Abstract
In the tropics, there are too few studies on isolation of Blastocystis sp. subtypes from water sources; in addition, there is also an absence of reported studies on the occurrence of Blastocystis sp. subtypes in water during different seasons. Therefore, this study was aimed to determine the occurrence of Blastocystis sp. subtypes in river water and other water sources that drained aboriginal vicinity of highly endemic intestinal parasitic infections during wet and dry seasons. Water samples were collected from six sampling points of Sungai Krau (K1–K6) and a point at Sungai Lompat (K7) and other water sources around the aboriginal villages. The water samples were collected during both seasons, wet and dry seasons. Filtration of the water samples were carried out using a flatbed membrane filtration system. The extracted DNA from concentrated water sediment was subjected to single round polymerase chain reaction and positive PCR products were subjected to sequencing. All samples were also subjected to filtration and cultured on membrane lactose glucuronide agar for the detection of faecal coliforms. During wet season, Blastocystis sp. ST1, ST2 and ST3 were detected in river water samples. Blastocystis sp. ST3 occurrence was sustained in the river water samples during dry season. However Blastocystis sp. ST1 and ST2 were absent during dry season. Water samples collected from various water sources showed contaminations of Blastocystis sp. ST1, ST2, ST3 and ST4, during wet season and Blastocystis sp. ST1, ST3, ST8 and ST10 during dry season. Water collected from all river sampling points during both seasons showed growth of Escherichia coli and Enterobacter aerogenes, indicating faecal contamination. In this study, Blastocystis sp. ST3 is suggested as the most robust and resistant subtype able to survive in any adverse environmental condition. Restriction and control of human and animal faecal contaminations to the river and other water sources shall prevent the transmission of Blastocystis sp. to humans and animals in this aboriginal community.
Keywords: Blastocystis sp., Water, Aboriginal settlements, Wet and dry seasons
Introduction
Blastocystis sp., a single-celled anaerobic enteroparasite inhabiting the lower gastrointestinal tract of humans and animals, has been reported to cause non-specific gastrointestinal symptoms (Souppart et al., 2010). The size of Blastocystis sp. is within the range of two known waterborne parasites viz, Giardia and Cryptosporidium (Suresh, Smith & Tan, 2005). Blastocystis sp. has been associated with two out of the 325 outbreaks (0.6%) of waterborne diseases caused by parasites worldwide (Karanis, Kourenti & Smith, 2007). In addition, Blastocystis sp. has been listed in the Water Sanitation and Health programmes of the World Health Organization and WHO Guidelines for Drinking-water Quality (World Health Organization, 2008; World Health Organization: microbial fact sheets, 2011).
Two studies in Malaysia have reported the occurrence of Blastocystis sp. in many water catchments, including recreational waters, rivers and lakes (Suresh, Tan & Illi, 2009; Ithoi et al., 2011). Absence of proper piped water supply was found to be a significant risk factor in the acquisition of Blastocystis sp. infection (Abdulsalam et al., 2012; Anuar et al., 2013). Drinking unboiled and untreated water have also been reported to be associated with Blastocystis infection (Leelayoova et al., 2004; Li et al., 2007).
Reports are still lacking on the occurrence of Blastocystis sp. subtypes from surface water and other water sources in various communities in Malaysia. The objective of this study was to ascertain the potential of water as source of acquiring Blastocystis sp. infections in these communities. Furthermore, the absence of studies on the seasonal influence on the occurrence of Blastocystis sp. subtypes in river water and other water sources has also steered the conduct of this research. It is hoped that this present study will add essential information on the occurrence of Blastocystis sp. subtypes in water and create awareness towards the role of surface water and other water sources in the dynamic transmission of Blastocystis sp. infections.
Materials and Methods
Study and sampling areas
Water samples were collected from a river, Sungai Krau from October 2014–November 2014 during wet season and June 2015 during dry season. Being located at the north-eastern part of Malaysia, the study areas were heavily flooded during wet season, where most of the houses located near the river were affected. The collection of the water samples in wet season was carried out 1 to 2 months before the heavy flood while the collection of water samples during dry season was carried out five months after the flood. February was documented as the month of the least amount of rainfall in Temerloh area in 2014 and 2015 as recorded by Malaysian Meterological Department. The north-eastern states of Peninsular Malaysia usually receives less rainfall from June to July (Malaysian Meterological Department (MetMalaysia), Ministry of Science, Technology Innovation (2015)). However, in certain regions of Malaysia, rainfall show different patterns because of several other factors, including geographical location. Although the study area is located in the north-eastern state of Peninsular Malaysia, based on the rainfall data, the study area receives minimum rainfall from June to July (first minimum rainfall) and in February (second minimum rainfall).
Due to heavy flooding which lasted until early of January 2015, collection of water samples within the second minimum rainfall period in February was not performed, since most of the villages in the study area were still affected by the flood and clean-up as well as rebuilding after massive floods took months to be accomplished.
Sungai Krau flows along the Malay and five aboriginal villages in Kuala Krau, Temerloh Pahang. Of all the aboriginal villages, the most upstream were Kampung Terbol, followed by Kampung Pian, Kampung Lubok Wong, Kampung Pasu and the most downstream Kampung Penderas. Most of the aborigines settled in Kampung Penderas with the widest land area. With the smallest land area, the least occupied village is Kampung Terbol.
