Significance
Laquinimod is an oral drug currently being evaluated for the treatment of relapsing, remitting, and primary progressive multiple sclerosis as well as Huntington’s disease. It is thought that laquinimod has a primary effect on the peripheral innate immune system and also acts directly on resident cells within the CNS. However, the exact mechanism of action of laquinimod has not been fully elucidated. We investigated gene expression in laquinimod-treated mice and show induction of genes downstream to activation of the aryl hydrocarbon receptor (AhR). In this paper, we examine the role of the AhR in laquinimod treatment of experimental autoimmune encephalomyelitis and demonstrate that AhR is the molecular target of laquinimod in this model.
Keywords: aryl hydrocarbon receptor, EAE, laquinimod
Abstract
Laquinimod is an oral drug currently being evaluated for the treatment of relapsing, remitting, and primary progressive multiple sclerosis and Huntington’s disease. Laquinimod exerts beneficial activities on both the peripheral immune system and the CNS with distinctive changes in CNS resident cell populations, especially astrocytes and microglia. Analysis of genome-wide expression data revealed activation of the aryl hydrocarbon receptor (AhR) pathway in laquinimod-treated mice. The AhR pathway modulates the differentiation and function of several cell populations, many of which play an important role in neuroinflammation. We therefore tested the consequences of AhR activation in myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) using AhR knockout mice. We demonstrate that the pronounced effect of laquinimod on clinical score, CNS inflammation, and demyelination in EAE was abolished in AhR−/− mice. Furthermore, using bone marrow chimeras we show that deletion of AhR in the immune system fully abrogates, whereas deletion within the CNS partially abrogates the effect of laquinimod in EAE. These data strongly support the idea that AhR is necessary for the efficacy of laquinimod in EAE and that laquinimod may represent a first-in-class drug targeting AhR for the treatment of multiple sclerosis and other neurodegenerative diseases.
Laquinimod is an oral drug that is currently in late-stage clinical development for the treatment of relapsing remitting multiple sclerosis (RRMS), primary progressive MS, and Huntington’s disease. Current knowledge indicates that laquinimod exerts activities both on the peripheral immune system and within the CNS. Laquinimod, at the 0.6-mg/d dose, has demonstrated efficacy in phase II and III MS clinical trials, in which it reduced relapse rate, disability progression, development of new and active MRI lesions, and brain atrophy (1–3). The clinical efficacy profile of laquinimod is characterized by a dissociation of the moderate magnitude of the effect on relapse reduction and its associated inflammatory MRI findings and the disproportionally large effect on disability progression. Such an efficacy profile in patients with RRMS may relate to a distinctive intracerebral activity potentially mediated via changes in CNS resident cell populations, potentially astrocytes and microglia.
The influence of laquinimod on the immune system was studied in experimental autoimmune encephalomyelitis (EAE) (4–12), an autoimmune disease mediated by proinflammatory myelin-reactive lymphocytes that cause CNS inflammation leading to demyelination and axonal loss. Laquinimod has also been effective in the treatment of other models of autoimmune diseases, specifically experimental autoimmune neuritis (13, 14), lupus nephritis (15), and colitis (16). A common characteristic of autoimmune diseases is that autoantigen-reactive T cells must undergo several discrete steps to cause disease. Initial signals that direct T-cell activation and differentiation are provided by antigen-presenting cells (APC), including monocytes, macrophages, and dendritic cells (DCs). It was reported that treatment of mice with laquinimod is associated with alterations in the frequency of myeloid subpopulations that included a reduction in CD4+ DCs. Laquinimod treatment also promoted the development of anti-inflammatory type II monocytes and DCs (6, 7, 9), which are likely associated with its immunomodulatory activities. These activities include reduced production of proinflammatory cytokines such as IL-17, reduced migration of lymphocytes (4, 7), augmentation of regulatory T-cell numbers (5, 7), and production of brain-derived neurotrophic factor (5, 8). Although no molecular target has been identified for laquinimod, it has been shown to modulate the T-cell response probably as a result of its effects on STAT1, MAPK, and NF-κB signaling in APCs (reviewed in ref. 17). To further elucidate laquinimod’s immunomodulatory mechanisms of action, in this paper we analyzed gene expression levels modulated by laquinimod versus vehicle-treated mice. We show that laquinimod induces genes known to be associated with the aryl hydrocarbon receptor (AhR). In the present study, we investigate whether laquinimod suppresses EAE via the AhR pathway by testing its efficacy in myelin oligodendrocyte glycoprotein (MOG)-induced EAE using AhR knockout mice.
Results
Transcriptome Analysis Reveals That Laquinimod Treatment Induces Genes Associated with Activation of the AhR.
