Significance
The growing tips of plants maintain a constant number of stem cells in shoot apical meristems despite a continuous differentiation of stem cell descendants. The levels of homeodomain transcription factor WUSCHEL (WUS) determine the number of stem cells. Although WUSCHEL has been demonstrated to move across cells, the protein signatures that control nuclear levels and spatial patterning have remained elusive. We show that transcriptional regulatory domains also influence nuclear levels of WUS through nuclear-cytoplasmic partitioning and determine the spatial distribution of WUS through DNA binding and homodimerization. Our data also suggest that maintenance of WUSCHEL levels involves protein destabilization. Utilization of the same protein domains to regulate transcription and protein concentration may provide robustness to the spatiotemporal regulation of gene expression.
Keywords: CLAVATA3, feedback, homodimerization, meristem, nuclear-cytoplasmic partitioning
Abstract
The homeodomain transcription factor WUSCHEL (WUS) promotes stem cell maintenance in inflorescence meristems of Arabidopsis thaliana. WUS, which is synthesized in the rib meristem, migrates and accumulates at lower levels in adjacent cells. Maintenance of WUS protein levels and spatial patterning distribution is not well-understood. Here, we show that the last 63-aa stretch of WUS is necessary for maintaining different levels of WUS protein in the rib meristem and adjacent cells. The 63-aa region contains the following transcriptional regulatory domains: the acidic region, the WUS-box, which is conserved in WUS-related HOMEOBOX family members, and the ethylene-responsive element binding factor-associated amphiphilic repression (EAR-like) domain. Our analysis reveals that the opposing functions of WUS-box, which is required for nuclear retention, and EAR-like domain, which participates in nuclear export, are necessary to maintain higher nuclear levels of WUS in cells of the rib meristem and lower nuclear levels in adjacent cells. We also show that the N-terminal DNA binding domain, which is required for both DNA binding and homodimerization, along with the homodimerization sequence located in the central part of the protein, restricts WUS from spreading excessively and show that the homodimerization is critical for WUS function. Our analysis also reveals that a higher level of WUS outside the rib meristem leads to protein destabilization, suggesting a new tier of regulation in WUS protein regulation. Taken together our data show that processes that influence WUS protein levels and spatial distribution are highly coupled to its transcriptional activity.
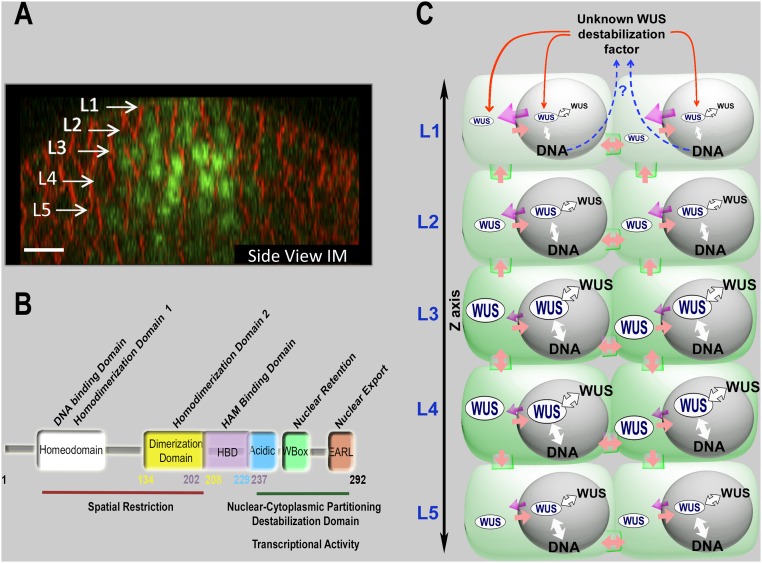
Inflorescence meristems (IMs) harbor a set of pluripotent stem cells in the central zone (CZ) (1). A subset of stem cell progeny that are displaced into the adjacent peripheral zone (PZ) differentiate as lateral organs whereas those that are displaced into the rib meristem (RM), located beneath the CZ, differentiate and become part of the stem (1). The inflorescence meristems are organized into three cell layers that are clonally distinct; the outermost-L1 and the subepidermal-L2 form monolayers, together referred to as the tunica (1). The cells located beneath the L2 layer are collectively referred to as the L3 layers/corpus (1).
In Arabidopsis inflorescence meristems, WUSCHEL (WUS), a homeodomain transcription factor (TF) synthesized in a few cells of the RM/L3/corpus (2, 3), migrates into adjacent cells (4). WUS accumulates at a lower level in the nuclei of cells in the L1 and L2 cell layers compared with the inner layers (Fig. 1 A–D) (4). WUS protein also diffuses radially and accumulates at a lower level in the peripheral zone (4). WUS regulates its own transcript levels by activating CLAVATA3 (CLV3) in the central zone (5). CLV3, a secreted peptide, activates a receptor kinase pathway to restrict WUS transcription (6–9). Previous studies have shown that ectopic activation of WUS in the central zone leads to meristem overproliferation (10, 11). Conversely, the depletion of WUS leads to premature differentiation of stem cell progeny (11–13), showing the importance of the regulation of WUS protein levels.
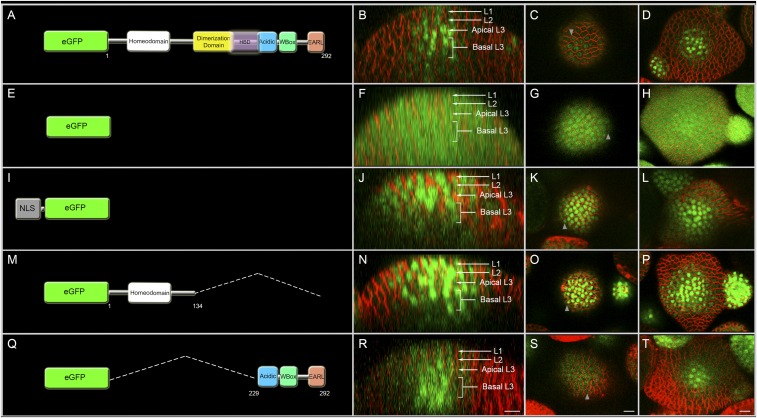
Fig. 1.
The C terminus of WUS is sufficient for the spatial patterning of WUS protein. The first column shows diagrams of various constructs expressed from the WUS promoter: (A) eGFP-WUS, (E) free (untagged) eGFP, and (I) nuclear localization tagged NLS-eGFP. (M) [eGFP-WUS (amino acids 1 to 134)] and (Q) [eGFP-WUS (amino acids 229 to 292)] show schematics of several deletions of WUS fused to eGFP and expressed from the WUS promoter. The break points of deletions are marked as amino acid positions. The second column (B, F, J, N, and R) shows side views of IMs showing eGFP protein distribution for the constructs shown in the corresponding panels in column 1. The third column (C, G, K, O, and S) shows the top views of the L1 layer of IMs for the constructs shown in the corresponding panels in column 1. The fourth column (D, H, L, P, and T) shows the top views of the apical L3 layers of IMs for the constructs shown in the corresponding panels in column 1. Gray arrowheads mark the spread of protein. All constructs were expressed from pWUS. eGFP (green) and FM4-64 (red). (Scale bars: 10 μm.)
The processes and mechanisms that control differences in WUS protein accumulation between cells of the rib meristem and adjacent cells are not well-understood. The predominant models for protein gradient formation in animal systems involve processes that control the amount of protein synthesis in the source, the rate of diffusion or movement of the protein, and degradation rates (14). The rate of protein diffusion or movement can be influenced by several factors, such as the constraints imposed by the subcellular compartments, the size of the protein, and the nature of its interactions with other molecules. Protein interactions can lead to the formation of homo-multimers or even hetero-multimers, which results in an increased size and decreased diffusion rates. In addition, the mobile proteins can form complexes with nonmobile molecules, such as the DNA or cytoskeleton elements, that can impede movement. Finally, the differential degradation rates of the protein in the source cells versus the surrounding cells can contribute to the differences in protein levels.