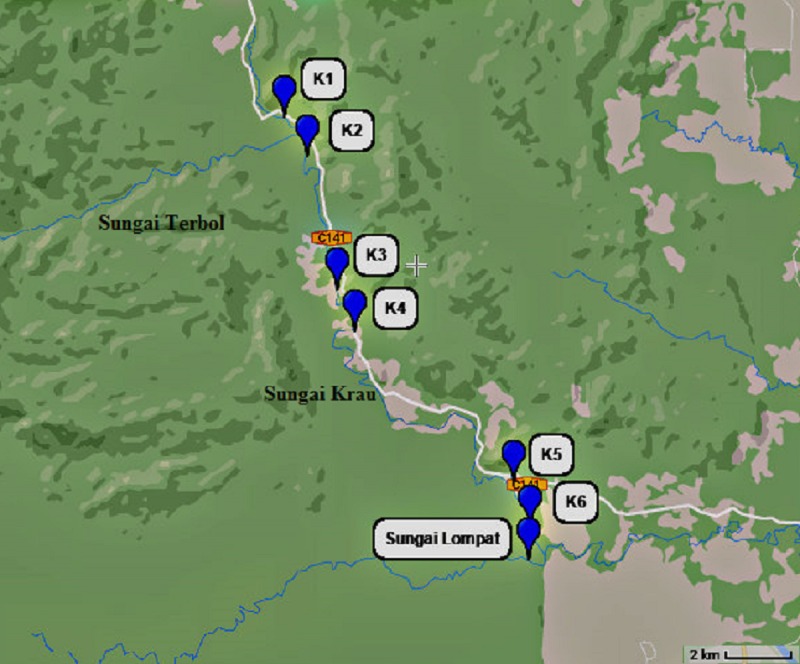
Six sampling points of the river were identified; K1 (1,000 m before Kampung Terbol, 3.83507°, 102.21404°), K2 (in the middle of Kampung Terbol, 3.81314°, 102.22804°), K3 (1,000 m before Kampung Lubok Wong, 3.78516°, 102.23596°), K4 (in the middle of Kampung Lubok Wong, 3.77014°, 102.23763°), K5 (1,000 m before Kampung Penderas, 3.74364°, 102.27091°) and K6 (in the middle of Kampung Penderas, 3.71301°, 102.28753°). Another sampling point was determined at Sungai Lompat (3.71259, 102.28839), a river which flow and meets Sungai Krau downstream in Kampung Penderas (Figure 1). Other water sources were also determined, inclusive of wells, water tank, tap water and others.
Figure 1. Map showing each six river sampling point at Sungai Krau and a point at Sungai Lompat.
Collections of water samples
The study protocol has been approved by the Research and Ethical Committee, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre (FF-2014-219). Permission to conduct the sampling was obtained from the Ministry of Rural and Regional Development Malaysia, reference number : JAKOA/PP.30.032Jld29(04). Using 10 litres water containers, water samples were collected from each seven sampling points of the rivers. The sampling were performed about 10–15 feet from the river bank. The river water was collected from the surface of the river with extra caution to avoid floating material at the water surface.
Besides the collection of 10 litres of river water samples, one thousand and five hundred millilitres of river water were also collected at the same points of all river water sampling for faecal coliforms count.
One thousand and five hundred millilitres bottles were used to collect water from various other water sources that are available in the villages. In Kampung Terbol, other sources was sampled from a water tank provided by the Malaysian government, tap water and well.
Tap water, stored water in a container and water in a fish pond were sampled in Kampung Lubok Wong. In Kampung Penderas water samples were collected from tap water, stored water, wells and small stream. All the water samples were brought back to the Community Laboratory in the Department of Parasitology and Medical Entomology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre with no addition of preservatives for processing.
Physicochemical parameter of the rivers
Data on pH, conductivity, temperature, dissolved oxygen, and total dissolved solid were recorded at each sampling point at Sungai Krau (K1, K2, K3, K4, K5, K6) and a single point at Sungai Lompat using multiparameter (Hanna, USA, model HI 9829). Measurement of turbidity was performed using microprocessor turbidity meter (model HI 93703; Hanna Instruments, Woonsocket, RI, USA). Using a colorimeter (Thermo Scientific, Singapore, model Orion AQ4000), chemical oxygen demand (COD) and sulfate concentrations were measured. Measurement of total chlorine was carried out using a multiparameter bench photometer (model HI 83200; Hanna Instruments, Woonsocket, RI, USA). The results of all physicochemical parameters were recorded for correlation analysis with the presence of Blastocystis sp. subtypes in water samples collected from the rivers.
Rainfall data
The wet and dry seasons were determined based on the monthly total rainfall data of 2010–2013 recorded from the nearest station to the study area (Temerloh station) and were obtained from the Malaysian Meteorological Department (MetMalaysia). The monthly total rainfall volume recorded during samples collection in the wet season were 116.8 mm–276.4 mm. Meanwhile, the total rainfall volume during samples collection in the dry season was 87.8 mm.
