, B. subtilis)] was generated by site-directed mutagenesis using oligonucleotide primers oCR523 and oCR524 and pCR094.
pCR257 [yhdG::PspoIIIA (2)-spoIIIAG (kan)] was generated by a two-way ligation of a BamHI-XhoI PCR product (oligonucleotide primers oCR486 and oCR433 on PY79 genomic DNA) containing the relevant DNA segment of into pBB283 (yhdG::kan) cut with BamHI-XhoI. pBB283 (yhdG::kan) is a double-crossover ectopic integration vector provided by B. Burton and D. Z. Rudner, Harvard Medical School.
pCR258 [yhdG::PspoIIIA (2)-spoIIIAGR209E (kan)] was generated by site-directed mutagenesis using oligonucleotide primers oCR515 and oCR516 and pCR257.
pCR259 [yhdG::PspoIIIA (2)-spoIIIAGK223E (kan)] was generated by site-directed mutagenesis using oligonucleotide primers oCR517 and oCR518 and pCR257.
Discussion
Here, we have shown that AG homologs from B. subtilis and two distantly related species that form endospores assemble into large rings with a cup-and-saucer architecture. Mutations in predicted interface residues in the AGBsu ring result in impaired ring assembly in vitro and reduced SigG activity and sporulation in vivo. Collectively, these results provide evidence for a conduit between the mother cell and forespore and support the idea that the A–Q complex could function as a channel or secretion complex.
Our modeling suggests that the AG cup results from the oligomerization of a region inserted within the ring-building motif (RBM) that is predicted to contain two long antiparallel β-strands. Intriguingly, the third RBM of FliF in the flagellar basal body also has an insertion region predicted to be rich in β secondary structures (21). Accordingly, we hypothesize that the FliF cup (called the “R region”) may be similarly composed of this insertion and form a large β-barrel. In the case of FliF, the cup faces the outer membrane where it engages the flagellar rod complex (16, 24). Although the orientation of the AG cup is not known, we favor a model in which it also faces toward the forespore (away from the mother cell membrane), because our data suggest that the D1 domain is part of the base of the saucer and this domain is directly preceded by AG’s transmembrane segment (Fig. 1B). In addition, this orientation of the ring is similar to that of the PrgK and EscJ rings in type III secretion systems (18, 20).
The structural similarities between the extracellular domain of AH and EscJ/PrgK proteins and in silico modeling suggested that AH together with Q oligomerize into a pair of rings (14, 15). Our finding that three distinct AG homologs assemble into rings strengthens and extends this model. Because Q is synthesized in the forespore and AH in the mother cell, the Q ring likely resides in close apposition to the forespore membrane followed by AH (Fig. 5). We have previously shown that the localization of AG to the membranes surrounding the forespore requires AH and Q, suggesting that the AG ring could stack against AH by analogy to the stacked rings found in type III secretion systems (2, 18). If, as our data suggest, the AG cup faces toward the forespore, then in the context of this model the cup region would contact the AH ring (Fig. 5). How it does so is unknown at present. To accommodate steric clashes between AH protomers (and, separately, Q protomers), AH–Q ring models containing 12, 15, and 18 subunits have been proposed (14, 15). A 15-member AH–Q ring has a pore size of ∼7 nm and could not accommodate the AG cup with an outer diameter of ∼11 nm. Accordingly, in this model, the “lip” of the cup would contact the AH ring. If instead the cup is inserted into the pore of the AH ring, then the latter ring must contain at least 18 protomers. An alternative possibility is that AH assembles into a 24-member ring, which would allow 1:1 interactions between AG and AH subunits, as is the case with PrgK and PrgH (20). It is noteworthy that, in addition to the mother cell-specific (SigE) promoter upstream of the spoIIIA operon, there is a second SigE-responsive promoter within the spoIIIAF (AF) gene (25). This promoter results in increased expression of AG and AH, suggesting that the stoichiometry of these two components is higher than that of the other SpoIIIA proteins in the complex and is consistent with a stacked ring model. Our attempts to generate heteromeric complexes with purified extracellular domains of AG, AH, and Q have been unsuccessful thus far (SI Materials and Methods). Addressing the precise stoichiometry and organization of the rings in the A–Q complex will ultimately require a high-resolution structure of the intact complex, ideally purified directly from sporulating cells.
