Significance
A crucial step in the pathogenesis of autoimmune diseases, such as multiple sclerosis (MS), is transmigration of pathogenic T cells across the blood–brain barrier. These T cells mediate inflammation and subsequent lesion formation in the CNS. However, molecular mechanisms underlying lesion distribution and formation are not well understood. We here show that genetic ablation of a single immunoregulatory molecule on T cells, B7-homolog 1 (B7-H1), causes local endothelial dysfunction and determines lesion topography in a spontaneous mouse model of CNS autoimmunity. These findings can lead to new therapeutic approaches in targeting pathogenic T cell responses in MS.
Keywords: neuroinflammation, multiple sclerosis, spontaneous EAE, CNS lesion distribution, blood–brain barrier
Abstract
Molecular mechanisms that determine lesion localization or phenotype variation in multiple sclerosis are mostly unidentified. Although transmigration of activated encephalitogenic T cells across the blood–brain barrier (BBB) is a crucial step in the disease pathogenesis of CNS autoimmunity, the consequences on brain endothelial barrier integrity upon interaction with such T cells and subsequent lesion formation and distribution are largely unknown. We made use of a transgenic spontaneous mouse model of CNS autoimmunity characterized by inflammatory demyelinating lesions confined to optic nerves and spinal cord (OSE mice). Genetic ablation of a single immune-regulatory molecule in this model [i.e., B7-homolog 1 (B7-H1, PD-L1)] not only significantly increased incidence of spontaneous CNS autoimmunity and aggravated disease course, especially in the later stages of disease, but also importantly resulted in encephalitogenic T-cell infiltration and lesion formation in normally unaffected brain regions, such as the cerebrum and cerebellum. Interestingly, B7-H1 ablation on myelin oligodendrocyte glycoprotein-specific CD4+ T cells, but not on antigen-presenting cells, amplified T-cell effector functions, such as IFN-γ and granzyme B production. Therefore, these T cells were rendered more capable of eliciting cell contact-dependent brain endothelial cell dysfunction and increased barrier permeability in an in vitro model of the BBB. Our findings suggest that a single immune-regulatory molecule on T cells can be ultimately responsible for localized BBB breakdown, and thus substantial changes in lesion topography in the context of CNS autoimmunity.
Multiple sclerosis (MS) is the most common chronic inflammatory demyelinating disease of the CNS. Disease pathogenesis is initiated by peripheral activation of autoimmune T lymphocytes by yet unknown mechanisms, followed by T-cell expansion and subsequent migration across the complex structure of the blood–brain barrier (BBB). Within the CNS, entry of this first wave of T cells elicits recruitment of other immune cells, which together evoke a local inflammatory process ultimately resulting in demyelination, as well as axonal and neuronal damage (1). Several histological MS subtypes have been described with regard to lesion distribution and cellular composition (2). The reasons underlying distinct lesion development at different anatomical sites still remain however largely elusive: Some authors have proposed that the nature and expression pattern of the target autoantigen might play a role (3, 4). Others have observed an influence of the HLA complex and its role in shaping antigen presentation, thus suggesting that T-cell antigen specificity might impact the location of inflammation (5). Additionally, T-cell polarization into distinct T helper subtypes, as well as their expression pattern of chemokine receptors and adhesion molecules, has been implicated in determining the localization of inflammatory lesions within the CNS (6–8).
With respect to the very onset of lesion development, imaging techniques have revealed that a local dysregulation of the BBB integrity already precedes lesion formation (9, 10). Moreover, in a recent study, Maggi et al. observed an association between early changes in BBB permeability and perivascular inflammatory cuffing (11). However, the mechanisms causing local BBB dysfunction as an initial step in MS lesion formation still remain to be resolved.
Animal models of CNS autoimmunity, especially the models of experimental autoimmune encephalomyelitis (EAE), have been of great value in elucidating crucial steps in disease pathogenesis. Up to now, a broad range of different models with distinct features have been available, the most commonly used of them induced by immunization of susceptible rodents with myelin antigens or adoptive transfer of highly activated myelin-reactive T cells. However, these models are of limited value for studying the very first steps of disease initiation and lesion development, mainly due to their dependence on microbial adjuvants for artificial breakdown of tolerance and promotion of BBB disruption (12–14). During the last years, novel models of spontaneous CNS autoimmunity have been developed by crossing myelin-specific T-cell receptor (TCR) transgenic mice and myelin-specific Ig heavy chain knock-in mice (3, 15). The offspring of those mice are characterized by spontaneous development of a severe form of EAE. Lesions here are confined to the spinal cord and optic nerves, making this model an interesting tool for further investigation of the determinants of lesion development and lesion distribution under “homeostatic”, non–vaccine-depending immune-regulatory conditions.
We investigated the hypothesis whether and how genetic modification of single immune-regulatory molecules could influence lesion distribution and phenotype variation in genetically susceptible hosts. We thus used the opticospinal EAE (OSE) model and genetically modulated an immune-regulatory molecule, B7-homolog 1 (B7-H1). Absence of B7-H1 increased incidence of spontaneous CNS autoimmunity and aggravated disease course. Importantly, B7-H1 ablation on T cells, but not on antigen-presenting cells (APCs), enhanced their capacity to elicit brain endothelial cell (EC) dysfunction in a cell contact-dependent fashion in an in vitro model of the BBB and increased BBB permeability both in vitro and in vivo. Our findings suggest that single immune regulatory molecules that alter the activation status of encephalitogenic T cells can influence lesion distribution, which is associated with an altered capacity of these cells to elicit transient focal EC dysfunction and barrier dysfunction as essential steps for lesion development.
Results
Ablation of B7-H1 Increases Disease Incidence and Severity in OSE Mice Associated with Altered Lesion Distribution.
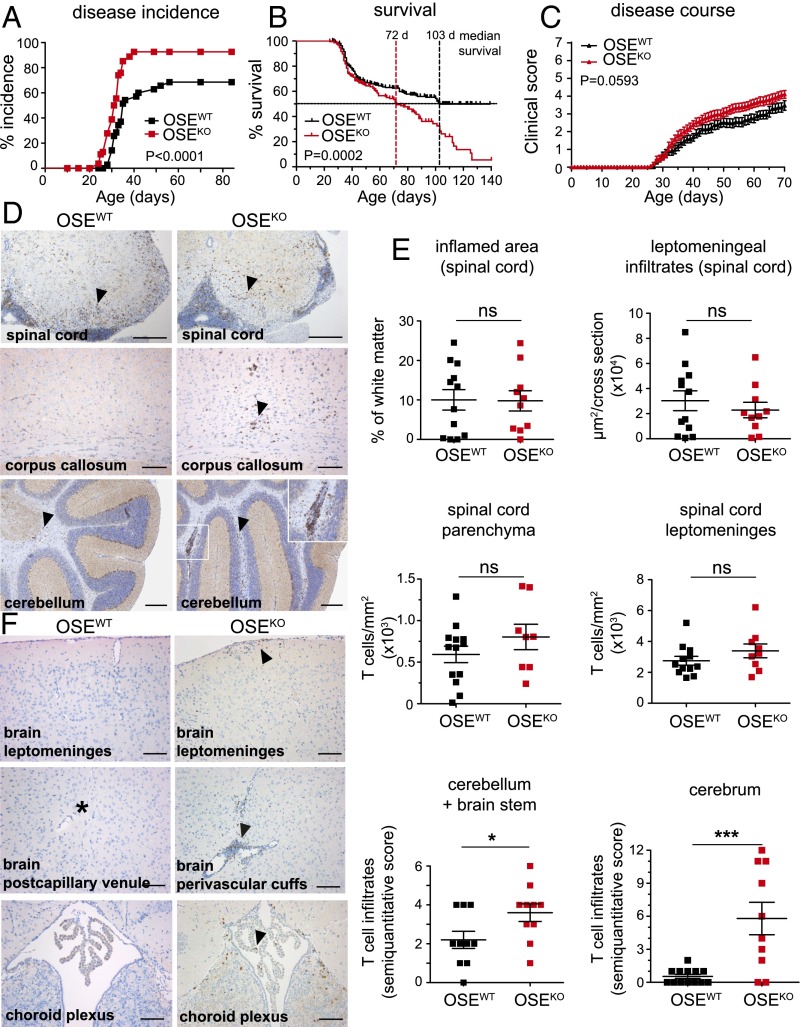
To assess the relevance of B7-H1 in the context of spontaneous CNS autoimmunity, we generated mice that both lack B7-H1 on all cells and harbor myelin oligodendrocyte glycoprotein (MOG)-specific T-cell receptor (TCR) transgenic T cells as well as MOG-specific Ig heavy-chain transgenic B cells on a C57BL/6 background (B7-H1KO × TCRMOG × IgHMOG, for simplification named OSEKO). As described in the literature, double transgenic TCRMOG × IgHMOG mice (here named OSEWT mice) develop spontaneous inflammatory demyelinating lesions in the spinal cord and the optic nerve, with an average disease incidence of ∼50 to 70% (3). Strikingly, additional lack of B7-H1 in these mice resulted in a highly significant increase in disease incidence, resulting in a nearly 100% disease penetrance (93% in OSEKO mice compared with 69% in OSEWT mice) (Fig. 1A). Moreover, OSEKO mice exhibited an aggravated disease course, especially in later disease stages, as reflected by a highly significant change in the median survival from 103 d in OSEWT mice to 72 d in OSEKO mice (Fig. 1B). These profound differences in disease severity have been observed in two independent cohorts of mice encompassing more than 215 mice per group (Fig. S1 and Table S1). However, early disease course was only slightly aggravated in OSEKO mice (Fig. 1C). Interestingly, quantitative histological analysis of spinal cord infiltrates from score-matched 12 OSEWT mice and 10 OSEKO mice (mean score 5.75) did not reveal any alterations with regard to the size of the inflamed area and leptomeningeal infiltrates, as well as T-cell numbers in spinal cord leptomeninges and parenchyma (Fig. 1 D and E). In contrast to that finding, detailed histological analysis of other brain regions classically spared in this animal model (3) revealed a significant increase in T-cell infiltrates within the cerebellum and brainstem regions in OSEKO mice compared with OSEWT mice and the presence of T-cell infiltrates in the cerebrum of 8 out of 10 OSEKO mice compared with 1 out of 12 OSEWT mice (Fig. 1E, Bottom). Together, these data indicate that OSEKO mice exhibit an enhanced disease severity at later stages, resulting in increased mortality combined with inflammatory lesion development in regions normally spared in this model.
Fig. 1.
B7-H1 restricts spontaneous CNS autoimmunity and prevents T-cell infiltration into the brain of sick OSE mice. (A) Percentages of OSEWT and OSEKO mice that spontaneously developed EAE symptoms over a period of 12 wk after birth. Forty-five OSEWT and 57 OSEKO mice were analyzed. (B) Survival of 246 OSEWT and 216 OSEKO mice was monitored over a period of 140 d after birth. Mice were recorded as dead when they reached the clinical score 5.5 or higher; mice eliminated for reasons unrelated to disease course (all with score below 5.5) were censored (indicated by vertical ticks). (C) Clinical scores of 18 OSEWT and 18 OSEKO mice until 70 d of age. (D–F) Immunohistochemical analysis of T-cell infiltration in different CNS regions of 12 OSEWT and 10 OSEKO mice (score-matched, mean clinical score 5.75). (D) Representative images of T-cell infiltrates in the spinal cord, corpus callosum, and cerebellum, as indicated. (E) Quantification of the extent of inflammation and T-cell infiltration in the spinal cord parenchyma and leptomeninges, as indicated (Top and Middle); semiquantitative scores of T-cell infiltration in the cerebellum and brainstem, and in the cerebrum (Bottom). (F) Representative images of T-cell infiltration in different brain areas of OSEWT and OSEKO mice. (D and F) T cells are indicated by arrowheads. The asterisk in F indicates a postcapillary venule. (Scale bars: D, spinal cord, 200 μm; corpus callosum, 100 μm; cerebellum, 200 μm; F, 100 μm.) (E) Each data point represents one mouse. (A and B) Log-rank (Mantel–Cox) test. (C) Two-way ANOVA with Bonferroni correction for multiple comparisons. (E) Unpaired, two-tailed Student’s t test. *P < 0.05; ***P < 0.001; ns, not significant.
