Significance
Tau-driven neurotoxicity occurs in multiple neurodegenerative diseases that have a severe impact on families and the society at large. However, its mode of toxicity is poorly understood, and therefore no effective drug treatments have been discovered. Here, we show that aggregate-prone Tau accumulates in axons where it causes presynaptic dysfunction and matching neuronal hypoactivity. The adenosine A1 receptor antagonist rolofylline, a drug developed for patients with acute heart failure and renal dysfunction, normalizes neuronal functioning in vitro and restores cognition in Tau-transgenic mice. We hypothesize that rolofylline could be used as a treatment by increasing bona fide neuronal activity, which is diminished in tauopathy patients. In turn, this should delay the onset or the progression of these neurodegenerative diseases.
Keywords: tauopathies, rolofylline, hypoactivity, axons, treatment
Abstract
Accumulation of Tau is a characteristic hallmark of several neurodegenerative diseases but the mode of toxic action of Tau is poorly understood. Here, we show that the Tau protein is toxic due to its aggregation propensity, whereas phosphorylation and/or missorting is not sufficient to cause neuronal dysfunction. Aggregate-prone Tau accumulates, when expressed in vitro at near-endogenous levels, in axons as spindle-shaped grains. These axonal grains contain Tau that is folded in a pathological (MC-1) conformation. Proaggregant Tau induces a reduction of neuronal ATP, concomitant with loss of dendritic spines. Counterintuitively, axonal grains of Tau are not targeted for degradation and do not induce a molecular stress response. Proaggregant Tau causes neuronal and astrocytic hypoactivity and presynaptic dysfunction instead. Here, we show that the adenosine A1 receptor antagonist rolofylline (KW-3902) is alleviating the presynaptic dysfunction and restores neuronal activity as well as dendritic spine levels in vitro. Oral administration of rolofylline for 2-wk to 14-mo-old proaggregant Tau transgenic mice restores the spatial memory deficits and normalizes the basic synaptic transmission. These findings make rolofylline an interesting candidate to combat the hypometabolism and neuronal dysfunction associated with Tau-induced neurodegenerative diseases.
The Tau protein is well known for stabilizing microtubules in neurons, although in a subset of neurodegenerative disorders called tauopathies [e.g., Alzheimer disease (AD), frontotemporal lobar degeneration (FTLD), Pick disease, etc.] Tau becomes modified (e.g., by hyperphosphorylation, acetylation, proteolytic processing, etc.), leading to neurofibrillary tangles (1). Alternatively, Tau can assemble as spindle-shaped grains, as in argyrophilic grain disease (AGD). Mutations within the repeat domain of the Tau protein can increase its β-sheet propensity (e.g., mutations P301L, ∆K280, and others), leading to missorting and aggregation of Tau (2, 3). In humans, such mutations can cause typical FTLD pathology with corresponding neurofibrillary tangles (4). In transgenic mice expressing human Tau with the ΔK280 mutation, the Tau protein is missorted into the somatodendritic compartment, (hyper)phosphorylated, and folded into a pathological conformation (MC-1 epitope) (5). These mice are still functionally impaired from ∼12 mo onward despite the absence of neurofibrillary tangles (6). Here, we use this transgenic human Tau model (ΔK280, proaggregant) in parallel with its antiaggregant counterpart (∆K280-PP line) where Tau cannot aggregate because of Ile-to-Pro mutations that serve as β-sheet breakers (7). Both types of Tau bind similarly to microtubules but differ in their aggregation potential (8). Noxious stimuli [e.g., hypoxia or amyloid-β (Aβ)] increase adenosine levels 30–100 times in the brain (9). Adenosine is a neuromodulator and has a depressant effect on neuronal activity when bound to the ubiquitously expressed adenosine A1 receptor, a Gi/G0-protein coupled receptor (10, 11). Hypometabolism (i.e., diminished neuronal activity) is strongly associated with neurodegeneration (12–14). Moreover, pathological Tau can disrupt ongoing network activity even at early asymptomatic stages (15). The relationship between hypometabolism seen in human tauopathies, Tau aggregation, and its effect on neuronal activity is not well established. Here, we show that moderate levels of aggregation-prone Tau protein induces hypoactivity of neurons with a reduction in neuronal ATP levels, loss of dendritic spines, and impaired synaptic functioning. The neuronal activity and impaired presynaptic compartment can be restored by application of the adenosine A1 receptor antagonist rolofylline in vitro and in vivo, suggesting that restoration of the diminished neuronal activity may be a yet-unexplored treatment strategy to combat cognitive impairment in tauopathies.
Results
High Aggregation Propensity Is Not Necessary for Tau Missorting but Causes Tau to Accumulate in Axons as Grains.
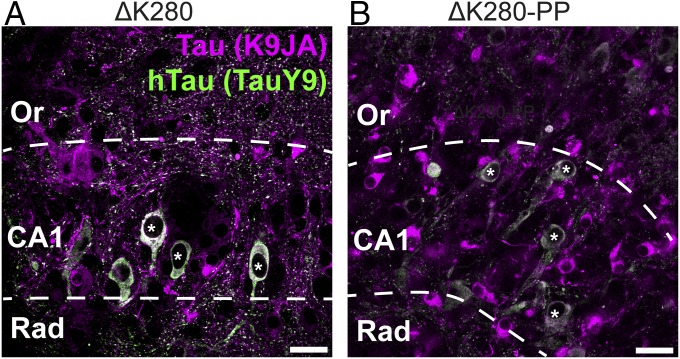
In organotypic hippocampal slices, both proaggregant and antiaggregant Tau is missorted to the somatodendritic compartment as has been shown before (Fig. 1 A and B, asterisks) (5). This missorted Tau in transgenic slices consists of, at least partially, transgenic human Tau (Fig. S1). Only proaggregant Tau transgenic slices reveal Tau-positive beaded structures in the neuropil oriented mostly perpendicular to the apical dendrites of the CA1 pyramidal cells (Fig. 1 C and D, arrowheads) resembling grains in human AGD (16). This suggests that proaggregant Tau accumulates in the axons as grains. Transgenic proaggregant Tau is expressed at 83% of wild-type Tau levels, whereas antiaggregant Tau is expressed at 50% of wild-type Tau levels in 30 d in vitro (DIV30) organotypic slices (Fig. 1E), which is approximately threefold less as has been found in the in vivo Tau transgenic mouse brain (6). The quantitative difference between proaggregant and antiaggregant Tau could be partially explained by the fact that proaggregant Tau accumulates in the neuropil as well, whereas antiaggregant Tau does not. To confirm the axonal nature of the grains of Tau, we performed a biolistic cotransfection of the red fluorescent protein TandemTomato (for morphology) and proaggregant Tau (Fig. 1 F–I). Single neurons are cotransfected, for example, in Fig. 1F, which shows a transfected CA1 neuron or a dentate gyrus granule cell in Fig. 1H and Fig. S2. The axons of transfected neurons (Fig. 1 G and I) clearly reveal small inclusions of Tau (∼1 µm in size, arrowheads), although presynaptic boutons (e.g., giant mossy fiber boutons) are only marginally stained for Tau (Fig. 1I and Fig. S2 A and B; arrow), indicating that Tau does not accumulate at presynaptic boutons in these slices. Furthermore, we did not see colocalization of the grains of Tau and presynaptic marker synaptophysin (Fig. S3). We also observed that Tau missorts into a subgroup of proximal dendrites, which correlates with a dramatic spine loss in the affected dendrites (Fig. S2 C and F). By contrast, dendrites that do not contain Tau are richly decorated with spines (>1 spines per µm), indicating that there is only local impairment of dendritic function in case of proaggregant Tau missorting.
Fig. 1.
Aggregate-prone Tau accumulates in the neuropil as axonal grains. (A and B) CA1 region of proaggregant Tau (ΔK280) and antiaggregant Tau (ΔK280-PP) transgenic organotypic hippocampal slices stained for neurons [NeuN and pan-Tau (K9JA)]. (Scale bar: 20 µm.) Asterisks indicate missorted Tau. (C and D) Higher magnification of the CA1 and neuropil of proaggregant Tau transgenic slices. [Scale bar: 20 µm (C) and 4 µm (D).] (E) Representative immunoblot of total Tau (antibody K9JA) and quantification. **P < 0.01 (one-way ANOVA with Tukey’s test). Error bar indicates SEM. (F–I) Littermate control slices cotransfected with the red fluorescent protein TandemTomato (TdTom.) and proaggregant Tau [Tau (K9JA)]. (F and H) A transfected CA1 pyramidal cell (F) and a transfected dentate gyrus granule cell (H) with schematic representations. (Scale bar: 200 µm.) Dotted boxes in F and H are magnified in G and I. (Scale bar: 10 µm.) Or, stratum oriens; Rad, stratum radiatum.
Fig. S1.
Missorted Tau in Tau transgenic organotypic hippocampal slices (partially) consists of human Tau. (A and B) Transgenic CA1 neurons costained for pan-Tau (K9JA) and transgenic human Tau (TauY9 antibody) in proaggregant Tau (A) and antiaggregant Tau (B) transgenic organotypic hippocampal slices. Asterisks indicate neurons with missorted Tau. Or, stratum oriens; Rad, stratum radiatum. (Scale bars: 20 µm.)
Fig. S2.
Transfection of Tau-ΔK280 leads to axonal grains of Tau in axons that do not colocalize with synaptic boutons and causes local spine loss when missorted in the dendrites. (A) CA1 pyramidal cell cotransfected with proaggregant Tau (ΔK280) and TandemTomato. (Scale bar: 200 µm.) (B) The axon accumulates grains of Tau when proaggregant Tau is expressed (arrowheads). Proaggregant Tau does not accumulate in presynaptic boutons (long arrow). (Scale bar: 10 µm.) (C) Tau in the dendrites causes loss of spines (arrowheads and black dendrite). Dendrites secondary to the one with missorted Tau or other primary dendrites are richly decorated with spines (long arrow). (Scale bar: 10 µm.) (D) Dentate gyrus granule cell cotransfected with proaggregant Tau (ΔK280) and TandemTomato. (Scale bar: 200 µm.) (E) Proaggregant Tau can be found in axonal dilatations (arrowheads). (Scale bar: 10 µm.) (F) The somatodendritic compartment shows missorting of Tau in the soma and dendrites (arrowheads). Dendritic spines are lost in case of missorting of Tau. The same cell still has dendrites decorated richly with spines (arrow). DG, dentate gyrus; Or, stratum oriens; Rad, stratum radiatum. (Scale bar: 10 µm.)
