Significance
Cocaine elicits a strong motor response when administered in rodents. These effects have been assigned to activation of D1 receptor-mediated signaling in striatal medium spiny neurons (dMSNs). However, we previously have shown that activation of motor activity and signaling in dMSNs was dampened in D2 receptor (D2R) knockout mice. Nevertheless, because of the wide distribution of D2Rs in different neuronal types, it was not possible to assess which D2R-expressing neurons allow cocaine-mediated effects. Using mice with neuron-specific deletion of D2R, we identify D2R+-MSNs (iMSNs) as those enabling the activation of dMSNs in response to cocaine. These studies identify the striatal iMSNs and D2R-mediated signaling as key regulators of intrastriatal functions required for the full expression of the motor and cellular activating effects of cocaine.
Keywords: dopamine, D2 receptor, cocaine, medium spiny neurons, signal transduction
Abstract
The psychomotor effects of cocaine are mediated by dopamine (DA) through stimulation of striatal circuits. Gabaergic striatal medium spiny neurons (MSNs) are the only output of this pivotal structure in the control of movements. The majority of MSNs express either the DA D1 or D2 receptors (D1R, D2R). Studies have shown that the motor effect of cocaine depends on the DA-mediated stimulation of D1R-expressing MSNs (dMSNs), which is mirrored at the cellular level by stimulation of signaling pathways leading to phosphorylation of ERKs and induction of c-fos. Nevertheless, activation of dMSNs by cocaine is necessary but not sufficient, and D2R signaling is required for the behavioral and cellular effects of cocaine. Indeed, cocaine motor effects and activation of signaling in dMSNs are blunted in mice with the constitutive knockout of D2R (D2RKO). Using mouse lines with a cell-specific knockout of D2R either in MSNs (MSN-D2RKO) or in dopaminergic neurons (DA-D2RKO), we show that D2R signaling in MSNs is required and permissive for the motor stimulant effects of cocaine and the activation of signaling in dMSNs. MSN-D2RKO mice show the same phenotype as constitutive D2RKO mice both at the behavioral and cellular levels. Importantly, activation of signaling in dMSNs by cocaine is rescued by intrastriatal injection of the GABA antagonist, bicuculline. These results are in support of intrastriatal connections of D2R+-MSNs (iMSNs) with dMSNs and indicate that D2R signaling in MSNs is critical for the function of intrastriatal circuits.
The intake of drugs of abuse generates a strong elevation of dopamine (DA) levels (1) in brain areas that belong to the limbic system (2, 3). DA signaling thus plays a central role in the mechanisms underlying the behavioral, cellular, and molecular effects of addictive drugs in the central nervous system (4). Indeed, knockout mice for the DA D1 and D2 receptors (D1R and D2R) have altered responses to the psychomotor effects of psychostimulants such as cocaine (5–7).
The psychomotor effects of cocaine strongly depend on the activation of the major striatal output neurons, the medium spiny neurons (MSNs). In particular, MSNs expressing D1R (dMSNs) but not those expressing D2R (iMSN) appear responsible for these effects. Indeed, mice lacking D1R fail to show the motor-stimulating effects of cocaine (7). Cocaine-mediated activation of dMSNs has been shown to activate the cAMP and ERK pathways (8) and to lead to c-fos induction (9). Both c-fos and extracellular signal-regulated kinase (ERK) activation require the simultaneous stimulation of D1R+-MSNs by DA and glutamate (10, 11). dMSNs form the direct pathway, which together with the indirect pathway, formed by iMSNs, are the only striatal output pathways. The balanced activity of these two pathways regulates movements. We have reported that the constitutive knockout of D2R in mice (D2RKO) (12) prevents the motor and cellular (c-fos) responses to acute cocaine administration (6). These results were at odds with the reported involvement of dMSNs and the direct pathway in cocaine’s effects, particularly in light of normal striatal D1R expression and signaling in D2RKO mice (6). We therefore hypothesized that loss of D2R unveils a D2R-mediated permissive control on dMSNs activity. We postulated that the inhibition may occur through the D2R-mediated presynaptic functions. However, D2Rs are expressed by striatal and cortical neurons (13, 14) and DA neurons (5, 15, 16).
Thus, to identify the neurons and the mechanism underlying the blunted response of D2RKO mice to cocaine, we performed experiments in which we compared the motor and cellular responses of the constitutive D2RKO with that of neuron-specific D2R mutants (5). For this process, we used mice lacking D2R either in the MSNs (MSN-D2RKO) or in the DA neurons (DA-D2RKO) (5) and compared their response to WT littermates. Interestingly, these analyses show that the absence of D2R in MSNs, but not in DA neurons, is responsible for the behavioral and cellular phenotype of the constitutive D2RKO. Importantly, we show that the cellular phenotype is rescued by the GABAA antagonist bicuculline. These results demonstrate that the activity of iMSNs affects responses of the dMSNs and the absence of D2R in MSN-D2RKO mice prevents the motor and cellular effects of cocaine. These effects are likely mediated by the presence of collaterals between subtypes of MSNs in the striatum (17) with consequences on their targets.
These data strongly support a leading role of D2 receptors in controlling the physiological response of striatal circuits to cocaine.
Results
Absence of D2R Signaling Affects the Motor Response to Cocaine.
We have reported that cocaine in mice carrying the constitutive deletion of D2R (D2RKO mice) (12) does not induce the robust activation of motor activity as it does in WT mice (6). These results indicated that the absence of D2R in the striatal circuits controlling locomotion abolishes the psychomotor effects of cocaine. However, the heterogeneous distribution of D2R in multiple neurons prevented the possibility to identify the neuronal type involved in the establishment of this phenotype. The recent generation of mice with cell-specific D2R knockout allowed us to address this question. Specifically, we tested the motor-activating effects of cocaine in MSN-D2RKO and in DA-D2RKO mutants, respectively, missing D2R either in striatal MSNs or in midbrain dopaminergic neurons (5).
