Significance
In mitophagy, damaged mitochondria stabilize PTEN-induced putative kinase 1 (PINK1) and recruit Parkin, an E3-ligase that ubiquitinates proteins on the outer membrane and targets mitochondria for degradation. The crucial roles of PINK1 phosphorylation of Parkin and ubiquitin in mitophagy are well-established. Other substrates of PINK1, however, have also been reported but the significance of those phosphorylations is less clear. We now show that Miro phosphorylations can regulate Parkin recruitment to Miro and trigger Miro degradation. The consequence of this branch of the PINK1/Parkin pathway is the disruption of mitochondrial motility, an event that may spatially restrict the deleterious effects of mitochondrial damage prior to the mitophagic removal of the organelle.
Keywords: PINK1, Parkin, Miro, mitochondrial transport, mitophagy
Abstract
The PTEN-induced putative kinase 1 (PINK1)/Parkin pathway can tag damaged mitochondria and trigger their degradation by mitophagy. Before the onset of mitophagy, the pathway blocks mitochondrial motility by causing Miro degradation. PINK1 activates Parkin by phosphorylating both Parkin and ubiquitin. PINK1, however, has other mitochondrial substrates, including Miro (also called RhoT1 and -2), although the significance of those substrates is less clear. We show that mimicking PINK1 phosphorylation of Miro on S156 promoted the interaction of Parkin with Miro, stimulated Miro ubiquitination and degradation, recruited Parkin to the mitochondria, and via Parkin arrested axonal transport of mitochondria. Although Miro S156E promoted Parkin recruitment it was insufficient to trigger mitophagy in the absence of broader PINK1 action. In contrast, mimicking phosphorylation of Miro on T298/T299 inhibited PINK1-induced Miro ubiquitination, Parkin recruitment, and Parkin-dependent mitochondrial arrest. The effects of the T298E/T299E phosphomimetic were dominant over S156E substitution. We propose that the status of Miro phosphorylation influences the decision to undergo Parkin-dependent mitochondrial arrest, which, in the context of PINK1 action on other substrates, can restrict mitochondrial dynamics before mitophagy.
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, and is closely linked to mitochondrial dysfunction (1, 2). Two hereditary forms of recessive PD are caused by mutations in PINK1 (PTEN-induced putative kinase 1), a Ser/Thr mitochondrial kinase, and Parkin, a cytosolic E3 ubiquitin ligase (3, 4). The realization that these proteins are in a single pathway, with PINK1 acting upstream of Parkin to influence mitochondrial properties, was a critical step in uncovering the underlying pathological mechanisms of PD (5–7). This pathway can trigger the selective autophagy of damaged mitochondria, termed mitophagy (8, 9), but additional cellular functions have also been indicated for PINK1 and Parkin (10–15). Much, however, remains unclear about how the PINK1/Parkin pathway is regulated.
In current models of PINK1/Parkin mitophagy (reviewed in ref. 1), healthy mitochondria import a PINK1 precursor constitutively to the inner membrane, where it is cleaved (16–18). The cleaved form then returns to the cytoplasm and is degraded by the N-end rule pathway (19). Mitochondrial depolarization, or protein misfolding in the matrix of energized mitochondria (20), prevent the import and degradation of PINK1, resulting in the accumulation of PINK1 on the outer mitochondrial membrane (OMM) (9, 21, 22). Once on the OMM, PINK1 kinase activity recruits Parkin from the cytosol (8, 9). Although Parkin adopts a self-inhibited conformation in solution (23–25), it becomes fully activated in a PINK1-dependent manner on the mitochondria (9, 21). Parkin ubiquitinates numerous proteins of the OMM (26, 27), and thereby recruits autophagy-related proteins to the damaged mitochondrion for autophagosome assembly (28–30).
How PINK1 recruits and activates Parkin on mitochondria remains incompletely understood, but two key components have been identified: PINK1 phosphorylation of both Parkin and ubiquitin (31–34). Together, these actions form a positive-feedback loop in which PINK1 activates Parkin by phosphorylating Serine 65. Activated Parkin adds ubiquitin to outer membrane-localized proteins at the mitochondrial surface, providing more substrates for PINK1 and the resulting phosphoubiquitin is an allosteric activator of Parkin, further increasing its activity. Recruitment and activation of Parkin, although distinct processes, are thus tightly linked (35, 36) by the positive feed-forward effect of ubiquitination by Parkin causing additional binding sites for Parkin, and additional binding causing further ubiquitination and allosteric activation of Parkin. Parkin substrates can also be de-ubiquitinated by Usp30 (37) and Usp15 (38).
PINK1, however, can phosphorylate other proteins, including the motor-adaptor protein Miro (also called RhoT1/2), Mitofusin 1/2, and Hsp75 (also known as Trap1) (15, 39–42), and the functional role of those modifications in regulating Parkin is unclear. Given that some of these proteins are also substrates of Parkin (15, 39), it is possible that modification of these proteins can act as a layer of regulation to modulate the overall levels of Parkin on mitochondria or to modulate specific aspects of mitochondrial dynamics, such as motility and fusion. Miro, an OMM GTPase involved in the regulation of mitochondrial traffic, is a well-established substrate of Parkin (15, 27, 43, 44). Data from our laboratory and others indicated that PINK1 can interact with Miro and cause Miro phosphorylation of S156 in vitro (15, 45). PINK1 and Parkin-mediated modifications of Miro result in the proteasomal degradation of Miro and, because Miro is required to tether kinesin and dynein to the mitochondrial surface, its degradation arrests mitochondrial movement. Genetic analysis indicated that, as in the mitophagic pathway, PINK1 acts upstream of Parkin in regulating motility (15). The arrest of mitochondrial movement may be a precursor to mitophagy or, independent of mitophagy, provide a means of regulating mitochondrial dynamics. In Drosophila axons, for example, knockdown of either PINK1 or Parkin increased mitochondrial transport in the absence of acute damage to the organelles (15). Moreover, Miro phosphorylations are needed for the survival of dopaminergic neurons and the proper development of the neuromuscular junction in Drosophila (46). Miro is thus a good candidate in which to explore the functional roles of PINK1 phosphorylations of Parkin substrates.
Previous experiments indicated that PINK1 can phosphorylate Miro in vitro not only on S156 but also on T298/299 (15). We generated phosphomimetic and nonphosphorylatable Miro mutants in all three sites. Mimicking phosphorylation on S156 stimulated the interaction of Miro with Parkin, promoted the recruitment of Parkin to the mitochondria, and induced mitochondrial arrest in axons, but was not sufficient alone to trigger mitophagy. Mimicking T298/T299 phosphorylation suppressed the effects of the phosphomimetic S156E. We conclude that Miro phosphorylations on S156 and T298/T299 can regulate the onset of Parkin signaling by modulating the levels of Parkin on mitochondria and can regulate mitochondrial motility.
Results
Phosphomimetic Mutant MiroS156E Enhances the Recruitment of Parkin to Mitochondria in a PINK1-Independent Manner.
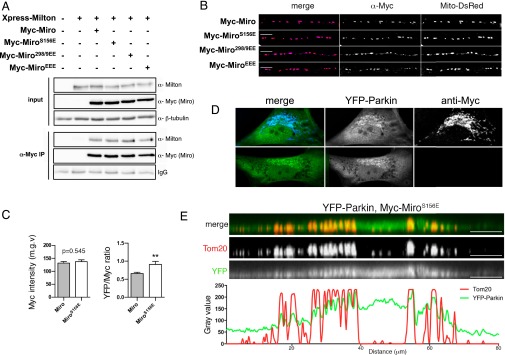
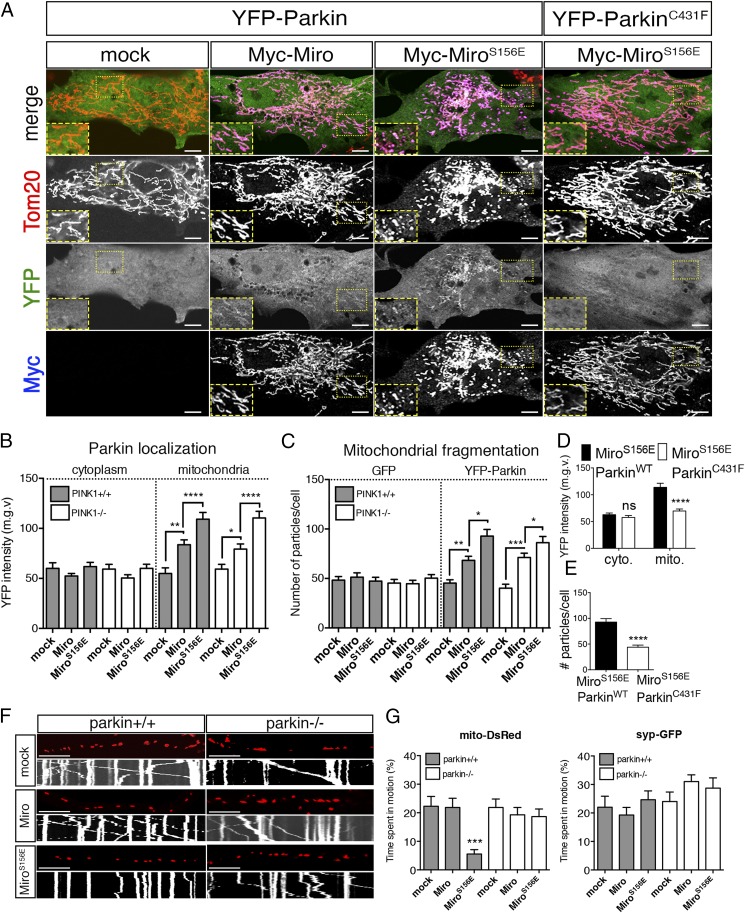
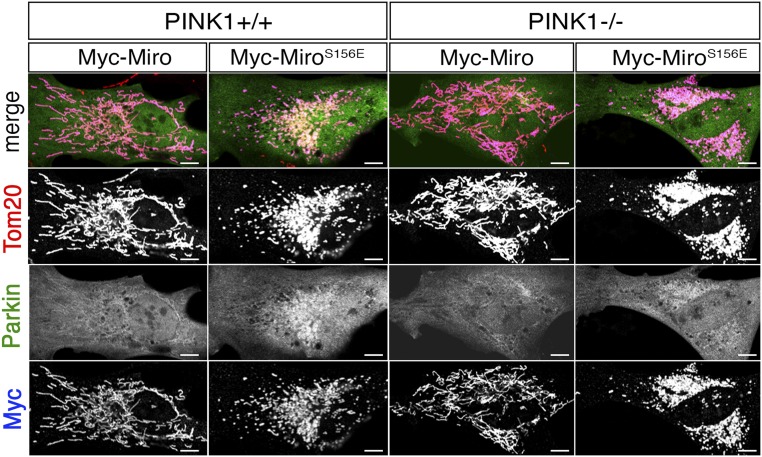