Fig. S3.
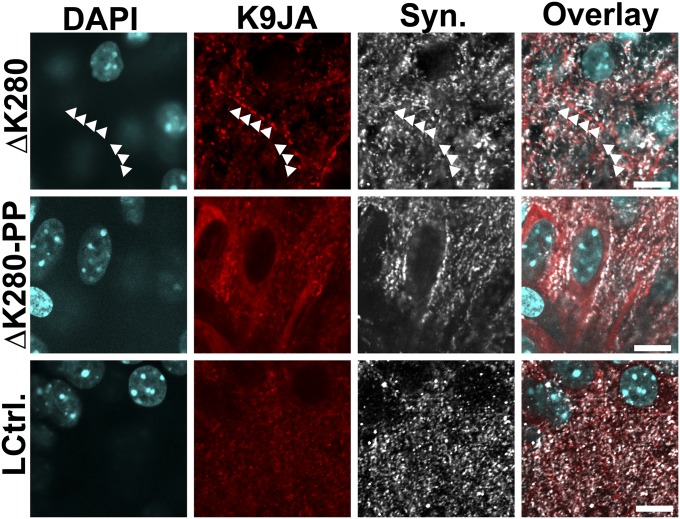
Grains of Tau found in proaggregant Tau transgenic slices do not colocalize with the presynaptic marker synaptophysin. The grains of Tau found in proaggregant Tau transgenic slices do not colocalize with the grains of synaptophysin (white arrowheads). Antiaggregant Tau or littermate control slices do not show grains of Tau in the neuropil. (Scale bars: 10 µm.)
Both Proaggregant and Antiaggregant Tau Are Phosphorylated, but Only the Grains of Proaggregant Axonal Tau Appear in a Pathological Conformation.
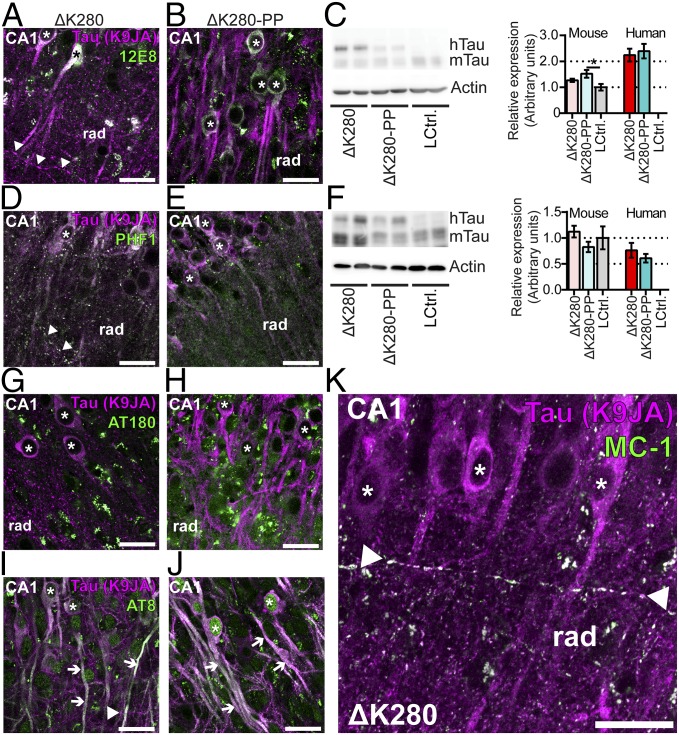
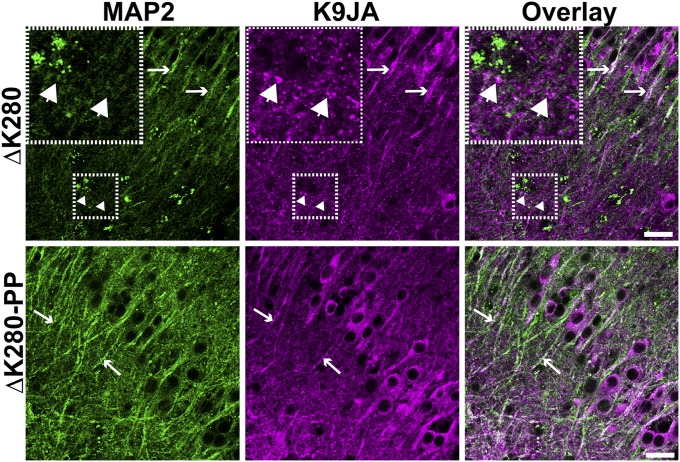
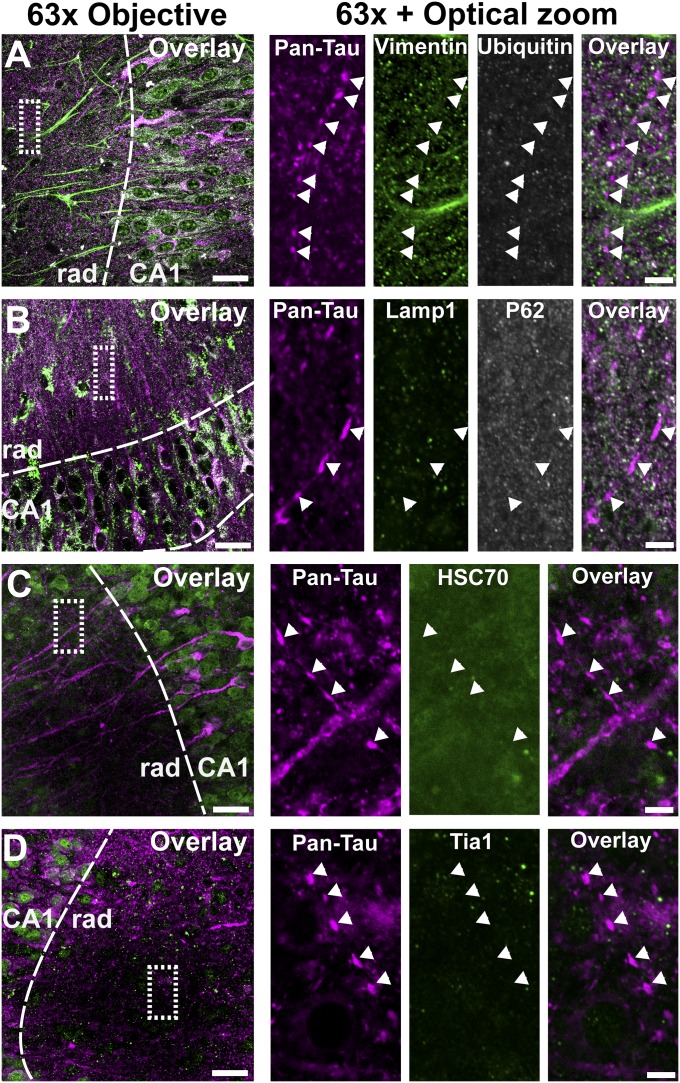
The proaggregant transgenic mice have aberrantly phosphorylated human Tau-ΔK280, although the relation between these posttranslational modifications and Tau toxicity is poorly understood (6). Therefore, to differentiate between toxic and nontoxic modifications of Tau, we compared the phosphorylation status of Tau in proaggregant (ΔK280) and antiaggregant (ΔK280-PP) transgenic organotypic hippocampal slices (Fig. 2 and Table S1). Slices from both types of transgenic mice show the 12E8 epitope (pSer262/pSer356, Fig. 2 A and B) in the somatodendritic compartment (asterisks). Phosphorylation of these serines is known to induce detachment of Tau from microtubules and to promote missorting of Tau (17). Indeed, the axonal grains of Tau are not 12E8 positive (arrowheads), emphasizing that 12E8 staining is found only in mislocalized Tau. When corrected for the difference in total Tau, 12E8 phosphorylation does not differ between proaggregant and antiaggregant Tau (Fig. 2C). The PHF-1 epitope (pSer396+pSer404, Fig. 2 D–F) is seen in both types of Tau transgenic slices where it appears in the somatodendritic compartment (asterisks) and in the axonal grains (arrowheads). PHF-1 phosphorylation levels are similar for antiaggregant or proaggregant Tau when corrected for total Tau input (Fig. 2F). The antibody AT180 (Tau pThr231) (18) shows (very) weak staining in the cell soma of both types of Tau transgenic slices (Fig. 2 G and H, asterisks) contrasting the high degree of Tau phosphorylated at Ser202/Thr205 [asterisks (somata) and long arrows (apical dendrites)] (AT8 antibody, Fig. 2 I and J). We also studied pathologically folded Tau using the MC-1 antibody (Fig. 2K). MC-1–positive Tau accumulates in the axonal grains of proaggregant Tau as described above (arrowheads), whereas antiaggregant slices remain unstained. By contrast, Tau missorted in the somatodendritic compartment in either proaggregant or antiaggregant Tau transgenic slices is negative for the MC-1 epitope (asterisks), consistent with the absence of grains in dendrites. We also attempted to costain Tau (K9JA) with dendritic marker MAP2. Although Tau frequently colocalized with MAP2 in dendrites (missorting), the grains never colocalized with MAP2, emphasizing the axonal nature of the grains (Fig. S4). Surprisingly, the axonal grains of Tau appear to resist protein degradation because they are negative for markers of degradation (vimentin, ubiquitin, Lamp1, Sqstm1/P62, Hsc70, and Tia-1) (Fig. S5). Taken together, most phosphoepitopes are present in both antiaggregant and proaggregant Tau transgenic slices. Grains of pathologically folded Tau accumulate only in the axons of proaggregant Tau transgenic neurons, which suggests that these grains play a critical role in Tau-induced neuronal dysfunction. However, these grains are not targeted for degradation, nor do they appear to induce an unfolded protein response within the axon.