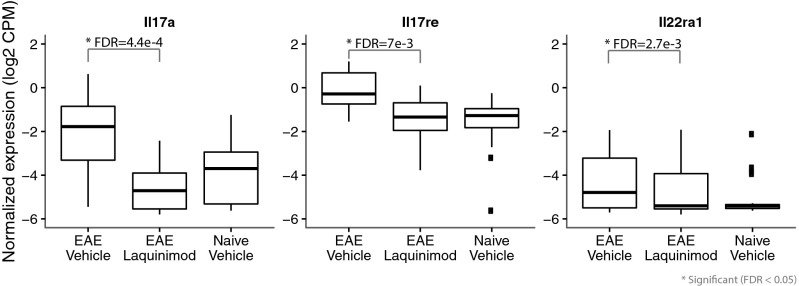
To gain insight into laquinimod’s immunomodulatory mechanisms of action, spleen gene expression profiles were compared between laquinimod- and vehicle-treated EAE mice, 6 d postdisease induction. Our analysis showed that 610 genes were differentially modulated between laquinimod- and vehicle-treated EAE mice. Of the 610 genes, 227 genes were down-regulated by laquinimod treatment, including key Th17-related cytokine genes, specifically IL-17a, IL-17re, and IL-22Ra1 (Fig. 1), which were significantly reduced in laquinimod-treated mice [log2 fold-changes of −2.91, −1.64, and −1.81, respectively; false-discovery rate (FDR) values of 4.4e-4, 7e-3, and 2.7e-3]. These findings are in line with published data showing the beneficial effect of laquinimod on Th17 (4, 7). A total of 383 genes were up-regulated by laquinimod in this analysis, and cytochrome P450 family member A1 (Cyp1a1) and Ahrr, prototypical genes associated with the AhR pathway (18, 19), were among the highest fold-change genes induced by laquinimod treatment in EAE mice (Fig. 2A). Analysis of laquinimod-induced genes in naive mice revealed a similar pattern (Fig. 2B), indicating that activation of AhR was inherent to drug and independent of disease state. However, many other AhR-associated genes, including Cyp1b1 (20), Tiparp (19), Ido1 and Ido2 (21, 22), Spint1, and Serpins (23) were induced by laquinimod in both naive and EAE mice. Laquinimod-induced activation of the AhR pathway was confirmed by assessing gene expression levels of the AhR biomarker, Cyp1a1 in mouse liver. As shown in Fig. 2C, the endogenous AhR ligand 2-(1H-Indol-3-ylcarbonyl)-4-thiazolecarboxylic acid methyl ester (ITE) (24) caused increases of up to 74- and 4.2-fold in hepatic Cyp1a1 and Cyp1a2 mRNA levels, respectively. Treatment of mice with 25 mg/kg laquinimod caused 539- and 21-fold increases in hepatic Cyp1a1 and Cyp1a2 mRNA expression levels, respectively, compared with vehicle-treated mice. These data verified the initial genomic findings and demonstrated that laquinimod consistently induces the expression of genes downstream to the activation of AhR.
Fig. 1.
Laquinimod treatment down-regulates genes associated with the Th17 pathway. These boxplots show the patterns of the expression of individual genes from splenocyte samples taken from naive and EAE mice at day 6 postinduction (n = 6 per treatment). It shows the patterns of expression across three conditions: EAE mice treated with vehicle, EAE mice treated with laquinimod, and naive mice treated with vehicle (n = 5). Laquinimod treatment significantly reduced the expression of IL-17a [log2 fold change (FC): −2.91; FDR: 4.4e-4], Il17re (log2 FC: −1.64; FDR 7e-3), and Il22ra1 (log2 FC: −1.81; FDR: 2.7e-3).
Fig. 2.
Transcriptome analysis reveals that laquinimod induces genes in the AhR pathway. (A) This heatmap was generated using the patterns of expression of splenocyte samples from EAE mice at day 6 postinduction. It shows the patterns of expression of 88 genes with higher levels of expression in EAE mice treated with laquinimod (green, n = 6) than in EAE mice treated with vehicle (orange, n = 6). (B) This heatmap was generated using the patterns of expression of splenocyte samples from naive mice at 6 d postinduction. It shows the patterns of expression of 45 genes with higher levels of expression in naive mice treated with laquinimod (red, n = 6) than in naive mice treated with vehicle (blue, n = 5). For both A and B, the filters used to define statistical significance were a fold-change > 2 and an FDR < 0.01. Rows were ordered from highest to lowest average fold-change. (C) In vivo induction of hepatic Cyp1a mRNA following 5-d treatment with 25 mg/kg laquinimod in C57BL/6 mice. This graph is taken from a representative experiment, which has been repeated at least four times.
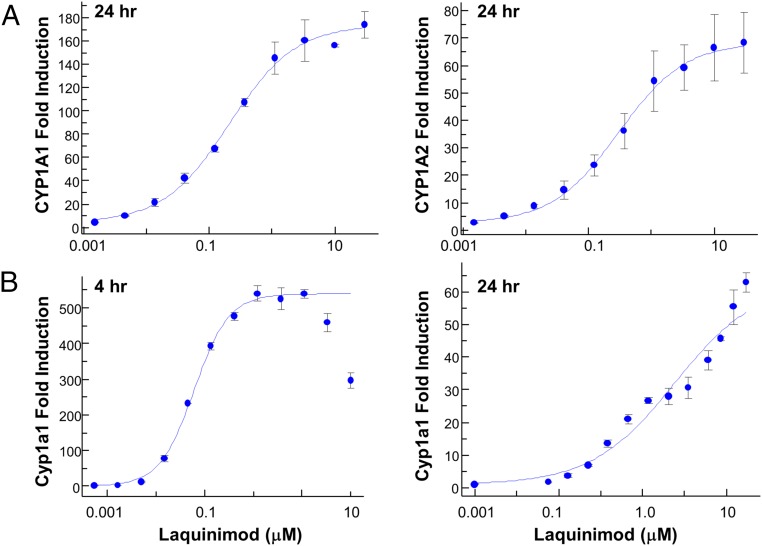
Additional experiments were performed to determine the concentration dependence of the laquinimod effect by quantifying the level of mRNA changes in primary hepatocytes, the most AhR-responsive tissue. Treatment of human hepatocytes with laquinimod for 24 h resulted in ∼180- and ∼80-fold induction of CYP1A1 and CYP1A2 mRNA, with an EC50 of 0.2 ± 0.04 μM and 0.3 ± 0.03 μM, respectively (Fig. 3A). Treatment with 100 μM omeprazole for 24 h was used as a positive control and resulted in 1,487 ± 34 and 188 ± 11 fold-induction of CYP1A1 and CYP1A2 mRNA. Treatment of primary mouse hepatocytes with laquinimod resulted in induction of Cyp1a1 mRNA with an EC50 of 0.06 ± 0.02 μM and 5.70 ± 0.20 μM at 4 and 24 h, respectively (Fig. 3B). Both the potency and magnitude of induction by laquinimod in mouse hepatocytes were larger at 4 h than at 24 h, presumably due to metabolism following longer incubation. Omeprazole does not activate mouse AhR, so 3-methylcholanthrene (3-MC) treatment (2 μM) for 24 h was used as a positive control and resulted in a 116- ± 1.5-fold induction of Cyp1a1 mRNA. In mouse hepatocytes, basal levels of Cyp1a2 mRNA were high and were not significantly augmented by treatment with either laquinimod or 3-MC.
