Significance
Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) are emerging as key regulators of cell fate in response to biochemical and biophysical cues, but their role in modulating vascular homeostasis in health and disease is not clearly understood. In studying the flow regulation of YAP/TAZ activities in vascular endothelial cells, we find that disturbed flow (without a clear direction), but not laminar flow (with a clear direction), activates YAP/TAZ to promote the atheroprone phenotypes (proliferation and inflammation). We also demonstrate that inhibition of YAP/TAZ attenuates the atheroprone phenotypes and lesion progression in atherosclerotic mice. These findings indicate that YAP/TAZ serve as important mechanotransducers in the disturbed flow-induced atherogenesis and provide insights to the development of therapeutic strategy for cardiovascular diseases.
Keywords: atherogenesis, disturbed flow, endothelial cells, mechanotransduction
Abstract
The focal nature of atherosclerotic lesions suggests an important role of local hemodynamic environment. Recent studies have demonstrated significant roles of Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) in mediating mechanotransduction and vascular homeostasis. The objective of this study is to investigate the functional role of YAP/TAZ in the flow regulation of atheroprone endothelial phenotypes and the consequential development of atherosclerotic lesions. We found that exposure of cultured endothelial cells (ECs) to the atheroprone disturbed flow resulted in YAP/TAZ activation and translocation into EC nucleus to up-regulate the target genes, including cysteine-rich angiogenic inducer 61 (CYR61), connective tissue growth factor (CTGF), and ankyrin repeat domain 1 (ANKRD1). In contrast, the athero-protective laminar flow suppressed YAP/TAZ activities. En face analysis of mouse arteries demonstrated an increased nuclear localization of YAP/TAZ and elevated levels of the target genes in the endothelium in atheroprone areas compared with athero-protective areas. YAP/TAZ knockdown significantly attenuated the disturbed flow induction of EC proliferative and proinflammatory phenotypes, whereas overexpression of constitutively active YAP was sufficient to promote EC proliferation and inflammation. In addition, treatment with statin, an antiatherosclerotic drug, inhibited YAP/TAZ activities to diminish the disturbed flow-induced proliferation and inflammation. In vivo blockade of YAP/TAZ translation by morpholino oligos significantly reduced endothelial inflammation and the size of atherosclerotic lesions. Our results demonstrate a critical role of the activation of YAP/TAZ by disturbed flow in promoting atheroprone phenotypes and atherosclerotic lesion development. Therefore, inhibition of YAP/TAZ activation is a promising athero-protective therapeutic strategy.
Atherosclerosis, the most common cause of acute myocardial infarction and ischemic stroke, results from endothelial dysregulation in the arterial wall (1, 2). The hemodynamic forces acting on the arterial wall play a crucial role in determining the function and phenotype of endothelial cells (ECs) (3, 4). In the straight part of the arterial tree, the blood flow pattern is laminar with a significant forward direction (laminar shear stress, LS), and the resulting gene expression and structural/functional consequences in ECs are athero-protective, i.e., antiproliferation and antiinflammation. In contrast, the local flow patterns at branch points and aortic arch are disturbed, having oscillations with little forward component (oscillatory shear stress, OS), and the EC phenotypes and gene expression are atheroprone, i.e., proproliferation and proinflammation (3, 4). Thus, the different mechanisms of mechanotransduction in response to flow patterns with or without a clear direction lead to the distinct EC phenotypes in different regions of the arterial tree. Multiple pathways mediating the flow-regulated functional outcomes (athero-protective vs. atheroprone) have been established (3), but there is a relative lack of information on the downstream functional effectors.
Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are emerging as master signaling regulators in organ growth control and tumor suppression (5–7). YAP and TAZ (YAP/TAZ) activities are canonically modulated by the Hippo pathway kinase large tumor suppressor (LATS)-dependent phosphorylation. The dysregulation of Hippo pathway and hyperactivation of YAP/TAZ has been reported in many types of human cancers (6–8). YAP/TAZ are retained in the cytoplasm and inactive in gene transcription when phosphorylated; in contrast, the dephosphorylated form of YAP/TAZ translocates into the nucleus to promote the target gene transcription via interactions with the transcription factor partners (e.g., TEA domain transcription factors, TEADs) (9). Many biochemical factors such as cytokines (e.g., IL-6), growth factors (e.g., EGF), and drugs (e.g., statins) have been shown to regulate YAP/TAZ activities and functional outcomes (10–13). Recent studies have reported that YAP/TAZ serve as major mechanotransducers responding to extracellular biophysical cues, such as cell geometry and matrix stiffness, in modulating stem cell fate (14). YAP has been shown to mediate vascular remodeling in the progression of carotid stenosis (15). Two of the YAP/TAZ transcriptional targets, cysteine-rich angiogenic inducer 61 (CYR61) and connective tissue growth factor (CTGF), are highly expressed in atherosclerotic or injured, but not in normal, human arteries (16, 17). However, it remains to be determined whether YAP/TAZ mediate a mechanosensitive pathway that plays a significant role in the hemodynamic regulation of vascular homeostasis in health and disease.
In this study, we provide evidence that atherogenic OS, but not athero-protective LS, resulted in YAP/TAZ phosphorylation and nuclear localization in ECs both in vitro and in vivo. Therefore, we hypothesized that OS signals through YAP/TAZ to induce the proproliferative and proinflammatory responses of ECs and the consequential atherogenesis. Our results have validated this hypothesis by demonstrating that knocking down YAP/TAZ repressed the OS induction of EC proliferation and inflammation; in contrast, overexpression of YAP/TAZ led to the expression of these atheroprone EC phenotypes. In addition, we found that statin, a cholesterol-lowering drug, enhanced athero-protection in ECs through YAP/TAZ inactivation. These findings provided a rationale for exploring the regulatory role of YAP/TAZ in the atherosclerotic mouse model. Our in vivo studies demonstrated that systemic inhibition of YAP/TAZ attenuated the development of atherosclerotic lesions induced by the partial carotid ligation (PL) procedure in apolipoprotein E-deficient (ApoE−/−) mice, suggesting that YAP/TAZ activation in ECs plays a causative role in the initiation and progression of atherosclerosis. Thus, our findings have established the role of YAP/TAZ activation in mediating the atherogenesis induced by disturbed flow and provided mechanistic and therapeutic insights for atherosclerosis.
Results
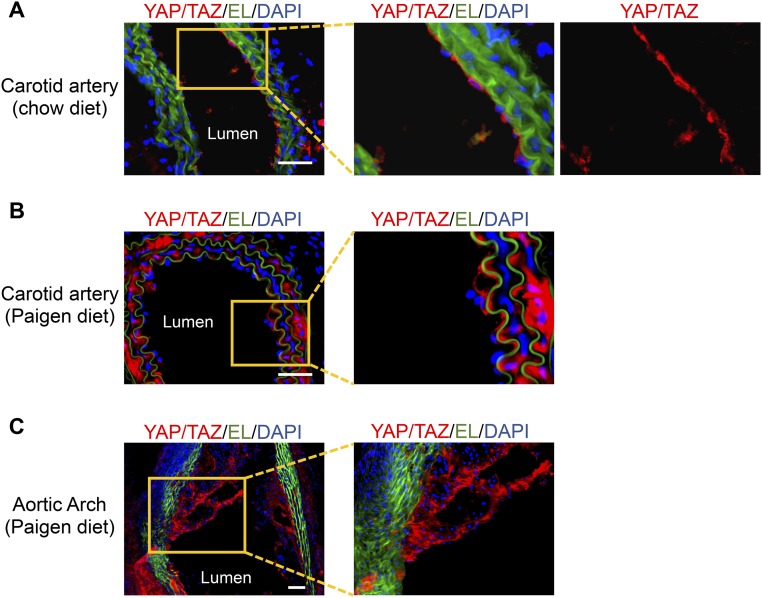
To assess the role of YAP/TAZ in regulating vascular homeostasis and diseases, we examined their expression and localization in normal and atherosclerotic arteries in the ApoE−/− mice. Immunofluorescence staining of the normal carotid artery showed that YAP/TAZ-positive cells were present in abundance in the endothelium in tunica intima, but few in the media layer (Fig. S1A). In contrast, in the atherosclerotic carotid artery and the aortic arch, YAP/TAZ staining was prominent in both the endothelium and the media layer, and the intimal hyperplasia plaque (Fig. S1 B and C). These results demonstrate that, although YAP/TAZ are enriched in ECs under normal physiological conditions, they are aberrantly elevated in atherosclerotic vessels, suggesting that YAP/TAZ may serve as important regulators of EC functions and that dysregulation of YAP/TAZ may contribute to the development of atherosclerosis. Therefore, we further investigated the mechanisms by which vascular YAP/TAZ are regulated and their functional role in modulating vascular phenotypes.
Fig. S1.
The LCA from the control ApoE−/− mice on a chow diet (A) and the LCA and aortic arch from atherosclerotic ApoE−/− mice fed the Paigen diet for 8 wk (B and C) were embedded in O.C.T. compound and subjected to frozen section. The expression of YAP/TAZ in the sections of LCAs (A and B) and aortic arch (C) was demonstrated by immunofluorescence staining of YAP/TAZ (red). Elastic lamina (EL, green) and nuclei (DAPI, blue) were shown to indicate vessel structure. Boxed regions are enlarged in the right images. Results are representative images from four animals with similar staining. (Scale bars: 100 µm.)