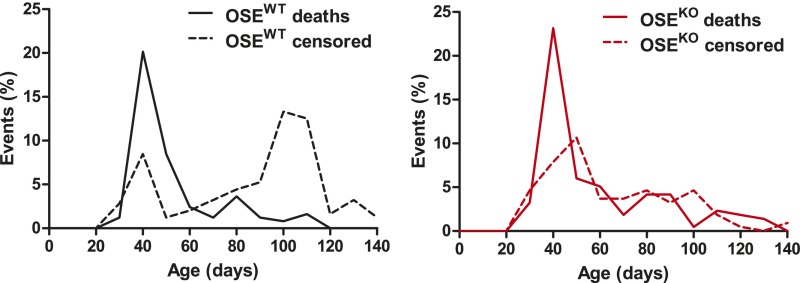
Fig. S1.
B7-H1 prolongs survival of OSE mice. Survival of OSEWT and OSEKO mice was monitored over a period of 140 d after birth. Mice were recorded as dead when clinical score 5.5 or higher was reached; mice eliminated for reasons unrelated to disease course (all with score below 5.5) were censored. In the OSEWT group, 246 mice were analyzed (102 deaths, 144 censored); in the OSEKO group, 216 mice were analyzed (116 deaths, 100 censored). Distribution of death and censored events over the observed period is shown, grouped by genotype.
Table S1.
B7-H1 limits spontaneous EAE incidence, ameliorates disease severity, and prolongs survival of OSE mice
| Cohort | OSEWT | OSEKO | P value |
| Cohort I | |||
| Total no. of mice | 45 | 57 | |
| Disease incidence | 31/45 (69%) | 53/57 (93%) | <0.0001* |
| Mean age of onset, d | 35.8 ± 7.1 | 31.2 ± 3.9 | <0.0001† |
| Mean clinical score (±SD) | 2.4 ± 1.7 | 3.7 ± 1.0 | <0.0001† |
| Cohort II | |||
| Total no. of mice | 246 (88 litters) | 216 (66 litters) | |
| No. of females | 134 | 100 | |
| No. of males | 112 | 116 | |
| Median survival (total), d | 103 | 72 | 0.0002* |
| Median survival (females), d | 126 | 88 | 0.0153* |
| Median survival (males), d | 103 | 59 | 0.0026* |
| Deaths (total) | 102 (41%) | 116 (54%) | 0.0890‡ |
| Censored (total) | 144 (59%) | 100 (46%) |
Log-rank (Mantel–Cox) test.
Unpaired, two-tailed Student’s t test.
Fisher’s exact test.
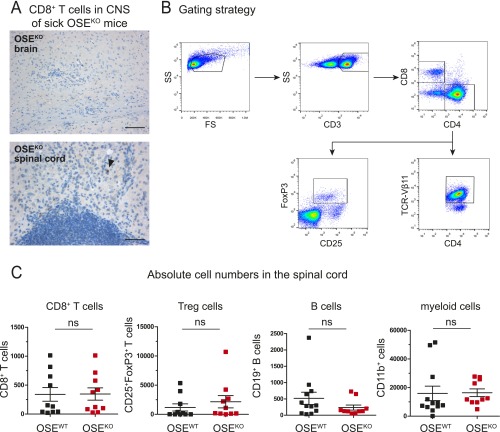
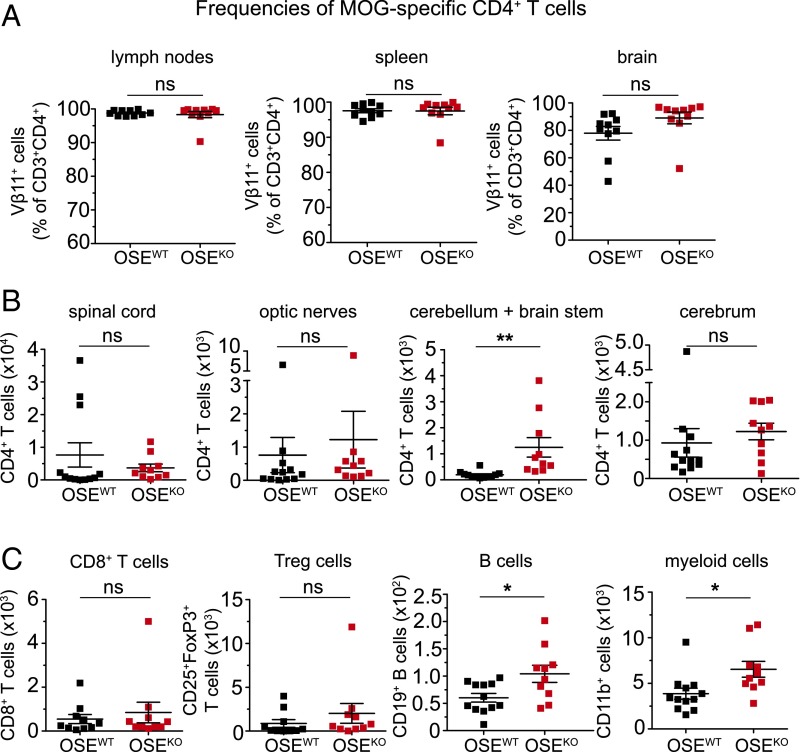
A detailed analysis of these brain infiltrates in OSEKO mice further revealed only a few T cells within the choroid plexus and leptomeninges and a clear predominance of T-cell infiltrates within the perivascular areas (Fig. 1F). These T-cell infiltrates did not contain CD8+ T cells (Fig. S2A). Our histological findings were accompanied by significantly increased numbers of CNS infiltrating CD4+ T cells in the cerebellum and brainstem of OSEKO compared with OSEWT mice. This finding was also determined by quantitative flow cytometry at the very early stage of disease (Fig. 2B) whereas CD4+ T-cell numbers in spinal cord and optic nerves—the classical predilection sites of CNS inflammation in this model—were again not different between OSEKO and OSEWT mice at this time point (Fig. 2B). There was a trend toward increased CD4+ T-cell numbers in the cerebrum of OSEKO mice compared with OSEWT mice; however, this trend did not reach statistical significance, probably due to the overall low number of isolated immune cells from this large brain area. Of note, frequencies of MOG-specific transgenic CD4+ T cells were not different in OSEKO mice compared with OSEWT mice, and these transgenic T cells accounted for more than 90% of all CD4+ T cells in both strains (Fig. 2A). Further flow cytometric analysis of cerebellum and brainstem areas, as well as spinal cords, revealed no differences in numbers of infiltrating CD8+ T cells and FoxP3+ regulatory T cells but a slight increase in B cells and myeloid cells in the cerebellum/brainstem of OSEKO mice, without any differences in spinal cords (Fig. 2C and Fig. S2C). Together, these data suggest that altered lesion topography in OSEKO mice is driven by MOG-specific CD4+ T cells, with a concomitant rise in other non–T-cell subpopulations, such as B cells and myeloid cells.
Fig. S2.
Immune cell infiltration in OSE mice. (A) Immunohistochemical analysis of CD8+ T-cell infiltration in the brain and spinal cord parenchyma of sick OSEKO mice (mean clinical score 6). [Scale bars: Upper, 100 µm (for the brain); Lower, 50 µm (for the spinal cord).] Arrowhead indicates CD8+ T cell. Representative images of four OSEKO mice are shown. (B and C) Flow cytometric analysis of different immune cell types in the periphery and CNS of age-matched OSE mice (mean age 32 d). (B) Gating strategy for the lymph nodes is shown. (C) Absolute numbers of infiltrating immune cells (as indicated) in the spinal cord, determined using cell-counting beads. Each data point represents one mouse. Data show mean ± SEM. Unpaired, two-tailed Student’s t test. ns, not significant.
Fig. 2.
B7-H1 limits T-cell infiltration in the cerebellum and brainstem of OSE mice at disease onset. (A) Frequencies of MOG-specific CD4+ T cells (percent of CD3+CD4+) in the lymph nodes, spleen, and brain, as determined by flow cytometry. (B) Absolute numbers of CD4+ T cells in different CNS regions of OSEWT and OSEKO mice at disease onset (mean age 28 d), as determined by flow cytometry and using cell-counting beads. (C) Absolute numbers of immune cells (as indicated) in the cerebellum and brainstem of OSEWT and OSEKO mice at disease onset (mean age 32 d), determined by flow cytometry and using cell-counting beads. Each data point represents one mouse. Unpaired, two-tailed Student’s t test. *P < 0.05; **P < 0.01; ns, not significant.
Ablation of B7-H1 on T Cells Augments T-Cell Effector Functions.