Fig. 5.
AG, AH, and Q form stacked rings in the intermembrane space. Schematic diagram showing the A–Q complex in the two membranes that surround the forespore. AH (in magenta) and Q (in purple) are shown as a double ring containing 18 protomers of each, as proposed by Meisner et al. (15). The AG D2 ring model fitted in the experimental EM map is shown in cyan. The other membrane proteins encoded in the spoIIIA operon (AB–AF) are shown schematically as a single complex (gray) with a predicted membrane pore. AA is shown as a hexamer by analogy to other secretion ATPases. Evidence suggests the existence of a pore in the forespore membrane (6) (shown schematically in gray); the identity of this protein is unknown.
Extending the analogy between the A–Q complex and type III secretion systems and the flagellar basal body, a needle or inner rod complex might be expected to reside in the pore generated by AG and AH. No proteins currently known to be required for SigG activity are similar to those that make up these tube-like structures. Accordingly, whether such a structure exists in the A–Q complex remains an outstanding question. The absence of any candidate protein for an inner rod or needle raises the possibility that the A–Q complex uses its type III-like proteins to generate a channel (4, 5). In line with this idea, AH, AG, and AF are the only proteins in the A–Q complex that share sequence similarity with proteins found in type III secretion systems. Indeed, AA most closely resembles secretion ATPases found in type IV secretion systems, and the polytopic membrane proteins AB and AE both have domains with remote homology to GspF from type II secretion systems. One popular model that accounts for the absence of an inner rod or needle is that the complex functions as a feeding tube (5) in which the ATPase component and associated integral membrane proteins act as a gate or energy source for transport of undefined molecules across the outer forespore membrane and into the AG/AH/Q channel.
Interestingly, recent work in the endospore former Clostridium difficile suggests that the A–Q complex may be dispensable for late forespore gene expression under SigG control and therefore may not play a direct role in maintaining transcriptional potential in the C. difficile forespore (8, 9). Intriguingly, both studies identified morphological defects in spore differentiation in the absence of the A–Q complex, suggesting that the complex could play a structural role in maintaining forespore development. These findings raise the possibility that this highly conserved transenvelope complex might not be a secretion complex or a feeding tube. One possibility that is compatible with the remote homologies and structural similarities described here is that the complex is a noncanonical piliation system and, for example, functions to adhere the two membranes together. In its absence, the forespore develops morphological defects, fails to mature, and, in the case of B. subtilis, loses transcriptional potential. Although currently there is no candidate pilin or pseudopilin protein that would connect the two cells, it is possible that, as in the case of the SpoIIIA proteins, this factor has remote or even undetectable homology to counterparts in canonical piliation and type II secretion systems. Future genetic and biochemical studies will be required to distinguish among these models, while structure characterization will help unravel the global architecture of the A-Q complex.
Materials and Methods
All B. subtilis strains were derived from the prototrophic strain PY79 (26). Sporulation assays and fluorescence microscopy were performed as previously described (7). All recombinant proteins were overexpressed in E. coli Rosetta (DE3) pLysS and were affinity purified as His-SUMO fusions (27) followed by size-exclusion chromatography. For cryo-EM, purified AGBsu was loaded onto a Quantifoil R2/1 holey grid and vitrified using a Mark IV Vitrobot; images were acquired on a Polara electron microscope. Detailed protocols are provided in SI Materials and Methods, and strains, plasmids, and oligonucleotide primers are listed in Table S1.
Table S1.
