Significance
We describe here the discovery that assemblies of O-fucosylated proteins localize to the nuclear membrane of Toxoplasma gondii, particularly in proximity to the nuclear pore complexes (NPCs). O-fucose is added to Ser and Thr residues found in some of the Phe-Gly (FG) domain-containing proteins that characterize the NPC channel as well as in Ser-rich sequences in many proteins predicted to have roles in transcription, mRNA processing, and cell signaling. O-fucosylation of nucleocytosolic proteins has not been described previously in any eukaryote and appears to be unique to T. gondii and closely related apicomplexans.
Keywords: toxoplasma, fucose, nuclear glycosylation, nuclear pore complex
Abstract
Toxoplasma gondii is an intracellular parasite that causes disseminated infections in fetuses and immunocompromised individuals. Although gene regulation is important for parasite differentiation and pathogenesis, little is known about protein organization in the nucleus. Here we show that the fucose-binding Aleuria aurantia lectin (AAL) binds to numerous punctate structures in the nuclei of tachyzoites, bradyzoites, and sporozoites but not oocysts. AAL also binds to Hammondia and Neospora nuclei but not to more distantly related apicomplexans. Analyses of the AAL-enriched fraction indicate that AAL binds O-linked fucose added to Ser/Thr residues present in or adjacent to Ser-rich domains (SRDs). Sixty-nine Ser-rich proteins were reproducibly enriched with AAL, including nucleoporins, mRNA-processing enzymes, and cell-signaling proteins. Two endogenous SRDs-containing proteins and an SRD-YFP fusion localize with AAL to the nuclear membrane. Superresolution microscopy showed that the majority of the AAL signal localizes in proximity to nuclear pore complexes. Host cells modify secreted proteins with O-fucose; here we describe the O-fucosylation pathway in the nucleocytosol of a eukaryote. Furthermore, these results suggest O-fucosylation is a mechanism by which proteins involved in gene expression accumulate near the NPC.
The apicomplexan parasite Toxoplasma gondii causes disseminated infections in humans, and these infections can lead to severe damage in immunocompromised individuals and fetuses (1, 2). There is no human vaccine against T. gondii, and recently the price of pyrimethamine, the drug used to treat toxoplasmosis in the United States, has increased more than 50-fold (2).
T. gondii has a complex life cycle, and the parasite’s ability to differentiate through its life stages in response to stresses and environmental conditions is fundamental for its pathogenicity and transmission (3). Transcriptome analyses have revealed that a large percentage of mRNAs show life stage-specific expression (4) and/or cell cycle regulation (5). Recent studies have increased our understanding of gene expression in T. gondii by identifying the AP2 family of transcription factors (6–8) and by describing posttranslational modifications (PTMs) of histones and some of the enzymes responsible for them (9–11). However, little is known about protein organization at the nuclear periphery, a subnuclear compartment that plays a critical role in transcriptional regulation in many eukaryotes. In particular, the gene-gating model (12) suggests that the nuclear pore complex (NPC) has a role in transcriptional regulation and chromatin organization as well as in protein and mRNA transport (13, 14).
In T. gondii chromodomain protein 1 localizes with heterochromatin at the nuclear periphery (15), and centromeres sequester to an apical nuclear region (16). Although the nuclear localization signal (NLS) and importin-α system are present, key nuclear import and export molecules are not easily identified (17–19). Furthermore, the NPC composition is divergent, so that only nucleoporins containing phenylalanine-glycine (FG) repeats (FG-Nups) and a putative Nup54 can be predicted by primary sequence homology (20).
Here we report the discovery of numerous assemblies of O-fucosylated proteins that associate with the nuclear membrane near the NPCs. These results improve our understanding of the architecture of the T. gondii nuclear periphery and highlight O-fucosylation as a PTM involved in assemblies associated with the NPC.
Results
The Fucose-Binding Aleuria aurantia Lectin Labels the Nuclei of T. gondii in a Stage-Specific, Species-Specific, but Cell Cycle-Independent Manner.
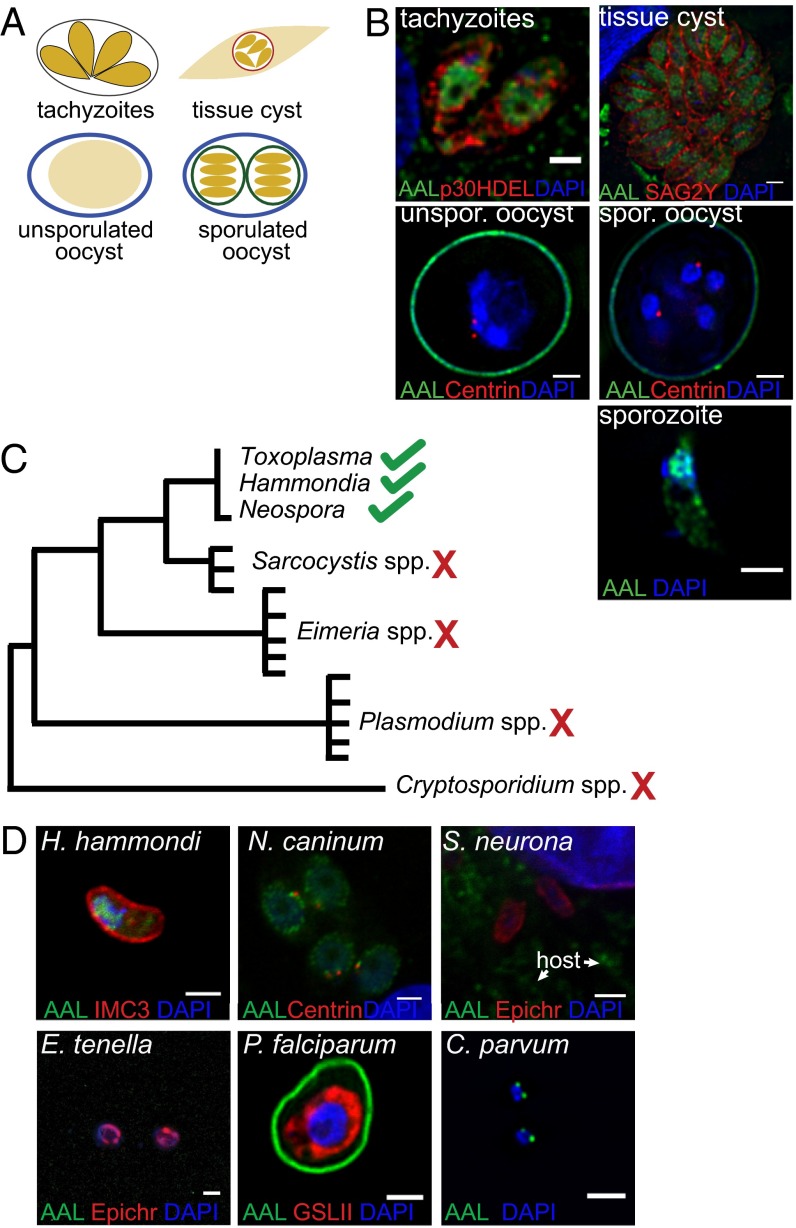
Fucose is a common monosaccharide in many eukaryote and prokaryote glycoconjugates (21). Because only one fucose-containing glycoconjugate, a pentasaccharide on S-phase kinase-associated protein 1 (Skp1) (22), was known in T. gondii, we used Aleuria aurantia lectin (AAL), a lectin that binds to terminal fucose, to search for fucosylated glycans in its various life stages (Fig. 1A). Unexpectedly, AAL strongly labels the nuclei of tachyzoites, bradyzoites, and sporozoites but fails to bind to the nuclei of oocysts (Fig. 1B). Binding of anti-centrin antibodies to centrosomes shows that oocyst walls are permeabilized (23). Transient expression of GAP40-YFP, which highlights the inner membrane complex (24), shows that AAL binds to T. gondii nuclei throughout the tachyzoite cell cycle (Fig. S1).
Fig. 1.
AAL binding to nuclei of T. gondii is stage- and species-specific. (A) Schematic of the T. gondii life cycle. (B) AAL binds to nuclei of tachyzoites, bradyzoites, and sporozoites but not oocysts. (C) The Apicomplexa phylogenetic tree. (D) AAL binds to closely related organisms (H. hammondi and N. caninum) but not to more distantly related apicomplexans (S. neurona, E. tenella, and P. falciparum). Binding to sporozoites is shown for all species, with the exception of P. falciparum for which a trophozoite is shown. IMC3 marks the inner membrane complex, centrin marks the centrosome, and epichromatin marks the nuclear periphery. Griffonia simplicifolia lectin II (GSLII) labels the short P. falciparum N-glycans. All images were taken by deconvolution fluorescence microscopy. (Scale bars: 2 μm.)
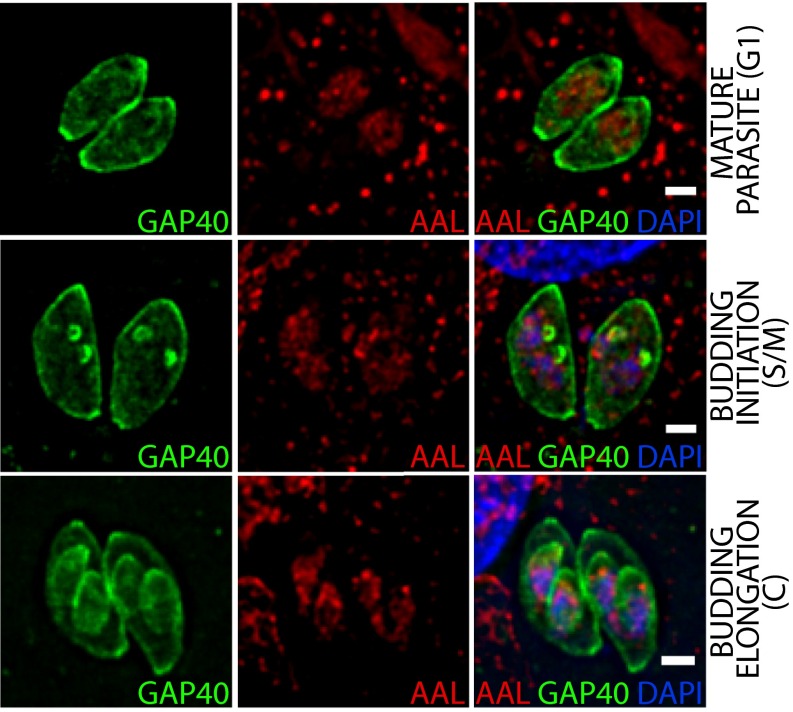
Fig. S1.
AAL binds to the nucleus of tachyzoites throughout the cell cycle. A cell line expressing GAP40-YFP was used to follow the inner membrane complex during replication using deconvolution fluorescence microscopy. (Scale bars: 2 μm.)
The apicomplexan phylogenetic tree based on housekeeping genes is cartooned in Fig. 1C. AAL binds to nuclei of T. gondii, Hammondia hammondi, and Neospora caninum, which are closely related species, but not to the nuclei of the more distantly related Sarcocystis neurona, Eimeria tenella, or Plasmodium falciparum (Fig. 1D). Although AAL labels numerous loci within the nucleus of T. gondii, the lectin labels only one or two spots in the perinuclear region of Cryptosporidium parvum sporozoites. These results show that strong labeling of nuclei by AAL is not a common property of apicomplexans but instead appears to have developed after the divergence of T. gondii and S. neurona.