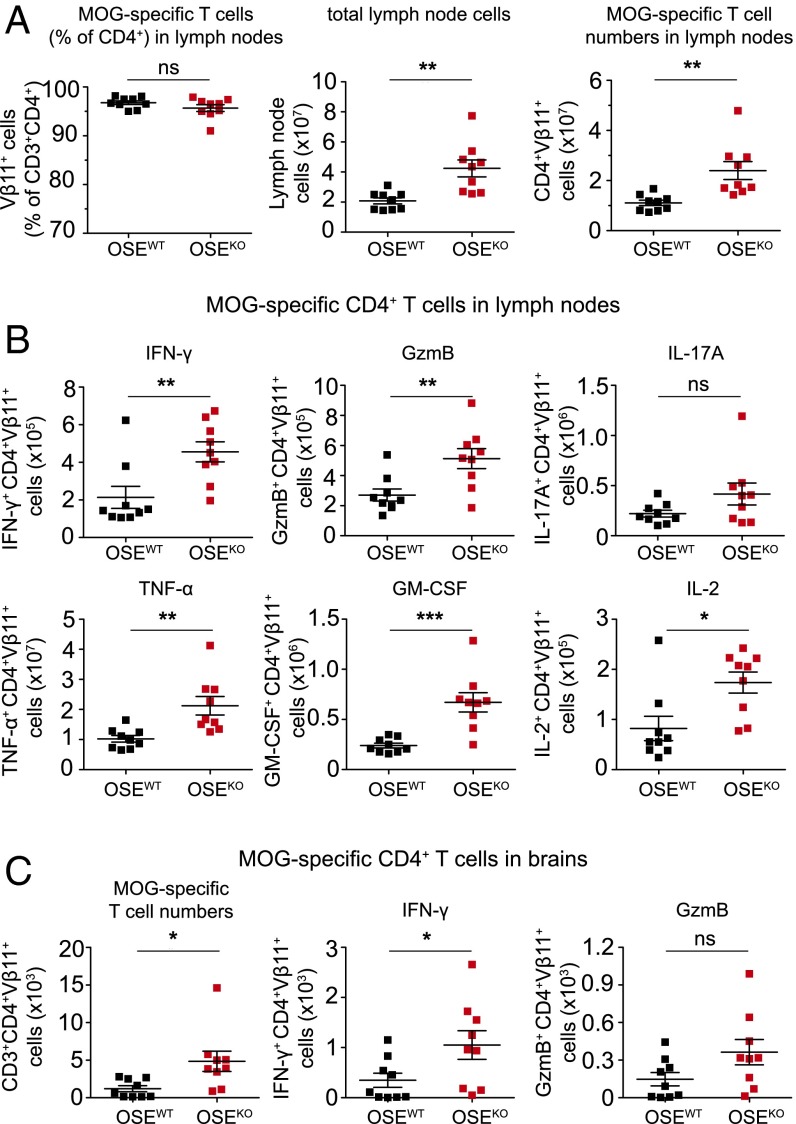
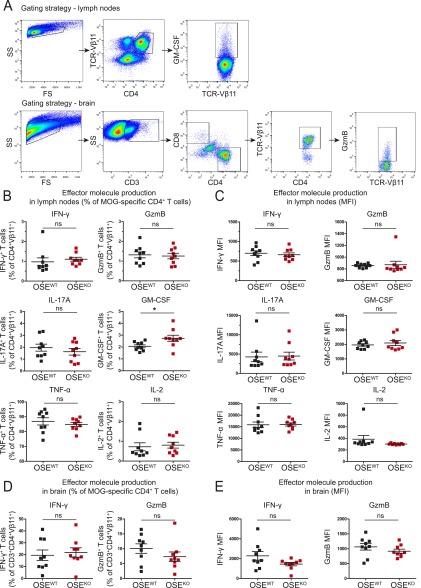
Based on our findings so far, we next characterized MOG-specific CD4+ T-cell responses in the periphery of OSEKO compared with OSEWT mice with established disease (score-matched mice with a mean score of 4). Also, in these score-matched mice, the frequency of MOG-specific transgenic T cells within the CD4+ T-cell population was not altered (Fig. 3A). However, diseased OSEKO mice exhibited significantly increased lymph node cell numbers, as well as absolute numbers of MOG-specific CD4+ T cells within lymph nodes, compared with OSEWT mice (Fig. 3A), suggesting a pronounced expansion of autoreactive T cells in the peripheral immune compartment of OSE mice lacking the immune-inhibitory molecule B7-H1. Along these lines, absolute numbers of cytokine and granzyme B-producing MOG-specific CD4+ T cells were significantly increased in the lymph nodes of OSEKO compared with OSEWT mice (Fig. 3B), which was observed for a broad range of effector molecules (including IFN-γ, granzyme B, TNF-α, GM-CSF, IL-2, and IL-17A), indicating that there is no shift in T helper-cell polarization in OSEKO mice (Fig. 3B). Percentages of effector molecule-producing MOG-specific T cells within the CD4+ T-cell population, as well as effector molecule production on a per cell basis assessed by mean fluorescence intensity (MFI), were not altered in OSEKO mice (Fig. S3 B and C).
Fig. 3.
B7-H1 inhibits expansion of cytokine-producing MOG-specific CD4+ T cells in lymph nodes and brains of sick OSE mice. (A) Frequencies and numbers of MOG-specific CD4+ T cells in lymph nodes of OSEWT and OSEKO mice with established disease (score-matched, mean clinical score 4) were analyzed. (Left) Frequencies of MOG-specific CD4+ T cells in lymph nodes, as determined by flow cytometry. (Center) Absolute cell numbers of total lymph nodes as determined by Neubauer cell-counting chamber. (Right) Absolute numbers of MOG-specific CD4+ T cells within total lymph nodes as determined by flow cytometry. (B and C) Flow cytometric analysis of MOG-specific CD4+ T cells producing effector molecules in total lymph nodes (B) and brains (C) of sick OSE mice (as described in A), after 4 h of ex vivo restimulation with PMA and ionomycin. Absolute numbers of brain-derived MOG-specific CD4+ T cells (C, Left), as well as numbers of IFN-γ and GzmB producing MOG-specific T cells (Center and Right). Each data point represents one mouse. Unpaired, two-tailed Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Fig. S3.
Effector molecule production per cell by MOG-specific CD4+ T cells is not limited by B7-H1 in the periphery and brain of sick OSE mice. (A) Gating strategy for flow cytometric analysis of lymph nodes and brains of score-matched OSE mice (mean clinical score 4). Examples of GM-CSF production in the lymph nodes (Top) and GzmB production in the brain (Bottom) by MOG-specific CD4+ T cells are shown. (B–E) Quantification of effector molecule production after 4 h of ex vivo restimulation with PMA, ionomycin, and Golgi Plug. (B and C) Production of IFN-γ, GzmB, IL-17A, GM-CSF, TNF-α, and IL-2 in the lymph nodes expressed as percent of MOG-specific CD4+ T cells (B) and MFI (C). (D and E) Production of IFN-γ and GzmB in the brain, expressed as percent of MOG-specific CD4+ T cells (D) and MFI (E). Each data point represents one mouse. Data show mean ± SEM. Unpaired, two-tailed Student’s t test. *P < 0.05; ns, not significant.
Along these lines, we also observed an increase in absolute numbers of MOG-specific CD4+ T cells in brains of score-matched OSEKO compared with OSEWT mice (Fig. 3C) and, accordingly, an increase in absolute numbers of MOG-specific T cells expressing IFN-γ and granzyme B (Fig. 3C) whereas percentages of transgenic T cells producing these effector molecules were again unchanged (Fig. S3 D and E). Together, these data indicate that OSEKO mice exhibit enhanced quantitative but not qualitative MOG-specific CD4+ effector T-cell responses both in the periphery and in the brain. These differences were not caused by differences in disease severity because score-matched mice were used for analysis.
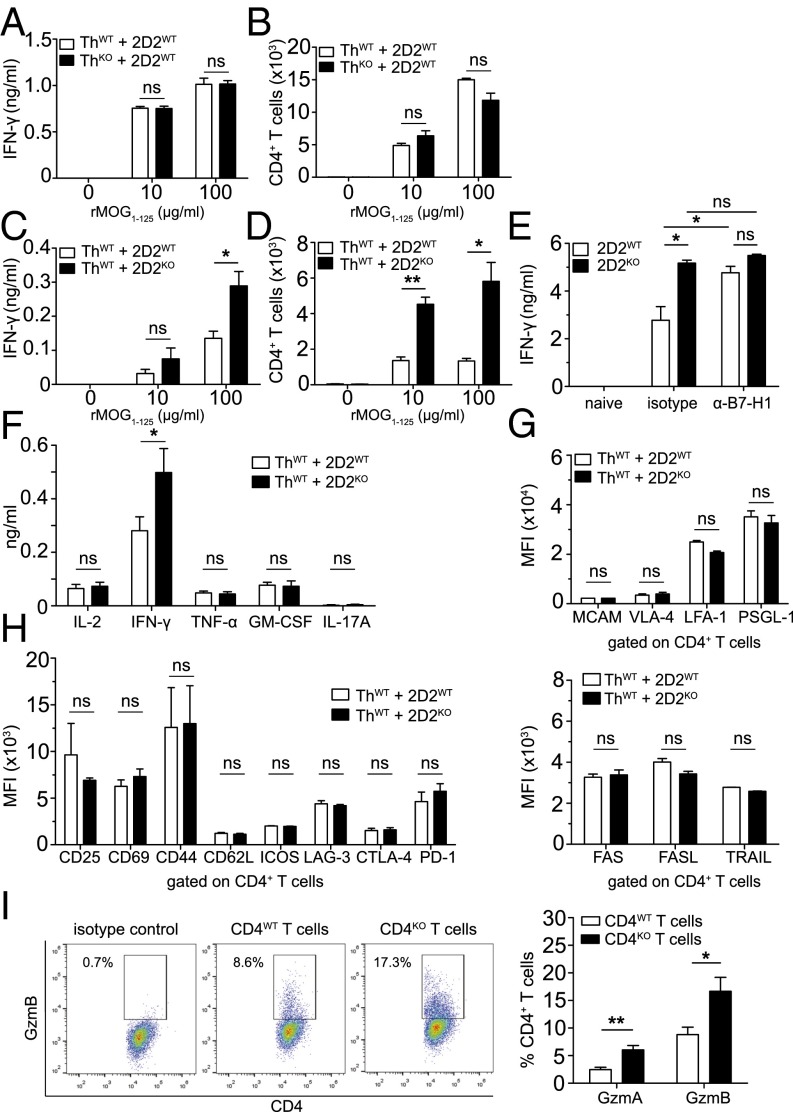
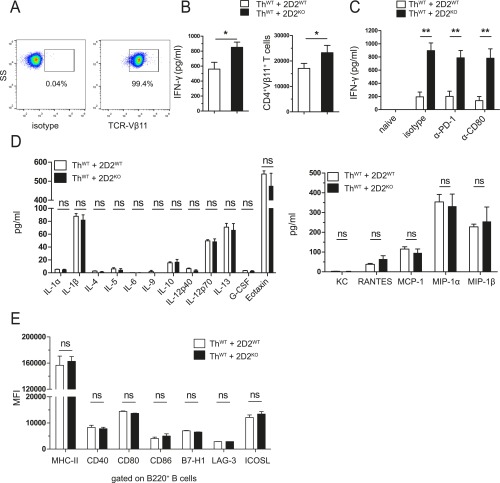
We next went on to evaluate how lack of B7-H1 might affect initial T-cell activation as a first putative step in disease pathogenesis. Based on the well-known role of B7-H1 on APCs in control of T-cell activation and T-cell–mediated autoimmunity (16–18), we performed coculture assays using MOG-specific B cells from Th mice as APCs and MOG-specific CD4+ T cells from 2D2 mice. Surprisingly, lack of B7-H1 on antigen-presenting B cells did not result in enhanced T-cell activation as evaluated by quantification of IFN-γ production (Fig. 4A) and T-cell expansion (Fig. 4B). In sharp contrast, lack of B7-H1 on T cells resulted in significantly enhanced T-cell responses reflected by increased IFN-γ production as well as augmented T-cell expansion (Fig. 4 C and D and Fig. S4B). Of note, WT T cells also displayed an enhanced IFN-γ production in the presence of a neutralizing antibody against B7-H1 (Fig. 4E). Evaluation of other key proinflammatory cytokines revealed that increased cytokine production by B7-H1KO T cells was basically confined to IFN-γ because levels of IL-2, TNF-α, GM-CSF, and IL-17A remained unchanged, thus indicating that also in vitro there was no shift in T helper-cell polarization in the absence of B7-H1 (Fig. 4F and Fig. S4D). Moreover, detailed analysis of a broad range of T-cell activation markers, as well as costimulatory molecules, did not reveal any differences between naïve, as well as activated, B7-H1KO T cells compared with WT T cells (Fig. 4 G and H and Fig. S4D). Along these lines, the activation status of B cells upon interaction with either B7-H1WT or B7-H1KO T cells was not differentially affected (Fig. S4E). In contrast, activated B7-H1KO T cells produced significantly increased numbers of cytotoxic molecules, such as granzyme A and granzyme B, compared with WT T cells (Fig. 4I). Taken together, lack of B7-H1 on T cells results in enhanced proinflammatory and lytic effector functions such as IFN-γ and granzyme A and B production upon T-cell activation.
Fig. 4.