Differential Regulation of YAP/TAZ Activities Under Different Flow Patterns in Vitro and in Vivo.
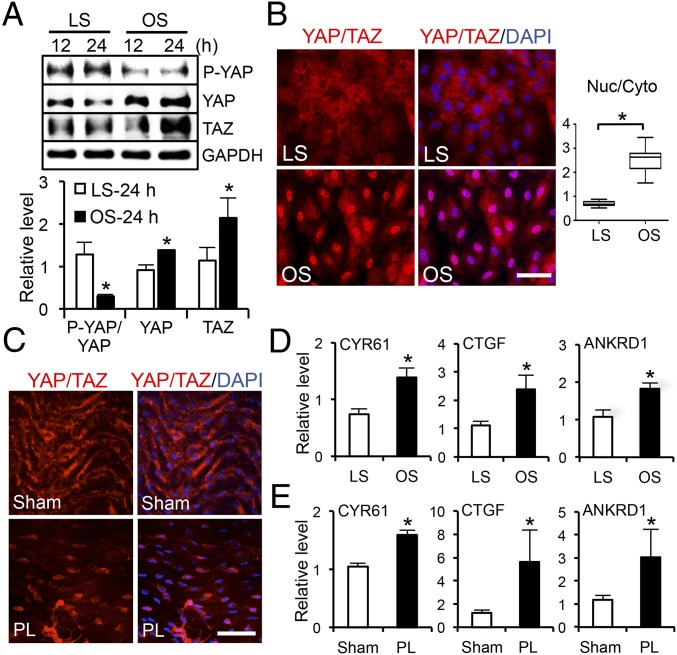
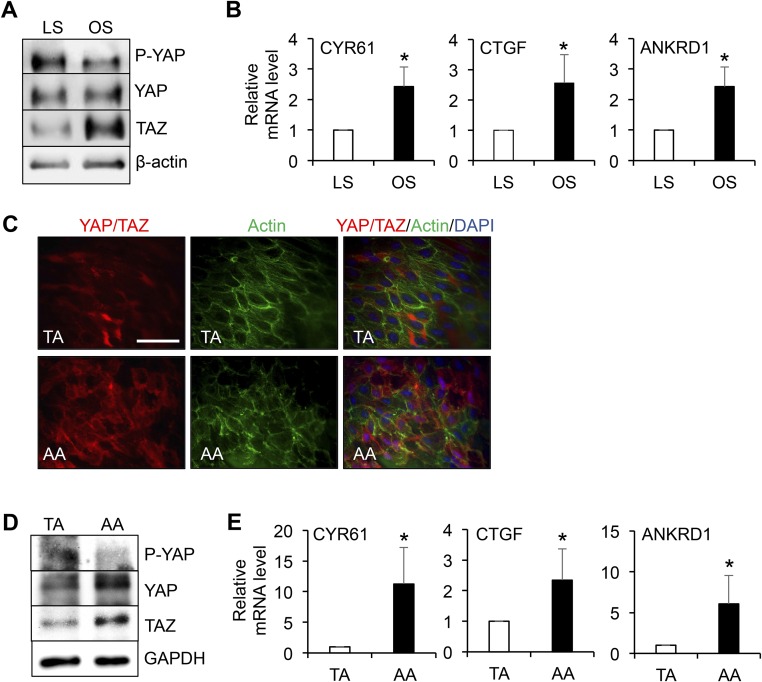
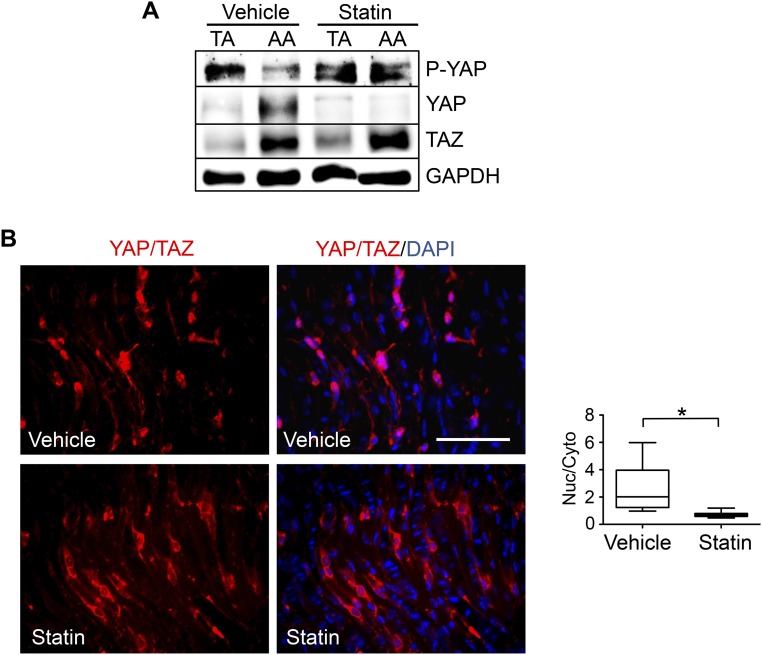
EC phenotypes and functions are modulated by the flow patterns generated by local hemodynamic forces. The activation of YAP/TAZ in ECs is determined by the hypophosphorylation of YAP at the Ser127 residue, increases of YAP/TAZ and their target gene expression, and nuclear localization of YAP/TAZ. To investigate whether the flow pattern plays a role in modulating YAP/TAZ activities in ECs, we performed in vitro shear stress experiments (OS vs. LS) by using the parallel flow chamber and in vivo flow perturbation experiments using the PL model in ApoE−/− mice (18, 19). Human umbilical vein ECs (HUVECs) were subjected to OS at 0.5 ± 4 dyn/cm2 or LS at 12 dyn/cm2 for 12 and 24 h, and the levels of YAP phosphorylation and YAP/TAZ expression were examined by Western blot analysis. Our results demonstrated that OS, but not LS, led to a significant decrease of YAP phosphorylation and a marked increase of YAP expression. We found that 24-h OS elevated TAZ expression, in comparison with the LS condition (Fig. 1A). The hypophosphorylation of YAP and induction of YAP/TAZ under OS were confirmed in human aortic ECs (HAECs) in vitro (Fig. S2A). These findings suggest that OS results in YAP/TAZ activation in both HUVECs and HAECs. The OS activation of YAP/TAZ was further demonstrated by the nuclear staining of YAP/TAZ that was detected by an antibody recognizing both YAP and TAZ, whereas LS caused cytoplasmic retention of the inactive YAP/TAZ (Fig. 1B). Quantitative analysis of the intensity of YAP/TAZ staining in the nuclear and cytoplasmic regions confirmed the nuclear localization of YAP/TAZ under OS, but not under LS. To elucidate the flow regulation of YAP/TAZ activation in vivo, we harvested the left common carotid arteries (LCA) from ApoE−/− mice subjected to PL and sham operation, where the endothelium had experienced atheroprone disturbed flow and athero-protective laminar flow, respectively. En face staining showed YAP/TAZ accumulation in the nuclei of ECs in the PL group, but a cytoplasmic and diffused distribution in ECs in the sham group (Fig. 1C). Quantitative RT-PCR (qRT-PCR) analysis revealed that the expression levels of YAP/TAZ target genes, including CYR61, CTGF, and ankyrin repeat domain 1 (ANKRD1), were significantly higher in ECs under OS than LS (Fig. 1D and Fig. S2B). In agreement with our in vitro results, the levels of YAP/TAZ target genes were higher in the PL than the sham group in our in vivo study (Fig. 1E). In addition, YAP/TAZ activation and expression were found to be higher in the disturbed flow region of the inner curvature of aortic arch (AA) than the laminar flow region of the straight segment of thoracic aorta (TA) (Fig. S2 C and D). Consistent with this finding, the expression of the YAP/TAZ target genes were also elevated in AA, in comparison with those in TA (Fig. S2E).
Fig. 1.
Disturbed flow induces activation and nuclear localization of YAP/TAZ. (A) Western blot analysis of P-YAP, YAP, TAZ, and GAPDH in ECs subjected to LS or OS for the indicated time. The graphic data are mean ± SD of band intensity normalized to its internal control and LS 12-h group, n = 3. *P < 0.05 vs. LS-24 h. (B and C) Immunofluorescence staining of YAP/TAZ in ECs subjected to LS or OS (B) and in the endothelium of PL- or sham-operated carotid arteries (C). (Scale bars: 100 µm.) n = 4. The graphic data in B are the YAP/TAZ intensity ratio (nuclear/cytoplasmic) from cells randomly selected from three independent experiments. *P < 0.05 vs. LS. (D and E) qRT-PCR analysis of YAP/TAZ target genes expression in ECs subjected to OS or LS (D) and in PL or sham groups (E) (mean ± SD, n = 3, *P < 0.05 vs. LS or sham).