Fig. 2.
Proaggregant and antiaggregant Tau are both phosphorylated in organotypic hippocampal slices, but only grains of proaggregant Tau can be stained for pathological (MC-1) Tau. (A and B) Pan-Tau and 12E8 (pSer262 and pSer356) costaining of proaggregant Tau (ΔK280) and antiaggregant Tau (ΔK280-PP) transgenic organotypic hippocampal slices. (C) Quantification of 12E8 phosphorylation by immunoblotting. Data are corrected for total Tau input. Error bar indicates SEM. *P < 0.05 (one-way ANOVA with Tukey’s test). (D and E) Costaining of pan-Tau with PHF-1 phosphorylated Tau (Ser396 and Ser404) in organotypic slices of both transgenic lines. (F) Quantification of PHF-1 phosphorylation by immunoblotting. Data are corrected for total Tau input. Error bar indicates SEM. (G and H) Immunostaining of organotypic slices for the AT180 (phospho-Tau pThr231) epitope and pan-Tau. (I and J) Immunostaining using the pan-Tau antibody (K9JA) and phospho-Tau epitope AT8 (Ser202 and Thr205). (K) Costaining for Tau conformation-dependent epitope MC-1 and pan-Tau (K9JA). Rad, stratum radiatum. (All scale bars: 25 µm.)
Table S1.
Summary of the immunofluorescence stainings of Tau species in organotypic slices transgenic for proaggregant Tau and antiaggregant Tau
| Antibody | Proaggregant Tau transgenic slices | Antiaggregant Tau transgenic slices | |
| Cell soma and apical dendrite | Axonal grains in neuropil | Cell soma and apical dendrite | |
| Pan-Tau (K9JA) | + | + | + |
| Human Tau (TauY9) | + | + | + |
| 12E8 (pSer262 and/or pSer356) | + | − | + |
| PHF1 (pSer396 and pSer404) | + | + | + |
| AT8 (pSer202 + pThr205) | + | − | + |
| AT180 (pThr231) | +/− | − | +/− |
| MC1 | − | + | − |
Results for proaggregant Tau are subdivided into those applicable to Tau in the somatodendritic compartment and Tau accumulating as grains in axons.
Fig. S4.
The (axonal) grains of Tau do not colocalize with the dendritic marker MAP2. Missorted Tau shows colocalization with MAP2 (long arrows), whereas the grains of Tau seen in proaggregant Tau transgenic organotypic slices do not (arrowheads). (Scale bars: 25 µm.)
Fig. S5.
The grains of Tau in the neuropil of proaggregant Tau transgenic slices are negative for classical inclusion markers. Overview image (Left) with dotted rectangle magnified on the Right. (Scale bars: in overview images, 20 µm; in magnified images, 5 µm.) (A) Triple staining of Pan-Tau (K9JA), vimentin, and ubiquitin. Proaggregant Tau grains indicated with arrowheads. (B) Triple staining of Pan-Tau (K9JA), Lamp1, and P62/Sqstm1. (C) Double staining of Pan-Tau (K9JA) and HSC70. (D) Double staining of Pan-Tau (K9JA) and Tia1. Rad, stratum radiatum.
Proaggregant Tau Causes Spine Loss, Reduces Axonal Mitochondria, and Lowers Cytoplasmic ATP.
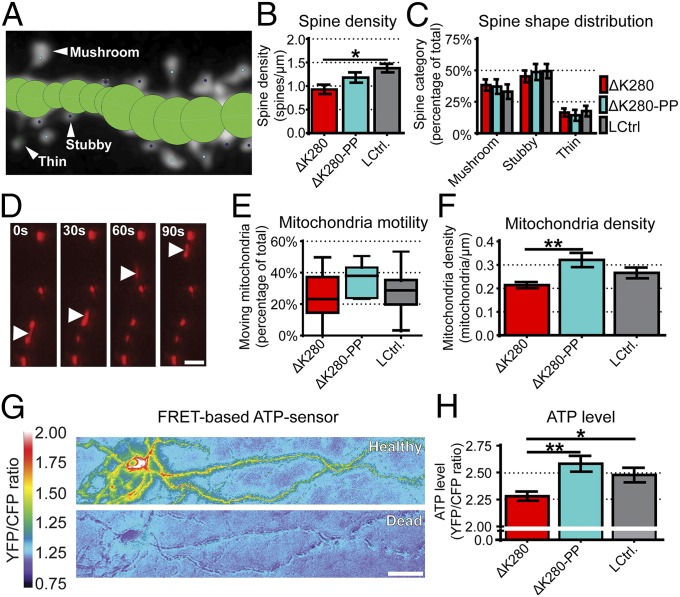
We labeled neurons of the organotypic slices diolistically with DiI to investigate the effect of the proaggregant and antiaggregant Tau on spine density and morphology. Proaggregant Tau transgenic slices showed a significant reduction of spines compared with littermate control slices, whereas spine density of antiaggregant Tau transgenic slices was similar to controls (Fig. 3 A and B). Dendritic spines are usually classified into different categories based on their shape, which represents different functional properties (19). We could not detect a difference in classes of spines between slices of both transgenic lines and littermate controls (Fig. 3C). We determined mitochondrial movements in live organotypic slices because aggregation-prone Tau is known to impair mitochondrial transport (Fig. 3 D and E). Mitochondria transport is similar in both kinds of Tau transgenic slices (Fig. 3E) with only a moderately lower mitochondrial density in proaggregant Tau transgenic slices compared with antiaggregant slices (Fig. 3F and Table S2). We also investigated the effect of proaggregant and antiaggregant Tau on energy status (ATP level). Transgenic slices were biolistically transfected with the FRET-based ATP sensor (ATeam) (Fig. 3 G and H) (20). ATP is reduced in the proaggregant transgenic slices, matching the lower mitochondrial density, compared with littermate controls or antiaggregant Tau transgenic slices (Fig. 3H). This suggests that the energy status of the neurons is compromised by proaggregant but not by antiaggregant Tau.
Fig. 3.
Proaggregant Tau transgenic slices have fewer spines, less axonal mitochondria, and reduced ATP levels compared with antiaggregant Tau transgenic or control littermates. (A) Example image of semiautomated spine counting. (B) Graph representing total number of spines per micrometer for proaggregant (ΔK280), antiaggregant (ΔK280-PP), and control littermate (LCtrl.) slices. *P < 0.05. (C) Graph showing the distribution of different spine categories obtained using NeuronStudio. Data are expressed as a percentage of total number of spines analyzed. (D) Images showing an example of a moving mitochondrion within an axon at different time points. (Scale bar: 10 µm.) (E and F) Graph representing the percentage of moving mitochondria in the different groups analyzed (E) and the density of mitochondria per micrometer of axon. **P < 0.01 (F). (G) Representative image of a neuron expressing the ATP sensor in a healthy state (Upper, YFP/CFP > 2) and after death (Lower, YFP/CFP ∼ 1). (Scale bar: 50 µm.) (H) ATP levels displayed as the background-corrected ratio between YFP and CFP. (**P < 0.01 and *P < 0.05, compared with Tau-ΔK280 slices.) All error bars indicate SEM. Significant differences determined by using one-way ANOVA with Tukey’s test.
Table S2.
Mitochondrial moving statistics of proaggregant and antiaggregant Tau transgenic slices and littermate controls
| Parameter | Littermate control ± SEM | Proaggregant ± SEM | Antiaggregant ± SEM |
| Mitochondrial density | 0.266 ± 0.023 | 0.214 ± 0.013 | 0.321 ± 0.030 |
| % Moving | 27.53 ± 4.31% | 24.85 ± 4.12% | 35.49 ± 3.79% |
| Ratio anterograde/retrograde | 1.892 ± 0.455 | 1.618 ± 0.341 | 2.117 ± 0.376 |
| Instantaneous speed anterograde | 0.284 ± 0.049 | 0.243 ± 0.035 | 0.368 ± 0.067 |
| Instantaneous speed retrograde | 0.194 ± 0.038 | 0.272 ± 0.046 | 0.538 ± 0.095 |
| No. of anterograde moving mitochondria per 100 µm | 2.6 ± 0.461 | 1.8 ± 0.316 | 3.5 ± 0.491 |
| No. of retrograde moving mitochondria per 100 µm | 1.1 ± 0.200 | 1.5 ± 0.315 | 2.1 ± 0.497 |
The Proaggregant Tau-Induced Phenotype Can Be Rescued with the Adenosine A1 Receptor Antagonist Rolofylline.