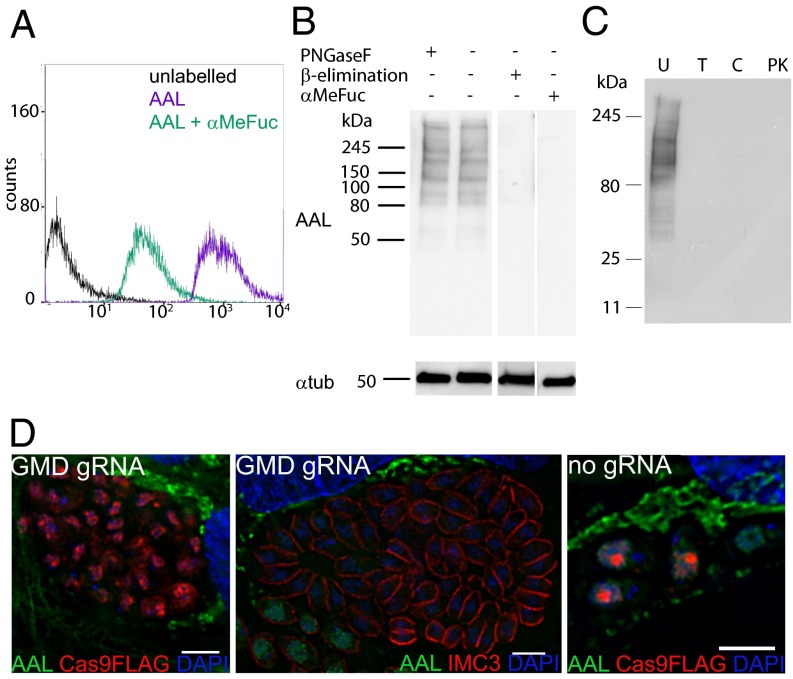
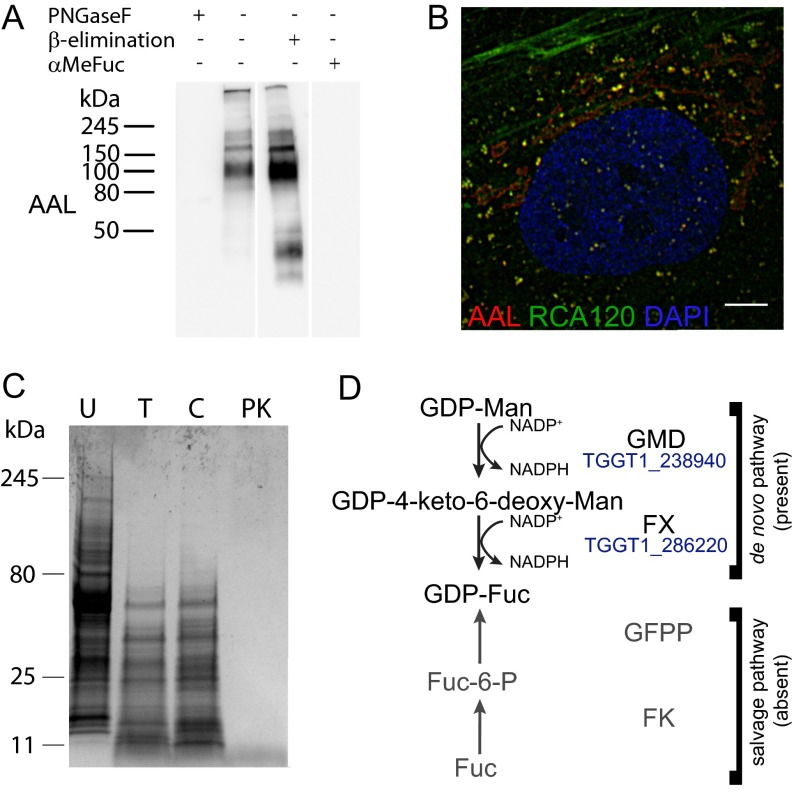
AAL binding to extracellular tachyzoites can be inhibited by preincubation with methyl-α-fucopyranoside (αMeFuc), as shown by flow cytometry and lectin blotting (Fig. 2 A and B), indicating the specificity of the carbohydrate–lectin interaction. Proteases and β-elimination treatments were also effective in blocking AAL binding to cell lysate, whereas peptide-N-glycosidase F (PNGase F) had no effect (Fig. 2 B and C and Fig. S2C), indicating that AAL binds to O-glycosylated proteins in T. gondii. In contrast, AAL binds to the N-glycans in the secretory pathway of human foreskin fibroblasts (HFFs), as shown by loss of binding on lectin blots only upon PNGase F treatment and by colocalization by immunofluorescence analysis with RCA120, a galactose-binding lectin (Fig. S2 A and B).
Fig. 2.
AAL specifically binds to O-glycans on T. gondii glycoproteins. (A) AAL binding to extracellular tachyzoites by flow cytometry shows inhibition by preincubation with αMeFuc. (B) β-Elimination, but not PNGase F treatment, inhibits AAL binding to tachyzoite cell lysates. Tubulin is shown as a loading control. (C) Protease treatments show that AAL binds exclusively to glycoproteins. C, chymotrypsin; PK, proteinase K; T, trypsin; U, untreated. (D) Disruption of GDP-fucose synthesis using CRISPR/Cas9 eliminates AAL binding by deconvolution fluorescence microscopy. (Scale bars: 4 μm.)
Fig. S2.
(A) AAL binds to N-glycans in the host cell because PNGase F inhibits AAL binding to the HFF lysate. (B) AAL-positive glycans are found in the ER and secretory vesicles of the host cells (deconvolution fluorescence microscopy). (Scale bar: 5 μm.) (C) Coomassie staining in a protease experiment on T. gondii tachyzoite cell lysate. C, chymotrypsin; PK, proteinase K; T, trypsin; U, untreated. (D) The de novo GDP-fucose biosynthesis pathway, composed of GDP-mannose-4,6-dehydratase (GMD, EC 4.2.1.47) and GDP-fucose synthase (FX, EC 1.1.1.271), is present in the predicted T. gondii proteome. No salvage pathway (shown in gray) can be identified: fucokinase (FK, EC 2.7.1.52) and GDP-fucose pyrophosphorylase (GFPP, EC 2.7.7.30) homologs were searched using Homo sapiens FK and GFPP or the bifunctional Leishmania major and Arabidopsis thaliana enzymes as templates. No homologs for the biosynthesis of GDP-Rhamnose or UDP/dTDP-Rhamnose could be found; dTDP/UDG-glucose-4,6-dehydratase (dTDG/UDG, EC 4.2.1.46/47), dTDP/UDP-Rhamnose synthase (RX, EC1.1.1.133), the trifunctional GDP-Rhamnose synthase (RHM1), and GDP-6-deoxy-mannose reductase (RMD, EC 1.1.1.281) from A. thaliana, Trypanosoma cruzi, Escherichia coli, or Pseudomonas aeruginosa were used as templates.
T. gondii Nuclear Fucosylation Is Dependent on the de Novo GDP–Fucose Biosynthetic Pathway.
Because T. gondii and other apicomplexans have no salvage pathway for fucose (25, 26), GDP-fucose is synthesized from GDP-mannose in a three-step process catalyzed by GDP-mannose dehydratase (GMD, TGGT1_238940) and GDP-fucose synthase (Fig. S2D) (21). Expression of Cas9 and a guide RNA (gRNA) targeting the first exon of the T. gondii gmd gene results in the loss of AAL binding to tachyzoite nuclei at 48 h post electroporation (Fig. 2D). Immunostaining against inner membrane complex protein 3 (IMC3) indicates that the subpopulation of AAL-negative cells still maintains the correct cell morphology (27). No loss of AAL binding was observed when cells were electroporated with Cas9 but without gRNA. So far we have been unable to isolate the gmd-depleted cells by cloning by limiting dilution with or without a selection agent (28), suggesting either that gmd is essential or that its deletion causes a strong growth phenotype in T. gondii tachyzoites.
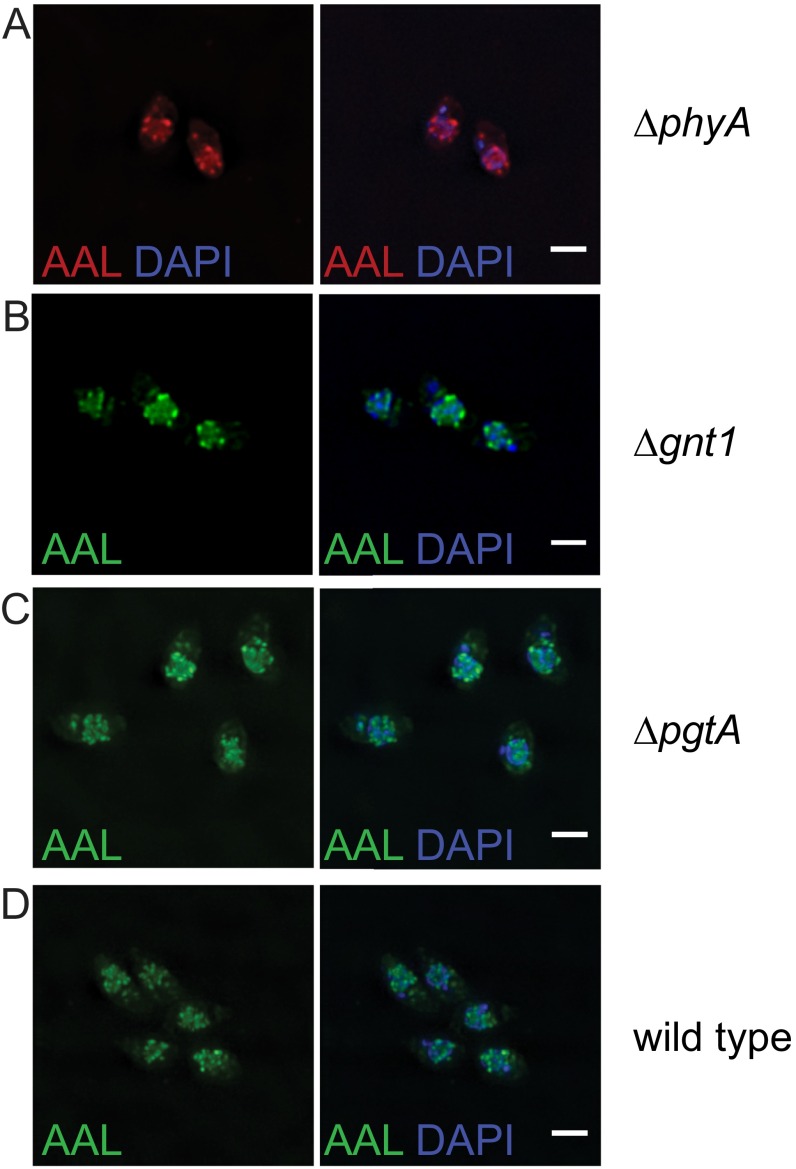
The only characterized downstream user of GDP-fucose (GDP-Fuc) in T. gondii is the Skp1 glycosylation pathway. T. gondii is one of a few organisms that add a pentasaccharide containing GlcNAc, Gal, and fucose to a hydroxyl-proline on Skp1, a E3 ubiquitin ligase adaptor (22, 29). Knockout of Skp1 proline hydroxylase (phyA), GlcNAc transferase (gnt1), and bifunctional galactose and fucose transferase (pgtA) did not affect AAL binding (Fig. S3), indicating that biosynthesis of the AAL-positive glycan is independent of the Skp1 glycosylation pathway.
Fig. S3.
The Skp1 glycosylation pathway is not involved in the generation of the AAL-positive glycans. (A–C) Knock out of the proline hydroxylase phyA (A), the GlcNAc transferase gnt1 (B), and the bifunctional Fuc/Gal transferase pgtA (C) does not affect the binding of AAL to extracellular tachyzoites by deconvolution fluorescence microscopy. (D) Wild type is shown for comparison. (Scale bars: 2 μm.)
