Significance
Cell-to-cell communication is critical for the coordination of developmental processes between cells and tissues within an organism. Mechanical signals, secreted peptides, and hormones can all convey information between cells. In plants, neighboring cells can directly communicate with each other via plasmodesmata (PD). It has long been assumed that the exchange of proteins and RNAs via PD is important for the regulation of plant development. However, the tools for large-scale testing of this hypothesis have been lacking. Here we exploit a tool that effectively blocks movement of proteins and small RNAs between cells to show that PD-dependent cell-to-cell signaling is important for the coordination of cell divisions, cell polarity, and cell expansion between cell layers in the Arabidopsis root.
Keywords: symplastic signaling, plasmodesmata, cell division, cell expansion, cell polarity
Abstract
Cell-to-cell communication is essential for the development and patterning of multicellular organisms. In plants, plasmodesmata (PD) provide direct routes for intercellular signaling. However, the role that PD-mediated signaling plays in plant development has not been fully investigated. To gain a comprehensive view of the role that symplastic signaling plays in Arabidopsis thaliana, we have taken advantage of a synthetic allele of CALLOSE SYNTHASE3 (icals3m) that inducibly disrupts cell-to-cell communication specifically at PD. Our results show that loss of symplastic signaling to and from the endodermis has very significant effects on the root, including an increase in the number of cell layers in the root and a misspecification of stele cells, as well as ground tissue. Surprisingly, loss of endodermal signaling also results in a loss of anisotropic elongation in all cells within the root, similar to what is seen in radially swollen mutants. Our results suggest that symplastic signals to and from the endodermis are critical in the coordinated growth and development of the root.
An enduring question in developmental biology is how cellular patterning and specification of cell fate are achieved. How are the processes of cell division, cell expansion, and cell differentiation coordinated over multiple cell distances to produce a functional organ? In plants, where nearly all cells are connected to their neighbors by plasmodesmata (PD; intercellular bridges), coordination of cellular patterning with growth and development could occur symplastically (through PD). Indeed, numerous groups have shown that proteins and RNAs are able to move between PD and that this movement is correlated with predictable changes in plant growth and development (reviewed in ref. 1). For example, in the patterning of root hairs, a mobile signal, CAPRICE (CPC) moves from the stele and from nonhair cells into the hair cells where it is sequestered by GLABRA3 to inhibit the nonhair cell fate (2–4). Likewise, during flowering, the mobile signal FLOWERING LOCUS T (FT) moves from leaf tissue into the shoot apical meristem to induce floral development (5–7). Consistent with an essential role for PD in plant development, rather mild increases or decreases in PD-mediated signaling are associated with seedling and/or embryonic lethality (e.g., INCREASED SIZE EXCUSION LIMIT 1 and 2 and GFP ARRESTED TRAFFICKING 1) (8–12). Tissue-specific defects in PD-mediated protein movement are associated with defects in stomatal patterning and spacing and defects in root patterning and cellular specification (13, 14).
One of the obstacles to understanding the function of PD in plant development has been a lack of tools for specifically modifying PD-mediated protein movement. There are no drugs that specifically inhibit PD-mediated protein trafficking, as there are for other processes like secretion and endocytosis. Recently, however, Vatén et al. designed a synthetic form of CALLOSE SYNTHASE 3 (icals3m) that can be used to inducibly block movement of proteins and RNA via PD (14). Induction of icals3m results in an increase in callose, which mechanically decreases the size of the PD aperture and blocks protein and RNA trafficking through PD. Previously, we showed that stele-specific expression of the icals3m transgene in the roots of Arabidopsis thaliana blocked movement of the SHORT-ROOT (SHR) protein from the stele into the endodermis and transport of miRNA 165/66 from the endodermis into the stele, which resulted in defects in xylem patterning and the normal development of the ground tissue (14). Since then, the icals3m system has been used to show that changes in symplastic connectivity are required for generation and emergence of lateral roots and proper development of the shoot apical meristem (15–18). A loss-of-function callose synthase system has been used to examine tropisms in the hypocotyl of A. thaliana (19). To address the role of PD in the coordination of tissue patterning and plant development, we use icals3m to specifically block PD-mediated movement to and from the endodermis in A. thaliana roots. By blocking trafficking to and from the endodermis, we restrict symplastic signals from the stele into outer cell layers and likewise from the cortex or epidermis to the stele. We find that in the root meristem, symplastic signals are required for proper specification of the endodermis, coordination of growth between the endodermis and the cortex, and maintenance of cell polarity in the ground tissue. Outside of the meristem, symplastic signals from the endodermis are required for normal anisotropic cell expansion in the root elongation zone.
Results and Discussion
Transient Induction of icals3m in the Endodermis Blocks both Targeted and Nontargeted Movement.
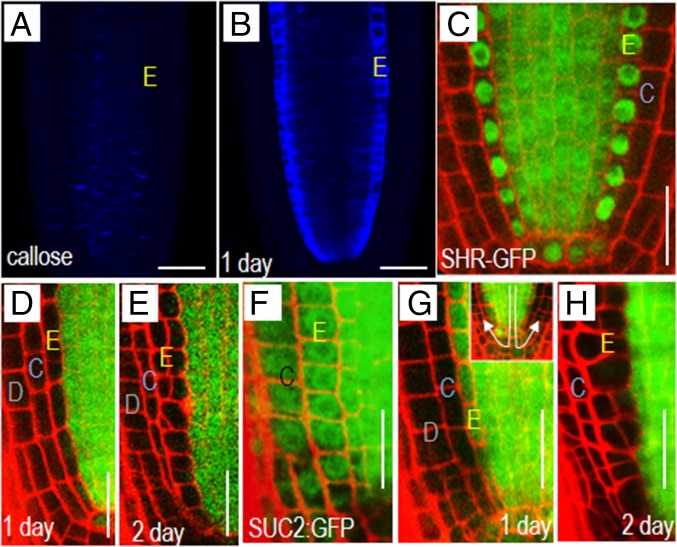
Previously we showed that expression of the icals3m transgene from the Cytokinin Response 1 (CRE1/aka WOODENLEG, WOL) promoter was sufficient to block movement of proteins out of the stele (14). To test the ability of icals3m to block movement into the endodermis, we expressed the icals3m transgene from the Endodermis 7 (EN7) promoter. The EN7 promoter is active in the endodermis, cortical endodermal initial (CEI) cells, and the cortical endodermal daughter (CED) cells, but absent from the quiescent center (QC) (20). As early as 3 h postinduction with estradiol, we saw a clear accumulation of callose in the endodermis of roots with the EN7:icals3m transgene (Fig. 1 A and B). Within 24 h of induction, the levels of callose were much higher in the endodermis of induced roots compared with controls. To determine whether this increase in callose blocked transport through PD, we introduced two markers: SHR:SHR-GFP and SUC2:GFP into the EN7:icals3m-expressing plants. SHR-GFP, which facilitates its own movement through PD, serves as a marker of targeted movement, whereas free GFP driven by the SUC2 promoter serves as a marker for nontargeted movement (i.e., diffusion) (21, 22). Before induction with estradiol, SHR-GFP was present in the stele, endodermis, and QC cells (Fig. 1C). After induction of EN7:icals3m, SHR-GFP in the endodermis diminished to background, but was still present in the QC, consistent with a lack of EN7 activity in QC cells (Fig. 1 D and E). The SUC2 promoter drives GFP expression in the phloem; from the phloem, GFP diffuses freely throughout the meristem (Fig. 1F) (14, 22). Induction of EN7:icals3m significantly limited movement of GFP into the endodermis and surprisingly all cells outside of the stele (Fig. 1 G and H). Because EN7 does not drive icals3m expression in QC cells (20), this suggests that there is very little shootward movement of GFP once it has trafficked through the QC into the columella (in the direction indicated by arrows in Fig. 1G, Inset). Therefore, our results show that the expression of icals3m in the endodermis can effectively block both targeted and nontargeted movement into this cell layer. Additionally, based upon the SUC2:GFP results, it appears that passive movement through the QC and then shootward into the lateral tissues (i.e., lateral root cap, epidermis, and cortex) may be generally limited.