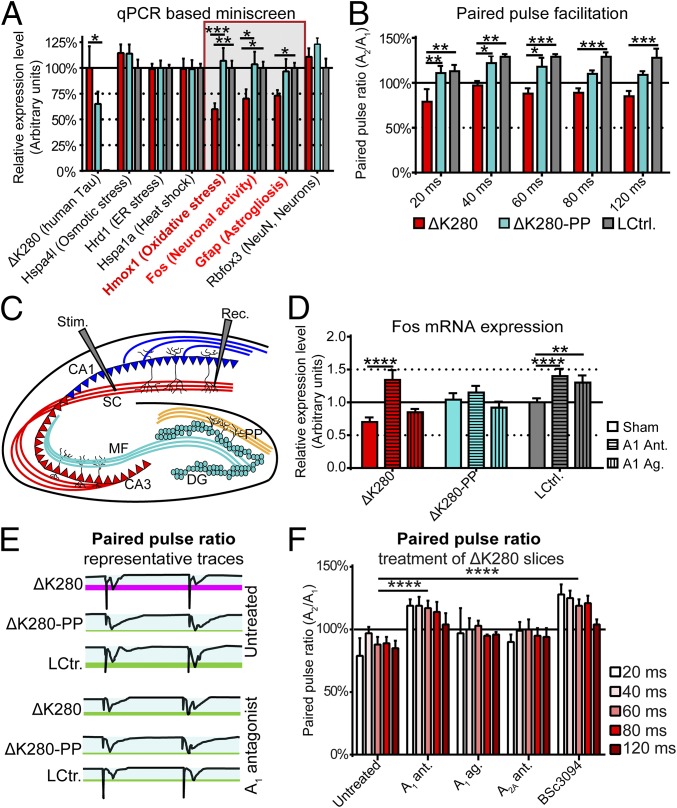
The markers described so far did not reveal any clear mode of action of toxic proaggregant Tau despite the functional impairment reported previously in transgenic mice (6). We therefore designed a quantitative PCR-based miniscreen of key genes known to be (up-)regulated at the mRNA level as a result of specific stressors serving as markers for insults (Table S3). Surprisingly, expression of neuronal activity marker cFos, astrocytic activity marker Gfap, and oxidative stress marker Hmox1 were reduced in the proaggregant Tau transgenic slices, whereas antiaggregant Tau transgenic slices were not different from littermate controls (Fig. 4A). Signs of molecular stress (e.g., protein misfolding, osmotic stress, oxidative stress, etc.) reflected by an increase in stress markers (Hspa1a, Osp94, Hmox1, etc.), however, could not be found in the proaggregant Tau transgenic slices. This confirms the lack of a classical cytotoxicity by this species of aggregate-prone Tau (e.g., chaperones, aggresomes, stress granules, etc.), as has been reported above (Fig. S5). Given that grains of Tau accumulate within axons (Fig. 1) and that axonal mitochondria density is reduced (Fig. 3), we tested next whether axonal (presynaptic) functioning is indeed impaired in organotypic hippocampal slices. We therefore measured the paired-pulse ratio (PPR) by applying a paired-pulse stimulus of the Schaffer collaterals (Fig. 4 B and C). We observed a typical paired-pulse facilitation (PPF) response in littermate controls and antiaggregant Tau transgenic slices, whereas in proaggregant Tau transgenic slices, the same stimulus paradigm resulted in a paired-pulse depression (Fig. 4B). This indicates that proaggregant Tau induces presynaptic impairment, whereas presynapses of antiaggregant Tau slices are unaffected. Adenosine down-modulates neuronal activity (cFos levels), impairs the presynapse, and attenuates long-term potentiation (LTP) via the A1 receptor (21). Because this resembles our presynaptic phenotype (Fig. 4B) and the outcome of the miniscreen, we attempted to counterbalance the observed phenotype by using the adenosine A1 receptor antagonist rolofylline. An adenosine A2A receptor antagonist (ZM-241385), an adenosine A1 receptor agonist (N6-cyclopentyladenosine), and a Tau aggregation inhibitor (BSc3094) were used as controls (Table S4) (22–24). Rolofylline increases neuronal activity (Fos mRNA) both in proaggregant Tau transgenic slices and controls, although in case of the proaggregant slices neuronal activity is almost doubled, yielding levels similar to those of treated littermate control slices (Fig. 4D). In line with these observations, the presynaptic impairment in proaggregant Tau transgenic slices can be reversed by rolofylline or BSc3094 without causing adverse effects in controls (Fig. 4F and Fig. S6).
Table S3.
Genes selected for the mRNA expression based miniscreen
| Gene | Parameter |
| FBJ osteosarcoma oncogene, c-Fos (Fos) | Neuronal activity |
| Glial fibrillary acidic protein (Gfap) | Astrogliosis |
| Heme oxygenase 1 (Hmox1) | Oxidative stress |
| Osmotic stress protein 94 (Osp94/Hspa4l) | Osmotic stress |
| E3 ubiquitin-protein ligase synoviolin (Syvn1) | Endoplasmic reticulum stress |
| Heat shock 70-kDa protein 1A (Hspa1a) | Heat shock response |
| RNA binding protein fox-1 homolog 3 (Rbfox3/NeuN) | Number of neurons |
Fig. 4.
Organotypic slices expressing proaggregant Tau show reduced neuronal and astrocytic activity and impaired axonal functioning. This can be alleviated by antagonizing Tau aggregation propensity or stimulation of cell activity with adenosine A1 receptor antagonist rolofylline. (A) The mRNA levels of transgenic Tau and stress-related genes in proaggregant (ΔK280), antiaggregant (ΔK280-PP), and control littermate (LCtrl.) organotypic hippocampal slices. Error bars indicate SEM. ***P < 0.001, **P < 0.01, and *P < 0.05 (two-way ANOVA and Dunnett's multiple-comparisons test). (B and C) Paired-pulse response in ΔK280, ΔK280-PP, and LCtrl. organotypic slices. Error bars indicate SEM. ***P < 0.001, **P < 0.01, and *P < 0.05 (two-way ANOVA with Tukey’s test). (C) Electrodes were placed in the stratum radiatum to excite the Schaffer collaterals. DG, dentate gyrus; MF, mossy fibers; PP, perforant pathway; Rec., recording electrode; SC, Schaffer collaterals; Stim., stimulation electrode. (D) The Fos mRNA levels in slices treated with adenosine A1 receptor antagonist rolofylline and adenosine A1 receptor agonist N6-cyclopentyladenosine. Error bars indicate SEM. ****P < 0.0001, **P < 0.01 (two-way ANOVA and Dunnett's multiple-comparisons test). (E) Representative traces of the paired-pulse response for proaggregant Tau (ΔK280), antiaggregant Tau (ΔK280-PP) transgenic slices and littermate controls (LCtrl.). (F) PPRs in proaggregant Tau transgenic organotypic hippocampal slices after treatment with compounds. Error bars indicate SEM. ****P < 0.0001 (two-way ANOVA with Tukey’s test).
Table S4.
Compounds used to treat organotypic hippocampal slices
| Designation | Compound | Concentration |
| Adenosine A1 receptor antagonist | Rolofylline (KW-3902) | 50 nM |
| Adenosine A1 receptor agonist | N6-Cyclopentyladenosine | 300 nM |
| Adenosine A2A receptor antagonist | ZM-241385 | 50 nM |
| Tau aggregation inhibitor | BSc3094 | 25 µM |
Fig. S6.
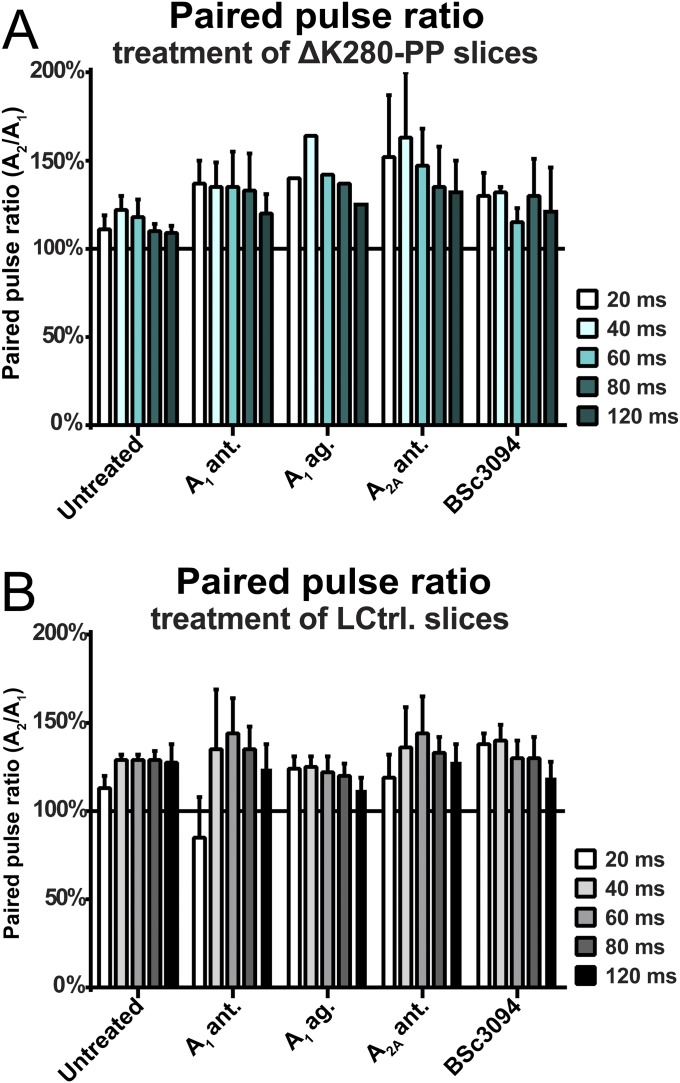
PPF in (treated) antiaggregant Tau and littermate control hippocampal organotypic slices. No adverse effects of the tested compounds on PPF in antiaggregant Tau (ΔK280-PP) (A) or littermate control (LCtrl.) slices (B). A1 ag., adenosine A1 receptor agonist N6-cyclopentyladenosine; A1 ant., rolofylline; A2A ant, A2A receptor antagonist ZM-241385; BSc3094, Tau aggregation inhibitor BSc3094. Error bars indicate SEM.
Rolofylline Treatment Restores Dendritic Spine Levels in Proaggregant Tau Transgenic Slices, Rescues Long-Term Memory Deficits, and Normalizes Basal Synaptic Transmission in Proaggregant Tau Transgenic Mice.