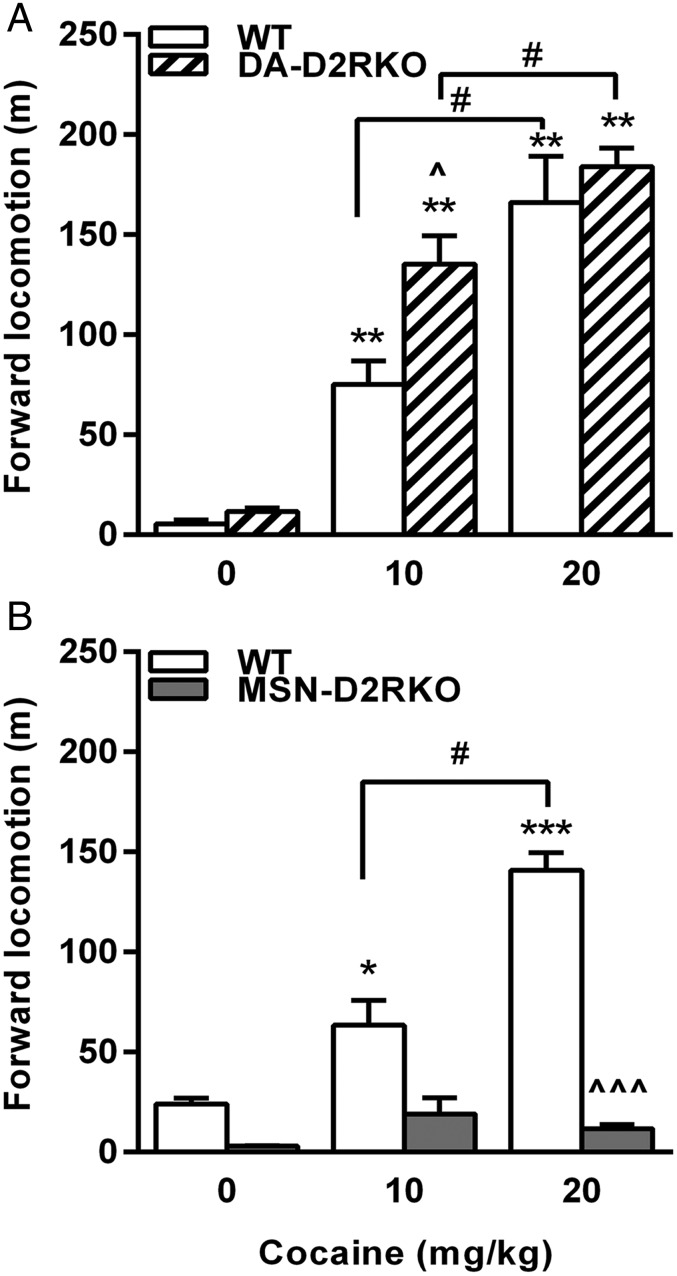
Motor behavior in response to 10–20 mg/kg (i.p.) of cocaine was tested in neuron-specific D2R mutants and respective WT littermates and compared with the response of the constitutive D2RKO mice at 20 mg/kg. MSN-D2RKO and DA-D2RKO together with their WT siblings were administered cocaine 2 h after habituation to a new home cage, and motor activity was recorded for 1 h. WT mice from all lines showed a robust dose-dependent increase of motor activity (P < 0.001) (Fig. 1 and Fig. S1) in response to cocaine. Interestingly, similar to WT siblings, a statistically significant dose-dependent increase of motor activity was observed in cocaine-treated DA-D2RKO mutants compared with saline-treated mice of the same genotype (Fig. 1A). Statistical analyses showed a genotype effect (P < 0.02) due to the stronger motor activation at the dose of 10 mg/kg in DA-D2RKO compared with equally treated WT mice (P < 0.05), likely due to the loss of D2 autoreceptors in these mice (5). In great contrast, MSN-D2RKO mice compared with WT littermates showed a blunted motor response to cocaine at both doses tested (Fig. 1B) [genotype × treatment, F(2, 51) = 24.79, P < 0.0001], in a manner similar to D2RKO mice [genotype × treatment, F(1,28) = 58.99, P < 0.0001] (Fig. S1) (6). Thus, D2R-mediated signaling in MSNs is critical for the psychomotor effects of cocaine.
Fig. 1.
The acute motor response to cocaine requires D2R signaling in MSNs. (A) Similar response to cocaine in DA-D2RKO and WT littermates [genotype × treatment F(2, 40) = 2.001; P > 0.05]; at the dose of 10 mg/kg DA-D2RKO showed a higher response than WT mice (P < 0.05). (B) MSN-D2RKO [genotype × treatment F(2, 51) = 24.79; P < 0.0001] have blunted response to cocaine as compared to WT mice. Values are mean ± SEM (n = 6–14 per treatment per genotype). ***P < 0.001, **P < 0.01, *P < 0.05 saline vs. cocaine; #P < 0.05 10 vs. 20 mg/kg cocaine; ^^^P < 0.001, ^P < 0.05 among genotypes.
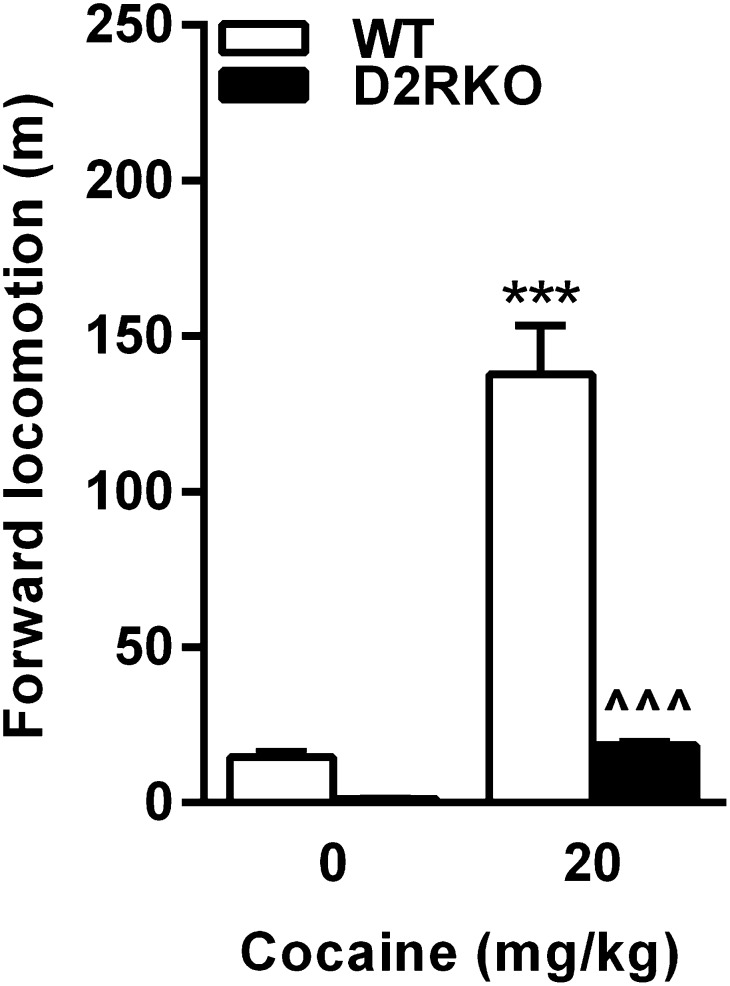
Fig. S1.