The factors and processes that can influence WUS movement are beginning to emerge. WUS is synthesized in the rib meristem, and its expression levels are limited by the receptor kinase signaling pathway mediated by the CLAVATA class of proteins (3, 5–8). WUS diffuses into the adjacent CZ and the PZ where it accumulates at a lower level (4). Transcription factors (TFs) in plants have been shown to migrate between cells through plasma membrane-lined pores in cell walls, plasmodesmata (PDs) (15). Several aspects, such as the density and distribution of PDs, may influence the rate of protein movement; however, they are poorly studied in shoot meristems of Arabidopsis. The PDs have also been shown to have a size exclusion limit (SEL), which allows movement of those TFs whose molecular size falls below the SEL (15). Therefore, the size of WUS protein and the WUS-containing protein complex may influence the rate of movement. An earlier study has shown that the movement of WUS from the rib meristem to adjacent cells could be inhibited by using a WUS protein of higher molecular weight (2XeGFP-WUS), suggesting that WUS likely moves between cells through plasmodesmata and that the SEL of PDs may influence mobility (4). A later study used an alternate approach, involving overexpression of CALLOSE SYNTHASE 3 to reduce the pore size of PDs (16), which also led to inhibition of WUS movement (17).
Earlier studies have shown that WUS can bind DNA, form homodimers, and interact with other proteins. WUS has been shown to homodimerize by using sequences between the N-terminal homeodomain and the C-terminal region (amino acids 100 to 249) (17). WUS lacking the homodimerization sequences not only complemented wus-1 mutants but also caused meristem overproliferation, showing that homodimerization restricts meristem growth (17). An independent study has shown that WUS also interacts with HAIRYMERISTEM (HAM) proteins that are required for meristem maintenance (18, 19). The HAM proteins haven been shown to bind the WUS sequence located between amino acids 203 and 236 (referred to as the HAM binding domain) (19). WUS lacking the HAM binding domain failed to complement the wus-1 mutant defects, showing that this region of WUS is critical for its function in promoting stem cell specification and shoot apical meristem (SAM) growth (19). Therefore, it is not clear whether homodimerization of WUS or HAM binding impedes WUS movement through the formation of larger homo-complexes or hetero-complexes. It is also unclear how the overproliferation of meristems observed in an earlier study (17) was possible with a large deletion (amino acids 100 to 249) that removed a domain of WUS that is critical for HAM binding and WUS function.
The subcellular localization of WUS may also contribute to the observed differences in WUS protein accumulation between cells of the rib meristem and cells located in adjacent domains. An earlier study has shown that increased nuclear targeting of WUS impedes its mobility into the outer cell layers of inflorescence meristems, suggesting that nuclear-cytoplasmic partitioning plays a critical role in intercellular movement of WUS (4). However, it is not known whether WUS protein contains intrinsic signals and/or whether any spatially localized factors exist that influence nuclear-cytoplasmic partitioning. Earlier studies have shown that ectopic overexpression of WUS in the central zone increases CLV3 expression and promotes meristem overproliferation (10, 11). A straightforward logic from these experiments suggests that WUS protein instability in the central zone may not be a contributing factor to the observed differences in WUS protein levels.
To understand the processes and decipher the mechanisms that influence the observed differences in WUS protein accumulation, we have used structure-function analysis and transient studies involving a hormone-inducible form of WUS. Our analysis reveals that the homodimerization and DNA binding mediated by the N-terminal DNA binding domain, together with the central part of the protein, restrict WUS from spreading excessively in inflorescence meristems. We also find that homodimerization of WUS is critical to promote meristem maintenance. The transcriptional regulatory domains located at the C terminus of WUS, the WUS-box and EAR-like domain, influence nuclear-cytoplasmic partitioning, thereby controlling the nuclear levels of WUS. Contrary to the perceived logic, we also find that ectopic overexpression of WUS destabilizes WUS protein, which adds another tier to the regulation of WUS protein levels. We discuss possible mechanisms and protein regulators that may influence multiple processes regulating WUS protein concentration, which is critical for transcriptional regulation and meristem maintenance [see the companion paper (20)].
Results
A 63-aa Stretch at the C Terminus Is Sufficient for Spatial Patterning of WUS.
A simple diffusion of WUS protein from the site of synthesis, the rib meristem, into adjacent cells could theoretically account for the observed variation in nuclear levels (Fig. 1 A–D). However, we observed the uniform distribution of fluorescence signal when a free (untagged) eGFP (Fig. 1 E–H) (n = 20) or nuclear-localized eGFP (NLS-eGFP) (Fig. 1 I–L) (n = 17) was expressed from the WUS promoter described in an earlier study (4). This result indicates that the cells of the inflorescence meristem are interconnected through their plasmodesmata. Thus, WUS may contain domains that limit its movement and are responsible for the observed variation in nuclear levels.
To identify the domains within WUS that are required to limit its movement and that determine its concentration in the nucleus, we expressed various truncations of WUS fused to eGFP from the WUS promoter. The deletion of the C-terminal half resulted in an intense and relatively uniform distribution of fluorescence in all cell layers, suggesting that the C terminus may contain information to control WUS spatial distribution (Fig. 1 M–P) (n = 20). A finer deletion within the C terminus showed that the last 63-aa stretch was sufficient to allow higher WUS accumulation in inner cell layers compared with the outer cell layers, showing that this part of the protein contains the necessary information to maintain spatial patterning of WUS protein in inflorescence meristems (Fig. 1 Q–T and Fig. S1 U–X) (n = 12).
Fig. S1.
The stability of WUS is determined by a 63-aa region in the C terminus. Column 1 shows various WUS truncations expressed from the WUS promoter: (A) eGFP-WUS (Δ amino acid 281 to amino acid 292), (E) eGFP-WUS (Δ amino acid 249 to amino acid 265), (I) eGFP-WUS (Δ amino acid 229 to amino acid 249), (M) eGFP-WUS (Δ amino acid 208 to amino acid 229), and (Q) eGFP-WUS ((Δ amino acid 134 to amino acid 208). The break points of deletions are marked as amino acid positions. Column 2 is the side views of inflorescence meristems (IMs) showing eGFP-WUS protein distribution for the various constructs shown in the corresponding panels in column 1: (B) eGFP-WUS (Δ amino acid 281 to amino acid 292), (F) eGFP-WUS (Δ amino acid 249 to amino acid 265), (J) eGFP-WUS (Δ amino acid 229 to amino acid 249), (N) eGFP-WUS (Δ amino acid 208 to amino acid 229), and (R) eGFP-WUS (Δ amino acid 134 to amino acid 208). The cell layers in IMs are indicated by white arrows and brackets. Column 3 shows the top views of the L1 layer of IMs for the constructs shown in the corresponding panels in column 1: (C) eGFP-WUS (Δ amino acid 281 to amino acid 292), (G) eGFP-WUS (Δ amino acid 249 to amino acid 265), (K) eGFP-WUS (Δ amino acid 229 to amino acid 249), (O) eGFP-WUS (Δ amino acid 208 to amino acid 229), and (S) eGFP-WUS (Δ amino acid 134 to amino acid 208). Column 4 shows the top views of the apical L3 layers of IMs showing eGFP-WUS protein distribution for the constructs shown in the corresponding panels in column 1: (D) eGFP-WUS (Δ amino acid 281 to amino acid 292), (H) eGFP-WUS (Δ amino acid 249 to amino acid 265), (L) eGFP-WUS (Δ amino acid 229 to amino acid 249), (P) eGFP-WUS (Δ amino acid 208 to amino acid 229), and (T) eGFP-WUS (Δ amino acid 134 to amino acid 208). All constructs were expressed from pWUS. eGFP-WUS (green) and FM4-64 (red). (Scale bars: 10 μm.) (U–X) Enhanced versions of main (Fig. 1 C and S) images with increase in intensity (U and V) and thresholding (W and X). Note expansion of domain with higher nuclear levels. (Scale bars: 10 μm.)
Mutations in the WUS-Box and the EAR-Like Domains Disturb Subcellular Accumulation of WUS.
Earlier work has shown that the C-terminal 63-aa stretch of WUS, which includes the acidic region, the WUS-box, and the EAR-like domains, is necessary for its biological function (21, 22). Loss-of-function analyses in transient transcriptional assays carried out in leaf protoplasts have implicated the acidic region in transcriptional activation and the EAR-like domain and WUS-box in transcriptional repression (22). A recent study using the loss-of-function and domain complementation studies has demonstrated that WUS-box is a transcriptional repressor domain essential for stem cell maintenance (23). Nevertheless, their role in the regulation of spatial patterning of WUS protein is still unclear. Therefore, we investigated the role of these C-terminal domains in spatial patterning of WUS protein by generating individual deletions of each of the three domains, which resulted in destabilization (Fig. S1 A–L), except for a few cells of the RM that revealed a faint nuclear signal in the case of WUS-box domain deletion (Fig. S1H) and acidic region deletion (Fig. S1L). These results suggest that deletion of any one of the C-terminal domains exposes the signatures necessary for destabilization of WUS.
