Significance
Although in several species of bird and animal, testosterone increases male–male aggression, in human males, it has been suggested to instead promote both aggressive and nonaggressive behaviors that enhance social status. However, causal evidence distinguishing these accounts is lacking. Here, we tested between these hypotheses in men injected with testosterone or placebo in a double-blind, randomized design. Participants played a modified Ultimatum Game, which included the opportunity to punish or reward the other player. Administration of testosterone caused increased punishment of the other player but also, increased reward of larger offers. These findings show that testosterone can cause prosocial behavior in males and provide causal evidence for the social status hypothesis in men.
Keywords: testosterone, aggression, generosity, human males, social status
Abstract
Although popular discussion of testosterone’s influence on males often centers on aggression and antisocial behavior, contemporary theorists have proposed that it instead enhances behaviors involved in obtaining and maintaining a high social status. Two central distinguishing but untested predictions of this theory are that testosterone selectively increases status-relevant aggressive behaviors, such as responses to provocation, but that it also promotes nonaggressive behaviors, such as generosity toward others, when they are appropriate for increasing status. Here, we tested these hypotheses in healthy young males by injecting testosterone enanthate or a placebo in a double-blind, between-subjects, randomized design (n = 40). Participants played a version of the Ultimatum Game that was modified so that, having accepted or rejected an offer from the proposer, participants then had the opportunity to punish or reward the proposer at a proportionate cost to themselves. We found that participants treated with testosterone were more likely to punish the proposer and that higher testosterone levels were specifically associated with increased punishment of proposers who made unfair offers, indicating that testosterone indeed potentiates aggressive responses to provocation. Furthermore, when participants administered testosterone received large offers, they were more likely to reward the proposer and also chose rewards of greater magnitude. This increased generosity in the absence of provocation indicates that testosterone can also cause prosocial behaviors that are appropriate for increasing status. These findings are inconsistent with a simple relationship between testosterone and aggression and provide causal evidence for a more complex role for testosterone in driving status-enhancing behaviors in males.
The gonadal steroid hormone testosterone has long been known to play a fundamental role in the development and maintenance of physical masculinization (1, 2). However, precisely determining its behavioral effects in human males has proven more challenging. Early animal research and contemporary mainstream views associate it principally with aggression and antisocial behavior (3–5). In humans, one influential line of supporting evidence for this association comes from studies that showed that male prisoners with high testosterone levels are more likely to have committed violent crimes and broken prison rules than those with low testosterone levels (6–8). The limited number of experimental studies that have manipulated male testosterone levels during economic games (9, 10) found that administration of testosterone caused participants to be less generous to others (10) and more likely to punish those who stole from them (9). These studies have, however, been criticized for methodological problems (11), and the causal evidence for an association between testosterone and aggression in human males remains weak (12).
In humans, it has been suggested that endogenous increases in testosterone facilitate aggression in competitive contexts with the function of maintaining social dominance and establishing access to mating opportunities (13). This proposition originates from the literature on the role of testosterone in birds and primates (14). It is supported by evidence of an association between testosterone levels and social rank in nonhuman primates (15) and observations that administration of testosterone to lambs and tropical birds selectively increases aggressive dominance behaviors when the status hierarchy is unstable (16, 17).
Although increased aggression may be critical in achieving social rank among other animal species, human social interactions are arguably more complex, and status may be obtained by nonaggressive, even prosocial, means, such as generosity (18–20). Although human generosity often occurs without an expectation of material benefit (21), experimental research has shown that generosity to others can also have a social signaling function; for example, it is increased when donations will be made public (22–24), and male generosity specifically is increased in the presence of female observers (25). This generosity has been repeatedly shown to increase ratings of the giver’s social status (19, 22, 26), leading to greater influence in group decision making (26) and election to leadership positions (27) as well as reciprocal generosity (22, 27).
In line with this observation, an alternative theory of testosterone’s effect on male behavior proposes that, instead of promoting only aggressive behaviors, testosterone promotes behaviors intended to achieve and maintain social status or dominance (28, 29). This theory predicts that, while in social contexts where status is threatened by perceived provocation, this motivation may indeed lead to increased aggression; in others, nonaggressive behaviors, such as generosity, will be more appropriate for advancing social status and will, therefore, be promoted by testosterone.
There is some evidence that, rather than giving rise to indiscriminate aggression, testosterone may indeed be associated with aggressive responses to perceived provocation, so-called reactive aggression, as the status theory predicts (30). A number of findings also links testosterone with nonaggressive status seeking. The work in ref. 31 found that the testosterone levels of dominant but nonviolent males were indistinguishable from those of their violent peers and that the testosterone levels of both groups were significantly higher than those of their nondominant peers, and the work in ref. 29 found that making a task relevant to status increased performance in a test of mathematical ability in high-testosterone males specifically. However, without a direct experimental manipulation of testosterone, it is not possible to rule out the possibility that another variable correlated with testosterone may be driving these nonaggressive behaviors.
The correlational nature of the supporting literature means that the distinguishing predictions of the status theory of testosterone for male behavior remain untested. First, it has not been shown that, rather than promoting indiscriminate aggression, testosterone selectively causes male reactive aggression in circumstances in which an individual’s status is threatened. Second, it has not been shown that testosterone may cause nonaggressive, even prosocial, behaviors in males if those behaviors are consistent with increasing status.
To address these questions, we injected testosterone or placebo in a double-blind, randomized procedure to a group of young males who then played a modified version of the Ultimatum Game (UG). The classic UG is an economic game in which two players must decide how to split a sum of money between them. In each round, the first player, the proposer, presents a proposal to the second player, the responder, which describes how this money should be divided. The responder may accept this proposal, in which case the split is implemented, or reject it, resulting in both players winning nothing. Our participants played the role of the responder in a UG that was modified so that, having accepted or rejected a proposed split, they had the option to reward or punish the proposer by increasing or decreasing their monetary payoff at a proportional cost to themselves.
According to testosterone’s proposed role in driving status-enhancing behaviors, the predicted effect of testosterone administration on participants’ choices would depend on the social context. Offers of small amounts of money would be perceived as unfair (32) and be punished more strongly by those administered testosterone, but reward of generous offers would not be decreased by treatment. In contrast, if testosterone simply increases indiscriminate aggression, we would expect to see both greater punishment of unfair offers and reduced reward of generous offers. Additionally, the status theory of testosterone predicts that offers of large amounts of money would be expected to facilitate status-enhancing displays of generosity and therefore, that, when men injected with testosterone were offered large amounts, they would reward the proposer more than those administered placebo. Alternatively, if testosterone causes status-enhancing reactive aggression but does not cause nonaggressive status-enhancing behaviors, we would expect to see no increase in reward of generous offers.
Concern has been raised (33) that ostensibly emotional behaviors in economic games among participants administered testosterone may, in fact, be driven by rational concerns. If testosterone administration influences participants’ beliefs about the likely strategy of their opponents, any difference in behavior associated with such a manipulation may simply be a strategic earnings-maximizing response to these changed beliefs. Uniquely, our design excludes this interpretation, because participants were aware that the proposers’ behavior had been recorded beforehand, and therefore, the proposers had no opportunity to respond to the participants’ own behavior. Thus, although participants believed that their choices to reject, punish, and reward had real financial consequences for the proposers, participants could not use these behaviors as instruments to influence the proposers’ offers, and they did not need to anticipate the proposers’ responses to their behavior. In fact, a player who wished to maximize his earnings on our task should simply accept all offers and never choose to punish or reward the other player.
Results
Effects of Treatment on UG Behavior.
After confirming that our administration of testosterone was successful in producing a clear increase in the serum testosterone levels of the treatment group relative to the placebo group (SI Results and Fig. S1), we analyzed participants’ choices to accept or reject proposers’ offers to divide the endowment (Fig. S2 and Table S1). We found a significant positive effect of the amount offered to the participant on the probability of acceptance () but no effect of treatment group or the interaction of treatment group and amount offered.
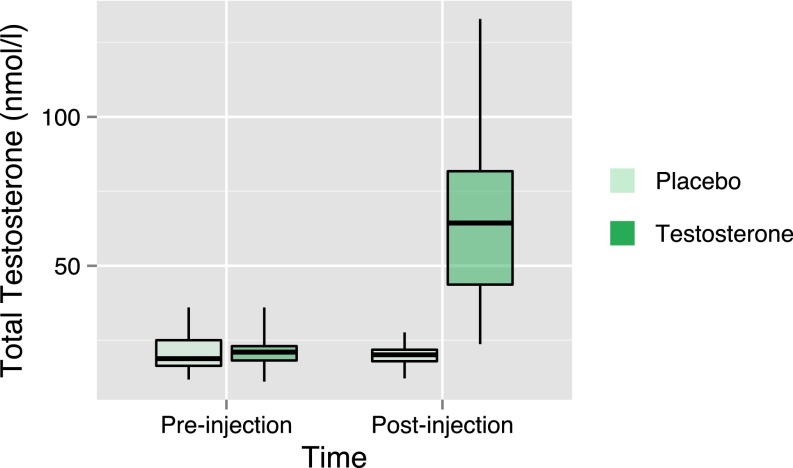
Fig. S1.