AAL Binds to O-Fucose in T. gondii.
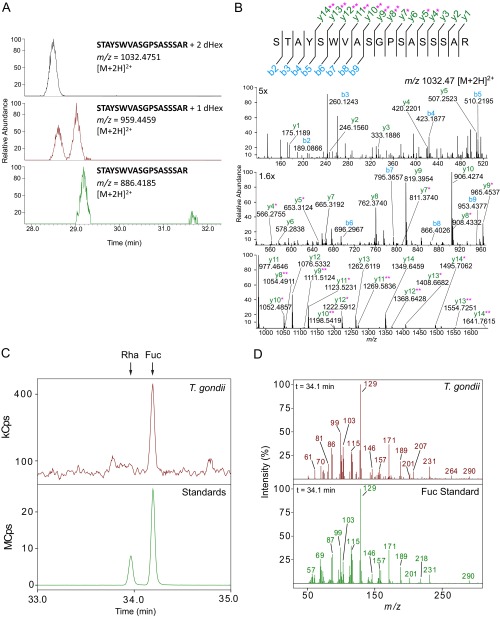
LC-MS/MS of tryptic peptides of AAL-enriched proteins from extracellular tachyzoites identified 69 unique glycopeptides containing one to six deoxyhexose(s) (dHexs), each linked to Ser or Thr (Dataset S1). These glycopeptides correspond to 50 different peptide sequences, and in many cases different numbers of dHexs were observed on the same peptide. All glycopeptides were manually reviewed and confirmed by either prompt neutral loss of at least one dHex, as indicated by the presence of peaks in the MS that correspond to different glycoforms detected at the same retention time, and/or by peaks in the MS/MS spectra that can be confidently assigned to peptide fragments containing dHex (Dataset S1). No sugars other than dHex were observed, indicating that AAL binds to O-fucose (O-Fuc). In a few cases, the same peptide was observed modified with a dHex and, at a later retention time, in its unglycosylated form, suggesting that the addition of O-Fuc may be a probabilistic event, i.e., that distinct pools of modified and unmodified protein may be present at that site (Dataset S1). The amino acid modified with O-Fuc could not be determined for most glycopeptides because of the labile nature of the O-Fuc and the low complexity of the amino acid sequence of most of the modified peptides (Fig. S4A). Also, we cannot exclude the possibility of heterogeneity in the glycosylation sites. Combining high-energy collision dissociation (HCD) and electron-transfer dissociation (ETD) MS/MS data, we often were able to narrow the modification sites to two to six likely Ser/Thr residues (Fig. S4B and Dataset S1). Furthermore, we were able to specify the modification site for one glycopeptide, T610 on TGGT1_203780 (Fig. 3B), a putative FG-Nup and one of the most abundant proteins identified in the AAL-enriched fraction (Dataset S2). GC-MS monosaccharide composition analysis of the sugars released by reductive β-elimination identified fucose as the only dHex present in the AAL-enriched fraction (Fig. S4 C and D).
Fig. S4.
AAL binds proteins modified by one or more O-Fuc on Ser/Thr residues, as verified by neutral loss of dHex, MS/MS fragmentation, and GC-MS monosaccharide analysis. (A) XICs obtained from LC-MS spectra of a glycopeptide from TGGT1_285190 show neutral loss of two to one dHex(s) and in smaller amounts of one to zero dHex. (B) MS/MS spectrum for the glycoform with two dHexs, m/z 1032.47 [M+2H]2+. The y and b series are marked in green and blue, respectively. Fragment ions plus one or two dHexs are marked with one and two pink asterisks, respectively. (C) GC-MS extracted ion chromatograms for the T. gondii sample and the standards mixture. The matched retention time compared with the standard indicates that fucose is the only dHex present after reductive β-elimination release of sugars from AAL-enriched T. gondii proteins. XICs were generated from the ions (129 and 171 m/z), which are abundant in the EI spectra from dHex alditol acetates. XICs from 33 to 35 min are shown; the arrows indicate the rhamnose (Rha) and fucose (Fuc) retention times. (D) GC-MS EI spectra of the alditol acetates from the T. gondii sample and Fuc alditol acetate standard. The retention time (34.1 min) from the XIC shown in C was used to generate the spectrum for each. Both the ions pattern and retention time match the Fuc standard and not that of Rha.
Fig. 3.
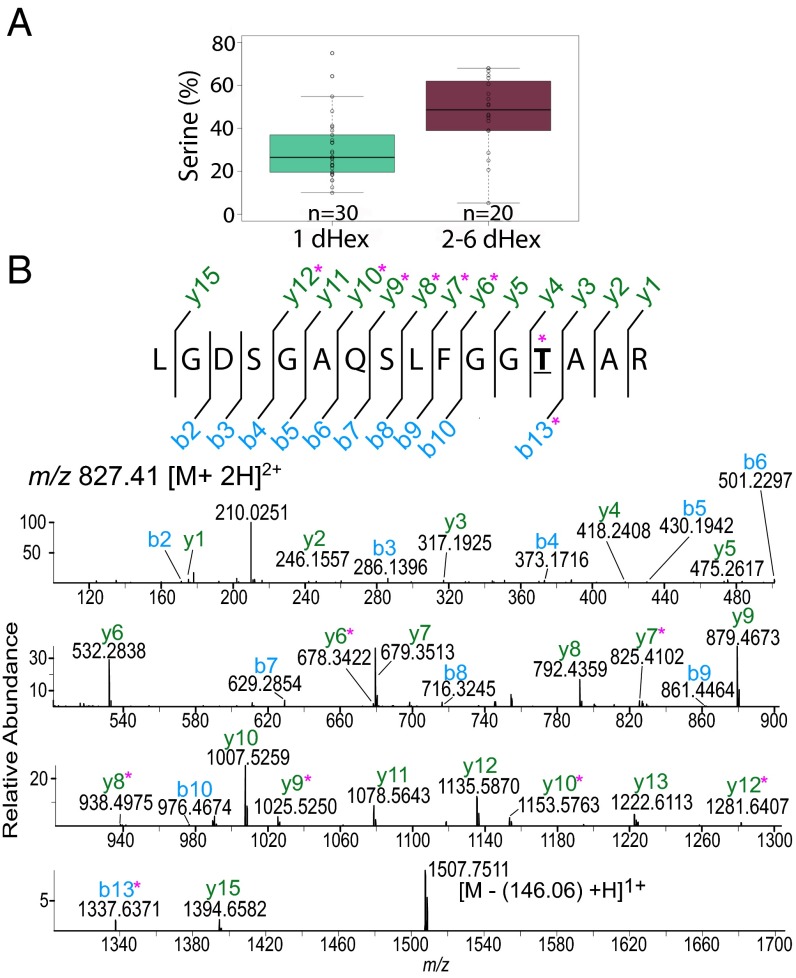
AAL-bound proteins are modified by O-Fuc on Ser/Thr residues. (A) Peptides with a higher Ser percentage are more likely to be modified with more than one O-Fuc (dHex) residue. (B) HCD MS/MS spectrum of a peptide from a FG-Nup (TGGT1_203780), m/z 827.41 [M+2H]2+. Ions b13+dHex and y6+dHex were observed, indicating that T610 (bold underlined) is the modified residue. Ions plus dHex are marked by a pink asterisk.
AAL Recognizes Proteins Involved in Gene Regulation.
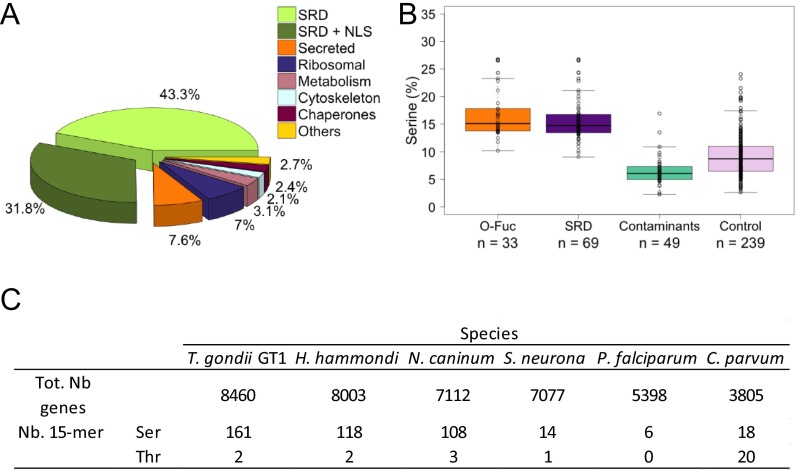
In most cases, O-Fuc is added to low-complexity Ser-rich domains (SRDs) or to sequences adjacent to these domains (Fig. 4 A and F), and peptides with long SRDs are likely to have more than one dHex (Fig. 3A and Dataset S1). We define SRDs as sequences with five or more Ser residues in tandem. More than 70% of the peptides identified in this study came from proteins that had one or more SRDs and/or contained more than 10% Ser (Fig. 3A, Fig. S5 A and B, and Dataset S2). Consistent with this observation, a higher number of proteins with Ser-15-mer are found in the apicomplexans that show nuclear AAL binding than in those that do not (Fig. 1D and Fig. S5C). Comparison of data from five biological repeats resulted in a set of 69 Ser-rich proteins reproducibly pulled down by AAL and/or for which we observed glycopeptides. We identified glycopeptides for 33 of the 69 AAL-enriched proteins (Table 1 and Dataset S2), and it is likely that numerous O-fucosylated peptides on long SRDs were not detected because of the paucity of flanking trypsin cleavage sites (Fig. 4A). In contrast, proteins that were present in AAL-enriched pull-downs but that are likely contaminants because of their high abundance (e.g., ribosomal proteins, cytoskeletal components, chaperones, and so forth) do not contain SRDs and have an average Ser content of 6%. A control set comprising T. gondii proteins associated with nuclear and cytoplasmic Gene Ontology (GO) terms was shown to have an average Ser content of 9%, compared with the 15% Ser present in the O-fucosylated proteins or the SRD set (Fig. S5B). About 40% of the 69 proteins with one or more SRDs contain a canonical NLS (cNLS), as identified by cNLS mapping (Table 1, Fig. S5A, and Dataset S2) (30). Analysis of PTMs showed that the majority of AAL-enriched proteins exhibit some degree of phosphorylation, but none is modified by ubiquitin. This result is in agreement with the datasets in refs. 31 and 32. AAL enriches five of seven predicted T. gondii nucleoporins (four FG-Nups and a Nup54 ortholog), each with one or more SRDs (Table 1 and Dataset S2). Also present in the AAL-enriched fraction are proteins predicted to be involved in mRNA processing, protein–protein interactions, ubiquitination, and enzymes that catalyze the addition/removal of phosphate groups from proteins and polyphosphate phosphatidylinositol (33, 34). Transcription regulators and proteins with nucleotide-binding and chromosome-binding domains are also present (Table 1 and Dataset S2). Numerous hypothetical proteins are found, and these often are conserved in T. gondii, H. hammondi, and N. caninum but are absent in the apicomplexans that do not bind AAL (Fig. 1).
Fig. 4.