Fig. 3.
In vitro verification of induction of CYP1A mRNA by laquinimod. (A) CYP1A1 (Left) and CYP1A2 (Right) fold-induction in human hepatocytes treated with laquinimod for 24 h compared with vehicle controls. Experiments were repeated at least four times with cells from different donors. (B) Cyp1a1 fold-induction in mouse hepatocytes treated with laquinimod for 4 (Left) or 24 h (Right). Experiments were repeated at least four times with different donors.
The Efficacy of Laquinimod in EAE Is Dependent on the AhR.
Given that laquinimod activates AhR, we wanted to determine the role of AhR in the efficacy of laquinimod in MOG-induced EAE. We found that AhR−/− mice were susceptible to EAE, albeit with slightly less severe disease. Whereas WT mice developed EAE with a mean day of onset of 12.8 ± 1.3, AhR−/− mice developed EAE with delayed onset (15.7 ± 4.5 d) and less severe disease (mean maximal score of 3.5 ± 1.1, compared with 4.2 ± 0.7 in AhR−/− and WT, respectively). As previously reported, prophylactic daily oral treatment with 25 mg/kg laquinimod inhibited MOG-induced EAE in WT C57BL/6 mice (Fig. 4). Laquinimod treatment reduced disease incidence from 100 to 20% and decreased the severity of disease in mice that show clinical severity from 4.2 ± 0.7 to 0.3 ± 0.6 (93% inhibition, P < 0.0001). In contrast, laquinimod completely lost its efficacy in AhR−/− mice (Fig. 4) that had comparable disease severity (3.3 ± 1.7) and a profile undistinguishable from vehicle-treated AhR−/− mice (3.5 ± 1.1).
Fig. 4.
Prophylactic treatment with laquinimod has no effect on clinical score in AhR−/− mice. The graph shows mean disease scores with SE from vehicle-treated (closed symbols) and laquinimod-treated (open symbols) WT or AhR−/− mice (n = 15 per group). The embedded table shows disease incidence, mean day of onset, and mean maximal score of disease. The data are representative of four independent experiments.
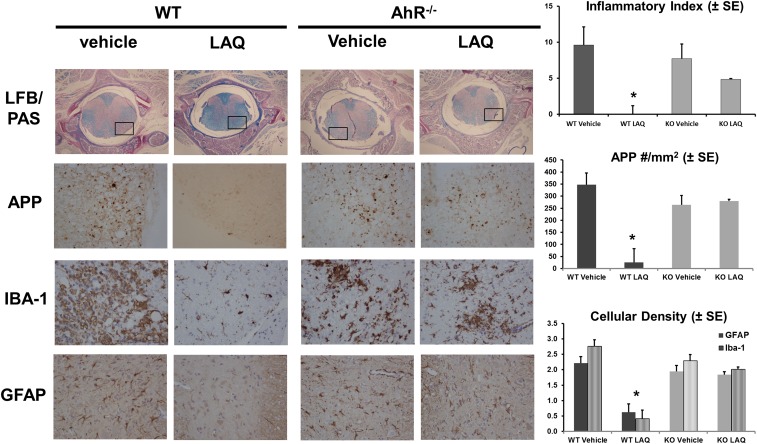
As previously shown (4), prophylactic treatment with laquinimod abrogated the extent of inflammation, demyelination, and acute axonal injury within the spinal cords from WT mice with MOG-induced EAE. The extent of damage in spinal cords from WT and AhR−/− mice was assessed by histological analysis. Quantification of inflammatory demyelination as determined in luxol-fast-blue (LFB)/periodic acid-Schiff (PAS) with H&E counterstaining revealed a significant protective effect of laquinimod in WT animals but not in AhR−/− animals (Fig. 5). Although widespread inflammatory demyelination was evident in vehicle-treated WT mice, these histopathological alterations were virtually absent in laquinimod-treated WT animals (P = 0.0003). Unpaired t test with Welch’s correction showed no significant difference between vehicle-treated and laquinimod-treated AhR−/− animals (P = 0.31). Quantification of acute axonal damage in amyloid precursor protein (APP)-stained slides revealed extensive axonal damage in vehicle-treated mice. As expected, the number of APP spheroids was most intense in regions with white matter inflammation. Laquinimod was protective in WT mice (P < 0.0001), but had no effect on acute axonal damage in AhR−/− mice (Fig. 5). Semiquantification of the extent of microglial and astrocytic activation was performed in Iba1- and GFAP-stained sections. Extensive microglial activation and astrogliosis were present in vehicle-treated mice, and both were significantly reduced by laquinimod in WT mice. In contrast, laquinimod had no effect on either parameter in AhR−/− mice (Fig. 5).
Fig. 5.
Prophylactic treatment with laquinimod has no effect on inflammatory demyelination, acute axonal damage, or microglial and astroglial activation in AhR−/− mice. All stains were performed using n = 15 per treatment group. The inflammatory index was quantified using LFB/PAS and H&E staining of spinal cords taken from vehicle- or laquinimod-treated WT or AhR−/− mice. (Upper panels) A cross-section of the whole spinal cord using 4× objective (Nikon Eclipse E200). (Lower panels) A higher magnification (40×) taken from the area marked by a rectangle. Quantification of acute axonal damage using APP staining showed a significant reduction (*P < 0.0001) in the number of APP+ spheroids in laquinimod-treated WT mice, but not in AhR−/− mice. Quantification of microglial and astrocyte activation using Iba-1 or GFAP staining showed a significant reduction (*P < 0.0001) in the number of Iba-1 (black bars) and GFAP (striped bars) cells in laquinimod-treated WT mice, but not in AhR−/− mice.