B7-H1 on MOG-specific T cells, but not on MOG-specific B cells, limits antigen-specific T-cell activation. (A and B) Coculture of MOG-specific 2D2 T cells and MOG-specific antigen-presenting Th B cells in the presence or absence of recombinant MOG1–125 protein for 4 d; antigen-presenting B cells either express B7-H1 (ThWT) or lack B7-H1 (ThKO). (A) IFN-γ production as determined by ELISA. (B) T-cell expansion at the end of coculture as determined by flow cytometry using cell-counting beads. (C and D) Coculture of MOG-specific 2D2 T cells and MOG-specific antigen-presenting Th B cells in the presence or absence of recombinant MOG1–125 protein for 4 d; antigen-specific T cells either express B7-H1 (2D2WT) or lack B7-H1 (2D2KO) (analysis as described above). (E) IFN-γ production (ELISA) by MOG-specific 2D2WT or 2D2KO CD4+ T cells after stimulation with precoated α-CD3 and soluble α-CD28 (both at 1 μg/mL) for 3 d in the presence of a blocking α-mouse B7-H1 antibody (added at 40 µg/mL each day) or the corresponding isotype control. (F–H) Cytokine production and expression of various T-cell markers on 2D2WT and 2D2KO T cells after coculture with ThWT B cells in the presence of MOG35–55 (20 μg/mL). (F) Cytokine production was analyzed after 5 d. (G) Expression levels of cell adhesion molecules and (H) T-cell activation markers, costimulatory and coinhibitory molecules, and apoptosis-inducing molecules on CD4+ T cells were analyzed by flow cytometry after 3 d (except for CD69—after 1 d). (I) Flow cytometric analysis of granzyme A- and granzyme B-producing CD4WT and CD4KO T cells. T cells were polyclonally stimulated with precoated α-CD3 and soluble α-CD28 (both at 1 μg/mL) for 2 d. (A–D) Results show technical triplicates and are representative of five independent experiments for each set-up. (E) Data show one representative experiment (five mice per genotype) out of three independent experiments. (F) Supernatants from at least nine coculture experiments were used (except for GM-CSF—from five coculture experiments). (G and H) Pooled results from at least two independent experiments. (I) Five mice per genotype were used. Unpaired, two-tailed Student’s t test. *P < 0.05; **P < 0.01; ns, not significant.
Fig. S4.
B7-H1 on T cells does not limit cytokine and chemokine production or B-cell marker expression in vitro. (A–C) For cocultures with ThWT B cells, MOG-specific CD4+ T cells from 2D2WT and 2D2KO mice were isolated using biotinylated α-mouse TCR-Vβ11 antibody and α-biotin microbeads. (A) Representative dot plots of MOG-specific CD4+ T cells after isolation (>97% TCR-Vβ11+). (B) IFN-γ production (Left) and expansion (Right) of MOG-specific CD4+ T cells from 2D2WT and 2D2KO mice, after 4 d of coculture with ThWT B cells in the presence of MOG35–55 (20 µg/mL). Absolute numbers of proliferated cells were determined by flow cytometry, using cell-counting beads. (C) IFN-γ production from cocultures as described in B, in the presence of blocking α-mouse PD-1 and CD80 antibodies (added at 40 µg/mL each day) or the isotype control. (D) Levels of cytokines and chemokines in the supernatants from cocultures of ThWT B cells and 2D2WT or 2D2KO T cells in the presence of MOG35–55 (20 µg/mL) for 5 d, as determined by Bio-Plex Mouse Cytokine 23-plex Assay. (E) MFI of costimulatory and coinhibitory molecules on ThWT B cells cocultured with 2D2WT or 2D2KO T cells in the presence of MOG35–55 (20 µg/mL) for 1 to 3 d. Flow cytometric results for CD40, CD80, and CD86 show expression levels after 1 d, and for B7-H1, LAG-3, and ICOSL after 3 d of coculture. (B and C) Five 2D2WT and five 2D2KO mice were used. (D) Nine mice per genotype were used. (E) Pooled results from three independent experiments (n = 3) are shown (except for MHC-II and CD80, n = 5). Data show mean ± SEM. Unpaired, two-tailed Student’s t test. *P < 0.05, **P < 0.01, ns, not significant.
Lack of B7-H1 on T Cells Augments Their Capacity to Elicit Brain Endothelial Barrier Dysfunction and Increased Permeability.
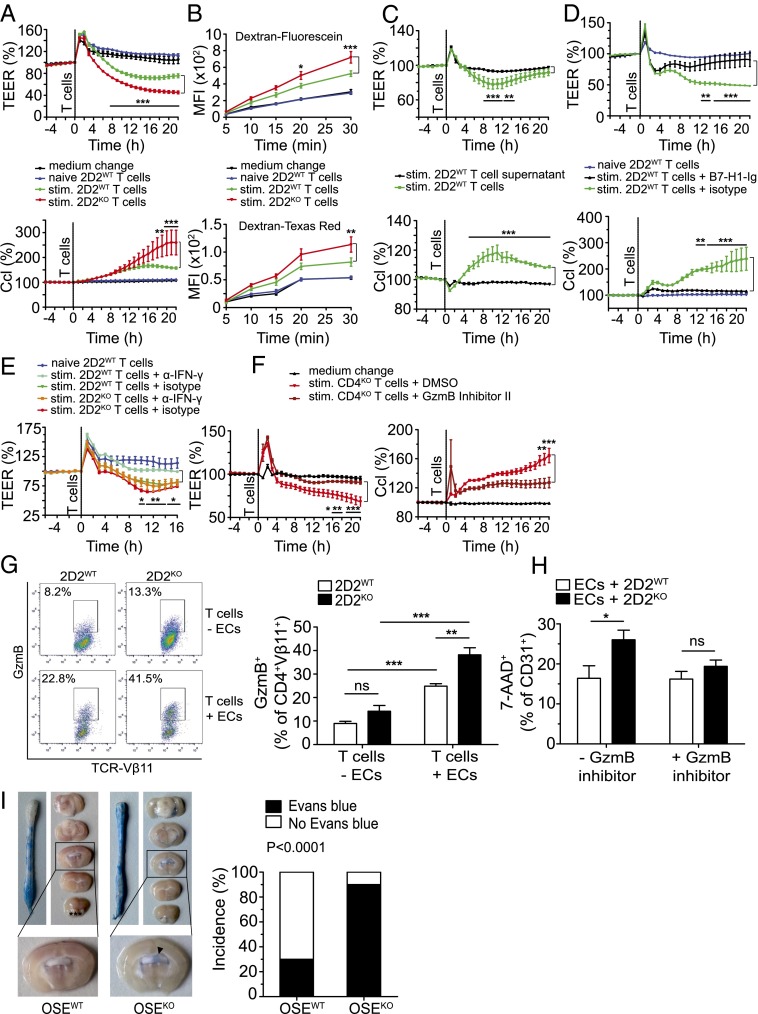
After having demonstrated that lack of B7-H1 doesn’t alter peripheral T-cell polarization but increases T-cell effector functions, we asked whether B7-H1KO T cells might exhibit an enhanced capacity to penetrate the endothelial barrier compared with B7-H1–competent T cells. To this end, we investigated whether B7-H1 ablation on T cells affects mouse brain microvascular endothelial cell (MBMEC) barrier properties upon coculture of brain endothelial cells with T cells by assessing changes in transendothelial electrical resistance (TEER). First, TEER was not affected upon interaction of naive CD4+ T cells with brain endothelial cells. Activated T cells, however, exhibited a distinct capacity to affect barrier integrity, as reflected by a significant decrease in TEER (Fig. 5A). Importantly, this drop in TEER was significantly more pronounced upon interaction with B7-H1KO T cells (Fig. 5A), which was associated with a persistent increase in capacitance (Fig. 5A), as well as enhanced MBMEC monolayer permeability to small (dextran-fluorescein) as well as larger molecules (dextran-Texas Red) (Fig. 5B), thus illustrating a relevant decrease in endothelial barrier integrity. Further experiments revealed that T-cell–endothelial cell contact is required and decisive for this effect because supernatants derived from activated CD4+ T cells were not sufficient to affect TEER and capacitance in this setup (Fig. 5C). Delivery of a strong immune-inhibitory signal to activated CD4+ T cells via use of a B7-H1–Ig fusion protein (16) was capable of abrogating T-cell–mediated disturbance of endothelial barrier integrity (Fig. 5D), suggesting that the strength of T-cell activation, shaped by the balance between costimulatory and coinhibitory signals, directly influences their capacity to impair endothelial barrier function upon contact.
Fig. 5.
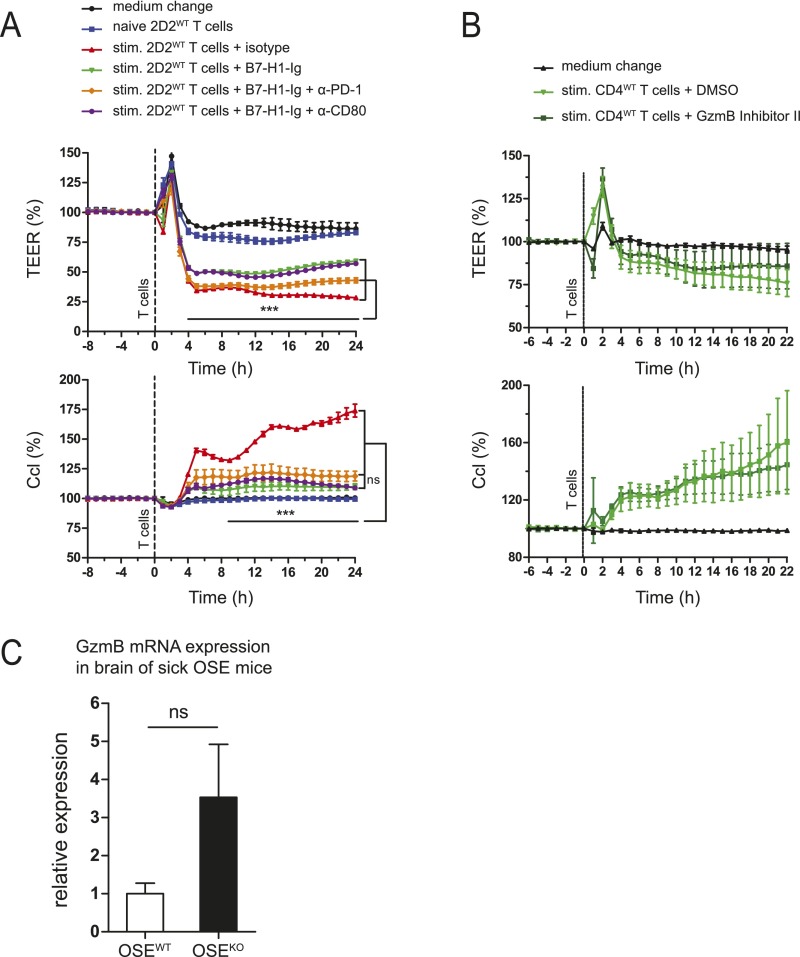
B7-H1 on CD4+ T cells restricts T-cell–mediated brain endothelial cell dysfunction, at least partly via inhibition of granzyme B function, and limits cerebral BBB permeability in vivo. (A and C–F) TEER measurements were performed by coculturing MBMECs (2 × 104 per well) with T cells (0.2 × 106 per well), their supernatants, or α–IFN-γ antibody, as indicated; TEER and Ccl were measured every hour for 1 d. (A) TEER measurement with 2D2WT or 2D2KO T cells, naive or preactivated by ThWT B cells and MOG35–55 (20 μg/mL) for 5 d. (B) Permeability of MBMECs to dextran-fluorescein and dextran-Texas Red using samples from A after TEER measurement. (C) TEER measurement with stimulated 2D2WT T cells or their supernatants. T cells were preactivated as in A. (D) TEER measurement with 2D2WT T cells, preactivated with precoated α-CD3 and soluble α-CD28 (both 1 μg/mL), in the presence of B7-H1–Ig fusion protein (10 μg/mL) or the corresponding isotype control, for 2 d. (E) TEER measurement with MOG-activated 2D2WT or 2D2KO T cells, in the presence of neutralizing α-IFN-γ antibody (20 μg/mL) or the corresponding isotype control, during the coculture of T cells and MBMECs. (F) TEER measurement with CD4KO T cells, preactivated as in D in the presence of granzyme B inhibitor II (10 μM) or its diluent DMSO. (G) Flow cytometric analysis of GzmB production by 2D2WT or 2D2KO T cells (%), alone or cocultured with MBMECs. T cells were stimulated with precoated α-CD3 and soluble α-CD28 (both at 1 μg/mL) for 3 d. Next, T cells were harvested and cultured either alone or with MBMECs for another 24 h. (H) Flow cytometric analysis of 7-AAD+ CD31+ MBMECs after 24 h of coculture with 2D2WT or 2D2KO T cells (stimulated as in G). T cells were cultured in the presence of GzmB inhibitor II (10 µM) or DMSO, added at the beginning of T-cell stimulation. (I) BBB leakage in brains and spinal cords of score-matched OSEWT and OSEKO mice (n = 10; mean clinical score 6), as visualized by Evans Blue dye. (I, Right) Incidence of mice (%) with Evans Blue leakage in the supratentorial brain regions. (A–E) Results show technical triplicates and are representative of three independent experiments each. (F) Results show three biological replicates and are representative of two independent experiments. (G and H) Five mice per genotype were used for each setup. (A–F) Two-way ANOVA with Bonferroni correction for multiple comparisons. (G and H) Unpaired, two-tailed Student’s t test. (I) Fisher’s exact test. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Further experiments revealed that neutralization of IFN-γ was partially effective in restoring TEER upon interaction with activated CD4+ T cells. This restoration was even less pronounced using B7-H1KO T cells (Fig. 5E), indicating that IFN-γ is involved but doesn’t act as the crucial part of T-cell–mediated endothelial cell dysfunction. In sharp contrast, interference with granzyme function using granzyme B inhibitor II was capable of completely restoring TEER in the presence of both WT and B7-H1KO T cells (Fig. 5F and Fig. S5B), indicating that these activated T cells indeed elicit endothelial barrier dysfunction via granzyme-mediated, cell contact-dependent endothelial barrier damage in vitro. Following this line, we wanted to further address the link between granzyme B production by activated CD4+ T cells and endothelial cell damage by performing coculture assays of preactivated CD4+ T cells and MBMECs. Interestingly, granzyme B production by CD4+ T cells was significantly enhanced upon coculture with brain endothelial cells, and this increase was much more pronounced in 2D2KO T cells compared with 2D2WT T cells (Fig. 5G). What is more, 2D2KO T cells displayed a greater capacity to elicit endothelial cell death than 2D2KO T cells, and this difference was completely abrogated in the presence of granzyme B inhibitor II (Fig. 5H). These data indicate that, indeed, activated CD4+ T cells can produce functionally relevant amounts of granzyme B—especially in the presence of endothelial cells—to cause EC death and that this production is modulated by B7-H1 on T cells.