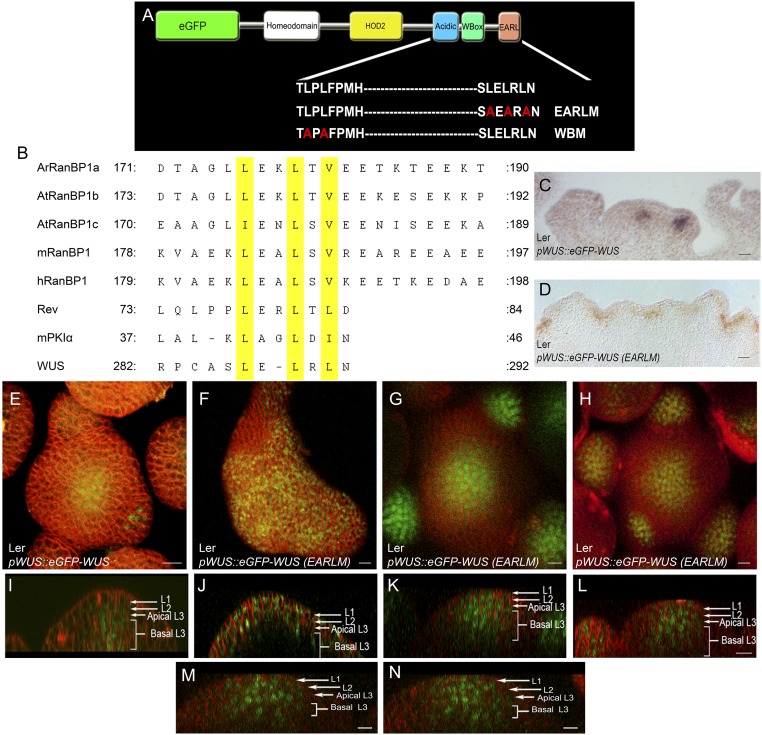
Because deletion of the three WUS domains destabilized the protein, we introduced point mutations in the WUS-box and the EAR-like domain (Fig. S2A). An earlier study has shown that the WUS-box contains two conserved amino acids at invariant positions (L255 and L257) in WOX family members (22). The L255A and L257A substitutions have been shown to abolish the biological functions of WUS, including the transcriptional regulation of the putative direct targets regulated by WUS: CLV3, ARABIDOPSIS RESPONSE REGULATOR 5 (ARR5), ARR6, and AGAMOUS (22). The expression of eGFP-WUS carrying these same point mutations in the WUS-box (referred to as WBM) from the pWUS led to a dramatic nonnuclear accumulation of WUS (Fig. 2 M–O) (n = 13). These results suggested that WUS-box is required for either nuclear import or nuclear retention. The EAR-like domain, which has been shown to contribute to the transcriptional activity of WUS (22), also resembles nuclear export signals (NESs), which are characterized by the presence of a short patch of hydrophobic amino acids (Fig. S2B) (24). Substituting hydrophobic leucine residues for alanine (L>A) has been shown to prevent nuclear exclusion (24). The expression of eGFP-WUS protein with L>A substitutions (L287A, L289A, and L291A) from the WUS promoter [pWUS::eGFP-WUS (EARLM)] led to a relatively higher level of WUS protein in the L1 and the L2 layers, and the protein was detected in a wider region in all cell layers of the inflorescence meristems (Fig. 2 D–F and Fig. S2 M and N) (n = 22) compared with WT (Fig. 2 A–C and Fig. S2 E and I).
Fig. S2.
The EAR-like domain influences nuclear accumulation of WUS. (A) A schematic of WUS protein domains showing amino acid sequences of the WT, the mutant WUS-box domain, and the mutant EAR-like domain (EARLM). Amino acid substitutions are shown in red. (B) Sequence alignment of nuclear export signals (NESs) of RAN binding proteins (RanBPs) from Arabidopsis, mouse, human, HIV1 protein Rev, and heat stable inhibitor of cAPK (mPKIα) along with the EAR-like domain of WUS. The leucine residues are highlighted in yellow. RNA in situ with WUS anti-sense probe in (C) pWUS::eGFP-WUS and (D) one of the enlarged IMs of pWUS::eGFP-WUS (EARLM). E and I are the 3D top and side views of IM expressing pWUS::eGFP-WUS. (F–H) and (J–L) are the 3D top and side views of three independent IMs expressing pWUS::eGFP-WUS (EARLM). M and N are side views of different sections of an IM expressing pWUS::eGFP-WUS (EARLM). The cell layers in SAMs are indicated by white arrows and brackets. eGFP-WUS (green) and FM4-64 (red). (Scale bars: 10 μm, except F, 15 μm and D, 30 μm.)
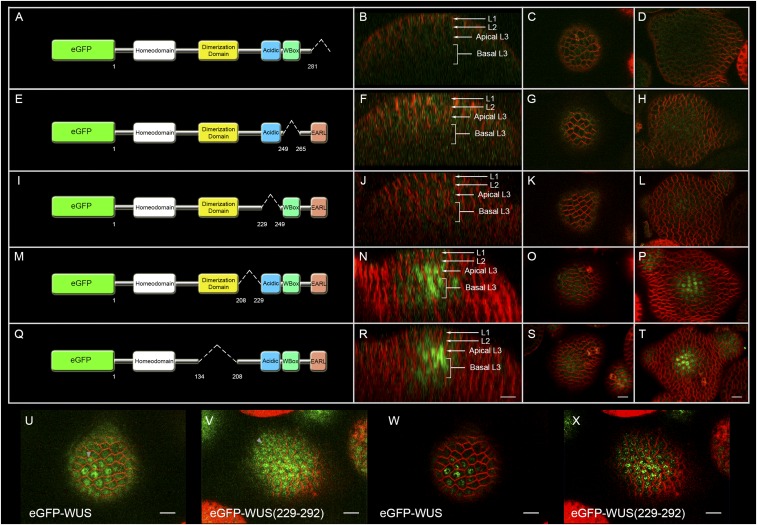
Fig. 2.
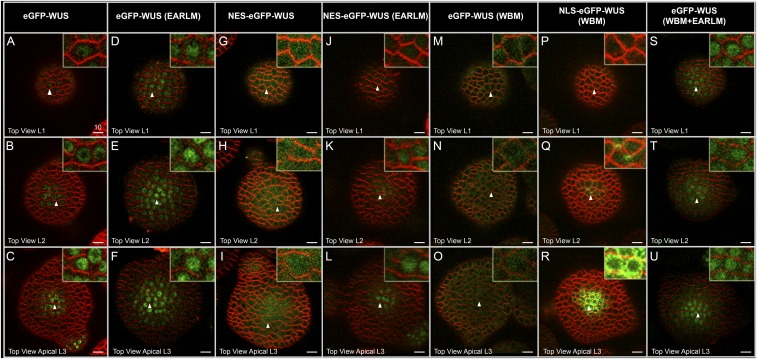
The transcriptional regulatory domains influence the subcellular localization of WUS. Horizontal rows represent (A, D, G, J, M, P, and S) L1 layers, (B, E, H, K, N, Q, and T) L2 layers, and (C, F, I, L, O, R, and U) apical L3 layers of IMs expressing various constructs: (A–C) eGFP-WUS, (D–F) eGFP-WUS (EARLM), (G–I) NES-eGFP-WUS, (J–L) NES-eGFP-WUS (EARLM), (M–O) eGFP-WUS (WBM), (P–R) NLS-eGFP-WUS (WBM), and (S–U) eGFP-WUS (WBM+EARLM). All constructs are expressed from the pWUS. Insets on each panel show a higher magnification (3×) view of the region identified by the white arrowheads. (Scale bars: 10 µm.)
Of the 48 T1 plants that expressed pWUS::eGFP-WUS(EARLM), only 6 of them showed variable enlargement of inflorescence meristems (Fig. S2 F–H and J–L). The RNA in situ analysis, using a WUS antisense probe on enlarged inflorescence meristems, revealed the expansion of the WUS domain (Fig. S2 C and D) in inner cell layers, which ruled out misexpression of the WUS promoter in the outer cell layer. None of the WBM and EARLM double mutant plants developed larger inflorescence meristems, which is consistent with the requirement of the WUS-box in the regulation of transcriptional activity (22).
Because we observed nonnuclear accumulation of the WBM, we hypothesized that the opposing activities of the WUS-box and EAR-like domains might determine nuclear levels of WUS. To test this hypothesis, we expressed a double mutant of the WBM and EARLM from the pWUS, which resulted in nuclear accumulation and resembled the WUS distribution pattern observed in single EARLM (Fig. 2 S–U) (n = 12). Taken together, these results suggest that WBM can translocate into the nucleus but that it cannot be retained in the nucleus in the presence of a functional EAR-like domain, thus implicating the WUS-box in nuclear retention and the EAR-like domain in nuclear export.