Fig. S2.
Representative Western blot analysis of P-YAP, YAP, TAZ, and β-actin (A), and qPCR analysis of the expression levels of YAP/TAZ target genes (CYR61, CTGF, and ANKRD1) (B) in HAECs subjected to 24-h LS or OS (mean ± SD, n = 3, *P < 0.05 vs. LS). (C–E) The thoracic aorta (TA) and aortic arch (AA) from the control ApoE−/− mice fed a chow diet were dissected for en face staining of YAP/TAZ (red), actin fibers (green), and nuclei (blue) (C); or lysed for Western blot analysis of P-YAP, YAP, TAZ, and GAPDH (D), and qRT-PCR analysis of CYR61, CTGF, and ANKRD1 (E). Results in C and D are representative images from four animals with similar results. The graphic data in E are mean ± SD of the relative expression levels from four animals. *P < 0.05 vs. TA. (Scale bar: 100 µm.)
Disturbed Flow Promotes EC Proliferation Through YAP/TAZ Activation.
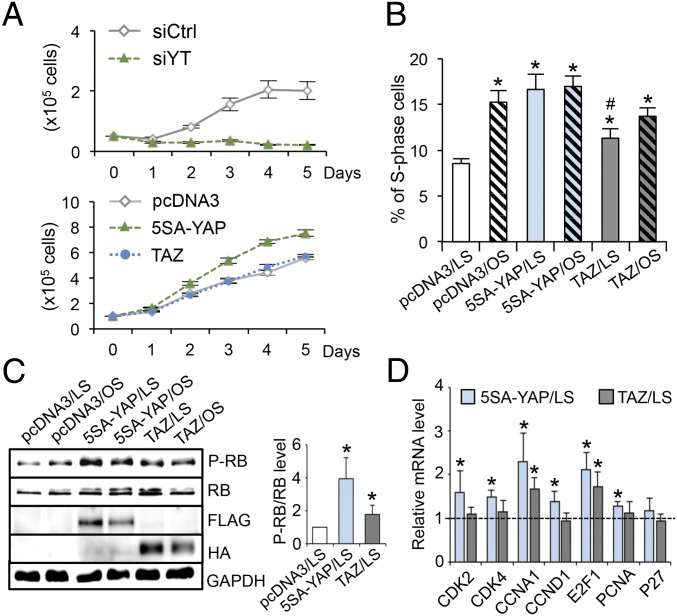
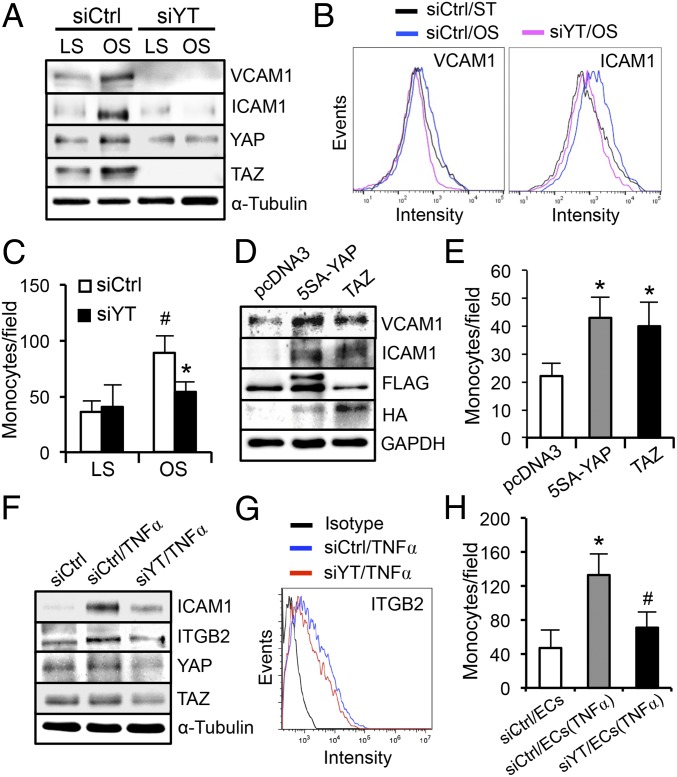
In light of such differential regulations of YAP/TAZ phosphorylation, localization, and target gene expression by flow patterns found both in vitro and in vivo, we further studied the functional role of YAP/TAZ in modulating EC phenotypes. YAP/TAZ are known to promote cell growth in many tissues. To determine the role of flow regulation of YAP/TAZ activities in modulating endothelial proliferation, we performed loss-of-function experiments in ECs by using silencing RNAs against YAP/TAZ (siYT) and gain-of-function experiments using plasmids overexpressing constitutively active FLAG-5SA-YAP mutant (5SA-YAP) and wild-type HA-TAZ (TAZ) to mimic the OS activation of YAP/TAZ. The results demonstrated that knocking down YAP/TAZ significantly inhibited EC growth (Fig. 2 A, Top), and that overexpression of 5SA-YAP, but not TAZ, markedly increased EC growth (Fig. 2 A, Bottom). In accordance with our previous report (18), exposure of ECs to long-term LS led to a reduction of cell numbers in the S phase and growth arrest in the G0/G1 phase (Fig. 2B). Flow cytometry analysis showed that overexpression of 5SA-YAP mitigated the LS-induced cell cycle arrest, with the percentage of S-phase cells increased from 8.54 ± 1.13% to 16.64 ± 3.23%; overexpression of TAZ under the same transfection condition had a lesser effect as the percentage of S-phase cells increased to only 11.31 ± 1.99% (Fig. 2B). To identify the molecules that mediate the YAP regulation of cell cycle progression, we examined the phosphorylation level of retinoblastoma protein (RB) and expression levels of cell cycle regulatory genes in G1-to-S transition (i.e., CDK2/4, CCNA1, CCND1, E2F1, p27, and PCNA). In agreement with the flow cytometry analysis, overexpression of 5SA-YAP exerted a stronger effect than TAZ in promoting the hyperphosphorylation of RB under both LS and OS conditions (Fig. 2C) and expressions of cell cycle regulatory genes (Fig. 2D). These results suggest that LS inactivates YAP/TAZ to exert the antiproliferative effect on ECs; in contrast, OS results in YAP/TAZ activation to program ECs to a proliferative phenotype. The inactivation of YAP/TAZ by LS and the resulting cell cycle suppression would contribute to the lower EC turnover rate and the quiescent EC phenotype in athero-protective regions of the arterial tree in comparison with the atheroprone regions.
Fig. 2.
YAP/TAZ regulate the EC growth and cell cycle progression. (A) EC growth curves with YAP/TAZ knockdown (Upper) and overexpression (Lower). ECs were transfected with silencing RNAs against YAP/TAZ (siYT) or scramble control (siCtrl) and plasmids expressing FLAG-5SA-YAP (5SA-YAP), HA-TAZ (TAZ), or pcDNA3, and then seeded onto six-well plates. The number of ECs were counted daily for 5 d. n = 3. (B–D) ECs were transfected with 5SA-YAP, TAZ, or pcDNA3 plasmids, followed by OS and LS experiments for the analysis of cell cycle (B), retinoblastoma (RB) phosphorylation (C), and cell cycle regulatory genes expression (D). (B–D) Mean ± SD, n = 3, *P < 0.05 vs. pcDNA3/LS and #P < 0.05 vs. 5SA-YAP/LS.
Disturbed Flow Promotes Inflammation Through YAP/TAZ Activation.