Mass spectrometry of recombinant Drosophila Miro phosphorylated in vitro by human PINK1 immunoprecipitated from HEK293T cells previously revealed two phospho-peptides, corresponding to amino acids 177–190 and 319–330 (15). The homologous peptides in human Miro1 (hMiro1) contained the conserved sites S156, T298, and T299. To establish the likely consequences of the S156 modifications at the cellular level, we asked whether expressing the phosphomimetic MiroS156E influenced Parkin recruitment to mitochondria and its consequent arrest of mitochondrial motility. The phosphomimetic substitution per se did not detectably disrupt Miro function. Myc-MiroS156E coprecipitated with Xpress-tagged Milton equivalently to Myc-Miro when expressed in HEK293T (Fig. S1A). In addition, when expressed in neurons, the MiroS156E construct correctly localized to axonal mitochondria (Fig. S1B). Because overexpression of Miro constructs probably does not displace the endogenous Miro but increases Miro levels on mitochondria, we used overexpressed wild-type Miro as a control for phosphorylation-independent effects of Miro. If Miro phosphorylation can recruit and activate Parkin, we anticipated that the phosphomimetic Miro would have a dominant effect over the endogenous Miro. To determine if the S156E mutation altered Parkin distribution, YFP-Parkin was cotransfected into primary rat embryonic fibroblasts with either mock DNA, Myc-Miro, or Myc-MiroS156E and the abundance of YFP-Parkin in cytoplasmic and mitochondrial compartments was evaluated automatically in a blinded fashion using Mitolyzer1.0, a script we developed for ImageJ (Materials and Methods) (Fig. 1 A and B). In the absence of Miro overexpression, YFP-Parkin was not noticeably concentrated on mitochondria. Miro and MiroS156E isoforms were expressed at equivalent levels judged by α-Myc immunofluorescence levels (Fig. S1C) and highly localized to mitochondria (Fig. 1A). Although expression of Myc-Miro increased slightly the levels of YFP-Parkin on mitochondria, MiroS156E caused a significantly greater mitochondrial accumulation of Parkin (Fig. 1 A and B), and consequently a higher ratio of Parkin to Miro on mitochondria (Fig. S1C). This effect cannot be attributed to cross-talk between fluorophores because the accumulation of YFP-Parkin is also apparent in the absence of the second fluorophore (Fig. S1D). Thus, mimicking phosphorylation of Miro on S156 can recruit Parkin to the mitochondria. To observe with higher detail the recruitment of Parkin to the mitochondria by MiroS156E, we collected stacks of confocal images of a MiroS156E, YFP-Parkin–expressing fibroblast, and performed a 3D reconstruction. We also performed a line scan of the same region from a confocal image (Fig. S1E). Parkin-YFP signal colocalizes with α-Tom20 signal in the reconstruction and in the line-scan. Because Parkin recruitment to mitochondria is thought to depend on its activation by PINK1 (35), we also examined the effects of MiroS156E expression in primary embryonic fibroblasts from PINK1−/− rats (47). MiroS156E caused equivalent mitochondrial localization of YFP-Parkin in these cells (Fig. 1B and Fig. S2). Thus, the phosphomimetic form of Miro was capable of recruiting Parkin to mitochondria even in the absence of PINK1.
Fig. S1.
(A) Milton was coexpressed with the indicated Miro isoforms, and the Miro complex was immunoprecipitated using anti-Myc antibody. (B) Rat hippocampal neurons were fixed after a time-lapse imaging session and stained with anti-Myc to reveal the localization of Myc-Miro constructs in axons. Note that the anti-Myc signal is localized to mitochondria, which are labeled with Mito-DsRed. (Scale bars, 10 μm.) (C) Quantification of anti-Myc fluorescence intensity and YFP/Myc ratio in the fibroblasts of the indicated genotypes. Myc-Miro is expressed at the same level as Myc-MiroS156E, but the YFP/Myc ratio is higher for the latter. **P < 0.01. (D) MiroS156E was coexpressed with YFP-Parkin in PINK1+/+ rat fibroblasts, and cells were either labeled with anti-Myc (blue, Upper) or not labeled with antibody (Lower) (magnification: 40×). (E) Three-dimensional reconstruction followed by an orthogonal projection of a cell of the indicated genotype. A line scan of a confocal slice of the same region shows that YFP-Parkin accumulates preferentially on mitochondria. (Scale bars, 10 μm.)
Fig. 1.
Cellular effects upon mimicking Miro phosphorylation on S156. (A) Rat embryonic fibroblasts were transfected with the indicated constructs and stained with α-Tom20 to label mitochondria and α-Myc to label Myc-Miro. YFP fluorescence was used to estimate Parkin levels and localization. (B) The average median gray value of YFP-Parkin in PINK1+/+ (gray bars) and PINK1−/− (white bars) cells in the cytoplasmic and the mitochondrial compartments plotted for each condition. Overexpression of Myc-MiroS156E, and to a lesser extent Myc-Miro, increased YFP intensity exclusively in the mitochondrial compartment. (C) Average number of small (0.2–5 μm in diameter) and rounded (circularity ∼0.5–1) mitochondria in cells expressing either GFP or YFP-Parkin in PINK1+/+ and PINK1−/− genetic backgrounds. (D and E) YFP intensity in cytoplasmic and mitochondrial compartments (D) and average number of mitochondrial fragments (E) in cells coexpressing MiroS156E with either YFP-ParkinWT or YFP-ParkinC431F. n = 40–50 cells for each condition from three independent transfections per genotype. (F) Mitochondrial movement in representative axons transfected with mito-DsRed along with indicated constructs and dissected from Parkin+/+ (Left) or Parkin−/− (Right) animals. The first frame of each time-lapse imaging series is shown above a kymograph generated from the movie. The x axis represents mitochondrial position, and the y axis is time (moving from top to bottom). Vertical lines are stationary mitochondria, whereas diagonal lines represent movement. (G) Average time spent in motion by each mitochondrion (mito-DsRed) or synaptic vesicle (syp-GFP) in axons of indicated genotypes. n = 100–130 mitochondria and n = 81–236 synaptic vesicles from nine axons/genotype and three independent biological replicates per genotype. (Scale bars, 10 μm.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See also Table S1.
Fig. S2.
Either MiroWT or MiroS156E was coexpressed with YFP-Parkin in PINK1+/+ or PINK1−/− rat fibroblasts and stained with anti-Myc (blue) and anti-Tom20 (red). YFP-Parkin accumulated on mitochondria when MiroS156E was expressed even in the absence of PINK1. (Scale bars, 10 μm.)
Phosphomimetic Mutant MiroS156E Enhances Mitochondrial Fragmentation.
The mitochondrial network also appeared fragmented in cells where MiroS156E was coexpressed with YFP-Parkin (Fig. 1A). To quantify this phenotype, we incorporated a particle analysis feature to Mitolyzer1.0 and determined the numbers of small (0.2–5 μm in diameter) and rounded (circularity ∼0.5–1) mitochondria in the fibroblasts. The average number of small rounded mitochondria per cell was ∼50 in control cells, but almost doubled with MiroS156E expression and this effect was equally apparent in PINK1−/− fibroblasts (Fig. 1C). Fragmentation also increased significantly when Miro was expressed, although to a lesser extent (∼30% of control). This fragmentation was dependent on Parkin activity because it did not occur when we substituted YFP-ParkinC431F, a catalytically dead form of Parkin that is not recruited to mitochondria (36). YFP-ParkinC431F was not significantly recruited to mitochondria by MiroS156E and the mitochondrial network was not fragmented by their coexpression (Fig. 1 A, D, and E). Thus, Myc-MiroS156E, and to a lesser extent Myc-Miro overexpression, promoted Parkin recruitment to the mitochondria and induced mitochondrial fragmentation as a consequence of the catalytic activity of Parkin, and these effects of MiroS156E did not require additional activation of Parkin by PINK1.
Phosphomimetic MiroS156E Can Arrest Axonal Transport of Mitochondria.
The PINK1/Parkin pathway, by triggering the degradation of Miro, halts the movement of axonal mitochondria (15). To determine if S156E similarly altered mitochondrial behavior in neurons, we coexpressed Mito-DsRed to label mitochondria, synaptophysin-GFP (Syp-GFP) to label axons, and either Myc-MiroWT or Myc-MiroS156E in either wild-type or Parkin KO mouse hippocampal neurons (48). We then imaged live neurons by time-lapse microscopy and calculated the average time mitochondria spent in motion in each condition. Although expression of Miro did not significantly affect mitochondrial motility, expression of MiroS156E diminished the percentage of mitochondria that were moving in either direction (Fig. 1 F and G and Table S1). Mitochondrial arrest induced by MiroS156E was specific to mitochondria, as the movement of Syp-GFP vesicles was not altered (Fig. 1G and Table S1). Moreover, in Parkin−/− neurons, expression of MiroS156E failed to induce mitochondrial arrest (Fig. 1 F and G and Table S1). Thus, mimicking PINK1 phosphorylation on S156 induced mitochondrial arrest in a Parkin-dependent manner, similar to the effect of PINK1 activation or overexpression and not through a nonspecific disruption of the transport apparatus.
Table S1.