Addition of an Exogenous Nuclear Localization Signal or Nuclear Exclusion Signals Supports the Roles of WUS-Box and EAR-Like Domains in Nuclear-Cytoplasmic Partitioning.
The nonnuclear accumulation of the WBM may be either due to a lack of protein import into the nucleus or due to a lack of retention of the protein in the nucleus. To further investigate whether the WUS-box functions in either nuclear retention or nuclear import, we expressed a WBM fused to a strong foreign nuclear localization signal (NLS) from the WUS promoter. An earlier study has shown that addition of a strong NLS resulted in nuclear enrichment and limited protein mobility into the L1 layer (4). The WBM that contained a strong NLS was not detected in the nucleus (Fig. 2 P–R) (n = 6), which supports that WBM is required for nuclear retention and not for nuclear import. The protein also failed to move into the L1 layer (Fig. 2P); this restricted distribution may be due to the efficient cycling of the protein into and out of the nucleus, which could limit the cytosolic pool.
To test further whether the EAR-like domain functions as a nuclear export signal (NES), we examined whether the EARLM can offset the nuclear export mediated by exogenous NES. The addition of an exogenous NES to WUS led to nonnuclear accumulation (Fig. 2 G–I) (n = 24). Conversely, the introduction of the EARLM into WUS carrying exogenous NES led to a nuclear accumulation of WUS in the L2 and L3 cell layers (Fig. 2 J–L) (n = 12), further supporting that the EAR-like domain may participate in mediating nuclear export. The EARLM carrying the NES was not detected in the nuclei of the L1 layer cells. Perhaps a relatively higher nuclear export activity in these cells might have prevented nuclear accumulation of the protein.
In summary, our results show that the WUS-box is necessary for nuclear retention and that the EAR-like domain is required for nuclear export. These results are not in agreement with an earlier study, which found that mutations in either the WUS-box or EAR-like domains did not significantly influence WUS protein distribution (17). Perhaps the C-terminal GFP fusion used in the earlier study might have masked the nuclear export activity of EAR-like domain, which is located at the very end of the C terminus. Masking of the EAR-like domain function, in turn, might have prevented the nonnuclear accumulation of the WUS-box mutant protein, which is consistent with our results showing that double mutants of the WUS-box and EAR-like domains resemble single EAR-like mutants. Taken together, these results show that the transcriptional regulatory domains also participate in nuclear-cytoplasmic partitioning.
DNA Binding and Homodimerization Restrict WUS Protein from Excessive Spreading.
The analysis presented in the previous section reveals that the C-terminal stretch of WUS contains necessary information for differential accumulation of WUS protein. Therefore, the C-terminal GFP fusion to WUS used in an earlier study might have produced a stable form (17). The use of the shorter form [WUS (amino acids 100 to 249)] of such a stable protein that can diffuse farther might have resulted in meristem overproliferation, despite lacking the HAM binding domain. To understand precisely the role of homodimerization in WUS function, protein mobility, and spatial patterning, and the role of the HAM binding domain in spatial patterning of WUS protein, we revisited the structure-function analysis of WUS.
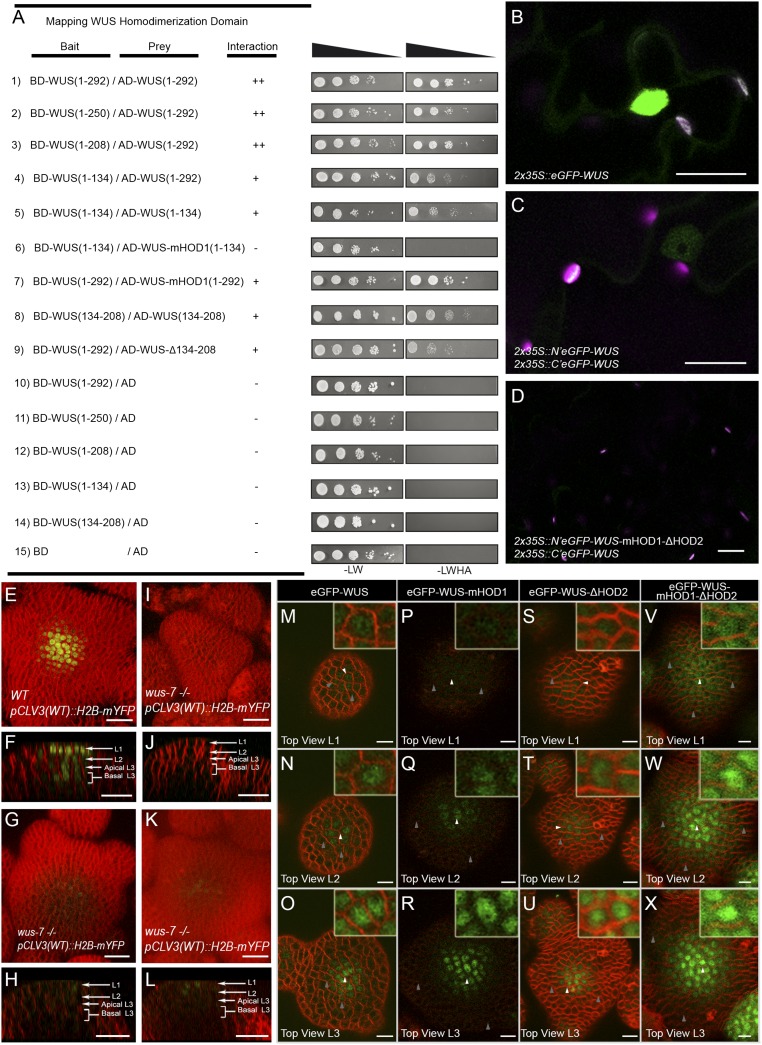
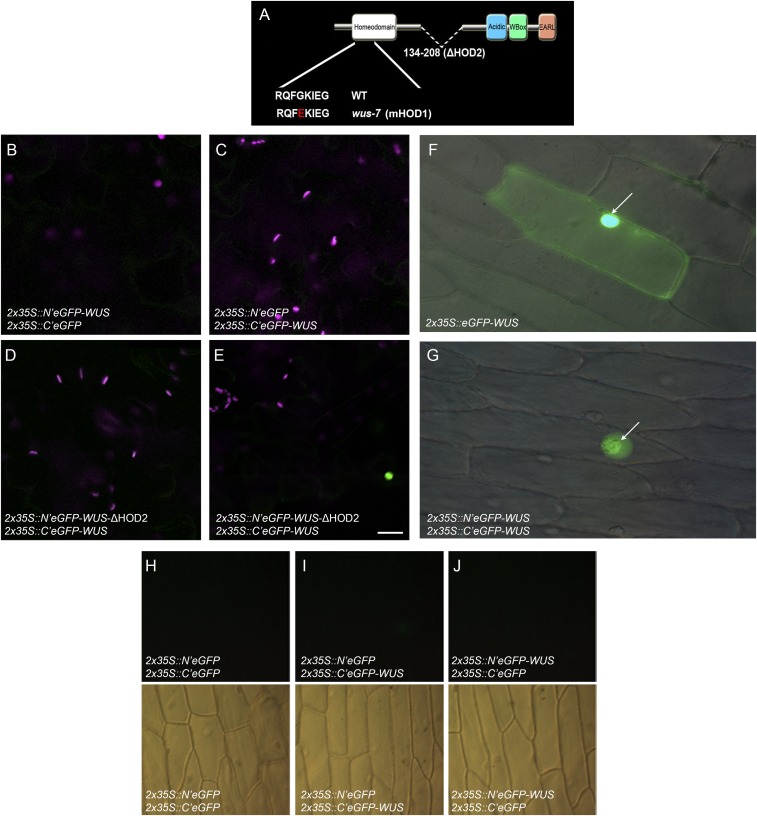
Our yeast two-hybrid (Y2H) analysis revealed two homodimerization contact points in WUS: a DNA binding homeodomain located at the N terminus and a 74-aa stretch (amino acids 134 to 208) in the central part of the protein (referred to as HOD2) (Fig. 3A and Fig. S3A). An earlier study isolated a wus-7 allele, which carries a missense mutation (G77E), in the loop that connects the second and third alpha helices of the homeodomain (25). The same point mutation (G77E) was introduced into the truncated WUS lacking the HOD2 (amino acids 1 to 134), which failed to homodimerize in Y2H assays, revealing the homodimerization residue in the DNA binding domain, referred to as HOD1 (Fig. 3A). The bimolecular fluorescence complementation (BiFC) assay, in Nicotiana benthamiana leaves and in onion epidermal cells, with the WT WUS protein, revealed a higher signal in nuclei than in the cytoplasm (Fig. 3 B–D and Fig. S3 B–J). The deletion of HOD2 alone failed to produce a fluorescence signal in 59 of the 60 cells tested (Fig. S3 D and E). The HOD1 mutation in the context of HOD2 deletion failed to produce a fluorescence signal (Fig. 3D) (n = 60). Taken together, these results show that both HOD1 and HOD2 are required for homodimerization.