Locomotor response to cocaine in D2RKO mice. D2RKO have blunted response to cocaine compared with WT mice [genotype × treatment F(1, 28)= 58.99; P < 0.0001]. ***P < 0.001 vs. WT saline, ^^^P < 0.001 vs. WT cocaine.
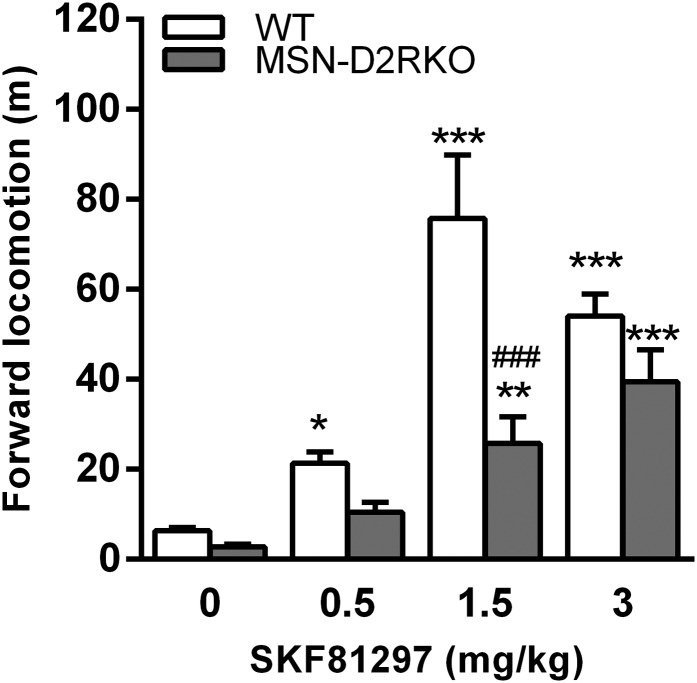
We showed that D1R mRNA and protein levels are not affected in MSN-D2RKO mice (5). However, to investigate whether the blunted response to cocaine might depend on altered D1R-mediated stimulation of MSNs, we tested the effects of SKF81297, a direct D1R-specific agonist, on locomotion in these mice. As in WT, MSN-D2RKO mice show a dose-dependent response to SKF81297 (Fig. S2), although of a lower magnitude because of their reduced motor activity under basal conditions. These results suggest that the loss of D2R in iMSNs partially influence dMSNs activity.
Fig. S2.
Locomotor response to a direct D1R agonist in MSN-D2RKO mice. WT and MSN-D2RKO mice were individually habituated for 1 h to a novel home cage and then injected with increasing concentrations of SKF81297. The locomotor activity was recorded for 1 h after injection. Values represent the mean ± SEM. Two-way ANOVA of data showed significant genotype × treatment F(3, 90) = 10.20; treatment effect F(3, 90) =70.82. P < 0.0001. ***P < 0.001, *P < 0.05 vs. saline; ###P < 0.001 among genotypes; **P < 0.01 vs. saline.
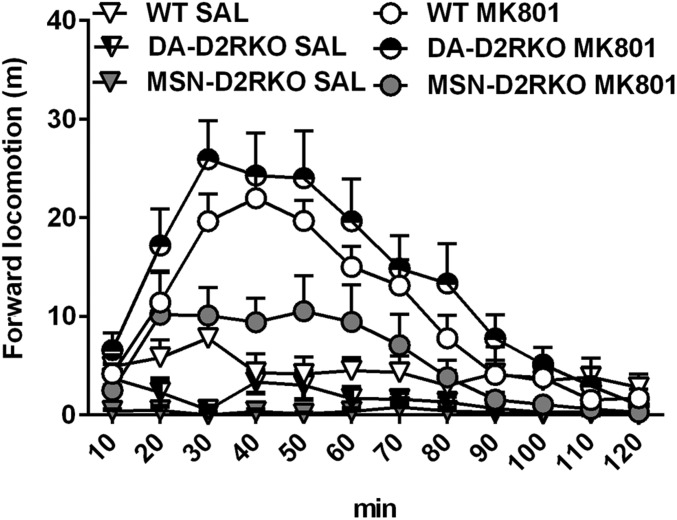
To assess the impact of D2R ablation in MSNs on the striatocortical loop, we tested the effect of the glutamate antagonist MK801 in WT and MSN-D2RKO mice. MK801 is a noncompetitive antagonist of NMDA receptors whose efficacy depends on the level of circulating glutamate (18); administration of MK801 induces a strong activation of motor activity. Thus, we hypothesized that MSN-D2RKO will have a lower response to the motor-activating effects of MK801 in comparison with WT littermates, but also to DA-D2RKO mice. MSN-D2RKO, DA-D2RKO, and WT littermates were thus administered with 0.3 mg/kg MK801, and their motor activity was analyzed in a NHC for 2 h. In agreement with our hypothesis, we found that whereas WT and DA-D2RKO mice show a significant and robust hyperactivity after MK801 administration (Fig. 2) [treatment effect F(11, 495) = 23.44; P < 0.0001], the response of MSN-D2RKO mice was minimal (Fig. 2) [genotype effect F(22, 495) = 38.97; P < 0.01].
Fig. 2.
Decreased response to the glutamate antagonist MK801 in MSN-D2RKO mice. Motor response (cumulative activity count every 10 min) to MK801 of MSN-D2RKO, DA-D2RKO, and WT littermates. MSN-D2RKO show decreased response to MK801 compared with WT and DA-D2RKO mice [genotype effect F(22, 495) = 38.97; P < 0.01]. Values are mean ± SEM (n = 7–11 per treatment per genotype).
These results show that, as expected, loss of D2Rs in iMSNs affects the normal functioning of the cortico-striatal pathway and may participate in the blunted response to cocaine of MSN-D2RKO mice.
Altered c-fos Induction in Striatum Parallels Less Response to Cocaine.