Testosterone levels. Box plot summarizing serum concentrations of TT at screening (preinjection) and the time of behavioral testing (postinjection) in the placebo (pale green) and testosterone (dark green) groups. Box centers correspond to median values, box bottoms and tops correspond to the first and third quartiles respectively, and whiskers represent the maximum and minimum concentrations. Pre- and postinjection testosterone levels did not differ significantly in the placebo group (Mann–Whitney U = 162, P = 0.64, two tailed), but in the treatment group, postinjection levels were significantly elevated (Mann-Whitney U = 4, P < 0.001, one sided).
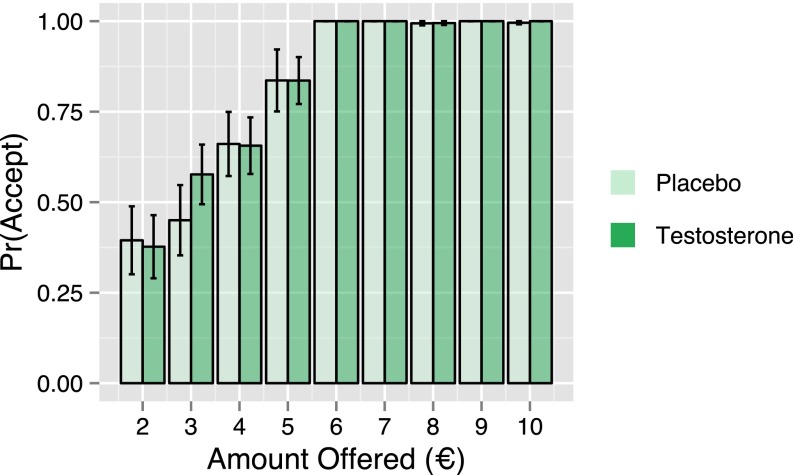
Fig. S2.
Rates of offer acceptance. Bar plot of participants’ average acceptance rates as a function of offer amount for the placebo (pale green) and testosterone (dark green) groups. Offer acceptance was not influenced by treatment.
Table S1.
Regressions of choices to accept/reject proposers’ offers
| Regressor | Binary probit (accept offer = 1, reject offer = 0) | |
| Group | Hormone | |
| Intercept | 3.21*** (0.43) | 4.56** (1.41) |
| Offer amount† | 0.93*** (0.07) | 0.75** (0.24) |
| Treatment group‡ | −0.24 (0.56) | |
| Treatment group × offer amount | −0.09 (0.08) | |
| Belief§ | −0.49 (0.77) | −0.33 (0.85) |
| Belief × offer amount | 0.31* (0.13) | 0.62** (0.19) |
| T¶ | 1.42 (1.34) | |
| T × offer amount | −0.18 (0.19) | |
| E# | −0.79 (0.74) | |
| E × offer amount | 0.04 (0.10) | |
| TBaseline‖ | −4.88 (5.04) | |
| TBaseline × offer amount | 0.72 (0.82) | |
β-Coefficients (SEs) from mixed effects probit models with random participant-level intercept. Models group (n = 40) and hormone (n = 37) include participants from both the placebo and testosterone groups.
P < 0.001.
P < 0.01.
Centered at mean (€6).
Testosterone = 1, placebo = 0.
Believed testosterone = 1, believed placebo = 0.
P < 0.05.
TT (nanomoles per liter × 100) at Appointment 4.
Estradiol (picomoles per liter × 100) at Appointment 4.
‖TT (nanomoles per liter × 100) at Appointment 2.
On the subsequent choice, at which participants decided whether to punish, do nothing to, or reward the proposer (Fig. 1 and Table S2), we again found a significant positive effect of the amount offered as well as a significant positive effect of the interaction of treatment group and offer amount. The results of this ordered probit regression indicate that participants administered testosterone were more likely to punish proposers who offered below-average amounts, whereas for offers of above-average amounts, they were more likely to reward the proposer.
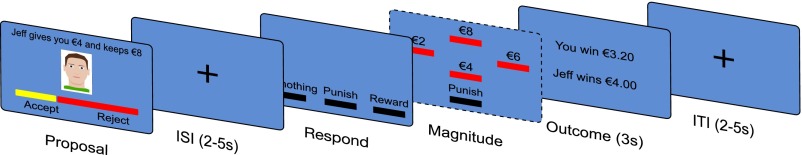
Fig. 1.
Illustration of trial. Participants accepted or rejected an offer to split a sum of €12. Participants then chose to punish or reward the proposer at a cost to themselves or do nothing. After an interstimulus interval (ISI), they specified the magnitude of punishment or reward. Finally, participants saw the net trial winnings of both players and an intertrial interval (ITI).
Table S2.
Regressions of choices to punish, do nothing, or reward the proposer
| Regressor | Ordered probit (punish, do nothing, or reward) | Binary probit (reward = 1, do nothing = 0, punish = 0) | Binary probit (punish = 1, do nothing = 0, reward = 0) | ||||||
| Group | Hormone | Placebo | Group | Hormone | Placebo | Group | Hormone | Placebo | |
| Intercept | −2.02*** (0.36) | −2.86** (1.11) | −7.34*** (1.68) | −2.11*** (0.25) | −3.10*** (0.82) | −2.98* (1.39) | |||
| Offer amount† | 0.38*** (0.02) | 0.27*** (0.04) | −0.01 (0.07) | 0.41*** (0.03) | 0.61*** (0.11) | 1.35*** (0.21) | −0.71*** (0.11) | −0.13 (0.23) | |
| Group‡ | −0.06 (0.14) | −0.21 (0.47) | 0.70* (0.32) | ||||||
| Group × offer amount | 0.05** (0.02) | 0.12** (0.04) | 0.18*** (0.04) | ||||||
| Belief§ | 0.16 (0.19) | 0.16 (0.51) | 0.17 (0.66) | −0.01 (0.66) | 0.03 (0.70) | −0.25 (0.79) | −0.43 (0.46) | −0.56 (0.54) | 0.06 (0.71) |
| Belief × offer amount | 0.0 (0.02) | 0.02 (0.03) | −0.06 (0.03) | 0.01 (0.05) | −0.01 (0.06) | 0.01 (0.09) | −0.13 (0.07) | −0.20* (0.09) | 0.12 (0.13) |
| T¶ | 0.81 (0.79) | 2.13 (9.24) | 1.07 (1.06) | 32.02** (11.72) | −0.50 (0.78) | 26.10** (9.41) | |||
| T × offer amount | 0.41*** (0.04) | 3.87*** (0.50) | 0.64*** (0.12) | −0.63 (1.83) | −0.40*** (0.10) | 2.82 (1.57) | |||
| E# | −0.55 (0.44) | −0.74 (1.14) | −0.37 (0.61) | −1.38 (1.42) | 0.68 (0.44) | −2.59* (1.12) | |||
| E × offer amount | −0.16*** (0.02) | −0.33*** (0.06) | −0.35*** (0.05) | −0.69** (0.26) | 0.22*** (0.05) | −0.68*** (0.17) | |||
| TBaseline‖ | 0.85 (3.00) | 1.49 (4.83) | 3.82 (4.06) | −0.50 (5.86) | 2.67 (3.03) | −9.94* (5.05) | |||
| TBaseline × offer amount | 0.78*** (0.16) | −0.19 (0.25) | 0.41 (0.36) | 0.05 (0.72) | 0.27 (0.39) | −2.05* (0.90) | |||
β-Coefficients (SEs) from mixed effects ordered probit and binary probit models with random participant-level intercept. Models group (n = 40) and hormone (n = 37) include participants from both the placebo and testosterone groups, whereas model placebo (n = 17) includes only participants from the placebo group.
P < 0.001.
P < 0.01.
P < 0.05.
Centered at mean (€6).
Testosterone = 1, placebo = 0.
Believed testosterone = 1, believed placebo = 0.
TT (nanomoles per liter × 100) at Appointment 4.
Estradiol (picomoles per liter × 100) at Appointment 4.
‖TT (nanomoles per liter × 100) at Appointment 2.
We carried out additional analyses to determine whether these effects of treatment were attributable to a difference between the groups in their propensity to punish only or a difference in their propensity to reward only. We performed two binary probit regressions of their choices on treatment group, amount offered, and their interaction: the first regression coding choices to punish as one and choices to do nothing or punish as zero, and the second coding choices to reward as one and choices to do nothing or reward as zero (Table S2). Null effects of treatment group in one or both of these analyses would indicate that testosterone administration did not influence rates of both punishment and reward. In both cases, however, we observed effects of treatment group. We found a positive main effect of treatment group () as well as a positive interaction of treatment group with offer amount on punishment rate ). Follow-up analyses show that this increasing rate of punishment with offer amount was restricted to below-average offer amounts (SI Results and Table S3), indicating that treatment with testosterone did indeed selectively increase punishment of unfair offers. We found a positive effect of the interaction between treatment group and amount offered on reward rate (), such that those in the treatment group were more likely to reward higher offers than those in the control group. Taken together, these results indicate that treatment with testosterone influenced rates of both punishment and reward.