Fig. 3.
The DNA binding and dimerization of WUS restrict its spatial localization. Mapping the homodimerization domains of WUS using a yeast two-hybrid analysis (A). The break points of WUS deletions are indicated by the amino acid positions. +, stronger interaction; −, no interaction. Expression of (B) 35S::eGFP-WUS and (C) BiFC signal of WT and (D) the double homodimerization mutant in N. benthamiana leaves. Shown are 3D top views of IMs expressing WT pCLV3 (H2B-mYFP) in (E) WT and (I, G, and K) wus-7 mutant plants. A side view of E is shown in F. Side views of I, G, and K are shown in J, H, and L, respectively. H2B-mYFP images in (G, H, K, and L) were taken at three times laser intensity used in E, F, I, and J. Horizontal rows represent (M, P, S, and V) the L1 layers, (N, Q, T, and W) the L2 layers, and (O, R, U, and X) the apical L3 layers of IMs expressing (M–O) eGFP-WUS, (P–R) eGFP-WUS-mHOD1 (G77E), (S–U) eGFP-WUS-ΔHOD2 (Δ amino acid 134 to amino acid 208), and (V–X) eGFP-WUS-mHOD1(G77E)-ΔHOD2 (Δ amino acid 134 to amino acid 208) from pWUS. Insets on each panel show a higher magnification (3×) view of region identified by the white arrowheads. WUS protein detected in a broader domain is indicated by gray arrows. H2B-mYFP (yellow), eGFP (green), and FM4-64 (red). (Scale bars: B–L, 20 µm; M–X, 10 µm.)
Fig. S3.
WUS contains two homodimerization domains. (A) A schematic of WUS protein domains showing amino acid sequences of the WT, the mutant HOD1 (G77E), a single amino acid mutated in wus-7 allele, and the deletion of HOD2 (Δ amino acid 134 to amino acid 208). Amino acid sequence is shown, and substitutions are shown in red. (B and C) Negative controls of BiFC in transfected Nicotiana benthamiana leaves. (D and E) Independent transfections carrying BiFC constructs N′eGFP-WUS-ΔHOD2 (Δ amino acid 134 to amino acid 208), which produce nuclear signal only once (E) in 60-cell analysis. Green signal is from GFP fluorescence whereas magenta is chlorophyll autofluorescence. Expression of (F) 35S::eGFP-WUS and (G) BiFC signal in onion epidermal cell. The white arrows point to the nuclei. (H–J) The negative controls for BiFC experiments. (H) (N′eGFP and C′eGFP), (I) (N′eGFP and C′eGFP-WUS), (J) (N′eGFP-WUS and C′eGFP). (Upper) Fluorescence images. (Lower) Differential interference contrast (DIC) images. All constructs were expressed from the 2X35S promoter. (Scale bar: 20 μm.)
Because HOD1 resides within the homeodomain, we tested whether it is also necessary for DNA binding. The analysis presented in the accompanying manuscript (20) shows that HOD1 is also required for DNA binding. Consistent with the compromised DNA binding ability of HOD1, wus-7 homozygous mutants failed to activate pCLV3::H2B-mYFP expression at levels comparable with the WT (Fig. 3 E–L) (n = 21). The expression of WUS forms carrying deletions in HOD2 from the WUS promoter in wus-1, a strong loss-of-function allele, resulted in plants with much smaller inflorescence meristems and incomplete flowers (Fig. S4 A–C) (n = 18). Remarkably, the expression of WUS carrying the mutated HOD1 residue that also lacked the HOD2 failed to rescue wus-1 mutant phenotypes (Fig. S4A). In summary, these results show that both HOD1 and HOD2 mediate homodimerization and that HOD1 also participates in DNA binding. These results also show that homodimerization is critical for WUS function in promoting meristem maintenance.
Fig. S4.
The two homodimerization domains are required for WUS function. (A) pWUS::WUS-ΔHOD2 (Δ amino acid 134 to amino acid 208) in wus-1 homozygous mutants showing partially rescued and partial flowers devoid of carpels. n = 18 (wus-1 homozygous plants derived from four independent lines). pWUS::WUS-mHOD1 (G77E)-ΔHOD2 (Δ amino acid 134 to amino acid 208) in wus-1 homozygous mutants showing lack of rescue (14 wus-1 homozygous plants were identified from six independent transgenic lines). White arrowheads indicate lack of auxiliary SAM development, and gray arrowheads indicates empty peduncles of partially rescued flowers. (Lower) Higher magnification images of flowers of each genotype indicated above. (B) A 3D reconstructed top view of a WT IM. (C) A 3D reconstructed top view of an IM of wus-1 carrying pWUS::WUS-ΔHOD2 (Δ amino acid 134 to amino acid 208), showing extremely small meristem. FM4-64 (red). (Scale bar: 10 μm.)
In light of these results, it is conceivable that Daum et al., despite using WUS lacking the HOD2 and the HAM binding region, were able to observe stem cell overproliferation because of the combined effects of improved protein stability caused by the defective C-terminal fusion and the higher mobility associated with the shorter form of the protein (17). Our work also shows that, at higher WUS levels, HOD1 alone is sufficient to mediate homodimerization (20). The redundant function of HOD1 in promoting homodimerization may explain the inflorescence meristem overproliferation observed in the earlier study (17), despite the use of a WUS form that lacked HOD2. Thus, our work shows that homodimerization of WUS is necessary to promote meristem growth rather than to restrict it, as suggested by an earlier study (17). Because the deletion used in that previous study (17) also included the HAM binding region, our work also suggests that the requirement of HAM binding may become dispensable at higher WUS levels, which needs to be tested in future studies with the WT WUS protein.
The discovery of homodimerization domain HOD1 and a better demarcation of HOD2 led us to investigate the individual and combined roles of both HOD domains on the spatial distribution of the WUS protein. The eGFP-WUS-mHOD1 mutant protein expressed from the WUS promoter was detected in a broader domain in the L2 and L3 layers, and it was relatively delocalized from the nucleus (Fig. 3 P–R) compared with the WT protein (Fig. 3 M–O), showing that DNA binding or homodimerization restricts spatial distribution and is also required for proper nuclear accumulation in the L1 and L2 cell layers. The eGFP-WUS-ΔHOD2 mutant protein expressed from the WUS promoter was delocalized from the nucleus, although to a lesser extent than the mHOD1, whereas no significant change in spatial distribution was observed (Fig. 3 S–U and Fig. S1 Q–T). The expression of eGFP fused to WUS carrying the HOD1 mutation that also lacked HOD2 was detected in a much broader domain and also accumulated at higher levels, and the protein was relatively delocalized from the nucleus compared with the WT protein, particularly in the L1 layer (Fig. 3 V–X). Interestingly, the expression of eGFP fused to a WUS deletion (amino acids 208 to 229), a region largely responsible for binding HAM proteins, did not significantly affect WUS protein localization (Fig. S1 M–P). Taken together, these results show that both DNA binding and homodimerization limit spatial distribution of WUS, presumably by restricting mobility and also by retaining the protein in the nucleus.
Ectopic Overexpression of WUS Leads to WUS Protein Destabilization.
Our results, thus far, show that a combination of mechanisms involving DNA binding and homodimerization, along with the C-terminally encoded nuclear-cytoplasmic partitioning domains, determine the spatial limits and observed variations in nuclear levels of WUS protein. Nevertheless, the variations in WUS protein levels may also be due to a reduction of protein accumulation caused perhaps by the targeted destabilization in cells located outside the rib meristem. To test this hypothesis, we first expressed eGFP-WUS from a central zone-specific promoter, which led to lower levels of the protein than the eGFP-WUS expressed from the WUS promoter (20). This result shows that ectopic overexpression of WUS in the central zone does not result in higher protein levels, suggesting a destabilization of WUS.