Detection of Blastocystis sp. subtypes
Filtration of water samples
Ten liters of water sample from each sampling point and 1.5 L of water sample collected from each of the water sources available in the villages were filtered using flatbed membrane filtration system (Masterflex I/P, model XX80EL230; Millipore, Billerica, MA, USA) through mixed cellulose esters (MCE) membrane filter with a 1.2 µm pore size and 14 mm diameter. Using a cell scrapper, the water concentrate on the membrane filter was removed thoroughly and rinsed three times with phosphate buffered saline (PBS). The washings were then centrifuged at 1, 400 × g at room temperature for 10 min to obtain water concentrate. The supernatant was discarded until 5mL was left and kept in the cold room (Lee et al., 2012).
DNA extraction and amplification of the DNA using single round polymerase chain reaction (PCR) and sequencing
Extraction of the DNA from all water samples were performed using QIAamp® Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) and followed the manufacturer’s instructions.
Amplification of the extracted DNA of all water samples were performed using BhRDr (genus-specific): GAGCTTTTTAACTGCAACAACG and RD5 (broad eukaryotic-specific): ATCTGGTTGATCCTGCCAGT primers (Scicluna, Tawari & Clark, 2006).
PCR was performed by 30 cycles of initial denaturing at 95 °C for 5 min, followed by denaturation at 95 °C for 1 min, annealing at 63.3 °C for 1 min and 30 s, extending at 72 °C for 1 min and an additional cycle of 10 min chain elongation at 72 °C. The PCR products were visualized in 1.5% agarose gel. PCR were carried out in duplicate to detect a possibility of mixed Blastocystis sp. subtype infections. Positive PCR products were then sent to Genomics Bioscience Taiwan for sequencing using the amplification primers to determine the subtypes. The sequences were then compared with the sequences available in GenBank™ using the BLASTN program on the National Center for Biotechnology Information Server (http://www.ncbi.nlm.nih.gov/BLAST). The sequences obtained from the sequencing were also deposited in GenBank™ (accession numbers: KX351998 –KX352032). The sequences were also queried into the “Blastocystis ST (18S) and Multi Locus Sequence Typing (MLST) Multi Locus Sequence Typing Databases” (www.pubmlst.org/blastocystis) which identified the sequences to 18S allele level (Stensvold, 2013). The exact or closest match allele to each sequence was identified.
Detection of faecal coliforms
Approximately 100 µL, 10 µL, 1 µL and 0.1 µL of water samples from the rivers in 100 mL of phosphate buffered saline (PBS) were filtered through 0.45 µm, 5 cm diameter nitrocellulose membrane filter. The filter was transferred onto membrane lactose glucuronide agar (MLGA) and incubated at 37 °C for 24–36 h. Plates were observed daily until 36 h for the presence of faecal coliforms indicated by colours ; Green (Escherichia coli) and yellow (Enterobacter aerogenes) colonies. The colonies of both faecal coliforms were counted and the results were expressed in number of colonies in every 100 ml water. Both bacteria were faecal coliforms.
Statistical analysis
A correlation analysis using Spearman’s rho was performed using a statistical software package (SPSS version 22) to determine the correlation between the physicochemical parameters and the occurrence of Blastocystis sp. subtypes.
Results
Physicochemical data and faecal coliforms count of the rivers
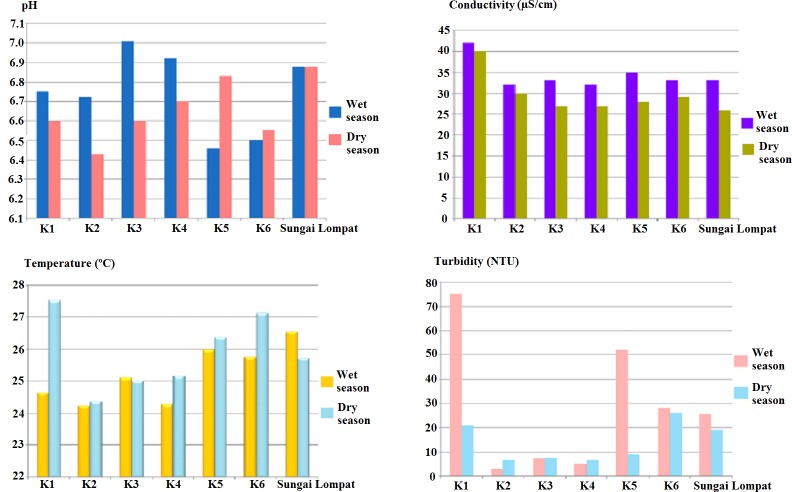
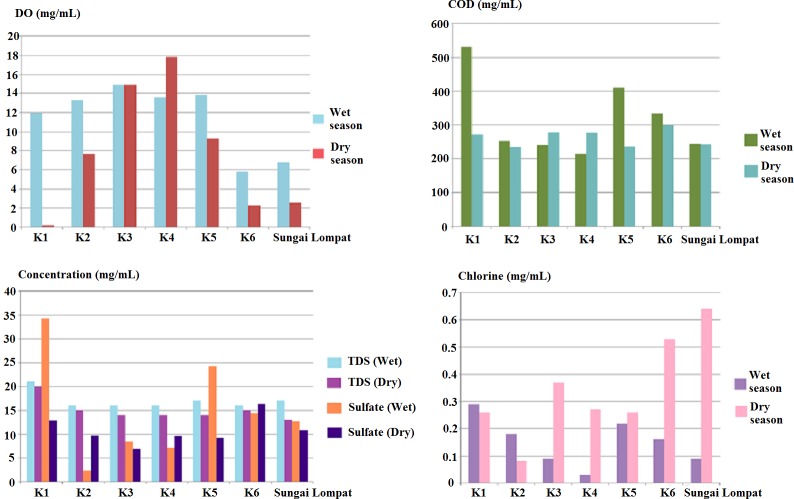
The physical parameters data of Sungai Krau and Sungai Lompat during both season are presented in Fig. 2 and chemical data of the rivers were as shown in Fig. 3. Physical parameters were pH, conductivity (µS/cm), temperature (°C) and turbidity (NTU). Meanwhile, chemical parameters measured were dissolved oxygen (DO) (mg/mL), chemical oxygen demand (COD) (mg/mL), total dissolved solids (TDS) (mg/mL), sulfate and total chlorine (mg/mL).