Fig. S5.
B7-H1 limits T-cell–mediated brain endothelial cell dysfunction, which is at least partly caused by granzyme B production. (A) MOG-specific CD4+ T cells from 2D2WT mice were stimulated with precoated α-CD3 and soluble α-CD28 (both at 1 µg/mL) for 2 d, in the presence of B7-H1–Ig (precoated for 3 h, at 10 µg/mL) or its isotype control. In addition, blocking α-mouse PD-1 and CD80 antibodies were added each day at 40 µg/mL as indicated, during both T-cell stimulation and the subsequent coculture of harvested T cells with MBMECs. Changes in TEER and Ccl during 24 h of the coculture are shown. (B) Changes in TEER and Ccl upon coculture of MBMECs with CD4WT T cells. T cells were stimulated as described in A, in the presence of granzyme B inhibitor II (10 µM) or its diluent DMSO. (C) Granzyme B mRNA expression in brains of score-matched OSE mice (mean clinical score 4). Brain mononuclear cells were isolated using discontinuous Percoll gradient, and GzmB mRNA levels were determined by RT-qPCR. Then, 18S rRNA was used as endogenous control. (A and B) Technical triplicates are shown. Results are representative of two independent experiments. (C) Five OSEWT and five OSEKO mice were used. Data show mean ± SEM. (A and B) Two-way ANOVA with Bonferroni correction for multiple comparisons. (C) Unpaired, two-tailed Student’s t test. ***P < 0.001, ns, not significant.
Lack of B7-H1 in OSE Mice Is Associated with Increased BBB Permeability in Supratentorial Brain Regions in Vivo.
Finally, we aimed to address whether B7-H1KO T cells preferentially cause blood–brain barrier breakdown in certain topographical regions: namely, supratentorial brain regions in the context of spontaneous CNS autoimmunity in vivo. We therefore evaluated blood–brain barrier integrity in 10 score-matched mice (mean score of 6) by assessing Evans Blue leakage from microvessels after intravenous injection into living mice. Indeed, whereas all animals displayed comparable Evans Blue leakage in the spinal cord, reflecting the predominance of spinal cord inflammation in this animal model, 9 out of 10 OSEKO mice, but only 3 out of 10 OSEWT mice, displayed Evans Blue leakage in supratentorial brain areas, especially the periventricular region (Fig. 5I), which further supports the notion of changes in regional encephalitogenicity of T cells lacking immune-regulatory B7-H1, thus strongly contributing to the altered lesion distribution and dissemination of CNS autoinflammation.
Discussion
Using a model system of spontaneous CNS autoimmunity, our study provides evidence that genetic deficiency of a single immune-regulatory molecule (i.e., B7-H1) in autoimmune-susceptible hosts can determine substantial changes in lesion topography critically influenced by regional breaching of the blood–brain barrier. In light of its relevance for maintenance of immune tolerance, the role of B7-H1 on APCs has been addressed in CNS autoimmunity before: In active MOG35–55-induced EAE, B7-H1 knock-out resulted in an enhanced disease severity, which was linked to B7-H1 expressed on APCs (18). Moreover, enhanced expression of B7-H1 was observed in MS lesions and attributed to macrophages/microglial cells, as well as astrocytes (18, 19). In the periphery, B7-H1 expression has been analyzed on B cells and monocytes from MS patients; here, an increased percentage of B7-H1–positive cells was found in stable versus active MS patients (20). In contrast to these studies, however, the relevance of B7-H1 on T cells has not been studied in the context of MS so far.
The advent of novel transgenic mouse models featuring spontaneous development of CNS inflammation offers the unique opportunity to study the very first steps in immune cell activation and lesion development (21, 22). This model is in contrast to conventional EAE models, where exogenous application of microbial adjuvants is indispensable for disease induction, which is known to affect the mechanisms of (auto-) immune cell activation and BBB permeability. Several hallmarks of the double-transgenic OSE mouse model used in our study deserve attention. First, autoreactive T-cell activation is triggered by MOG-presenting B cells serving as the main source of professional APCs in this particular animal model (3). Second, T-cell responses in these mice are predominantly of a TH1 phenotype, with a minor role of IL-17A production (3, 15). And, third, inflammatory demyelinating CNS lesions are confined to the spinal cord and optic nerves, but not the brain, brainstem, or cerebellum (3, 15). With regard to the first aspect, we addressed the impact of lack of B7-H1 on either APCs or CD4+ T cells by performing coculture assays of MOG-specific B cells and MOG-specific CD4+ T cells in the presence of recombinant MOG protein and observed that B7-H1 was completely dispensable on antigen-presenting B cells but augmented CD4+ T-cell expansion and effector functions when lacking on the T-cell side. The B7-H1–PD-1 pathway has received major attention over the last years as a key component of the B7-homologs involved in the regulation of immune tolerance, tumor surveillance, and autoimmunity (18, 23–25). Despite its well-known role in immune regulation by APCs via interaction with its canonical receptor PD-1 on T cells, only a few studies so far have investigated the relevance of B7-H1 on T cells: Talay et al. observed that expression of B7-H1 was required for T-cell–mediated conditioning of dendritic cell maturation in the context of infections (26). However, when evaluating classical maturation markers of APCs, such as expression of costimulatory molecules, we did not observe any difference in expression levels, at least in vitro. Another publication described in trans control of TH17 differentiation via B7-H1 expression on adjacent CD4+ T cells (27). In contrast to these findings, we did not observe a particular increase in TH17 responses due to lack of B7-H1 on T cells, either in vitro or in vivo. Instead, we here provide evidence that lack of B7-H1 on T cells boosts their expansion in vitro and promotes particular effector functions, such as production of IFN-γ and granzyme A and B. The interaction partner of B7-H1 expressed on T cells, however, remains unclear because both PD-1 and CD80 do not seem to be critically involved (Figs. S4C and S5A), which is in line with a previous publication by our group (16) and which argues in favor of a novel yet unknown receptor for B7-H1, as has also been proposed by other groups (28, 29).
Ultimately, we cannot rule out that, in our in vivo model during spontaneous CNS autoimmunity, lack of B7-H1 on non-T cells might additionally contribute to the overall phenotype of these mice. There are nonetheless several arguments that argue against a strong relevance of such a contribution in our model. Despite accumulation of MOG-specific CD4+ T cells in the periphery, we did not observe an increase in the percentages of cytokine-producing CD4+ T cells, both in lymph nodes and within the CNS of sick OSEKO vs. OSEWT mice, indicating that cytokine production on a per cell basis is not affected in these animals (Fig. S3). Moreover, absolute numbers of cytokine-producing CD4+ T cells were unaffected within the classical predilection sites of inflammatory CNS lesions in this animal model: i.e., spinal cord and optic nerves. Instead, we observed T-cell infiltrates in brain areas of OSEKO mice that are classically spared in this model, such as brainstem, cerebellum, and cerebrum, especially in the corpus callosum and periventricular regions, which at least suggests that these T cells acquired functions that are distinct from the functions of T cells derived from OSEWT mice. Further analysis of these brain infiltrates by histology and flow cytometry, respectively, revealed that these lesions predominantly consisted of MOG-specific effector CD4+ T cells, and we did not find significant numbers of CD8+ T cells or altered frequencies of FoxP3+ regulatory T cells within these infiltrates.
Several factors have been discussed to determine lesion distribution in CNS inflammation and might favor nonspinal manifestation of disease. First, the density of antigen expression in different target (CNS) areas, in conjunction with the antigen specificity of CNS-autoreactive T cells, has been suggested to influence lesion topography (3–5). However, in light of the particular animal model used in our study, we can exclude variations of antigen density or T-cell antigen specificity contributing to the observed changes in lesion topography. Second, T helper-cell polarization, especially the ratio between autoreactive TH1 and TH17 cells, has been shown to determine lesion topography in different animal models (6, 7, 30), with TH1 cells favoring typical spinal disease manifestation and TH17 cells rather favoring cerebellar and supratentorial lesion development. However, as also described before, we observed a strong predominance of TH1 responses in the OSE animal model, and, more importantly, this predominance was preserved upon additional lack of B7-H1, thus ruling out that atypical lesion topography in these mice might be associated with a preponderance of TH17 responses. Along these lines, it has been proposed that TH17 cells can serve as gatekeepers of other T cells by entering the CNS via the choroid plexus in a CCR6-dependent fashion; however, the authors of that study did not make use of a spontaneous model of CNS autoimmunity (8). We therefore investigated the potential entry route of these brain-infiltrating T cells in sick OSEKO mice and observed that most T-cell infiltrates were located around perivascular areas whereas leptomeningeal T cells and T cells in the choroid plexus were scarcely present. These histological findings nicely correlated with focal breakdown of the BBB in these areas, confined to OSEKO mice but not OSEWT mice, as demonstrated by our Evans Blue leakage experiments in score-matched mice. Of note, also in these leakage experiments, the difference in blood–brain barrier breakdown was confined to the brain, especially in the periventricular areas, whereas leakage in the spinal cord was not altered in OSEKO mice. Following this line, our in vitro experiments using MOG-specific B7-H1KO T cells vs. B7-H1WT T cells clearly demonstrate that B7-H1 expression on T cells determines their capacity to affect BBB integrity as reflected by the extent of TEER drop upon direct contact of T cells with primary brain endothelial cells. These data thus indicate that expression of B7-H1 on T cells is decisive for the extent of certain T-cell effector functions, especially IFN-γ and granzyme production, which in turn might affect their capacity to inflict local BBB dysfunction.