Treatment with laquinimod has been shown to decrease Th1 and Th17 responses with a corresponding increase in CD4+CD25+FoxP3+ regulatory T cells both in the periphery and within the CNS (4, 5, 7, 11). To investigate whether these immunomodulatory effects of laquinimod are also mediated by the AhR pathway, we analyzed the production of Th1 and Th17 cytokines and the frequency of regulatory T cells in spleens taken from WT and AhR−/− mice. Treatment with laquinimod in WT mice significantly increased the percentage of CD4+CD25+FoxP3+ regulatory T cells (Fig. 6A) compared with vehicle-treated mice (0.80 ± 0.06 vs. 0.37 ± 0.04; P < 0.05), whereas in AhR−/− mice no difference was observed (0.33 ± 0.04 vs. 0.38 ± 0.06). As expected, laquinimod treatment resulted in decreased production of IL-17, GM-CSF, and IFNγ in MOG-reactivated splenocytes from WT mice, albeit only significantly for GM-CSF (P < 0.04). In contrast, laquinimod treatment did not reduce MOG-specific IL-17, GM-CSF, and IFNγ production in splenocytes taken from AhR−/− mice. In all mice, the recall response to purified protein derivative (PPD) was not affected by laquinimod, and the level of cytokine release was not statistically different between WT and AhR−/− mice (Fig. 6B). These data demonstrate that laquinimod expands regulatory T cells and limits T-effector cells in an AhR-dependent manner in EAE.
Fig. 6.
Laquinimod immunomodulation in EAE is AhR-dependent. (A) MOG EAE mice were treated daily with laquinimod (25 mg/kg, n = 6 per group) or vehicle (water, n = 6 per group), and 15 d after immunization, spleen cells were removed and evaluated by FACS for expression of CD25 and Foxp3 by CD4+ cells. (Left) A representative dot plot from a vehicle-treated mouse, where CD4+CD25+ cells were gated, and the percentage of FoxP3+ cells was calculated from within that gate (Middle). The individual data from each animal demonstrates that laquinimod significantly increases the percentage of CD4+CD25+FoxP3+ regulatory T cells in WT and not AHR−/− mice. Data are representative of two independent experiments. (B) The remaining spleen cells were restimulated with PPD or MOG in vitro, and after 48 h the culture supernatants were analyzed for IL-17, GM-CSF, and IFNγ release. Under some conditions, the cytokine levels were below the level of detection and are marked as “BQL.” Laquinimod treatment reduced MOG-specific GM-CSF (P < 0.04), IL-17, and IFNγ. Data are representative of two independent experiments.
AhR Deletion in the Immune System Is Necessary and Sufficient to Negate the Effect of Laquinimod in EAE.
Current knowledge indicates that laquinimod exerts activities both on the peripheral immune system and within the CNS. The data above clearly show that AhR is required for the efficacy of laquinimod in EAE; however, it is not clear whether AhR is required in the peripheral immune system, in the CNS, or in both. To address this, we made chimeras by transplanting bone marrow (BM) cells into busulfan-conditioned recipients: In one set of animals, AhR−/− (CD45.2) BM cells were transplanted into congenic B6 CD45.1 recipients to create mice with an AhR−/− peripheral immune system and AhR+/+ CNS; and in another set of animals B6 CD45.1 BM cells were transplanted into AhR−/− recipients to create mice with an AhR+/+ peripheral immune system and an AhR−/− CNS. Chimerism was tracked using FACS analysis of peripheral blood, and at 13 wk posttransplant recipient mice had >80% donor-derived blood cells. At that point, chimeric mice were induced with MOG EAE and treated with vehicle or laquinimod. In chimeras with an AhR+/+ immune system, laquinimod treatment reduced disease severity from a mean clinical score of 2.5 ± 0.8–0.7 ± 0.8 (72% inhibition, Fig. 7), although the effect of laquinimod was not as efficacious as seen in WT C57BL/6 mice where it inhibited disease by 93% (Fig. 4). In contrast, laquinimod completely lost its efficacy in chimeric mice with an AhR−/− immune system (Fig. 7) where disease severity (2.9 ± 1.1) was comparable to vehicle-treated AhR−/− mice (3.5 ± 0.5). These data indicate that AhR is fully required in the peripheral immune system for the effect of laquinimod and may be partially dependent on the expression of AhR for the effect of laquinimod within the CNS.
Fig. 7.
Lack of AhR in the peripheral immune system is sufficient to negate the effect of laquinimod. Bone marrow chimeras were made from WT or AhR−/− mice into preconditioned AhR−/− or WT recipients. MOG EAE was induced in the chimeric mice 13 wk posttransplant, and the graph shows mean disease scores with SE from vehicle-treated (closed symbols) and laquinimod-treated (open symbols) mice (n = 9 or 10 per group). The embedded table shows disease incidence, mean day of onset, and group mean score of disease. The data are representative of a single experiment.
Discussion
In this paper we used transcriptome analysis to further elucidate the mechanism of action (MoA) of laquinimod, and we demonstrate that laquinimod induces genes associated with the AhR pathway. The prototypical AhR genes Cyp1a1 and Ahrr were among those with the highest average fold-change in both naive and EAE mice treated with laquinimod (Fig. 2 A and B). We verified the in vivo induction of Cyp1a1 using qPCR of mRNA levels from livers of treated mice (Fig. 2C) and demonstrated that laquinimod is a potent inducer of Cyp1a in vitro (Fig. 3). Together, these findings demonstrate that laquinimod is an activator of AhR, and the exact molecular events that result in activation are currently being investigated. This raised the possibility that the therapeutic effect of laquinimod is dependent on AhR activation. Indeed, we report here that the effect of laquinimod was completely lost in MOG-induced EAE in AhR−/− mice, as depicted by clinical score (Fig. 4) and histopathological findings (Fig. 5). These data indicate that deletion of AhR is necessary and sufficient to abrogate the effect of laquinimod in EAE.