Fig. 1.
Transient induction of icals3m in the endodermis blocks symplastic communication between cells. Aniline blue staining showing the accumulation of callose in the root tips of seedlings (A) before and (B) after induction of the EN7:icals3m transgene. Note the endodermal-specific accumulation of callose in B. (C–H) Confocal images of roots expressing the (C–E) SHR:SHR-GFP transgene and (F–H) SUC2:GFP (C and F) before and (D, E, G, and H) after induction of the EN7:icals3m expression. Induction of the EN7:icals3m blocks movement of (D and E) SHR-GFP and (G and H) free GFP throughout the root meristem. Times after induction are marked. E, endodermal lineage; D, epidermis; C, cortex. In all cropped half-root images (e.g., D–H) the epidermis is oriented to the left side of the image. (Scale bars, 25 μm.)
Symplastic Isolation of the Endodermis Causes Defects in Root Growth and Patterning and a Lack of Coordinated Cell Divisions Between the Endodermis and Cortex.
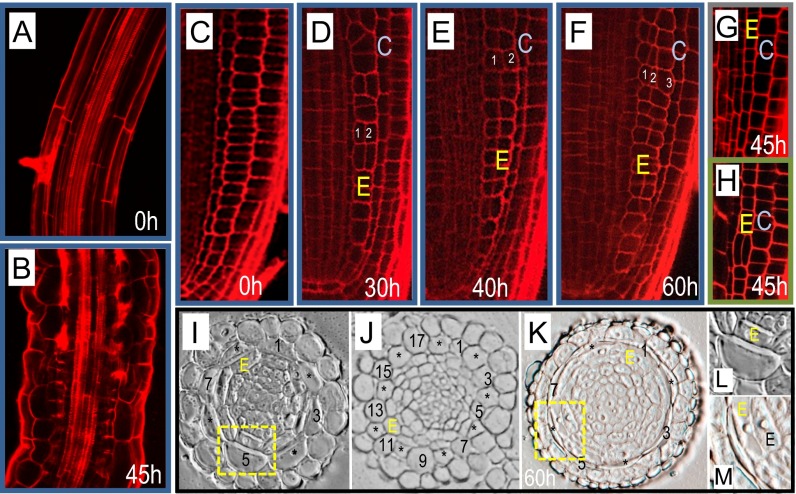
To determine how a loss of PD-mediated endodermal signaling affects root development, seedlings containing the EN7:icals3m transgene were transferred to estradiol-containing media or control media. The transferred seedlings were then imaged at various time points, up to 60 h after transfer. During normal root development, cells expand anisotropically as they exit the meristem (Fig. 2A). When SHR moves from the stele into the endodermis where it turns on miRNA165/166; miRNA165/66 in turn moves from the endodermis into the stele, where it patterns xylem in a concentration-dependent manner (23). Consistent with a loss of SHR or miRNA165/166 movement, the xylem was mispatterned in EN7:icals3m roots treated with estradiol (Fig. 2 A and B) (14, 23). In addition, the cells in the elongation zone were shorter and wider than controls, having an appearance similar to radially swollen mutants. These results indicate that endodermal signaling is required to maintain normal developmental patterning and polarized cell elongation in multiple cell layers in the root.
Fig. 2.
Disruption of symplastic signaling in the endodermis alters endodermal cell expansion and radial patterning. (A and B) Confocal images of the elongation zone of roots (A) before and (B) after activation of the EN7:icals3m transgene. White arrowheads in B point to areas of aberrant xylem development. (C–F) Confocal micrographs of the root meristem (C) before and (D–F) after induction with estradiol. (G and H) Two different roots 45 h after induction of the CRE1:icals3m transgene. Note that compared with D–F, the ground tissue (G and H) is normally patterned following induction of CRE1:icals3m (quantified in Fig. S1). (I–M) Cross-sections through (I) uninduced EN7:icals3m roots, (J) roots expressing the SCR:SHR-nlsGFP transgene, and (K) induced EN7:icals3m roots; every other cortical cell is numbered. L and M are magnified images of the same cells (yellow boxes) in I and K, respectively. E, endodermal lineage; C, cortex. Confocal images of roots of the EN7:icals3m genotype are outlined in blue. Those expressing CRE1:icals3m are outlined in green. Times indicate duration of induction. (Scale bars, 25 μm.)
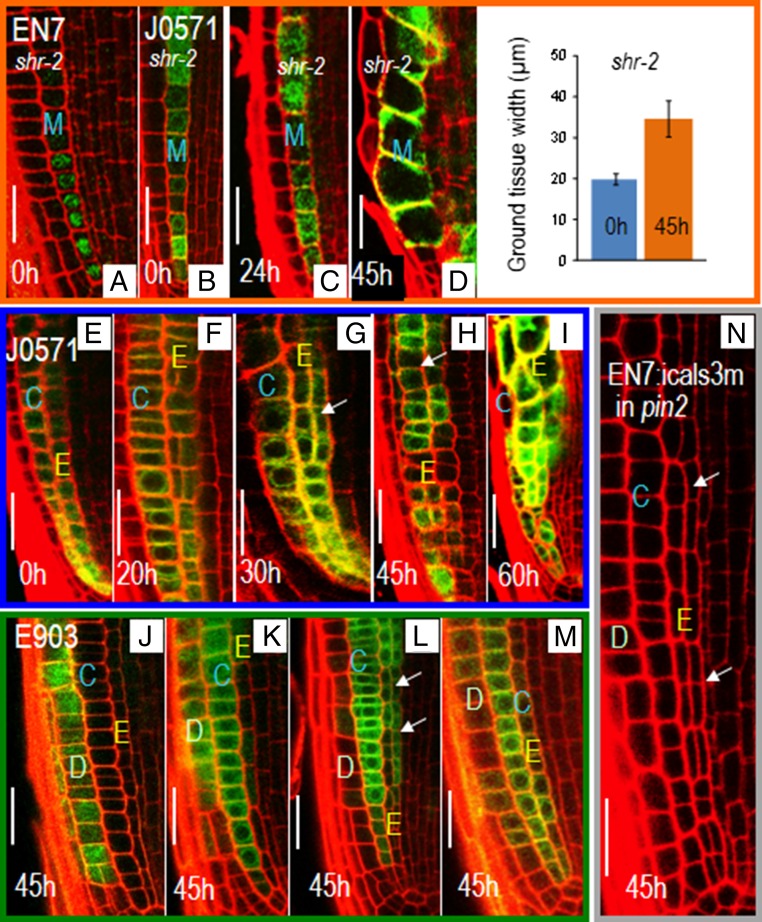
In the meristem, activation of EN7:icals3m resulted in the expansion of endodermal cells and defects in radial patterning (Fig. 2 C–F). In wild-type roots, the endodermal cell lineage occupies a single cell file (Fig. 2C). However, after induction of EN7:icals3m, the endodermal lineage expanded into multiple cell files. Sixty hours after induction, nearly all cells within the endodermal cell lineage had divided, creating one to two additional cell layers (Fig. 2 D–F and Fig. S1A). We observed no defects in the patterning of the cortex or the epidermis; these remained as single cell layers, suggesting that the effect on radial patterning is specific to the endodermis. To test whether the defects observed require a bona fide endodermis, we blocked PD-mediated signaling in shr-2 roots, which lack a typical endodermis (24). To drive the expression of the icals3m transgene in the ground tissue of shr-2 roots, we tried both the EN7 promoter and the J0571:GAL4 enhancer trap system (https://www.arabidopsis.org/abrc/haseloff.jsp) (24). The EN7 promoter showed poor expression in shr-2 roots (Fig. 3A). However, the levels of J0571:GAL4; UAS:erGFP in the ground tissue of shr-2 roots was similar to wild-type (Fig. 3B). J0571:GAL4 was therefore used to inducibly express UAS:icals3m in shr-2 roots. shr-2 roots expressing the J0571:GAL4; UAS:icals3m transgenes showed increased cell expansion in the mutant cell layer, but no change in radial patterning (Fig. 3 B–E). Likewise wild-type roots expressing the J0571:GAL4; UAS:icals3m transgenes showed expansion of both the endodermis and cortex cell layers, but no change in the orientation of the cell division in the cortex (Fig. 3 F–J). By about 45 h postinduction, the phenotypes of the wild-type roots expressing the EN7:icals3m or the J0571:GAL4; UAS:icals3m transgenes were similar (Fig. 3I). These results suggest that the effect of the icals3m transgene on cell division in the ground tissue is specific to the endodermis.