Table S3.
Regressions of choices to punish for unfair and fair offers
| Regressor | Binary probit (punish = 1, do nothing = 0, reward = 0) | |
| Offer amounts < €6 | Offer amounts ≥ €6 | |
| Intercept | −0.43 (0.25) | −4.88*** (1.20) |
| Offer amount† | −0.34*** (0.07) | 0.75 (0.24) |
| Treatment group‡ | 0.25 (0.32) | 1.40 (0.90) |
| Treatment group × offer amount | 0.26*** (0.07) | −0.18 (0.33) |
| Belief§ | −0.13 (0.45) | |
| Belief × offer amount | −0.22* (0.10) | |
β-Coefficients (SEs) from mixed effects binary probit models (n = 40) of choices to punish (coded as one) vs. do nothing or reward (coded as zero) with random participant-level intercept. Separate regressions were carried out on choices in response to unfair offers (<€6) and fair offers (≥€6). Only one participant who believed that he had received testosterone punished offers ≥€6. Regressors representing participants’ beliefs about treatment were, therefore, omitted, because their effects cannot be estimated.
P < 0.001.
Centered at mean.
Testosterone = 1, placebo = 0.
Believed testosterone = 1, believed placebo = 0; testosterone (nanomoles per liter × 100) at Appointment 2.
P < 0.05.
When participants indicated that they wished to reward or punish their proposer, they subsequently chose the magnitude of that punishment or reward. Our regression analyses of these choices revealed a significant effect of the interaction of treatment group and the amount offered to the participant on the amount that they rewarded the proposer () (Fig. 2B and Table S4). Specifically, increasing the amount offered to the participant was associated with a greater increase in reward magnitude among those administered testosterone than among those in the placebo group. We found no significant main or interaction effects of treatment group on punishment magnitude.
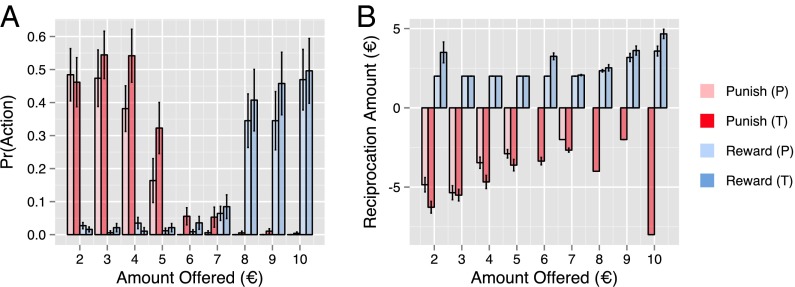
Fig. 2.
Treatment with testosterone influenced punishment and reward. (A) Bar plot of participants’ proportion of choices to reward (blue) and punish (red) the proposer as a function of the amount offered to the participant for the placebo (pale) and testosterone (dark) groups. Treatment with testosterone increased rates of punishment (main effect: ; interaction with offer amount: ) as well as rates of reward of large offers (), with increasing testosterone levels specifically associated with increased rates of punishment of low offers () (Table S2) and reward of high offers (). (B) Bar plot of the average magnitudes of reward (blue) and punishment (red) that participants chose as a function of offer amount for the placebo (pale) and testosterone (dark) groups. Linear regressions of these choices revealed a significant effect of the interaction of treatment group and offer amount on the amount that they rewarded the proposer (). Specifically, increasing the amount offered to the participant was associated with a greater increase in reward magnitude among those administered testosterone than among those in the placebo group. This effect was also associated specifically with increased levels of testosterone (). We found no significant main or interaction effects of treatment group on punishment magnitude. All error bars represent SEM. P, placebo; T, testosterone.
Table S4.
Regressions of choices of punishment amount and reward amount
| Regressor | Reward amount linear regression | Punishment amount linear regression | ||||
| Group | Hormone | Placebo | Group | Hormone | Placebo | |
| Intercept | 2.11*** (0.30) | 0.21 (1.03) | −0.55 (1.80) | −1.92*** (0.52) | 0.19 (1.70) | −2.83 (3.50) |
| Offer amount† | 0.41*** (0.05) | 0.82*** (0.20) | 1.56*** (0.33) | 0.96*** (0.11 | 1.19*** (0.35 | 0.85 (0.72) |
| Group‡ | 0.11 (0.39) | −0.84 (0.64) | ||||
| Group × offer amount | 0.15* (0.07) | 0.01 (0.13) | ||||
| Belief§ | −0.16 (0.55) | 0.01 (0.62) | 0.22 (0.80) | 0.87 (0.97) | 0.29 (1.12) | 1.18 (2.01) |
| Belief × offer amount | −0.18* (0.08) | −0.17 (0.10) | −0.30* (0.14) | −0.20 (0.21) | −0.46 (0.25) | −0.32 (0.51) |
| T¶ | −0.46 (0.85) | 4.29 (12.66) | 4.00** (1.47) | −13.59 (22.06) | ||
| T × offer amount | 0.46** (0.16 | −1.34 (2.78) | 0.85** (0.26) | −4.30 (4.88) | ||
| E# | 0.83 (0.58) | 1.45 (1.49) | −2.29** (0.82) | 1.27 (2.85) | ||
| E × offer amount | −0.33** (0.13) | −0.80* (0.34) | −0.33* (0.14) | 0.65 (0.76) | ||
| TBaseline‖ | 4.51 (3.43) | −0.99 (6.03) | −4.31 (6.33) | 11.12 (12.22) | ||
| TBaseline × offer amount | −0.22 (0.63) | 0.56 (1.22) | −0.34 (1.30) | 1.58 (2.70) | ||
β-Coefficients (SEs) from mixed effects linear models with random participant-level intercept. Models group (n = 40) and hormone (n = 37) include participants from both the placebo and testosterone groups, whereas model placebo (n = 17) includes only participants from the placebo group.
P < 0.001.
Centered at mean (€6).
Testosterone = 1, placebo = 0.
P < 0.05.
Believed testosterone = 1, believed placebo = 0.
TT (nanomoles per liter × 100) at Appointment 4.
P < 0.01.
Estradiol (picomoles per liter × 100) at Appointment 4.
‖TT (nanomoles per liter × 100) at Appointment 2.
Importantly, all effects of testosterone treatment that we found in our previous analysis survive the inclusion of regressors representing treatment belief and its interaction with offer amount. The inclusion of these regressors also revealed distinct effects of treatment belief on participants’ behavior (SI Results and Fig. S3).
Fig. S3.
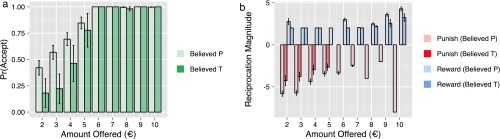
Effects of participants’ beliefs. (A) Bar plot of participants’ average acceptance rates as a function of offer amount for participants who believed that they had received placebo (pale green) and testosterone (dark green). Participants’ beliefs about treatment interacted with offer amount , such that participants who believed that they had received testosterone (n = 6) were more likely to reject low offers than those who believed that they had received placebo (n = 34). (B) Bar plot of the average magnitudes of reward (blue) and punishment (red) that participants chose as a function of offer amount for participants who believed that they had received placebo (pale) and testosterone (dark). Participants who believed that they had received testosterone reciprocated generous offers with rewards of lesser magnitude than those who believed that they had received placebo . The effect of participants’ treatment beliefs on punishment magnitude was not significant. All error bars represent SEM. Believed P, believed placebo; believed T, believed testosterone.
Effects of Treatment Are Attributable to Testosterone and Estradiol.
Our administration of testosterone was successful in producing a clear increase in the serum testosterone levels of the experimental group. However, testosterone is converted to the estrogen estradiol by the enzyme aromatase, a relationship that is reflected in a concomitant rise in the estradiol levels of participants in our testosterone group relative to those in our placebo group (SI Results). This relationship between testosterone and estradiol has led to suggestions in the literature that certain physiological effects previously attributed to testosterone may, in fact, be mediated by estradiol (34).
To assess whether the behavioral effects of our manipulation should be attributed to increases in the testosterone levels of those in the treatment group, their raised estradiol levels, or both, we reanalyzed participants’ choices. We included regressors representing their levels of testosterone and estradiol measured immediately before they performed the task as well as their levels of testosterone measured during their medical screening to account for any effects of baseline testosterone. According to testosterone’s proposed role in driving status-enhancing behaviors, we would expect to find that increasing testosterone levels would be associated with increasing punishment of low offers and reward of high offers after accounting for the effects of other hormonal measurements.
We indeed found that, when choosing whether to punish or reward their proposer, those with high levels of testosterone were more sensitive to the amount offered by the proposer, such that they were more likely to punish below-average offers () (Table S2) and more likely to reward above-average offers () as measured by separate binary probit regressions of choices to punish and choices to reward. This effect of the interaction between offer amount and testosterone level was present whether choices to punish or reward the proposer were modeled using a single ordered probit model or separate binary probit models.