Treatment with laquinimod has been shown to shift the balance from pathogenic Th17 cells toward an increase in the number of CD4+CD25+FoxP3+ regulatory T cells both in the periphery and in situ within the CNS (4, 5, 7). Our RNA-Seq data showed 227 genes had decreased expression levels in laquinimod-treated mice, including key Th17-related cytokine genes (Fig. 1). The mechanism by which AhR signaling modulates T-regulatory (T-reg) biology includes shaping T-reg differentiation by dictating the state of FoxP3 promoter methylation and enhancing FoxP3 expression; mediating methylation of the il17 promoter and decreasing the expression of IL-17; and inducing tolerogenic DCs that promote the generation of T regs (25–30). Although we do not have experimental evidence regarding the effect of laquinimod on FoxP3 promoter methylation, laquinimod has been shown to reduce IL-17 production (5, 7) and induce tolerogenic DCs (7, 9). Furthermore, we show that both the reduction of proinflammatory cytokines and the increase in CD4+CD25+FoxP3+ regulatory T-cell numbers by laquinimod is AhR-mediated (Fig. 6). Considering the link of AhR with other regulatory T-cell populations, specifically Tr1 cells (31–34), it will be interesting to investigate whether laquinimod also modulates Tr1 cells. Various compounds acting on the AhR pathway regulate adaptive immune responses through effects on both APCs and T cells (29). In contrast, no direct effects of laquinimod on T cells have been detected (7). Laquinimod, like other AhR activators, can induce the differentiation of APCs with a tolerogenic phenotype. The mechanisms responsible for this tolerogenic phenotype may result from the up-regulation of indoleamine 2,3-dioxygenase expression, resulting in increased synthesis of immunosuppressive kynurenines (21, 22). Indeed, our transcriptome data showed up-regulation of both Ido1 and Ido2 (Fig. 2). Taken together, these data suggest that the effects of laquinimod on the encephalitogenic T-cell response in EAE involve different cellular and potentially molecular mechanisms from other AhR ligands. Similar to other ligand-activated transcription factors, AhR-mediated biological responses have been shown to be compound, cell type, and species-dependent (35). Studies to delineate in which target cells and by what mechanism laquinimod activates AhR are currently ongoing in our laboratory.
Laquinimod inhibits astrogliosis in EAE (Fig. 5), and astrocytes have been proposed to play an important role in the protective effect of laquinimod in cuprizone-induced CNS demyelination (36). Laquinimod treatment reverses cuprizone-induced astrogliosis and leads to decreased production of proinflammatory factors and reduced NF-κB activation in cultured murine and human astrocytes (36). Interestingly, cross-talk between AhR and NF-κB has been described in other cell types (37, 38), suggesting that laquinimod-mediated AhR activation may lead to down-regulation of NF-κB in astrocytes and possibly other immune-related cells. In an attempt to elucidate the importance of AhR in the peripheral immune system versus in resident CNS cells like astrocytes, we performed a cross-over bone marrow transplant experiment. In chimeras with an AhR−/− immune system, laquinimod completely lost efficacy (Fig. 7), indicating that the AhR in the immune system is sufficient and necessary for the effect of laquinimod. The exact population of immune cells that requires AhR is currently being investigated. In chimeras with an AhR+/+ immune system, laquinimod treatment inhibited disease by 72% (Fig. 7), although the effect of laquinimod was not as strong as the almost complete inhibition seen in WT C57BL/6 mice (Fig. 4). This finding supports the idea that laquinimod may be partially dependent on the expression of AhR within the CNS, where lack of expression of AhR in resident astrocytes may explain the less-than-expected efficacy of laquinimod in chimeras with an AhR+/+ immune system. Indeed, our data would seem to corroborate a very recent publication that reports that expression of AhR within astrocytes limits CNS inflammation (39). Although our data strongly suggest that the anti-inflammatory effects of laquinimod are mediated by AhR, the precise mechanism of its neuroprotective activities remains to be further investigated.
In conclusion, we have demonstrated that laquinimod activates AhR, which is necessary for its therapeutic efficacy in the MOG-induced EAE model of MS. AhR has been known for its ability to mediate the biochemical, metabolic, and toxic effects of environmental chemicals. Recently, a paradigm shift has occurred with the understanding that AhR has endogenous roles and is an important regulator of cell development, differentiation, and function (40). AhR is an important regulator of the development and function of both innate and adaptive immune cells, mediated by the ability of AhR to respond to endogenous ligands generated from the host cell, diet, and microbiota (41–44). This recent paradigm shift has opened new avenues of research in the possibility of targeting AhR to treat inflammatory diseases in which a role of AhR has been found, including lupus (45) and colitis (46). Laquinimod may represent a first-in-class drug targeting AhR for MS and other diseases with inflammatory or neuroinflammatory components.
Materials and Methods
Test Compounds and Formulation.
Laquinimod was synthesized at Teva Pharmaceutical Industries, Ltd. The compound was dissolved at 2.5 mg/mL in purified water and administered orally by gavage in a volume of 0.2 mL. ITE was purchased from Tocris Biosciences and was freshly prepared daily in corn oil and then administered intraperitoneally at a volume of 0.2 mL. Omeprazole and 3-MC were purchased from Sigma-Aldrich. Busuflex (Busulfan) (6 mg/mL) was purchased from Otsuka America Pharmaceutical Inc.
Mice.
Healthy C57BL/6 mice at 6–8 wk of age were obtained from the Harlan Animal Breeding Center, Rehovot, Israel. AhR knockout (AhR−/−) mice on a C57BL/6 background (C57BL/6-Ahrtm1.2Arte) were obtained from Taconic. Congenic C57BL/6 B6.SJL-Ptprca Pepcb/BoyJ (B6 CD45.1) mice were obtained from Jackson Laboratories. The mice were housed at 22–24 °C, and food and water were available ad libitum. All experimental procedures conformed to accepted ethical standards for use of animals in research and were in accordance with Committee for the Care and Use of Experimental Animal guidelines and approved by the Teva Institutional Animal Care and Use Committee.