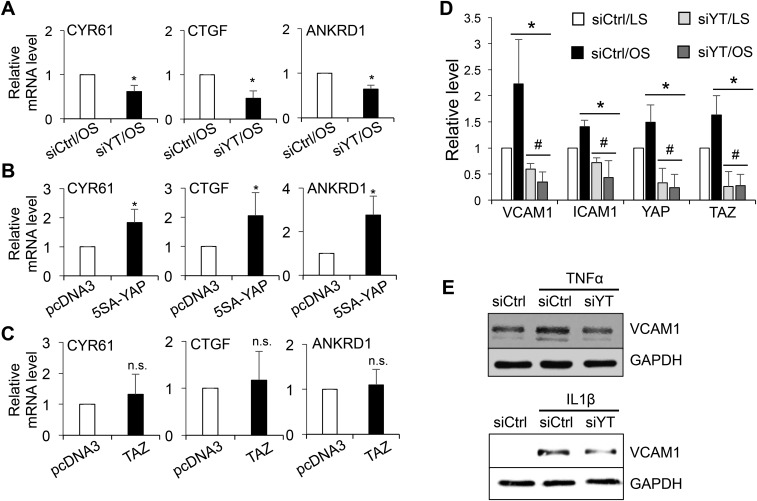
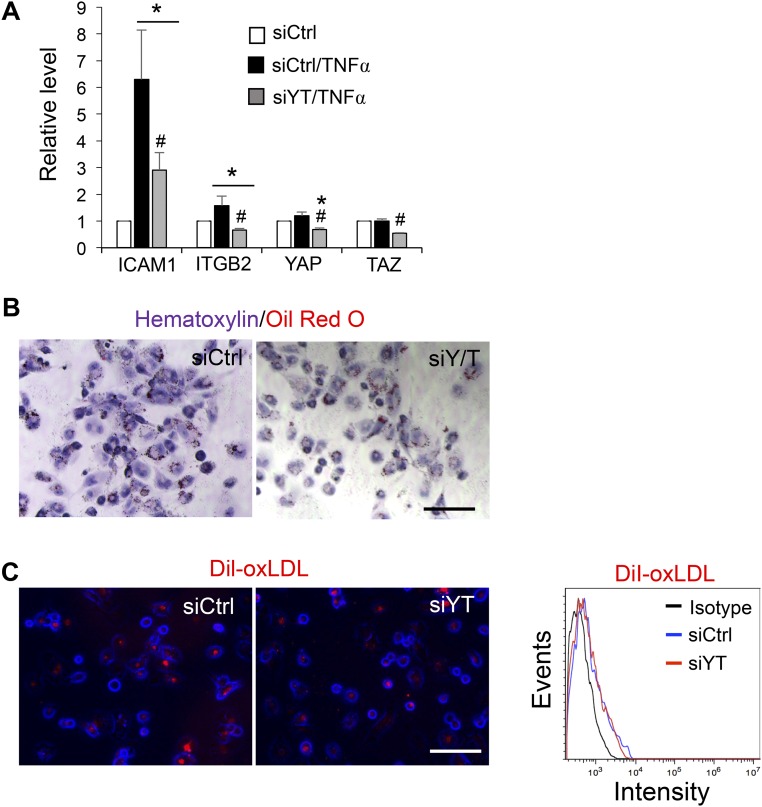
We investigated whether YAP/TAZ mediates the proinflammatory responses in ECs under disturbed flow. The effects of YAP/TAZ knockdown or overexpression on their target gene expressions were examined by qRT-PCR analysis (Fig. S3 A–C). Application of OS, but not LS, up-regulated the levels of VCAM1 and ICAM1 in EC lysates (Fig. 3A and Fig. S3D) and on EC surfaces (Fig. 3B), and the OS-induction of adhesion molecules was accompanied by an increase in the number of THP1 monocytes attached to ECs (Fig. 3C). YAP/TAZ knockdown decreased the OS-induced proinflammatory responses, as evidenced by the reductions of EC adhesion molecule expression and monocyte attachment (Fig. 3 A–C), whereas overexpression of 5SA-YAP and TAZ increased the proinflammatory responses under static condition (Fig. 3 D and E). Moreover, YAP/TAZ knockdown attenuated the VCAM1 expressions in ECs induced by inflammatory cytokines (TNFα and IL1β) (Fig. S3E). A previous report identified β2 integrin (ITGB2) as one of the potential targets induced by YAP/TAZ activation (9). Because of the unique role of ITGB2 in the chemosensory adhesion machinery of bloodborne cells, we further examined the role of YAP/TAZ in monocytes. YAP/TAZ knockdown blunted the up-regulation of β2 integrin and ICAM1 induced by TNFα in THP1 cells and reduced their adherence to the activated ECs (Fig. 3 F–H and Fig. S4A) without altering the plasticity of THP1 monocyte-to-macrophage differentiation (Fig. S4 B and C). Taken together, these results support our hypothesis that the YAP/TAZ activation by disturbed flow and cytokines enhance monocyte–EC interaction and contribute, at least partially, to atherogenesis.
Fig. S3.
The expression levels of CYR61, CTGF, and ANKRD1 in ECs subjected to YAP/TAZ knockdown (A) and overexpression (B and C) were determined by qRT-PCR. The graphic data are mean ± SD of the relative expression levels. n = 3. *P < 0.05 vs. siCtrl/OS (A) or vs. pcDNA3 (B and C). n.s., not significant. (D) Quantification analysis of the Western blot result shown in Fig. 3A (mean ± SD, n = 3, *P < 0.05 vs. siCtrl/LS and #P < 0.05 vs. siCtrl/OS). (E) ECs transfected with siYT or siCtrl were treated with TNFα and IL1β for 6 h and then collected for Western blot analysis of VCAM1 and GAPDH. Results are representative images from three independent experiments with a similar trend.
Fig. 3.
YAP/TAZ activation promotes the disturbed flow-induced proinflammatory responses. (A–C) ECs were transfected with siYT or siCtrl, subjected to 24-h LS or OS, and followed by Western blot analysis of VCAM1, ICAM1, and YAP/TAZ (A), flow-cytometric analysis of VCAM1 and ICAM1 surface expression (B), and THP1 monocyte adhesion assay (C), *P < 0.05 vs. siCtrl/OS and #P < 0.05 vs. siCtrl/LS. (D and E) ECs were transfected with 5SA-YAP, TAZ, or pcDNA3 plasmids and subjected to the Western blot analysis of VCAM1 and ICAM1 (D) and monocyte adhesion assay (E). *P < 0.05 vs. pcDNA3. (F–H) THP1 monocytes were transfected with siYT or siCtrl, treated with tumor necrosis factor-α (TNFα, 10 ng/mL) for 6 h, and then subjected to Western blot analysis of ICAM1 and ITGB2 (F), flow cytometric analysis of ITGB2 surface expression (G), and adhesion assay to the TNFα-activated ECs (H). *P < 0.05 vs. siCtrl/EC and #P < 0.05 vs. siCtrl/EC(TNFα).
Fig. S4.
(A) THP1 monocytes were transfected with siYT or siCtrl, treated with TNFα (10 ng/mL) for 6 h, and then subjected to Western blot analysis of ICAM1 and ITGB2 (Fig. 3F). The graphic data are the quantification analysis of the Western blot results shown in Fig. 3F and presented as mean ± SD of band intensity normalized to its internal control and the respective control group. (n = 3, *P < 0.05 vs. siCtrl and #P < 0.05 vs. siCtrl/TNFα). (B and C) THP1 monocytes were transfected with siYT or siCtrl, differentiated to macrophages with phorbol myristate acetate (PMA, 25 ng/mL) for 48 h, followed by 0.5% serum starvation for 6 h, and then incubated with oxidized LDL (B) or DiI-oxLDL (C) for 12 h. The LDL uptake levels were determined by Oil Red O staining (B) and fluorescence microscopy and flow cytometric analyses of Dil-positive cells (C). (Scale bars: 100 µm.)
Statin Exerts Athero-Protective Effects Through Inactivation of YAP/TAZ.
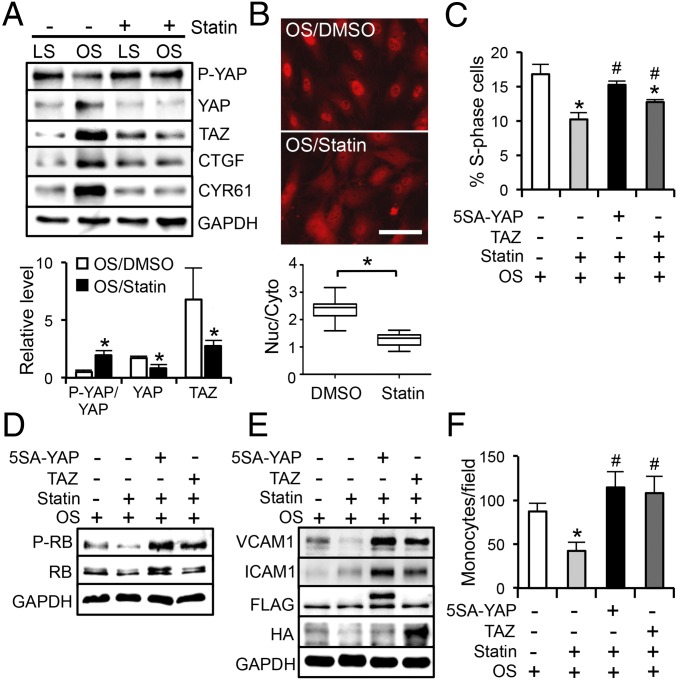
Statin, a commonly used antiatherosclerotic drug, has been shown to repress YAP activity and prevent YAP-mediated transcription in tumor growth (12, 13). Similar to the LS, statin treatment exerts athero-protective effects on vascular ECs (20). We further investigated whether YAP/TAZ mediate the statin-modulated athero-protective effects. Our results demonstrated that exposure of ECs to OS in the presence of 1 µM simvastatin (statin), but not the solvent control (dimethyl sulfoxide, DMSO), resulted in hyperphosphorylation of YAP, reductions of YAP/TAZ and their target genes (Fig. 4A), and a decrease of nuclear YAP/TAZ (Fig. 4B), suggesting that statin treatment inactivates YAP/TAZ and may lead to inhibitory effects on EC proliferation and inflammation. Indeed, reconstitution of YAP/TAZ activities by overexpression of 5SA-YAP or TAZ attenuated the statin suppression of EC cell cycle progression (Fig. 4 C and D). Statin treatment also decreased the VCAM1 expression and the monocyte attachment to ECs induced by OS. Introduction of 5SA-YAP and TAZ reversed these inhibitory effects exerted by statin (Fig. 4 E and F). In agreement with the in vitro findings, our in vivo results demonstrated that daily statin administration (15 mg/kg, 3 d) resulted in YAP inactivation and reduction, but did not alter TAZ expression, in the AA inner curvature and the TA straight segment (Fig. S5A). En face staining of the PL-operated LCAs showed YAP/TAZ accumulation in the nuclei of ECs from the vehicle control group, but a cytoplasmic and diffused YAP/TAZ distribution pattern in ECs from the statin-treated group (Fig. S5B). Taken together, our results suggest that the pleiotropic effects of statin on the suppression of the atheroprone EC phenotypes are mediated, at least in part, through YAP/TAZ inactivation.