We also found that those with high testosterone levels were more sensitive to the amount offered when choosing the magnitude of punishment () and reward (), responding to low offers with punishments of greater magnitude and high offers with rewards of greater magnitude (Table S4).
In contrast, the effects of participants’ estradiol levels that we found were antagonistic to those of testosterone (Tables S2 and S3), reducing the effect of the amount offered on both the rate () and magnitude () of punishment and the rate () and magnitude () of reward. Those with high levels of estradiol were less likely to punish and reward low and high offers, respectively, and when they did, they chose punishment and reward amounts of lesser magnitude.
Endogenous Testosterone Predicts Effects.
Although these results indicate that increasing males’ testosterone levels was associated with both increased punishment of unfair offers and reward of high offers, our manipulation raised testosterone to supraphysiological levels. It is possible that testosterone only influences these behaviors when it reaches levels not typically seen in young males. To assess whether this association is present among those with typical hormonal levels, we repeated our analyses of punishment and reward behavior including only participants from the placebo group.
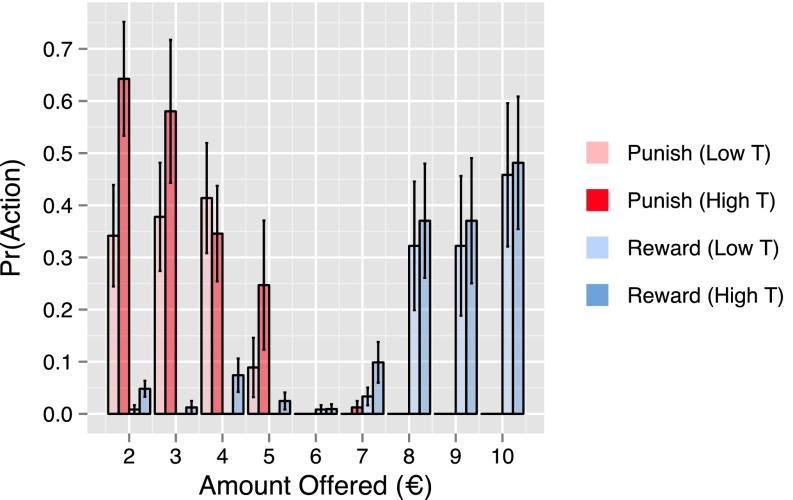
We found that those in the placebo group with high levels of testosterone were more likely to both punish () and reward () their proposer than those with low levels of testosterone (Fig. S4 and Table S2). These effects indicate that, even among those with typical endogenous levels, high testosterone is associated with increased rates of both retaliation and generosity. We did not find an effect of testosterone within the placebo group on the magnitudes of punishment or reward chosen by participants (Table S4). However, these regressions were carried out with a smaller number of observations, being restricted to not only the placebo group but also, the subset of trials in which participants first chose to punish or reward the proposer. Therefore, the null effects that we obtain may be attributable to a lack of power (effects of estradiol are discussed in SI Results).
Fig. S4.
High testosterone levels in the placebo group were associated with more frequent punishment and reward. Bar plot of the proportion of choices to reward (blue) and punish (red) the proposer as a function of offer amount for participants in the placebo group with high (dark) and low (pale) testosterone as determined by a median split. Rates of both punishment and reward increased significantly with testosterone. High T, high testosterone; low T, low testosterone.
SI Results
Hormone Levels.
As illustrated in Fig. S1, baseline serum concentration levels of TT and FT in the treatment [meanTT (MTT) = 21.06 nmol/L, SDTT = 5.66; MFT = 0.48 nmol/L, SDFT = 0.12] and the placebo groups (MTT = 20.46 nmol/L, SDTT = 5.88; MFT = 0.49 nmol/L, SDFT = 0.16) did not differ significantly (TT: Mann–Whitney U = 162, P = 0.64, two tailed; FT: Mann–Whitney U = 169, P = 0.79, two tailed). In contrast, the postinjection testosterone levels of the treatment group (MTT = 66.08 nmol/L, SDTT = 29.60; MFT = 1.92 nmol/L, SDFT = 0.97) were elevated relative to those of the placebo group (MTT = 20.44 nmol/L, SDTT = 4.10; MFT = 0.45 nmol/L, SDFT = 0.08). These differences were statistically significant (TT: Mann–Whitney U = 4, P < 0.001, one sided; FT: Mann–Whitney U = 0, P < 0.001, one sided), confirming the efficacy of treatment. We also found a concomitant increase in the estradiol levels of participants in our testosterone group (M = 185.38 pmol/L, SD = 39.55) relative to those in our placebo group (M = 101.58 pmol/L, SD = 31.05). This difference was statistically significant (Mann–Whitney U = 373, P < 0.001, one sided).
Treatment Selectively Increased Punishment of Unfair Offers.
Our binary probit regression of punishment yielded a positive effect of the interaction between treatment group and offer amount, suggesting that participants treated with testosterone were increasingly likely to punish offers of increasing magnitude. We performed follow-up analyses to determine whether this effect of treatment was restricted to unfair offers or generous offers or if it was common to both. By performing separate regressions of participants’ choices in response to below- and above-average (€6) offer amounts, we found (Table S3) that treatment influenced rates of punishment for below-average offer amounts only (), with increasing punishment with offer amount for unfair offer amounts below €6, and had no effect on punishment of generous offers.
Beliefs About Treatment Do Not Explain Effects of Treatment.
To determine whether participants were truly blind to their treatment group assignation, we examined their self-reported beliefs regarding whether they had received testosterone or placebo; 6 of 40 participants reported believing that they had received testosterone. This low number may result from the difference between the popular beliefs about the effects of behavior and its actual effects as illustrated in this manuscript. Of the participants who believed they had received testosterone, only two actually received testosterone. As in a previous between-subjects design (40), participants’ beliefs were not significantly correlated with the treatment that they had actually received (r = −0.16, P = 0.32); therefore, we conclude that participants were indeed blind to their treatment.
Although participants did not have insight into which substance that they had received, even erroneous beliefs about treatment can influence task responses (75). This influence is particularly relevant in the case of testosterone, for which there exists a strong folk belief linking it to aggression and violence (40). Importantly, all effects of testosterone treatment that we found in our previous analysis survive the inclusion of regressors representing treatment belief and its interaction with offer amount. The inclusion of these regressors also revealed several distinct effects of treatment belief on participants’ behavior (Fig. S1).
We found that those who believed that they had received testosterone were more likely to reject low offers (Table S1), with a negative main effect of treatment belief and a positive interaction with offer amount . In addition, participants’ beliefs about treatment influenced their choices of reward magnitude (Table S4), with a negative interaction between treatment belief and offer amount on reward amounts . Thus, when those who believed that they had received testosterone chose to reward high offers, they did so with rewards of lesser magnitude than those who had believed that they had received placebo.
Hormone Levels Were Not Correlated with Digit Ratio.
It has been suggested that the rate of aromatization of testosterone into estradiol may be related to in utero exposure to sex hormones (38), with greater exposure to estradiol being associated with faster metabolism of testosterone. Relative prenatal levels of testosterone and estradiol are thought to influence the hands’ 2D:4D, with a high 2D:4D indicating low prenatal testosterone exposure relative to estradiol (76). Therefore, to test this hypothesis, we tested for correlations between participants’ hormone levels and their right hands’ 2D:4D. We found no significant correlations between participants’ digit ratio and their levels of TT , the change in their TT relative to baseline measurement , their levels of estradiol , or the ratio of TT to estradiol . The same qualitative results are obtained if we restrict the analysis to participants in the placebo or testosterone group. Although we find no evidence of a relationship between digit ratio and hormone levels in this dataset, these analyses were performed with a subset of the participants (22 of 37), and the absence of a significant effect may, therefore, be attributable to a lack of power.
Effects of Endogenous Estradiol.
The effects of estradiol in the placebo group were similar to those found in previous analyses (Table S2), with estradiol levels reducing the effect of the amount offered on the rates of punishment (β = −0.68, SE = 0.17, P < 0.001) and reward (β = −0.69, SE = 0.26, P = 0.006) and the magnitude of reward chosen (Table S3). Estradiol levels in the placebo group did not influence choices of punishment magnitude (Table S3).
Reaction Times.