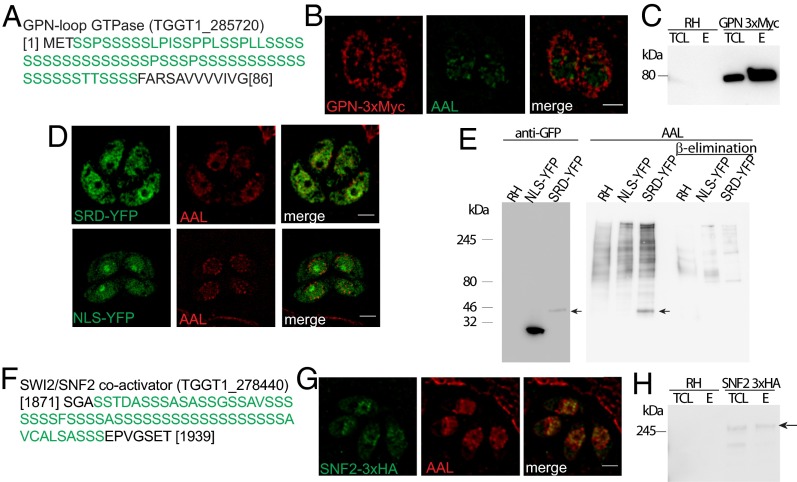
O-fucosylation directs proteins to the nuclear periphery. (A) The N-terminal SRD of GPN. (B) An ectopic copy of the protein localizes to the cytoplasm and to the nuclear periphery with AAL by ELYRA SIM. (C) AAL pull-down followed by anti–c-MYC Western blotting shows GPN enrichment. (D) SIM shows that the GPN SRD fused to YFP (SRD-YFP) partially colocalizes with AAL, but NLS-YFP does not. (E) AAL recognizes an additional band only in the SRD-YFP cell lysate, consistent with the molecular weight of SRD-YFP as defined by anti-GFP blot (black arrows). (F) The SRD of the SNF2 transcriptional coactivator. (G) SNF2 colocalizes with AAL at the nuclear periphery. (H) AAL pull-down followed by anti-HA blotting shows that SNF2 is present in the AAL-bound fraction (black arrow). E, elution; RH, wild-type cell lysate; TCL, total cell lysate. (Scale bars: 2 μm.)
Fig. S5.
(A) Peptides identified in a representative AAL enrichment. More than 70% of the peptides belong to proteins with SRDs (light and dark green). Of the proteins containing SRDs, 40% are predicted to have a nuclear localization signal (dark green). (B) Comparison of the percentage of Ser in proteins for which glycopeptides were identified (O-Fuc), Ser-rich proteins with no predicted signal peptide in the AAL enrichment (SRD), other proteins in the enrichment (Contaminants), and a control set of predicted cytosolic and nuclear proteins (Control). (C) Number of Ser or Thr 15-mer residues present in various apicomplexans. Ser-15-mer residues are present in higher numbers in the species that are bound by AAL, as shown in Fig. 1D.
Table 1.
Proteins containing Ser-rich domains identified by AAL pull-down and grouped by function (putative or annotated)
| Protein family | Nuclear localization signal | Unique O-fuc peptides (proteins*) | |
| + | − | ||
| Autophagy | 1 | 0 | 1 (1) |
| DNA-binding proteins | 3 | 2 | 3 (3) |
| Hypothetical proteins | 9 | 15 | 29 (13) |
| Kinases/phosphatases | 2 | 4 | 2 (1) |
| mRNA processing | 5 | 2 | 2 (2) |
| NPC/nuclear transport | 2 | 4 | 14 (4) |
| Nucleotide-binding domain | 0 | 2 | |
| Protein–protein interaction | 5 | 5 | 16 (7) |
| Transcription regulators | 2 | 1 | 1 (1) |
| tRNA synthesis | 2 | 0 | |
| Ubiquitination related | 1 | 2 | 1 (1) |
| 32 | 37 | 69 (33) | |
| Total | 69 | ||
Number of proteins from the corresponding family for which glycopeptides were observed.
O-Fucosylation Directs Glycoproteins to AAL-Labeled Assemblies Associated with the Nuclear Membrane in Close Proximity to the NPC.
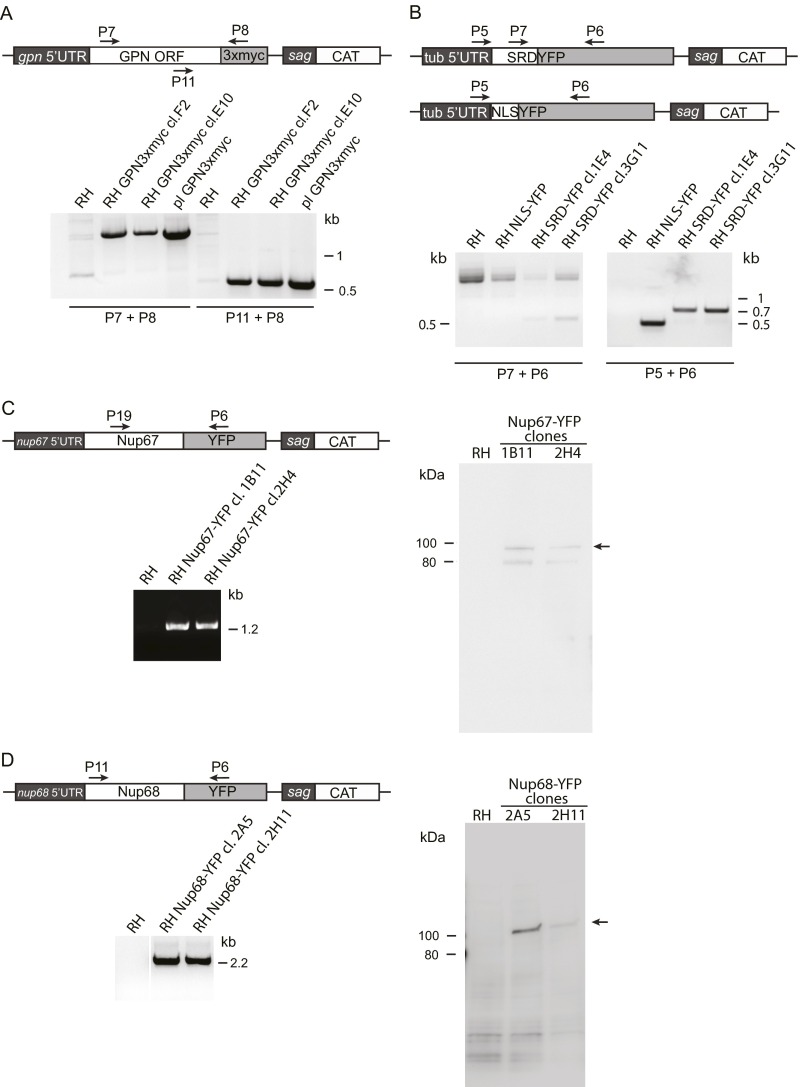
Two AAL-enriched proteins were chosen for further studies: a putative Gly-Pro-Asn-loop GTPase (hereafter “GPN”) and the transcriptional activator SWI/SNF2 (hereafter “SNF2”). GPN has a long N-terminal SRD but lacks an NLS (Fig. 4A). The protein was tagged with 3×MYC at its C terminus (Fig. S6A) and shown to be concentrated, like AAL, at the nuclear periphery but also was present in the cytosol, where no AAL labeling is observed (Fig. 4B). The tagged protein was also enriched by AAL (Fig. 4C). Addition of O-Fuc as a probabilistic event might explain why fucosylated GPN is present in the nucleus but unmodified GPN remains in the cytosol.
Fig. S6.
Characterization of the cell lines generated in this study. (A) An ectopic copy of GPN-loop GTPase (TGGT1_285720) was expressed under its endogenous promoter, and clones were selected for chloramphenicol resistance and analyzed by PCR. Experiments in Fig. 4 were performed with clone E10. (B) PCR analysis of clonal populations of SRD-YFP and NLS-YFP cell lines. Experiments in Fig. 4 and Fig. S7 were performed with clone 3G11 for SRD-YFP. (C) Nup67-YFP was expressed under its endogenous promoter. Two clonal populations were analyzed by PCR and Western blot. Detection with anti-GFP shows expression of the full-length fusion protein (arrow). A second, lower-molecular-weight band is also present in the clones but not in the wild type (RH). Microscopy in Fig. 5 was performed using clone 1B11. (D) Nup68-YFP clones were genotyped by PCR, and protein expression was analyzed by Western blot using anti-GFP antibody. Microscopy in Fig. 5 was performed using clone 2H11. Primer sequences are listed in Table S1.
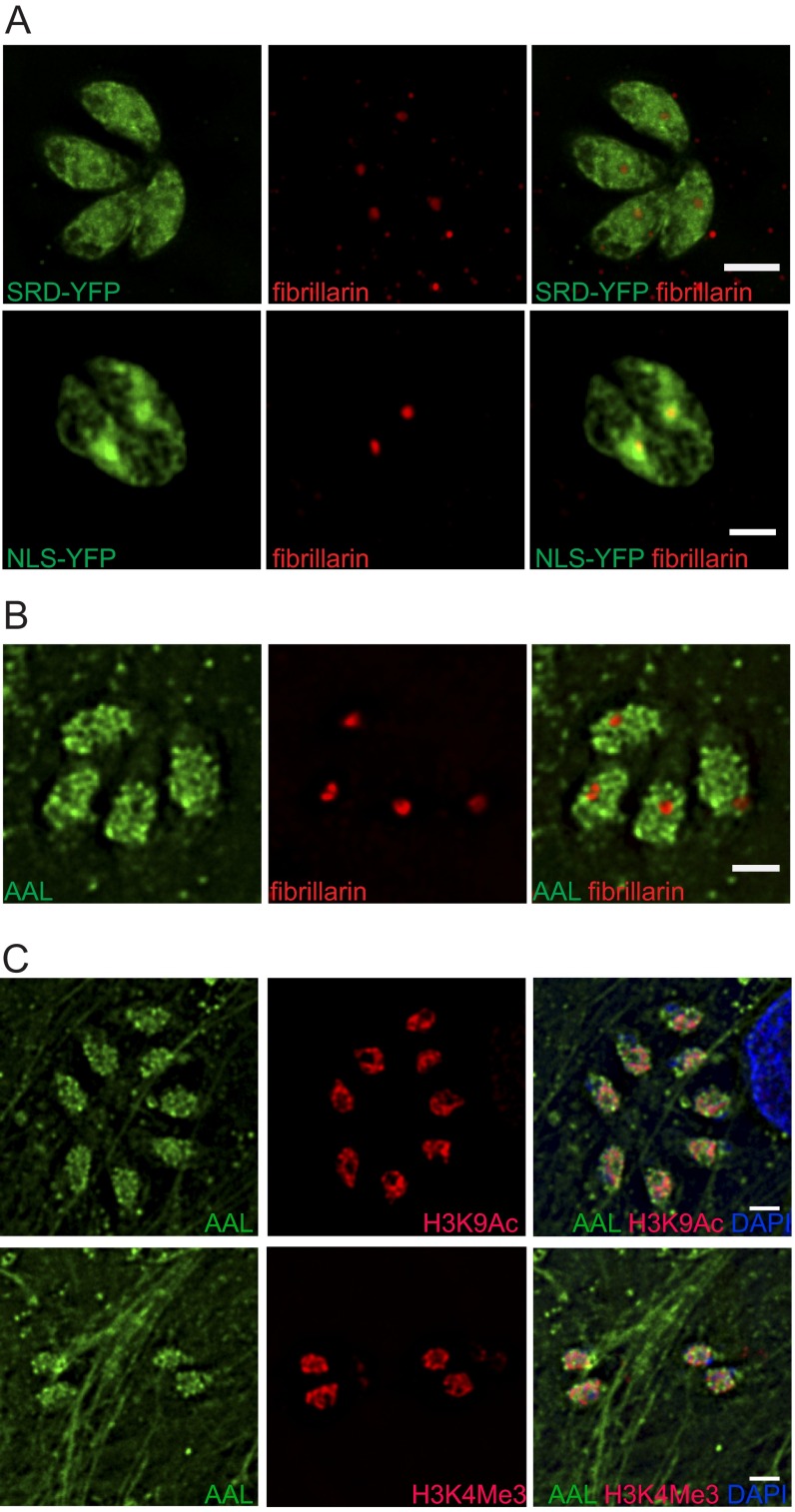
The SRD from GPN was fused to the N terminus of YFP (SRD-YFP) and expressed in tachyzoites (Fig. S6B). SRD-YFP localizes to the nucleus and to a lesser extent to the cytosol (Fig. 4D). Both SRD-YFP and AAL are excluded from the nucleolus, as shown by costaining with anti-fibrillarin antibodies (Fig. S7 A and B). A band corresponding to the observed molecular weight of SRD-YFP was labeled by AAL on Western blots, and AAL reactivity was eliminated upon β-elimination (Fig. 4E), indicating that SRD-YFP is modified with O-Fuc. In contrast, when the NLS from TgGNC5 (17) was added to the N terminus of YFP, it targeted the fluorescent protein to the nucleoplasm, including the nucleolus (Fig. 4D and Fig. S7A). Western blots showed that NLS-YFP, despite its relative abundance as compared with SRD-YFP, does not bind AAL, indicating that it is not O-fucosylated (Fig. 4E).