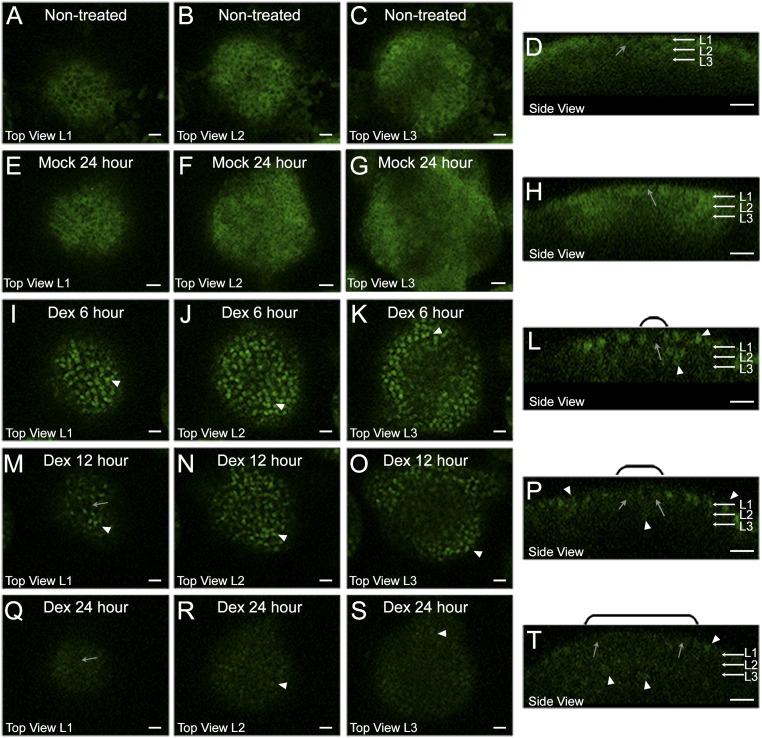
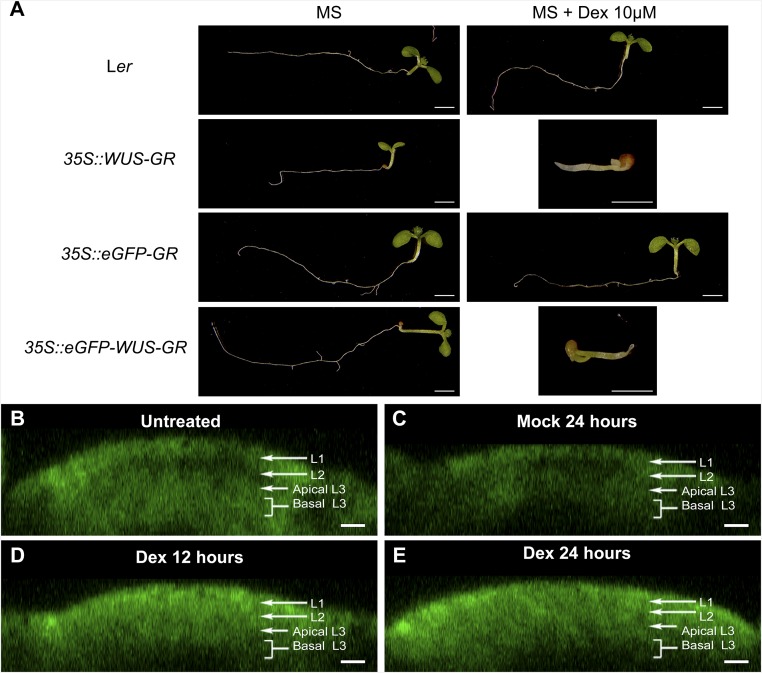
The destabilization of WUS protein observed upon misexpression of eGFP-WUS in the central zone could also be due to an indirect consequence of meristem overproliferation (10, 11, 20). To exclude such a possibility, we transiently overexpressed WUS, using a dexamethasone (Dex)-inducible form of WUS protein where eGFP-WUS was fused with the hormone binding domain of the rat glucocorticoid receptor (GR). The GR fusion allows visualization of the protein fate before and immediately after the Dex-induced release of WUS from the HSP-90–mediated cytoplasmic sequestration and subsequent translocation of the protein into the nucleus. The eGFP-WUS-GR expressed from the ubiquitous promoter (35S::eGFP-WUS-GR), before the Dex treatment, was detected mostly uniformly in the cytoplasm of all cells (Fig. 4 A–D) (n = 4). Upon 6 h of Dex treatment, the protein was detected in the nucleus in all cells, except for a few cells of the central zone, which accumulated relatively lower levels of the protein (Fig. 4 I–L). The region of lower protein accumulation expanded radially within 12 h of Dex treatment (Fig. 4 M–P). Within 24 h of Dex treatment, the protein was undetectable in cells located in the central part of the inflorescence meristem, except for a few cells that were located in the lateral edge of the peripheral zone and in the rib meristem (Fig. 4 Q–T). Conversely, mock treatment did not alter subcellular localization or levels of eGFP-WUS-GR (Fig. 4 E–H). A similar time course experiment with the 35S::eGFP-GR (Fig. S5 B–E) (n = 4), lacking the coding sequences of the WUS protein, revealed the stable nuclear accumulation of the protein upon Dex treatment, showing that the protein destabilization is specific to WUS. As seen with the 35S::WUS-GR–expressing seedlings, the 35S::eGFP-WUS-GR seedlings also failed to develop on Dex-containing plates and activated CLV3 (20), upon Dex application, confirming its functionality (Fig. S5A). Taken together, these results show that ectopic induction of WUS activity leads to instantaneous destabilization of WUS protein.
Fig. 4.
Ectopic overexpression of WUS destabilizes WUS protein. Vertical columns represent (A, E, I, M, and Q) the L1 layers, (B, F, J, N, and R) the L2 layers, (C, G, K, O, and S) the apical L3 layers, and (D, H, L, P, and T) the side views of inflorescence meristems expressing 35S::eGFP-WUS-GR. Shown are (A–D) untreated and (E–H) 24 h mock treatment. Dex treatment for (I–L) 6 h, (M–P) 12 h, and (Q–T) 24 h. White arrowheads indicate high nuclear levels, and gray arrows indicate cells with low nuclear accumulation. The domains of low nuclear accumulation, shown in side views, are noted with black brackets. Green channel is enhanced in all images to increase visibility of the protein. eGFP-WUS-GR (green) and FM4-64 (red). (Scale bars 15 µm.)
Fig. S5.
Characterization of a Dex-inducible eGFP-WUS system. (A, Left) Genotypes of seedlings shown in A: Ler, 35S::WUS-GR, 35S::eGFP-GR, and 35S::eGFP-WUS-GR. (A, Middle and Right) Representative seedling images that were grown on MS media without and with Dex, respectively. (Scale bars: 1 mm.) (B–E) Side views of IMs showing expression of untreated 35S::eGFP-GR (B), mock-treated (C), treated with Dex for 12 h (D), and treated with Dex for 24 h (E). eGFP-GR (green). The cell layers in SAMs are indicated by white arrows and brackets. (Scale bar: 10 µm.)
Discussion
The regulation of WUS protein levels is critical for maintaining the central zone identity and for the regulation of meristem proliferation (3, 5, 10, 11). Our work shows that multiple processes control nuclear levels and the spatial pattern of WUS protein accumulation in inflorescence meristems (Fig. 5 A and B). Both binding of WUS to DNA and homodimerization may sequester WUS. Our analysis reveals that DNA promotes homodimerization of WUS (20). Therefore, DNA-mediated sequestration may reduce the free pool of WUS that is available for nuclear export into the cytosol and subsequent migration into adjacent cells, thus influencing the spatial distribution of WUS protein (Fig. 5).
Fig. 5.
A sketch illustrating the control of WUSCHEL levels and spatial patterning in IMs. (A) A side view of the IM showing the spatial pattern of eGFP-WUS expressed from the WUS promoter. White arrows show different cell layers. eGFP (green) and FM4-64 (red). (Scale bar: 10 μm.) (B) The function of individual domains of WUS protein as inferred from the structure-function analysis and the ectopic overexpression of WUS. The functions of individual domains are depicted on WUS protein. The numbers indicate position of amino acids, starting with the N terminus. (C) A sketch illustrating the maintenance of WUS levels and spatial patterning, through DNA-dependent homodimerization, nuclear-cytoplasmic partitioning, and WUS protein destabilization represented on the inflorescence meristem cell layers. WUS is synthesized in a few cells of the rib meristem and migrates into adjacent cells, where it accumulates at a lower level in the nuclei of cells in the L1 and the L2 layers compared with the inner layers. Our analysis suggests a relatively higher nuclear export (indicated by the strength of magenta colored arrows) and a lower nuclear retention of WUS, which could be due to the lower affinity to the DNA (indicated by the strength of the white arrows) and a lower dimerization propensity (indicated by the strength of the white arrows with black outline) in the top layers than in the inner layers. WUS could be destabilized in outer layers (indicated by red arrows). We hypothesize that this destabilizing factor may be WUS-dependent (represented by the blue dashed arrows), which needs to be tested in future studies.