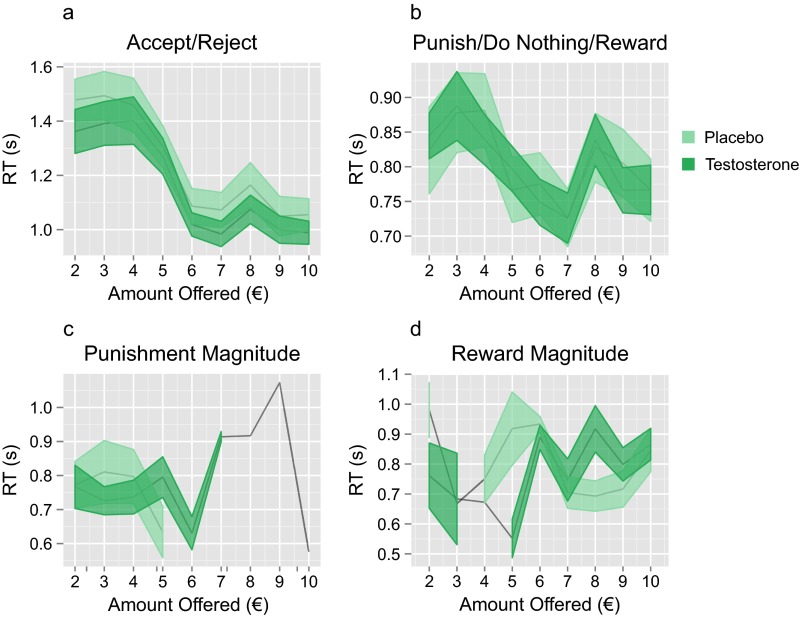
Treatment with testosterone had no effect on participants’ reaction times in the UG task when choosing to accept or reject the proposer’ offers or when choosing to punish, do nothing, or reward. This absence of a reduction in reaction times suggests that injection with testosterone did not render participants more impulsive. Treatment also had no effect on response speed when choosing the magnitude of reward. Participants treated with testosterone were slower when choosing the magnitude of punishment . This difference decreased with decreasing offer amount . However, it is not possible to determine whether these effects on reaction time when choosing the magnitude of punishment are directly attributable to treatment or the greater frequency with which participants in the testosterone group chose to punish the proposer (Fig. S5 and Table S5).
Fig. S5.
Reaction times. Line graphs of average reaction times (RT) in the placebo (pale green) and testosterone (dark green) groups as a function of offer amount when participants chose (A) whether to accept/reject offers; (B) whether to punish, do nothing to, or reward proposers; (C) what magnitude of punishment to impose; or (D) what magnitude of reward to impose. Shadow represents SEM. Points without shadows represent offer amounts for which only a single participant responded, thereby resulting in an SEM of zero. The reaction times of participants treated with testosterone differed only when choosing the magnitude of punishment, when they were slower for average-sized offers . This difference between the groups decreased with decreasing offer amount . These differences in reaction time may not be a direct effect of treatment but may instead be attributable to the greater frequency with which participants in the testosterone group chose to punish the proposer.
Table S5.
Regressions of response speeds
| Regressor | Accept/reject | Punish/do nothing/reward | Punish magnitude† | Reward magnitude |
| Intercept | 9.96e-1*** (6.08e-2) | 1.58*** (8.06e-2) | 3.44*** (0.65) | 1.91*** (0.29) |
| Offer amount‡ | 3.84e-2*** (3.28e-3) | −1.07e-3 (7.61e-3) | 0.43* (0.17) | 0.05 (0.04) |
| |Offer amount|§ | −2.68e-2*** (6.35e-3) | −1.02e-2 (1.46e-2) | −0.08 (0.08) | |
| Group¶ | 4.96e-2 (7.87e-2) | −1.35e-2 (1.05e-1) | −2.16** (0.78) | −0.36 (0.37) |
| Group × offer amount | 8.69e-4 (4.28e-3) | 1.10e-2 (9.91e-3) | −0.53** (0.20) | 0.01 (0.07) |
| Group × |offer amount| | 8.25e-3 (8.28e-3) | −1.79e-2 (1.91e-2) | 0.14 (0.10) | |
| Belief# | 9.68e-2 (1.10e-1) | 1.65e-1 (1.46e-1; 0.51) | 7.21*** (1.24) | 0.40 (0.51) |
| Belief × offer amount | 1.38e-2* (5.90e-3) | 3.49e-2* (1.36e-2) | 1.91*** (0.33) | 0.15* (0.08) |
| Belief × |offer amount| | 1.76e-3 (1.15e-2) | 2.00e-2 (2.63e-2) | 0.06 (0.02) |
β-Coefficients (SEs) from mixed effects linear regressions with random participant-level intercept of response speeds (the inverse of reaction times) for choices to accept or reject; choices to punish, do nothing, or reward; and choices of punishment and reward magnitude (n = 40).
|Offer amount| regressor omitted because of collinearity with offer amount regressor.
P < 0.001.
Centered at mean (€6).
P < 0.05.
Centered at mean (€6) and absolute valued.
Testosterone = 1, placebo = 0.
P < 0.01.
Believed testosterone = 1, believed placebo = 0.
Questionnaire Measures.
To determine whether the effects of treatment on participants’ choices were driven by testosterone-induced biasing of the participants’ judgments of the proposers, we analyzed participants’ ratings of the proposers’ faces. We found no effects of treatment on participants’ ratings of the proposers’ trustworthiness, dominance, frustration, angriness, friendliness, happiness, and attractiveness made immediately before and after performing the UG task. We found an effect of treatment on the Machiavellianism subscale of the IPIP, such that the average change in the score of participants in the placebo group (M = 1.44, SD = 2.62) after treatment were greater [t(35.36) = 2.16, P = 0.04, two tailed)] than that of those in the treatment group (M = −0.65, SD = 3.34). This effect does not survive a Holm–Bonferroni multiple comparisons correction for the number of subscales within the questionnaire. We found no effects of treatment on the dominance, anger, emotional stability, leadership, empathy, risk-taking, anxiety, conformity, social confidence, fun-seeking, drive, and reward-responsiveness subscales of the IPIP; the tension, depression, anger, fatigue, confusion, vigor, or total mood disturbance subscales of the POMS; the psychoticism, extraversion, neuroticism, or lie subscales of the EPQ-R; the SADI; the BDI; the BAI; or the Mach IV.
Discussion
In this study, we sought to expand on what is known about the influence of testosterone on male social behavior. Although empirical research and popular opinion center on its role in driving aggressive and antisocial behaviors, direct causal evidence for this link is weak in men (11, 12, 35). Some have suggested (12, 28, 29) that testosterone instead promotes both aggressive and nonaggressive behaviors that enhance and maintain social status. Here, we experimentally manipulated the testosterone levels of young males and tested the fundamental predictions of these theories against behavior in a two-player economic bargaining game.
We found that administration of testosterone caused participants to punish their opponents more frequently than those administered placebo and that higher testosterone levels were specifically associated with increased punishment of opponents who made unfair offers. Importantly, this punishment was costly to the participant and could not be used as an instrument to coerce their opponent into offering them larger amounts, because their opponents’ behavior was known by participants to be predetermined. Thus, unlike previous studies, we can conclude that testosterone can indeed cause male aggression (13) and that this aggression was not mediated by an increased motivation to maximize task earnings or altered beliefs about the strategic influence of their actions on others (33).
Testosterone has been suggested to selectively potentiate aggression that is reactive, or in response to provocation (30). Our results support such an interpretation, showing that, in the absence of provocation, as when they received large offers, participants in the treatment group were not less likely to reward these offers than those in the control group. Rather than giving rise to indiscriminate aggression, testosterone seemed to intensify aggression in social contexts where social status may be under threat. This effect is consistent with the idea that testosterone-induced aggression may be a tool to achieve social dominance and garner reproductive opportunities (13).
However, our results indicate that testosterone’s influence on male social behavior is not limited to reactive aggression. Participants who received testosterone were in fact more likely to offer monetary rewards to proposers who offered them large amounts of money. Furthermore, they chose rewards of greater magnitude than those administered placebo. Again, the task design excludes the possibility that this behavior can be interpreted as being motivated by a strategic intention to influence their opponents’ future offers. This increase in generosity represents a demonstration that testosterone can cause male behavior that is prosocial or beneficial to others. In addition, this behavior satisfies a distinguishing prediction of the status theory of testosterone (28), namely that testosterone should stimulate nonaggressive behaviors in males if, like generosity, those behaviors are status enhancing.
The increase that we observe in both punishment of small offers and reward of large offers may raise the concern as to whether administration of testosterone caused participants to simply become more impulsive. However, we found that our treatment had no effect on the immediate decision of whether to reject the offer, which they made before deciding whether to punish or reward the proposer. Treatment with testosterone also had no effect on the speed with which participants chose to punish or reward the proposer (SI Results, Fig. S5, and Table S5). The absence of an effect on reaction times suggests that testosterone does not simply enhance general emotional responsiveness but has a more restricted effect that is consistent with increasing status-enhancing aggressive and nonaggressive behaviors. The increase that we observe in reward of large offers does not seem to result from an enhancement of their hedonic value, because participants treated with testosterone do not accept large offers more frequently or more rapidly than those treated with placebo. The choices of participants’ between monetary gambles in the nonsocial certainty equivalents task were also unaffected by treatment. Thus, it seems that testosterone specifically altered the social motivations underlying participants’ behavior.
Although the double-blind, placebo-controlled treatment procedure is a vital tool for determining whether hormones exert a causal influence on human behavior (28), it is not without potential limitations. We performed a number of precautionary analyses not previously used in the literature to determine the robustness of our results.