Fig. S7.
Costaining of SRD-YFP, NLS-YFP, and RH with additional nuclear markers. (A and B) SRD-YFP is excluded from the nucleolus (A), whereas NLS-YFP is enriched in this subnuclear compartment (B). (C) AAL binds to nuclear subdomains distinct from euchromatin, as defined by antibodies against two histone modifications associated transcriptional activation: H3K9Ac (Upper) and H3K4Me3 (Lower). (Scale bars: 2 μm.) All images were taken on a deconvolving fluorescence microscope.
SNF2 has an SRD at amino acids 1871–1935 (Fig. 4F) and three predicted NLS (amino acids 1005–1016, 2402–2410, and 2507–2517). SNF2, which was endogenously tagged with a 3×HA tag, localizes to the nucleus with AAL (Fig. 4G). AAL pull-down confirmed that SNF2 is O-fucosylated (Fig. 4H).
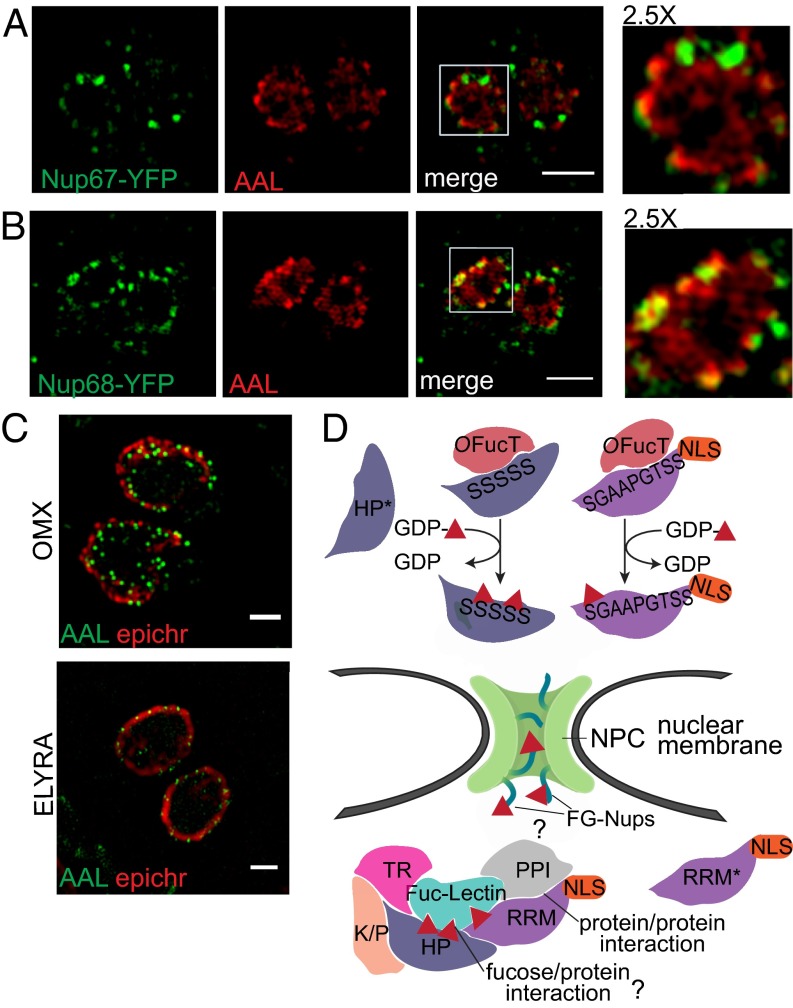
Superresolution microscopy shows that AAL binds to punctate structures that are adjacent to epichromatin (35), a conformational epitope of DNA in complex with histones H2A and H2B that marks the nuclear periphery (Fig. 5C). AAL binding is also found in proximity to euchromatin (Fig. S7C), as defined by antibodies against two histone modifications associated with active transcription, H3K9Ac and H3K4Me3 (9). Last, two predicted FG-Nups—Nup68, which is enriched by AAL, and Nup67, which is not—were expressed with a C-terminal YFP under their endogenous promoters (Fig. S6 C and D). Both fusion proteins partially colocalize with AAL at the nuclear membrane (Fig. 5 A and B), and in both cases, a subset of the AAL-labeled assemblies partially overlaps with the NPCs (see Insets in Fig. 5 A and B). The glycopeptides observed in Nup68 and other two FG-Nups (TGGT1_203780 and TGGT1_313430) indicate that O-Fuc modifies the FG repeats region (Dataset S1).
Fig. 5.
O-fucosylated proteins localize in assemblies at the nuclear membrane near the NPCs. (A and B) ELYRA SIM shows partial association between AAL-labeled assemblies and Nup67-YFP (A) and Nup68-YFP (B), one of the FG-Nups bound by AAL. For both fusion proteins, YFP was detected using anti-GFP. Boxed areas are shown at higher magnification. (Scale bars: 2 μm.) (C) SIM superresolution microscopy using either OMX or ELYRA shows that AAL labels punctate assemblies at the nuclear periphery. (Scale bars: 1 μm.) (D) Model for O-fucosylation of nuclear proteins in T. gondii: An as yet unidentified OFucT transfers fucose (red triangle) to Ser/Thr residues on SRD-containing proteins. O-fucosylated proteins then are shuttled to the nuclear periphery where they associate in assemblies, via protein–protein interactions and, possibly, an endogenous fucose-binding lectin (question mark). How the assembly of O-fucosylated proteins interacts with the NPC remains to be determined (question mark). According to the model, nonfucosylated proteins (asterisks) will stay in the cytosol or go to nucleus depending on the presence of an NLS. HP, hypothetical protein; K/P, kinase or phosphorylase domain; PPI, protein–protein interaction domain; RRM, RNA-recognition motif; TR, transcriptional regulator.
Discussion
Almost all eukaryotes present a glycosylation pathway dedicated to the modification of cytosolic and nuclear proteins in which N-acetylglucosamine is transferred to Ser/Thr in disordered domains by O-GlcNAc transferase (OGT) (36). Yeast, one of the few organisms lacking an OGT, recently has been shown to use O-mannose (O-Man) instead of O-GlcNAc to modify its nucleocytosolic proteins (37). In contrast, T. gondii has three cytosolic glycosylation pathways: an OGT (38), the hydroxylase and glycosyltransferases (GTs) that modify Skp1 with a pentasaccharide (22), and the nucleocytosolic O-fucosylation system described here. In the host and presumably in the parasite, the donor for the OGT reaction, UDP-GlcNAc, is sensitive to the metabolic state of the cell, and modification by O-GlcNAc affects protein activity (36). Similarly, glycosylation of Skp1 is required for normal growth of tachyzoites in culture, and proline hydroxylation on Skp1 is sensitive to the redox status of T. gondii (22, 29). In contrast, this study suggests that the addition of O-Fuc targets T. gondii proteins to assemblies closely associated with the nuclear membrane and that targeting of endogenous and exogenous proteins to the AAL-labeled assembly may occur in the absence of an NLS. SRD-YFP and two endogenous proteins, one containing a predicted NLS, are modified with O-Fuc and localize to the nuclear periphery, whereas the addition of an NLS to either Cas9 or YFP targets proteins to the nucleoplasm, including the nucleolus. Whether the addition of O-Fuc affects the activity of T. gondii proteins or protein–protein interactions was not determined.
Although O-fucosylation of Ser/Thr residues in secreted proteins of eukaryotic cells has been described previously, here we identify this modification on nuclear proteins. AAL staining suggests this pathway is conserved only in T. gondii, H. hammondi, and N. caninum. Both human fibroblasts (Fig. S2B) and bovine turbinate cells (Fig. 1D, S. neurona) did not show nuclear staining by AAL. Further studies should be performed in different taxonomic groups, but the limited data so far suggest that nuclear O-fucosylation may be restricted to these three species. Recognizing the limitations we have encountered thus far in defining the modification site(s) precisely, it appears that the unidentified T. gondii O-fucosyltransferase (OFucT) differs from host protein O-fucosyltransferases (POFUT1 and POFUT2) in its location and acceptor specificity. First, POFUTs are glycosyltransferases resident in the endoplasmic reticulum (ER) (21), whereas we predict that the T. gondii OFucT is either cytosolic or nuclear. Second, the host enzymes transfer O-Fuc to epidermal growth factor-like (POFUT1) or thrombospondin type I (POFUT2) repeats, both of which are characterized by conserved disulfide bonds (39, 40), whereas disulfides do not form in cytosolic and nuclear proteins.
Our working model of protein O-fucosylation in T. gondii is shown in Fig. 5D. AAL enrichment and the identified glycopeptides suggest that for the most part the acceptors of the putative OFucT are SRDs or proteins containing such domains. However, it seems likely that not all nucleocytosolic proteins with SRDs were identified in the AAL enrichment. In our model, we speculate that an as-yet-unidentified fucose-binding lectin would recognize O-fucosylated proteins and participate in their accumulation in assemblies closely associated with the nuclear membrane. This idea would be similar to the host cell secretory pathway in which lectins bind glucosylated and mannosylated N-glycans (41). Many hypothetical proteins, with no homology to any known conserved domains and specific to T. gondii, have been identified in the AAL pull-downs, and they may be important in forming the assemblies, i.e., the hypothesized fucose-binding lectin. The AAL-labeled assembly is also likely to contain proteins that are not O-fucosylated; in our model, these proteins would associate via protein–protein interactions. Protein–protein interactions, disrupted during lysis, reform during lectin enrichment, and we therefore cannot exclude the possibility that some of the proteins isolated in the pull-down that were categorized as contaminants are actually nonfucosylated members of the assemblies. Isolation of the intact assemblies will be required to discriminate between nonfucosylated proteins that are members of the assemblies and true contaminants. This distinction might be complicated further by the possibility that the protein composition of the assembly is itself heterogeneous. Furthermore, we observed the addition of O-Fuc to be a probabilistic event for ∼20% of the identified glycopeptides. According to our model, the fucosylated form of the protein might be present in the AAL-labeled assembly, and the nonfucosylated form might diffuse into the nucleus if an NLS is present or remain in the cytosol if an NLS is absent, as suggested by the localization of GPN-3×MYC (Fig. 4D). Whether addition of fucose as a probabilistic event is due to the OFucT mechanism and/or kinetics or is observed because nuclear O-Fuc, like O-GlcNAc, is a reversible modification cannot be clearly stated at this point. No putative O-fucosidase could be identified so far in T. gondii.