From a more general point of view, our data reveal that the activation status of encephalitogenic CD4+ T cells directly influences their capacity to evoke BBB dysfunction. This hypothesis is supported by several datasets here: (i) Naive CD4+ T cells did not affect BBB function at all in comparison with activated CD4+ T cells; (ii) B7-H1KO T-cell–induced BBB dysfunction was much more pronounced than B7-H1WT T-cell–induced dysfunction, as reflected by TEER measurement as well as our permeability assays; and (iii) inhibition of T-cell activation via delivery of a soluble B7-H1–Ig molecule profoundly restricted their capacity to elicit BBB dysfunction (16). Although an impact of immune cells on BBB integrity in the context of CNS autoimmunity is conceivable, very few publications so far have addressed direct T-cell–mediated influence on BBB integrity: Kebir et al. demonstrated that human TH17 cells preferentially promote BBB disruption compared with human TH1 cells (31), and Huppert et al. provided additional evidence that IL-17A is critically involved in such immune cell-mediated endothelial barrier dysfunction (32). Our system didn’t study specifically TH17 cells, and IL-17A seems not to be a critical player for endothelial dysfunction: we did not observe that activated MOG-reactive CD4+ T cells produced substantial amounts of IL-17A, at least in vitro, and, moreover, lack of B7-H1 did enhance T-cell–mediated BBB dysfunction despite similar IL-17A production by these cells. Additionally, at least in our hands, soluble factors, such as proinflammatory cytokines, seem to play only a minor role because supernatants from highly activated T cells were not sufficient to induce relevant BBB dysfunction, in stark contrast to direct interaction of activated T cells and brain endothelial cells. However, it should be pointed out that Kebir et al. (31) described pronounced granzyme B expression by these human TH17 cells. Although they did not investigate whether granzyme B was involved in TH17-mediated endothelial dysfunction, they described pronounced TH17-mediated killing of primary neurons.
We now extend these findings as we provide clear evidence of CD4+ T-cell–mediated BBB dysfunction, independent from IL-17A, that is cell contact-dependent and at least partly depends on granzyme B expression by these T cells because the granzyme B inhibitor II profoundly ameliorated T-cell–mediated BBB dysfunction by both B7-H1KO T cells and B7-H1WT T cells. Following this line, we could show that, at least in vitro, brain endothelial cells enhance granzyme B production by activated CD4+ T cells and that activated B7-H1KO T cells elicit brain endothelial cell death upon coculture in a granzyme B-dependent fashion. Interestingly, granzyme release has already been implicated in the context of immune cell transmigration across endothelial cells—including CD4+ T cells—albeit not in the context of multiple sclerosis (33). Mechanistically, such granzyme release by T cells has been linked to endothelial cell apoptosis in vivo in mouse models of atherosclerosis and transplant vascular disease (34, 35). The functional relevance of granzyme B production in T-cell–mediated myocarditis was also suggested by demonstration of a link between reduced granzyme expression and reduced myocardial injury upon antiinflammatory intervention (36). In human cases of T-cell–mediated skin eruptions, there was a strong correlation between granzyme expression and endothelial cell apoptosis (37). Together, these findings imply a link between granzyme released by effector T cells, endothelial cell apoptosis, and immune cell transmigration in vivo.
What is the relevance of our findings with regard to the understanding of human CNS autoimmunity? We here provide a proof-of-concept that subtle alterations in T-cell function caused by dysregulation of single immune-regulatory molecules might explain interindividual, as well as intraindividual, changes in lesion topography and phenotypes of MS. One plausible cause for such interindividual variations could be the presence of single nucleotide polymorphisms in immune-relevant genes in individuals (38). On the other hand, especially, intraindividual changes might be caused as bystander effects due to altered expression of costimulatory or coinhibitory molecules in the context of bacterial or viral infections (39, 40). Following this line, such changes in expression levels of immune-regulatory molecules or adhesion molecules with their consequences with regard to T-cell invasion into the CNS might also account for so far unexplained decreases in the individual treatment response during the course of therapy, as observed in the context of natalizumab treatment. In this context, it is important to note that the B7-H1/PD-1 pathway is obviously key to susceptibility and disease type/course of several autoimmune disorders, including MS (41–43), which puts our findings into the overriding context of what functional consequences certain risk genes can have. This study demonstrates that single immune regulatory molecules affecting the activation status of (encephalitogenic) T cells can influence lesion distribution, which is associated with an altered capacity of these cells to elicit transient focal EC dysfunction and barrier dysfunction as essential steps for lesion development.
Finally, these findings imply that therapeutic options specifically targeting the expression level and/or function of single immune-regulatory molecules on CD4+ T cells might selectively affect their capacity to compromise BBB integrity without impairing their capacity for immune surveillance under homeostatic conditions, as observed in the context of drugs interfering with immune cell trafficking (44). The feasibility of such an approach could be illustrated by our experiment, using a soluble B7-H1–Ig molecule interfering with activation of CD4+ T cells, that profoundly diminished their capacity to impair BBB integrity.
Materials and Methods
Detailed methods are provided in SI Materials and Methods.
Animals.
C57BL/6 mice were purchased from Harlan Laboratories. Transgenic lines were on a C57BL/6 background and were purchased from The Jackson Laboratory. B7-H1KO mice (45) were provided by L. Chen, Johns Hopkins University School of Medicine, Baltimore. Th (IgHMOG) mice (46) were crossed to 2D2 (TCRMOG) mice (47) to create OSEWT mice (opticospinal EAE, also referred to as Devic), as previously described (3). Th B7-H1KO mice were crossed to 2D2 B7-H1KO mice to create OSEKO mice. For all experiments, mice were bred and maintained under specific pathogen-free conditions in the central animal facility at the University of Münster, according to German guidelines for animal care. All experiments were performed according to guidelines of the animal experimental ethics committee and approved by the local authorities of North Rhine-Westphalia, Germany.
Spontaneous EAE Incidence, Disease Severity, and Survival of OSE Mice.
OSEWT and OSEKO mice were monitored daily for the presence and severity of EAE symptoms. Disease severity was determined on a scale from 0 to 8: 0, healthy; 1, limp tail; 2, ataxia or unilateral hindlimb paralysis; 3, moderate unilateral or mild bilateral hindlimb paralysis; 4, moderate bilateral hindlimb paralysis; 5, complete hindlimb paralysis; 6, complete hindlimb and partial forelimb paralysis; 7, complete quadriplegia; 8, moribund. Mice with a score of 6 or higher were immediately taken out of the experiment and euthanized. Accordingly, for analysis of survival of OSEWT and OSEKO mice, a death event was recorded when a clinical score of at least 5.5 was reached. Mice eliminated for reasons unrelated to disease severity were recorded as censored (all with a score of less than 5.5) and were also included in the survival analysis.
Immunohistochemistry.
Score-matched OSEWT and OSEKO mice (mean clinical score 5.75) were killed under deep anesthesia and transcardially perfused with PBS and 4% (wt/vol) paraformaldehyde (PFA) in PBS. Spleen, spinal cords, and brains were dissected and fixed in 4% (wt/vol) PFA overnight. Two days later, 4% (wt/vol) PFA was removed, and the CNS tissue was kept in PBS at 4 °C until further processing. The brain was cut into three coronar sections whereas the cervical, thoracic, and higher lumbar spinal cord was cut into 8–11 3-mm-thick transverse segments before embedding. To perform semiquantitative analysis of infiltrates in cerebrum and brainstem, the brain was sagitally cut in the midline. Five-micrometer-thick sections were stained for hematoxylin/eosin and Luxol-fast blue (LFB)/periodic acid-Schiff (PAS). To detect macrophages/microglia, immunohistochemistry was performed using a biotin-streptavidin peroxidase technique (K5001; Dako) and an automated immunostainer (AutostainerLink 48; Dako). Sections were pretreated in a steamer (treatment solutions pH 6.0 or 9.0; Dako) before incubation with the primary antibodies anti-Mac3, (clone M3/84, 553322, 1:100; BD Pharmingen), α-CD3 (MCA 1477, 1:50; Serotec), or α-CD8 (clone C8/144B, 1:100; Dako). DAB was used as a chromogen, and sections were counterstained using hematoxylin.
Preparation of CNS Mononuclear Cells.
For flow cytometric analysis of immune cell infiltration in the CNS, mononuclear cells were isolated as previously described (48). Briefly, anesthetized mice were transcardially perfused with 20 mL of PBS, and CNS tissue was removed, homogenized with scissors, and digested with Collagenase Type IA (0.4 mg/mL; Sigma Aldrich) for 30 min in a water bath at 37 °C. Digested tissue was passed through a cell strainer and extensively washed before Percoll density centrifugation. Mononuclear cells were collected from the interphase of the discontinuous Percoll gradient, washed twice with PBS containing 1% FCS, and resuspended in FACS buffer for flow cytometry.
B- and T-cell Isolation and Culture.
For ex vivo analysis of lymph node-derived T cells from OSE mice, the following lymph nodes were used: inguinal, brachial, axillary, and cervical (referred to as total lymph nodes). For in vitro studies of cytokine production and proliferation of T cells activated by B cells, cocultures of B and T cells were set up as follows: B cells from spleens of Th or Th B7-H1KO mice were isolated using CD19 MACS MicroBeads (Miltenyi). Cells were resuspended in B-cell medium, containing X-VIVO 15 medium (Lonza), 10% (vol/vol) FCS, 1% penicillin/streptavidin (Sigma Aldrich), 1% l-glutamine, and 50 µM β-mercaptoethanol (Gibco). Recombinant mouse MOG1–125 protein (AnaSpec) was incubated with B cells (5 × 106 in 500 µL total volume) for 30 min on ice; cells were then washed with PBS (Sigma Aldrich) and incubated in 1 mL of B-cell medium at 37 °C for 2 h. CD4+ T cells from spleens and lymph nodes of 2D2 or 2D2 B7-H1KO mice were isolated using CD4 MACS MicroBeads (Miltenyi). For isolation of MOG-specific CD4+ T cells (Figs. 4E and 5 G and H and Figs. S4 A–C and S5A), biotinylated α-mouse TCR-Vβ11 antibody (clone RR3-15; BD Biosciences) was used (at 10 µg/mL), which was followed by MACS separation with α-biotin MicroBeads (Miltenyi). B and T cells were seeded onto a round-bottom 96-well plate at a 1:1 ratio (0.1 × 106 each). For TEER measurements, MOG35–55 (Biotrend) at 20 µg/mL was used instead of rMOG1–125 protein. B and T cells were cocultured for 4 to 5 d at a 1:2 ratio (0.05 × 106 B and 0.1 × 106 T cells per well).