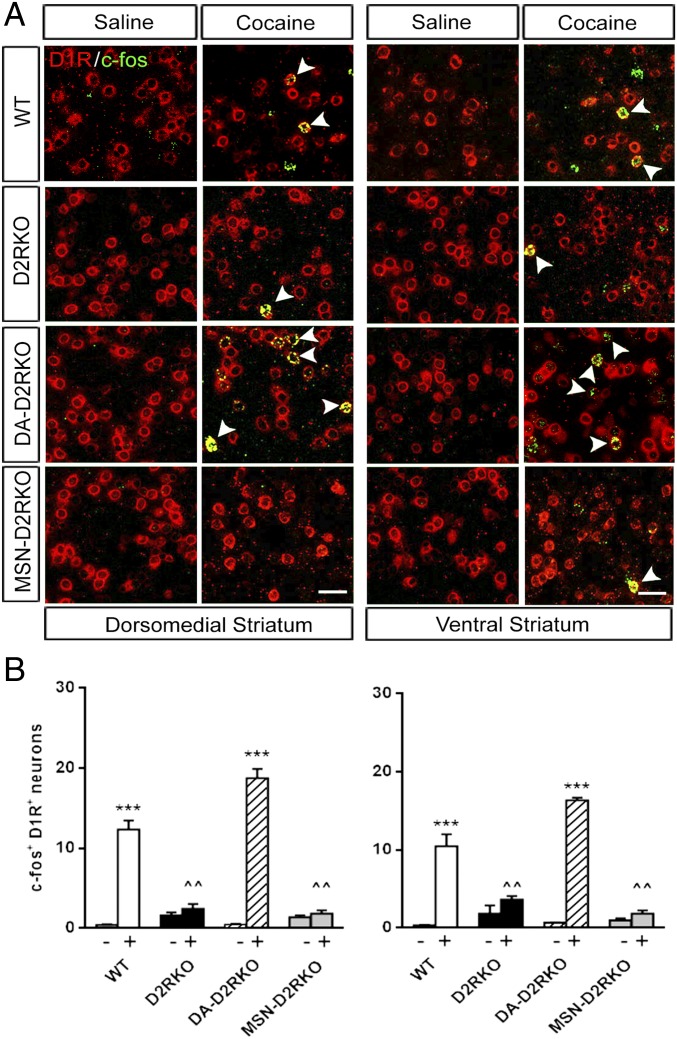
How do these behavioral phenotypes translate at the cellular level? Acute administration of psychostimulants induces the transcriptional activation of immediate early genes, such as c-fos (19) in the dMSNs (2, 20). The contrasting effects of cocaine on the motor activity of D2R mutants brought us to hypothesize that these differences would be mirrored by the c-fos activation pattern in the dMSNs of the mutants. We thus performed double in situ hybridization (dISH) using probes for c-fos and for markers of dMSNs (D1R) and iMSNs (Enkephalin; Enk). c-fos mRNA expression was evaluated in the dorso-medial striatum (DMS) and ventral striatum (VS) of each mutant 1 h after cocaine (20 mg/kg) administration.
The results of these analyses showed the expected robust induction of c-fos expression in dMSNs of the DMS and VS of cocaine-treated WT mice compared with saline-treated controls (Fig. 3). Analyses of sections from cocaine-treated DA-D2RKO mice showed similar c-fos induction in both the DMS and VS as in WT littermates (Fig. 3). Thus, absence of D2Rs from DA neurons, despite the resultant loss of autoreceptor-mediated regulation of DA release (5), does not affect c-fos expression in dMSNs.
Fig. 3.
Blunted induction of c-fos expression in the absence of D2R in MSNs. (A) Representative dISH using D1R (red) and c-fos (green) specific probes hybridized to striatal sections containing either the DS or VS, as indicated. (Scale bars, 25 μm.) Arrowheads indicate cells that are both c-fos+ and D1R+. (B) Quantifications of the number of neurons positive for D1R and c-fos in the DMS (Left) and in the VS of D2R mutants (Right). DMS: F(3, 10) = 27.20, P < 0.0001 and VS: F(3, 10) = 39.27, P < 0.0001. The (−) and (+) symbols indicate absence or presence of cocaine, respectively. Values are mean ± SEM (n = 3 per treatment per genotype). ***P < 0.001 vs. saline-treated mice of the same genotype; ^^P < 0.01 vs. WT cocaine-treated mice.
In contrast, the cocaine-mediated c-fos induction in the dMSNs of MSN-D2RKO striatal sections was completely prevented (Fig. 3). These results strongly resemble those obtained in striata of cocaine-treated D2RKO mice (Fig. 3) (6).
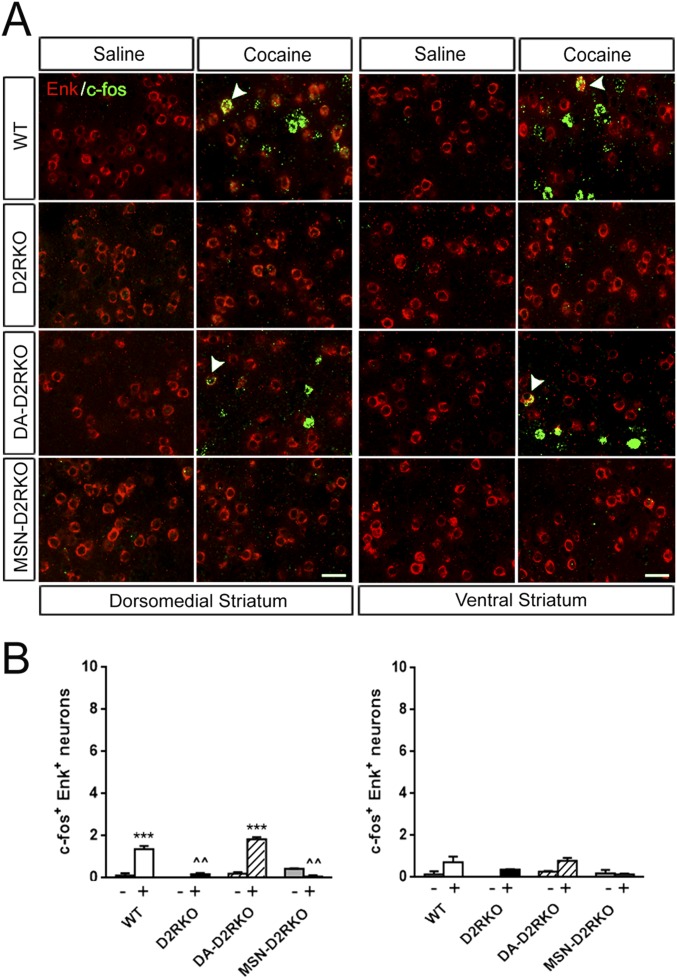
Cocaine has also been reported to stimulate c-fos in a small population of iMSNs (Enk+) (21). In agreement with these findings, we observed a significant induction of c-fos in the iMSNs in the DMS of WT and DA-D2RKO mice compared with saline-treated controls of each genotype (Fig. S3). Conversely, no induction was observed in the same anatomical district of MSN-D2RKO and D2RKO mice (Fig. S3). Cocaine failed to increase c-fos in the iMSNs of the VS in all genotypes.