Multiple pieces of evidence point to the potential importance of the AAL-labeled assemblies in the nucleus of T. gondii. First, proteins in AAL pull-downs include numerous putative nucleoporins, mRNA processing enzymes, transcription regulators, and signaling proteins. Second, disruption of GDP-Fuc biosynthesis, which eliminates the binding of AAL to nuclei, appears to affect growth severely, as suggested by our inability to clone the AAL-negative cells. The absence of AAL binding to oocyst nuclei suggests that these proteins are either absent or not glycosylated in these life stages, because electron microscopy showed that the nuclear membrane of sporulating T. gondii remains intact (42). Last, five of seven predicted nucleoporins are present in the AAL enrichments, and tagged versions of Nup68 and Nup67 partially colocalize with AAL-labeled assemblies. Furthermore, MS showed that O-Fuc is found on three FG-Nups: Nup68, TGGT1_203780 [the second most abundant protein in the pull-down (Dataset S2)], and TGGT1_313430 [the T. gondii ortholog of yeast Nup98/96 (43)]. In all three instances, the sugar modifies the FG repeats region. FG regions are the disordered sequences that characterize the NPC channel and interact with karyopherins to mediate nuclear transport (19). In higher eukaryotes and yeast, FG regions are highly decorated with O-GlcNAc and O-Man, respectively, and evidence suggests that this PTM could affect cargo selectivity (36, 37).
O-fucosylation thus appears to be a mechanism by which T. gondii proteins involved in gene expression and mRNA processing (gating hypothesis) are gathered at the nuclear membrane, often in close proximity to the NPCs (14), and components of the NPC itself are O-fucosylated.
Methods
Parasite Cell Culture and Manipulation.
T. gondii type I RH tachyzoites culture, manipulation, and in vitro differentiation to bradyzoites were performed as previously described (7, 44). The RH Δku80 (44) transgenic cell line has been described previously. RH Δku80 ΔphyA, Δgnt1, and ΔpgtA (22) were kind gifts from Christopher West, University of Georgia, Athens, GA and Ira Blader, SUNY at Buffalo School of Medicine, Buffalo, NY. RH SNF2-3xHA was a kind gift from William Sullivan, Jr, Indiana University School of Medicine, Indianapolis. The generation of transgenic cells expressing GPN-3xMYC, SRD-YFP, NLS-YFP, Nup68-YFP, and Nup67-YFP is described in SI Methods. All animal work was approved by the Institutional Animal Care and Use Committee at Boston University. C. parvum (Iowa strain) oocysts were purchased from Bunch Grass Farm. S. neurona SN3 was a kind gift from Michael Grigg, NIH, Bethesda, and P. falciparum was a kind gift from Jeffrey Dvorin, Harvard Medical School, Boston. N. caninum Nc-1 and H. hammondi were kind gifts of Jon Boyle, University of Pittsburgh, Pittsburgh. For oocyst excystation and fixation protocols, see SI Methods.
DNA Manipulation.
All primers used in this study are listed in Table S1. Cloning strategies and plasmids used are described in SI Methods.
Table S1.
List of primers used in this study
| Primer | Sequence (5′–3′) | Restriction site |
| P1 | AAGTTGCCCAGACGCGCCCTTATTACG | |
| P2 | AAAACGTAATAAGGGCGCGTCTGGGCA | |
| P3 | GACTCAAAATGAGGAAGCGTGTGAAGCGCC | BglII |
| P4 | CTAGGGCGCTTCACACGCTTCCTCATTTTA | AvrII |
| P5 | CGTAGAGAACAAGCACTCG | |
| P6 | CGTCCTTGAAGAAGATGGTG | |
| P7 | CTAAAATGGAGACCTCTTCTC | |
| P8 | CACCGTTCAAGTCTTCCTCGG | |
| P9 | CGTTTAAACCGACACAAGCGACGCAC | PmeI |
| P10 | CCAGATCTGTCTGTGAAGTGAGGTG | BglII |
| P11 | CTAGATCTATGTTCGGCAACACGGC | BglII |
| P12 | CTCCTAGGTTCACCATGTTTCGCATC | AvrII |
| P13 | CTGTTTAAACGTTCTCTGGCGAAGCAG | PmeI |
| P14 | CTGGATCCCCCAGATCTGAAGAGAAG | BamHI |
| P15 | CCCAGATCTATGTTTAGCTCCGCTACC | BglII |
| P16 | CTCCTAGGCTTCGCTCCAAATAAGCC | AvrII |
| P17 | CTGTTTAAACCCTCGAATAACTCGAGGTG | PmeI |
| P18 | CCCAGATCTCGTCAGAGACAAAGAACAAG | BglII |
| P19 | GCTCTCGAACTCCGAGAGA |
Immunofluorescence Microscopy.
Fixation, permeabilization, blocking, and labeling conditions are described in SI Methods. Deconvolution microscopy was performed on a Olympus XI70 inverted microscope and DeltaVision SoftWoRx as previously described (45). Superresolution microscopy was performed on an OMX-3D, V3 type (Applied Precision) or on an ELYRA (Zeiss) microscope. In both cases images were acquired with a 100×/1.4 oil immersion objective, 0.125-µm z sections at room temperature. For the OMX microscope, images were processed with OMX SoftWoRx software, and projections of 10× z stacks are shown. For the ELYRA microscope, images were processed for structured illumination (SIM) using ZEN software, and single optical sections were selected using Fiji (46).
Lectin Pull-Down.
Proteins were pulled down from total cell lysate by incubation with biotinylated AAL (Vector Labs) and Dynabeads MyOne Streptavidin T1 (Thermo Fisher) and were eluted by incubation with αMeFuc (Carbosynth). See SI Methods for details (47).
MS.
Proteins in the elution were reduced, alkylated, and digested with trypsin either in solution or in gel. Peptides and glycopeptides were analyzed by LC-MS/MS using a ChIP or an ultra-performance LC (UPLC) C18 column on a 6550-QTOF (Agilent Technologies), an LTQ-Orbitrap-XL-ETD, or a Q Exactive Plus Quadrupole hybrid Orbitrap (Thermo Scientific) MS system. The PTM search function within the analysis software PEAKS (Bioinformatic Solutions) (48) was used to produce a list of putative O-fucosylated peptides that was manually verified. Scaffold (Proteome Software) (49) was used to compare the five biological repeats, and further analyses were performed using RStudio. Monosaccharide composition analysis was performed on a Bruker Scion-SQ GC-MS (Bruker). See SI Methods for details.
SI Methods
Oocyst Excystation.
T. gondii type III VEG oocysts were used to infect mice. Cats were infected with tissue cysts from mice brains, and oocysts were isolated and sporulated as previously described (45, 50). E. tenella oocysts were isolated from infected chicken ceca and sporulated as described in ref. 45. H. hammondi, T. gondii, E. tenella, and C. parvum sporulated oocysts were excysted as described in refs. 50 and 51. Sporulated and unsporulated oocysts were subjected to three freeze/thaw cycles in 4% paraformaldehyde (PFA) in phosphate buffer (PB) and were stored overnight at 4 °C in fixative. All sporozoites were fixed in 4% PFA in PB for 20 min at room temperature and permeabilized and blocked using the same conditions as tachyzoites.
DNA Manipulations.
The SRD from TGGT1_285720 (nucleotides 1–237) and the whole TGGT1_285720 ORF (GPN ORF) were sent for gene synthesis (GenScript). The SRD was cloned in BglII/AvrII-digested ptubYFP2(MCS)/sagCAT (27) to generate ptubSRDYFP/sagCAT. For TgNLS, primers P3 and P4 were annealed and phosphorylated, and the resulting dsDNA oligo was ligated in the BglII/AvrII-digested plasmid to obtain ptubNLSYFP/sagCAT. The GPN ORF was cloned in ptubCactinCD1-3xmyc/sagCAT using BglII and AvrII to replace CactinCD1. A region about 1 kb upstream of the start codon was amplified by PCR and cloned in place of the tub promoter using PmeI and BglII to obtain the final plasmid pgpnGPN3xmyc/sagCAT. The Nup68 (TGGT1_273850) and Nup67 (TGGT1_306560) ORFs were amplified by PCR from cDNA using primers P11/P12 and P15/P16 and were cloned in ptubYFP2(MCS)/sagCAT using BglII and AvrII. As above, a region about 1 kb upstream of the Nup68 start codon was amplified from genomic DNA using primers P13 and P14, digested with PmeI/BamHI, and cloned in ptubNup68YFP/sagCAT linearized with PmeI and BglII, resulting in the final plasmid pnup68Nup68YFP/sagCAT. Nup67 5′ UTRs were amplified as above but using primers P17 and P18, digesting both insert and vector with PmeI/BglII, and were cloned into ptubNup67YFP/sagCAT to obtain pnup67Nup67YFP/sagCAT. Both ptubYFP2(MCS)/sagCAT and ptubCactinCD1-3xmyc/sagCAT were the kind gift of Marc-Jan Gubbels, Boston College, Boston. ptubGAP40YFPHA/sagCAT has been described previously (24). After electroporation, RH cells were selected with chloramphenicol. Clonal cell lines were obtained by cloning by limiting dilution and were characterized by PCR and Western blot.
The pU6Universal plasmid was used for gene disruptions (52). Primers P1 and P2 were annealed and phosphorylated, and the resulting dsDNA was cloned in the BsaI-digested pU6 plasmid. Transient gene disruption by CRISPR/Cas9 was performed as previously described. Cells were fixed at 24, 48, and 72 h post electroporation.
Immunofluorescence Microscopy.
Intracellular tachyzoites were fixed in 4% PFA in PB for 20 min at room temperature, permeabilized in 0.25% Triton X-100 in 1× PBS for 15 min at room temperature, and blocked in 3% BSA in 1× PBS for 1 h at room temperature. Extracellular parasites were filtered through 3-μm polycarbonate membranes and harvested by centrifugation. After washing with 1× PBS, the cells were resuspended in 4% PFA in PB to 2 × 107 cells/mL and fixed as above. Fixed extracellular tachyzoites were air dried on coverslips, permeabilized in 0.1% Triton X-100 for 10 min at room temperature, and blocked as above. Lectins (all from Vector Labs) were labeled with either Alexa Fluor 488 or Alexa Fluor 594 succinimidyl esters (Molecular Probes) following the manufacturer’s instructions. Primary antibodies were used at the following concentrations: rabbit anti-TgCentrin 1:40,000; mouse anti-SAG2Y 1:5,000; mouse anti-SAG1 DG52 1:1,000; rat anti-IMC3 1:2,000; mouse anti-epichromatin 1:100; mouse anti-fibrillarin 1:500; mouse anti-H3K9Ac Y28 (Millipore) 1:250; mouse anti-H3K4Me3 MC315 (Millipore) 1:1,000; mouse anti-FLAG (Sigma) 1:100; mouse anti-GFP (Roche) 1:300; mouse anti–c-myc 9E10 (Santa Cruz Biotechnology) 1:50; and mouse anti-HA (Pierce) 1:500. Antibodies to TgCentrin, IMC3, epichromatin, and fibrillarin were kind gifts from Marc-Jan Gubbels, and anti-SAGY2 and SAG1 were kind gifts from Jeroen Saeij, University of California, Davis, CA. Goat Alexa Fluor-conjugated anti-mouse, anti-rat, and anti-rabbit antibodies (Molecular Probes) were used at 1:800. AAL-Alexa Fluor 488/594 was used at 1:200; GSLII-Alexa Fluor 594 was used at 1:250; and RCA-Alexa Fluor 488 was used at 1:125. Cells were labeled with 2 μg/mL DAPI for 10 min at room temperature. Coverslips were mounted on glass slides using Prolong Gold with DAPI (Molecular Probes) for deconvolution microscopy or Vectashield (Vector Labs) for SIM.