Bone Marrow Transplantation.
Ten congenic B6 CD45.1 or AhR−/− (CD45.2) recipient mice were preconditioned with three intraperitoneal injections of 10 mg/kg busulfan on days −5, −3, and −1 before transplantation. Donor mice were killed, and bone marrow cells were isolated from the femurs and tibias of all four limbs. Following lysis of RBCs, 9–10 × 106 BM cells were injected i.v. into the recipient mice. Peripheral blood was removed, lysed, and resuspended in FACS buffer and then stained using CD45.1-PE or CD45.2-APC (Miltenyi Biotec). After 13 wk, mice were induced with MOG EAE.
Induction of EAE and Clinical Evaluation.
Mice were immunized in the flanks with 300 μg/mouse pMOG35–55 peptide (Novetide) in normal saline emulsified in an equal volume of Complete Freund’s Adjuvant containing 500 μg per mouse Mycobacterium tuberculosis. Two injections of pertussis toxin (175 ng/0.2 mL per mouse, intraperitoneally) were given at the time of immunization and 48 h later. Animals were scored for clinical signs of disease on a daily basis using the following scores: 0 = normal behavior, 1 = distal limp tail, 1.5 = complete limp tail, 2 = disturbed righting reflex, 3 = ataxia, 4 = early paralysis (hind legs), 5 = full paralysis, and 6 = moribund or death. To calculate mean disease onset, animals that did not develop disease were considered to have onset on day 31. Laquinimod was dosed orally at 25 mg⋅kg⋅d beginning on the day of immunization until the end of the experiment (days 0–30). Placebo-treated mice were similarly administered a volume of 0.2 mL water orally 6 d a week. Each group contained between 12 and 15 mice.
RNA Sequencing of Splenocytes from Naive and EAE Mice.
Naive female C57BL/6 mice or mice with MOG EAE were treated with vehicle or 25 mg/kg laquinimod for 25 d. After 6 d, spleens from six mice per treatment arm were removed, and RNA was isolated using the Qiagen miRNeasy Mini Kit. Globin mRNA-depleted RNA samples were converted into cDNA libraries using the TruSeq Stranded mRNA Sample Prep Kit (Illumina, #RS-122-2103). Final cDNA libraries were analyzed for size distribution and, using an Agilent 2200 TapeStation (D1000 Screentape, Agilent # 5067–5582), quantitated by qPCR (KAPA Library Quant Kit, KAPA Biosystems # KK4824) and then normalized to 2 nM in preparation for sequencing. Sequencing was performed using an Illumina TruSeq Paired-End Cluster Kit V4 (Illumina # PE-401-4001), and a clustered flowcell was generated using the normalized cDNA libraries as templates. The cDNA templates were denatured using fresh 0.1 N NaOH, diluted to a final loading concentration of 13 pM, and placed on an Illumina cBot (v1.5.12.0) for cluster generation. Templates were attached to the flowcell via a dense lawn of oligonucleotides that bind to the sequencing adapters added during sample preparation, which are extended and then denatured. The flowcell was then sequenced through 51 bases, paired end, with an 8-base index cycle on an Illumina HiSEq. 2000 (HiSeq Control Software v1.5.15.1). During sequencing cycles, fluorescent reversible terminator dNTPs were added to the clusters with only a single base per target being incorporated. Following imaging of the clusters, the terminator and fluorescent tags were cleaved so that the next base could be incorporated.
RNA Sequencing Analysis.
Gene expression values were obtained by aligning the sequencing reads to the mouse genome (GRCm38) using STAR (47) and using featureCounts (48) to quantify the number of reads that aligned uniquely to genes specified in the GENCODE mouse transcriptome M7 (49). The resulting count matrix was filtered by removing genes with less than 20 cumulative counts across splenocyte samples. The filtered matrix was normalized with Limma Voom (50) using the mouse type (EAE or naive) and treatment (laquinimod or vehicle) variables to estimate the model coefficients. Differential expression between EAE treated with laquinimod and EAE treated with vehicle was performed using Limma-moderated t tests (51). The thresholds used to define statistical significance were a Benjamini-Hochberg–corrected (FDR) P < 0.05 and an absolute fold-change > 2.0. The raw sequence data can be accessed with the accession no. PRJNA319255 at www.ncbi.nlm.nih.gov/bioproject/319255.
Histopathology of Spinal Cords from EAE.
At the end of the EAE study, animals were perfused with PBS solution containing 4% (vol/vol) paraformaldehyde. Thereafter, the entire vertebral column was carefully dissected, and isolated vertebral columns were incubated overnight at 4 °C for the purpose of tissue post fixation. For histological and immunohistochemical studies, spinal cords were decalcified for 48 h (37 °C), and then samples were washed for 12 h under running water to remove the decalcification solution. Spinal cords were embedded in paraffin, and 5-µm-thick transverse sections were prepared and stained for myelination/inflammatory index, microglial and astrocytic activation, and acute axonal damage. Intact and damaged myelin plus inflammatory infiltrates were visualized using LFB/PAS stains and counterstained with H&E. The extent of inflammatory demyelination was quantified by assessing the inflammatory-demyelination index, which is defined as the area covered by inflammatory demyelination in relation to the entire white matter area of each slide. For the visualization and quantification of microglia/monocyte and astrocyte activation, paraffin-embedded sections were dewaxed, washed in PBS, and incubated overnight with the respective primary antibody diluted in blocking solution. For the visualization of epitope-primary antibody complexes, HRP-coupled polymer secondary antibodies were used (EnVision, Dako), and 3,3′-diaminobenzidine (DAB) was used as a chromogenic substrate. Quantification of the extent of microgliosis/monocytosis (anti-Iba1) and astrogliosis (anti-GFAP) was performed using a blinded staging approach. The following scoring system was used: 0 = normal cellular density; 1 = moderate increase in cellular density; 2 = intermediate increase in cellular density; 3 = high cellular density; and 4 = maximum cellular density. Anti-APP stains were performed to visualize acute axonal damage. To quantify the extent of acute axonal damage, the number of APP+ spheroids was counted in four randomly chosen fields of the white matter part of the corpus callosum irrespective of the presence or absence of lesions. The number of positive spheroids was counted under high-power magnification and quantified as the number of spheroids per square millimeter.