Figure 2. Physical parameters (pH, conductivity, temperature and turbidity) at all river sampling points.
Figure 3. Chemical parameters (concentration of Dissolved Oxygen (DO), Chemical Oxygen Demand (COD), Total Dissolved Solids (TDS), Sulfate & Chlorine) at all river sampling points.
The highest reading of pH (7.01), conductivity (42.00 µS/cm) and turbidity (75.00 NTU) were recorded in K1 during the wet season. Temperature of the river water was highest in Sungai Lompat (26.55 °C). During the dry season, K1 showed the highest reading of conductivity (40.00 µS/cm) and temperature (27.53 °C). pH reading was highest in Sungai Lompat with the reading of 6.88 and the most turbid point during the dry season was K6 (26.23 NTU).
During the wet season, K1 is the point with the highest reading of chemical oxygen demand (531.89 mg/mL), total dissolved solids (21.00 mg/mL) concentration of sulfate (34.20 mg/mL) and total chlorine (0.29 mg/mL). Dissolved oxygen was highest in K3 with a reading of 14.90 mg/mL. Meanwhile, during the dry season, the highest reading in K1 was total dissolved solids (19.98 mg/mL). The reading of chemical oxygen demand (300.16 mg/mL) and sulfate (16.34 mg/mL). Dissolved oxygen was highest in K4 with reading of 17.82 mg/mL. Sungai Lompat were recorded as the sampling point of the river with the highest total chlorine reading (0.64 mg/mL) were highest in K6.
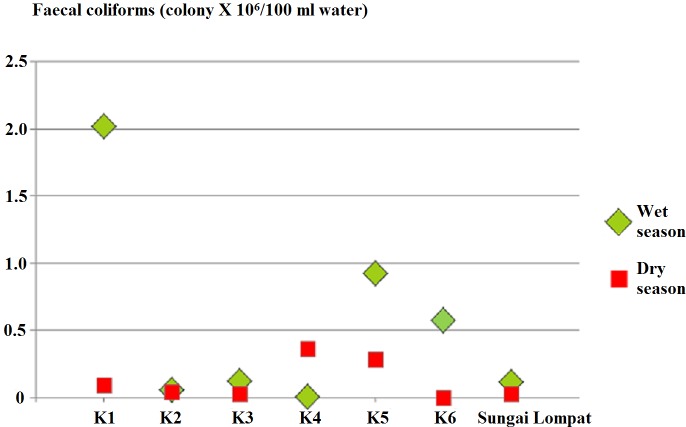
Figure 4 showed faecal coliforms counts in all sampling points with the highest count in K4 during the wet season (2.0180 × 106) CFU per 100 mL of water . Similar to wet season faecal coliforms were positive at all sampling points in the dry season with the highest count in K4 (0.3685 × 106) CFU per 100 mL.
Figure 4. Faecal coliforms count at all river sampling points.
Detection of Blastocystis sp. subtypes
During the wet season, all water samples collected from the seven points of the two rivers were positive for Blastocystis sp. (Table 1). Only three subtypes of Blastocystis sp. were isolated from the water samples and they were Blastocystis sp. ST1, ST2 and ST3. Blastocystis sp. ST3 was isolated from river water samples in all seven points of the two rivers 100.0% (7/7); single occurrence of Blastocystis sp. ST3 was observed in K3 and K4. No single occurrence of Blastocystis sp. ST1 and ST2 was observed in the water samples; mixed subtypes of Blastocystis sp. ST2 and ST3 were detected in K1 and K5, whereby mixed subtypes of Blastocystis sp. ST1 and ST3 were examined in K2, K6 and Sungai Lompat.
Table 1. Blastocystis sp. subtypes isolated from river water samples during wet and dry seasons.
| Point of sampling | Blastocystis sp. subtypes | |||||
|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | ||||
| Wet | Dry | Wet | Dry | Wet | Dry | |
| Sungai Krau K1 | − | − | + | − | + | + |
| Sungai Krau K2 | + | − | − | − | + | + |
| Sungai Krau K3 | − | − | − | − | + | + |
| Sungai Krau K4 | − | − | − | − | + | + |
| Sungai Krau K5 | − | − | + | − | + | + |
| Sungai Krau K6 | + | − | − | − | + | + |
| Sungai Lompat | + | − | − | − | + | + |
| Total | 3/7 (42.9%) | 0/7 (0.0%) | 2/7 (28.6%) | 0/7 (0.0%) | 7/7 (100.0%) | 7/7 (100.0%) |
Notes.