Strains, plasmids, and oligonucleotides
| Construct | Genotype/description/sequence | Source or reference |
| B. subtilis strains | ||
| PY79 | Prototrophic wild type | (26) |
| BCR267 | spoIIQ::kan | This work |
| BCR776 | spoIIIAG::markerless | |
| BCR859 (AHB1331) | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat | (4) |
| BCR877 | ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat | This work |
| BCR878 | spoIIIAG::markerless, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat | This work |
| BCR879 | spoIIIAH::erm, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat | This work |
| BCR896 | ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, pbpG(∆R147-K148)Ωphleo | This work |
| BCR900 | spoIIIAH::erm, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, pbpG(∆R147-K148)Ωphleo | This work |
| BCR929 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIAH::erm | This work |
| BCR935 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIA::kan | This work |
| BCR946 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIAG::markerless | This work |
| BCR959 | spoIIIAG::markerless, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, pbpG(∆R147-K148)Ωphleo | This work |
| BCR961 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIAH::erm, pbpG(∆R147-K148)Ωphleo | This work |
| BCR964 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIA::kan, pbpG(∆R147-K148)Ωphleo | This work |
| BCR976 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIAG::markerless, pbpG(∆R147-K148)Ωphleo | This work |
| BCR990 | ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, spoIIIA::kan | This work |
| BCR991 | ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, pbpG(∆R147-K148)Ωphleo, spoIIIA::kan | This work |
| BCR992 | spoIIIGΔ1, amyE::PspoIIQ-sigG::spec, ΔcsfB::tet, ywrK::Tn917::amyE::PsspB-cfp(Bs)::cat, pbpG(∆R147-K148)Ωphleo | This work |
| BCR1434 | spoIIIAG::markerless, yhdG::PspoIIIA (2)-spoIIIAG (kan) | This work |
| BCR1435 | spoIIIAG::markerless, yhdG::PspoIIIA (2)-spoIIIAGR209E (kan) | This work |
| BCR1436 | spoIIIAG::markerless, yhdG::PspoIIIA (2)-spoIIIAGK223E (kan) | This work |
| BCR1437 | spoIIIAG::markerless, ycgO::PsspB-RBSopt-gfp (spec) | This work |
| BCR1438 | spoIIIAG::markerless, yhdG::PspoIIIA (2)-spoIIIAG (kan), ycgO::PsspB-RBSopt-gfp (spec) | This work |
| BCR1439 | spoIIIAG::markerless, yhdG::PspoIIIA (2)-spoIIIAGR209E (kan), ycgO::PsspB-RBSopt-gfp (spec) | This work |
| BCR1440 | spoIIIAG::markerless, yhdG::PspoIIIA (2)-spoIIIAGK223E (kan), ycgO::PsspB-RBSopt-gfp (spec) | This work |
| Plasmids | ||
| pCR094 | His6-SUMO-spoIIIAG (51-229, B. subtilis) | This work |
| pCR133 | His6-SUMO-spoIIIAG (90-229, B. subtilis) | This work |
| pCR134 | His6-SUMO-spoIIIAG (66-201, A. longum) | This work |
| pCR137 | His6-SUMO-spoIIIAG (78-212, G. thermodenitrificans) | This work |
| pCR244 | His6-SUMO-spoIIIAGR209E (51-229, B. subtilis) | This work |
| pCR245 | His6-SUMO-spoIIIAGK223E (51-229, B. subtilis) | This work |
| pCR247 | His6-SUMO-spoIIIAGR209A (51-229, B. subtilis) | This work |
| pCR248 | His6-SUMO-spoIIIAGK223A (51-229, B. subtilis) | This work |
| pCR257 | yhdG::PspoIIIA (2)-spoIIIAG (kan) | This work |
| pCR258 | yhdG::PspoIIIA (2)-spoIIIAG R209E (kan) | This work |
| pCR259 | yhdG::PspoIIIA (2)-spoIIIAGK223E (kan) | This work |
| Oligonucleotides | ||
| oCR160 | gctcacagagaacagattggtggttcttcacctgagaaaactgaaaacg | This work |
| oCR161 | tcgacggagctctgctcttctaccttatgaatcctcctttatttttttag | This work |
| oCR302 | gctcacagagaacagattggtggtgctgactcgatcgatgactatgaa | This work |
| oCR433 | cgcggatccttatgaatcctcctttatttttttag | This work |
| oCR486 | gcgctcgagcttttcaagacagaccccgaagt | This work |
| oCR515 | accattatcgaagcggtgacagaggtcctggatgttccaagccac | This work |
| oCR516 | gtggcttggaacatccaggacctctgtcaccgcttcgataatggt | This work |
| oCR517 | caccgggttgcggttgcccctgaaaaaataaaggaggattca | This work |
| oCR518 | tgaatcctcctttattttttcaggggcaaccgcaacccggtg | This work |
| oCR521 | accattatcgaagcggtgacagcggtcctggatgttccaagccac | This work |
| oCR522 | gtggcttggaacatccaggaccgctgtcaccgcttcgataatggt | This work |