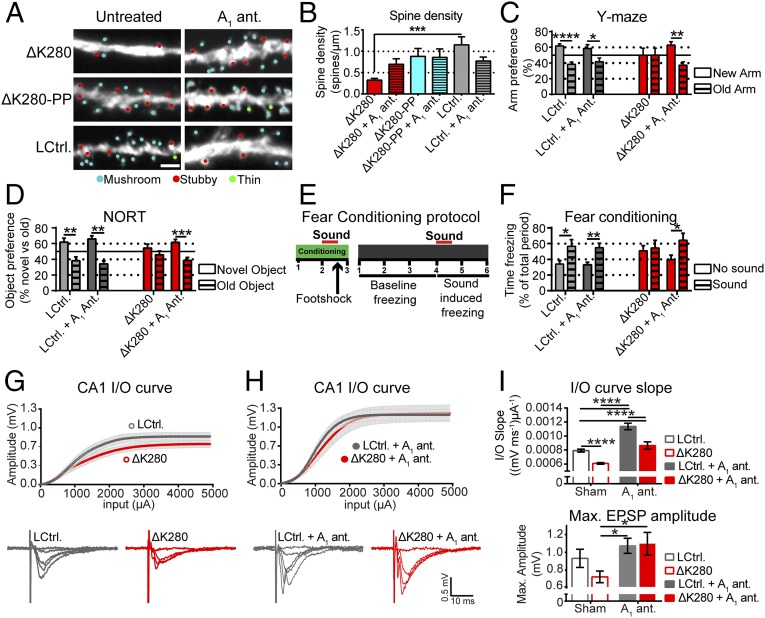
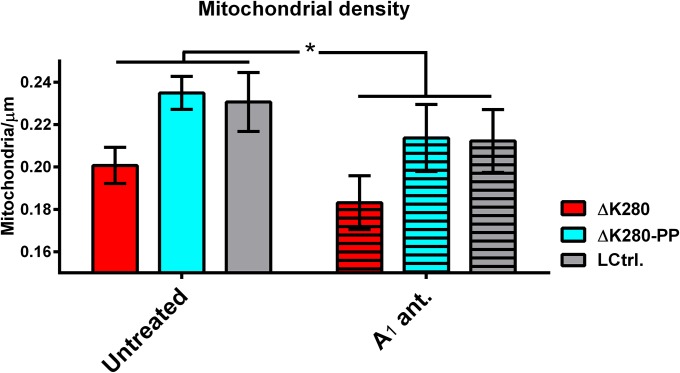
Because the electrophysiological parameters in the proaggregant Tau transgenic slices were normalized by rolofylline treatment, we investigated whether it would also restore the level of dendritic spines in these slices. Indeed, the reduced level of spines seen in proaggregant Tau transgenic slices are normalized when treated with rolofylline, whereas no significant changes are found in antiaggregant Tau transgenic slices or littermate controls (Fig. 5 A and B). The axonal density of mitochondria, which is slightly lower in proaggregant compared with antiaggregant Tau transgenic slices, is marginally decreased by rolofylline treatment albeit in a genotype-independent manner (Fig. S7). Having observed that rolofylline restores presynaptic functioning (i.e., PPF, Fig. 4F), neuronal activity (induction of Fos, Fig. 4D), and dendritic spine levels in proaggregant Tau transgenic organotypic slices (Fig. 5 A and B), we tested whether we could restore long-term spatial memory in proaggregant Tau transgenic mice as well. We therefore performed the Y-maze test, novel object recognition test (NORT), and the fear conditioning test with 14-mo-old proaggregant Tau transgenic and littermate control mice within 10–20 d of oral rolofylline treatment. In the Y-maze test, (treated) control mice spent more time in the novel arm, whereas untreated proaggregant mice did not show any arm preference (Fig. 5C). Rolofylline reestablished novel arm preference in proaggregant mice, suggesting that rolofylline restores spatial memory in these animals. In the NORT, (treated) control mice explored the novel object more compared with the old object, whereas the proaggregant mice did not show any preference for the new or the old object (Fig. 5D). Rolofylline treatment improved long-term object recognition memory in proaggregant Tau transgenic mice, as shown by increased novel object preference. For fear conditioning testing, the effects of systemic rolofylline administration on different stages of contextual and clue-based (sound) fear learning were investigated 24 h after the training session (Fig. 5 E and F). Contextual memory was unaltered as all groups showed similar freezing when reintroduced into the chamber. Control groups and the rolofylline-treated proaggregant group showed a clue-induced freezing response, whereas no effect was seen in the untreated proaggregant mice (Fig. 5F). This result suggests an impaired learning association between the sound and the foot shock in proaggregant mice, which can be rescued by rolofylline treatment. Ten weeks of posttreatment (starting at 14 mo), we assessed the electrophysiological properties of the CA1 region of the hippocampus in treated proaggregant Tau transgenic animals and controls (Fig. 5 G and H). Compared with untreated proaggregant Tau transgenic mice, treated mice (proaggregant Tau transgenics and littermate controls) have significantly larger maximal excitatory postsynaptic potential amplitudes (Fig. 5 G–I). The slope of the input/output (I/O) curve is significantly reduced in proaggregant Tau transgenic mice compared with controls, indicative of impaired basal synaptic transmission (Fig. 5I). Treatment with rolofylline increases the slope of the I/O curve in both proaggregant Tau transgenic slices and littermate controls (Fig. 5I). The impairment of the presynapse, as determined by application of a paired-pulse protocol is ambiguous in the acute slices (Fig. S8A). However, at the shortest pulse interval (20 ms), PPF is normally strongly suppressed by the feedforward inhibition (25). For the proaggregant Tau transgenic slices, feedforward inhibition seems to be impaired because the PPR is as high as the ratios seen with larger pulse intervals. Synaptic plasticity, measured as the ability to elicit LTP by theta-burst stimulation, is not altered in any condition compared with untreated littermate controls (Fig. S8B).
Fig. 5.
Treatment with rolofylline restores the dendritic spine level in proaggregant Tau transgenic slices and reverses spatial memory deficits and normalizes basal synaptic transmission in proaggregant Tau transgenic mice. (A and B) Quantification of dendritic spines of in rolofylline (A1 ant.) or sham-treated organotypic slices. Error bars indicate SEM. ***P < 0.001 (one-way ANOVA with Tukey’s test). (Scale bar in A: 2 µm.) (C–F) Outcome of behavior testing after 10–20 d of rolofylline treatment for the Y-maze test (C), novel object recognition test (NORT) (D), and fear conditioning test (E and F). Error bars indicate SEM. ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05. (G and H) Basal synaptic transmission (I/O curve) in acute slices from littermate control and proaggregant Tau transgenic. Representative traces of the I/O curves of proaggregants and littermate controls are displayed. Sham treated in G and rolofylline (A1 ant.) treated in H. Representative traces of the I/O curves are displayed. (I) The slope and maximum amplitude of the I/O curves of CA1 of rolofylline (A1 ant.) and sham-treated acute slices of proaggregant Tau transgenic and littermate controls. Error bars indicate SEM. ****P < 0.0001, *P < 0.05 (two-way ANOVA with Tukey’s test).
Fig. S7.
Rolofylline treatment of organotypic hippocampal slices decreases mitochondrial density slightly, which is independent of genotype. Mitochondrial density in axons of rolofylline treated (striped bars) and untreated (open bars) organotypic hippocampal slices of proaggregant (red), antiaggregant (cyan), or nontransgenic (gray) mice. There is a statistically significant decrease of mitochondria density (by ∼10%) as an effect of rolofylline treatment (two-way ANOVA, *P < 0.05).
Fig. S8.
PPRs and LTP for (treated) acute slices of proaggregant Tau transgenics (ΔK280) and littermate controls (LCtrl.). (A) PPR (EPSP2/EPSP1) for multiple interstimulus intervals of untreated (open bars) and rolofylline-treated (A1 ant., closed bars) organotypic slices. Representative averaged traces are displayed. PPF, green bar; pulse inhibition, magenta bar. Error bars indicate SEM. *P < 0.05 and **P < 0.01. (B) The ability to elicit LTP in [rolofylline treated (A1 ant.)] slices of proaggregant Tau transgenic (ΔK280) and littermate controls (LCtrl). Arrow labeled TBS indicates moment of theta-burst stimulation.