- +
- Positive for Blastocystis sp
- −
- Negative for Blastocystis sp
During dry season, all seven sampling points of the rivers were positive for Blastocystis sp. ST3 (100.0%, 7/7). Blastocystis sp. ST1 and ST2 at all river sampling points were absent (Table 1).
Water samples collected from various water sources during wet season showed 68.8% (11/16) were contaminated with Blastocystis sp. ST3, 18.8% (3/16) were contaminated with Blastocystis sp. ST1 while 6.3% (1/16) was contaminated with Blastocystis sp. ST2 and ST4 respectively (Table 2). In Kampung Terbol, during wet season, Blastocystis sp. ST3 was found in 2/4 of the tap water samples and in a water tank, namely Life Saver M1 System. Blastocystis sp. ST1 was found in the Life Saver M1 System and in 1/4 of the water samples collected from tap water. During wet season in Kampung Lubok Wong, Blastocystis sp. ST3 was detected in a tap water, stored water and fish pond. In Kampung Penderas, none of the tap water samples collected from government tap water was positive for Blastocystis sp. subtypes. However, there was a mixed subtype of Blastocystis sp. ST1 and ST3 and Blastocystis sp. ST2 and ST3 in water samples collected from wells. Blastocystis sp. ST3 was found in 3/3 of water stored in closed and opened containers. Blastocystis sp. ST4 was detected in 1/3 of the stored water (Table 2).
Table 2. Blastocystis sp. subtypes in water from other sampling sources during wet and dry seasons.
| Positive for Blastocystis sp. subtypes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total number of samples | ST1 | ST2 | ST3 | Other ST | ||||||
| Point of sampling | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry |
| Tap water | 8 | 13 | 1 | 0 | 0 | 0 | 3 | 6 | 0 | 0 |
| Water in tank (Life Saver) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Well | 2 | 3 | 1 | 0 | 1 | 0 | 2 | 3 | 0 | 0 |
| Stored water | 4 | 2 | 0 | 0 | 0 | 0 | 4 | 1 | 1 (ST4) | 1 (ST8) |
| Small stream | 0 | 1 | NA | 0 | NA | 0 | NA | 0 | NA | 0 |
| Fish pond | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 (ST10) |
| Total (%) | 16 | 21 | 3(18.8%) | 1(4.8%) | 1(6.3%) | 0(0.0%) | 11(68.8%) | 10(47.6%) | 1(6.3%) | 2(9.5%) |
Notes.
- NA
- Not applicable
During the dry season, the occurrence rate of Blastocystis sp. ST1 in the water samples collected from various water sources were reduced compared to wet season. In addition, none of the water samples collected in dry season was positive for Blastocystis sp. ST2 and ST4. The highest occurrence rate of Blastocystis sp. ST3 was observed (47.6%, 10/21), followed by Blastocystis sp. ST1 (4.8%, 1/21) and other subtypes which include Blastocystis sp. ST8 and ST10 (9.5%, 2/21). In tap water samples, Blastocystis sp. ST3 was detected in Kampung Terbol and Kampung Lubok Wong, whereby no occurrence of any Blastocystis sp. subtypes was observed in tap water samples of Kampung Penderas. Blastocystis sp. ST3 and ST8 were detected in the water stored outside a house in Kampung Penderas, and mixed subtype infections of Blastocystis sp. ST1 and ST10 were detected in the water collected from a small fish pond in Kampung Lubok Wong. Blastocystis sp. ST3 was detected in water from wells collected in Kampung Penderas and Kampung Terbol.
Tables 3A and 3B show the details of the water samples collected with the accession number and allele identity.
Table 3.
(A) Subtyping of Blastocystis sp. for water samples collected during the wet season. (B) Subtyping of Blastocystis sp. for water samples collected during the dry season.