Motility parameters of mitochondria and synaptic vesicles in Parkin+/+ and Parkin−/− axons
| Construct | #mito or #syp-GFP | Time spent in motion (%) | Anterograde (%) | Anterograde speed (μm/s) | Retrograde (%) | Retrograde speed (μm/s) | Stop frequency (%) | Reversal frequency (%) | Total travel length (μm) |
| Mitochondrial motility parameters in Parkin+/+ mouse hippocampal axons coexpressing Mito-DsRed with indicated constructs | |||||||||
| Mock | 106 | 22.29 ± 35.14 | 10.20 ± 25.48 | 0.16 ± 0.1 | 12.06 ± 27.22 | 0.18 ± 0.06 | 1.45 ± 2.53 | 0.16 ± 0.86 | 10.83 ± 21.09 |
| Myc-Miro | 121 | 21.85 ± 33.34 | 9.94 ± 25.38 | 0.17 ± 0.09 | 11.94 ± 25.14 | 0.35 ± 0.74 | 1.49 ± 2.49 | 0.14 ± 0.62 | 10.35 ± 21.37 |
| Myc-MiroS156E | 113 | 5.53 ± 16.23 | 0.70 ± 5.00 | 0.19 ± 0.27 | 4.84 ± 15.72 | 0.2 ± 0.14 | 0.63 ± 2.09 | 0 | 2.54 ± 9.05 |
| Motility parameters of synaptic vesicles in Parkin+/+ mouse hippocampal axons coexpressing syp-GFP with indicated constructs | |||||||||
| Mock | 81 | 21.58 ± 34.82 | 12.20 ± 27.82 | 0.14 ± 0.08 | 8.82 ± 25.28 | 0.12 ± 0.04 | 2.12 ± 4.37 | 0 | 6.64 ± 11.71 |
| Myc-Miro | 135 | 19.30 ± 30.76 | 10.71 ± 25.38 | 0.15 ± 0.08 | 8.6 ± 21.50 | 0.14 ± 0.14 | 1.9 ± 3.89 | 0.03 ± 0.24 | 7.35 ± 16.84 |
| Myc-MiroS156E | 124 | 24.65 ± 34.52 | 15.69 ± 31.44 | 0.19 ± 0.26 | 8.97 ± 22.06 | 0.12 ± 0.07 | 2.26 ± 4.48 | 0 | 9.57 ± 19.00 |
| Mitochondrial motility parameters in Parkin−/− mouse hippocampal axons coexpressing Mito-DsRed with indicated constructs | |||||||||
| Mock | 140 | 21.84 ± 35.22 | 10.09 ± 27.76 | 0.3 ± 0.38 | 11.75 ± 26.37 | 0.2 ± 0.16 | 1.0 ± 2.33 | 0.03 ± 0.3 | 11.84 ± 26.96 |
| Myc-Miro | 179 | 19.28 ± 34.45 | 7.35 ± 21.38 | 0.22 ± 0.27 | 11.93 ± 29.26 | 0.26 ± 0.22 | 0.67 ± 1.66 | 0.1 | 9.60 ± 22.67 |
| Myc-MiroS156E | 149 | 18.64 ± 32.86 | 9.68 ± 23.52 | 0.88 ± 2.77 | 8.96 ± 24.91 | 0.25 ± 0.37 | 1.2 ± 3.34 | 0.2 ± 1.03 | 12.41 ± 27.49 |
| Motility parameters of synaptic vesicles in Parkin−/− mouse hippocampal axons coexpressing syp-GFP with indicated constructs | |||||||||
| Mock | 106 | 23.87 ± 34.36 | 11.84 ± 27.06 | 0.10 ± 0.05 | 12.05 ± 26.96 | 0.13 ± 0.11 | 1.77 ± 2.53 | 0 | 7.82 ± 14.23 |
| Myc-Miro | 239 | 31.4 ± 37.06 | 16.22 ± 31.20 | 0.15 ± 0.18 | 15.18 ± 29.60 | 0.11 ± 0.09 | 2.45 ± 3.45 | 0.07 ± 0.52 | 10.20 ± 15.99 |
| Myc-MiroS156E | 117 | 28.72 ± 38.68 | 17.03 ± 33.09 | 0.12 ± 0.07 | 11.71 ± 27.65 | 0.12 ± 0.06 | 1.73 ± 3.14 | 0.12 ± 0.78 | 9.5 ± 14.63 |
This table is related to Fig. 1G.
Phosphomimetic MiroS156E Does Not Induce Mitophagy.
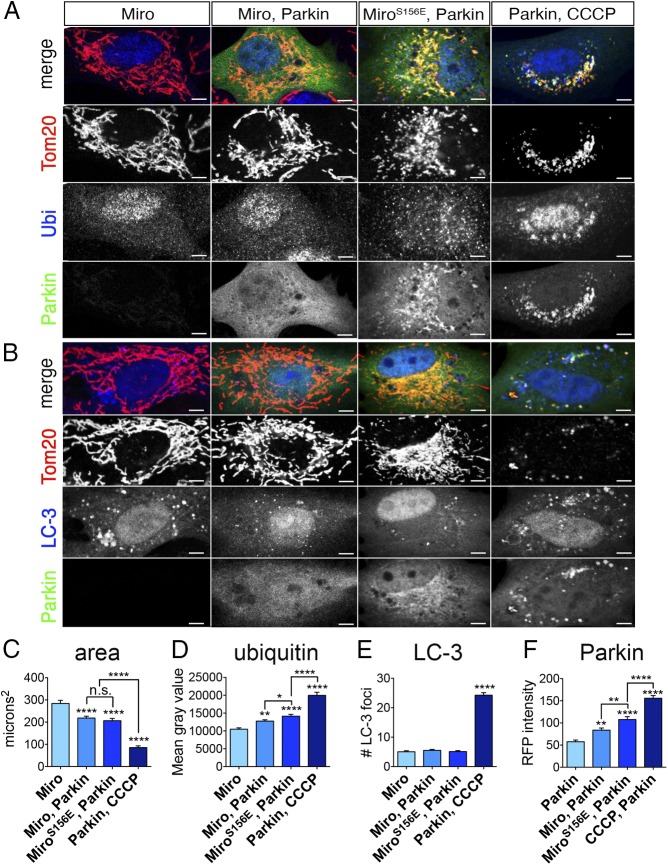
When mitochondrial damage causes PINK1 stabilization on the OMM, Parkin-recruitment to mitochondria and mitochondrial fragmentation are a prelude to mitophagy (8). To assess if mimicking phosphorylation on S156 induced mitophagy, we expressed Myc-Miro, either wild-type or S156E, along with Parkin in fibroblasts and examined three mitophagy markers: mitochondrial content, mitochondrial α- ubiquitin staining, and colocalization of LC-3 with mitochondria (Fig. 2). We also quantified the levels of Parkin recruitment to the mitochondria. We used cells only expressing Miro as a negative control, and cells that overexpressed Parkin and were treated with carbonyl cyanide 3-chlorophenyl-hydrazone (CCCP) as a positive control. As expected, in CCCP-treated cells, mitochondrial content, as judged by α-Tom20 immunoreactivity, was drastically reduced (Fig. 2C), and α-ubiquitin immunoreactivity, LC-3, and Parkin colocalization with mitochondria increased (Fig. 2 B and D–F) (49). In contrast, although Myc-MiroS156E coexpression with Parkin increased Parkin and α-ubiquitin colocalization with mitochondria relative to Myc-MiroWT coexpression with Parkin, the S156E mutation did not cause LC-3 recruitment. Miro coexpression with Parkin caused a modest decrease in mitochondrial content, but there was no significant further effect of the S156E mutation. The extent of mitochondrial ubiquitination induced by the S156E mutation presumably reflects Parkin activity, but at levels significantly lower than those induced by CCCP (Fig. 2 A and D). Thus, Parkin recruitment and activation by MiroS156E, although sufficient to induce mitochondrial fragmentation, was inadequate to trigger the full pathway for mitophagy; PINK1 activity on other substrates, such as ubiquitin and Parkin itself, is probably needed for mitophagy to proceed.
Fig. 2.
MiroS156E does not induce mitophagy. (A) Rat fibroblasts were transfected with the indicated constructs and stained with α-Tom20 (red) and α-ubiquitin (blue). YFP fluorescence was used to estimate Parkin levels and localization. (B) Fibroblasts were transfected as in A, but m-Cherry Parkin was used instead of YFP-Parkin (false-colored in green for consistency) and autophagic vesicles were labeled by transfection of LC-3–GFP, (false-colored in blue). (C–F) Quantification of mitochondrial content (expressed as average area of Tom-20 immunoreactivity), α-ubiquitin immunoreactivity on mitochondria (expressed as average mean gray value from 16-bit images) from cells transfected as in A, the number of LC-3–GFP vesicles coextensive with mitochondria from cells transfected as in B, and the intensity of YFP-Parkin on mitochondria from 8-bit images of cells transfected as in A. CCCP was added at a final concentration of 20 μM for 3 h before fixation. n = 60–70 cells per genotype from three independent transfections. (Scale bars, 10 μm.) Statistical comparisons are to Miro-only cells, except where otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001.
Miro Phosphomimetic S156E Promotes the Interaction of Parkin with Miro.