| oCR523 | caccgggttgcggttgcccctgcaaaaataaaggaggattca | This work |
| oCR524 | tgaatcctcctttatttttgcaggggcaaccgcaacccggtg | This work |
Acknowledgments
We thank members of the Vernet, D.Z.R., Dessen, and Bernhardt laboratories for advice and encouragement; Janet Iwasa for figure preparation; Daphna Fenel for negative-stain EM imaging; Amy Camp for sharing strains; and Christine Ebel and Aline Le Roy for analytical ultracentrifugation analyses. Support for this work came from NIH Grant GM086466 (to D.Z.R.) and Agence Nationale de la Recherche (ANR) Grant ANR-11-BSV8-005-01 PILIPATH. This work used the platforms of the Grenoble Instruct Centre (Integrated Structural Biology Grenoble, UMS 3518 CNRS-Commissariat à l’Energie Atomique et aux Energies Alternatives-Université Grenoble Alpes-EMBL), with support from the French Infrastructure for Integrated Structural Biology Initiative (FRISBI) Grant ANR-10-INSB-05-02 and the Grenoble Alliance for Integrated Structural Cell Biology (GRAL) Grant ANR-10-LABX-49-01 within the Grenoble Partnership for Structural Biology (PSB).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The cryo-EM 3D reconstruction map of the D1+D2 rings of SpoIIIAG from Bacillus subtilis has been deposited in the EMDataBank (EMDB ID code EMD-4072).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609604113/-/DCSupplemental.
References
- 1.Costa TR, et al. Secretion systems in Gram-negative bacteria: Structural and mechanistic insights. Nat Rev Microbiol. 2015;13(6):343–359. doi: 10.1038/nrmicro3456. [DOI] [PubMed] [Google Scholar]
- 2.Burkinshaw BJ, Strynadka NC. Assembly and structure of the T3SS. Biochim Biophys Acta. 2014;1843(8):1649–1663. doi: 10.1016/j.bbamcr.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Tan IS, Ramamurthi KS. Spore formation in Bacillus subtilis. Environ Microbiol Rep. 2014;6(3):212–225. doi: 10.1111/1758-2229.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camp AH, Losick R. A novel pathway of intercellular signalling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol Microbiol. 2008;69(2):402–417. doi: 10.1111/j.1365-2958.2008.06289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camp AH, Losick R. A feeding tube model for activation of a cell-specific transcription factor during sporulation in Bacillus subtilis. Genes Dev. 2009;23(8):1014–1024. doi: 10.1101/gad.1781709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meisner J, Wang X, Serrano M, Henriques AO, Moran CP., Jr A channel connecting the mother cell and forespore during bacterial endospore formation. Proc Natl Acad Sci USA. 2008;105(39):15100–15105. doi: 10.1073/pnas.0806301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doan T, et al. Novel secretion apparatus maintains spore integrity and developmental gene expression in Bacillus subtilis. PLoS Genet. 2009;5(7):e1000566. doi: 10.1371/journal.pgen.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fimlaid KA, Jensen O, Donnelly ML, Siegrist MS, Shen A. Regulation of Clostridium difficile spore formation by the SpoIIQ and SpoIIIA proteins. PLoS Genet. 2015;11(10):e1005562. doi: 10.1371/journal.pgen.1005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano M, et al. The SpoIIQ-SpoIIIAH complex of Clostridium difficile controls forespore engulfment and late stages of gene expression and spore morphogenesis. Mol Microbiol. 2016;100(1):204–228. doi: 10.1111/mmi.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues CD, Marquis KA, Meisner J, Rudner DZ. Peptidoglycan hydrolysis is required for assembly and activity of the transenvelope secretion complex during sporulation in Bacillus subtilis. Mol Microbiol. 2013;89(6):1039–1052. doi: 10.1111/mmi.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaylock B, Jiang X, Rubio A, Moran CP, Jr, Pogliano K. Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization. Genes Dev. 2004;18(23):2916–2928. doi: 10.1101/gad.1252704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doan T, Marquis KA, Rudner DZ. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol Microbiol. 2005;55(6):1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]