AAL Pull-Downs.
For LC-MS/MS analyses, 1.5 × 109 extracellular tachyzoites were harvested by centrifugation and washed four times in 1× PBS. Cells were lysed in 2% SDS, 0.15 M NaCl, 0.1 mM DTT in 40 mM Tris⋅HCl (pH 7.4), heated for 20 min at 50 °C, and cooled to room temperature. After dilution to 0.03% SDS in PD buffer [0.8% (wt/vol) N-octyl-glucopyranoside, 0.15 M NaCl, 40 mM DTT, 20 mM Tris⋅HCl (pH 7.4)] plus EDTA-free complete protease inhibitor tablets (Roche), the lysate was incubated for 2 h with rotation at 4 °C with 30 μg biotinylated AAL followed by incubation for 30 min with Dynabeads MyOne Streptavidin T1. Beads were collected and washed five times in PD buffer. For elution 1 (E1), proteins were eluted by incubation with 0.2 M αMeFuc in PD buffer for 16 h with shaking at 4 °C. Elution 2 (E2) was performed by incubating the beads for an additional 2 h in 0.2 M αMeFuc in PD buffer. For analysis of tagged cell lines, pull-downs were performed on 4.5 × 108 tachyzoites, and amounts of biotinylated-AAL and beads were adjusted accordingly.
Cell Lysate for Western Blot.
Extracellular tachyzoites were harvested by centrifugation, washed in 1× PBS, and lysed in 1× reducing SDS/PAGE loading buffer. For protease treatments, after washing in 1× PBS, tachyzoites were resuspended in 0.1% Nonidet P-40, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 10% glycerol, 20 mM Tris⋅HCl (pH 7.4) and subjected to five freeze/thaw cycles. Lysate was incubated with 200 nM trypsin, chymotrypsin, or proteinase K for 2 h at 37 °C.
Western Blots.
For total cell lysate, the equivalent of 5 × 106 cells was loaded in each lane with the exception of the Nup68-YFP blot, in which the equivalent of 107 cells was loaded. For the elutions from the AAL pull-downs, E1 and E2 were combined, and half of the total volume was loaded in one lane. Samples were run on 4–15% Tris-glycine gradient gels (Bio-Rad) and were blotted on PVDF. β-Elimination on blots was performed as described. Membranes were blocked in 50 mM Tris⋅HCl, 0.15 M NaCl, 0.25% BSA, 0.05% Nonidet P-40 (pH 7.4) (47). Primary and secondary antibodies were diluted in blocking buffer at the following concentrations: biotinylated AAL (Vector Labs) 1:2,000; mouse anti–c-myc 9E10 1:200; mouse anti-GFP 1:2,000; mouse anti-HA 1:1,000; ExtrAvidin HRP-conjugated (Sigma) 1:10,000; and anti-mouse HRP-conjugated (Bio-Rad) 1:1,000. For sugar inhibition experiments, biotinylated AAL was preincubated for 30 min at room temperature with 0.2 M αMeFuc. Blots were developed by ECL (Pierce) using an ImageQuant LAS4000 imager.
LC-MS/MS.
Biological replicates 1–3.
E1 was run on a 4–20% TGX gel (Bio-Rad) until the front was about 2 cm from the well. After staining with colloidal Coomassie, the smear was excised, and reduction, alkylation, and trypsin digestion were performed in-gel. The extracted peptides were separated using a Polaris-HR-Chip-3C18 composed of a trapping column and a 75 μm × 150 mm analytical column. Peptides were trapped at a flow rate of 2 µL/min for 4 min at 2% mobile phase B [A: 1% acetonitrile (ACN), 0.1% formic acid (FA); B: 99% ACN, 0.1% FA]. A 25-min linear gradient from 2–40% B, followed by a 10-min gradient from 40–60% B was used to separate peptides. The column then was washed with 95% B for 5 min and re-equilibrated at 2% B for 15 min. The chip was coupled to an Agilent 6550 QTOF mass spectrometer to acquire MS and collision-induced MS/MS spectra. The top 20 MS peaks in the range between m/z 295–1,700 were selected for MS/MS fragmentation, and a custom ramped collisional energy (CE) table was used to determine the CE, based upon precursor charge. Nitrogen was used as the collisional gas.
Biological replicates 4 and 5.
E1 was precipitated in cold methanol (MeOH) with 0.1 M ammonium acetate for at least 18 h at −20 °C. The precipitate was washed in MeOH/ 0.1 M ammonium acetate, and any remaining solvent was removed by speed vacuum (SpeedVac Plus; Savant). The dried samples were resuspended in 50 mM ammonium bicarbonate (pH 8.0) and then were reduced, alkylated, and digested with trypsin. The resulting digest was dried by speed vacuum and desalted on C18 ZipTip concentrators (EMD Millipore). Samples were reconstituted in 2% ACN, 0.1% FA and were separated on a UPLC capillary system (Waters), composed of a nanoAcquity 5-μm Symmetry C18 180 μm × 20 mm trap and a 1.7-μm BEH130C18 150 μm × 10 cm analytical column. The TriVersa NanoMate ion source (Advion) was coupled to either an LTQ-Orbitrap-XL-ETD or QE Plus. Samples were loaded on the trapping column for 4 min at 4 µL/min and then were separated at 0.5 µL/min using the following conditions: 2–40% B linear gradient for 43 min, held at 40% B for 9 min, washed to 98% B, held at 98% B for 5 min, and re-equilibrated to 2% B for 18 min. Alternatively, samples were reconstituted in 1% B and injected on the trapping column at 4 µL/min for 3.75 min. Analytical separation was performed as follows: 0.5 µL/min flow, 1–60% B linear gradient for 90 min, followed by holding at 60% B for 7 min, ramping to 95% B for 3 min, holding for 5 min, and re-equilibration at 1% B for 15 min. On the QE Plus, MS spectra were obtained by scanning over the range m/z 350–2,000 with one microscan and a maximum injection time of 50 ms. MS/MS parameters were the top 20 MS/MS isolation window, 1.4 m/z, one microscan, maximum injection time 45 ms, scan range m/z 100–2,000. HCD fragmentation was performed at either 27 or 45 eV. ETD experiments were performed on an LTQ-Orbitrap-XL-ETD. MS scan parameters were one microscan, range m/z 300–2,000. MS/MS scan parameters were isolation width 3 m/z, maximum injection time 25 ms per microscan, fragmentation using ETD with supplemental activation with a 180-ms reaction time, four microscans averaged per MS/MS, detection in the linear quadrupole ion trap. The resulting spectra were searched using Mascot against the T. gondii GT1 predicted proteome (trypsin as the enzyme with a maximum of three missed cleavages, 10 ppm precursor, and 0.8 Da fragmentation mass tolerances). Carbamidomethyl cysteine was specified as fixed modification, and dHex (on Ser/Thr) or methionine oxidation were specified as variable modifications. For HCD MS and MS/MS, spectra were acquired on the Orbitrap as follows: MS: range m/z 300–2,000; one microscan; maximum 500-ms injection time. MS/MS: two microscans; maximum 500-ms injection time; 2 m/z isolation window; and 35 eV HCD fragmentation. The MS data have been deposited in the ProteomeXchange Consortium database with the dataset identifier PXD004426.
Data Analysis.
All MS/MS spectra acquired in the five biological repeats, with the exception of the ETD data, were first analyzed with PEAKS software (48). The biological samples (including technical replicates) were analyzed individually. The following parameters were specified for the de novo search: parent and fragment mass error tolerances, 8 ppm and 0.05 Da, respectively; carbamidomethyl cysteine as a fixed modification; a maximum of eight variable PTMs per peptide. For the database (DB) search, the above settings were used with ToxoDB-24_TgondiiGT1_RH as the database (combined predicted proteins for GT1 and RH, with redundant protein sequences removed) and false-discovery rate (FDR) estimation enabled. For the PTM search, all parameters were identical to those above except for a maximum of five variable PTMs per peptide, with all built-in Unimod PTMs considered and dHex (on Ser/Thr) or methionine oxidation as variable modifications. Data acquired on the Agilent 6550 were analyzed as above, but a monoisotopic parent ion tolerance of 12 ppm and a fragmentation tolerance of 0.1 Da were specified. A list of putatively fucosylated peptides was generated from the PEAKS PTM search (5% FDR maximum threshold for identified peptide–spectrum matches). Each peptide assignment was manually reviewed by examining mass spectra for peaks spaced at intervals corresponding to the mass of one or more dHexs in the same MS scan (within ±5 mmu) that could be attributed to neutral losses from a single precursor. If peaks having mass differences corresponding to one or more dHexs were observed at different retention times, these were considered to be indicators of PTM heterogeneity on a single peptide. Raw data were examined manually using the XCalibur Qual Browser (Thermo Scientific) or MassHunter Qualitative Analysis B.06.00 (Agilent). A qualitative assessment of each MS/MS spectrum on the list of peptides containing one or more PTMs was performed based on signal intensity, peptide coverage, and parent ion accuracy. The higher-quality spectra were sequenced manually with the goal of specifying the locations of the dHex(s) on the peptide. The PEAKS PTM results from each biological replicate were imported as unique biological samples into Scaffold 7.5 (Proteome Software) with all technical replicates contained within their respective biological sample group (49). The data were filtered to show 99% protein threshold and a minimum of 10 unique peptides. A 3.6% peptide FDR was determined using the Prophet model. Proteins listed in Dataset S2 and used to build Table 1 were either Ser-rich (either ≥10% Ser content or the presence of one or more SRD as defined by five or more tandem Ser residues, with no predicted signal peptide and present in at least four out of five biological samples) and/or had at least one observed glycopeptide confirmed by either neutral loss or the presence of fragment ions plus dHex in the MS/MS spectrum.
GC-MS Monosaccharide Analysis.
E1 (starting material 2.5 × 108 tachyzoites) was precipitated in cold methanol/0.1 M ammonium acetate as described above. Reductive β-elimination was performed by dissolving the protein precipitate in 200 μL of 50 mM NaOH containing 1 M NaBD4 and incubation in an oven set to 55 °C for 16 h. Residual NaBD4 was eliminated by adding glacial acetic acid drop-wise until effervescence ceased. The sample was subsequently dried in a SpeedVac. The boric acid then was removed by extensive drying cycles in 10% acetic acid/MeOH, followed by 100% MeOH. To isolate the released sugars, the dried sample was resuspended in 2% ACN, 0.1% TFA and loaded on a Sep-Pak C18 cartridge (Waters). Four volumes of H2O 0.1% TFA were washed through the cartridge, and the eluate was collected. The sample was lyophilized and then per-acetylated to generate alditol acetates. Alditol acetates were generated by adding equal volumes of pyridine and acetic anhydride to the lyophilized sample and heating at 110 °C for 40 min. Once the sample was cooled, water was added to quench the remaining acetic anhydride. The alditol acetates were extracted into ethyl acetate, concentrated in a SpeedVac, and analyzed on a Bruker Scion-SQ equipped with a 436-GC gas chromatography system using helium as a carrier gas. The concentrate was diluted into hexane, and 2 μL was injected. The injector temperature was set for 220 °C, and a constant column flow rate of 1 mL/min was maintained for the duration of the analysis. The initial split-less sample injection was followed by a 100 mL/min split flow for 1 min and then a 50 mL/min split flow for 59 min. The GC temperature was held at 60 °C for 1 min, then was raised to 250 °C at a rate of 4 °C/min, then to 300 °C at a rate of 20 °C/min, and was held for 10 min. Separation was achieved on a Restek Rxi 5-ms capillary column. Electron impact (EI) ionization was performed with a 70-eV source, and spectra were acquired in positive mode after a 5-min solvent delay, scanning the m/z range 50–500 with a scan time of 500 ms. Spectra were background subtracted, and five scan averages were used to make a composite spectrum. The spectra and retention times were compared with those of genuine deutero-reduced alditol acetate standards analyzed on the same column under identical conditions. To differentiate between rhamnose and fucose, extracted ion chromatograms (XICs) (129 + 171 m/z) were compared with those of the genuine standards. The software MS Data Review 8.0 (Bruker) was used for data analysis.