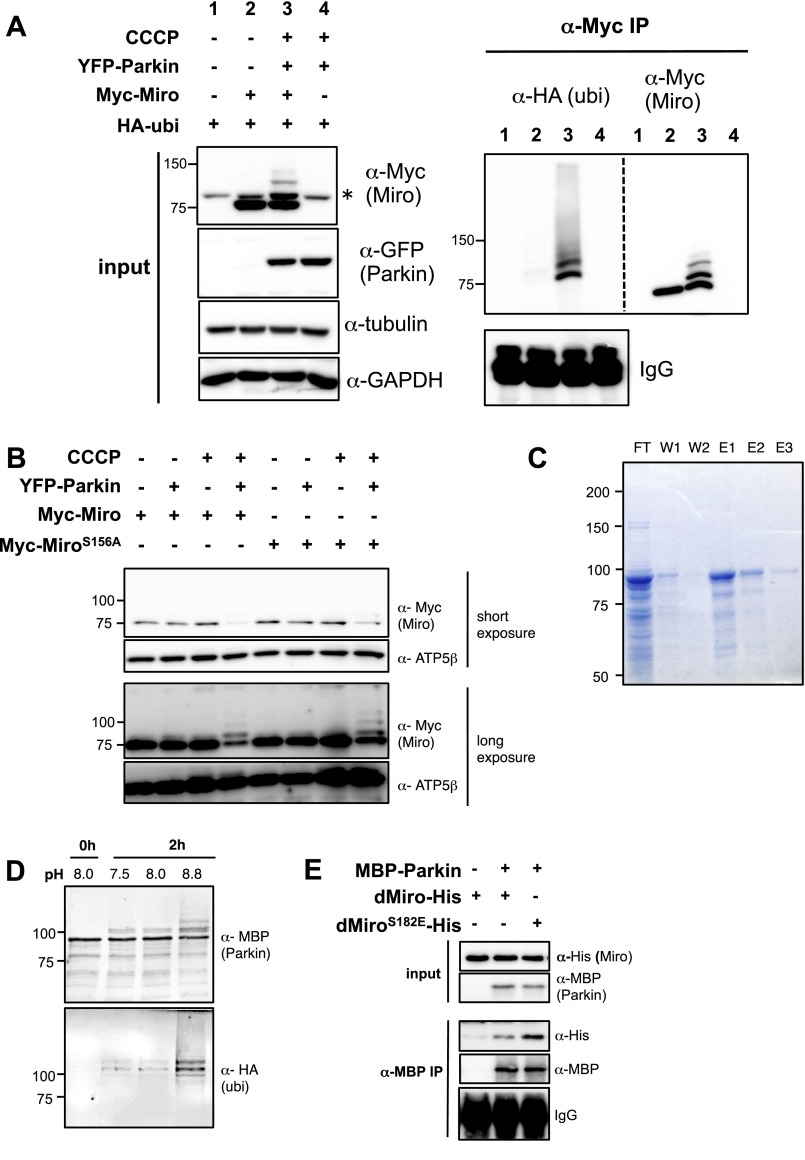
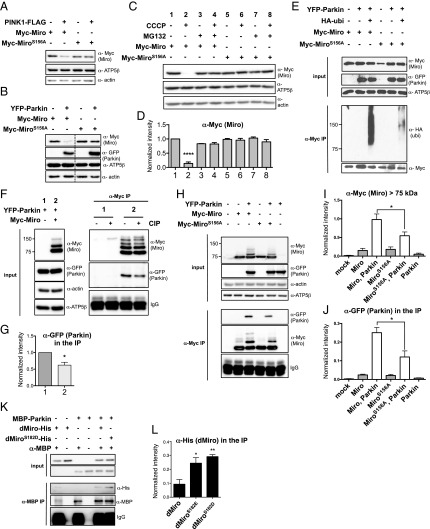
To explore the mechanism underlying MiroS156E-induced Parkin recruitment, we turned to established cell lines. When wild-type Miro (Myc-Miro) was coexpressed with Parkin and HA-ubiquitin in HeLa cells and the PINK1/Parkin pathway was activated by CCCP, Miro immunoreactivity appeared in higher molecular-mass bands than that of 75 kDa unmodified Myc-Miro (Fig. S3A). When we immunoprecipitated Myc-Miro, the high molecular-mass bands were positive for both α-Myc and α-HA–ubiquitin. Thus, Parkin-induced ubiquitination of Myc-Miro can be detected as α-Myc+ bands migrating more slowly than 75 kDa. The apparent synergism of CCCP, an activator of PINK1, and Parkin-expression in promoting Miro ubiquitination could be because of PINK1 phosphorylation of either Parkin or Miro or both. Therefore, to examine the contribution of phosphorylation of MiroS156 we compared phosphoresistant Myc-MiroS156A (where Serine 156 was changed to Alanine) to Myc-Miro. Several groups have failed to detect differences in Myc-MiroS156A ubiquitination levels compared with Myc-Miro (44, 50). In agreement with these groups, we also see that, under some circumstances, Myc-MiroS156A high molecular-mass bands can be induced by Parkin (Fig. S3B). Our results indicate that in conditions where Parkin is strongly activated, the status of S156 is of lesser importance. However, if levels of Parkin activity are moderate to low, as in the following experiments, the phosphorylation status of S156 can determine the extent of Miro ubiquitination and degradation. After coexpression of either Myc-Miro or S156A with either PINK1 or Parkin in HEK cells, the Myc-Miro band had significantly less immunoreactivity compared with Myc-MiroS156A (Fig. 3 A and B), suggesting that phosphorylation on S156 indeed facilitates Miro degradation. The efficacy of the phosphoresistant mutation in also preserving Miro levels in the absence of PINK1 overexpression or activation with CCCP suggests that this site may undergo phosphorylation even in their absence. If CCCP was used to activate the endogenous PINK1/Parkin pathway, the influence of the S156 site was also apparent. When we transfected low levels of Myc-Miro or Myc-MiroS156A in HEK293T and treated the cells with CCCP for 1 h before lysis, Myc-Miro underwent more proteasome-dependent degradation than Myc-MiroS156A (Fig. 3 C and D). To test the significance of S156 for ubiquitination of Miro, either wild-type or MiroS156A was expressed together with Parkin and HA-ubiquitin in HEK293T cells and Miro was immunoprecipitated with α-Myc. Myc-MiroS156A had less anti–HA-ubiquitin immunoreactivity than Myc-Miro (Fig. 3E). We then asked if phosphorylation stimulated the interaction of Miro with Parkin. We coexpressed Miro and Parkin, immunoprecipitated Miro with α-Myc, and then treated the immunoprecipitate with calf alkaline phosphatase (CIP). The interaction of Miro with Parkin was significantly weaker upon the treatment with the phosphatase (Fig. 3 F and G), Thus, Miro–Parkin interaction is promoted by phosphorylations. To test if the interaction depended on Miro phosphorylated on S156, we coexpressed either Myc-Miro or Myc-MiroS156A with YFP-Parkin in HEK293T cells and examined the amount of Parkin coprecipitated with Miro. Less YFP-Parkin coprecipitated with Myc-MiroS156 than with Myc-Miro (Fig. 3 G and H), and Myc-MiroS156A also had significantly less of the slowly migrating and presumably ubiquitinated bands (Fig. 3 H and I). Thus, the interactions of Parkin with Miro in HEK293T cells depend, at least in part, on phosphorylation of Miro on S156.
Fig. S3.
(A) HeLa cells transfected with the indicated constructs and treated with 20 μM CCCP for 2 h before lysis followed by immunoprecipitation of Myc-Miro. Membranes were probed with anti-HA (ubiquitin), then stripped of secondary antibody and incubated with anti-Myc (Miro). HA+ bands are only present in the CCCP-treated condition and run at the same position as the higher molecular-mass species of Myc-Miro. An asterisk indicates nonspecific band detected by anti-Myc antibody. (B) HeLa cells transfected with the indicated constructs at the concentrations used by Liu et al. (44) and treated with 20 μM CCCP for 2 h before lysis. With both Parkin overexpression and CCCP treatment, but wild-type and nonphosphorylatable MiroS156A appear to be ubiquitinated and degraded, as indicated by the decrease in the 75-kDa Miro band and the appearance of a ladder of higher molecular-mass species upon longer exposure. (C) Coomasie staining of samples taken during purification of MBP-Parkin on an MBP column. E1–3, eluates 1–3 from the column; FT, flow through; W1-2, first and second wash. E1 was used for the experiments. (D) In vitro ubiquitination reaction to test whether the MBP-Parkin used for in vitro assays was catalytically active. MBP-Parkin self-ubiquitinates at an alkaline pH in the presence of E1 and E2 ligases, ubiquitin, and ATP (63). (E) In vitro interactions of MBP-Parkin with dMiroWT and dMiroS182E.
Fig. 3.
MiroS156 promotes the interaction of Parkin with Miro. (A and B) Transfection of HEK293T cells with either 1 μg of DNA for PINK1-FLAG (A) or 1 μg of DNA for YFP-Parkin (B) caused preferential degradation of Myc-Miro compared with Myc-MiroS156A (expressed equivalently from 2 μg of DNA). Dashed lines in B indicate the place where the order of the lanes on the gel was altered for clarity of presentation. (C and D) HEK293T cells expressing 0.1 μg of either Myc-Miro or Myc-MiroS156A for 24 h were pretreated with 5 μM MG132 for 12 h and with 10 μM CCCP for 2 h before lysis. CCCP caused preferential proteasomal degradation of Myc-Miro compared with Myc-MiroS156A. A representative immunoblot (C) and quantification from three independent replicates (D) are shown. (E) α-Myc–Miro immunoprecipitates from cells expressing YFP-Parkin and HA-ubiquitin show greater α-HA–ubiquitin immunoreactivity on Myc-Miro than Myc-MiroS156A. (F and G) α-Myc immunoprecipitates from lysates of HEK293T cells transfected with 1 μg of Myc-Miro and 2 μg of YFP-Parkin were incubated with CIP (30 U) for 30 min. α-GFP staining of the immunoprecipitates showed less intensity of YFP-Parkin in the CIP-treated sample. A representative example (F) and quantification (G) from three independent replicates are shown. (H–J) Cells transfected with 1 μg of Myc-Miro or MycMiroS156A and 2 μg of YFP-Parkin as indicated. YFP-Parkin expression caused a ladder of high molecular mass species immunoreactive for α-Myc. This ladder was more abundant in cells expressing Myc-Miro than Myc-MiroS156A, as quantified in I, after normalization to actin. Upon immunoprecipitation with α-Myc, YFP-Parkin was more abundant in Myc-Miro than in Myc-MiroS156A-expressing cells, as quantified in J after normalization to the 75 kDa myc-immunoreactive band. n = 3. (K) The indicated purified recombinant proteins were combined and incubated for 30 min at 37 °C before immunoprecipitation for 2 h with α-MBP antibody. (L) Quantification of immunoprecipitated dMiro (anti-His immunoreactivity) from four experiments as in K. Background-subtraction and normalization to MBP levels as in J. *P < 0.05, **P < 0.01, **** P < 0.0001.
The effects of the S156A mutation suggested that phosphorylation of this site promotes Parkin–Miro interactions. To test this hypothesis directly, we affinity-purified recombinant human Parkin tagged with maltose binding protein (MBP) (Fig. S3C), which is catalytically active (Fig. S3D), and incubated it with wild-type and phosphomimetic forms of bacterially expressed Miro. Because recombinant Drosophila Miro can be successfully expressed and purified, we used Drosophila Miro with glutamate or aspartate substitutions at S182 (dMiroS182E or -D), the site equivalent to mammalian S156. After incubation of the proteins for 30 min at 37 °C, we immunoprecipitated MBP-Parkin and evaluated the levels of dMiro present in the precipitate. The interaction was significantly greater for dMiroS182E and dMiro182D than for dMiroWT (Fig. 3 K and L and Fig. S3E). Collectively, these lines of evidence suggested that Miro phosphorylation on S156 promotes the ubiquitination and degradation of Miro by stimulating its interaction with Parkin.
Miro T298E and T299E Phosphomimetics Render Miro Resistant to Parkin-Induced Ubiquitination and Degradation.