| Samples code | Source of samples | Subtype | Identity (%) | Accession number | 18S rRNA allele |
|---|---|---|---|---|---|
| (A) | |||||
| K1 B4 KT | River | 2 | 99 | KX352014 | 15 |
| K2 in KT | River | 1 | 99 | KX352015 | 4 |
| K2 in KT | River | 3 | 99 | KX352016 | 34 |
| K3 B4 LW | River | 3 | 99 | KX352017 | 34 |
| K4 in LW | River | 3 | 99 | KX352018 | 38 |
| K5 B4 KP | River | 2 | 99 | KX352019 | 15 |
| K5 B4 KP | River | 3 | 99 | KX352020 | 34 |
| K6 in KP | River | 1 | 99 | KX352021 | 4 |
| K6 in KP | River | 3 | 99 | KX352022 | 34 |
| SL | River | 1 | 99 | KX352023 | 4 |
| 22 KP | Tap water | 3 | 99 | KX352024 | 38 |
| Stored water ASPLW | Stored water | 3 | 99 | KX352025 | 38 |
| Well Abu3 | Well | 3 | 99 | KX352030 | 38 |
| Fish pond3 | Fish pond | 3 | 99 | KX352032 | 38 |
| Stored water Selamat3KP | Stored water | 3 | 99 | KX352027 | 31 |
| Untreated tap water hill KT | Tap water | 3 | 99 | KX352028 | 31 |
| Well KP | Well water | 1 | 99 | KX352029 | 4 |
| Well Abu2 | Well water | 2 | 99 | KX352031 | 15 |
| Stored water Selamat4 | Stored water | 4 | 99 | KX352026 | 94 |
| (B) | |||||
| K1 B4 KTA | River | 3 | 99 | KX351998 | 34 |
| K2 in KTA | River | 3 | 99 | KX351999 | 34 |
| K3 B4 LWA | River | 3 | 99 | KX352000 | 34 |
| K4 in LWA | River | 3 | 99 | KX352001 | 34 |
| K5 B4 KPA | River | 3 | 99 | KX352002 | 34 |
| K6 in KPA | River | 3 | 99 | KX352003 | 34 |
| SLa | River | 3 | 99 | KX352004 | 34 |
| Well water KPA | Well water | 3 | 99 | KX352005 | 36 |
| Stored water Selamat3 | Stored water | 3 | 99 | KX352009 | 34 |
| TBLWA | Tap water | 3 | 99 | KX352010 | 33 |
| Tap water LWA | Tap water | 3 | 99 | KX352011 | 33 |
| Well water KTA | Well water | 3 | 99 | KX352012 | 34 |
| Fish pond LWA10 | Fish pond | 10 | 99 | KX352006 | 43 |
| Fish pond LWA1 | Fish pond | 1 | 99 | KX352007 | 4 |
| Stored water Selamat8 | Stored water | 8 | 99 | KX352008 | 21 |
Correlation of Blastocystis sp. subtypes with physicochemical parameters, faecal coliforms and monthly total rainfall volume
The correlation of Blastocystis sp. subtypes occurrence in the river with physicochemical parameters, faecal coliforms and monthly total rainfall data are shown in Table 4. During the wet season, there is a significant correlation of the occurrence of Blastocystis sp. ST2 and conductivity (rs = 0.828, P < 0.05), turbidity (rs = 0.791, P < 0.05), chemical oxygen demand (rs = 0.791, P < 0.05), total chlorine (rs = 0.798, P < 0.05), sulfate (rs = 0.791, P < 0.05) and faecal coliforms (rs = 0.791, P < 0.05). However, there is no significant correlation between Blastocystis sp. ST1 occurrence and the other physicochemical parameters, faecal coliform and monthly total rainfall. Correlations cannot be performed for Blastocystis sp. ST3 with all the parameters measured since Blastocystis sp. ST3 was detected at all river sampling points. During the dry season, correlation analysis cannot be performed for the occurrence of Blastocystis sp. ST1 and ST2 with the physicochemical parameters, faecal coliforms and monthly rainfall because of the absence of both subtypes at all river sampling points. Due to detection of Blastocystis sp. ST3 in all sampling points of the rivers, no correlation analysis was performed with all the parameters measured.
Table 4. Correlations between different Blastocystis sp. subtypes with physicochemical parameters, faecal coliforms and monthly total rainfall at Sungai Krau and Sungai Lompat (at 0.05 level, 2-tailed).
| Correlations | ||||||
|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | ||||
| Parameter | Wet | Dry | Wet | Dry | Wet | Dry |
| Physical | ||||||
| pH | rs = − 0.289 | NA | rs = − 0.474 | NA | NA | NA |
| P > 0.05 | P > 0.05 | |||||
| Conductivity | rs = − 0.378 | NA | rs = 0.828* | NA | NA | NA |
| P > 0.05 | P < 0.05 | |||||
| Temperature | rs = 0.144 | NA | rs = 0.158 | NA | NA | NA |
| P > 0.05 | P > 0.05 | |||||
| Turbidity | rs = − 0.289 | NA | rs = 0.791* | NA | NA | NA |
| P > 0.05 | P < 0.05 | |||||
| Chemical | ||||||
| Dissolved oxygen (DO) | rs = − 0.722 | NA | rs = 0.158 | NA | NA | NA |
| P > 0.05 | P > 0.05 | |||||
| Chemical oxygen demand (COD) | rs = 0.000 | NA | rs = 0.791* | NA | NA | NA |
| P > 0.05 | P < 0.05 | |||||
| Total dissolved solid (TDS) | rs = 0.000 | NA | rs = 0.683 | NA | NA | NA |
| P > 0.05 | P > 0.05 | |||||
| Total chlorine | rs = − 0.073 | NA | rs = 0.798* | NA | NA | NA |
| P > 0.05 | P < 0.05 | |||||
| Sulfate | rs = − 0.289 | NA | rs = 0.791* | NA | NA | NA |
| P > 0.05 | P < 0.05 | |||||
| Faecal coliform | rs = − 0.289 | NA | rs = 0.791* | NA | NA | NA |
| P > 0.05 | P < 0.05 | |||||
| Monthly total rainfall | rs = − 0.433 | NA | rs = 0.000 | NA | NA | NA |
| P > 0.05 | P > 0.05 | |||||
Notes.
- rs
- Correlation coefficients
- P
- Probability level
significant correlation at 0.05 level.