First, testosterone is converted to the estrogen estradiol by aromatase, which has led to suggestions that some effects of testosterone administration may be mediated by raised estradiol levels and not by testosterone per se (34, 36). We found that, in addition to raising their levels of testosterone, administering testosterone to our participants indeed caused a concomitant rise in their estradiol levels. However, by including participants’ hormone levels as covariates in our behavioral analyses, we confirmed that greater punishment of unfair offers and reward of generous ones are attributable to participants’ testosterone levels and not to their levels of estradiol. In fact, the effects of estradiol were antagonistic to those of testosterone, with increased estradiol levels associated with a reduction in the rate and magnitude of both punishment of unfair offers and reward of generous offers.
Second, we show that high levels of testosterone among those in the placebo group were associated with higher rates of both punishment of proposers who made low offers and greater generosity toward those who made large offers, showing that the behavioral effects that we observe are not limited to the supraphysiological levels of testosterone caused by our treatment.
It should be noted that, although correlating participants’ choices with their peripheral levels of testosterone and estradiol provides insight into the role of each in driving behavior, future research on testosterone would benefit from the use of a hormonal manipulation that does not perturb estradiol levels. One possibility for future studies would be to suppress the conversion of testosterone to estradiol with the administration of an aromatase inhibitor.
Although this study is one of the only placebo-controlled pharmacological studies focusing on the role of testosterone in male behavior, the effects of testosterone on women’s behavior have received considerably more experimental attention (12, 37–39). It has been argued that testosterone may also promote status concerns in women (33, 39, 40), and a number of studies has shown that testosterone’s effects in women are not limited to promoting aggression (38–40). In fact, our study extends to men recent findings suggesting that testosterone has important prosocial effects by increasing cooperation in the public goods game (38) and increasing generosity when repaying trust (39). There is some evidence, however, that there may be sex differences in the effects of testosterone. Although in males, testosterone has been associated with decreased UG offers (10), administering testosterone to women increases (39) or does not change (37) UG offers. In addition, sex differences have been observed in the responsiveness of testosterone levels to social stimuli (41). These findings may reflect fundamental differences in the function of testosterone in men and women or differences between the genders in the behaviors that are considered to increase status (42). Alternatively, we suggest that, in the light of our results, some of the sex variability in the effects of testosterone may be attributable to typically unmeasured effects of estradiol.
Neuroimaging studies have associated elevated testosterone with exaggerated blood oxygen level dependent (BOLD) responses in amygdala (43–45) and decreased amygdala–orbitofrontal cortex (OFC) coupling during processing of angry and fearful facial expressions (46, 47), with these mechanisms being suggested to mediate recruitment of aggressive behavior by testosterone in response to such threatening social stimuli (48). One interesting question for future research is whether this pathway may also mediate the prosocial effects of testosterone that we observed given that the roles of amygdala and OFC in regulating social behavior are not limited to aggression (49, 50). Estrogen receptors are also known to be present in amygdala and other components of the reward system (51, 52), suggesting that testosterone and estradiol might influence behavior by binding to their respective receptors in the same set of neural structures. Alternatively, given the opposing behavioral effects of estradiol and testosterone in this task, estradiol may have influenced behavior in the task by reducing the activity of androgen receptors by binding to the receptor (53) or down-regulation of receptor expression (54, 55).
Evolutionary game theories have established how the combination of two types of incentives (rewards and punishments) is efficient to lead to a population where defectors are punished and cooperation is promoted (56). Our study suggests that testosterone, by playing on both positive and negative incentives, could have played a key evolutionary role in not only promoting aggressive behavior but also, increasing generous behavior to maintain a high social status. Observations in nonhuman primates also indicate that the social hierarchy may be maintained by alpha males—having higher testosterone levels (57)—by not only aggressive behavior but also, sharing resources, such as access to food and females.
Our findings flatly contradict a simple link between testosterone and male aggression, a theory that would have predicted increased rejection and punishment of unfair offers and reduced reward of generous offers in those who had received testosterone. Instead, we find that testosterone’s effect on male behavior depended on the social context, and we show in a single experiment that testosterone can enhance both reactive aggression and generosity. This pattern of behavior cannot be explained by altered strategic beliefs (33) and is consistent with testosterone’s proposed role in promoting male behaviors that will increase social status (58), providing causal evidence for this theory.
Materials and Methods
Participants.
Forty-seven participants were recruited by advertisements posted at Trinity College Dublin and St. James’s Hospital. The study was approved by two local ethics committees (Trinity College Dublin and St. James’s Hospital) in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants. Four participants were excluded after clinical screening, whereas three participants who passed screening subsequently withdrew from the study before completion. Forty right-handed healthy men [ages from 18 to 30 y old; mean (M) = 21.25, SD = 2.97] completed the study. Participants’ self-reported sexual orientations were heterosexual (n = 37), bisexual (n = 1), or not indicated (n = 2).
Overview.
Participants who completed the study attended a total of five appointments, detailed below, at which they provided their consent to participate, were screened medically by a clinician, received injections of testosterone or placebo in a double-blind procedure, completed behavioral testing, and attended the clinician for a final check-up. Additional details are in SI Materials and Methods.
Modified UG.
Participants played a modified version of the UG, a simple economic game in which two players, the proposer and the responder, are given the opportunity to split a sum of money (Fig. 1). Here, participants always assumed the role of the responder and played with one of four proposers on each trial. Participants were endowed with €10 that they could use during the game. Participants were explicitly instructed that the proposers’ offers were prerecorded and therefore, independent of the choices of the participant. The sum of money to be divided was fixed at €12 on all trials. The first proposer always offered €2, €3, or €4; the second proposer always offered €5, €6, or €7; and the third proposer always offered €8, €9, or €10. A fourth proposer was associated with a control condition, in which the participant was instructed in the responses that they should make. A small number (3 of 40) of participants played the task without these control trials.
Every trial began with the presentation of the image of a proposer along with the offer to split the sum of money shown both in text form and using a colored horizontal bar, where the proportion colored yellow indicated the proportion of the sum being offered to the responder. The responders chose one of two responses: accept or reject. If they chose to accept, the sum of money was divided according to the offer, whereas if they chose to reject, the sum of money was returned to the experimenter. After a variable duration interstimulus interval (ISI) [∼U(2, 5)] and irrespective of whether they had chosen to accept or reject the offer, responders were then given the opportunity to punish or reward the proposer by increasing or decreasing the proposer’s payout for the trial. Participants could also choose to “do nothing” and leave the proposer’s earnings unchanged. If they chose to punish or reward, they specified its magnitude (€2, €4, €6, or €8) at the following screen. The cost of punishment/reward to the participant was set at 1/5 of its magnitude. Finally, participants were shown their net winnings and those of the proposer for the trial for 3 s. Each trial was followed by a variable duration interval intertrial interval (ITI) [∼U(2, 5)]. No maximum response times were enforced. Participants who played the task with control trials completed 108 trials, whereas those without control trials completed 90 trials. Because of technical problems, two participants completed 60 and 72 trials, respectively. After completing the task, participants received their €10 endowment plus the summed earnings/losses from three randomly selected trials.
Behavioral Data Analysis.
Participants’ choices in the modified UG task were analyzed using mixed effects regression analyses in R 3.0.3 (59), with participant identity modeled as a random intercept effect. Our first set of analyses modeled the following as fixed effects: offer amount [centered to the mean (€6)], participants’ treatment group (testosterone = 1, placebo = 0), the treatment group that they believed they had been assigned to (testosterone = 1, placebo = 0) , and the interactions of the two previous variables with offer amount. Our second set of analyses modeled the following as fixed effects: offer amount [centered to the mean (€6)], participants’ levels of total testosterone and estradiol at the time of testing, their baseline levels of total testosterone measured at screening (Appointment 2), the treatment group that they believed that they had been assigned to, and the interactions of the previous four regressors with offer amount. Our third set of analyses used the same model as the second set but was restricted to participants in the placebo group.
Participants’ accept/reject responses to each offer were modeled with mixed effects probit regression in the lme4 package (60); their subsequent choices to punish, do nothing, or reward their proposer were modeled with mixed effects ordered probit regression in the ordinal package (61) and mixed effects probit regression in lme4, and their final choices of punishment or reward amount as well as their reaction times were modeled with mixed effects linear regression in lme4. The Satterthwaite approximation implemented by the lmerTest package (62) was used to obtain P values after mixed effects linear regression in lme4.
SI Materials and Methods
Appointment 1: Consent and Questionnaires.
At the first appointment, all participants provided written informed consent and completed a battery of questionnaires, namely the Sexual Arousal and Desire Inventory (SADI) (63), the Beck Depression Inventory (BDI) (64), the Profile of Mood Status (POMS) (65), the International Personality Item Pool (IPIP) (66), the Machiavellianism inventory (Mach IV) (67), the Eysenck Personality Questionnaire Revised (EPQ-R) (68), and the Beck Anxiety Inventory (BAI) (69). Measurements of probability weighting were obtained by eliciting participants’ certainty equivalents for gambles for all using an iterative bisection procedure (70). Participants were paid the outcome of a randomly selected trial or €15, whichever was greater.
Appointment 2: Medical Screening.