Fig. 4.
Statin inhibits YAP/TAZ to exert antiproliferative and antiinflammatory effects on ECs. (A) Western blot analysis of P-YAP, YAP, TAZ, CTGF, and CYR61 in ECs subjected to LS or OS in the presence of 1 µM simvastatin (statin) or DMSO control. *P < 0.05 vs. OS/DMSO. (B) Staining of YAP/TAZ localization in ECs subjected to 24-h OS in the presence of statin or DMSO. (Scale bar: 100 µm.) The graphic data are the nuclear/cytoplasmic ratio of YAP/TAZ intensity from cells randomly selected from three independent experiments. *P < 0.05 vs. OS/DMSO. (C and D) ECs transfected with 5SA-YAP, TAZ, or pcDNA3 were subjected to OS in the presence of statin or DMSO, followed by the analyses of cell cycle (C) and RB phosphorylation (D). (E and F) The transfected ECs were subjected to OS in the presence of statin or DMSO, followed by the analysis of VCAM1, ICAM1, and GAPDH (E) and monocyte adhesion assay (F). (C and F) Mean ± SD, n = 3, *P < 0.05 vs. OS/DMSO/pcDNA3 and #P < 0.05 vs. OS/statin/pcDNA3.
Fig. S5.
ApoE−/− mice fed a chow diet were subjected to the PL of LCAs and followed by the i.p. injection of simvastatin (statin) with a daily dose of 15 mg/kg. Three days after PL, the TA and the inner curvature of AA were harvested for the Western blot analysis of P-YAP, YAP, TAZ, and GAPDH (A); and the LCAs were fixed and cut longitudinally for the en face staining of YAP/TAZ (red) and nuclei (blue) (B). Results in (A) and (B) are representative images from three animals with similar results. The graphic data in B are the nuclear/cytoplasmic ratio of the YAP/TAZ intensity in randomly selected cells from three animals, *P < 0.05 vs. vehicle control. (Scale bar: 100 µm.)
YAP/TAZ Inhibition Attenuates Endothelial Activation and Atherosclerotic Lesion Development in PL-Operated LCAs.
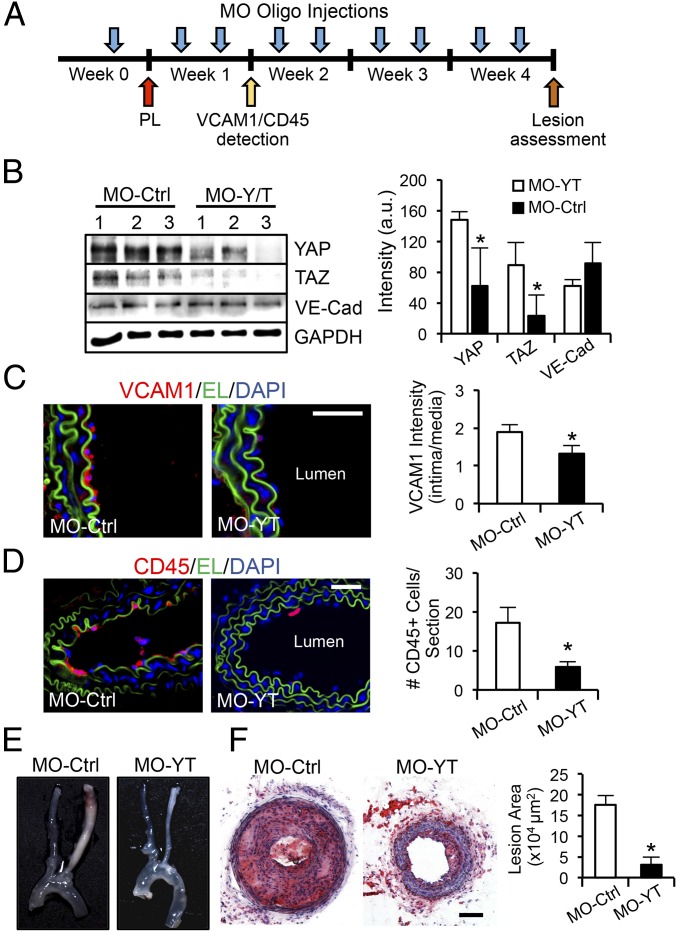
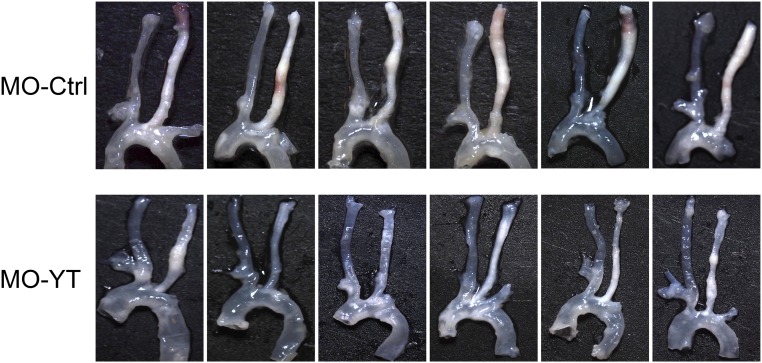
We next investigated the role of disturbed flow-induced YAP/TAZ activation on the development of atherosclerotic lesions. The PL-induced flow disturbance in LCAs of ApoE−/− mice results in severe carotid atherosclerosis in 4 wk (19). To perturb the disturbed flow activation of YAP/TAZ in vivo, we designed the morpholino (MO)-based oligos to inhibit YAP/TAZ translation (SI Materials and Methods). ApoE−/− mice fed the Paigen diet were given MO-YAP/TAZ (MO-YT) or MO-Control (MO-Ctrl) at a dose of 10 nmol (∼5 mg/kg) through tail-vein injections 72 h before and immediately after the PL procedure; the oligos were given twice a week after the operation for up to 4 wk (Fig. 5A). The expression levels of YAP/TAZ and endothelial inflammation were examined 1 wk after operation. The results showed that administration of MO-YT, but not MO-Ctrl, significantly diminished YAP/TAZ protein levels in the arteries (Fig. 5B). Functionally, this reduction of YAP/TAZ decreased the PL-induced adhesion molecule expression in the intima (Fig. 5C) and the consequential leukocyte attachment (Fig. 5D), as indicated by the immunofluorescence staining analysis of VCAM1 and CD45, respectively. After 4 wk of MO-oligos injections, the levels of cholesterol, HDL, and LDL in the serum of ApoE−/− mice fed the Paigen diet were comparable between the MO-Ctrl and MO-YT groups, but significantly higher than the control littermates that were fed a regular chow (Table S1). These results demonstrate that the YAP/TAZ modulation of vascular functions is not mediated through the blood lipids. Importantly, in vivo blockade of YAP/TAZ translation significantly reduced the overall size of atherosclerotic plaques in the PL-operated LCAs (Fig. 5E and Fig. S6). Histological analysis of the frozen sections and quantification of luminal lesion size confirmed that YAP/TAZ inhibition by MO-YT strongly attenuated the disturbed flow-induced carotid atherosclerosis (from 175,820 to 30,964 µm2) (Fig. 5F). These results demonstrate that the YAP/TAZ activation induced by disturbed flow synergizes with atherogenic factors, such as hypercholesterolemia, to promote the atheroprone phenotypes and atherosclerosis development.
Fig. 5.
YAP/TAZ play important roles in atherogenesis. (A) Experimental design of morpholino oligo treatments (MO-Ctrl vs. MO-YT, 10 nmol) in ApoE−/− mice fed the Paigen diet. The oligos were i.v. injected into animals 72 h before the PL, immediately after the PL, and twice a week afterward. (B) One-week post-PL, thoracic aortas were harvested for the analysis of YAP, TAZ, VE-Cadherin, and GAPDH expression. n = 3. (C and D) The sections of LCAs were examined by the immunofluorescence staining of VCAM1 (red) (C) and CD45 (red) (D). Elastic lamina (EL, green) and nuclear staining (DAPI, blue) are shown to indicate vessel structure. The VCAM1 intensity ratio (intima/media) and the number of CD45-positive cells attached to intima layer were quantified. n = 4. (E and F) Four-week post-PL, the arterial tissues were isolated to examine the atherosclerotic lesions (E), and the LCAs were sectioned for immunohistochemistry analysis (Oil Red O and Hematoxylin) and quantification of lesion area. n = 6 (F). (B–D and F): mean ± SD, *P < 0.05 vs. MO-Ctrl. (Scale bars: 100 µm.)