Fig. S3.
c-fos expression in iMSNs after acute cocaine. (A) Representative dISH using Enk (red) and c-fos (green) specific probes hybridized to striatal sections. (Scale bars, 25 μm.) Arrowheads indicate cells that are both c-fos+ and D1R+. (B) Quantifications of the number of neurons double-positive for Enk and c-fos in the dorsomedial striatum (Left) and in the ventral striatum (Right); genotypes are indicated. Two-way ANOVA of data in the DMS: genotype × treatment F(3, 10) = 57.92, P < 0.0001. In the VS, no significant c-fos induction was observed in any of the genotypes: F(3, 10) = 2.75, P > 0.05. Values are mean ± SEM of the number of c-fos-Enk+ positive cells per frame (387.5 × 387.5 μm). Bonferroni’s post hoc test, ***P < 0.001 vs. saline-treated mice of the same genotype; ^^P < 0.01 vs. WT cocaine-treated mice.
These results indicate that the presence of D2Rs in iMSNs is required not only for the psychomotor response to cocaine, but also for the induction of c-fos in dMSNs.
Reduced ERK and GluR1 Activation Correlates with Less c-fos Activation.
Cocaine-mediated c-fos induction in dMSNs is downstream of DA- and glutamate-activated signaling (8, 11, 21). Both neurotransmitters are also required for the activation of the ERK (9, 10).
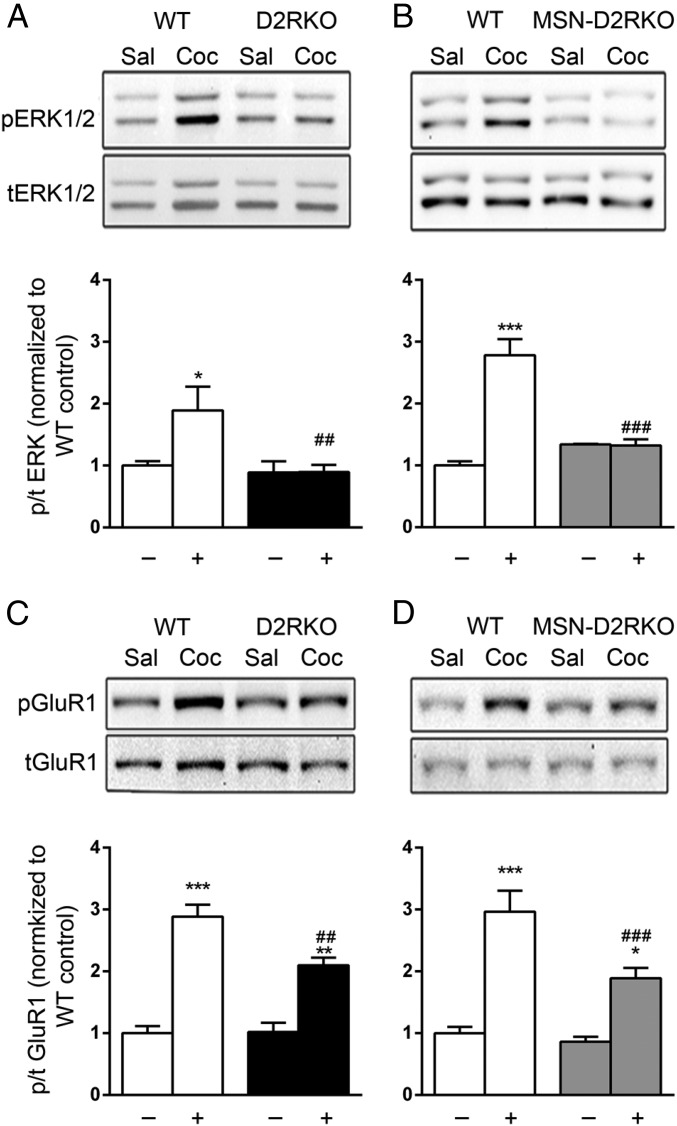
The observation of the blunted response to cocaine but also the altered response to SKF81297 and MK801 suggests that the signaling from either neurotransmitter and possibly both is affected by loss of D2R on iMSNs. Analyses of the induction of the ERK phosphorylation by cocaine (20 mg/kg) in the striatum of D2RKO and MSN-D2RKO showed that in line with the behavioral data, activation of ERK phosphorylation was abolished in both genotypes compared with WT controls (Fig. 4 A and B).
Fig. 4.
Cocaine differentially induces ERK and GluR1 phosphorylation in D2RKO and MSN-D2RKO mice. Blunted pERK1/2 induction by cocaine in D2RKO [F(1, 18) = 5.897, P < 0.05] (A) and in MSN-D2RKO striata (B) [F(1, 8) = 39.07, P < 0.001] compared with WT. Cocaine induced GluR1 Ser845 phosphorylation in D2RKO (C) and MSN-D2RKO striatal extracts (D) compared with WT. [D2RKO: genotype x treatment, F(1, 15) = 6.8, P < 0.05; MSN-D2RKO: genotype × treatment, F(1, 14) = 16.69, P < 0.01]. The (−) and (+) symbols indicate absence or presence of cocaine, respectively. Values are expressed as mean ± SEM (n = 4–5 per treatment per genotype). ***P < 0.001, **P < 0.01, *P < 0.05 vs. saline-treated mice; ###P < 0.001, ##P < 0.01 vs. WT cocaine-treated.
Acute cocaine administration also leads to phosphorylation of the glutamate receptor 1 (GluR1) subunit of the AMPA receptor on Serine845 (Ser845) residue, via activation of the protein kinase A pathway (10).
Analyses of GluR1 phosphorylation at Ser845 in striatal extracts of animals acutely treated with cocaine (20 mg/kg) showed increased phosphorylation in extracts of both genotypes compared with the saline-treated samples [Fig. 4 C and D: C, treatment effect F(1,14) = 91.82, P < 0.001; D, treatment effect F(1,14) = 70.11, P < 0.001]. However, GluR1 phosphorylation in the striatum of cocaine-treated D2RKO [genotype × treatment F(1, 15) = 6.803, P < 0.05] and MSN-D2RKO [genotype × treatment F(1, 14) = 16.69, P < 0.01] extracts was significantly attenuated compared with WT littermates in same conditions. These results indicate that in D2RKO and MSN-D2RKO, the glutamate and DA signaling are altered. This finding strongly points to a defective function of the striato-cortical loop in the phenotype observed in these mice when administered cocaine.
GABA Antagonism Reestablishes the Cellular Response to Cocaine.
Nevertheless, a comparison between the response of MSN-D2RKO mice to cocaine or to SKF81297 and MK801 shows that whereas the latter drugs are still able to induce a significant motor response, cocaine does not. This finding suggests the presence of an additional direct negative control exerted by iMSNs on dMSNs in MSN-D2RKO mice. iMSNs are gabaergic neurons; previous studies have demonstrated that these neurons modulate the activity of the indirect pathway and send collaterals to dMSNs (17).