Flow Cytometry.
For the detection of cell surface markers, single-cell suspensions were stained with fluorochrome-conjugated anti-mouse monoclonal antibodies (mAbs) or the appropriate isotype controls in FACS buffer (PBS, 1% FCS, 2 mM EDTA, 0.1% NaN3) for 30 min at 4 °C, protected from light. For intracellular cytokine staining, cells were first stimulated for 4 h at 37 °C with phorbol 12-myristate 13-acetate (PMA) (0.25 ng/µL) and ionomycin (0.4 ng/mL) (both from Sigma Aldrich), in the presence of GolgiPlug (1 µL/mL; BD Pharmingen). Next, cells were stained for cell surface markers, fixed, and permeabilized using Cytofix/Cytoperm and Perm/Wash buffer (BD Biosciences) according to the manufacturer’s instructions and stained for intracellular cell markers. CountBright absolute counting beads (Life Technologies) were used to determine absolute numbers of proliferated T cells, according to the manufacturer’s instructions. Measurements were performed on a Gallios flow cytometer (Beckman Coulter), and analysis was done using FlowJo7.6.5 software (Tree Star).
ELISA.
Cytokine production by CD4+ T cells activated by B cells in the presence of MOG (as described above) was determined by mouse ELISA Ready-Set-Go! kit (eBioscience) according to the manufacturer’s instructions. The kits were used for the following cytokines: IL-2, IFN-γ, IL-17A, TNF-α, and GM-CSF. OD values of supernatants were measured with a microplate reader (Thermo) at 450 nm, and wells with only B-cell medium were used as blank. Supernatants from cocultures with naive CD4+ T cells (cocultures without MOG) were used as negative controls.
MBMEC Isolation and Culture.
MBMECs were isolated as previously described (49–51). After isolation, cells were cultured at 37 °C, 5% CO2, and fresh MBMEC medium without Puromycin was added after 4 d. The next day, when the cells reached confluence, they were trypsinized, washed, and counted for subsequent TEER experiments.
For flow cytometric analysis of cocultures of MBMECs and T cells, MBMECs were cultured on a precoated flat-bottom 96-well plate. When confluence was reached, MOG-specific CD4+ T cells from 2D2WT or 2D2KO mice (prestimulated for 2 d with α-CD3/CD28, both at 1 µg/mL) were added to MBMECs (0.1 × 106 T cells per well); 24 h later, MBMECs and T cells were harvested by trypsinization, washed, and analyzed by flow cytometry.
TEER Measurement.
Five days after isolation, MBMECs were trypsinized, resuspended in MBMEC medium, and seeded onto precoated Transwell inserts (pore size 0.4 µm; Corning) at 2 × 104 cells per insert, and placed in cellZscope. TEER measurements were performed using the cellZscope 24-cell module and cellZscope v2.2.2 Software (nanoAnalytics GmbH) essentially as described before (52). Automated TEER and Ccl measurements were made every hour for 4 to 5 d until the cell monolayer reached full confluence, as determined by stable Ccl at 0.7 µF/cm2 and TEER at its maximum level for at least a few hours. Next, MBMECs were treated with preactivated MOG-specific CD4+ T cells or their supernatants. Then, 0.2 × 106 T cells per insert were added directly on top of MBMECs, and the measurement was resumed for another 24 h. Acquired data were exported and analyzed using GraphPad Prism 5 software.
Permeability Assay.
Permeability assays were performed as previously described (52, 53), with certain modifications. Fluorescently labeled dextran conjugates fluorescein (3 kDa) and Texas Red (70 kDa) (both from Molecular Probes) were added at 0.1% in Hepes buffer on top of each insert and let to diffuse through the MBMEC monolayer. After indicated time points, flow-through was collected from the lower compartment and transferred to a black 96-well plate. An Infinite M200 PRO Tecan plate reader was used to measure fluorescence intensity (fluorescein, excitation 494 nm, emission 521 nm; Texas Red, excitation 595 nm, emission 625 nm). Wells with only Hepes buffer served as blank.
In Vivo BBB Permeability Assay.
For visualization of the BBB leakage in sick OSEWT and OSEKO mice, Evans Blue dye was used as described (52), with certain modifications. One-hundred microliters of 2% (wt/vol) Evans Blue (Sigma Aldrich) in PBS was injected intravenously into the tail vein of score-matched (mean score 6) OSEWT and OSEKO mice. One hour later, mice were killed and transcardially perfused with 20 mL of PBS. The spinal cord and brain were dissected, and 2-mm-thick brain sections were made using acrylic brain matrix (World Precision Instruments), photographed, and assessed for the presence of the dye in the supratentorial brain areas.
Statistical Analysis.
All statistical tests were performed using GraphPad Prism 5 software. Values are presented as mean ± SEM. For the analysis of survival of OSEWT and OSEKO mice and EAE incidence, a log-rank (Mantel–Cox) test was used. Two-way ANOVA with Bonferroni correction for multiple comparisons was used for analysis of clinical scores of OSE mice, TEER measurements, and permeability assays. Fisher’s exact test was used for the analysis of contingency tables, as indicated. An unpaired, two-tailed Student’s t test was used for comparisons of means between two groups (*P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant).
SI Materials and Methods
Immunohistochemistry.
To quantify the inflamed white matter, we measured the percentage of white matter infiltrated by Mac3-positive macrophages in all transverse spinal cord sections of one animal and determined the mean. The extent of inflammation in spinal cord leptomeninges was measured by quantification of the area of leptomeningeal inflammation, and the mean area per transverse spinal cord section per mouse was determined. T-cell infiltrates in the brain were semiquantitatively estimated by quantification of T cells per coronal brain section (0–5 cells, score 0; 6–33 cells, score 1; 34–67 cells, score 2; 68–100, score 3; more than 100 cells, score 4). The scores of the three coronal sections per mouse were added to the final score. To semiquantitatively determine the number of T cells in the brainstem and cerebellum, the following score was used: for cerebellum, no infiltrates, score 0; single infiltrate, score 1; multiple infiltrates, score 2; for brainstem parenchyma, no infiltrates, score 0; infiltrates, score 1; for brainstem leptomeninges, no infiltrates, score 0; infiltrates consisting of one or two cell layers, score 1; infiltrates consisting of three or four layers, score 2; infiltrates consisting of more than four layers, score 3. The scores of these three anatomical sites were added, and the final score per mouse was determined.
B- and T-cell Isolation and Culture.
For polyclonal stimulation of T cells, round-bottom 96-well plates were precoated with purified α-CD3 (145-2C11; BioLegend) at 1 µg/mL for 3 h at 37 °C and then washed with PBS. Next, T cells were mixed with soluble purified α-CD28 (37.51; BD Pharmingen) at 1 µg/mL and seeded at 0.1 × 106 cells per well in medium containing Iscove's Modified Dulbecco's Medium (IMDM) plus l-glutamine (Gibco), 1% penicillin/streptavidin, 10% (vol/vol) FCS, and 50 µM β-mercaptoethanol. Cells were analyzed at different time points as indicated. When indicated, neutralizing low endotoxin, azide-free (LEAF) purified α-mouse B7-H1 (10F.9G2), PD-1 (29F.1A12), and CD80 (16-10A1) antibodies (all from BioLegend) were added to T-cell culture each day at 40 µg/mL For experiments with granzyme inhibitor, polyclonally stimulated T cells were incubated with or without Granzyme B Inhibitor II (10 µM; Calbiochem) for 2 d. For analysis of T-cell proliferation, T cells were labeled with cell proliferation dye eFluor670 (eBioscience) at 5 µM before seeding as described by the manufacturer. On the day of analysis, cocultured cells were stained with anti-mouse CD4 and/or anti-mouse TCR-Vβ11 and analyzed by flow cytometry.
Flow Cytometry.
For the detection of cell surface markers, the following mAbs were used: CD3 (17A2), CD4 (GK1.5 and RM4-4), CD8a (53-6.7), TCR-Vβ11 (KT11), CD25 (PC61), CD31 (390), CD62L (MEL-14), CD80 (16-10A1), CD86 (GL-1), LFA-1 (H155-78), VLA-4 (R1-2), MCAM (ME-9F1), B7-H1 (10F.9G2), PD-1 (RMP1-30), ICOSL (HK5.3), CTLA-4 (UC10-4B9), and TRAIL (N2B2), CD19 (6D5) (all from BioLegend); CD40 (1C10), CD45 (30-F11), CD69 (H1.2F3), FAS (15A7), FASL (MFL3) (all from eBioscience); CD11b (M1/70), B220 (RA3-6B2), MHC-II (M5/114.15.2), ICOS (7E.17G9), PSGL-1 (2PH1), and LAG-3 (C9B7W) (all from BD Pharmingen); and CD44 (KM201) (Beckman Coulter).
For intracellular cytokine staining, the following mAbs or their isotype controls were used: IL-2 (JES6-5H4), TNF-α (MP6-XT22), IFN-γ (XMG1.2), GzmA (3G8.5), and GzmB (GB11) (all from BioLegend); IFN-γ (XMG1.2) and GM-CSF (MP1-22E9) (BD Pharmingen); and IL-17A (17B7) and FoxP3 (FJK-16s) (eBioscience).
For flow cytometric analysis of endothelial cell death, 7-AAD (BD Biosciences) was used, according to the manufacturer’s instructions.
MBMEC Isolation and Culture.
MBMECs were isolated as follows. Brains from 20 C57BL/6 mice were removed, and the cortex was separated from the rest of the brain tissue, rolled over autoclaved blotting paper to remove meninges, homogenized with scissors, and digested with collagenase type 2 (1 mg/mL; Worthington) and DNase I (10 µg/mL; Sigma Aldrich) for 1 h at 37 °C on a shaker. After washing with DMEM (Gibco) (10 min at 4 °C, 1,000 × g), tissue was resuspended in 20% (wt/vol) BSA (in DMEM) and centrifuged for 20 min at 1,000 × g at 4 °C. Pellet was resuspended in DMEM and digested with collagenase/dispase (1 mg/mL; Roche) and DNase I (10 µg/mL) for another hour at 37 °C on a shaker. After washing with DMEM, resuspended cells were added on top of continuous Percoll gradient [containing 10 mL of Percoll (Sigma Aldrich), 19 mL of 1× PBS, 1 mL of 10× PBS, 1 mL of FCS; ultracentrifuged for 1 h at 16,000 rpm (SORVALL RC-6 PLUS centrifuge, Thermo Fisher Scientific; rotor: FIBERLite F21-8x50y, Thermo Scientific) at 4 °C, acceleration 9, deceleration 0]. After centrifugation (10 min at 4 °C, 1,000 × g, acceleration 0, deceleration 0), fragments of capillaries were collected and washed twice with DMEM. The final pellet was resuspended in MBMEC medium [80% (vol/vol) DMEM, 20% (vol/vol) FCS, 1% penicillin/streptomycin, 0.1% heparin (Sigma Aldrich), 0.05% human basic FGF (PeproTech), 0.1% puromycin (Sigma Aldrich)] and seeded onto a precoated 24-well plate [3 h at 37 °C with coating solution consisting of 50% (vol/vol) dH2O, 40% (vol/vol) collagen from human placenta type IV (Sigma Aldrich), and 10% (vol/vol) fibronectin (Sigma Aldrich)]. Cells were cultured at 37 °C, and fresh MBMEC medium without puromycin was added after 4 d. Next day, when the cells reached confluence, they were trypsinized, washed, and counted for subsequent experiments.
TEER Measurement.