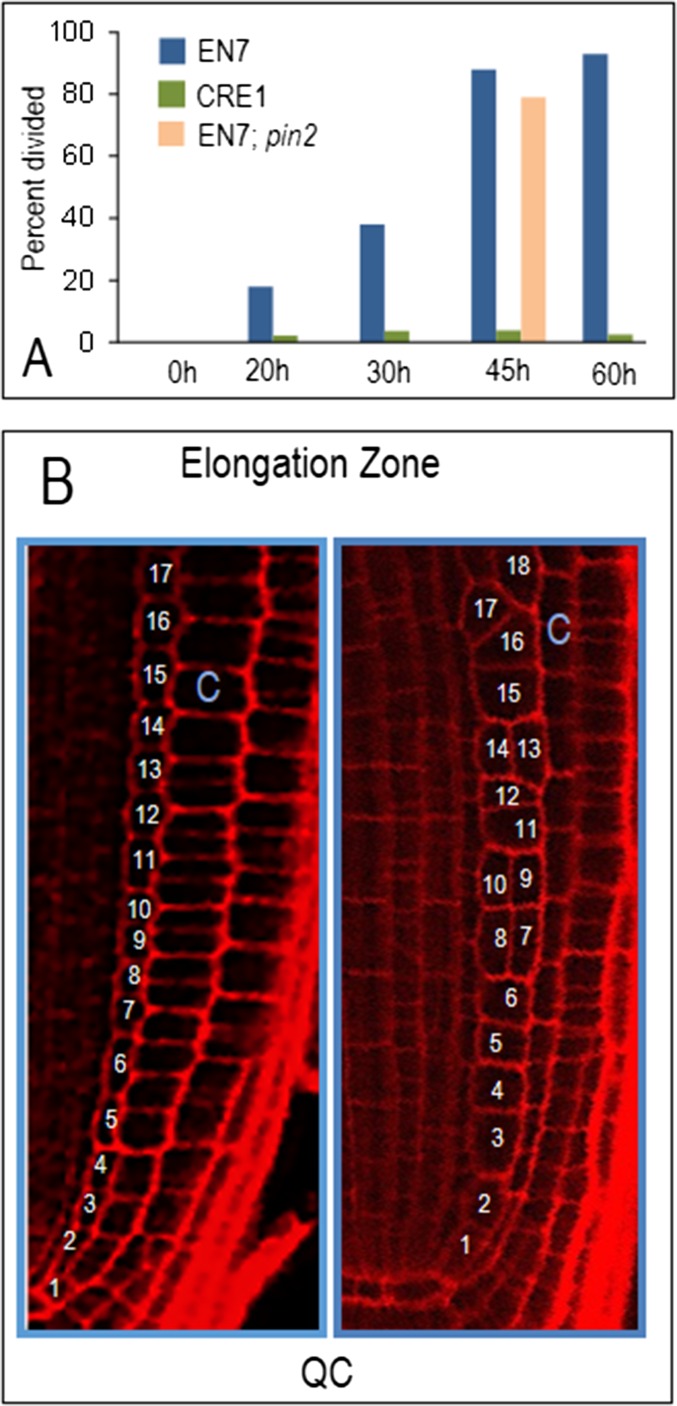
Fig. S1.
Loss of PD signaling to and from the endodermis but not the stele affects the orientation of cell divisions in the endodermis. (A) Quantification of cells within an endodermal file that had divided periclinally. One hundred percent would mean that all cells in the endodermal file had undergone at least one periclinal cell division. EN7 and CRE1 represent EN7:icals3m and CRE1:icals3m, respectively; EN7;pin2 represents EN7:icals3m in the pin2 background. (B) Diagram showing how the number of cells in the endodermal lineage was counted. All cells in the endodermal cell file from the QC to the elongation zone were counted.
Fig. 3.
Symplastic regulation of radial patterning is specific to the endodermis. Confocal images of shr-2 roots showing expression of EN7:H2B-GFP (A) and UAS:erGFP driven by the J0571 enhancer trap line (B–D). shr-2 roots expressing the J0571:GAL4; UAS:icals3m and UASerGFP transgenes (B) before and (C and D) after induction with estradiol. (D) Quantification of ground tissue width in shr-2 roots expressing the J0571:GAL4; UAS:icals3m; UASerGFP transgenes. (E–I) Wild-type roots expressing the J0571:GAL4; UAS:icals3m transgenes (F) before and (F–I) after induction with estradiol (times as indicated). (J–M) Wild-type roots expressing the E903:GAL4; UAS:icals3m transgenes. The variable expression pattern of E903 is shown by the variability in erGFP expression: (J) only in the epidermis; (K) epidermis and cortex; (L) cortex and endodermis; and (M) epidermis and cortex with weak expression in endodermis. Note that only roots with significant expression in the endodermis (L, arrows) show changes in radial patterning in response to induction of the icals3m transgene. (N) Induction of EN7:icals3m transgenes in pin2 roots causes defects in radial patterning. Arrows point to the periclinal cell divisions. E, endodermal lineage; M, mutant ground tissue layer in shr-2 mutants; C, cortex; D, epidermis.
Previously, Helariutta et al. showed that expression of SHR directly in the endodermis resulted in an increase in the number of endodermal cell layers and the number of cortex cells (24). Likewise Nakajima and colleagues showed in argonaute 1 (ago1-101) roots that an increase in endodermal cell layers (25) is accompanied by an increase in the number of cortex cells. In the SCR:SHR-GFP and the ago1-101 roots the cortex cell layer divides anticlinally (in the longitudinal dimension) to produce additional cortex cells (an increase from the normal eight cells per layer), which accommodates the expansion in endodermal cell layers (24, 25). Shown here, the expression of SHR-nlsGFP (21) from the SCR promoter results in the same multilayered endodermal phenotype observed by Helariutta et al. (24) (Fig. 2J). Likewise, the number of cortex cells in the roots expressing SCR:SHR-nlsGFP increases (in the root shown from 8 to 18; Fig. 2J). Surprisingly in the estradiol-induced roots, there is an increase in the number of cell layers in the endodermis, but no compensatory change in cortex cell numbers (Fig. 2 K and M). The cortex maintains the eight-cell number but the cells appear stretched, presumably to accommodate the increased root girth (Fig. 2 K and M). These cortex cells are alive; there is no penetration of propidium iodide into the cell (Fig. 2 D–F). These results suggest that symplastic signals (as opposed to mechanical signals, for example) from the endodermis are required to induce compensatory changes in cell division in the cortex that balance the increase in cell number in the endodermis.
