The small size, and thus coding capacity, of viral genomes necessitates the repetitive use of identical building blocks in constructing the viral capsid. This genetic economy gives rise to highly symmetric structures, typically with either helical or icosahedral geometries. Assembly of symmetric viral capsids is believed to proceed via “conformational switching” of individual protein subunits (1–3). In this model, a special nucleation event initiates the assembly process, and in subsequent assembly steps, each protein that binds to the growing structure undergoes a conformational change that creates a new binding site for the next protein to assemble. Although the use of icosahedral symmetry in capsid assembly provides an elegant solution to the need for genetic economy, assuming the entire capsid obeys this symmetry is an oversimplification that does not adequately reflect the complexity of biology (4, 5). Indeed, the viral genome itself is present as only a single copy and thus cannot assume the icosahedral symmetry of the surrounding capsid. Additionally, it is imperative that regions of the virus shell deviate from the global icosahedral symmetry of the capsid to successfully accomplish critical aspects of the viral life cycle such as genome packaging/release and host cell recognition/attachment (4, 6–9). Until recently, structural biology has largely ignored these essential deviations from symmetry. For technical reasons, X-ray and cryo-electron microscopy (cryo-EM) structures of viruses typically impose icosahedral symmetry and are thus incapable of illuminating the subtle structural rearrangements of the capsid necessary to successfully navigate the viral life cycle. In PNAS, Gorzelnik et al. (10) present an asymmetric cryo-EM reconstruction of the bacterial virus Qβ that provides insights into the assembly pathway of the virus, the organization of the genetic material within the viral capsid, and the potential coupling of host cell recognition and genome release.
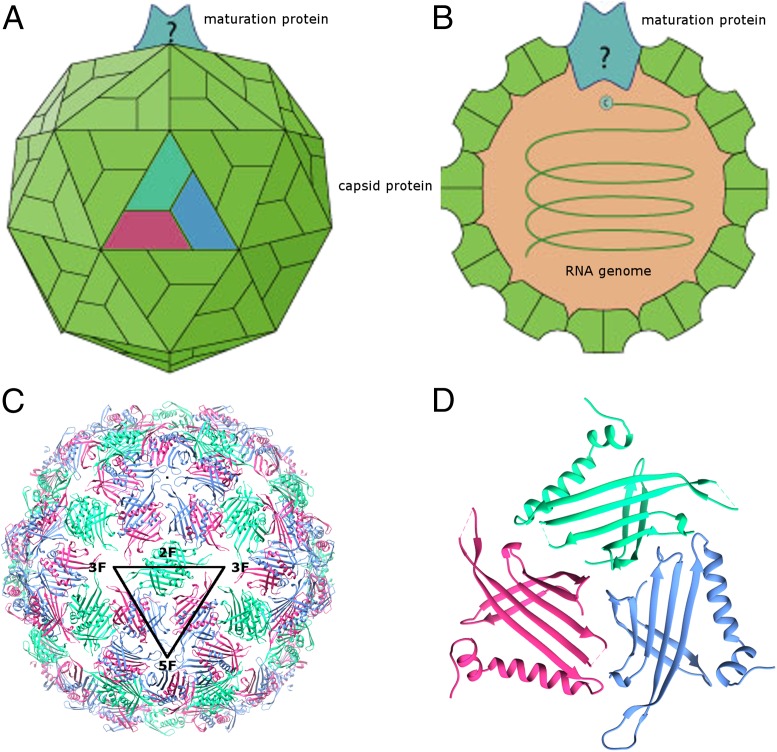
Bacteriophage Qβ belongs to the Leviviridae family of small, positive-sense, single-stranded RNA bacteriophages (11) (Fig. 1 A and B). These viruses infect their Gram-negative bacterial hosts via adsorption to bacterial pili. The Leviviridae have small, ∼4-kb, genomes that encode a maturation protein, the capsid protein, and a subunit of an RNA-dependent RNA replicase (12, 13). MS2-like Leviviridae phages encode an additional lysis protein, whereas in the Qβ-like phages, lysis is carried out by the maturation protein (called A2 in Qβ). Within the viral capsids of Leviviridae viruses, the RNA forms a branched network of stem-loops adjacent to the inner surface of the capsid, primarily binding to coat protein dimers that are located predominantly in one-half of the shell (14). Thus, although the RNA genome is single-stranded, it adopts specific secondary structures within capsids with many double-stranded regions; these regions are thought to play essential roles in translational regulation and replication (14). The highly structured RNA genome is encapsidated by an icosahedral protein shell with T = 3 quasisymmetry (12, 13) (Fig. 1 A, C, and D). The capsid contains 178 copies of the coat protein (CP) and a single copy of the maturation protein, which replaces one CP dimer in the icosahedral lattice (14) (Fig. 1B). The maturation protein is attached to the encapsidated genomic RNA and is believed to bind to the host receptor.
Fig. 1.