At the second appointment, participants were screened by an endocrinologist. Exclusion criteria were active medical disease: history of stroke; epilepsy/seizure disorder; heart attack; blackouts and episodes of unexplained loss of consciousness; head injury if they had experienced posttraumatic amnesia greater than 24 h; loss of consciousness for more than 1 h; significant posttraumatic sequelae or any evidence of cerebral damage on the computed tomography; clinically significant abnormalities on ECG including but not limited to conduction abnormalities; heart rate less than 55 beats per minute as judged by the investigator; major psychiatric illness; current intake of psychotropic medications, benzodiazepines, or corticosteroids; current alcohol abuse/dependency; scoring above cutoffs (8) on the Hospital Anxiety and Depression Scale (71); any contraindication to taking testosterone as specified in the Summary of Products Characteristics; were or had been taking leuprolide acetate, finasteride, spironolactone, or cimetidine; reproductive dysfunction; previous or current prostate cancer; elevated prostate-specific antigen (PSA) levels; abnormal renal or hepatic function tests; sleep apnea; and previous testosterone or other androgen replacement. Three participants were excluded because of high scores on the Hospital Anxiety and Depression Scale and advised to attend their general practitioner. Another participant was excluded because of low testosterone concentrations. He was fully evaluated in regards to his hormonal status, his repeat testosterone concentration was within normal range, and we arranged to see him again in the metabolic research unit in 6 mo for additional follow-up. Participants provided blood samples for the measurement of serum testosterone concentrations. Samples for all participants were obtained between 8:00 AM and 9:00 AM. If candidates agreed to participate after being found eligible, they were randomly assigned to the treatment (n = 21) or placebo (n = 19) group in a double-blind procedure using an online randomization program (https://www.randomizer.org/).
Appointment 3: Injection.
At the third appointment, participants received a single i.m. injection. Participants in the treatment group were administered a 1-mL dose of testosterone enanthate (250 mg; Androtardyl/Testoviron Depot), whereas participants in the placebo group were administered 1 mL saline; i.m. testosterone enanthate is a long-acting ester of testosterone. The pharmacokinetics of testosterone enanthate yields supraphysiological testosterone levels in serum as early as 2 h after injection, reaching peak levels four to five times above basal between 8 and 24 h after injection (72, 73).
Appointment 4: Testing.
At the fourth appointment, which took place on the following day, blood samples were collected for the measurement of serum testosterone concentrations. These samples were collected 17.5–20 h after the injection of testosterone or placebo. All participants were then given oral and written instructions for both the modified UG task (Fig. 1) (detailed below) and a gambling task not presented here. Participants rated pictures of the proposers’ faces for trustworthiness, dominance, frustration, angriness, friendliness, happiness, and attractiveness and played a short practice session before undergoing MRI. The scanning lasted ∼80 min (15 min of anatomical imaging and 65 min of functional imaging), during which participants completed both the modified UG task and the gambling task. After scanning, participants again rated the proposers for trustworthiness, dominance, frustration, angriness, friendliness, happiness, and attractiveness and completed the SADI, the BDI, the POMS, the IPIP, the Mach IV, the EPQ-R, the BAI, and the certainty equivalents task. Participants also reported whether they believed they had received testosterone and described the effects that they would expect testosterone administration to have on themselves and others. Participants were paid their summed earnings from the UG task, the gambling task, and the outcome of a randomly selected trial from the certainty equivalents task or €80, whichever was greater. The analysis of the neuroimaging data will be presented in a separate paper.
Appointment 5: Medical Follow-Up.
Finally, participants attended the endocrinologist again 4–6 wk after the injection. A physical examination was carried out, and blood samples were collected and analyzed for hematocrit, lipid profile, PSA, liver, renal profile, and hormonal status to assess any potential changes after testosterone administration. Paired sample t tests were used to compare the parameters at baseline and follow-up. There were no significant changes in hemoglobin, hematocrit, total cholesterol and its fractions, and PSA concentrations after either of the injections. Two men enrolled in the study reported pain at the injection sites, which fully resolved after 2 d. One participant in the placebo group reported increased libido after injection. Participants were paid €50 for attending this appointment.
Laboratory Measurements.
Using blood samples obtained at Appointments 2 and 4, we determined serum concentrations of total testosterone (TT) by the electrochemiluminescence immunoassay (ECLIA) kit on a cobas e analyzer (Roche Diagnostic Systems). Serum concentrations of sex hormone-binding globulin (SHBG) were measured by ECLIA kit on a cobas e analyzer. Serum albumin was measured by colorimetric assay (ALB2; cobas e analyzer). Apparent concentrations of free testosterone (FT) were calculated from values of TT, SHBG, and albumin using the method described and validated by Vermeulen et al. (74). Estradiol was measured by ECLIA (cobas e analyzer).
Blood samples were not obtained from two participants at Appointment 2 and one participant at Appointment 4 because of experimenter error. Those participants are omitted from figures and analyses involving measurements of testosterone at the respective time points.
Digit Ratio Measurements.
To determine whether prenatal hormonal exposure was associated with the metabolism of testosterone to estradiol, we recalled participants to obtain their second-to-fourth digit length ratios (2D:4D); 22 of 37 participants complied with this request (11 placebo and 11 testosterone), and 2D:4D was calculated from a digital photograph of the right hand by measuring the length of the index and ring finger from the ventral proximal crease to the tip of the finger using the GNU Image Manipulation Program (GIMP, version 2.8.10). The rater, who was blind to the hypothesis and the participants’ treatment, measured 2D:4D twice with a time interval of 1 wk. These two measurements were highly correlated (r = 0.98, P < 0.0001). The mean value of the two measurements was used for analysis.
Questionnaires.
We carried out a mixed design multivariate analysis of variance (MANOVA) on participants’ pre- and posttask ratings of the UG proposers’ trustworthiness, dominance, frustration, angriness, friendliness, happiness, and attractiveness with factors treatment, time (pre- or posttask), and proposer identity. We conducted mixed design ANOVAs on participants’ scores on the SADI (63), the BDI (64), the POMS (65), the PIP (66), the Mach IV inventory (67), the EPQ-R (68), and the BAI (69) that were administered at Appointment 1 (preinjection) and Appointment 4 (postinjection) to test for effects of treatment.
Acknowledgments
We thank Pierre Wydoodt for his assistance with the early stages of data analysis. This research was funded by the FP7-People Intra-European Fellowship 235076 (to J.-C.D.). It was also performed within the framework of the Laboratory of Excellence (LABEX) ANR-11-LABEX-0042 of Université de Lyon within the program Investissements d'Avenir (ANR-11-IDEX-0007) operated by the French National Research Agency (to J.-C.D.). This work was also supported by grants from the Agence Nationale pour la Recherche (ANR 'Brain Choice’ n°14-CE13-0006) (to J.-C.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608085113/-/DCSupplemental.
References
- 1.Wilson JD, George FW, Griffin JE. The hormonal control of sexual development. Science. 1981;211(4488):1278–1284. doi: 10.1126/science.7010602. [DOI] [PubMed] [Google Scholar]
- 2.Goy RW, Bercovitch FB, McBrair MC. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav. 1988;22(4):552–571. doi: 10.1016/0018-506x(88)90058-x. [DOI] [PubMed] [Google Scholar]
- 3.Allee WC, Collias NE, Lutherman CZ. Modification of the social order in flocks of hens by the injection of testosterone propionate. Physiol Zool. 1939;12(4):412–440. [Google Scholar]
- 4.Mitchell D. 2008. Trading on Testosterone. N Y Times. Available at www.nytimes.com/2008/04/19/business/19online.html. Accessed November 13, 2015.
- 5.Antonakis J. 2014. Does Power Lead to Corruption? The Guardian. Available at https://www.theguardian.com/sustainable-business/2014/dec/17/does-power-lead-to-corruption-research-testosterone. Accessed November 13, 2015.
- 6.Rada RT, Laws DR, Kellner R. Plasma testosterone levels in the rapist. Psychosom Med. 1976;38(4):257–268. doi: 10.1097/00006842-197607000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Dabbs JM, Jr, Frady RL, Carr TS, Besch NF. Saliva testosterone and criminal violence in young adult prison inmates. Psychosom Med. 1987;49(2):174–182. doi: 10.1097/00006842-198703000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Dabbs JM, Jr, Carr TS, Frady RL, Riad JK. Testosterone, crime, and misbehavior among 692 male prison inmates. Pers Individ Dif. 1995;18(5):627–633. [Google Scholar]
- 9.Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug Alcohol Depend. 1995;40(1):73–79. doi: 10.1016/0376-8716(95)01192-7. [DOI] [PubMed] [Google Scholar]
- 10.Zak PJ, et al. Testosterone administration decreases generosity in the ultimatum game. PLoS One. 2009;4(12):e8330. doi: 10.1371/journal.pone.0008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenegger C, Haushofer J, Fehr E. No sound evidence for a gender-specific effect of testosterone administration on aggressive motivation exists: Reply to Josephs et al. Trends Cogn Sci. 2011;15(11):510–511. [Google Scholar]
- 12.Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends Cogn Sci. 2011;15(6):263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “Challenge Hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136(6):829–846. [Google Scholar]
- 15.Sapolsky RM. Testicular function, social rank and personality among wild baboons. Psychoneuroendocrinology. 1991;16(4):281–293. doi: 10.1016/0306-4530(91)90015-l. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-de-la-torre JL, Manteca X. Effects of testosterone on aggressive behaviour after social mixing in male lambs. Physiol Behav. 1999;68(1-2):109–113. doi: 10.1016/s0031-9384(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 17.Collias NE, Barfield RJ, Tarvyd ES. Testosterone versus psychological castration in the expression of dominance, territoriality and breeding behavior by male village weavers (Ploceus cucullatus) Behaviour. 2002;139(6):801–824. [Google Scholar]
- 18.Harbaugh WT. What do donations buy?: A model of philanthropy based on prestige and warm glow. J Public Econ. 1998;67(2):269–284. [Google Scholar]
- 19.Anderson C, Kilduff GJ. The pursuit of status in social groups. Curr Dir Psychol Sci. 2009;18(5):295–298. [Google Scholar]
- 20.Nichols M. 2010. Philanthropy Becoming New Status Symbol for Wealthy. Reuters. Available at www.reuters.com/article/us-wealth-philanthropy-status-idUSTRE67A2WB20100811. Accessed January 18, 2016.