When the EN7:icals3m transgene is activated, symplastic signals emanating from the cortex and epidermis into the endodermis are blocked. Likewise symplastic signals coming from the stele into the endodermis are also blocked. To determine which cell layers are responsible for the phenotype seen in the EN7:icals3m roots, we expressed the icals3m transgene in the stele or in the cortex and epidermis. Induction of CRE1:icals3m results in the disruption of symplastic movement out of the stele (14). However, even after 60 h of induction of the CRE1:icals3m transgene, no significant changes in the orientation of cell divisions in the endodermis (Fig. 2 G and H and Fig. S1) were observed. These results suggest that a lack of stele to endodermis signaling alone is not what drives the EN7:icals3m phenotype. To determine whether a loss of symplastic signals from the cortex or epidermis could induce periclinal cell divisions in the endodermis we made use of the E903 enhancer trap line (https://www.arabidopsis.org/abrc/poethig.jsp) to drive inducible expression of UAS:icals3m. The E903 lines show variable expression of the UAS:erGFP marker in the epidermis, cortex, and endodermis. In most roots, as marked by erGFP, expression is in the epidermis only (Fig. 3J) or epidermis and cortex (Fig. 3K). In these roots, activation of the UAS:icals3m has no effect on the patterning of the endodermis. However, in roots where expression of UAS:erGFP is driven also in the endodermis (Fig. 3L), activation of UAS:icals3m correlated with periclinal divisions in the endodermis. Weak expression in the endodermis had no effect on patterning (Fig. 3M). These results strongly suggest that a loss of symplastic signaling from the cortex or epidermis into the endodermis is not sufficient to induce periclinal cell division in the endodermis. Instead the phenotype observed in the EN7:icals3m roots is the result of a loss of PD-mediated signals from both the stele and the cortex into the endodermis or signals between endodermal cells.
Signals from the Endodermis Are Not Required to Maintain the Activity of the CED Cells or for QC Maintenance or Function.
Previous results by van den Berg et al. showed that isolation of CED cells from their progeny in the cortex and endodermis (via laser ablation) resulted in a loss of periclinal cell divisions in the CED cells (26). This prompted the “top-down signaling hypothesis,” a specific model, which posited that top-down (hence the name) signals derived from more mature tissues were transmitted into the CED cells where they were required to maintain the formative periclinal cell divisions that produce the separate endodermis and cortex (26, 27). Because only complete ablation of all cortex and endodermal cells in direct contact with the CED affected periclinal cell divisions in the CED, the top-down signal was thought to be symplastic, juxtacrine, or mechanical. However, in our assays, neither activation of EN7:icals3m (no symplastic signals from the endodermis to the CED; Fig. 2 D–F) nor activation of J0571:GAL4; UAS:icals3m (no symplastic signals from the endodermis or cortex to the CED; Fig. 3 G–J) caused a loss of periclinal cell division in the CED cells. This finding argues against symplastic conveyance of the top-down signal required to maintain the proper behavior of the CED stem cell populations, and instead for some other form of signaling.
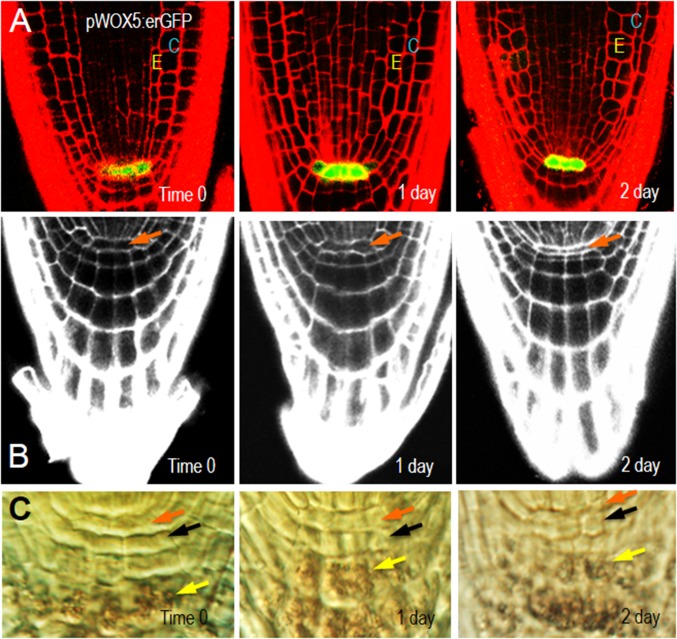
The QC functions as an organizer of cellular patterning and inhibits the differentiation of surrounding stem cells. The QC sits at the apex of the cortical endodermal lineage and is in direct contact with the CEIs (28). To determine whether symplastic signaling from the endodermis or the CEIs is required for maintenance of the QC, we examined QC morphology and expression of the QC marker, WOX5:erGFP after induction of EN7:icals3m (Fig. S2A) (29). Similar to what we saw in the cortex, the cells of the QC appeared stretched or compressed after induction of the EN7:icals3m transgene (Fig. S2 A–C). Despite the extensive defects in ground tissue patterning and changes in QC morphology, the expression of WOX5 was well maintained after induction of EN7:icals3m (Fig. S2A). Consistent with this finding, the organization of the columella was largely normal with a lack of starch in the columella initials and starch accumulation in the columella (Fig. S2C) (29). These results suggest that symplastic signals from the endodermis are not required for QC function.
Fig. S2.
Induction of EN7:icals3m has no effect on QC function. (A) Expression of WOX5-erGFP is not affected by induction of EN7:icals3m. (B) The expression of EN7:icals3m results in morphological changes in the QC (orange arrows), but no changes in the normal differentiation of the columella, as shown by (C) the accumulation of starch in the progeny of the columella stem cells (yellow arrows). Columella stem cells are marked by black arrows. Times after induction are noted on the images.
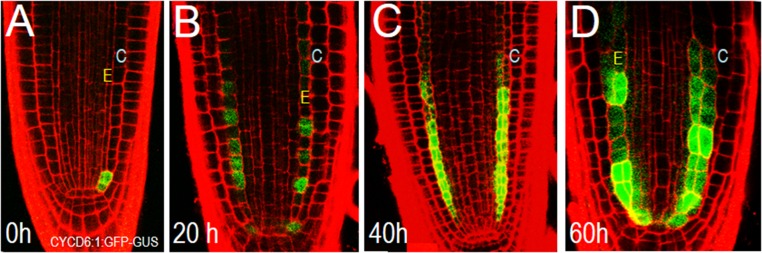
Periclinal cell divisions of the CED cells requires the expression of CYCD6;1 (30). Following asymmetric division of the CED cell, expression of CYCD6;1 is turned off and the cells in the newly formed endodermis and cortex divide symmetrically in a series of transit amplifying cell divisions. To determine whether expression of CYCD6;1 is affected by a loss of endodermal signaling, the CYCD6;1:GFP-GUS marker was crossed into roots expressing EN7:icals3m. As expected, before estradiol induction, CYCD6;1 expression was found predominately in the CEI and CED cells (Fig. S3A). After 20 h on estradiol, CYCD6;1 expression was present in the endodermal cell lineage (Fig. S3B). The occurrence of elevated levels of CYCD6;1 appeared before the ectopic periclinal cell divisions in the endodermis. Longer treatment with estradiol correlated with a further expansion of CYCD6;1 expression and increased numbers of periclinal cell divisions within the endodermis (Fig. S3 C and D). In no instances was CYCD6;1 expression found in cortical or epidermal cells. These results indicate that symplastic signals to the endodermis or between endodermal cells are required to inhibit inappropriate expression of CYCD6;1. The persistence of CYCD6;1 expression in the endodermis suggests that symplastic signals (perhaps from more mature cells in the lineage) are required to promote the transition from a CED cell fate to an endodermal precursor cell fate. Collectively, these results suggest that, whereas symplastic signals from the ground tissue are dispensable for asymmetric division of the CEDs and the function of the QC, signals from the endodermis may be important in triggering a switch from CED cell fate to endodermal cell fate.