CYP1A Induction in Primary Human and Murine Hepatocytes.
Cryopreserved murine (Bioreclamation In Vitro Technologies) and human (Celsis In Vitro Technologies) hepatocytes were thawed and plated on 24 multiwell plates coated with collagen type I substratum in William’s E medium (GIBCO-32551) supplemented with 5 µg/mL insulin, 0.1 µM dexamethasone, and 10% (vol/vol) FBS. Cells were for 3–4 h, and the medium was changed to Hepatocyte Basal Medium (HBM-Lonza CC-3199) supplemented with CC-4182 (Lonza). Plates were maintained at 37 °C for 24 h before treatment with compounds. Prototypical CYP1A inducers 3-MC and omeprazole were dissolved in DMSO and added to the culture medium at a final solvent concentration of 0.1% (vol/vol). After 4 or 24 h of treatment, total RNA was extracted from duplicate samples using an RNA extraction kit (RNeasy 96 kit, 74181, Qiagen). Quantitative mRNA analysis was performed by real-time qRT-PCR in the ABI Prism 7900 HT Sequence Detection System (TaqMan, Perkin-Elmer-Applied Biosystem). EC50 values were calculated by nonlinear regression using XLfit 4.2 (IDBS).
CYP1A mRNA Induction in Livers of WT Mice.
Female C57BL/6 mice were treated for 5 d with vehicle, laquinimod (25 mg/kg, orally), or ITE (10 mg/kg, i.p.). Mouse liver samples were taken 4 or 24 h after the final dose of compound, and tissue samples were lysed and total RNA purified. Single-stranded cDNA was prepared from RNA with the RT Master Mix using the AB 7900HT Fast Real Time PCR System thermocycling program (Applied Biosystems). Probes for murine Beta-Actin (Mm01205647_g1), Cyp1a1 (Mm00487218_m1), and Cyp1a2 (Mm00487224_m1) were purchased from Thermo Fischer Scientific. The relative quantity of the target cDNA compared with that of the control cDNA (β-actin) was determined by the ΔΔCt method. The results of this method are expressed as fold-change with respect to the target transcript expression in the untreated control.
T-Cell Phenotyping in WT and AhR−/− Mice.
Following EAE induction, WT and AhR−/− mice (n = 6 per group) were treated with vehicle or laquinimod for 15 d. Spleens were removed under sterile conditions, crushed in PBS to create a suspension, and lysed with ammonium-chloride-potassium (ACK) lysis buffer, and the cell pellet was resuspended in FACS buffer or cell culture medium. Cells (1 × 106) were stained for CD4+CD25+Foxp3+ using the eBioscience mouse regulatory T-cell staining kit according to the manufacturer’s instructions. The percentage of FoxP3+ cells was calculated from within the CD4+CD25+ lymphocyte gate. The remaining cells were resuspended in Stimulation Medium (RPMI; 2.5% FCS, Antibiotics, l-glutamine and β−mercaptoethanol) and seeded at 0.5 × 106 cells per well in a 96-well, flat-bottom plate to a final volume of 0.25 mL/well. Cells were exposed to medium, PPD (2 μg per well), or MOG35–55 (10 μg per well) for 48 h. Cell culture supernatants were then analyzed for cytokine levels using the R&D mouse magnetic luminex kit LXSAMSM-14 according to the manufacturer’s instructions.
Acknowledgments
We thank Prof. Markus Kipp of ProMyelo GmbH, who performed all of the histology analysis, and Dr. Annalise Di Marco from IRBM Science Park, who performed the AhR induction experiments in hepatocytes.
Footnotes
Conflict of interest statement: The authors are employees of Teva Pharmaceutical Industries Ltd. or Immuneering Corporation.
This article is a PNAS Direct Submission.
Data deposition: The raw data are available at the National Center for Biotechnology Information (accession no. PRJNA319255).