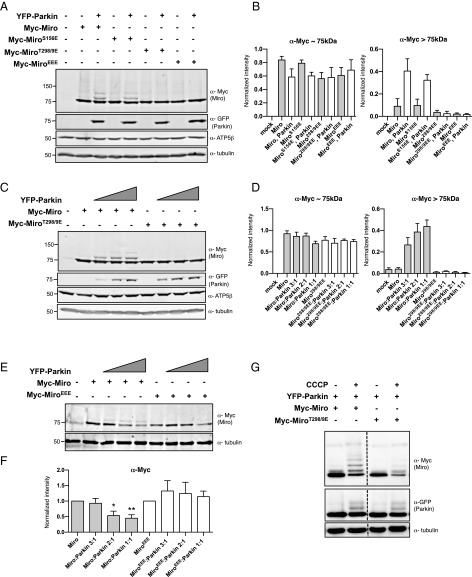
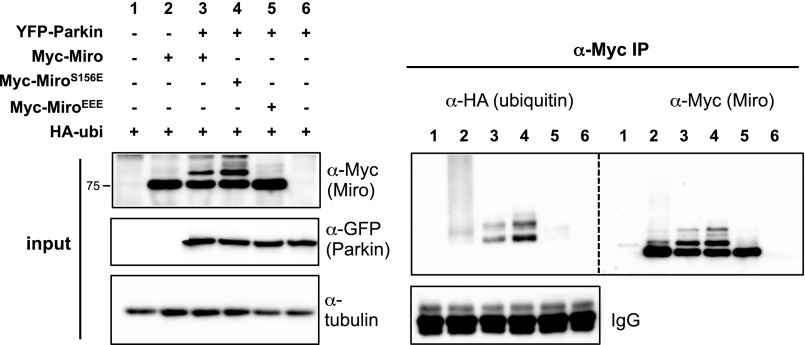
To determine the consequences of phosphorylation of T298 and T299, we replaced both residues with a phosphomimetic glutamate (Miro298/9EE) mutant. We also generated a triple Miro phosphomimetic mutant (MiroEEE = MiroS156E,T298E,T299E). The mutations did not affect the ability of Miro to localize to mitochondria or bind to Milton (Fig. S1). Although we anticipated that coexpression of the mutants with YFP-Parkin would promote the slower-migrating and presumably ubiquitinated bands of Miro similar to S156E, Miro298/9EE, and MiroEEE, mutants were resistant to the modification (Fig. 4 A and B), even at high ratios of Parkin:Miro transfection (Fig. 4 C and D). Moreover, Parkin expression conditions that caused wild-type Miro levels to be significantly reduced did not reduce levels of MiroEEE (Fig. 4 E and F). We confirmed that the slower-migrating bands of MiroWT and S156E were immunoreactive for HA-ubiquitin but no HA-ubiquitin was observed in the immunoprecipitate when MiroEEE was coexpressed with Parkin (Fig. S4). Thus, mimicking phosphorylation on T298 and T299 likely suppresses the ubiquitination and degradation of Miro by Parkin and does so even when S156 also has been replaced by a glutamate. However, PINK1 activation of Parkin upon depolarization of mitochondria with CCCP could override the effect of T298/9EE; Miro levels were reduced and the slower-migrating Miro bands appeared, consistent with ubiqutination (Fig. 4G). The effect of Miro phosphomimetic mutations thus depends on the level of Parkin activation.
Fig. 4.
MiroT298/9EE is resistant to Parkin-mediated ubiquitination and degradation. (A and B) HEK293T cells cotransfected with Myc-Miro mutant constructs and YFP-Parkin DNA as indicated. Intensities of the ∼75-kDa band and bands higher than ∼75 kDa in size were normalized to α-ATPase5β intensity. Bar graphs represent the mean normalized intensity from four independent transfections. (C and D) Increasing YFP-Parkin DNA concentrations were cotransfected with either Myc-Miro or Myc-Miro298/9EE and quantified as in B. (E and F) Increasing amounts of YFP-Parkin was coexpressed with the indicated constructs in HEK293T cells. Bar graphs represent α-Myc intensities in four independent transfections. (G) HeLa cells transfected with the indicated constructs and treated with 40 μM CCCP for 3 h before lysis. Dashes indicate places where the ordering of the lanes on the gel was altered for clarity of presentation. *P < 0.05, **P < 0.01.
Fig. S4.
HEK cells transfected with the indicated constructs and subjected to lysis followed by immunoprecipitation with anti-Myc antibody. Membranes were stained with anti-HA (ubiquitin), then stripped of secondary antibody and incubated with anti-Myc (Miro). HA+ bands are present in lysates of MiroWT- (lane 3) and MiroS156E- (lane 4) expressing cells but not in MiroEEE- (lane 5) expressing cells. Note that HA+ bands migrated at the same position as the high molecular-mass Myc+ bands.
MiroT298E,T299E Inhibits Parkin Effects on Mitochondrial Dynamics.
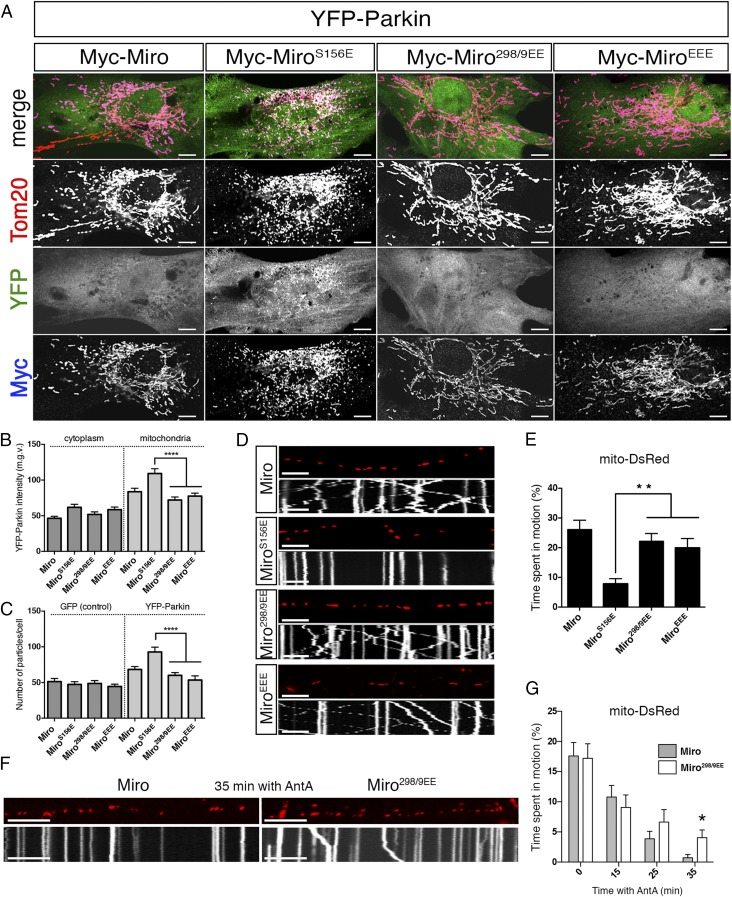
The partial resistance of MiroEEE mutants to degradation in cell lines suggested that 298/9EE might also suppress the effects of Parkin on mitochondrial dynamics. In primary rat fibroblasts, Miro298/9EE prevented S156E from recruiting Parkin; the triple phosphomimetic induced neither mitochondrial accumulation of Parkin nor fragmentation (Fig. 5 A–C). Consistent with these findings, the 298/9EE mutant also suppressed the effect of S156E on mitochondrial movement in axons; neither Myc-Miro298/9EE nor EEE arrested their transport (Fig. 5 D and E and Table S2).
Fig. 5.
MiroT298/9EE suppresses S156E-induced effects on mitochondrial dynamics. (A) Rat embryonic fibroblasts transfected with the indicated constructs and stained with α-Tom20 (red) and α-Myc (blue). (B) Average median gray value of YFP signal and (C) average number of small, α-Tom20+ mitochondrial particles per cell in cells transfected with the indicated constructs. n = 40–50 cells from three biological replicates. (D) Kymographs from distal axons of mouse hippocampal neurons transfected with mito-DsRed and the indicated constructs. (E) Average time each mitochondrion (mito-DsRed) spent in motion in axons transfected with the indicated constructs and average time each synaptic vesicle (syp-GFP) spent in motion in the same axons. n = 125–149 mitochondria and 171–240 synaptic vesicles/axon from nine axons and three biological replicates (see also Table S2). (F) Representative kymographs of mitochondrial motility in axons transfected with the indicated constructs and imaged after 35 min incubation with 10 μM Antimycin A. (G) Average time spent in motion by mitochondria at different times after application of 10 μM Antimycin A. n > 100 mitochondria and time points from nine axons and four biological replicates per time point. (Scale bars, 10 μm.) *P < 0.05, **P < 0.01, ****P < 0.0001. See also Table S3.
Table S2.
Motility parameters in mouse hippocampal axons coexpressing Mito-DsRed with indicated constructs
| Construct | #mito or #syp-GFP | Time spent in motion (%) | Anterograde (%) | Anterograde speed (μm/s) | Retrograde (%) | Retrograde speed (μm/s) | Stop frequency (%) | Reversal frequency (%) | Total travel length (μm) |
| Mitochondrial motility parameters in mouse hippocampal axons coexpressing Mito-DsRed with indicated constructs | |||||||||
| Myc-Miro | 125 | 26.1 ± 35.31 | 10.23 ± 25.43 | 0.16 ± 0.1 | 15.90 ± 29.65 | 0.35 ± 0.46 | 2.42 ± 3.67 | 0.18 ± 1.57 | 16.44 ± 29.5 |
| Myc-MiroS156E | 127 | 7.83 ± 20.3 | 1.16 ± 7.6 | 0.13 ± 0.16 | 6.66 ± 19.18 | 0.19 ± 0.14 | 0.70 ± 1.64 | 0 | 4.22 ± 11.94 |
| Myc-Miro298/9EE | 149 | 22.17 ± 31.96 | 10.9 ± 25.57 | 0.12 ± 0.05 | 11.29 ± 24.13 | 0.27 ± 0.4 | 1.99 ± 3.33 | 0.11 ± 1.09 | 11.40 ± 22.51 |
| Myc-MiroEEE | 126 | 19.91 ± 35.65 | 10.30 ± 28.33 | 0.30 ± 0.33 | 9.62 ± 25.32 | 0.32 ± 0.40 | 0.89 ± 2.30 | 0.03 ± 0.36 | 12.81 ± 32.00 |
| Motility parameters of synaptic vesicles in mouse hippocampal axons coexpressing Syp-GFP with indicated constructs | |||||||||
| Myc-Miro | 171 | 23.54 ± 32.04 | 12.78 ± 27.16 | 0.52 ± 2.13 | 10.76 ± 22.95 | 0.44 ± 2.07 | 2.42 ± 3.67 | 2.46 ± 3.42 | 14.18 ± 32.38 |
| Myc-MiroS156E | 240 | 25.58 ± 32.61 | 13.17 ± 26.12 | 0.20 ± 0.28 | 12.42 ± 26.18 | 0.21 ± 0.39 | 2.43 ± 3.69 | 0.06 ± 0.45 | 11.93 ± 21.05 |
| Myc-Miro298/9EE | 214 | 27.35 ± 37.10 | 13.94 ± 30.05 | 0.16 ± 0.18 | 13.41 ± 29.09 | 0.13 ± 0.09 | 2.24 ± 3.94 | 0 | 11.66 ± 21.88 |
| Myc-MiroEEE | 185 | 28.82 ± 35.65 | 16.09 ± 30.69 | 0.15 ± 0.14 | 12.73 ± 27.29 | 0.10 ± 0.05 | 2.15 ± 3.450 | 0.01 ± 0.22 | 11.64 ± 19.17 |
This table is related to Fig. 5E.