Table S1.
Serum lipid profile of ApoE−/− mice injected with MO oligos and fed the Paigen diet for 4 wk
| Lipid panel (mg/dL) | Chow diet/MO-Ctrl | Paigen diet/MO-Ctrl | Paigen diet/MO-Y/T |
| Cholesterol | 344 ± 56 | 3,446 ± 508 | 2,572 ± 292 |
| HDL | 90 ± 11 | 1,430 ± 157 | 1,097 ± 100 |
| LDL | 215 ± 43 | 1,982 ± 354 | 1,437 ± 212 |
| Triglyceride | 187 ± 35 | 171 ± 38 | 195 ± 33 |
All values are expressed as mean ± SD of three animals.
Fig. S6.
Reduction of the PL-induced carotid atherosclerosis with in vivo inhibition of YAP/TAZ. ApoE−/− mice injected with MO oligos (MO-Ctrl and MO-YT, six mice each group) were subjected to PL and followed by the Paigen diet for 4 wk, as described in Fig. 5A. The arterial tissues (from carotid bifurcation to descending aorta) were isolated and examined for atherosclerotic lesions.
SI Materials and Methods
Cell Culture and Flow Channel Experiments.
HUVECs and HAECs (purchased from Lonza) were cultured in medium M199 supplemented with 10% (vol/vol) endothelial cell growth medium, 10% (vol/vol) FBS, 1% sodium pyruvate, 1% l-glutamine, and 1% penicillin-streptomycin. HUVECs were used in most of the in vitro studies except the experiments using HAECs in Fig. S2 A and B. THP1 monocytes (ATCC) were maintained in RPMI medium 1640 supplemented with 10% (vol/vol) FBS and 1% penicillin-streptomycin. ECs within passages 4–8 were seeded onto collagen I-coated glass slides and grown until confluence. The slides were assembled into flow chambers and connected to the flow system for the shear experiments. ECs were exposed to steady laminar shear stress (LS, 12 dyn/cm2), oscillatory shear stress (OS, 0.5 ± 4 dyn/cm2), or kept as static control. The system was kept at 37 °C, and the circulating medium was equilibrated with humidified 5% (vol/vol) CO2-95% (vol/vol) air.
Reagents.
Antibodies against P-YAP (Ser-127), YAP, TAZ, LATS1, FLAG-tag, HA-tag, and GAPDH used in Western blot analysis were purchased from Cell Signaling Technology; antibodies against YAP/TAZ (63.7) used in immunofluorescence staining and Western blot and CTGF, CYR61, α-tubulin, and β-actin used in Western blot analysis were purchased from Santa Cruz Biotechnologies; antibodies against P-RB and RB were purchased from BD Pharmingen. Silencing RNAs against YAP/TAZ (siYAP/TAZ or siYT), LATS1 (siLATS1), and the negative control molecule (siCtrl) were purchased from Dharmacon. The plasmids expressing FLAG-5SA-YAP (5SA-YAP) and HA-TAZ (TAZ) were acquired from Addgene. Morpholino-based (MO) oligomers blocking YAP/TAZ translation (MO-YAP: 5′-CTCCATGGCTGCGGCCTCGTTCGAC-3′; MO-TAZ: 5′-TTGAATTATGCATGACCTCCTGGCC-3′) and the control oligo (MO-Ctrl: 5′-CCTCTTACCTCAGTTACAATTTATA-3′) used in the animal study were synthesized by Gene Tools. The morpholino oligos contain an octa-guanidine dendrimer as an in vivo delivery moiety and are dissolved in saline and ready for in vivo use. Simvastatin purchased from Abcam was dissolved in dimethyl sulfoxide (DMSO) and activated with NaOH before use. Tumor necrosis factor-α (TNFα) and interlukin-1-β (IL1β) were purchased from R&D Systems. The primer sets listed in Table S2 were synthesized by ValueGene.
Table S2.
List of the primers used in this study
| Gene | Species | Sequence (5′ → 3′) |
| CYR61 | Human | Forward: ATGGTCCCAGTGCTCAAAGA |
| Reverse: GGGCCGGTATTTCTTCACAC | ||
| CTGF | Human | Forward: CAGCATGGACGTTCGTCTG |
| Reverse: AACCACGGTTTGGTCCTTGG | ||
| ANKRD1 | Human | Forward: ACGCCAAAGACAGAGAAGGA |
| Reverse: TTCTGCCAGTGTAGCACCAG | ||
| CYR61 | Mouse | Forward: CTGCGCTAAACAACTCAACGA |
| Reverse: GCAGATCCCTTTCAGAGCGG | ||
| CTGF | Mouse | Forward: GGGCCTCTTCTGCGATTTC |
| Reverse: ATCCAGGCAAGTGCATTGGTA | ||
| ANKRD1 | Mouse | Forward: GGATGTGCCGAGGTTTCTGAA |
| Reverse: GTCCGTTTATACTCATCGCAGAC | ||
| CDK2 | Human | Forward: CATTCCTCTTCCCCTCATCA |
| Reverse: CAGGGACTCCAAAAGCTCTG | ||
| CDK4 | Human | Forward: TTCTGGTGACAAGTGGTGGA |
| Reverse: CTGGTCGGCTTCAGAGTTTC | ||
| CCNA1 | Human | Forward: GTCACCACATACTATGGACATG |
| Reverse: AAGTTTTCCTCTCAGCACTGAC | ||
| CCND1 | Human | Forward: AGACCTGCGCGCCCTCGGTG |
| Reverse: GTAGTAGGACAGGAAGTTGTTC | ||
| E2F1 | Human | Forward: TGCAGAGCAGATGGTTATGG |
| Reverse: AGATGATGGTGGTGGTGACA | ||
| PCNA | Human | Forward: GGCGTGAACCTCACCAGTAT |
| Reverse: TCTCGGCATATACGTGCAAA | ||
| P27 | Human | Forward: TGCAACCGACGATTCTTCTACTC |
| Reverse: CAAGCAGTGATGTATCTGAT |
Transfection.
Silencing RNAs and plasmids (5SA-YAP, TAZ, and pcDNA3) were transfected with Amaxa Nucleofector (Lonza) into ECs or THP1 monocytes according to the manufacturer’s protocol. Forty-eight hours after transfection, the ECs on slides were subjected to the shear experiment and/or statin treatment. The efficiencies of YAP/TAZ knockdown and overexpression were determined by Western blot and qRT-PCR analyses.
Western Blot and qRT-PCR Analyses.
ECs or THP1 cells were lysed in the ice-cold lysis buffer (25 mM Hepes, pH 7.4, 1% deoxycholate, 1% Nonidet P-40, 125 mM NaCl, and 5 mM EDTA) supplemented with protease/phosphatase inhibitor mixture (Roche). Snap-frozen arterial tissues from mice were thawed and homogenized in the lysis buffer as described above. The protein concentration was determined with Pierce 660 nm Protein Assay by using a spectrophotometer. Equal amounts of protein were loaded and resolved by SDS/PAGE. After electrophoresis, the proteins were transferred to nitrocellulose membranes, followed by blocking with 5% (wt/vol) BSA, incubation with the primary antibodies and secondary antibodies, and detection with a chemiluminescence kit. The blot image was acquired using Alpha Innotech imaging system and the band density was quantified by using ImageJ software. For qRT-PCR analysis, total RNA was isolated from ECs and homogenized arterial tissues by using TRIzol (Invitrogen) according to the manufacturer’s protocol. The concentration of RNA was determined by using a NanoDrop spectrophotometer. Five hundred nanograms of total RNA was reversely transcribed to cDNA by using the oligo-dT primer (Fermentas) and MLV reverse transcriptase (Invitrogen). The real-time PCR was carried out with iQ SYBR Green supermix on Bio-Rad iCycler detection system. Primer sets used for qRT-PCR are listed in Table S2. The relative gene expression was determined by 2-∆∆CT method.
Cell Growth and Cell Cycle Analyses.
ECs transfected with siRNAs or plasmids were seeded at a density of 5 × 104 cells per well, and the cell growth curves were determined by counting cell numbers using a hemocytometer every 24 h over a 5-d period. For cell cycle analysis, ECs collected from shear and statin experiments were fixed in 70% (vol/vol) ice-cold ethanol overnight. Fixed ECs were treated with RNase A (100 µg/mL) and labeled with propidium iodide (50 µg/mL) for 1 h at 37 °C. The labeled ECs were then assayed by using BD Accuri C6 cytometer. Distributions of ECs in different cell cycle stages were determined by the Watson Pragmatic algorithm by using FlowJo software.
Monocyte Adhesion Assay.
THP-1 monocytes maintained in suspension culture were pelleted by centrifugation, labeled with 5 mM Calcien-AM at 37 °C for 30 min, and resuspended to the concentration of 5 × 105 cells per mL in M199 medium. The adhesion assay was performed by adding the labeled THP-1 cells to the sheared (Figs. 3C and 4F), plasmid-transfected (Figs. 3E and 4F), or TNFα-treated (Fig. 3H) EC monolayers for 10 min at 37 °C. After removing the unbound cells by three times of gentle washing with M199 medium, the THP-1 cells attached to ECs were fixed with 4% (wt/vol) paraformaldehyde for 10 min. Fluorescent images of the THP1 cells attached to the ECs were taken under an Olympus IX70 microscope. ImageJ was used to subtract background noise and quantify the number of adherent THP1 cells.