Our results assign functions for the EAR-like domain in mediating nuclear export and for the WUS-box in mediating nuclear retention. Earlier studies have shown that the WUS-box is both necessary and sufficient to mediate transcriptional repression activity of WUS (22). The WUS-box has been shown to be critical for the interaction of WUS with TOPLESS (TPL) and TOPLESSRELATED (TPR) proteins (21). The TPL and TPR proteins have been shown to interact with HISTONE DEACETYLASE19 to form a transcriptional repression complex (26). Our work showing the requirement of the WUS-box for nuclear retention suggests that it could tether WUS to chromatin by assembling a repressor complex (Fig. 5C). Perhaps the WUS pool that is not part of the repressor complex may be exported out of the nucleus by the hypothetical regulators that use the EAR-like domain. Our results also show that the EARLM was able to migrate into adjacent cells, which suggests that the nuclear export within the rib meristem was not affected to an extent that it could trap the mutant protein. Therefore, the nuclear export machinery may be highly active in cells that are located outside the rib meristem. In addition, the nuclear retention may be relatively weaker in cells located outside the rib meristem because our work shows that the WUS that fails to bind DNA and homodimerize was more highly nonnuclear in the L1 layer than in inner layers (Fig. 3 P–R and V–X). Therefore, a combination of potent nuclear export and a weaker nuclear retention may decrease WUS levels in the nuclei of cells located outside the rib meristem (Fig. 5C).
The destabilization of WUS, observed upon ectopic overexpression, suggests an additional layer of control in maintaining different WUS protein levels between the rib meristem and the adjacent regions. Moreover, the destabilization of WUS observed upon its nuclear translocation suggests a possibility of self-destabilization. The variability observed in the accumulation of EARLM of WUS in different transgenic lines (Fig. S2 E–N) could be due to self-destabilization, which might depend on WUS levels. However, a transient analysis involving the transcriptionally inactive versions and mutant versions that alter subcellular distribution of WUS is required to test whether WUS destabilizes itself and the importance of WUS concentration in self-destabilization. The instability that has been observed upon deletion of each of the acidic domain, the EAR-like domain, and the WUS-box suggests that this region may fold as a single module that can either engage or disengage with destabilization machinery. Taken together, our work shows that the transcriptional regulatory domains also influence the processes that regulate nuclear levels as well as the spatial patterning of WUS protein (Fig. 5 B and C). We find that WUS activates the transcription of CLV3 at a lower level and represses at a higher level by binding the same cis-elements as monomers and as dimers, respectively (20). The utilization of transcriptional regulatory domains in fine tuning the nuclear levels and the spatial pattern of WUS accumulation allows a cross-talk between regulation of protein concentration and transcriptional regulation, which could lead to the spatiotemporal control of gene expression.
Experimental Procedures
Plant Growth, Genotypes, and Microscopy.
Arabidopsis plant growth procedures for imaging and phenotypic analysis were followed as described in earlier studies (4, 11). All transgenic lines were generated in Landsberg erecta (Ler) background. Both wus-7 (25) and wus-1 have been previously described (2). For 35S::eGFP-WUS-GR and 35S::eGFP-GR analysis on Dex plates, seedlings were germinated on MS plates for 7 d containing 10 μM Dex. Preparations for imaging, Dex treatment, the optics, microscopy platform (510 LSM; Zeiss), image acquisition, and image reconstruction have been described in earlier studies (4, 11). Plants were germinated on MS-agar plates and allowed to grow for 10 d before they were transferred into clear plastic boxes containing MS-agar. For single time point observations, plants were grown on soil. Upon bolting, when the shoot apex emerged out of the rosette, the plants were prepared for time-lapse imaging. The MS-agar surface was overlaid with 1% (wt/vol) agarose to minimize contamination. The older floral buds were removed to expose SAMs. The rosette was stabilized by applying 1.5% (wt/vol) molten agarose onto the stem. FM4-64 (50 μg/mL) was applied directly onto the shoot meristems 30 min before imaging. eGFP-tagged constructs were imaged with 488 nm excitation, and emission was collected between 500 nm and 550 nm by using a Zeiss 510 confocal microscope.
Plasmid Construct and Generation of Transgenic Lines.
Deletions, mutations, and addition of an extra NES coding sequence, identified previously as a potent nuclear export signal in Rev protein from HIV-1 (24), in eGFP-WUS were generated by inverse PCR using 5′ phosphorylated oligos listed in Table S1, using eGFP-WUS in a PCR4 vector as template (4). All mutations, deletions, and WUS-containing exogenous NLSs and NESs were introduced into the WUS promoter in pCAMBIA2300 (4). To generate the CLV3::LhG4, 6XOP::eGFP-WUS, the coding sequences of eGFP-WUS were cloned into the pENTR vector (Invitrogen). The recombination reaction was carried out with the Gateway 6XOP pzp222 binary vector to create 6XOP::eGFP-WUS. Twenty independent transgenic lines carrying these constructs were crossed to the driver lines CLV3::LhG4 used in an earlier study (11). The constructs 35S::eGFP-WUS-GR and 35S::eGFP-GR were generated by adding the GR sequence to the eGFP-WUS in the PCR4 vector. Then, the eGFP-WUS-GR and eGFP-GR were cloned in the pENTR cloning vector. Finally, LR reaction was carried out with the pMDC32 binary vector containing the 2X35S promoter (27). The number of independent transgenic lines analyzed for each transgene is shown as “n” in parentheses at appropriate places in the paper.
Table S1.
Primers and probes
| Construct | Primer name | Sequence |
| eGFP-WUS deletion and mutation for structure-function analyses | ||
| eGFP-NES-WUS | NES-WUS-Fw | AACGATTAACCCTCGATATGGAGCCGCCACAGCATCAGC |
| NES-WUS-Rev | CTAATGGTGGTAATTGTAAGGATCCCTTGTACAGCTCGTCC | |
| Free eGFP and free NLS-eGFP | DWUS-Fw | TGAACTAGGCCTGCAAGGGCG |
| DWUS-Rev | GGATCCCTTGTACAGCTCGTC | |
| eGFP–WUS (1-134) | SP/ WUS1/1-1147 | CTGGCGCGCCATGGTGAGCAAGGGCGAGGAGCTGTTCA |
| ASP/WUS1/1-1147 | CCAGGCCTTCAGGGAACACCGTGATGATGGTGAAGTAG | |
| eGFP–WUS (281-292) | DEARL-Fw | CTAGGCCTGCAAGGGCGAATTCGCG |
| DEARL-Rev | CGAACTTCCGATTGGCCATACTTCC | |
| eGFP–WUS (249-265) | DWB-Fw | CACATCAACGGTGGTAGTGGTGCCATC |
| DWB-Rev | TTCCAGATAAGCATCGCCACCACATTC | |
| eGFP–WUS (230-246) | PEST Frw | GCTTATCTGGAACATCGACGTACGC |
| PEST rev lg | CTTTGCTCTATCGAAGAAGTTGTAAGG | |
| eGFP–WUS (208-227) | IFF-PEST-1 forward | GCAAAGCCTCTGTTTGGTCTAGAAGG |
| IFF PEST1 rev | ACCTACGTTGTTGTAATTCATAGAACAG | |
| eGFP–WUS (134-208) | 5′pIFF-F | GGATGGGCAAACATGGATCATCATTAC |
| 5′pIFF-R | GGGAACACCGTGATGATGGTGAAG | |
| EARLM | EARLM-Fw | TTCTGCTGAGGCACGTGCGAACTAG |
| EARLM-Rev | GCGCAAGGGCGAACTTCCGATTGGCC | |
| WBM | WBM-Fw | CGACGTACGGCTCCTGCCTTCCCTATGCAC |
| WBM-Rev | ATGTTCCAGATAAGCATCGCCACCACATTC | |
| BiFC cloning | ||
| pENTR cloning | N′eGFP-Fw-GW | CACCATGGTGAGCAAGGGCGAGGAGCTGTTC |
| C′eGFP-Fw-GW | CACCATGGACAAGCAGAAGAACGGCATCAAG | |
| WUS-5′-Rev | CTAGTTCAGACGTAGCTCAAGAGAAGC | |
| Yeast two hybrid | ||
| 134-ECORI | 134 Rev | CAATTCCGTGATGATGGTGAAGTAGAGGATG |
| 208-ECORI | 208 rev | CTGGAATTCCTATGCCCATCCTCCACCTACGTTG |
| 250-ECORI | 250 rev | GAATTCCTATTCCAGATAAGCATCGCCACCAC |
| FL-ECORI | FL REV | CTGGAATTCCTAGTTCAGACGTAGCTCAAGAG |
| 134-NDE-F | 134 forward | CAG CATATG CCCATGCAGAGACCTGCTAATT CCG |
| Cloning of WUS deletion and mutation in pET-28a for protein expression in Escherichia coli | ||
| WUS-NDE | Forward | CATATGATGGAGCCGCCACAGCATCA |
| WUS-FL-XHO | REVERSE | CTCGAGCTAGTTCAGACGTAGCTCA |
| WUS-208-XHO | REVERSE | CTCGAGTCCACCTACGTTGTTGTAATTCATAG |
| WUS-134-XHO | REVERSE | CTCGAGCGTGATGATGGTGAAGTAGAGGATG |
| WUS-7M | WUS-7M-FW | GAGACAGTTCGAAAAGATTGAGG |
| WUS-7M-REV | AGCCTTGCAGTGATCTTC | |
| Expressing eGFP-WUS in L2 layer | ||
| pHDG4 | SP/At4g17710/1–3115 | AAGGTACCGGATCCTGATTAGAGCAATTAGCCCGT |
| ASP/At4g17710/1–3115 | ATGGCGCGCCAGACAAAGAGAAGACTGAGTTTAAA |
Yeast Two-Hybrid Interaction.