To test the effects of 298/9EE on mitochondrial dynamics when endogenous PINK1 was activated, we transfected rat hippocampal neurons with either with Myc-Miro or Myc-Miro298/9EE and applied 10 μM antimycin A to activate the PINK1/Parkin pathway (51). Antimycin A caused mitochondrial arrest in both populations (Fig. 5G), although a low amount of axonal mitochondrial movement was visible in Miro298/9EE-expressing neurons at late time-points (Fig. 5 F and G and Table S3) that was significantly higher than in control neurons expressing Myc-Miro. This experiment indicated that even upon depolarization, the presence of the 298/9EE mutation can modestly delay the onset of Parkin activity with regard to Miro. Therefore, we concluded that the presence of a phosphate-mimicking modification on T298 and T299 can, via the suppression of Miro ubiquitination and degradation, negatively regulate the ability of Parkin to regulate mitochondrial dynamics.
Table S3.
Mitochondrial motility parameters in rat hippocampal axons coexpressing Mito-DsRed with indicated constructs upon treatment with 10 μM antimycin A
| Time with 10 μM AntA (min) | Construct | #mito | Time spent in motion (%) | Anterograde (%) | Anterograde speed (μm/s) | Retrograde (%) | Retrograde speed (μm/s) | Stop frequency (%) | Reversal frequency (%) | Total travel length (μm) |
| 0 | Myc-Miro | 176 | 17.59 ± 29.74 | 8.29 ± 22.68 | 0.23 ± 0.27 | 9.28 ± 20.30 | 0.25 ± 0.12 | 1.49 ± 3.87 | 0.19 ± 0.93 | 4.89 ± 8.73 |
| Myc-Miro298/9EE | 139 | 17.21 ± 28.42 | 8.86 ± 12.19 | 0.23 ± 0.15 | 8.37 ± 21.29 | 0.27 ± 0.19 | 1.51 ± 3.67 | 0.09 ± 0.6 | 5.01 ± 10.07 | |
| 5 | Myc-Miro | 140 | 14.05 ± 26.45 | 5.88 ± 18.33 | 0.25 ± 0.43 | 7.46 ± 18.51 | 0.20 ± 0.13 | 1.66 ± 4.34 | 0.13 ± 0.72 | 3.6 ± 10.55 |
| Myc-Miro298/9EE | 111 | 11.99 ± 28.51 | 5.77 ± 20.46 | 0.18 ± 0.16 | 5.31 ± 19.66 | 0.21 ± 0.07 | 0.79 ± 2.55 | 0 | 2.11 ± 5.24 | |
| 10 | Myc-Miro | 148 | 12.67 ± 25.24 | 6.09 ± 19.3 | 0.16 ± 0.08 | 6.57 ± 17.85 | 0.36 ± 0.52 | 1.47 ± 4.17 | 0.03 ± 0.29 | 3.92 ± 12.37 |
| Myc-Miro298/9EE | 111 | 10.43 ± 23.09 | 3.68 ± 13.43 | 0.17 ± 0.08 | 5.84 ± 17.40 | 0.16 ± 0.06 | 1.27 ± 2.96 | 0.04 ± 0.27 | 1.78 ± 3.4 | |
| 15 | Myc-Miro | 143 | 10.79 ± 23.17 | 5.33 ± 17.23 | 0.29 ± 0.18 | 5.46 ± 15.62 | 0.29 ± 0.56 | 1.49 ± 4.33 | 0.1 ± 0.53 | 3.11 ± 10.65 |
| Myc-Miro298/9EE | 110 | 9.08 ± 21.44 | 4.37 ± 16.56 | 0.17 ± 0.06 | 4.72 ± 14.78 | 0.17 ± 0.06 | 1.14 ± 3.65 | 0.01 ± 009 | 2.00 ± 5.28 | |
| 20 | Myc-Miro | 137 | 6.66 ± 18.77 | 3.56 ± 13.98 | 0.14 ± 0.06 | 3.09 ± 10.75 | 0.23 ± 0.14 | 1.00 ± 3.09 | 0.12 ± 0.80 | 1.33 ± 3.87 |
| Myc-Miro298/9EE | 117 | 11.83 ± 25.48 | 6.17 ± 21.71 | 0.15 ± 0.04 | 5.67 ± 15.09 | 0.18 ± 0.08 | 1.17 ± 3.11 | 0.03 ± 0.29 | 2.39 ± 5.36 | |
| 25 | Myc-Miro | 130 | 3.85 ± 14.24 | 0.72 ± 3.76 | 0.18 ± 0.18 | 3.13 ± 13.69 | 0.2 ± 0.1 | 0.33 ± 1.13 | 0.04 ± 0.44 | 0.76 ± 2.95 |
| Myc-Miro298/9EE | 105 | 6.64 ± 21.16 | 2.02 ± 11.79 | 0.15 ± 0.04 | 4.62 ± 18.10 | 0.21 ± 0.12 | 0.39 ± 1.70 | 0 | 1.55 ± 5.44 | |
| 30 | Myc-Miro | 130 | 3.46 ± 13.80 | 0.85 ± 8.5 | 0.11 ± 0.02 | 2.60 ± 11.07 | 0.2 ± 0.09 | 0.45 ± 2.18 | 0 | 0.73 ± 2.83 |
| Myc-Miro298/9EE | 106 | 6.9 ± 20.58 | 2.67 ± 12.93 | 0.13 ± 0.06 | 4.22 ± 16.71 | 0.20 ± 0.09 | 0.14 | 0 | 1.15 ± 3.43 | |
| 35 | Myc-Miro | 123 | 0.72 ± 6.09 | 0.72 ± 6.09 | 0.12 ± 0.04 | 0 | 0 | 0.08 ± 0.42 | 0 | 0.12 ± 0.99 |
| Myc-Miro298/9EE | 111 | 4.05 ± 13.53 | 2.58 ± 11.14 | 0.12 ± 0.03 | 1.48 ± 8.17 | 8.84 ± 21.31 | 0.48 ± 2.30 | 0 | 1.56 ± 9.86 |
This table is related to Fig. 5G.
Discussion
The physiological role of PINK1 modification of Parkin substrates has been unclear. We report here that: (i) mimicking phosphorylation of the Parkin substrate Miro at S156 can recruit Parkin to mitochondria; (ii) Parkin can be activated by phosphomimetic Miro in a PINK1-null background; (iii) substrate-based activation of Parkin can cause mitochondrial fragmentation and mitochondrial arrest, but will not proceed to mitophagy without PINK1 activation of Parkin; and (iv) the effect of the modification of S156 is reversible by phosphomimetic substitutions on two other Miro residues.
Solving how PINK1 recruits Parkin to damaged mitochondria will be instrumental for the rational design of Parkin-modulatory therapies. Besides modifying ubiquitin and Parkin, PINK1 phosphorylates several known Parkin substrates on the mitochondrial outer membrane. Phosphorylation-dependent recruitment of E3 ligases to their substrates is a common regulatory theme (52), and we propose that Parkin is no exception to this pattern. To circumvent PINK1 phosphorylation of Parkin and isolate the consequences of the substrate phosphorylation, we used phosphomimetic substitutions either in wild-type fibroblasts where PINK1 was not activated pharmacologically or in rat embryonic fibroblasts lacking PINK1, where even background activity of the kinase would be lacking. In either case, mimicking PINK1 phosphorylation of Miro on S156 promoted Parkin recruitment to mitochondria in fibroblasts (Fig. 1 A and B and Fig. S2). In vitro, mimicking this modification enhanced the interaction of Miro with Parkin (Fig. 3 K and L and Fig. S3E). Thus, the phosphorylation state of a Parkin substrate can stimulate Parkin interaction with that substrate and was sufficient to recruit Parkin to the mitochondria. In neurons, the cell type most affected by pathological mutations in PINK1 and Parkin, mimicking S156 phosphorylation was sufficient to cause Parkin-dependent arrest of mitochondria (Fig. 1 F and G), indicating that the S156E modification can stimulate endogenous Parkin. Substrate phosphorylation has also been reported to cause Mitofusin1/2 degradation upon Parkin recruitment (41), and thus may influence a variety of Parkin targets. Because Parkin-mediated degradation of Miro and Mitofusin1/2 happens before bulk clearance of mitochondrial proteins by mitophagy (15), selective phosphorylation-promoted recruitment of Parkin to particular high-priority targets may play a role in the early steps of Parkin recruitment, whereas ubiquitin phosphorylation is instrumental in driving the subsequent feed-forward loop that broadly ubiquitinates many proteins at the mitochondrial surface and induces mitophagy.
What role, if any, does PINK1 modification of Parkin substrates play in regulating the catalytic activity of Parkin? Parkin is a self-inactivated enzyme whose activity is stimulated by two PINK1 phosphorylations: (i) phosphorylation of S65 in the ubiquitin-like domain of Parkin and (ii) phosphorylation of an equivalent serine in ubiquitin, which can allosterically activate Parkin. Our data indicate that PINK1 phosphorylation of Miro also can activate Parkin, even in the absence of the other two events. Overexpression of wild-type Miro itself could cause Parkin to translocate to mitochondria, but the effect was enhanced by the phosphomimetic mutation (Fig. 1 A and B). The action of the phosphorylation, therefore, is less like an on/off switch than like an increase in the affinity for Parkin, and hence an increase in its ability to activate Parkin. Because Parkin catalytic activity is needed for Parkin to remain on mitochondria after mitochondrial depolarization (36, 53), Parkin recruitment to mitochondria can be used as a proxy for Parkin activity. Consistent with activation of Parkin by the MiroS156E phosphomimetic, wild-type Parkin, but not catalytically inactive Parkin, accumulated on mitochondria. Further evidence for catalytic activation of Parkin by Miro S156E was the fragmentation of the mitochondrial network upon its coexpression with Parkin in fibroblasts, a phenotype that also did not occur when the catalytically dead Parkin was expressed (Fig. 1 A and B). Importantly, PINK1 was not needed in these experiments, as we observed the same phenotypes in PINK1-null cells (Fig. S2). Thus, PINK1 phosphorylations of ubiquitin or Parkin are not absolutely required for Parkin activation. It will be interesting to know if substrate-driven activation is specific to Miro or can extend to other Parkin substrates as well.