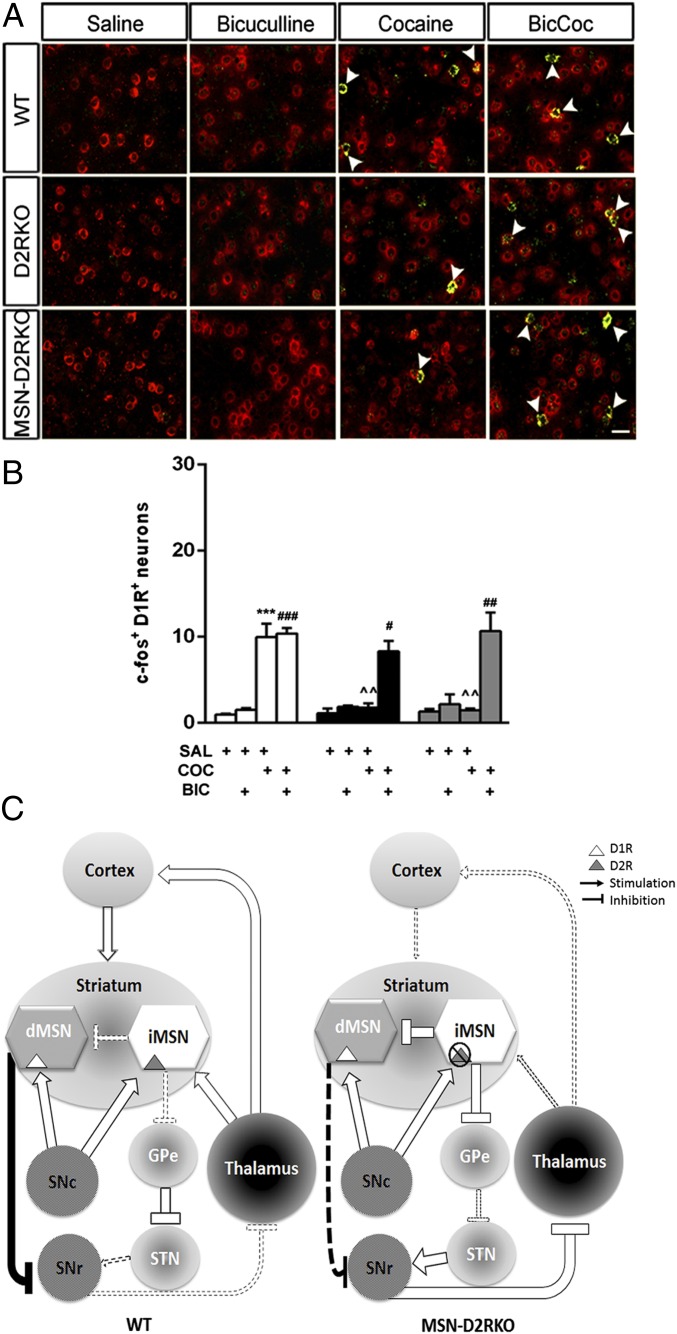
Thus, we hypothesized that absence of D2Rs on iMSNs eliminates the inhibitory control exerted by DA on the activity of these cells with consequences on dMSNs. To test this hypothesis, we blocked the GABAA receptors by using the antagonist bicuculline. To observe the effects of the drug in the striatum, bicuculline was stereotaxically injected in the DMS, the area showing the most robust induction of c-fos in cocaine-treated WT mice. D2RKO, MSN-D2RKO, and WT littermates were divided into four groups/genotype (n = 3 per group): (i) saline, (ii) saline/cocaine (20 mg/kg, i.p.), (iii) bicuculline/saline, (iv) bicuculline/cocaine. Bicuculline was injected 5 min before systemic administration of cocaine in freely moving mice; brains were collected 1 h later to perform dISH by using probes specific for c-fos together with the marker of dMSNs.
Importantly, antagonism of GABAA receptor in the absence of cocaine did not induce c-fos in the striatum; however, when bicuculline was injected before cocaine, c-fos induction was efficiently restored in the dMSNs of the DMS (Fig. 5) of D2RKO and MSN-D2RKO, in a manner comparable to WT (P > 0.05). These results advocate toward an aberrant iMSNs GABA-mediated inhibition of dMSNs in the striatum of D2RKO and MSN-D2RKO mice due to loss of D2Rs in these neurons.
Fig. 5.
GABAA antagonism restores c-fos induction in the striatum of D2RKO and MSN-D2RKO mice. Bicuculline restored c-fos induction to WT levels in D1R+ cells of the DMS of both D2RKO and MSN-D2RKO mice. (A) Representative images of in situ hybridization for c-fos (green) and D1R (red) in the DMS of WT, D2RKO, and MSN-D2RKO following the different treatments as indicated on the top. (Scale bar, 25 μm.) Arrowheads indicate cells that are both c-fos+ and D1R+. (B) Values are mean ± SEM of c-fos-D1R+ cells per frame (385.7 × 385.7 μm) in the DMS (n = 3 per treatment per genotype). ***P < 0.001 vs. saline-treated mice. ###P < 0.001, ##P < 0.01, #P < 0.05 vs. bicuculline-treated mice; ^^P < 0.01 vs. WT cocaine-treated mice. (C) Model of D2R-mediated control of the acute effects of cocaine in the DMS. (Left) In WT mice, acute cocaine administration increases the release of DA from dopaminergic neurons of the substantia nigra compacta (SNc), activating D1R in MSNs of the direct pathway (dMSNs) and D2R in MSNs of the indirect pathway (iMSNs). As a result, there is a potentiation of the direct pathway and the consequent reduction of the inhibitory input of the substantia nigra reticulata (SNr) to the thalamus, which, in return, stimulates the cortex and the striatum. This chain of events is responsible for the increased locomotor response to cocaine in WT mice. (Right) In MSN-D2RKO mice, absence of D2R in iMSNs prevents the inhibition of these neurons, leading to a potentiation of their inhibitory tone that results in an increased activation of the indirect pathway of SNr neurons. In intrastriatal circuits, the absence of D2Rs in iMSNs leads to an increased collateral inhibition by these neurons of dMSNs activity; this results into decreased inhibition from the direct pathway of SNr activity. Activation of SNr neurons inhibits the thalamus, resulting into a reduced stimulation of the cortex and striatum. The final outcome is reduced motor response to cocaine in MSN-D2RKO mice and altered signal transduction and gene expression in dMSNs. Solid lines indicate a potentiation of the effect whether excitatory or inhibitory, whereas broken lines indicate a reduced effect.