- 21.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425(6960):785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 22.Hardy CL, Van Vugt M. Nice guys finish first: The competitive altruism hypothesis. Pers Soc Psychol Bull. 2006;32(10):1402–1413. doi: 10.1177/0146167206291006. [DOI] [PubMed] [Google Scholar]
- 23.Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. J Cogn Neurosci. 2010;22(4):621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- 24.Izuma K. The social neuroscience of reputation. Neurosci Res. 2012;72(4):283–288. doi: 10.1016/j.neures.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Iredale W, Van Vugt M, Dunbar R. Showing off in humans: Male generosity as a mating signal. Evol Psychol. 2008;6(3):386–392. [Google Scholar]
- 26.Willer R. Groups reward individual sacrifice: The status solution to the collective action problem. Am Sociol Rev. 2009;74(1):23–43. [Google Scholar]
- 27.Milinski M, Semmann D, Krambeck H-J. Donors to charity gain in both indirect reciprocity and political reputation. Proc Biol Sci. 2002;269(1494):881–883. doi: 10.1098/rspb.2002.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazur A, Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21(3):353–363. [PubMed] [Google Scholar]
- 29.Josephs RA, Newman ML, Brown RP, Beer JM. Status, testosterone, and human intellectual performance: Stereotype threat as status concern. Psychol Sci. 2003;14(2):158–163. doi: 10.1111/1467-9280.t01-1-01435. [DOI] [PubMed] [Google Scholar]
- 30.Josephs RA, Mehta PH, Carré JM. Gender and social environment modulate the effects of testosterone on social behavior: Comment on Eisenegger et al. Trends Cogn Sci. 2011;15(11):509–510. doi: 10.1016/j.tics.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenkranz J, Bliss E, Sheard MH. Plasma testosterone: Correlation with aggressive behavior and social dominance in man. Psychosom Med. 1974;36(6):469–475. doi: 10.1097/00006842-197411000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Güth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. J Econ Behav Organ. 1982;3(4):367–388. [Google Scholar]
- 33.Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E. Eisenegger et al. reply. Nature. 2012;485(7399):E5–E6. doi: 10.1038/nature08711. [DOI] [PubMed] [Google Scholar]
- 34.Nathan L, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: Critical role of aromatase. Proc Natl Acad Sci USA. 2001;98(6):3589–3593. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert DJ, Walsh ML, Jonik RH. Aggression in humans: What is its biological foundation? Neurosci Biobehav Rev. 1993;17(4):405–425. doi: 10.1016/s0149-7634(05)80117-4. [DOI] [PubMed] [Google Scholar]
- 36.Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269(1493):823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zethraeus N, et al. A randomized trial of the effect of estrogen and testosterone on economic behavior. Proc Natl Acad Sci USA. 2009;106(16):6535–6538. doi: 10.1073/pnas.0812757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Honk J, Montoya ER, Bos PA, van Vugt M, Terburg D. New evidence on testosterone and cooperation. Nature. 2012;485(7399):E4–E5. doi: 10.1038/nature11136. [DOI] [PubMed] [Google Scholar]
- 39.Boksem MAS, et al. Testosterone inhibits trust but promotes reciprocity. Psychol Sci. 2013;24(11):2306–2314. doi: 10.1177/0956797613495063. [DOI] [PubMed] [Google Scholar]
- 40.Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E. Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature. 2010;463(7279):356–359. doi: 10.1038/nature08711. [DOI] [PubMed] [Google Scholar]
- 41.Salvador A. Coping with competitive situations in humans. Neurosci Biobehav Rev. 2005;29(1):195–205. doi: 10.1016/j.neubiorev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 42.van Anders SM, Steiger J, Goldey KL. Effects of gendered behavior on testosterone in women and men. Proc Natl Acad Sci USA. 2015;112(45):13805–13810. doi: 10.1073/pnas.1509591112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derntl B, et al. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34(5):687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biol Psychiatry. 2008;63(3):263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Bos PA, van Honk J, Ramsey NF, Stein DJ, Hermans EJ. Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology. 2013;38(6):808–817. doi: 10.1016/j.psyneuen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 46.van Wingen GA, et al. Testosterone increases amygdala reactivity in middle-aged women to a young adulthood level. Neuropsychopharmacology. 2009;34(3):539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- 47.Spielberg JM, et al. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc Cogn Affect Neurosci. 2015;10(3):408–415. doi: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carré JM, Olmstead NA. Social neuroendocrinology of human aggression: Examining the role of competition-induced testosterone dynamics. Neuroscience. 2015;286:171–186. doi: 10.1016/j.neuroscience.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 49.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11(4):168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877(1):242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 52.Donahue JE, et al. Cells containing immunoreactive estrogen receptor-α in the human basal forebrain. Brain Res. 2000;856(1-2):142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- 53.Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: A new pathway for sex hormones in prostate. Proc Natl Acad Sci USA. 1998;95(10):5527–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richter CA, Taylor JA, Ruhlen RL, Welshons WV, Vom Saal FS. Estradiol and Bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect. 2007;115(6):902–908. doi: 10.1289/ehp.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stover EP, Krishnan AV, Feldman D. Estrogen down-regulation of androgen receptors in cultured human mammary cancer cells (MCF-7) Endocrinology. 1987;120(6):2597–2603. doi: 10.1210/endo-120-6-2597. [DOI] [PubMed] [Google Scholar]
- 56.Sigmund K. Punish or perish? Retaliation and collaboration among humans. Trends Ecol Evol. 2007;22(11):593–600. doi: 10.1016/j.tree.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21(1):68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta PH, Josephs RA. Testosterone change after losing predicts the decision to compete again. Horm Behav. 2006;50(5):684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 59.R Core Team 2014. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
- 60.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. arXiv:14065823.
- 61.Christensen RHB. 2013. Ordinal—Regression Models for Ordinal Data (R Package, version 2013.9-30). Available at https://cran.r-project.org/web/packages/ordinal/index.html. Accessed September 10, 2016.
- 62.Kuznetsova A, Brockhoff PB, Christensen RHB. 2013. lmerTest: Tests for Random and Fixed Effects for Linear Mixed Effect Models (lmer Objects of lme4 Package) (R Package, version 2.0-29). Available at https://cran.r-project.org/web/packages/lmerTest/index.html. Accessed September 10, 2016.
- 63.Toledano R, Pfaus J. The Sexual Arousal and Desire Inventory (SADI): A multidimensional scale to assess subjective sexual arousal and desire. J Sex Med. 2006;3(5):853–877. doi: 10.1111/j.1743-6109.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 64.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 65.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- 66.Goldberg LR, et al. The international personality item pool and the future of public-domain personality measures. J Res Pers. 2006;40(1):84–96. [Google Scholar]
- 67.Christie R, Geis FL, Berger D. Studies in Machiavellianism. Academic; New York: 1970. [Google Scholar]
- 68.Eysenck SB, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Pers Individ Dif. 1985;6(1):21–29. [Google Scholar]
- 69.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 70.Fox CR, Poldrack RA. Prospect theory and the brain. In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editors. Neuroeconomics: Decision Making and the Brain. Academic Press; San Diego: 2009. pp. 145–173. [Google Scholar]
- 71.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 72.Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51(6):1335–1339. doi: 10.1210/jcem-51-6-1335. [DOI] [PubMed] [Google Scholar]
- 73.Schürmeyer T, Nieschlag E. Comparative pharmacokinetics of testosterone enanthate and testosterone cyclohexanecarboxylate as assessed by serum and salivary testosterone levels in normal men. Int J Androl. 1984;7(3):181–187. doi: 10.1111/j.1365-2605.1984.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 74.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 75.Colagiuri B. Participant expectancies in double-blind randomized placebo-controlled trials: Potential limitations to trial validity. Clin Trials. 2010;7(3):246–255. doi: 10.1177/1740774510367916. [DOI] [PubMed] [Google Scholar]
- 76.Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13(11):3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]