Animal Experiments.
All animal experiments performed in this study were in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the University of California at San Diego. Adult ApoE−/− mice (12–16 wk old) originally obtained from The Jackson Laboratory were euthanized for tissue collection or subjected to PL as described (18, 19). The animals were anesthetized with Ketamine (100 mg/kg body weight)-Xylazine (10 mg/kg body weight) mixture through i.p. injection. In brief, the PL was performed by ligating three branches (left external artery, left internal artery, and occipital artery) of the LCA with 7–0 silk suture, whereas the superior thyroid artery was left intact. Postoperative ApoE−/− mice were fed a chow or the Paigen diet containing 15.8% (wt/wt) fat, 1.25% (wt/wt) cholesterol, and 0.5% sodium cholate (TD. 88051; Harlan Laboratories) to induce atherosclerosis in partially ligated LCAs within 4 wk. To inhibit YAP/TAZ translation in vivo, the MO-YT or MO-Ctrl diluted in saline were administered at a dose of 10 nmol (∼5 mg/kg) through tail-vein injections at 72 h before the PL, immediately after the PL, and twice a week until killing. PL was performed on 20 ApoE−/− mice. Ten of those mice received the MO-YT, and the other 10 received MO-Ctrl. Four animals from each group were killed at the end of week 1 to examine the efficiency of YAP/TAZ knockdown and the atheroprone phenotypes. The remaining animals were killed at the end of week 4 to examine the progression of atherosclerotic lesions in LCAs. At the end of experiments, the animals were euthanized by CO2 inhalation, and blood was collected for lipid analysis (Table S1). Residual blood in the cardiovascular system was removed by PBS perfusion, and the arterial tissues were excised for the subsequent biochemical and histological analysis. For biochemical analyses, the tissues were homogenized and subjected to protein or RNA extraction as described above. For histological analysis and lesion quantifications, the arterial tissues were fixed with 4% (wt/vol) paraformaldehyde in PBS at 4 °C for 16 h. For histological examination and lesion quantification, LCAs were excised from the fixed arterial tissues and cross-cut in the middle into two fragments. The fragments were then cut longitudinally for en face staining or embedded with Tissue-Tek O.C.T. compound for frozen sectioning. Serial sectioning was performed starting at the middle of each fragment and extending for ∼1 mm, using a Cryotome Cryostat machine (Leica). Multiple sections (10 μm per section) were randomly selected from each fragment and stained with Oil Red O and hematoxylin for quantification of the atherosclerotic lesions, and the luminal lesion areas were averaged for each animal (six animals in each group). The lesion area was measured as the Oil Red O-positive area between the inner boundary of the internal elastic layer and the narrowed lumen by using ImageJ software.
Immunofluorescence and Histology Staining.
For in vitro experiments, ECs subjected to shear and statin experiments were fixed with 4% (wt/vol) paraformaldehyde in PBS for 10 min. The cells were permeabilized with 0.1% Triton X-100 and blocked with 3% (wt/vol) BSA in PBS for 1 h, followed by incubation with the primary antibody against YAP/TAZ (63.7) at 4 °C for 12 h. For en face staining, fixed arterial tissues were cut longitudinally and pinned on wax pad to lay flat (with intima layer face-up). The pinned tissues were then permeabilized, blocked, and incubated with primary antibodies against YAP/TAZ at 4 °C for 12 h. For tissue-section samples, frozen sections were rinsed with PBS to remove the O.C.T. compound, permeabilized, blocked, and incubated with primary antibodies against YAP/TAZ, VCAM1, or CD45, at 4 °C for 12 h. After the incubation with primary antibodies, Alexa Fluor 594-conjugated anti-rabbit, anti-mouse, and anti-goat IgG antibodies were used (1:200) as the secondary antibodies, and DAPI was used for counterstaining. After mounting, the images of en face staining were acquired under an Olympus IX70 Confocal Microscope and the rest of images were taken under an epi-fluorescence microscope.
Statistical Analysis.
For comparisons between two groups, statistical analyses were performed by two-tailed unpaired Student’s t test. Comparison of multiple groups was made by one-way ANOVA, and statistical significance among multiple groups was determined by Tukey’s post hoc test (for pair-wise comparisons of means). All results are presented as mean ± SD from at least three independent experiments.
Discussion
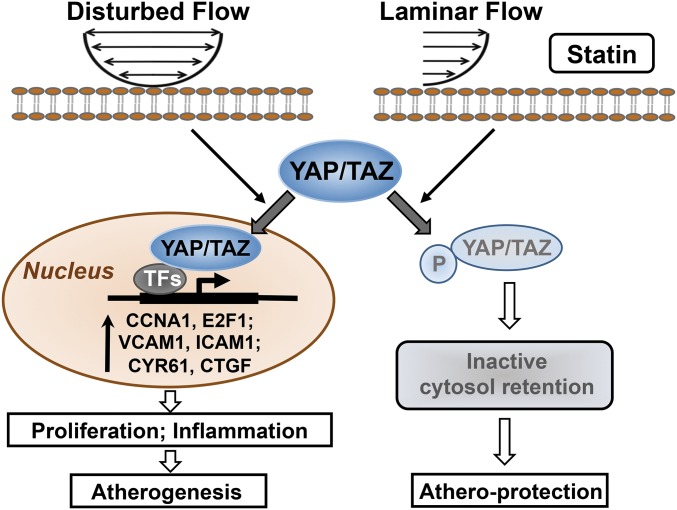
The detailed mechanisms for atheroprone EC phenotypes, such as dysregulated turnover and maladaptive inflammation, induced by the disturbed flow patterns at the arterial branches and curvature, remain to be elucidated (3, 4). Recent reports demonstrated that, in addition to their responses to chemical stimuli, YAP/TAZ serve as important transducers relaying mechanical signals to modulate gene expression profiles and functional consequences through their transcription factor partners (14, 21, 22). Our current findings elucidate the mechanotransducing role of YAP/TAZ in hemodynamic regulation of endothelial homeostasis and diseases. As summarized in Fig. 6, we have revealed a mechanism that YAP/TAZ are critical regulators in mediating the disturbed flow-induced atheroprone EC phenotypes and the consequential development of atherosclerosis. This conclusion is supported by the in vitro and in vivo findings that (i) the disturbed flow, but not steady laminar flow, induces YAP/TAZ activation, as indicated by hypophosphorylation of YAP, nuclear localization of YAP/TAZ, and up-regulations of YAP/TAZ and their target genes; (ii) the disturbed flow activation of YAP/TAZ promotes the cell cycle progression, proinflammatory gene expression, and monocyte adhesion; (iii) the statin suppression of endothelial proliferation and inflammation is mediated by inhibition of YAP/TAZ activities; (iv) the inhibition of YAP/TAZ attenuates the PL-induced endothelial inflammation and the consequential progression of carotid atherosclerosis in ApoE−/− mice.
Fig. 6.
Schematic diagram of YAP/TAZ signaling and their modulation of EC phenotypes in response to disturbed flow, laminar flow, and statin.
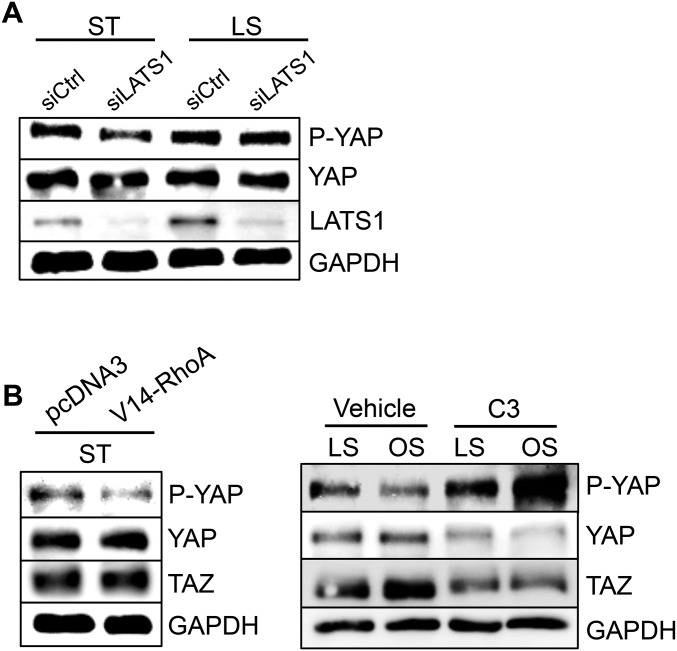
The regulation of YAP/TAZ activities, as indicated by the phosphorylation status of YAP and the resulting cytoplasmic/nuclear localization, are controlled canonically by LATS kinases (7). Recent reports have shown that mechanical stimuli modulate YAP/TAZ activities via a Rho-GTPases/actomyosin-dependent, but LATS-independent, manner (14, 22). In our hands, depletion of LATS1 was not sufficient to reduce the LS-induced YAP phosphorylation in ECs (Fig. S7A); instead, modulations of Rho-GTPase activities with an active RhoA (V14-Rho) and a Rho inhibitor (C3 exoenzyme) strongly regulated YAP/TAZ activities (Fig. S7B), suggesting the flow regulation of YAP/TAZ activities does not solely depend on LATS kinases but also through the modulation of Rho-GTPase activities. In addition to the Hippo and Rho-GTPase pathways, other known mechanosensors, such as G protein-coupled receptors and integrins (23, 24), and mechanotransduction pathways, including AMP-activated protein kinase, and VE-cadherin–mediated phosphatidylinositol 3-kinase (PI3K)/AKT signaling (25, 26), have been shown to modulate YAP/TAZ activities through phosphorylation. It would be worthwhile to further delineate the Hippo signaling cascade and the cross-talk between the flow-sensitive pathways that modulate YAP/TAZ activities. YAP/TAZ–TEADs complex has been shown to induce expressions of its target genes controlling cell growth and cell cycle progression (9, 27). It is likely that the OS-activated YAP/TAZ act through TEADs to promote EC proliferation. Further investigation will be required to dissect the transcriptional regulation of YAP/TAZ-target gene expressions and the functional network in ECs in response to different flow patterns.