Discussion
Drugs of abuse such as cocaine modify synaptic plasticity and generate long-term changes in the physiology of striatal neurons (22). In particular, DA induction of dMSNs has been shown to be responsible for the motor-activating effects of psychostimulants (23). These results are also inferred by genetic evidence using D1R knockout mice (7) in which cocaine fails to induce motor activity and activation of intracellular signaling in dMSNs. The contribution of D2R activation in the response to cocaine has thus been thought to only marginally participate in the motor and cellular activation of striatal neurons. Nevertheless, mice carrying the constitutive knockout of D2R do not show the acute motor-activating effects of cocaine nor the induction of c-fos expression in dMSNs (6). These results are in agreement with previously reported pharmacological studies showing that D2R antagonism blocked the acute motor-activating effect of psychostimulants (24). The similarities between the behavioral response to cocaine using D2R antagonists in WT or the use of D2RKO mice suggest the absence of compensatory mechanisms able to replace D2R-mediated signaling in vivo.
The response to cocaine of constitutive D2R knockout mice suggested the presence of a D2R-mediated inhibitory control in specific neurons that affects dMSNs. However, the identity of the neurons responsible for these inhibitory effects was unclear because of the wide expression of D2R in several neuronal types of the striatum but also in other brain regions (i.e., cortex).
In this work, we have addressed this question by comparing the behavioral and cellular response to cocaine in MSN-D2RKO and DA-D2RKO mice, lacking D2Rs specifically in iMSNs or DA neurons, respectively. We demonstrate that the blunted motor and cellular response of D2RKO mice is generated by the loss of D2R signaling in the iMSNs. Indeed, whereas DA-D2RKO mice respond to cocaine in a manner similar to WT littermates, MSN-D2RKO behave as D2RKO mice and do not show a significant response to the motor-activating effect of cocaine. Interestingly, MSN-D2RKO mice also present a reduced motor response to SKF81297 and MK801 compared with both WT and DA-D2RKO mice. These results suggest that the ablation of D2R from iMSNs by relieving the inhibition of the indirect pathway lead to a reduced stimulation of the striato-cortical loop. In addition, lack of inhibition of iMSNs might also negatively influence the activity of DA neurons (25) and DA release (5) as observed by the reduced stimulation of GluR1 phosphorylation on Ser845 (Fig. 4). Thus, reduced input to dMSNs from cortical and dopaminergic neurons might partly explain the absence of ERK phosphorylation (8–10) and c-fos (11) in the striatum of these mutants compared with WT mice.
Nevertheless, D2RKO and MSN-D2RKO mice do respond to SKF81297 and MK801, but to a lesser extent, suggesting the presence of additional factors inhibiting dMSNs when mice are exposed to cocaine. Thus, we turned our attention to GABA signaling because iMSNs send collaterals to dMSNs (17). In MSN-D2RKO, the absence of D2R in iMSNs might result into an increased GABA-mediated inhibition of dMSN activity. Importantly, in line with this hypothesis, we show that an intracerebral injection of the GABAA antagonist, bicuculline, in the DMS restores the cellular response to cocaine in dMSNs.
It might be argued that the GABAergic inhibition could originate from DAergic neurons that also release GABA (26); however, the response of DA-D2RKO mice to cocaine is similar to that of WT littermates, making this possibility unlikely. Similarly, striatal GABAergic interneurons do not express D2Rs; furthermore, in D2RKO and MSN-D2RKO mice, these neurons likely receive lower cortical stimulation because of the lack of inhibition of iMSNs activity on the indirect pathway. The full rescue of c-fos induction by GABA antagonism supports the view of a major intrastriatal effect of iMSNs on dMSNs via collaterals (17, 27). We propose that deletion of D2R specifically in iMSNs is at the basis of the inhibition of signaling in dMSNs after cocaine. Thus, D2R-mediated signaling in iMSNs is critical for the full expression of psychomotor response to cocaine through modulation of the indirect pathway (Fig. 5C) but importantly through a direct inhibition of dMSNs signaling and activity (Fig. 5C). This study asserts a facilitatory role of D2R signaling in iMSNs in regulating the acute response to cocaine.
Materials and Methods
Animals.
Eight- to 12-wk-old D2RKO, DA-D2RKO, and MSN-D2RKO mice and WT littermates were used. D2RKO are constitutive knockout mice (12). D2Rfloxflox mice (5) were used and mated with En1Cre mice to generate DA-D2RKO mice (previously named D2Rfloxflox/En1Cre/+) (5) and with D1Cre mice to generate MSN-D2RKO mice (previously named D2Rfloxflox/D1Cre/+) (5). The En1 promoter is specifically expressed in midbrain neurons (28), resulting in the completed deletion of D2R in SN and ventral tegmental area (5). The use of dopamine D1R promoter in D1CRE mice (29) allowed for the complete deletion of D2R in MSNs (5) despite the restricted expression of D1R in the MSNs of the direct pathway in adults. However, D1R and D2R are coexpressed during embryonal development, as shown in primary striatal cultures of MSNs (30) obtained from late embryonic stages, thus allowing the use of this line to delete D2R in MSNs of the indirect pathway (5). Mice were maintained in standard conditions (12 h light/dark cycle) with food and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee in accordance with the NIH guidelines.
Drugs.
Cocaine hydrochloride, MK801, SKF 81297 hydrobromide (Sigma-Aldrich), and bicuculline methiodide (Fluka) were dissolved in saline [0.9% NaCl (wt/vol)].
Behavioral Analyses.
Locomotor activity was recorded by using a video-tracking system (Viewpoint). Animals were handled (5 min) 2 d before the experiment and habituated to novel home cages (20 × 30-cm transparent plastic box) for 2 h before cocaine (10, 20 mg/kg, i.p.) or MK801 (0.3 mg/kg, i.p.). Activity was recorded for 1 (cocaine) or 2 (MK801) h after injection. For SKF81297 (0.5–3 mg/kg) mice were habituated for 1 h and recorded for 1 h.
Stereotaxic Injections.