Discussion
Most rural and remote communities still use untreated water either from streams, rivers and wells for drinking and other daily activities (Jagals, 2006; Whelan & Willis, 2007). Many studies have implicated the role of contaminated water, especially drinking water, surface water and others as a source of Blastocystis sp. infections (Tan, Suresh & Smith, 2008). Cysts of Blastocystis sp. were detected in sewage samples collected from Kuala Lumpur, Malaysia (Suresh, Smith & Tan, 2005). A study was performed to determine the presence of Blastocystis sp. in water from rivers located in recreational areas in Malaysia namely Sungai Congkak and Sungai Batu. The study reported the average percentage of Blastocystis sp. detections of 33.3% in Sungai Congkak and 22.1% in Sungai Batu, with the highest detection rate in the downstream sampling point (Ithoi et al., 2011). Blastocystis sp. ST1, ST3 and ST5 were detected in rivers and lakes around Klang Valley, Malaysia (Suresh, Tan & Illi, 2009). Two previous studies in Malaysia have reported absence of a proper piped water supply and drinking unboiled or untreated water as the significant risk factors in the acquisition of Blastocystis sp. infections (Abdulsalam et al., 2012; Anuar et al., 2013). As one of the most common intestinal parasitic infections among the aborigines (Abdulsalam et al., 2012; Anuar et al., 2013), the occurrence of Blastocystis sp. in the water sources should be implicated as the source of Blastocystis sp. infections in humans and animals. In addition, certain Blastocystis sp. subtypes including Blastocystis sp. ST1 (Moosavi et al., 2012), ST2 (Ramírez et al., 2014), ST3 (Tan, Suresh & Smith, 2008) and ST4 (Dominguez-Marquez et al., 2009) have been reported to be pathogenic. Therefore, there is a need to detect the occurrence of the Blastocytis sp. subtypes in the water samples used by the aborigines in this study.
This study reveals that river water used by the villagers are highly contaminated with human and animal faecal materials as shown by the detection of high faecal coliform counts in all seven sampling points in Sungai Krau and Sungai Lompat. Interestingly, Blastocystis sp. ST1, ST2 and ST3 were the subtypes identified in the river water samples of which Blastocystis sp. ST3 being the most predominant subtype isolated in all water sampling points. Besides that, Blastocystis sp. ST3 was the only subtype that was persistently isolated during wet and dry seasons. In contrast, a study in Nepal highlighted four of the river water samples were positive for Blastocystis sp. ST1 and ST4 (Lee et al., 2012). Abdulsalam (2013) in her thesis reported Blastocystis sp. ST4 was the only subtype isolated from river water samples. Of the nine Blastocystis sp. subtypes reported in humans, four subtypes (ST1–ST4) are common in humans (Clark et al., 2013). However, Blastocystis sp. ST4 was rarely reported outside Europe (Alfellani et al., 2013). This study detected ST4 in one of the samples collected from water stored in a container during the wet season. Based on the lack of reports of ST4 in Malaysia, the potential of faeces of the aborigines to contaminate the water sample in the container was low. The presence of Blastocystis sp. ST1, ST2 and ST3 isolated from river water samples during wet season and Blastocystis sp. ST3 during dry season in all of the sampling points in this study, in addition to the detection of faecal coliforms in all river water samples indicates that the sources of river water contamination by Blastocystis sp. are most possibly from humans and a small amount from animal faeces. These findings were supported by many molecular studies in humans that revealed the common Blastocystis sp. subtypes were ST1–ST4 with the most dominant subtype being ST3 (Yoshikawa et al., 2004; Özyurt et al., 2008; Tan, Ong & Suresh, 2009; Yakoob et al., 2010; Nithyamathi, Chandramathi & Kumar, 2016).
Although the community in Kampung Penderas is equipped with proper tap water supply and toilet facilities by the government, data gathered from the questionnaires and from our own observations revealed that many of the villagers still practise open defaecation and collect water from the rivers and wells for daily activities. Water is kept in a container for daily usage and sometimes kept for further usage during a shortage of water. The less-structured villages of Kampung Terbol and Kampung Lubok Wong are not equipped with safe pipe water supply and toilets. The aboriginal community in these two villages built up their own piping system directly from streams at the hilly areas since the water from the upstream is cleaner and less polluted than the Sungai Krau itself. Some of the aborigines still collect water from Sungai Krau for storage and to be used when there is a shortage of water from their own piping system. Besides own-built piping system, Kampung Terbol and Kampung Lubok Wong are equipped with Life Saver M1 System. The tank operated without chemicals or power supply and filtered any water sources including rain water, well water and water from the nearby river. Realizing the chances of contamination of other water sources with Blastocystis sp., other water sources used by the community including water in wells, tank, fish pond, stored containers and tap waters were also sampled. In this study, most of the water collected from many sources during wet season was contaminated by Blastocystis sp. ST1, ST2 and ST3, with Blastocystis sp. ST3 as the most prevalent subtype in the water within the vicinity of the aboriginal dwellings.
Blastocystis sp. ST3 has been nailed down as the pathogenic subtype of Blastocystis sp. (Jones Ii et al., 2008). Therefore, the detection of this particular subtype persistently in the river water samples and other water sources including tap water, wells and water stored in container during both seasons may be an important point to be raised. Further investigations need to be performed to determine Blastocystis sp. infections in the aborigines, so that the possibility of waterborne transmission of the subtype can be ruled out.