Schematic of a typical Leviviridae virus and the X-ray crystallographic structure of bacteriophage Qβ. (A) Schematic of the T = 3 capsid of a typical Leviviridae virus. Three quasiequivalent subunits are colored red, green, and blue, respectively. (B) Schematic cross-section of a typical Leviviridae virus showing the encapsidated single-stranded positive-sense RNA genome. The capsid protein, maturation protein, and RNA genome are labeled. (C) Ribbon diagram of the X-ray crystallographic structure of the T = 3 bacteriophage Qβ capsid (12) [Protein Data Bank (PDB) ID code 1QBE]. Quasiequivalent subunits in the T = 3 icosahedral lattice are colored red, green, and blue, respectively. The icosahedral asymmetric unit is shown as a black triangle with the positions of twofold, threefold, and fivefold symmetry axes indicated. (D) Crystallographic asymmetric unit of the bacteriophage Qβ X-ray crystal structure (12) (PDB ID code 1QBE). Ribbon diagrams in C and D were rendered using CHIMERA (21).
Like any virus, Qβ faces the challenge of assembling an environmentally stable structure that must partially disassemble upon host infection to release its genome and initiate a new cycle of virus replication. Unlike the double-stranded DNA bacteriophages that actively package their genomes into preassembled capsids using phage-encoded molecular motors (7, 15), Qβ assembles its capsid around the condensed viral genome. Capsid assembly and genome packaging are thus interlaced, raising the question as to how the viral capsid proteins select and interact with their own genomes among the multitude of nucleic acids present within the bacterial cytoplasm. Additionally, there is a topological requirement that knots or tangles in the genome cannot be tolerated during encapsidation so that the RNA can exit the capsid freely and smoothly during genome release. Thus, genome release must result from a carefully orchestrated process that proceeds in a coordinated fashion from a specific location in the capsid. To understand how the complex processes of genome encapsidation and release proceed, it is necessary to understand how structural differences at various positions on the capsid lattice facilitate these critical aspects of the viral life cycle. Unfortunately, these essential structural rearrangements are not visible in most X-ray and cryo-EM structures of viruses due to the imposition of icosahedral symmetry (4). By definition, symmetry requires that each asymmetric unit of the icosahedron is identical, and thus icosahedral symmetry averaging eliminates any functionally important structural differences.
Recent advances in cryo-EM microscopes (16, 17), including the introduction of direct electron detectors, and improvements in cryo-EM image reconstruction software have revolutionized the cryo-EM field. Coupled with the availability of powerful computing resources, these advances have made it possible to approach atomic resolution for certain well-ordered macromolecular assemblies and to reconstruct 3D volumes of viruses to subnanometer resolution without imposing any symmetry (6, 14, 18, 19). Building on these advances, Gorzelnik et al. have used cryo-EM to determine the structure of phage Qβ to 7-Å resolution without imposing any symmetry.
To evaluate the quality of their data and obtain the highest possible resolution for those capsid proteins that do obey icosahedral symmetry, the authors initially imposed this symmetry to generate a 3.7-Å reconstruction of the Qβ capsid. In addition to demonstrating the high quality of the dataset, the resulting reconstruction also delineated the structure of loops adjacent to icosahedral fivefold axes of symmetry that were not visible in the X-ray structure of Qβ (12). Next, the authors selected a high-contrast, homogeneous subset of their initial ∼52,000-particle dataset for an asymmetric reconstruction. The ∼13,000-particle subset yielded a ∼7-Å electron density map of Qβ. One of the most striking features of the resulting map is the extent of RNA within the virus that adopts easily recognizable secondary and tertiary structures. For example, the major and minor grooves of A-form double-stranded RNA helices are easily recognized, demonstrating the quality of the reconstruction. More importantly, the ability to reconstruct these secondary and tertiary structural
Gorzelnik et al. present an asymmetric cryo-EM reconstruction of the bacterial virus Qβ that provides insights into the assembly pathway of the virus, the organization of the genetic material within the viral capsid, and the potential coupling of host cell recognition and genome release.
elements indicates that the genome adopts essentially the same structure in each individual particle. This is in stark contrast to the structurally well-characterized double-stranded DNA bacteriophages, where the DNA is visible only as closely packed concentric spheres within the capsid (9, 18, 20), suggesting that, in these phages, the DNA is arranged differently in each individual virion. Because the RNA is structured in Qβ, it is also possible to visualize how the genome interacts with the inner surface of the capsid, providing insight into how the capsid protein recognizes and interacts with the viral genome to assemble an environmentally stable protein shell. Additionally, the asymmetric reconstruction of Qβ clearly shows that the maturation protein, A2, replaces a CP dimer at a single icosahedral 2F-symmetry axis. Importantly, the quality of the map is good enough that α-helices and β-sheets are easily recognizable, unambiguously indicating that this symmetry-breaking density is actually a protein rather than a bit of RNA poking through the shell. Interestingly, the genomic RNA strongly interacts with an α-helical bundle at one end of the maturation protein, resulting in a disruption in the packing of surrounding capsid proteins in the icosahedral lattice. The authors propose that this deviation from icosahedral symmetry establishes a weak point in the lattice that subsequently provides an exit route for the viral RNA upon infection. Because the maturation protein both occupies this special vertex of the virus and recognizes the bacterial pili upon infection, this hypothesis suggests a logical and simple means of coupling of host cell infection to genome release. Together with results from a recent asymmetric reconstruction of the related phage MS2 (14), the asymmetric structure of phage Qβ presented here provides a structural and conceptual framework for understanding how these ubiquitous ssRNA phages transition between various stages of their life cycles.