Although PINK1 phosphorylation of S156 is supported both by previous data from our laboratory and the functional experiments presented here, other groups have failed to observe a role of PINK1-induced S156 phosphorylation in the ubiqutination of Miro (44, 50). One reason for the disparity may lie in the high levels of Parkin used in these experiments. In agreement with those studies, we also find that nonphosphorylatable Miro gets ubiquitinated by Parkin in those conditions (Fig. S3B). However, in those experiments where only endogenous Parkin was present, we observe a clear effect of the S156A mutation (Fig. 3 A, and C and D). Therefore, although PINK1-mediated S156 phosphorylation is not required for Miro degradation, the functional effect of mutating this site can be revealed when Parkin activity is low to moderate. The in vivo significance of the phosphorylation site was observed in Drosophila (46) and is also supported by our previous observations on mitochondrial motility in hippocampal neurons (15).
Once Parkin has been recruited to Miro, it could have access to other mitochondrial substrates. The fragmentation of mitochondria we observed in MiroS156E could, for example, reflect the secondary loss of proteins that support fusion (39, 41, 54), although it might also be because of the loss of kinesin from mitochondria when Miro is degraded (55). However, MiroS156E recruitment of Parkin did not induce detectable mitophagy, at least in the time frame that we analyzed (Fig. 2). Therefore, PINK1 modification of this single substrate, although able to recruit Parkin to Miro and even to activate Parkin for Miro ubiquitination, was not sufficient to induce the widespread ubiquitination of proteins on the mitochondrial surface, and thereby cause the recruitment of adaptor proteins and mitophagy (29, 30, 36, 56, 57). When PINK1 is activated in the context of mitochondrial damage, it will act on multiple targets, including Parkin and ubiquitin, and thereby achieve full enzymatic activity of Parkin and trigger mitophagy.
The possibility of limited activation of the PINK1/Parkin pathway offers a potential mechanism to separate mitochondrial arrest from mitophagy: some PINK1 and Parkin substrates, including Miro and Mitofusin1/2 (26, 39), may undergo proteasomal degradation rather than autophagic degradation because of their special relationship with PINK1 and Parkin. We do not know whether the degradation of Miro in mammalian cells under normal physiological conditions is always an early “pro-clearance” step in a series that will ultimately produce mitophagy, or if there are levels of PINK1 activation that will stop the process with Miro degradation and a decrease in mitochondrial motility. In Drosophila, however, alterations in mitochondrial motility can be seen with manipulations of PINK1 and Parkin that do not appear to involve widespread mitophagy (15, 46, 58). If Miro degradation is a step toward clearance of either a segment of the OMM or mitophagy of the entire organelle (8, 59), it is likely to be important, along with Mitofusin1/2 degradation, as a means to quarantine damaged mitochondria rapidly before the slower steps of autophagosome-dependent mitophagy or mitochondria-derived vesicle-based quality control.
To our surprise, phosphomimetic modifications on T298 and T299 acted in an opposite manner to S156. The physiological significance of these sites is currently less clear. There is uncertainty at present concerning the kinase that acts at these sites in vivo; it may not be PINK1. However, the unexpected finding that phophomimetics at these residues can prevent the effects of S156 phosphorylation raises the possibility that they are part of a negative regulatory pathway requiring further exploration.
Better understanding of the regulation of Parkin by PINK1 may lead to rational strategies for treating Parkinson’s disease. Although many studies have focused on Parkin and ubiquitin phosphorylation by PINK1, our findings highlight the need to consider also the role that PINK1 substrates like Miro play in the onset of Parkin activity. The PINK1/Parkin mechanism now appears more complex and can involve proteasomal degradation of specific substrates as well as lysosomal clearance of either all or part of a mitochondrion. Because Parkin can be activated and recruited or inhibited by substrate phosphorylations, the state of particular substrates may influence the end results of the pathway.
Materials and Methods
Reagents.
Myc-Miro mutants were generated by mutagenizing Myc-Miro plasmid (60) with a QuikChange Site Directed Mutagenesis Kit (Agilent). YFP-ParkinC431F mutant was generated as above using YFP-Parkin (8) as backbone. All mutants were sequence-verified. Sequences used for mutagenesis are in SI Materials and Methods. For antibody and chemical reagents information, see SI Materials and Methods.
Cell Culture and Transfection.
DNA transfections in HEK293T cells were performed with the calcium phosphate method and Lipofectamine2000 (ThermoFisher Scientific) or GenJet (SignaGen Laboratories) were used for transfections in HeLa cells. Immunocytochemistry was done on fibroblasts of Long Evans wild-type or Long Evans or PINK1−/− rats (47). Rat and mouse hippocampal neurons were isolated according to standard procedures and cultured as in ref. 61. Rat and mouse procedures were approved by the Institutional Animal Care Committee at the Boston Children's Hospital and by the Harvard HMA Standing Committee on Animals, respectively. See SI Materials and Methods for details.
Image Acquisition and Image Analysis.
Live-cell imaging of axonal mitochondria was performed as described in ref. 61 (see also SI Materials and Methods). For fixed samples, cells were fixed in 2.5% (vol/vol) PFA 24 h after transfection, permeabilized in 0.5% (wt/vol) saponin, blocked in 1% (wt/vol) BSA, 0.5% (wt/vol) saponin, and incubated overnight at 4 °C with primary antibodies. Confocal images were taken on a Zeiss LSM700 confocal microscope using 63× Zeiss plan-Apochromat oil, 1.4 NA or 100× Zeiss plan-Apochromat oil, 1.3 NA objectives. To analyze YFP-Parkin and ubiquitin intensity as well as mitochondrial morphology, images were processed in batch using a custom ImageJ script (SI Materials and Methods).
In Vitro Experiments, Immunoprecipitations, and Western Blotting.
dMiro constructs were mutagenized using standard procedures and then expressed in bacteria, purified, and stored as described in ref. 62. MBP-Parkin was purified according to ref. 63. MBP-Parkin was used within 2 wk after purification for in vitro reactions. Validation of MBP-Parkin activity was carried out following the protocols described in ref. 63. See SI Materials and Methods for details.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism v6.0. All samples were first subjected to a D’Angostino–Pearson omnibus normality test. If values came from a Gaussian distribution, t-test analysis was used for paired comparisons and one-way ANOVA along with Bonferroni correction for multiple comparisons. If values came from a non-Gaussian distribution, a Mann–Whitney U test was used for paired comparisons and Kruskall–Wallis nonparametric ANOVA test was used for multiple comparisons. Graph bars represent the mean ± SEM P values: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
SI Materials and Methods
DNA Constructs.
The following primers were used to generate Miro mutants: CCTGAAGAACATAGAAGAGCTCTTTTATTACG (MiroS156E forward primer), CGTAATAAAAGAGCTCTTCTATGTTCTTCAGG (MiroS156E reverse primer), CCTCCTGATTGCGAAGAAGAATTAAATCATC (MiroT298/9EE forward primer), GATGATTTAATTCTTCTTCGCAATCAGG (MiroT298/9EE reverse primer). The following primers were used to generate YFP-ParkinC431F mutant: AATGGAGGCTTCATGCACATG (YFP-ParkinC431F forward primer), TTTTTCCACTGGTACATGG (YFP-ParkinC431F reverse primer).
Antibodies and Small Molecules.
Primary antibodies were used at following dilutions for Western blots: rabbit anti-GFP (ThermoFisher Scientific, catalog no. A11122) at 1:3,000, mouse anti–β-tubulin (Sigma-Aldrich, catalog no. T6199) at 1:5,000, rabbit anti-ATPase (Sigma HPA001520) at 1:5,000, chicken anti-HA (EMD Millipore, catalog no. AB3254) at 1:1,000, rabbit anti-HA (Cell Signaling Technology, catalog no. 3724), mouse anti-HIS (ThermoFisher Scientific, Waltham, MA, catalog number MA1-21315) at 1:1,000, rabbit anti-MBP (New England Biolabs, catalog no. E8030S) at 1:10,000. Mouse anti-Myc, clone 4A6 (EMD Millipore, catalog no. 05724), detected ubiquitinated bands of Myc-Miro when this construct was highly expressed, and was used at 1:500. This antibody, however did not detect the upper-running bands when the amount of transfected DNA of Myc-Miro was lower than 1 μg. The following secondary antibodies were used for quantitative Western blots at 1:5,000 dilution, all from GE Healthcare Life Sciences: ECL-plex goat anti-mouse Cy5 (catalog no. PA45009V), ECL-plex goat anti-rabbit Cy5 (catalog no. PA45011V), ECL-plex goat anti-mouse Cy2 (catalog no. 28901108V), ECL-plex goat anti-rabbit Cy2 (catalog no. 28901110V).
For immunocytochemistry, the following antibodies were used: rabbit anti-Tom20 (Santa Cruz Biotechnology, catalog no. SC-11415) at 1:500, chicken anti-Myc (ThermoFisher Scientific, catalog no. A21281) at 1:500, rabbit anti-Myc (ThermoFisher Scientific, catalog no. 2272S) at 1:500, mouse anti-Ubiquitin (Santa Cruz Biotechnology, catalog no. P4D1) at 1:500, mouse anti-Parkin (Santa Cruz Biotechnology, catalog no. SC-32282) at 1:500. CCCP (Sigma-Aldrich, catalog no. C2759), Antimycin A (Enzo Life Sciences, catalog no. 380-075-M005), and MG132 (EMD Millipore, catalog no. 474790) were prepared and stored as instructed by the manufacturer.
Biochemistry in Established Cell Lines.
Cells were cultured in DMEM supplemented with l-glutamine, penicillin/streptomycin (ThermoFisher Scientific), and 10% FBS (Atlanta Biologicals). Calcium phosphate transfections were done at 80–90% confluence and cells were harvested 48 h after the transfection, except when indicated otherwise. Transfection efficiency in HEK293T cells usually was ∼90%. Cells were lysed 48 after the transfection. Although the final concentrations of plasmids varied form experiment to experiment, total DNA amount transfected in the wells of the same experiment was equilibrated with GFP-coding plasmid. Because the polyubiquitinated state of Miro is an intermediate on the pathway to degradation, the extent to which it was present in any given experiment depended closely on the rates of Miro synthesis, modification, and degradation, which vary with the levels of transfection and expression of each protein. For immunoprecipitations, cells were harvested in lysis buffer (0.05 M Tris, pH 7.5, 150 mM NaCl, 1% Triton, 0.1 mM PMSF, 1:500 Calbiochem protease inhibitor mixture set III), tumbled with 1–2 μg of primary antibody for 2 h at 4 °C followed by 1-h incubation with 6 mg of Sepharose protein A beads (GE Healthcare). CIP (New England Biolabs) was incubated in CutSmart buffer (New England Biolabs). For quantitative Western blot, membranes were incubated with fluorescent secondary antibodies and images were acquired on Typhoon laser scanner (GE Healthcare) within the linear range. When HRP-coupled secondary antibodies were used, images of the membranes were acquired either using ImageQuant scanner (GE Healthcare) within the linear range (for quantification) or film developer (for qualitative evaluation). All images were analyzed on ImageJ1.48q using the “Gels” tool. If not indicated, Western blots are representative of at least three independent transfections.