Bioinformatics and Statistical Analyses.
Predicted and known functions for the enriched proteins were assigned based on annotation on ToxoDB (26) and/or the presence of conserved PFAM domains identified by BlastP searches. Statistical analyses were performed in RStudio with a custom script using the packages Biostrings, ggplot2, and plotrix. For the box plot in Fig. 3A, only nonredundant peptide sequences were used, and the maximum number of dHex observed was used to assign the peptide sequence to either group. The control set in Fig. S5B was obtained from a GO term search on ToxoDB using the following terms: DNA replication (GO: 0006260), RNA processing (GO: 0006397), nucleus (GO: 0005634), and nucleoplasm (GO: 0005654).
Supplementary Material
Acknowledgments
We thank Dr. Aparajita Chatterjee and Dr. Carolina Agop-Nersesian for invaluable assistance with oocyst excystations; Dr. Christopher West for helpful discussions; Dr. Eliza Vasile at the Swanson Biotechnology Center Microscopy Core Facility for technical assistance with OMX microscopy; and the Harvard Centre for Biological Imaging (HCBI) for infrastructure and support. The ELYRA microscope was acquired through NIH Shared Instrumentation Grant Award S10 RR27990 (to the HCBI). Support for this study came from NIH Grants R01 AI110638 (to J.S.), R01 GM031318 (to P.W.R.), and P41 GM104603 (to C.E.C.), NIH-National Heart, Lung, and Blood Institute Contract HHSN268201000031C (to C.E.C.), and a grant from the Mizutani Foundation of Glycoscience (to J.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The proteomic data reported in this paper have been deposited in the ProteomeXchange Consortium database (dataset identifier PXD004426).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613653113/-/DCSupplemental.
References
- 1.Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: A systematic review. Bull World Health Organ. 2013;91(7):501–508. doi: 10.2471/BLT.12.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torrey EF, Yolken RH. Toxoplasma oocysts as a public health problem. Trends Parasitol. 2013;29(8):380–384. doi: 10.1016/j.pt.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzi H, et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun. 2016;7:10147. doi: 10.1038/ncomms10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behnke MS, Zhang TP, Dubey JP, Sibley LD. Toxoplasma gondii merozoite gene expression analysis with comparison to the life cycle discloses a unique expression state during enteric development. BMC Genomics. 2014;15(1):350. doi: 10.1186/1471-2164-15-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radke JR, et al. The transcriptome of Toxoplasma gondii. BMC Biol. 2005;3:26. doi: 10.1186/1741-7007-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005;33(13):3994–4006. doi: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker R, et al. The Toxoplasma nuclear factor TgAP2XI-4 controls bradyzoite gene expression and cyst formation. Mol Microbiol. 2013;87(3):641–655. doi: 10.1111/mmi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker R, et al. Toxoplasma transcription factor TgAP2XI-5 regulates the expression of genes involved in parasite virulence and host invasion. J Biol Chem. 2013;288(43):31127–31138. doi: 10.1074/jbc.M113.486589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nardelli SC, et al. The histone code of Toxoplasma gondii comprises conserved and unique posttranslational modifications. MBio. 2013;4(6):e00922–e13. doi: 10.1128/mBio.00922-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatti MM, Livingston M, Mullapudi N, Sullivan WJ., Jr Pair of unusual GCN5 histone acetyltransferases and ADA2 homologues in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2006;5(1):62–76. doi: 10.1128/EC.5.1.62-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saksouk N, et al. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol. 2005;25(23):10301–10314. doi: 10.1128/MCB.25.23.10301-10314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blobel G. Gene gating: A hypothesis. Proc Natl Acad Sci USA. 1985;82(24):8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon MR, Pope BD, Sima J, Gilbert DM. Many paths lead chromatin to the nuclear periphery. BioEssays. 2015;37(8):862–866. doi: 10.1002/bies.201500034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns LT, Wente SR. From hypothesis to mechanism: Uncovering nuclear pore complex links to gene expression. Mol Cell Biol. 2014;34(12):2114–2120. doi: 10.1128/MCB.01730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gissot M, et al. Toxoplasma gondii chromodomain protein 1 binds to heterochromatin and colocalises with centromeres and telomeres at the nuclear periphery. PLoS One. 2012;7(3):e32671. doi: 10.1371/journal.pone.0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks CF, et al. Toxoplasma gondii sequesters centromeres to a specific nuclear region throughout the cell cycle. Proc Natl Acad Sci USA. 2011;108(9):3767–3772. doi: 10.1073/pnas.1006741108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatti MM, Sullivan WJ., Jr Histone acetylase GCN5 enters the nucleus via importin-alpha in protozoan parasite Toxoplasma gondii. J Biol Chem. 2005;280(7):5902–5908. doi: 10.1074/jbc.M410656200. [DOI] [PubMed] [Google Scholar]
- 18.Dixon SE, Bhatti MM, Uversky VN, Dunker AK, Sullivan WJ., Jr Regions of intrinsic disorder help identify a novel nuclear localization signal in Toxoplasma gondii histone acetyltransferase TgGCN5-B. Mol Biochem Parasitol. 2011;175(2):192–195. doi: 10.1016/j.molbiopara.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel MB, Knoll LJ. The ins and outs of nuclear trafficking: Unusual aspects in apicomplexan parasites. DNA Cell Biol. 2009;28(6):277–284. doi: 10.1089/dna.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field MC, Koreny L, Rout MP. Enriching the pore: Splendid complexity from humble origins. Traffic. 2014;15(2):141–156. doi: 10.1111/tra.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 22.Rahman K, et al. The e3 ubiquitin ligase adaptor protein SKP1 is glycosylated by an evolutionarily conserved pathway that regulates protist growth and development. J Biol Chem. 2016;291(9):4268–4280. doi: 10.1074/jbc.M115.703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C-T, Gubbels M-J. The Toxoplasma gondii centrosome is the platform for internal daughter budding as revealed by a Nek1 kinase mutant. J Cell Sci. 2013;126(Pt 15):3344–3355. doi: 10.1242/jcs.123364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouologuem DT, Roos DS. Dynamics of the Toxoplasma gondii inner membrane complex. J Cell Sci. 2014;127(Pt 15):3320–3330. doi: 10.1242/jcs.147736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanz S, et al. Biosynthesis of GDP-fucose and other sugar nucleotides in the blood stages of Plasmodium falciparum. J Biol Chem. 2013;288(23):16506–16517. doi: 10.1074/jbc.M112.439828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gajria B, et al. ToxoDB: An integrated Toxoplasma gondii database resource. Nucleic Acids Res. 2008;36(Database issue):D553–D556. doi: 10.1093/nar/gkm981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson-White B, et al. Cytoskeleton assembly in Toxoplasma gondii cell division. Int Rev Cell Mol Biol. 2012;298:1–31. doi: 10.1016/B978-0-12-394309-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio. 2014;5(3):e01114–e14. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, et al. The Skp1 protein from Toxoplasma is modified by a cytoplasmic prolyl 4-hydroxylase associated with oxygen sensing in the social amoeba Dictyostelium. J Biol Chem. 2012;287(30):25098–25110. doi: 10.1074/jbc.M112.355446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosugi S, et al. Six classes of nuclear localization signals specific to different binding grooves of importin alpha. J Biol Chem. 2009;284(1):478–485. doi: 10.1074/jbc.M807017200. [DOI] [PubMed] [Google Scholar]
- 31.Silmon de Monerri NC, et al. The ubiquitin proteome of Toxoplasma gondii reveals roles for protein ubiquitination in cell-cycle Transitions. Cell Host Microbe. 2015;18(5):621–633. doi: 10.1016/j.chom.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe. 2011;10(4):410–419. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashburner M, et al. The Gene Ontology Consortium Gene ontology: Tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchler-Bauer A, et al. CDD: Conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2013;41(Database issue):D348–D352. doi: 10.1093/nar/gks1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanagas L, et al. Epichromatin is conserved in Toxoplasma gondii and labels the exterior parasite chromatin throughout the cell cycle. Parasitology. 2013;140(9):1104–1110. doi: 10.1017/S0031182013000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bond MR, Hanover JA. A little sugar goes a long way: The cell biology of O-GlcNAc. J Cell Biol. 2015;208(7):869–880. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halim A, et al. Discovery of a nucleocytoplasmic O-mannose glycoproteome in yeast. Proc Natl Acad Sci USA. 2015;112(51):15648–15653. doi: 10.1073/pnas.1511743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee S, Robbins PW, Samuelson J. Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum. Glycobiology. 2009;19(4):331–336. doi: 10.1093/glycob/cwn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valero-González J, et al. A proactive role of water molecules in acceptor recognition by protein O-fucosyltransferase 2. Nat Chem Biol. 2016;12(4):240–246. doi: 10.1038/nchembio.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Shareffi E, et al. 6-alkynyl fucose is a bioorthogonal analog for O-fucosylation of epidermal growth factor-like repeats and thrombospondin type-1 repeats by protein O-fucosyltransferases 1 and 2. Glycobiology. 2013;23(2):188–198. doi: 10.1093/glycob/cws140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson DJ, Birch-Andersen A, Siim JC, Hutchison WM. An ultrastructural study on the excystation of the sporozoites of Toxoplasma gondii. Acta Pathol Microbiol Scand [B] 1979;87(5):277–283. doi: 10.1111/j.1699-0463.1979.tb02439.x. [DOI] [PubMed] [Google Scholar]
- 43.Iwamoto M, Asakawa H, Hiraoka Y, Haraguchi T. Nucleoporin Nup98: A gatekeeper in the eukaryotic kingdoms. Genes Cells. 2010;15(7):661–669. doi: 10.1111/j.1365-2443.2010.01415.x. [DOI] [PubMed] [Google Scholar]
- 44.Huynh M-H, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8(4):530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bushkin GG, et al. β-1,3-glucan, which can be targeted by drugs, forms a trabecular scaffold in the oocyst walls of Toxoplasma and Eimeria. MBio. 2012;3(5):e00258–e12. doi: 10.1128/mBio.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Güther MLS, et al. Fate of glycosylphosphatidylinositol (GPI)-less procyclin and characterization of sialylated non-GPI-anchored surface coat molecules of procyclic-form Trypanosoma brucei. Eukaryot Cell. 2009;8(9):1407–1417. doi: 10.1128/EC.00178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, et al. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics. 2012;11(4):M111.010587–M111.010587. doi: 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Searle BC. Scaffold: A bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics. 2010;10(6):1265–1269. doi: 10.1002/pmic.200900437. [DOI] [PubMed] [Google Scholar]
- 50.Walzer KA, et al. Hammondia hammondi, an avirulent relative of Toxoplasma gondii, has functional orthologs of known T. gondii virulence genes. Proc Natl Acad Sci USA. 2013;110(18):7446–7451. doi: 10.1073/pnas.1304322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee A, et al. Evidence for mucin-like glycoproteins that tether sporozoites of Cryptosporidium parvum to the inner surface of the oocyst wall. Eukaryot Cell. 2010;9(1):84–96. doi: 10.1128/EC.00288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One. 2014;9(6):e100450. doi: 10.1371/journal.pone.0100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.