Fig. S3.
A loss of PD signaling in the endodermis results in ectopic expression of CYCD6;1. (A–D) Confocal micrographs of wild-type roots expressing the CYCD6;1:GFP-GUS marker (A) before and (B–D) after induction of the EN7:icals3m transgene. Blue C, cortex cell layer; yellow E, endodermis. Times indicated are after induction.
Symplastic Communication Is Essential for Specifying Cell Identity in the Ground Tissue.
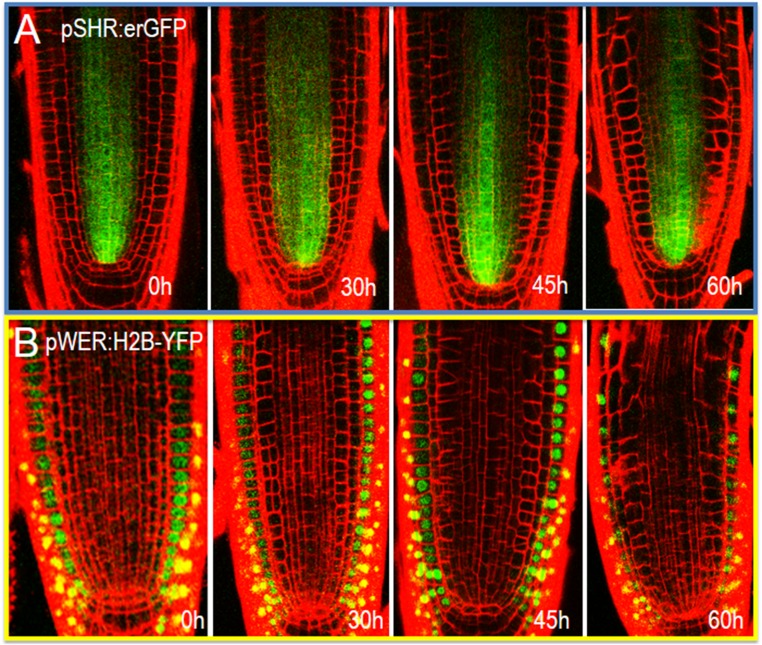
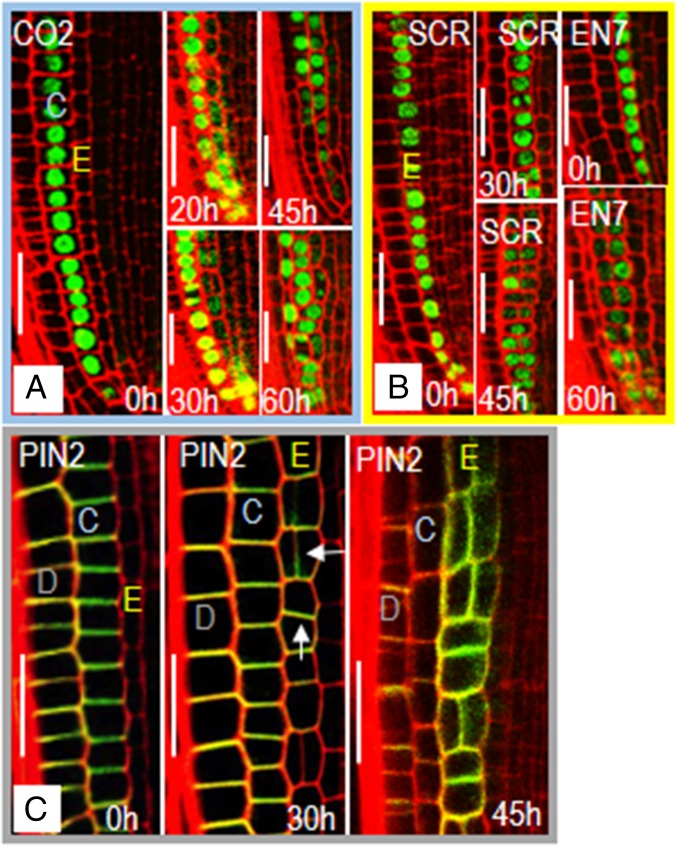
Plant cells rely heavily on spatial context, rather than lineage to determine cell fate. To determine whether loss of endodermal signaling affects cell fate in the radial dimension, we examined markers of stele (SHR:erGFP; Fig. S4A), epidermal (WER:H2B-YFP; Fig. S4B), cortical (CO2:H2B-YFP; Fig. 4A), and endodermal (EN7:H2B-YFP and SCR:H2B-YFP; Fig. 4B) cell fate (20, 24, 31). We saw no significant changes in the expression of SHR:erGFP (stele) or WER:H2B-YFP (epidermis) after induction (up to 60 h) of EN7:icals3m (Fig. S4 A and B). In contrast, the expression of CO2:H2B-YFP, which is usually restricted to the cortex and CED cells, is turned on in the endodermis (Fig. 4A) (20). Expression of the CO2:H2B-YFP marker in the endodermis preceded large-scale changes in radial patterning. After division, most of the cell layers derived from the endodermal cell lineage expressed the CO2:H2B-YFP marker. However, expression of CO2:H2B-YFP in these cells was generally lower than in the cortex and not exclusive of EN7:H2B-YFP or SCR:H2B-YFP expression (Fig. 4B). Instead, most of the cells in the endodermal lineage expressed the markers of both cortex and endodermis. Although CO2:H2B-YFP became active in the endodermis, we did not see the expression of endodermal markers in cortex, which is seen in the shr-2 mutants (Fig. 3A). These results suggest that symplastic signals to or between endodermal cells are required to maintain ground tissue cell fates.
Fig. S4.
A loss of symplastic signals to and from the endodermis has no effect on markers of the stele or epidermis. (A) Expression of SHR:erGFP in the stele and (B) WER:H2B-YFP in the epidermis. Times are after induction.
Fig. 4.
A lack of endodermal signaling affects cell identity and PIN2 localization. Confocal images of roots expressing markers of the (A) cortex (CO2:H2B-GFP; grouped and outlined in blue) and (B) endodermis (SCR:H2B-GFP and EN7:H2B-GFP; grouped and outlined in yellow) before and after induction of EN7:icals3m as labeled. After induction of the EN7:icals3m transgene, expression of the (A) CO2:H2B-GFP marker expands into the endodermal cell file. (C) PIN2:PIN2-GFP expression (grouped and outlined in gray) in the epidermis and cortex in uninduced and induced roots (as labeled). Expression of PIN2-GFP is absent from the endodermis in roots before induction (0 h) of icals3m. PIN2 expression turns on in the endodermis (arrows) after induction of icals3m, increasing in expression up to 45 h (the focal plane was chosen to highlight the PIN2-GFP in endodermis). E, endodermal lineage; C, cortex; D, epidermis. All times are after induction. (Scale bars, 25 μm.)
Auxin Signaling Is Affected by Symplastic Isolation of the Endodermis.