Discussion
In the present paper, we compared an aggregation-prone species of Tau with its antiaggregant counterpart as well as nontransgenic littermates. We used an organotypic hippocampal slice model to show that both proaggregant and antiaggregant Tau is (hyper)phosphorylated and mislocalized to the somatodendritic compartment in the absence of frank Tau aggregation. Although missorting and/or hyperphosphorylation of Tau may be a prerequisite for Tau pathology to occur, we clearly show here that it is not sufficient to cause neuronal dysfunction. This argues that increased aggregation propensity (the only difference between proaggregant and antiaggregant Tau) is essential for Tau toxicity. Despite of the high (>99.5%) homology of the two Tau species, only proaggregant Tau accumulates as axonal spindle-shaped grains in a pathological “pretangle” conformation, similar to argyrophilic grains in humans. Proaggregant Tau, but not antiaggregant Tau, decreases dendritic spine number and the ATP levels in neurons, which further supports the role of aggregation propensity as the mode of toxic action. Surprisingly, the axonal aggregates of Tau do not colocalize with known aggregation markers. Instead, presynapses are impaired in proaggregant Tau transgenic slices, giving rise to a general reduction of neuronal activity, which has been reported previously for Tau P301L-expressing mice as well (15). The reduced ATP levels, dendritic spine loss, diminished neuronal activity, and impaired presynaptic functioning are reminiscent of adenosine A1 receptor signaling. We therefore hypothesized that inhibition of the adenosine A1 receptor signaling may be able to restore neuronal functioning. Indeed, presynaptic functioning, neuronal activity, as well as the reduction of dendritic spines in the proaggregant Tau transgenic organotypic slices are normalized by application of the highly selective adenosine A1 receptor antagonist rolofylline. When administrated orally to proaggregant Tau transgenic mice, rolofylline restores cognitive functioning and strengthens basal synaptic transmission, which is known to be subdued as a result of adenosine signaling (26). Adenosine, when bound to the A1 receptor, has an inhibitory function on many organs including the brain where it reduces neurotransmitter release (11). Adenosine is normally produced both extracellularly and intracellularly where adenosine is formed by degradation of AMP. Because intracellular ATP levels are 50 times higher than AMP levels, small changes in the ATP catabolism lead to dramatic changes in AMP and subsequently adenosine levels. The exact mechanism by which Tau is able to reduce presynaptic functioning and subdue neuronal activity remains to be determined. However, we see a reduction of ATP in proaggregant Tau transgenic neurons, which may be caused by a release of ATP from the neurons or a shift from ATP to AMP intraneuronally, both leading to high extracellular adenosine levels (27). The role for adenosine in the CNS is ambiguous. Adenosine is very important for the circadian rhythm and for neuroprotective effects when bound to the adenosine A1 receptor (28). Concomitantly, the impaired neurotransmitter release by adenosine A1 receptor signaling blocks memory formation (29). So adenosine A1 signaling seems to drive neuronal networks from the (highly) excitable state to the rest and repair state, both of which are important for maintaining synaptic functioning as well as learning and memory. However, prolonged activation of the adenosine A1 receptor (due to pathological Tau, Aβ, or other chronic stressors) may bring the neurons in a permanent state of hypoexcitability impairing neuronal functioning. In this study, we provide evidence that antagonizing the adenosine A1 receptor can restore the Tau-induced neuronal dysfunction in a tauopathy mouse model. Rolofylline has never been tested as a treatment for any human neurodegenerative disease. As a diuretic, it failed in a phase III trial for patients suffering from acute heart failure due to unimproved renal function. Adverse effects were, however, limited (30). It has been reported that adenosine receptors are increased in neurons in the degenerating human brain and that administration of an adenosine A1 receptor agonist induces Aβ production, Tau phosphorylation, and Tau missorting in vitro (31). Down syndrome patients, known to suffer from early-onset AD, have higher levels of adenosine than aged matched controls (32). However, due to the very short half-life of adenosine (<10 s in blood), there have been no studies on adenosine levels in human brain (33). Brain hypometabolism (i.e., neuronal hypoactivity), on the other hand, is a characteristic hallmark during and preceding neurodegeneration (34, 35). Cognitively normal ApoE4 homozygous subjects show reduced glucose metabolism as is seen in AD patients (36). The same reduction of glucose metabolism occurs in preclinical individuals with a genetic predisposition for familial AD, long before the onset of cognitive decline (37). Systemic administration of GABAA agonists (benzodiazepines), which inhibit neuronal activity, almost double the risk for AD when taken for more than 6 mo (38). On the other hand, the most common psychoactive drug in the world (caffeine), an adenosine receptor antagonist that boosts neuronal activity, protects against AD (39). Stimulation of the perforant path in an Aβ-based mouse model is sufficient to restore memory retrieval (40). Similarly, transcranial magnetic stimulation in humans increases brain network activity and performance of associative memory, emphasizing the benefit of increased bona fide network activity (41). In conclusion, we show that Tau protein impairs neurons through its ability to aggregate, which in turn leads to reduced neuronal activity, lowered ATP levels, and dendritic spine loss. In both the organotypic slice model as well as in transgenic mice, one can alleviate the process of neuronal dysfunction by administration of the adenosine A1 receptor antagonist rolofylline, a compound that is proven to be safe in humans. Since neuronal hypoactivity/hypometabolism precedes human neurodegenerative diseases as well, restoration of normal neuronal activity by rolofylline administration may prove to be a successful treatment to counteract the Tau-induced brain dysfunction.
Materials and Methods
See SI Materials and Methods for detailed descriptions. All experiments were approved by an animal welfare committee of the agency for Nature, Environment, and Consumer Protection in North Rhine-Westphalia, Germany.
Slices were analyzed at DIV30 to DIV35. The localization of (phosphorylated) Tau was examined by immunofluorescence in organotypic hippocampal slices. Axonal localization of Tau, intraneuronal ATP levels, and mitochondrial motility were studied by using biolistic transfection of organotypic hippocampal slices. The mRNA quantification was performed by using real-time PCR. The synaptic transmission was analyzed by assessing the field excitatory postsynaptic potentials applied in a paired-pulse protocol. Dendritic spine levels in organotypic slices were quantified by biolistic transfection of TandemTomato or diolistic labeling using DiI. Organotypic proaggregant Tau transgenic mice and age-matched controls of 14 mo of age were used to test the effectiveness of rolofylline as a treatment for Tau-induced dysfunction by oral administration. The behavioral performance of mice treated with rolofylline was tested using the Y-maze, novel object recognition task, and fear conditioning testing. The basic synaptic transmission in acute slices was assessed by measuring the I/O responses of field excitatory postsynaptic potentials. All results are presented as mean ± SEM. Statistical comparisons between two groups were tested using Student’s t test. Comparisons among groups were tested using one-way or two-way ANOVA and Tukey’s test or Dunnett’s test for post hoc testing. P < 0.05 was considered significant.
SI Materials and Methods
Animals.
Transgenic mice coexpressing the human full-length Tau protein (2N4R, the largest isoform in human CNS) with the FTDP-17 mutation ΔK280 (deletion of lysine 280) and the reporter firefly luciferase gene under control of a bidirectional tetO-responsive CMV promoter were crossed with a CaMKIIα-tTA transgenic mouse described before (5). This yielded a neuron-specific repressible mutant (ΔK280) human Tau transgenic mouse expressing luciferase as a reporter. In parallel, another Tau transgenic mouse was generated with the same constructs as the human Tau ΔK280 transgenic mouse albeit with two additional prolines in the hexapeptide motifs of the repeat domain of the Tau protein, which serve as β-sheet breakers (human Tau ΔK280-PP) (5). The human Tau ΔK280 and human Tau ΔK280-PP transgenic mice are dubbed proaggregant and antiaggregant Tau transgenic mice, respectively. Before experiments, we have selected transgenic mice with comparable Tau expression by in vivo bioluminescence imaging to reduce interindividual variations (6). All animals were housed and tested according to standards of the German Animal Welfare Act.
Organotypic Hippocampal Slice Preparation.
Organotypic slices of the hippocampus were prepared using a protocol published previously (42). Briefly, mice (6–10 d old) were killed by decapitation, and brains were rapidly extracted, after which hippocampi were dissected and sliced in 400-µm slices with a McIlwain tissue chopper. Slices were immediately transferred to semiporous cell culture inserts (Merck Millipore) in six-well plates containing 1 mL of preincubated culture medium [50% (vol/vol) MEM, 25% (vol/vol) horse serum, 25% (vol/vol) Hank’s balanced salt solution, 4.5 mg/mL glucose, and 50 U/mL penicillin/streptomycin]. The genotype was determined by detection of the bioluminescence due to luciferase activity by using the IVIS system (PerkinElmer) the next day after which medium was changed. The medium was subsequently changed every 3 d. Analysis of the slices was done after 30 d in vitro (DIV30), unless stated otherwise.
Pharmacological Treatments of Organotypic Hippocampal Slice Cultures and Animals.
Organotypic slices of proaggregant and antiaggregant Tau transgenic mice with matching littermate controls were treated from the first medium change (DIV1) onward until time of analysis (DIV30). One exception was the for the dendritic spine count of the rolofylline and untreated slices, which were treated for 7 d from DIV30 onward. Compounds were replaced with every medium change. The compounds and concentrations are listed in Table S4. Mice were treated with custom-made food pellets (Ssniff Spezialdiäten GmbH) containing 1.5 mg adenosine A1 receptor antagonist rolofylline (KW-3902) per kg. The dosage of rolofylline was 0.2 mg⋅kg−1⋅d−1. About eight to nine control littermates and proaggregant mice were treated with rolofylline during 10 d, and then behavior analysis was started, followed by electrophysiological analysis. Animals were continuously treated until the end of the electrophysiological study.
Immunohistochemistry.
Organotypic slices were fixed while being attached to the Millicell membrane in 4% formaldehyde in PBS for 1 h at 4 °C. After washing with PBS and 0.1 M glycine in PBS, slices were incubated overnight in 0.5% Triton X-100 at 4 °C. The slices were incubated in 20% horse serum for 4 h at room temperature and subsequently incubated with primary antibodies in PBS for 72 h at 4 °C (Table S5 for concentrations and sources). After washing with PBS, slices were incubated with secondary antibody for 24–48 h. After rinsing and counterstaining with DAPI, slices were mounted using Permafluor mounting solution (Thermo Scientific).
Table S5.
Antibodies, epitope, concentration, and source
| Antibody | Epitope | Concentration | Brand/source |
| MAP2 | Rat brain microtubule-associated proteins (MAPs) | 1:200 | Sigma |
| K9JA (Pan Tau) | C-terminal part of human Tau (amino acids 243–441) | 1:1,000 | DAKO |
| TauY9 (human Tau) | N-terminal part of human 2N4R Tau (amino acids 12–27) phosphorylated at Tyr18 | 1:1,000 | Enzo |
| 12E8 | Tau phosphorylated at S262 and/or Ser356 | 1:500 | P. Seubert, Alan Farma |
| PHF-1 | Epitope around Ser396 and Ser404 phosphorylated sites (human Tau) | 1:250 | Peter Davies |
| AT8 | PHF-Tau (pSer202/pThr205) | 1:500 | Thermo |
| Vimentin | Recombinant human vimentin | 1:100 | Acris Antibodies |
| Ubiquitin | Bovine ubiquitin | 1:100 | DAKO |
| Lamp1 | NIH/3T3 mouse embryo fibroblast tissue culture cell membranes | 1:100 | Santa Cruz |
| SQSTM1 (P62) | Amino acids 151–440 of human SQSTM1 | 1:100 | Santa Cruz |
| HSC70 | Full length native Hsc70 protein (Hamster) | 1:100 | Abcam |
| MC-1 | N terminus (amino acids 7–9) and amino acids 313–322 (human Tau) | 1:5 | Peter Davies |
| α-NeuN | Purified cell nuclei from mouse brain | 1:1,000 | Millipore |
| AT-180 | Amino acids around pThr231 of Tau | 1:500 | Thermo |
| TIA-1 | C terminus of human TIA-1 | 1:100 | Santa Cruz |
| Actin | C-terminal peptide of β-Actin | 1:10,000 | Sigma |
| Synaptophysin | Synaptosome preparation from rat retina | 1:500 | Sigma |
Biolistic Transfection.