Acknowledgments
M.C.M.’s research is supported by NIH/National Institute of Allergy and Infectious Diseases Grant R01 AI110637, NIH/National Institute of General Medical Sciences Grants R01 GM095516 and R01 GM059604, and National Science Foundation/Division of Molecular and Cellular Biosciences Grant 1243963.
Footnotes
The author declares no conflict of interest.
See companion article on page 11519.
References
- 1.Caspar DL. Movement and self-control in protein assemblies. Quasi-equivalence revisited. Biophys J. 1980;32(1):103–138. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King J, Griffin-Shea R, Fuller MT. Scaffolding proteins and the genetic control of virus shell assembly. Q Rev Biol. 1980;55(4):369–393. doi: 10.1086/411981. [DOI] [PubMed] [Google Scholar]
- 3.Wood WB, Conley MP. Attachment of tail fibers in bacteriophage T4 assembly: Role of the phage whiskers. J Mol Biol. 1979;127(1):15–29. doi: 10.1016/0022-2836(79)90455-8. [DOI] [PubMed] [Google Scholar]
- 4.Morais MC, et al. Cryoelectron-microscopy image reconstruction of symmetry mismatches in bacteriophage phi29. J Struct Biol. 2001;135(1):38–46. doi: 10.1006/jsbi.2001.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San Martín C. Structure and assembly of complex viruses. Subcell Biochem. 2013;68:329–360. doi: 10.1007/978-94-007-6552-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao S, et al. Insights into the structure and assembly of the bacteriophage 29 double-stranded DNA packaging motor. J Virol. 2014;88(8):3986–3996. doi: 10.1128/JVI.03203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao H, et al. Structural and molecular basis for coordination in a viral DNA packaging motor. Cell Reports. 2016;14(8):2017–2029. doi: 10.1016/j.celrep.2016.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson AA, et al. Structure of the bacteriophage phi29 DNA packaging motor. Nature. 2000;408(6813):745–750. doi: 10.1038/35047129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Y, et al. Structural changes of bacteriophage phi29 upon DNA packaging and release. EMBO J. 2006;25(21):5229–5239. doi: 10.1038/sj.emboj.7601386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorzelnik KV, et al. Asymmetric cryo-EM structure of the canonical Allolevivirus Qβ reveals a single maturation protein and the genomic ssRNA in situ. Proc Natl Acad Sci USA. 2016;113:11519–11524. doi: 10.1073/pnas.1609482113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolakofsky D. A short biased history of RNA viruses. RNA. 2015;21(4):667–669. doi: 10.1261/rna.049916.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golmohammadi R, Fridborg K, Bundule M, Valegård K, Liljas L. The crystal structure of bacteriophage Q beta at 3.5 Å resolution. Structure. 1996;4(5):543–554. doi: 10.1016/s0969-2126(96)00060-3. [DOI] [PubMed] [Google Scholar]
- 13.Golmohammadi R, Valegård K, Fridborg K, Liljas L. The refined structure of bacteriophage MS2 at 2.8 Å resolution. J Mol Biol. 1993;234(3):620–639. doi: 10.1006/jmbi.1993.1616. [DOI] [PubMed] [Google Scholar]
- 14.Koning RI, et al. Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ. Nat Commun. 2016;7:12524. doi: 10.1038/ncomms12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morais MC. The dsDNA packaging motor in bacteriophage ø29. Adv Exp Med Biol. 2012;726:511–547. doi: 10.1007/978-1-4614-0980-9_23. [DOI] [PubMed] [Google Scholar]
- 16.Carroni M, Saibil HR. Cryo electron microscopy to determine the structure of macromolecular complexes. Methods. 2016;95:78–85. doi: 10.1016/j.ymeth.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramaniam S, Earl LA, Falconieri V, Milne JL, Egelman EH. Resolution advances in cryo-EM enable application to drug discovery. Curr Opin Struct Biol. 2016;41:194–202. doi: 10.1016/j.sbi.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo F, et al. Visualization of uncorrelated, tandem symmetry mismatches in the internal genome packaging apparatus of bacteriophage T7. Proc Natl Acad Sci USA. 2013;110(17):6811–6816. doi: 10.1073/pnas.1215563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, et al. DNA poised for release in bacteriophage phi29. Structure. 2008;16(6):935–943. doi: 10.1016/j.str.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers CG, Pettitt BM. Communication: Origin of the contributions to DNA structure in phages. J Chem Phys. 2013;138(7):071103. doi: 10.1063/1.4791708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]