When indicated, neutralizing α–IFN-γ antibody (XMG1.2; eBioscience) at 20 µg/mL or its isotype control was first mixed with T cells and then added to MBMECs. For TEER measurements with B7-H1–Ig fusion protein, T cells were first polyclonally stimulated for 2 d in the presence of precoated B7-H1–Ig (10 µg/mL) or its isotype control IgG2a. Next, T cells were harvested and added to MBMECs as described above. When indicated, blocking LEAF purified α-mouse PD-1 and CD80 antibodies were added to the T-cell culture each day at 40 µg/mL during both T-cell activation and the subsequent TEER measurement.
mRNA Extraction, cDNA Synthesis, and quantitative RT-PCR.
For analysis of granzyme B mRNA expression in brains of sick OSEWT and OSEKO mice, total RNA was extracted from brain mononuclear cells (prepared as described above) by using an RNeasy Micro Kit (Qiagen) and cleaned by using RNA Clean & Concentrator (Zymo Research); cDNA was made from 200 ng of total RNA by using a Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific), all performed according to the manufacturers’ instructions. For quantitative RT-PCR (RT-qPCR), a mouse GzmB TaqMan Gene Expression Assay (Mm00442837_m1) was used. Then, 18S rRNA was used as endogenous control. RT-qPCR was performed by using the StepOnePlus System (Applied Biosystems), and the analysis was done via the deltadelta Ct method.
Bio-Plex Cytokine Mouse 23-Plex Assay.
Cytokine and chemokine production in the supernatants from cocultures of CD4+ T cells and B cells in the presence of MOG35–55 (20 g/mL) was determined using a Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad), according to the manufacturer’s instructions. Samples were measured using the Bio-Plex MAGPIX System, standard curves were created for each molecule separately, and the analysis was performed for the following cytokines: IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, Eotaxin, G-CSF, KC, MCP-1, MIP-1α, MIP-1β, and RANTES.
Acknowledgments
We thank Annika Engbers, Andrea Pabst, Frank Kurth, and Claudia Kemming for excellent technical support. This work was supported by DFG SFB1009 Project A03 (to H.W. and L.K.); CRC TR128 Projects A08, Z1, and B01 (to L.K., T.K., and H.W.); and Interdisciplinary Center for Clinical Research (Medical Faculty of Münster) Grant Kl2/2015/14 (to L.K.).
Footnotes
Conflict of interest statement: L.K. received compensation for serving on Scientific Advisory Boards for Genzyme and Novartis; received speaker honoraria and travel support from Novartis, Merck Sorono, and CSL Behring; and receives research support from Novartis and Biogen Idec. C.C.G. received speaker honoraria and travel expenses for attending meetings from Genzyme (2014), Novartis Pharma GmbH (2011), and Bayer Health Care (2014). N.S. received speaking honoraria from Novartis and Biogen and travel expenses from Biogen. S.G.M. received honoraria for lecturing and travel expenses for attending meetings and financial research support from Bayer, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Omniamed, Novo Nordisk, Sanofi-Aventis, and Teva. T.K. received honoraria for lectures from Novartis and Biogen Idec Canada and served as a consultant for Genzyme Corporation. H.W. received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Genzyme, Merck Serono, Novartis, and Sanofi Aventis; received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen Idec, CSL Behring, Fresenius Medical Care, Genzyme, Glaxo Smith Kline, GW Pharmaceuticals, Lundbeck, Merck Serono, Omniamed, Novartis, and Sanofi Aventis; received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, and Sanofi Aventis; and received research support from Bayer Vital, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi Aventis Germany, and Sanofi US.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601350113/-/DCSupplemental.
References
- 1.Bennett JL, Stüve O. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: Therapeutic implications. Clin Neuropharmacol. 2009;32(3):121–132. doi: 10.1097/WNF.0b013e3181880359. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H. The pathology of multiple sclerosis. In: Alastair C, editor. McAlpine’s Multiple Sclerosis. 3rd Ed. Churchill Livingstone; Hong Kong: 1998. pp. 323–358. [Google Scholar]
- 3.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116(9):2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger T, et al. Experimental autoimmune encephalomyelitis: The antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest. 1997;76(3):355–364. [PubMed] [Google Scholar]
- 5.Fukazawa T, et al. Both the HLA-CPB1 and -DRB1 alleles correlate with risk for multiple sclerosis in Japanese: Clinical phenotypes and gender as important factors. Tissue Antigens. 2000;55(3):199–205. doi: 10.1034/j.1399-0039.2000.550302.x. [DOI] [PubMed] [Google Scholar]
- 6.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothhammer V, et al. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J Exp Med. 2011;208(12):2465–2476. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F, Ropele S, Enzinger C, Seifert T, Strasser-Fuchs S. Quantitative magnetization transfer imaging of pre-lesional white-matter changes in multiple sclerosis. Mult Scler. 2002;8(6):479–484. doi: 10.1191/1352458502ms860oa. [DOI] [PubMed] [Google Scholar]
- 10.Werring DJ, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: A serial diffusion MRI study. Brain. 2000;123(Pt 8):1667–1676. doi: 10.1093/brain/123.8.1667. [DOI] [PubMed] [Google Scholar]
- 11.Maggi P, et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann Neurol. 2014;76(4):594–608. doi: 10.1002/ana.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J Immunol. 2002;169(1):117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- 13.Brückener KE, el Bayâ A, Galla H-J, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J Cell Sci. 2003;116(Pt 9):1837–1846. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- 14.Linthicum DS, Munoz JJBA, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73(2):299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Baeten D, Jäger A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116(9):2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold M, et al. B7-H1 selectively controls TH17 differentiation and central nervous system autoimmunity via a novel non-PD-1-mediated pathway. J Immunol. 2015;195(8):3584–3595. doi: 10.4049/jimmunol.1402746. [DOI] [PubMed] [Google Scholar]
- 17.Klotz L, et al. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor gamma is mediated by up-regulation of B7H1. J Immunol. 2009;183(1):129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- 18.Ortler S, et al. B7-H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: Implications for the lesion pathogenesis of multiple sclerosis. Eur J Immunol. 2008;38(6):1734–1744. doi: 10.1002/eji.200738071. [DOI] [PubMed] [Google Scholar]
- 19.Pittet CL, Newcombe J, Antel JP, Arbour N. The majority of infiltrating CD8 T lymphocytes in multiple sclerosis lesions is insensitive to enhanced PD-L1 levels on CNS cells. Glia. 2011;59(5):841–856. doi: 10.1002/glia.21158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trabattoni D, et al. Costimulatory pathways in multiple sclerosis: Distinctive expression of PD-1 and PD-L1 in patients with different patterns of disease. J Immunol. 2009;183(8):4984–4993. doi: 10.4049/jimmunol.0901038. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Nun A, et al. From classic to spontaneous and humanized models of multiple sclerosis: Impact on understanding pathogenesis and drug development. J Autoimmun. 2014;54:33–50. doi: 10.1016/j.jaut.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Bittner S, et al. Effects of glatiramer acetate in a spontaneous model of autoimmune neuroinflammation. Am J Pathol. 2014;184(7):2056–2065. doi: 10.1016/j.ajpath.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290(1):72–79. doi: 10.1016/j.cellimm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Kroner A, et al. Accelerated course of experimental autoimmune encephalomyelitis in PD-1-deficient central nervous system myelin mutants. Am J Pathol. 2009;174(6):2290–2299. doi: 10.2353/ajpath.2009.081012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 26.Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci USA. 2009;106(8):2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirahara K, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36(6):1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23(1):515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, et al. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197(9):1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5(11):e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huppert J, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 33.Manes TD, Pober JS. Polarized granzyme release is required for antigen-driven transendothelial migration of human effector memory CD4 T cells. J Immunol. 2014;193(12):5809–5815. doi: 10.4049/jimmunol.1401665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyaw T, et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127(9):1028–1039. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- 35.Choy JC, et al. Granzyme B induces endothelial cell apoptosis and contributes to the development of transplant vascular disease. Am J Transplant. 2005;5(3):494–499. doi: 10.1111/j.1600-6143.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 36.Konishi M, et al. Imaging granzyme B activity assesses immune-mediated myocarditis. Circ Res. 2015;117(6):502–512. doi: 10.1161/CIRCRESAHA.115.306364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verneuil L, et al. Endothelial damage in all types of T-lymphocyte-mediated drug-induced eruptions. Arch Dermatol. 2011;147(5):579–584. doi: 10.1001/archdermatol.2011.104. [DOI] [PubMed] [Google Scholar]
- 38.Beecham AH, et al. International Multiple Sclerosis Genetics Consortium (IMSGC) Wellcome Trust Case Control Consortium 2 (WTCCC2) International IBD Genetics Consortium (IIBDGC) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou L, et al. Type 1 interferon-induced IL-7 maintains CD8+ T-cell responses and homeostasis by suppressing PD-1 expression in viral hepatitis. Cell Mol Immunol. 2015;12(2):213–221. doi: 10.1038/cmi.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ndejembi MP, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177(11):7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 41.Kroner A, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58(1):50–57. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 42.Huang C-H, et al. Effects of genetic polymorphisms of programmed cell death 1 and its ligands on the development of ankylosing spondylitis. Rheumatology (Oxford) 2011;50(10):1809–1813. doi: 10.1093/rheumatology/ker211. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell AL, et al. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison’s disease and Graves’ disease susceptibility. J Clin Endocrinol Metab. 2009;94(12):5139–5145. doi: 10.1210/jc.2009-1404. [DOI] [PubMed] [Google Scholar]
- 44.Schwab N, Schneider-Hohendorf T, Wiendl H. Therapeutic uses of anti-α4-integrin (anti-VLA-4) antibodies in multiple sclerosis. Int Immunol. 2015;27(1):47–53. doi: 10.1093/intimm/dxu096. [DOI] [PubMed] [Google Scholar]
- 45.Latchman YE, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101(29):10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litzenburger T, et al. B lymphocytes producing demyelinating autoantibodies: Development and function in gene-targeted transgenic mice. J Exp Med. 1998;188(1):169–180. doi: 10.1084/jem.188.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197(9):1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider-Hohendorf T, et al. Regulatory T cells exhibit enhanced migratory characteristics, a feature impaired in patients with multiple sclerosis. Eur J Immunol. 2010;40(12):3581–3590. doi: 10.1002/eji.201040558. [DOI] [PubMed] [Google Scholar]
- 49.Ruck T, Bittner S, Epping L, Herrmann AM, Meuth SG. Isolation of primary murine brain microvascular endothelial cells. J Vis Exp. 2014;(93):e52204. doi: 10.3791/52204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weidenfeller C, Schrot S, Zozulya A, Galla H-J. Murine brain capillary endothelial cells exhibit improved barrier properties under the influence of hydrocortisone. Brain Res. 2005;1053(1-2):162–174. doi: 10.1016/j.brainres.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 51.Deli MA, Abrahám CS, Niwa M, Falus A. N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine increases the permeability of primary mouse cerebral endothelial cell monolayers. Inflamm Res. 2003;52(Suppl 1):S39–S40. doi: 10.1007/s000110300045. [DOI] [PubMed] [Google Scholar]
- 52.Bittner S, et al. Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nat Med. 2013;19(9):1161–1165. doi: 10.1038/nm.3303. [DOI] [PubMed] [Google Scholar]
- 53.De Bock M, et al. Low extracellular Ca2+ conditions induce an increase in brain endothelial permeability that involves intercellular Ca2+ waves. Brain Res. 2012;1487:78–87. doi: 10.1016/j.brainres.2012.06.046. [DOI] [PubMed] [Google Scholar]