Mice were placed in stereotaxic frame (David Kopf Instruments) under anesthesia (ketamine 100 mg/kg and xylazine 10 mg/kg, i.p.) and guide cannulae (26G; Plastic One) were implanted in the dorsal striatum 0.5 mm above the site of microinjection (anterior-posterior, 0.98 mm; medial-lateral, ± 1.2 mm; dorso-ventral, −3 mm, from Bregma) according to the mouse brain atlas (31) (Fig. S4). Guide cannulae were fixed to the skull by using dental cement and dummy cannulae inserted. Following surgery, mice were monitored and handled daily for 7–10 d. Four groups were made receiving the following: group 1: saline (intracerebral) + saline (intraperitoneal); group 2: bicuculline (intracerebral) + saline (intraperitoneal); group 3: saline (intracerebral) + cocaine (intraperitoneal); group 4: bicuculline (intracerebral) + cocaine (intraperitoneal). Injector cannulae (33G) were inserted into the guide cannulae and bicuculline methiodide (0.01 μg/0.3 μL per side) or vehicle (sterile 0.9% saline 0.3 μL per side) were infused bilaterally over a period of 3 min. Injector cannulae were left for additional 2 min to ensure proper delivery of the drugs.
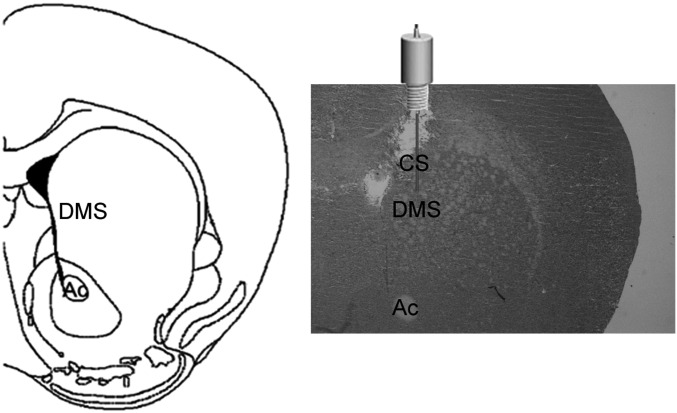
Fig. S4.
Schematic representation of the intrastriatal injection sites. (Left) Schematic drawing of the striatal coronal section where injections were made (0.98 anterior-posterior from Bregma). (Right) Representative coronal striatal section of the site of injection from one of the cannulated animals used in experiments shown in Fig. 5. Ac, anterior commissure; CS, cannula site; DMS, dorsomedial striatum.
In Situ Hybridization.
Brain sections were obtained and hybridized with fluorescein-labeled c-fos and digoxigenin (DIG)-labeled D1R or Enkephalin riboprobes (RNA labeling mix; Roche), as previously described (5), followed by anti–Fluorescein-POD (1:1000, Roche) and anti–DIG-AP (1:5000, Roche) antibodies. To amplify the signal the TSA PLUS fluorescein System (PerkinElmer) and HNPP (2-hydroxy-3-naphtoic acid-2'-phenylanilide phosphate) fluorescent Detection Set (Roche) were used.
Cell Counting.
Pictures were taken and analyzed by confocal microscopy (DMRE; SP5 microscope, Leica). Regions of interest (387.5 × 387.5 μm) were defined bilaterally in the dorso-medial striatum (DMS, 1.42–0.7 mm anterior to Bregma) and ventral striatum containing both core and shell regions (VS, 1.54–1.10 mm anterior to Bregma). Four sections per animal were counted in three animals/genotype per treatment.
Western Blot.
Brains were rapidly collected 20 min after saline or cocaine (20 mg/kg). Striatal tissue was sonicated in lysis buffer (Tris 25 mM, pH 6.8; β-mercaptoethanol 175 mM, SDS 1%) containing protease and phosphatase inhibitors (Complete EDTA-free; PhosSTOP; Roche) and boiled for 5 min. Protein content was determined by using the BCA kit (Thermo). Thirty micrograms of extracts were loaded onto 10% (vol/vol) SDS/PAGE and transferred to PVDF membranes (Millipore). Antibodies directed against phosphorylated ERK1/2 (p42/p44; Cell Signaling, 1:1000) and GluR1-Ser845 (Millipore; 1:1000) followed by the appropriate secondary antibodies were used; bands were revealed by using the ECL Plus reagent (Millipore). Membranes were then stripped and reprobed with antibodies directed against total ERK (Cell Signaling, 1:2000) and GluR1 (Santa Cruz; 1:1000). Quantifications of band intensity were performed by using the ImageJ (version 1.42q) software. Data for pERK1/2/ERK1/2 and pGluR1/GluR1 in D2R mutants were adjusted for comparisons to WT saline values arbitrarily set at 1.
Statistical Analysis.
All values are mean ± SEM. Statistical analyses were made by using GraphPad Prism. Data were analyzed by two-way ANOVA followed by Bonferroni’s post hoc or Student’s t test, as appropriate; P < 0.05 was considered statistically significant.
Acknowledgments
We thank C. Kinoshita, M. Ramos, C. De Mei, and B. Halbout for their interest in this study; J. Liu and S. Nolen for technical help; and K. Brami-Cherrier for discussions. This work was supported by funds from NIH Grants DA 024689, DA033554, and DA035600, and INSERM.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608362113/-/DCSupplemental.
References
- 1.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 3.Di Chiara G, et al. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 4.De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: Presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9(1):53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anzalone A, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welter M, et al. Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA. 2007;104(16):6840–6845. doi: 10.1073/pnas.0610790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79(6):945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 8.Valjent E, et al. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20(23):8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girault JA, Valjent E, Caboche J, Hervé D. ERK2: A logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7(1):77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102(2):491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16(13):4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baik JH, et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377(6548):424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 13.Bamford NS, et al. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24(43):9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson AB, et al. Striatal cholinergic interneurons drive GABA release from dopamine terminals. Neuron. 2014;82(1):63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centonze D, et al. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology. 2002;27(5):723–726. doi: 10.1016/S0893-133X(02)00367-6. [DOI] [PubMed] [Google Scholar]
- 16.Rouge-Pont F, et al. Changes in extracellular dopamine induced by morphine and cocaine: Crucial control by D2 receptors. J Neurosci. 2002;22(8):3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn JS, Aizenman E, Lipton SA. Central mammalian neurons normally resistant to glutamate toxicity are made sensitive by elevated extracellular Ca2+: Toxicity is blocked by the N-methyl-D-aspartate antagonist MK-801. Proc Natl Acad Sci USA. 1988;85(17):6556–6560. doi: 10.1073/pnas.85.17.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88(4):1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertran-Gonzalez J, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestler EJ, Barrot M, Self DW. DeltaFosB: A sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98(20):11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38(2):95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 25.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490(7419):262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cazorla M, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81(1):153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmel RA, et al. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000;14(11):1377–1389. [PMC free article] [PubMed] [Google Scholar]
- 29.Lemberger T, et al. Expression of Cre recombinase in dopaminoceptive neurons. BMC Neurosci. 2007;8:4. doi: 10.1186/1471-2202-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizman O, et al. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3(3):226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- 31. Paxinos G, Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates (Academic Press, New York), 2nd Ed.