The auxin efflux carrier PIN2, is a functional marker of cortical and epidermal cell identity in the root (Fig. 4C). Together with other PIN family proteins, PIN2 facilitates the polarized flow of auxin via asymmetric localization within the plasma membrane of cortical and epidermal cells. Whereas PIN2 localization is variable in the epidermis and cortex, the predominant localization is shootward in epidermal cells and rootward in the cortex (Fig. 4C and Fig. S5). PIN2 is not expressed in the endodermis (Fig. 4C). After 30 h on estradiol, and before ectopic periclinal cell divisions, PIN2:PIN2-GFP was expressed in the endodermis of EN7:icals3m roots (Fig. 4C and Fig. S5). In the endodermis, PIN2 showed preferential (but not exclusive) localization to the rootward end of the cell (Fig. S5 B, E, and E′). At 30 h, we saw mild increases in the localization of PIN2 to the longitudinal walls of the cortex and epidermis. In the cells created by the periclinal division in the endodermis, PIN2 was also expressed and largely apolar (Fig. 4C and Fig. S5). This apolar localization of PIN2 became more obvious with longer disruption (45 h) of symplastic communication in the endodermis (Fig. 4C). These results indicate that symplastic communication is required to maintain ground tissue identity and for cell polarity.
Fig. S5.
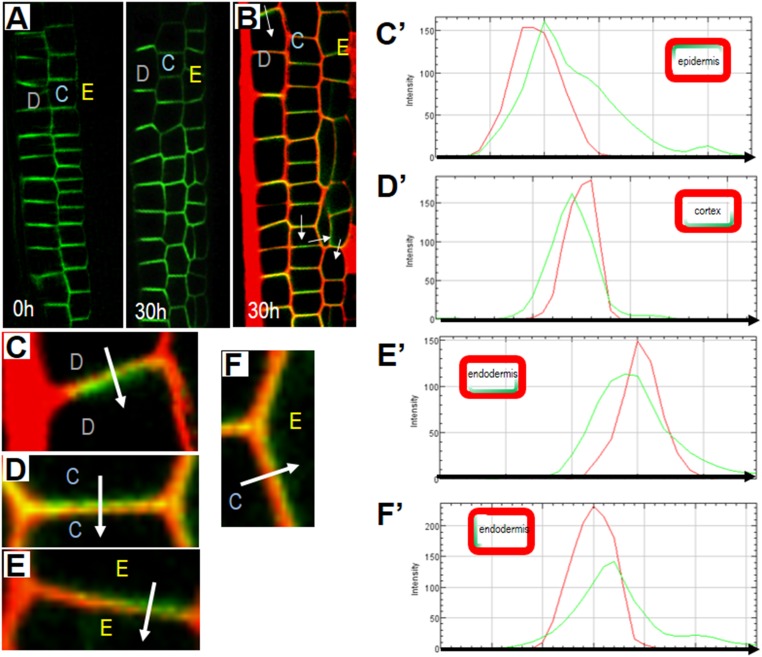
Analysis of PIN2-GFP expression and polarity in EN7:icals3m roots. (A) Overview of PIN2-GFP expression in EN7:icals3m roots (0 h) before and (30 h) after estradiol induction. (B) Expression of PIN2-GFP in PI-stained EN7:icals3m roots 30 h after estradiol treatment. (C–F) Magnified views of cells shown in B: (C) epidermis to epidermis; (D) cortex to cortex; (E) endodermis to endodermis; (F) cortex to endodermis. White arrows in B–F indicate the direction of measurement of the red (PI) and green (PIN2-GFP) signals. (C′–F′) Graphical display of the spatial distribution of the green and red signals (from the blunt end to the tip of the arrow) across the indicated cell walls that are shown in C–F, respectively. The cartoon Insets in the graphs (C′–F′) show the predominant relative positions of PIN2 (green) and the cell wall (red) in the epidermis, cortex, and endodermis (as labeled). Yellow E, endodermis; blue C, cortex; gray D, epidermis.
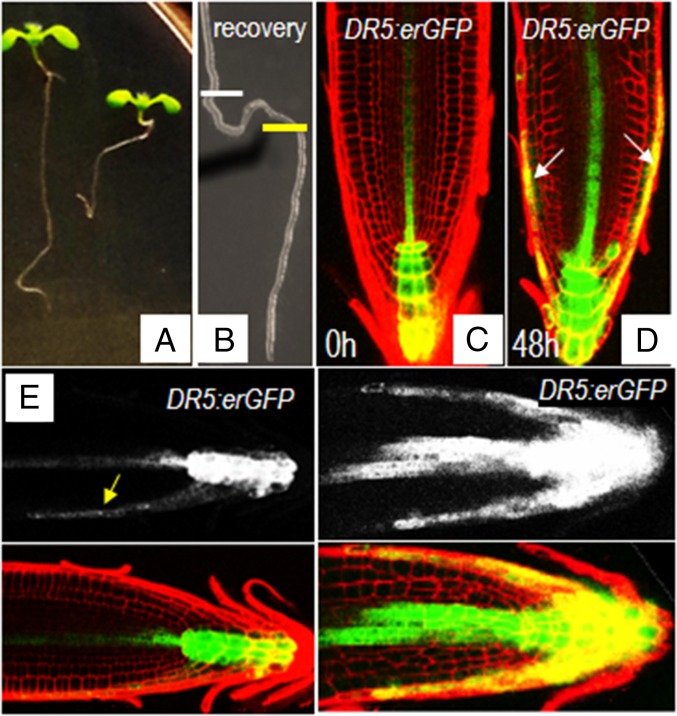
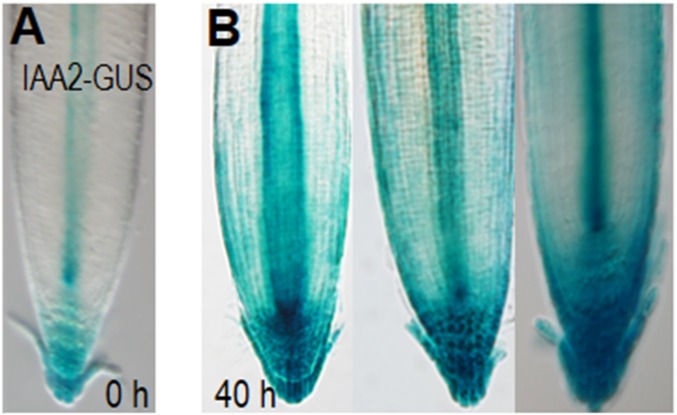
Because polar transport of auxin governs root gravitropism (32, 33), we examined whether the activation of EN7:icals3m affects root gravitropic responses. Upon shifting EN7:icals3m roots to estradiol-containing medium, the roots became noticeably less gravitropic (Fig. 5 A and B). After 3 days on estradiol, some roots had grown in circles, whereas others grew in opposition to gravity. To test whether this defect was caused by abnormal auxin distribution, we examined DR5:erGFP reporter in both wild-type (Fig. 5C) and EN7:icals3m (Fig. 5D) lines (34). In uninduced roots, grown vertically on plates, the fluorescence maximum of DR5:erGFP was in the distal region of the root tip, with no signal in the epidermis or the lateral root cap (Fig. 5C). In the induced roots, expression of DR5:erGFP is altered; the signal in the stele is increased with signals now in the epidermis and lateral root cap (Fig. 5D). IAA2:GUS showed a similar trend (Fig. S6 A and B) (35). Treatment of wild-type roots with auxin changes the pattern of DR5:erGFP signal so that there are peaks in expression in the outermost cell layers of the root meristem similar to what we observed in the roots with activated EN7:icals3m expression (Fig. 5D) (34). This result indicates that symplastic signals are necessary for proper gravity response through the control of PIN2-expressing cells.