A Gal4-based two-hybrid system was used in this study. A WUS coding sequence was used as a template to generate truncations, deletions, and mutant versions using the oligonucleotides listed in Table S1. All WUS fragments were cloned in yeast two-hybrid vectors pGBKT7 and pGADT7 (Clontech) and cotransformed into yeast two-hybrid strain AH109 (Clontech) using standard procedures. Transformants were selected on solid synthetic drop-out (SD) medium that lacked both leucine and tryptophan, (SD-Leu-Trp). WUS–WUS protein interaction was tested on solid SD medium that lacked leucine, tryptophan, histidine, and adenine (SD-Leu-Trp-His-Ade). To perform a semiquantitative growth assay, suspensions of each cotransformed yeast cell were prepared at standardized densities. Serial dilutions (10−1, 10−2, 10−3 and 10−4) were made and spotted onto solid selective media: SD-Leu-Trp and SD-Leu-Trp-His-Ade.
Bimolecular Fluorescence Complementation.
For BiFC analyses in N. benthamiana cells, the N-terminal fragment of eGFP (N-eGFP) from nucleotides 1 to 465 (amino acids 1 to 155) and the C-terminal fragment of eGFP (C-eGFP) from nucleotides 466 to 723 (amino acids 156 to 241) were fused to a WUS coding sequence and cloned into pENTR, along with N-eGFP and C-eGFP fragments that were used as negative controls. They were then recombined with the destination vector pMDC32 under the control of a 2X35S promoter. Mutations for homodimerization were introduced with primers from Table S1. Agrobacterium tumefaciens GV3101 transformed with WUS BiFC constructs was introduced in leaves of 3- to 4-wk-old Nicotiana by agroinfiltration. In all cases, agrobacterium transformed with the corresponding two BiFC plasmids along with the p19 suppressor of silencing was introduced into N. benthamiana leaves in a 1:1:1 ratio. The fluorescence was monitored by using an inverted Leica TCS-SP5 confocal system.
RNA in Situ Analysis.
Tissue preparation, sectioning of plants for RNA in situ analysis, probe synthesis, hybridization, and detection were performed as described earlier (http://www.its.caltech.edu/∼plantlab/protocols/insitu.htm). The WUS probes used in this study have been described in an earlier study (28).
Acknowledgments
We thank Thomas Laux, University of Freiburg, for sharing driver lines and wus mutants; Jacqueline Le for supporting experimental work; and members of the G.V.R. laboratory for comments on the manuscript. This work was supported by NSF Grant IOS-1456725 (to G.V.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607673113/-/DCSupplemental.
References
- 1.Steeves TA, Sussex IM. Patterns in Plant Development: Shoot Apical Meristem Mutants of Arabidopsis thaliana. Cambridge Univ Press; New York: 1989. [Google Scholar]
- 2.Laux T, Mayer KF, Berger J, Jürgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122(1):87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Mayer KF, et al. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95(6):805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 4.Yadav RK, et al. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 2011;25(19):2025–2030. doi: 10.1101/gad.17258511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoof H, et al. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100(6):635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 6.Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89(4):575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science. 1999;283(5409):1911–1914. doi: 10.1126/science.283.5409.1911. [DOI] [PubMed] [Google Scholar]
- 8.Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science. 2000;289(5479):617–619. doi: 10.1126/science.289.5479.617. [DOI] [PubMed] [Google Scholar]
- 9.Kondo T, et al. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science. 2006;313(5788):845–848. doi: 10.1126/science.1128439. [DOI] [PubMed] [Google Scholar]
- 10.Brand U, Grünewald M, Hobe M, Simon R. Regulation of CLV3 expression by two homeobox genes in Arabidopsis. Plant Physiol. 2002;129(2):565–575. doi: 10.1104/pp.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav RK, Tavakkoli M, Reddy GV. WUSCHEL mediates stem cell homeostasis by regulating stem cell number and patterns of cell division and differentiation of stem cell progenitors. Development. 2010;137(21):3581–3589. doi: 10.1242/dev.054973. [DOI] [PubMed] [Google Scholar]
- 12.Müller R, Borghi L, Kwiatkowska D, Laufs P, Simon R. Dynamic and compensatory responses of Arabidopsis shoot and floral meristems to CLV3 signaling. Plant Cell. 2006;18(5):1188–1198. doi: 10.1105/tpc.105.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav RK, et al. Plant stem cell maintenance involves direct transcriptional repression of differentiation program. Mol Sys Biol. 2013;9(1):654. doi: 10.1038/msb.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers KW, Schier AF. Morphogen gradients: From generation to interpretation. Annu Rev Cell Dev Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 15.Crawford KM, Zambryski PC. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol. 2000;10(17):1032–1040. doi: 10.1016/s0960-9822(00)00657-6. [DOI] [PubMed] [Google Scholar]
- 16.Vatén A, et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21(6):1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Daum G, Medzihradszky A, Suzaki T, Lohmann JU. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc Natl Acad Sci USA. 2014;111(40):14619–14624. doi: 10.1073/pnas.1406446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulze S, Schäfer BN, Parizotto EA, Voinnet O, Theres K. LOST MERISTEMS genes regulate cell differentiation of central zone descendants in Arabidopsis shoot meristems. Plant J. 2010;64(4):668–678. doi: 10.1111/j.1365-313X.2010.04359.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, et al. Control of plant stem cell function by conserved interacting transcriptional regulators. Nature. 2015;517(7534):377–380. doi: 10.1038/nature13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perales M, et al. Threshold-dependent transcriptional discrimination underlies stem cell homeostasis. Proc Natl Acad Sci USA. 2016;113:E6298–E6306. doi: 10.1073/pnas.1607669113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieffer M, et al. Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell. 2006;18(3):560–573. doi: 10.1105/tpc.105.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell. 2009;21(11):3493–3505. doi: 10.1105/tpc.109.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolzblasz A, et al. Stem cell regulation by Arabidopsis WOX genes. Mol Plant. 2016;9(7):1028–1039. doi: 10.1016/j.molp.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Haasen D, Köhler C, Neuhaus G, Merkle T. Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear export signals in Arabidopsis thaliana. Plant J. 1999;20(6):695–705. doi: 10.1046/j.1365-313x.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 25.Graf P, et al. MGOUN1 encodes an Arabidopsis type IB DNA topoisomerase required in stem cell regulation and to maintain developmentally regulated gene silencing. Plant Cell. 2010;22(3):716–728. doi: 10.1105/tpc.109.068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szemenyei H, Hannon M, Long JA. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science. 2008;319(5868):1384–1386. doi: 10.1126/science.1151461. [DOI] [PubMed] [Google Scholar]
- 27.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133(2):462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy GV, Meyerowitz EM. Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science. 2005;310(5748):663–667. doi: 10.1126/science.1116261. [DOI] [PubMed] [Google Scholar]