Fig. S7.
(A) ECs transfected with silencing RNA against LATS1 (siLATS1) or siCtrl were subjected to 24-h LS or kept under static condition, followed by the Western blot analysis of P-YAP, YAP, LATS1, and GAPDH. (B) ECs transfected with the constitutively active form of RhoA (V14-RhoA) or a Rho inhibitor (C3 exoenzyme) followed by 24-h LS or OS, were subjected to the Western blot analysis of P-YAP, YAP, TAZ, and GAPDH. Results are representative images from three independent experiments with similar trend.
CYR61 and CTGF, the best known targets of YAP/TAZ, have been associated with the chemosensory adhesion and migration of leukocytes (16, 17). Our in vitro and in vivo data demonstrate that disturbed flow patterns increased the expressions of CYR61 and CTGF in ECs, in comparison with steady laminar flow (Fig. 1 D and E). CYR61 and CTGF have also been found to be highly expressed in atherosclerotic arteries (16, 17). In our studies, we have found that, just as CYR61 and CTGF, YAP/TAZ are highly expressed transmurally in the atherosclerotic arteries, but their expression is only restricted to the intima layer at the lesion-free regions (Fig. S1). Furthermore, perturbations of YAP/TAZ by the silencing RNAs and the plasmids overexpressing YAP resulted in down- and up-regulations of CYR61 and CTGF, respectively (Fig. S3 A and B). Taken together, our findings indicate that the dysregulation of YAP/TAZ induced by disturbed flow and other atherogenic factors are responsible for the CYR61 and CTGF overexpressions and, hence, play a proatherogenic role in promoting the inflammatory-fibrotic responses in atherosclerosis.
Dysregulation of YAP/TAZ causes aberrant cell growth, leading to a series of developmental disorders and cancers (5, 7). In the vascular system, smooth muscle-specific deletions of YAP result in profound cardiac defects and vascular abnormalities (28). The inductions of YAP expression and activation following arterial injury enhance neointima formation and the subsequent stenosis as a result of the unrestrained smooth muscle cell proliferation (15). We demonstrate that perturbations of YAP/TAZ in ECs resulted in significant changes in EC growth, indicating that YAP/TAZ are essential for proliferation and cell cycle progression of ECs. The OS activation of YAP/TAZ induced strong proinflammatory responses in ECs (Fig. 3), and the loss of YAP/TAZ attenuated the induction of VCAM1 expression by the inflammatory cytokines (Fig. S3E); these findings indicate that YAP/TAZ activation exerts a proinflammatory effect in response to the elevation of cytokines at the atherosclerotic lesions, as well as the atheroprone flow conditions. However, the inactivation of YAP/TAZ by athero-protective LS counteracted the effects resulted from these risk factors associated with atherosclerosis. Our findings establish that YAP/TAZ activities modulate endothelial proliferation and inflammation, with the disturbed flow activation of YAP/TAZ playing a causative role in mediating the atheroprone phenotypes.
In addition to the blockade of cholesterol synthesis, statin exerts the beneficial pleiotropic effects on vascular cells, including the improvement of endothelial function and attenuation of vascular inflammation (20). Our findings provide a mechanistic explanation for such pleiotropic effects of statin on vascular cells. We found that statin repressed the OS-induced EC proliferation and inflammation, and that statin caused the reduction of YAP/TAZ activities and cytoplasmic retention of YAP/TAZ both in vitro and in vivo, similar to the findings in cancer cells (12, 13). Most importantly, reconstitution of the YAP/TAZ activities blocked the statin suppressions of EC proliferation and inflammation (Fig. 4). These findings demonstrate that inactivation of YAP/TAZ plays a critical role in the athero-protective effects of statin on ECs. It has been reported that statin therapy also inhibits the activities of proatherogenic Rho-GTPase/kinase (ROCK) pathway and improves the outcome in patients with atherosclerosis (29). One may speculate that the beneficial effects of statin inhibition of Rho/ROCK on atherosclerosis are attributable to YAP/TAZ inactivation in vascular cells. In this regard, YAP/TAZ inhibition may serve as a potential antiatherosclerotic strategy with better specificity and fewer off-target effects.
The proatherogenic role of YAP/TAZ activation in the pathogenesis and progression of atherosclerosis was further established by our in vivo loss-of-function studies. Morpholino-based antisense oligomers have been developed and tested in a range of disease models, including that for coronary artery restenosis after angioplasty, through translational inhibition (30). Using the rodent model to induce carotid atherosclerosis by disturbed flow patterns, we showed that the i.v. injections of morpholino oligos against YAP/TAZ reduced the PL-induced atheroprone EC phenotypes within the 1-wk treatment period, and that prolonged inhibition of YAP/TAZ (4 wk) attenuated the atherosclerotic lesion formation (Fig. 5). These findings provide the preclinical evidence that YAP/TAZ are potential therapeutic targets for atherosclerosis.
In conclusion, using the in vitro cell perfusion system and the in vivo PL-induced atherosclerotic mouse model, we have demonstrated that YAP/TAZ serve as critical mechanotransducers in modulating endothelial proliferation and inflammation in response to local hemodynamics, and that YAP/TAZ activation mediates the disturbed flow-induced atheroprone phenotypes and atherosclerotic lesion formation. Our findings provide strong evidence for the atherogenic role of YAP/TAZ activation and insights for the development of therapeutic intervention for atherosclerosis.
Materials and Methods
The sources of reagents and antibodies, and the methods for cell culture, flow channel experiments, transient transfection, qRT-PCR, Western blot, flow cytometric analysis of cell cycle, monocyte adhesion assay, animal experiments, histological characterization of the arterial tissues, quantification of atherosclerotic lesions, and statistical analysis are described in SI Materials and Methods. The primer sets used in this study are listed in Table S2. All animal experiments performed in this study were in accordance with NIH guidelines and approved by the Animal Care and Use Committee of the University of California at San Diego. The details are provided in SI Materials and Methods.
Acknowledgments
This work is supported by the National Institutes of Health under Award Nos. HL108735, HL106579, and HL121365 (to S.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613121113/-/DCSupplemental.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23) Suppl 1:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 3.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20(6):638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 8.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA. 2013;110(7):2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorrentino G, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA. 2014;111(1):E89–E98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. The induction of yes-associated protein expression after arterial injury is crucial for smooth muscle phenotypic modulation and neointima formation. Arterioscler Thromb Vasc Biol. 2012;32(11):2662–2669. doi: 10.1161/ATVBAHA.112.254730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schober JM, et al. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): Immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99(12):4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- 17.Cicha I, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25(5):1008–1013. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- 18.Wang KC, et al. MicroRNA-23b regulates cyclin-dependent kinase-activating kinase complex through cyclin H repression to modulate endothelial transcription and growth under flow. Arterioscler Thromb Vasc Biol. 2014;34(7):1437–1445. doi: 10.1161/ATVBAHA.114.303473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam D, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297(4):H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26(1):54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. doi: 10.1038/ncomms3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo JS, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17(4):500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi HJ, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 27.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17(9):1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. Deletion of yes-associated protein (YAP) specifically in cardiac and vascular smooth muscle cells reveals a crucial role for YAP in mouse cardiovascular development. Circ Res. 2014;114(6):957–965. doi: 10.1161/CIRCRESAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nohria A, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205(2):517–521. doi: 10.1016/j.atherosclerosis.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kipshidze N, et al. First human experience with local delivery of novel antisense AVI-4126 with Infiltrator catheter in de novo native and restenotic coronary arteries: 6-month clinical and angiographic follow-up from AVAIL study. Cardiovasc Revasc Med. 2007;8(4):230–235. doi: 10.1016/j.carrev.2007.04.002. [DOI] [PubMed] [Google Scholar]