Fig. 5.
A loss of endodermal signaling results in changes in auxin distribution and root gravitropism. (A) Seedlings were grown for 5 d on MS medium before transfer for 3 d to estradiol containing medium. (B) Roots from A were transferred back to MS medium and allowed to grow for 3 d during which time normal growth was recovered; the white bars mark the position of the root tip at the time of transfer to estradiol containing medium; the yellow bar marks the position of the root tip at the time of transfer to medium without estradiol. (C–F) Expression of DR5:erGFP in vertically grown roots with the EN7:icals3m transgene. DR5:erGFP in (C) uninduced and (D) induced roots. (D) Arrows note the expression of DR5:erGFP in the lateral root cap after a 2-d block to endodermal signaling. Vertically grown (E) uninduced and (F) induced EN7:icals3m roots expressing the DR5:erGFP marker were gravistimulated (turned 90°). Yellow arrow points to the expression of DR5:erGFP in the downward flank of the root meristem of the uninduced root following gravistimulation. (F) There is no change in DR5:erGFP expression in the induced root in response to gravistimulation.
Fig. S6.
Loss of symplastic signaling in the endodermis alters auxin responses. (A and B) Expression of the IAA2-GUS reporter in (A) uninduced and (B) induced EN7:icals3m roots.
When wild-type roots are rotated by 90°, there is a redistribution of auxin toward the lower edge of the root, as indicated by an increased level of DR5:erGFP signal (Fig. 5E). This precedes differential expansion of the root to reorient growth downward. Following rotation of the EN7:icals3m roots, no changes in the auxin concentration were observed; both the upper and lower sides of the root maintained high levels of auxin as indicated by the expression of the DR5:erGFP reporter (Fig. 5F). These results suggest that an inability to differentially localize auxin in the EN7:icals3m roots underlies their inability to respond to gravity. Interestingly, gravitropic defects caused by activation of EN7:icals3m can be recovered. Treatment in estradiol for 2–3 d followed by recovery in normal Murashige and Skoog (MS) medium restored root gravitropism, suggesting that patterning can be recovered (Fig. 4B).
Recent reports by Han et al. looking at hypocotyl tropism suggest that PD can serve as conduits for auxin movement (19). They found that loss of GLUCAN SYNTHASE-LIKE 8 (GSL8) activity (via RNAi) resulted in a significant reduction in hypocotyl accumulation of PD-localized callose and a loss of normal phototropic responses. They conclude that restricted movement of auxin through PD allows for the formation of an auxin gradient that regulates phototropic responses. In the EN7:icals3m roots the loss of a normal gravitropic response is more likely the result of aberrant PIN expression and localization rather than a restriction of PD-mediated auxin flow. Han et al. show normal localization of PIN3 in the hypocotyl of their gsl8 RNAi lines; however, because PIN4 and PIN7, which play significant roles in the phototropic responses, are not examined, an effect on PIN proteins cannot be eliminated (19).
To determine the extent to which ectopic expression of the PIN2 protein in the endodermal cell file drives the patterning defects after induction of EN7:icals3m, we expressed the EN7:icals3m transgene in pin2 (eir1) mutants (36). In these roots the frequency of periclinal cell divisions in the endodermal lineage is very similar to wild type (79% divided in pin2 mutants versus 88% divided in wild type at 45 h; Fig. S1A). The significant difference in the response of the pin2 mutants to induction of the icals3m transgene is a lack of radial swelling in the endodermis before periclinal cell division (Fig. 3O). Whereas root cells expand in all directions following cell division, most expansion occurs in the apical–basal axis; this allows for directional growth of the root. In wild-type plants, expression of EN7:icals3m resulted in a doubling of the width of endodermal cells compared with controls (uninduced EN7:icals3m versus EN7:icals3m after 1 d on estradiol; Student’s t test P values = 0.00038, n = 13–15); this increase in girth, preceded divisions in the endodermal cell file. In the pin2 mutants, periclinal cell divisions were induced in the endodermis without prior cell swelling (Fig. 3O). These results indicate that the cell expansion phenotype in the wild-type plants expressing EN7:icals3m is dependent upon an auxin response, whereas the ectopic periclinal divisions are not. In addition, these results uncouple the cell swelling phenotype from division, indicating that the periclinal cell divisions in the endodermis are not a response to an increase in cell girth. Collectively, our results show that symplastic signaling is critical for the regulation of cell divisions, cell polarity, and cell expansion in the endodermis of A. thaliana.
Materials and Methods
Details on materials and methods, including the plant material and chemicals, plasmid construction, confocal imaging, and histology, as well as other analyses and tools used are provided in SI Materials and Methods.
SI Materials and Methods
Plant Material and Growth Conditions.
Arabidopsis thaliana ecotype Col-0 was used as the wild type. All plants were grown on 1% agar plates with 0.5× Murashige and Skoog (MS) medium (Caisson Laboratories) containing 0.05% wt/vol morpholinoethansulfonic acid monohydrate (Mes) (pH 5.7), 1.0% wt/vol sucrose, and 1.0% granulated agar (Difco Laboratories) under a 16-h light/8-h dark cycle at 23 °C. Plants were used 5 d after plating unless otherwise stated. All fluorescent marker lines were crossed into plants expressing EN7:icals3m transgene. Homozygous lines that express both the fluorescent marker and EN7:icals3m transgene were selected based on fluorescence, PCR genotyping, and the root phenotype after induction.
Plasmid Construction and Plant Transformation.
The icals3m fragment containing XVE, rbcST, lexA + mini35S, and cals3m was amplified by PCR using previously published plasmid as a template (14) and then digested by XbaI/MfeI and inserted into XbaI/EcoRI sites of pCambia3300 (www.cambia.org/daisy/cambia/585). A DNA fragment containing the attR1/2 recombination sites flanking the chloramphenicol and ccdb selection cassettes was cloned by PCR using pMDC7 (37) as a template. This fragment was then inserted in front of the icals3m sequence to create a destination vector. The EN7 promoter in the pENTR3C vector was then recombined into the destination vector using standard gateway protocols (Invitrogen/Life Technologies, http://www.thermofisher.com/us/en/home/life-science/cloning/gateway-cloning.html). The resulting binary vectors were introduced into Agrobacterium tumefaciens strain GV3101-pSoup-pMP and Arabidopsis thaliana ecotype Col-0 was transformed using standard floral dip method. Transgenic plants were screened for resistance to glufosinate ammonium (Basta) in soil. For all of the transgenes, at least three independently transformed lines were analyzed. The EN7:histone2B-YFP, CO2:histone2B-YFP, and WER:histone2B-YFP lines are as described (20).
Confocal Microscopy and Imaging.
Roots were imaged and the measurement of fluorescence intensity and the calculation of the fluorescence ratios were performed as described (38–40). Callose staining was performed as described (41). For figure preparation, images were cropped in Photoshop and in some cases, empty areas of background were filled with black at 100% opacity to square the images. For PIN2 localization analysis, PIN2:PIN2-GFP expressing roots were stained with PI and then imaged on the Zeiss 880 confocal using the airy-scan function. The high-resolution images were then analyzed by ImageJ. A line was drawn across the cell wall of interest and red, green, blue (RGB) color intensity distribution was plotted using RGB profile plot function of the graphics plugin in ImageJ.
Histology and Histochemistry.