Live Cell Imaging.
Coverslips were transferred to HibernateE (BrainBits), axons were traced using Synaptophysin-GFP, and imaged two fields away of the axon terminal on either Zeiss LSM510 META or Zeiss LSM700 confocal microscopes using a 63× Zeiss Apochromat water, 1.0 NA objective. Time-lapse imaging was performed every 3.9 s for ∼300 s. Movies were analyzed using Kymolyzer macro for ImageJ developed by the laboratory (61). In all kymographs, anterograde motility is to the right, retrograde movement is to the left.
Mitolyzer1.0.
The script performs the following pipeline automatically: first, the image is separated into channels and regions of interest (ROIs) are established using the “default” threshold. For the cytoplasm the (ROI) is established upon a cytoplasmic signal (GFP or YFP-Parkin) and the mitochondrial ROI established using “default” threshold on the mitochondrial channel. Nuclear ROI is subtracted from the cytoplasmic ROI by “subtract” tool. Then, measurements of mean and median gray values, SD, skewedness, and kurtosis are extracted for each channel of interest from both cytoplasmic and mitochondrial ROIs. The same procedure was used in Figs. 1B and 2 C and D. Next, a conversion to binary of the mitochondrial channel is followed by counting particles, restricting the analysis to particles ranging from 0.1 to 5 μm in size and from 0.5 to 1 in roundness. In Fig. 2C, area measurement was added to the particle count measurement. To determine the coextension of GFP–LC-3 with α-Tom20 in Fig. 2, a “default” threshold was used to determine mitochondrial ROI, and the number of GFP particles located within the mitochondrial ROI was established using “analyze particles” tool. Results were exported as .csv files and statistical analysis was performed in GraphPad Prism v6.0. Source code for Mitolyzer1.0 is available upon request.
Primary Fibroblast and Neuron Culture.
Fibroblasts were obtained from embryonic day (E) 18 rat embryos by standard methods and cultured in DMEM+20% FBS. Cells were used between passages P1 and P5: 20 × 103 cells were seeded in 12-mm glass coverslips (Bellco Glass), plasmids transfected with Lipofectamine2000 and cells were fixed 24 h after transfection. Hippocampal neurons were obtained from E18 rat embryos, plated at 150 × 103 on 12-mm glass coverslips (Bellco Glass) coated with 20 μg/mL poly-l-Lysine (Sigma-Aldrich) and 3.5 μg/mL laminin (ThermoFisher Scientific), and maintained in Neurobasal (ThermoFisher Scientific) medium supplemented with B27 (ThermoFisher Scientific), l-glutamine, and penicillin/streptomycin. Neurons were transfected at day in vitro (DIV) 7 and imaged at DIV9–DIV10. The coexpression of multiple constructs transfected into neurons was validated by retrospective immunostaining following live-cell imaging. Hippocampal neurons form Parkin−/− animals and their littermate controls were isolated at E17 and plated, maintained, and transfected as above.
In Vitro Interactions.
To evaluate MBP-Parkin–dMiro interactions, the proteins were quick-thawed at 37 °C, centrifuged for 10 min at 600,000 × g to remove precipitates, and then dialyzed into the reaction buffer: 25 mM Hepes, pH 7.5, 150 mM NaCl, 0.5 mM MgCl2, 0.5 mM Tris (2-carboxyethyl) phosphine (TCEP), 20 μM GTP, 40 μM ATP, 0.5% Nonidet P-40, and protease inhibitors (mixture III from Calbiochem). Reaction buffer was prepared fresh before each experiment. Protein concentration was measured after dialysis and before the experiment using the Lowry technique. Approximately 0.5 μg of each protein was added to a final volume of 30 μL. dMiroWT and mutant proteins were incubated in parallel with MBP-Parkin with for 30 min time at 37 °C. Six independent replicates of the reaction were performed on separate days.
Acknowledgments
We gratefully acknowledge the gift of plasmid constructs from Drs. P. Aspenstrom for myc-hMiro1/2; G. Hajnoczky for Mito-DsRed; R. Youle for Parkin constructs; D. Selkoe for PINK1-FLAG; Sheila Thomas for GFP–LC-3; Noriyuki Matsuda for MBP-Parkin; G.W. Hart for Xpress-hMilton1; H. T. Cline for syp-GFP; and S. Rice for dMiro proteins. We thank S. Vasquez for assistance with primary hippocampal neuron cultures; K. Apaydin for assistance with neuron cultures and protein purification; Dr. K. Kapur for help with statistical analyses; A. Jackson for the help coding Mitolyzer1.0; Drs. G. Pekkurnaz and M. Cronin for help with the initial experiments and for fruitful discussions; the Intellectual and Developmental Disabilities Research Center (IDDRC) Molecular Genetics and Imaging Cores and L. Mkhitarian for general help; and Drs. C. Gutierrez, E. Martinez-Salas, and P. Gutierrez-Martinez for critically reading the manuscript. This work was funded by NIH Grants P30 HD018655 (to the IDDRC Imaging, Proteomics, and Molecular Genetics Cores) and NS065013 (to M.J.L); PDF-FBS-1320 from the Parkinson’s Disease Foundation (to E.S.); and the Mathers Foundation and NIH Grant R01GM069808 (to T.L.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612283113/-/DCSupplemental.
References
- 1.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: Molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31(14):3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitada T, et al. Mutations in the Parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 4.Valente EM, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 5.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105(5):1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with Parkin. Nature. 2006;441(7097):1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 7.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by Parkin. Nature. 2006;441(7097):1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 8.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morais VA, et al. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344(6180):203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- 11.Morais VA, et al. Parkinson’s disease mutations in PINK1 result in decreased complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1(2):99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger AK, et al. Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum Mol Genet. 2009;18(22):4317–4328. doi: 10.1093/hmg/ddp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henn IH, et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J Neurosci. 2007;27(8):1868–1878. doi: 10.1523/JNEUROSCI.5537-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller-Rischart AK, et al. The E3 ligase Parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol Cell. 2013;49(5):908–921. doi: 10.1016/j.molcel.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147(4):893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin SM, et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191(5):933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK. The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem. 2011;117(5):856–867. doi: 10.1111/j.1471-4159.2011.07253.x. [DOI] [PubMed] [Google Scholar]
- 18.Greene AW, et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012;13(4):378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamano K, Youle RJ. PINK1 is degraded through the N-end rule pathway. Autophagy. 2013;9(11):1758–1769. doi: 10.4161/auto.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin SM, Youle RJ. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy. 2013;9(11):1750–1757. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda N, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189(2):211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vives-Bauza C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107(1):378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley BE, et al. Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases. Nat Commun. 2013;4:1982. doi: 10.1038/ncomms2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trempe JF, et al. Structure of Parkin reveals mechanisms for ubiquitin ligase activation. Science. 2013;340(6139):1451–1455. doi: 10.1126/science.1237908. [DOI] [PubMed] [Google Scholar]
- 25.Wauer T, Komander D. Structure of the human Parkin ligase domain in an autoinhibited state. EMBO J. 2013;32(15):2099–2112. doi: 10.1038/emboj.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan NC, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20(9):1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarraf SA, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci. 2012;125(Pt 6):1488–1499. doi: 10.1242/jcs.094110. [DOI] [PubMed] [Google Scholar]
- 29.Yamano K, Fogel AI, Wang C, van der Bliek AM, Youle RJ. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife. 2014;3:e01612. doi: 10.7554/eLife.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in Parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA. 2014;111(42):E4439–E4448. doi: 10.1073/pnas.1405752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane LA, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205(2):143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazlauskaite A, et al. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460(1):127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyano F, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510(7503):162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 34.Ordureau A, et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014;56(3):360–375. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22(2):320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazarou M, et al. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol. 2013;200(2):163–172. doi: 10.1083/jcb.201210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bingol B, et al. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510(7505):370–375. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- 38.Cornelissen T, et al. The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum Mol Genet. 2014;23(19):5227–5242. doi: 10.1093/hmg/ddu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka A, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191(7):1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5(7):e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340(6131):471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai YC, et al. Phosphoproteomic screening identifies Rab GTPases as novel downstream targets of PINK1. EMBO J. 2015;34(22):2840–2861. doi: 10.15252/embj.201591593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazlauskaite A, et al. Phosphorylation of Parkin at Serine65 is essential for activation: elaboration of a Miro1 substrate-based assay of Parkin E3 ligase activity. Open Biol. 2014;4(3):130213. doi: 10.1098/rsob.130213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, et al. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8(3):e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48(9):2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai P-I, et al. PINK1-mediated phosphorylation of Miro inhibits synaptic growth and protects dopaminergic neurons in Drosophila. Sci Rep. 2014;4:6962. doi: 10.1038/srep06962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baptista MA, et al. A strategy for the generation, characterization and distribution of animal models by The Michael J. Fox Foundation for Parkinson’s Research. Dis Model Mech. 2013;6(6):1316–1324. doi: 10.1242/dmm.011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg MS, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278(44):43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- 49.Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6(8):1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birsa N, et al. Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J Biol Chem. 2014;289(21):14569–14582. doi: 10.1074/jbc.M114.563031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206(5):655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter T. The age of crosstalk: Phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28(5):730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Zheng X, Hunter T. Parkin mitochondrial translocation is achieved through a novel catalytic activity coupled mechanism. Cell Res. 2013;23(7):886–897. doi: 10.1038/cr.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30(12):4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, et al. Dynamic tubulation of mitochondria drives mitochondrial network formation. Cell Res. 2015;25(10):1108–1120. doi: 10.1038/cr.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell. 2015;60(1):7–20. doi: 10.1016/j.molcel.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazarou M, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devireddy S, Liu A, Lampe T, Hollenbeck PJ. The organization of mitochondrial quality control and life cycle in the nervous system in vivo in the absence of PINK1. J Neurosci. 2015;35(25):9391–9401. doi: 10.1523/JNEUROSCI.1198-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33(4):282–295. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278(8):6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 61.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158(1):54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klosowiak JL, et al. Structural coupling of the EF hand and C-terminal GTPase domains in the mitochondrial protein Miro. EMBO Rep. 2013;14(11):968–974. doi: 10.1038/embor.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuda N, et al. Diverse effects of pathogenic mutations of Parkin that catalyze multiple monoubiquitylation in vitro. J Biol Chem. 2006;281(6):3204–3209. doi: 10.1074/jbc.M510393200. [DOI] [PubMed] [Google Scholar]