The biolistic transfection of the ATP sensor [ATeam (AT1.03)], RFP-mito, TandemTomato, and the cotransfection of TandemTomato+ΔK280 Tau was done as was described previously using the Helios gene gun (Bio-Rad) (43). In short, the plasmid(s) were diluted in 50 µL of PCR-grade water with a final concentration of 1 µg/µL. The gold bullets (6–8 mg, 1.6-µm diameter) were mixed with 100 µL of 50 mM spermidine after which the DNA solution was added. Next, 100 µL of 1 M CaCl2 was added to this solution in a dropwise manner followed by brief sonication. After 10 min of incubation at room temperature, coated gold particles were rinsed in 100% ethanol for three times and resuspended in 3 mL of polyvinylpyrrolidone (PVP) (40 µg/mL ethanol). The gold-coated cartridges were generated using the tube prepping station according to the previously described protocol (44). For the cotransfection, we used the competitiveness between the two plasmids to favor the expression of TandemTomato at the expense of Tau to get human Tau levels similar to what is seen in neurons of Tau transgenic slices. The slices were transfected at DIV25 to DIV27 (DIV35 for TandemTomato) using the Helios gene gun. The pressure used for transfection was 20.7 bar (300 psi) with a shooting distance of 4 cm. The Millicell membrane inserts containing the slices were transferred to a glass-bottom culture dish (ø, 50 mm; Harvard Apparatus), which contained 1 mL of culture medium after 2–4 d for live-cell analysis. A long–working-distance 20× objective (Olympus) and 2.5× Optivar magnification combined with the Cell Observer microscope (Zeiss) and MetaMorph software (Molecular Devices) were used for image acquisition.
Dendritic Spines Quantification.
Organotypic hippocampal slices of Tau transgenic and littermate control mice (DIV30) were labeled using DiI-labeled gold particles using a previously described protocol with slight modifications (44). In brief, a suspension was made consisting of 60-mg gold particles in dimethylformamide containing 20 mg/mL DiI. The suspension was transferred to a glass Petri dish and left to dry, after which the DiI/gold was cut to a fine powder using a razor blade. The DiI/gold powder was sonicated in water and resuspended in 2.5 mL of a 60 µg/mL PVP solution. A circle was drawn on the microcarriers using a hydrophobic barrier pen after which 50 µL of the gold suspension was pipetted into the center of the circle. The organotypic slices were labeled using the PDS-1000/He System (Bio-Rad). The bombarded organotypic slices were immediately fixed with 4% formaldehyde in PBS and stored at 4 °C for 72 h to allow the DiI to diffuse. The TandemTomato-transfected slices were fixed with 4% formaldehyde in PBS and stored at 4 °C until further processing. All slices (DiI stained and transfected) were rinsed in PBS and counterstained using DAPI. Images were acquired using the FV1000 microscope (Olympus) with a 63× objective and 5× digital zoom. Image Z stacks were acquired and processed by deconvolution applying the 3D Huygens Deconvolution and Analysis Software (Scientific Volume Imaging), after which spines were counted using the trainable spine classifier of the NeuronStudio software (Computational Neurobiology and Imaging Center).
FRET Analysis of Transfected (Transgenic) Organotypic Slices.
For measuring neuronal ATP, we transfected (transgenic) organotypic hippocampal slices with a plasmid coding for the ATeam (AT1.03) ATP sensor (a kind gift of Dr. H. Imamura, Kyoto University, Kyoto, Japan). Transfected slices were analyzed using a Cell Observer microscope (Zeiss) with a matching Definite Focus unit and a DV2 Dual-View system (Photometrics). Neurons were selected based on their morphology. To measure CFP and YFP emission simultaneously, the region of interest (axon, dendrite, or soma) was delineated as well as a neighboring untransfected region of the slice for background subtraction. Background-subtracted measurements were used for determining the net YFP/CFP ratio. In addition, we transfected organotypic slices with a plasmid coding for a mitochondrial target sequence fused to RFP (RFP-mito) (45). RFP-mito was measured for analysis of mitochondrial morphology and trafficking using the Cell Observer microscope.
Quantitative PCR.
Total RNA was extracted by using the standard TRIzol/1-bromo-2 chloropropane extraction protocol with subsequent generation of cDNA using the first-strand cDNA synthesis kit (Thermo Scientific). Primers were designed using the Primer3Plus program. They were required to be intron spanning and yielding a PCR product of 70–120 bp in size. Primer sequences are listed in Table S6. The best three housekeeping genes (Mprip, Gapdh, and Rs27a) were selected from a selection of five by using Gnorm. The IQ5 real-time PCR machine (Bio-Rad) and the Maxima SYBR Green qPCR Master Mix (Thermo) were used for the actual quantitative PCR (qPCR). Raw fluorescence data were exported from the IQ5 software and subsequently loaded in the LinRegPCR program where relative mRNA expression levels were quantified based on an assumption-free analysis.
Table S6.
Genes and their respective oligo sequences (qPCR)
| Gene | Full name | Alias | Sequence forward oligo | Sequence reverse oligo |
| Mprip | Myosin phosphatase Rho interacting protein | CTGACGCAGGCAAAACCC | GATGAAGAATCGTCGCTGCC | |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | AAAAGGGTCATCATCTCCGC | ATTTCTCGTGGTTCACACCC | |
| Rps27a | Ribosomal protein S27a | CAGAGGCTGATCTTTGCTGG | GTCTCAACACCAGATGAAGGG | |
| Fos | FBJ osteosarcoma oncogene | cFOS | TCCTACTACCATTCCCCAGC | ATAAAGTTGGCACTAGAGACGG |
| Hspa1a | Heat shock protein 1A | HSP70 | CCTGAACAAGAGCATCAACCC | ACGTTCTCCGACTTGTCCC |
| Hmox1 | Heme oxygenase 1 | Ho-1 | TCAGGTGTCCAGAGAAGGC | TGGGTTCTGCTTGTTGCG |
| Syvn1 | Synovial apoptosis inhibitor 1, synoviolin | Hrd-1 | ACACATTCCCACTCTTTGCC | GTACAGTGTGTTCATGTTGCG |
| Hspa4l | Heat shock protein 4 like | Osp94 | TGGAAATGCAGCTAAGAGCC | CAAATGATCGCCCATGAAGC |
| Gfap | Glial fibrillary acidic protein | AAGAGACAGAGGAGTGGTATCG | CGATAGTCGTTAGCTTCGTGC | |
| Rbfox3 | RNA binding protein, fox-1 homolog (C. elegans) 3 | NeuN | GTAGAGGGACGGAAAATTGAGG | ATAGACTGTTCCTACCACAGGG |
All oligos were designed to work with 50 mM monovalent cations, 500 nM oligo, 3 mM Mg2+, and 0.8 mM dNTPs at a Tm of 60 °C.
Electrophysiology.
Organotypic hippocampal slices of proaggregant Tau transgenic and littermate control mice were excised from the culture insert at DIV30. The excised pieces of membrane were transferred to a Petri dish containing gelled medium (0.5% agarose). The slices were left to recover in the incubator, after which they were transferred to the recording chamber and submerged in carbogenated aCSF (126 mM NaCl, 21 mM NaHCO3, 3 mM KCl, 2 mM CaCl2, 1.8 mM MgSO4, 1.25 mM NaH2PO4, and 10 mM glucose, pH 7.4, 32 °C) at a flow rate of 4 mL/min. The field excitatory postsynaptic potentials were recorded in the stratum radiatum of the CA1 using a broken tip filled with aCSF as a stimulus electrode and a 3-mΩ aCSF filled glass pipette as a recording electrode. Recordings were sampled at 10 kHz, 10 times preamplified with a custom-made preamplifier, and processed using a HEKA double patch-clamp EPC 10 USB amplifier (HEKA Elektronik Dr. Schulze GmbH).
Acute Slices.
Mice were sedated using isoflurane and killed by decapitation, after which the brain was rapidly extracted from the scull and submerged into cold (4 °C) carbonated aCSF (formulation as mentioned for the organotypic slices). Transverse hippocampal slices (350 µm thick) were cut using a Leica VT 1200S vibratome and transferred to a storage chamber where they were left to recover for at least 1 h at room temperature in carbonated aCSF. Up to four slices at a time were transferred to recording chambers of the multiple slice evaluation system Synchroslice [Lohmann Research Equipment (LRE)]. Slices were perfused with heated (32 °C ± 0.5 °C) aCSF with a flow rate of 1 mL per min. A monopolar (impedance, <100 kΩ) electrode was placed in the stratum radiatum and a quartz glass insulated platinum/tungsten fiber electrode (Thomas Recording) was placed afferently from the stimulating electrode under visual control through a CCD camera system. Data acquisition (sampling rate, 10 kHz per channel), electrical stimulation, and off-line analysis were done using the automated software (SynchroBrain; LRE). The I/O curve amplitudes were averaged and fitted by a Boltzmann equation. Excitatory postsynaptic potential (EPSP) slopes were determined for PPR and LTP protocols.
Behavior.
Y-maze test.
The Y-maze task was used to analyze hippocampus-dependent memory in 13- to 15- mo-old mice. The dimensions of the used Y-maze were 30 × 6 × 15 cm (length by width by height) (Panlab). In the training session, one arm was closed (novel arm), and mice were placed in the stem arm of the Y (home arm) and allowed to explore this arm and the other available arm (old arm) for 10 min. The mice were placed back in their home cage after exploration. To assess long-term memory, 4 h postexploration, the closed arm was opened, and mice were placed in the stem arm of the Y-maze and allowed to freely explore all three arms for 5 min. Arm preference was determined by calculating the following: time spent in each individual arm*100/total time spent in both arms (both old and novel arm).
Novel object recognition test.
Mice were tested in a square open field (45 cm long) (Panlab) located in a room with dim lighting. NORT was performed as previously described (46). Briefly, mice were habituated to the open field in the absence of the objects for 10 min/d over 2 d. During the training period, mice were placed in the open field with two identical objects for 10 min. The retention test was performed 24 h posttraining (long-term memory) by placing the mice back to the open field for 5-min session, and by randomly exchanging the familiar object for a novel one.
Fear conditioning.