- 13.Söding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33(Web Server issue):W244-8. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levdikov VM, et al. Structure of components of an intercellular channel complex in sporulating Bacillus subtilis. Proc Natl Acad Sci USA. 2012;109(14):5441–5445. doi: 10.1073/pnas.1120087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meisner J, Maehigashi T, André I, Dunham CM, Moran CP., Jr Structure of the basal components of a bacterial transporter. Proc Natl Acad Sci USA. 2012;109(14):5446–5451. doi: 10.1073/pnas.1120113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Yonekura K, Namba K. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J Mol Biol. 2004;337(1):105–113. doi: 10.1016/j.jmb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Combet C, Blanchet C, Geourjon C, Deléage G. NPS@: Network protein sequence analysis. Trends Biochem Sci. 2000;25(3):147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- 18.Yip CK, et al. Structural characterization of the molecular platform for type III secretion system assembly. Nature. 2005;435(7042):702–707. doi: 10.1038/nature03554. [DOI] [PubMed] [Google Scholar]
- 19.Bergeron JR, et al. The modular structure of the inner-membrane ring component PrgK facilitates assembly of the type III secretion system basal body. Structure. 2015;23(1):161–172. doi: 10.1016/j.str.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Schraidt O, Marlovits TC. Three-dimensional model of Salmonella’s needle complex at subnanometer resolution. Science. 2011;331(6021):1192–1195. doi: 10.1126/science.1199358. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron JR. Structural modeling of the flagellum MS ring protein FliF reveals similarities to the type III secretion system and sporulation complex. PeerJ. 2016;4:e1718. doi: 10.7717/peerj.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordoli L, et al. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2009;4(1):1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- 23.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DR, Francis NR, Xu C, DeRosier DJ. The three-dimensional structure of the flagellar rotor from a clockwise-locked mutant of Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188(20):7039–7048. doi: 10.1128/JB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillot C, Moran CP., Jr Essential internal promoter in the spoIIIA locus of Bacillus subtilis. J Bacteriol. 2007;189(20):7181–7189. doi: 10.1128/JB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youngman PJ, Perkins JB, Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci USA. 1983;80(8):2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uehara T, Parzych KR, Dinh T, Bernhardt TG. Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J. 2010;29(8):1412–1422. doi: 10.1038/emboj.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harwood CR, Cutting SM. Molecular Biological Methods for Bacillus. Wiley, Hoboken; NJ: 1990. [Google Scholar]
- 29.Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broder DH, Pogliano K. Forespore engulfment mediated by a ratchet-like mechanism. Cell. 2006;126(5):917–928. doi: 10.1016/j.cell.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142(3):334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 32.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 33.Frank J, et al. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116(1):190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 34.van Heel M, Schatz M. Fourier shell correlation threshold criteria. J Struct Biol. 2005;151(3):250–262. doi: 10.1016/j.jsb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Scheres SH. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180(3):519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wriggers W. Conventions and workflows for using Situs. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 4):344–351. doi: 10.1107/S0907444911049791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. J Struct Biol. 2007;157(1):281–287. doi: 10.1016/j.jsb.2006.06.010. [DOI] [PubMed] [Google Scholar]