References
- 1.Comi G, et al. ALLEGRO Study Group Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med. 2012;366(11):1000–1009. doi: 10.1056/NEJMoa1104318. [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, et al. ALLEGRO Study Group Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry. 2014;85(8):851–858. doi: 10.1136/jnnp-2013-306132. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer TL, et al. BRAVO Study Group A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J Neurol. 2014;261(4):773–783. doi: 10.1007/s00415-014-7264-4. [DOI] [PubMed] [Google Scholar]
- 4.Wegner C, et al. Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;227(1-2):133–143. doi: 10.1016/j.jneuroim.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Aharoni R, et al. Oral treatment with laquinimod augments regulatory T-cells and brain-derived neurotrophic factor expression and reduces injury in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2012;251(1-2):14–24. doi: 10.1016/j.jneuroim.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Mishra MK, Wang J, Silva C, Mack M, Yong VW. Kinetics of proinflammatory monocytes in a model of multiple sclerosis and its perturbation by laquinimod. Am J Pathol. 2012;181(2):642–651. doi: 10.1016/j.ajpath.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Schulze-Topphoff U, et al. Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that modulate central nervous system autoimmunity. PLoS One. 2012;7(3):e33797. doi: 10.1371/journal.pone.0033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thöne J, et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol. 2012;180(1):267–274. doi: 10.1016/j.ajpath.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Jolivel V, et al. Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain. 2013;136(Pt 4):1048–1066. doi: 10.1093/brain/awt023. [DOI] [PubMed] [Google Scholar]
- 10.Ruffini F, et al. Laquinimod prevents inflammation-induced synaptic alterations occurring in experimental autoimmune encephalomyelitis. Mult Scler. 2013;19(8):1084–1094. doi: 10.1177/1352458512469698. [DOI] [PubMed] [Google Scholar]
- 11.Moore S, et al. Therapeutic laquinimod treatment decreases inflammation, initiates axon remyelination, and improves motor deficit in a mouse model of multiple sclerosis. Brain Behav. 2013;3(6):664–682. doi: 10.1002/brb3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra MK, et al. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann Clin Transl Neurol. 2014;1(6):409–422. doi: 10.1002/acn3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou LP, et al. Suppression of experimental autoimmune neuritis by ABR-215062 is associated with altered Th1/Th2 balance and inhibited migration of inflammatory cells into the peripheral nerve tissue. Neuropharmacology. 2002;42(5):731–739. doi: 10.1016/s0028-3908(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 14.Pitarokoili K, et al. Laquinimod exerts strong clinical and immunomodulatory effects in Lewis rat experimental autoimmune neuritis. J Neuroimmunol. 2014;274(1-2):38–45. doi: 10.1016/j.jneuroim.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Lourenço EV, Wong M, Hahn BH, Palma-Diaz MF, Skaggs BJ. Laquinimod delays and suppresses nephritis in lupus-prone mice and affects both myeloid and lymphoid immune cells. Arthritis Rheumatol. 2014;66(3):674–685. doi: 10.1002/art.38259. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, et al. Laquinimod ameliorates spontaneous colitis in interleukin-10-gene-deficient mice with improved barrier function. Int Immunopharmacol. 2015;29(2):423–432. doi: 10.1016/j.intimp.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Varrin-Doyer M, Zamvil SS, Schulze-Topphoff U. Laquinimod, an up-and-coming immunomodulatory agent for treatment of multiple sclerosis. Exp Neurol. 2014;262(Pt A):66–71. doi: 10.1016/j.expneurol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol. 2007;71(6):1475–1486. doi: 10.1124/mol.106.032748. [DOI] [PubMed] [Google Scholar]
- 19.MacPherson L, et al. Aryl hydrocarbon receptor repressor and TiPARP (ARTD14) use similar, but also distinct mechanisms to repress aryl hydrocarbon receptor signaling. Int J Mol Sci. 2014;15(5):7939–7957. doi: 10.3390/ijms15057939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutros PC, et al. Hepatic transcriptomic responses to TCDD in dioxin-sensitive and dioxin-resistant rats during the onset of toxicity. Toxicol Appl Pharmacol. 2011;251(2):119–129. doi: 10.1016/j.taap.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Jaronen M, Quintana FJ. Immunological relevance of the coevolution of IDO1 and AHR. Front Immunol. 2014;5:521. doi: 10.3389/fimmu.2014.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen NT, et al. Aryl hydrocarbon receptor and kynurenine: Recent advances in autoimmune disease research. Front Immunol. 2014;5:551. doi: 10.3389/fimmu.2014.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao N, et al. Xenobiotics and loss of cell adhesion drive distinct transcriptional outcomes by aryl hydrocarbon receptor signaling. Mol Pharmacol. 2012;82(6):1082–1093. doi: 10.1124/mol.112.078873. [DOI] [PubMed] [Google Scholar]
- 24.Quintana FJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107(48):20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 26.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One. 2013;8(11):e79819. doi: 10.1371/journal.pone.0079819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes MD, Ovcinnikovs V, Smith AG, Kimber I, Dearman RJ. The aryl hydrocarbon receptor: Differential contribution to T helper 17 and T cytotoxic 17 cell development. PLoS One. 2014;9(9):e106955. doi: 10.1371/journal.pone.0106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana FJ. Regulation of central nervous system autoimmunity by the aryl hydrocarbon receptor. Semin Immunopathol. 2013;35(6):627–635. doi: 10.1007/s00281-013-0397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pot C. Aryl hydrocarbon receptor controls regulatory CD4+ T cell function. Swiss Med Wkly. 2012;142:w13592. doi: 10.4414/smw.2012.13592. [DOI] [PubMed] [Google Scholar]
- 31.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhi R, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11(9):846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu HY, et al. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One. 2011;6(8):e23618. doi: 10.1371/journal.pone.0023618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascanfroni ID, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21(6):638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brück W, et al. Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012;124(3):411–424. doi: 10.1007/s00401-012-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruby CE, Leid M, Kerkvliet NI. 2,3,7,8-Tetrachlorodibenzo-p-dioxin suppresses tumor necrosis factor-alpha and anti-CD40-induced activation of NF-kappaB/Rel in dendritic cells: p50 homodimer activation is not affected. Mol Pharmacol. 2002;62(3):722–728. doi: 10.1124/mol.62.3.722. [DOI] [PubMed] [Google Scholar]
- 38.Vogel CF, et al. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: A role for nuclear factor-κB. J Biol Chem. 2014;289(3):1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothhammer V, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67(2):259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013;25(6):335–343. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- 42.Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013;65(4):1148–1161. doi: 10.1124/pr.113.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cella M, Colonna M. Aryl hydrocarbon receptor: Linking environment to immunity. Semin Immunol. 2015;27(5):310–314. doi: 10.1016/j.smim.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L. AHR function in lymphocytes: Emerging concepts. Trends Immunol. 2016;37(1):17–31. doi: 10.1016/j.it.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorgham K, et al. Ultraviolet light converts propranolol, a nonselective β-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand. Eur J Immunol. 2015;45(11):3174–3187. doi: 10.1002/eji.201445144. [DOI] [PubMed] [Google Scholar]
- 46.Lamas B, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao Y, Smyth GK, Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 49.Harrow J, et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]