Mice were tested in the fear conditioning apparatus as described elsewhere (47). Briefly, during the training, mice were introduced into the chamber and allowed to explore it during 2 min. A tone (sound clue) was then presented at a level of 80 dB for 30 s. A mild foot shock (0.5 mA) was administered during the last 2 s of the tone presentation and coterminates with the tone. After the shock presentation, mice were kept for 30 s in the chamber to allow memory consolidation. Contextual and cued fear conditionings were tested 24 h after the training. For contextual conditioning test, mice were placed into the chamber and the percentage of freezing per minute was analyzed during 4 min. After 4 min, the clue conditioning test was performed by presenting the tone during 30 s. Freezing in the presence and after the tone was analyzed during 2 min. Results were expressed as the mean percentage of freezing in the presence and absence of tone.
Statistics.
For immunoblot quantification of total Tau (antibody K9JA), phospho-Tau (12E8), and phospho-Tau (PHF-1), we performed a one-way ANOVA with Tukey’s test for multiple comparisons as a post hoc test to test for statistical significance. The spine density, mitochondrial density, and ATP levels were also tested for statistical significance by applying a one-way ANOVA with Tukey’s test for multiple comparisons as a post hoc test. For the qPCR-based miniscreen data, we used a two-way ANOVA and Dunnett's multiple-comparisons test for post hoc testing. We applied a two-way ANOVA with Tukey’s test for multiple comparisons for post hoc testing to test differences in PPRs for statistical significance. For the qPCR of the treated organotypic slices, we used a two-way ANOVA and Dunnett's multiple-comparisons test for post hoc testing. We applied a two-way ANOVA with Tukey’s test for multiple comparisons for post hoc testing to test for the treatment effect of the treated organotypic slices. For behavior testing (Y-maze, NORT, and fear conditioning), statistical analysis was performed using Student’s t tests. Statistical analysis of the electrophysiology data of acute slices (I/O curve slope, maximum EPSP amplitude, and the treatment effect in the PPR testing), we applied a two-way ANOVA with Tukey’s test for multiple comparisons for post hoc testing.
Acknowledgments
We thank Dr. C. Ginkel and her team of the German Center for Neurodegenerative Diseases (DZNE) animal facility as well as Dr. A. Haemisch and his team at the animal facility at the University of Hamburg Medical School for their continuous help in mouse breeding. We gratefully acknowledge reagents from Prof. Dr. E. Kandel (Columbia University; CaMKIIα-tTA transgenic mice), Dr. P. Seubert (Elan Pharma; 12E8 antibody), Dr. P. Davies (Albert Einstein College; MC1 and PHF1 antibodies), and Dr. H. Imamura (Kyoto University) for the ATeam ATP sensor plasmid. This research was supported by the Max Planck Society, DZNE, Wellcome Trust/Medical Research Council, Katharina-Hardt-Stiftung, and Tau Consortium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603119113/-/DCSupplemental.
References
- 1.Takashima A. Tauopathies and tau oligomers. J Alzheimers Dis. 2013;37(3):565–568. doi: 10.3233/JAD-130653. [DOI] [PubMed] [Google Scholar]
- 2.Rizzu P, et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in The Netherlands. Am J Hum Genet. 1999;64(2):414–421. doi: 10.1086/302256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barghorn S, et al. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39(38):11714–11721. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- 4.Momeni P, et al. Clinical and pathological features of an Alzheimer’s disease patient with the MAPT Delta K280 mutation. Neurobiol Aging. 2009;30(3):388–393. doi: 10.1016/j.neurobiolaging.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckermann K, et al. The beta-propensity of Tau determines aggregation and synaptic loss in inducible mouse models of tauopathy. J Biol Chem. 2007;282(43):31755–31765. doi: 10.1074/jbc.M705282200. [DOI] [PubMed] [Google Scholar]
- 6.Van der Jeugd A, et al. Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol. 2012;123(6):787–805. doi: 10.1007/s00401-012-0987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Bergen M, et al. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming beta structure. Proc Natl Acad Sci USA. 2000;97(10):5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocanu MM, et al. The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J Neurosci. 2008;28(3):737–748. doi: 10.1523/JNEUROSCI.2824-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Lubitz DK. Adenosine and cerebral ischemia: Therapeutic future or death of a brave concept? Eur J Pharmacol. 1999;371(1):85–102. doi: 10.1016/s0014-2999(99)00135-1. [DOI] [PubMed] [Google Scholar]
- 10.Reddington M, Lee KS, Schubert P. An A1-adenosine receptor, characterized by [3H] cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neurosci Lett. 1982;28(3):275–279. doi: 10.1016/0304-3940(82)90070-2. [DOI] [PubMed] [Google Scholar]
- 11.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Renard D, et al. Brain FDG-PET changes in ALS and ALS-FTD. Acta Neurol Belg. 2011;111(4):306–309. [PubMed] [Google Scholar]
- 13.Diehl J, et al. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Aging. 2004;25(8):1051–1056. doi: 10.1016/j.neurobiolaging.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Ciarmiello A, et al. 18F-FDG PET uptake in the pre-Huntington disease caudate affects the time-to-onset independently of CAG expansion size. Eur J Nucl Med Mol Imaging. 2012;39(6):1030–1036. doi: 10.1007/s00259-012-2114-z. [DOI] [PubMed] [Google Scholar]
- 15.Menkes-Caspi N, et al. Pathological tau disrupts ongoing network activity. Neuron. 2015;85(5):959–966. doi: 10.1016/j.neuron.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez RD, Grinberg LT. Argyrophilic grain disease: An underestimated tauopathy. Dement Neuropsychol. 2015;9(1):2–8. doi: 10.1590/S1980-57642015DN91000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004;167(1):99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amniai L, et al. Alzheimer disease specific phosphoepitopes of Tau interfere with assembly of tubulin but not binding to microtubules. FASEB J. 2009;23(4):1146–1152. doi: 10.1096/fj.08-121590. [DOI] [PubMed] [Google Scholar]
- 19.Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 20.Imamura H, et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA. 2009;106(37):15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastião AM. Adenosine: Setting the stage for plasticity. Trends Neurosci. 2013;36(4):248–257. doi: 10.1016/j.tins.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Williams M, Braunwalder A, Erickson TJ. Evaluation of the binding of the A-1 selective adenosine radioligand, cyclopentyladenosine (CPA), to rat brain tissue. Naunyn Schmiedebergs Arch Pharmacol. 1986;332(2):179–183. doi: 10.1007/BF00511410. [DOI] [PubMed] [Google Scholar]
- 23.Bulic B, Pickhardt M, Mandelkow E. Progress and developments in tau aggregation inhibitors for Alzheimer disease. J Med Chem. 2013;56(11):4135–4155. doi: 10.1021/jm3017317. [DOI] [PubMed] [Google Scholar]
- 24.Palmer TM, Poucher SM, Jacobson KA, Stiles GL. 125I-4-(2-[7-amino-2-[2-furyl][1,2,4]triazolo[2,3-a][1,3,5] triazin-5-yl-amino]ethyl)phenol, a high affinity antagonist radioligand selective for the A2a adenosine receptor. Mol Pharmacol. 1995;48(6):970–974. [PMC free article] [PubMed] [Google Scholar]
- 25.Bartley AF, Dobrunz LE. Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells. Eur J Neurosci. 2015;41(11):1402–1415. doi: 10.1111/ejn.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunwiddie TV, Hoffer BJ. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980;69(1):59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latini S, Pedata F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J Neurochem. 2001;79(3):463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 28.Pedata F, et al. Purinergic signalling in brain ischemia. Neuropharmacology. 2016;104:105–130. doi: 10.1016/j.neuropharm.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Normile HJ, Barraco RA. N6-Cyclopentyladenosine impairs passive avoidance retention by selective action at A1 receptors. Brain Res Bull. 1991;27(1):101–104. doi: 10.1016/0361-9230(91)90288-u. [DOI] [PubMed] [Google Scholar]
- 30.Massie BM, et al. PROTECT Investigators and Committees Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363(15):1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 31.Angulo E, et al. A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol. 2003;13(4):440–451. doi: 10.1111/j.1750-3639.2003.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stocchi V, Magnani M, Cucchiarini L, Novelli G, Dallapiccola B. Red blood cell adenine nucleotides abnormalities in Down syndrome. Am J Med Genet. 1985;20(1):131–135. doi: 10.1002/ajmg.1320200116. [DOI] [PubMed] [Google Scholar]
- 33.Möser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256(4 Pt 1):C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- 34.Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(4):a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kljajevic V, Grothe MJ, Ewers M, Teipel S. Alzheimer’s Disease Neuroimaging Initiative Distinct pattern of hypometabolism and atrophy in preclinical and predementia Alzheimer’s disease. Neurobiol Aging. 2014;35(9):1973–1981. doi: 10.1016/j.neurobiolaging.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Reiman EM, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy AM, et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer’s disease. Neurosci Lett. 1995;186(1):17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- 38.Billioti de Gage S, et al. Benzodiazepine use and risk of Alzheimer’s disease: Case-control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 1):S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 40.Roy DS, et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531(7595):508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JX, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 43.Woods G, Zito K. Preparation of gene gun bullets and biolistic transfection of neurons in slice culture. J Vis Exp. 2008;2008(12):e675. doi: 10.3791/675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seabold GK, Daunais JB, Rau A, Grant KA, Alvarez VA. DiOLISTIC labeling of neurons from rodent and non-human primate brain slices. J Vis Exp. 2010;2010(41):e2081. doi: 10.3791/2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat Methods. 2007;4(7):559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- 46.Anglada-Huguet M, et al. Prostaglandin E2 EP1 receptor antagonist improves motor deficits and rescues memory decline in R6/1 mouse model of Huntington’s disease. Mol Neurobiol. 2014;49(2):784–795. doi: 10.1007/s12035-013-8556-x. [DOI] [PubMed] [Google Scholar]
- 47.Curzon P, Rustay NR, Browman KE. 2009. Cued and contextual fear conditioning for rodents. Methods of Behavior Analysis in Neuroscience, Frontiers in Neuroscience, ed Buccafusco JJ (CRC, Boca Raton, FL), 2nd Ed. [PubMed]