The seedlings were fixed for 1 h in FAA and then embedded in agarose blocks as described previously (42). Sections were directly visualized using an upright Olympus BX51 microscope under differential interference contrast (DIC) optics. To visualize starch granules in the columella root cap, roots were incubated in a mixture of chloral hydrate and Lugol’s solution (6:1) for 1 min and mounted in the same solution for imaging. Whole roots were imaged using DIC on an upright Olympus BX51 microscope. Seedlings for β-glucuronidase (GUS) staining were incubated in cold acetone for 15 min and then incubated in staining solution (100 mM sodium phosphate buffer pH 7.0, 10 mM EDTA, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 0.5% Triton X-100, and 2 mM X-glucuronide) at 37 °C for 1 h. Seedlings were washed three times with the above solution without the substrate and then mounted in clearing solution for DIC imaging.
Estrogen Induction and Chemical Treatments.
Five days after plating, the seedlings were transferred to 0.5× MS (Caisson) agar (Difco-BBL) plates containing 20 µM estradiol (Sigma), and the same medium containing the estradiol carrier (DMSO) as controls. The seedlings were then grown under a 16-h light/8-h dark cycle at 23 °C until observation.
Acknowledgments
R.O. was supported by NIH Cell and Molecular Biology Training Grant GM-007229-37. S.W. and Y.S. were partially supported by Na-tional Science Foundation Grant 1243945 (to K.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610358113/-/DCSupplemental.
References
- 1.Gallagher KL, Sozzani R, Lee CM. Intercellular protein movement: Deciphering the language of development. Annu Rev Cell Dev Biol. 2014;30:207–233. doi: 10.1146/annurev-cellbio-100913-012915. [DOI] [PubMed] [Google Scholar]
- 2.Kurata T, et al. Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development. 2005;132(24):5387–5398. doi: 10.1242/dev.02139. [DOI] [PubMed] [Google Scholar]
- 3.Saint Savage N, et al. A mutual support mechanism through intercellular movement of CAPRICE and GLABRA3 can pattern the Arabidopsis root epidermis. PLoS Biol. 2008;6(9):e235. doi: 10.1371/journal.pbio.0060235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang YH, Song SK, Schiefelbein J, Lee MM. Nuclear trapping controls the position-dependent localization of CAPRICE in the root epidermis of Arabidopsis. Plant Physiol. 2013;163(1):193–204. doi: 10.1104/pp.113.221028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316(5827):1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17(12):1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Turnbull C. Long-distance regulation of flowering time. J Exp Bot. 2011;62(13):4399–4413. doi: 10.1093/jxb/err191. [DOI] [PubMed] [Google Scholar]
- 8.Burch-Smith TM, Zambryski PC. Loss of INCREASED SIZE EXCLUSION LIMIT (ISE)1 or ISE2 increases the formation of secondary plasmodesmata. Curr Biol. 2010;20(11):989–993. doi: 10.1016/j.cub.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim I, Hempel FD, Sha K, Pfluger J, Zambryski PC. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development. 2002;129(5):1261–1272. doi: 10.1242/dev.129.5.1261. [DOI] [PubMed] [Google Scholar]
- 10.Stonebloom S, et al. Loss of the plant DEAD-box protein ISE1 leads to defective mitochondria and increased cell-to-cell transport via plasmodesmata. Proc Natl Acad Sci USA. 2009;106(40):17229–17234. doi: 10.1073/pnas.0909229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benitez-Alfonso Y, et al. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA. 2009;106(9):3615–3620. doi: 10.1073/pnas.0808717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benitez-Alfonso Y, Jackson D. Redox homeostasis regulates plasmodesmal communication in Arabidopsis meristems. Plant Signal Behav. 2009;4(7):655–659. doi: 10.4161/psb.4.7.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guseman JM, et al. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8) Development. 2010;137(10):1731–1741. doi: 10.1242/dev.049197. [DOI] [PubMed] [Google Scholar]
- 14.Vatén A, et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21(6):1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 15.J Maule A, Gaudioso-Pedraza R, Benitez-Alfonso Y. Callose deposition and symplastic connectivity are regulated prior to lateral root emergence. Commun Integr Biol. 2013;6(6):e26531. doi: 10.4161/cib.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermeer JE, Geldner N. Lateral root initiation in Arabidopsis thaliana: A force awakens. F1000Prime Rep. 2015;7:32. doi: 10.12703/P7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeer JE, et al. A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science. 2014;343(6167):178–183. doi: 10.1126/science.1245871. [DOI] [PubMed] [Google Scholar]
- 18.Daum G, Medzihradszky A, Suzaki T, Lohmann JU. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc Natl Acad Sci USA. 2014;111(40):14619–14624. doi: 10.1073/pnas.1406446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, et al. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev Cell. 2014;28(2):132–146. doi: 10.1016/j.devcel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Heidstra R, Welch D, Scheres B. Mosaic analyses using marked activation and deletion clones dissect Arabidopsis SCARECROW action in asymmetric cell division. Genes Dev. 2004;18(16):1964–1969. doi: 10.1101/gad.305504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher KL, Paquette AJ, Nakajima K, Benfey PN. Mechanisms regulating SHORT-ROOT intercellular movement. Curr Biol. 2004;14(20):1847–1851. doi: 10.1016/j.cub.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 22.Stadler R, et al. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 2005;41(2):319–331. doi: 10.1111/j.1365-313X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- 23.Carlsbecker A, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465(7296):316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helariutta Y, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101(5):555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- 25.Miyashima S, Hashimoto T, Nakajima K. ARGONAUTE1 acts in Arabidopsis root radial pattern formation independently of the SHR/SCR pathway. Plant Cell Physiol. 2009;50(3):626–634. doi: 10.1093/pcp/pcp020. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature. 1995;378(6552):62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- 27.Wysocka-Diller JW, Benfey PN. Root development: Signaling down and around. BioEssays. 1997;19(11):959–965. doi: 10.1002/bies.950191105. [DOI] [PubMed] [Google Scholar]
- 28.Dolan L, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119(1):71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar AK, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446(7137):811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 30.Sozzani R, et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466(7302):128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MM, Schiefelbein J. WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell. 1999;99(5):473–483. doi: 10.1016/s0092-8674(00)81536-6. [DOI] [PubMed] [Google Scholar]
- 32.Rahman A, et al. Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells. Plant Cell. 2010;22(6):1762–1776. doi: 10.1105/tpc.110.075317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukumar P, Edwards KS, Rahman A, Delong A, Muday GK. PINOID kinase regulates root gravitropism through modulation of PIN2-dependent basipetal auxin transport in Arabidopsis. Plant Physiol. 2009;150(2):722–735. doi: 10.1104/pp.108.131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottenschläger I, et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA. 2003;100(5):2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casimiro I, et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13(4):843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12(14):2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133(2):462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koizumi K, Hayashi T, Wu S, Gallagher KL. The SHORT-ROOT protein acts as a mobile, dose-dependent signal in patterning the ground tissue. Proc Natl Acad Sci USA. 2012;109(32):13010–13015. doi: 10.1073/pnas.1205579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koizumi K, Wu S, MacRae-Crerar A, Gallagher KL. An essential protein that interacts with endosomes and promotes movement of the SHORT-ROOT transcription factor. Curr Biol. 2011;21(18):1559–1564. doi: 10.1016/j.cub.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Gallagher KL. Intact microtubules are required for the intercellular movement of the SHORT-ROOT transcription factor. Plant J. 2013;74(1):148–159. doi: 10.1111/tpj.12112. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell. 2011;23(9):3353–3373. doi: 10.1105/tpc.111.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S, Baskin TI, Gallagher KL. Mechanical fixation techniques for processing and orienting delicate samples, such as the root of Arabidopsis thaliana, for light or electron microscopy. Nat Protoc. 2012;7(6):1113–1124. doi: 10.1038/nprot.2012.056. [DOI] [PubMed] [Google Scholar]