During dry season, there was a marked reduction in the detection rate of Blastocystis sp. ST1 and absence of Blastocystis sp. ST2 and ST4 in the water samples collected from various water sources in the villages. However, there were additional Blastocystis sp. subtypes of ST8 and ST10 detected in the water samples collected from stored water and fish pond. Blastocystis sp. ST8 was rarely reported in humans, whereby Blastocystis sp. ST10 was identified in primates and artiodactyls (Stensvold et al., 2009; Ramírez et al., 2014). Therefore, we postulate that both Blastocystis sp. subtypes were from animal source which might contaminate the water. The Blastocystis-positive stored water which was used for washing hands and legs was left outside the house in an uncovered container. Therefore, the chance of any animals to contaminate the water cannot be avoided which might explain the discovery of Blastocystis sp. ST8 in one of the stored water collected. The occurrence of Blastocystis sp. ST10 in the water collected from fish pond was postulated to be from the faeces of fishes. However, since Blastocystis sp. ST10 is found mostly in primates and cattle, this could suggest accidental colonisation of the subtype in fishes or contamination of the water used to fill the fish pond with Blastocystis sp. ST10 by the faeces of primates or cattles.
Our findings revealed that there were no Blastocystis sp. subtypes detected in water samples collected from treated governmental tap water in Kampung Penderas although most of the tap water supplies in Malaysia originated from river waters. Sedimentation and chlorination in the water treatment process was found to be able to remove and kill Blastocystis sp. cyst although a study done by Zaki, Zaman & Khan (1996) reported that this protist showed resistance to chlorination. In contrast, Blastocystis sp. ST1 and ST3 were detected in untreated tap water from Kampung Terbol during wet season and single occurrence of Blastocystis sp. ST3 in untreated tap water from Kampung Lubok Wong. During dry season, Blastocystis sp. ST3 was detected in untreated tap water in Kampung Terbol and Kampung Lubok Wong. Therefore, the untreated tap water could be one of the sources of Blastocystis sp. infection in these two villages. Since many of the rural communities may still use untreated water from streams, rivers and wells, so this study may highlight the importance of drinking treated and boiled water.
To the best of our knowledge, this is the first study done in Malaysia to provide data on the seasonal influence on the presence of Blastocystis sp. in water sources. The worst floods in Malaysia which were affected by the new moon phenomenon and perigee hit the north-eastern parts of the country starting from mid of December 2014 to early January 2015 (Abdullah et al., 2014 ; Wei, 2014; Baharuddin et al., 2015; Ambu, 2015) might have changed the distribution of Blastocystis sp. subtypes in the study area. During wet season, Blastocystis sp. isolated in six sampling points of Sungai Krau and a point at Sungai Lompat, as well as in water samples collected from various water sources in the villages were of Blastocystis sp. ST1–ST3. However, to our surprise, there was a predominance of Blastocystis sp. ST3 at all river sampling points and in water samples collected from various other water sources in the villages during dry season. In addition, there was an absence of Blastocystis sp. ST1 and ST2 in the river water samples. In other water sources, there was a reduction in Blastocystis sp. ST1 occurrence and absence of Blastocystis sp. ST2. These findings lead to our postulation that Blastocystis sp. ST1 and ST2 might not be able to withstand the adverse condition and were flushed during the heavy flood. The detection of Blastocystis sp. ST3 as the most prevalent subtype especially during dry season, which was five months after the heavy flood suggest that it is the most resistant subtype since it is able to survive harsh environmental conditions especially during the heavy flood.
The possibility of waterborne transmission of Blastocystis sp. to humans in this study is not only restricted to the aboriginal community, however, since Sungai Krau flows along the Malay community which is located before Kampung Terbol (at the more upstream area) and after Kampung Penderas (at the more downstream area) as well, so the chances of waterborne transmission to the communities living along the rivers were possible. In addition, during water sampling in both seasons, we observed few groups of people from various other places went to the Sungai Krau and Sungai Lompat for fishing, since most of them search for large freshwater fishes. Therefore, the possibility of infection from the fishes which might as well become infected with Blastocystis sp. is high in those groups of people, especially when the fishes are not properly cleaned and cooked. However, since we did not take samples from the fishes, we do not know whether the fishes pose a risk as a source of Blastocystis sp. infections to these groups of people.
The primer BhRDr and RD5 were chosen in this study since they can identify Blastocystis sp. both at ST levels and 18S allele analysis with no chance of any subtype being missed in the detection (Stensvold, 2013). Different subtypes display various level of intra-subtype diversity where Blastocystis sp. ST3 is known to exhibit the most substantial intra-subtype genetic diversity (Stensvold, Alfellani & Clark, 2012; Stensvold, 2013). This study indicated that there were three ST3 allele found in the water samples collected during the wet season (ST3 allele 31, 34 and 38), meanwhile in the dry season, there were also three allele identified; ST3 allele 33, 34 and 36. Blastocystis sp. ST3 allele 34 was reported as a quite common allele in humans (Alfellani et al., 2013; Pandey et al., 2015; Das et al., 2016), therefore the detection of the allele in the water samples during both seasons in this study might indicate the contamination of water sources with human faecal samples. Blastocystis sp. ST3 allele 34, 36 and 38 have been reported in human and non-human primates (Alfellani et al., 2013), therefore both human and animal faecal samples might contribute to the contamination of the water samples with Blastocystis sp. during the wet and dry seasons.
Correlations between Blastocystis sp. subtypes occurrence in the rivers and physicochemical parameters and faecal coliforms showed significant positive correlation between the presence of Blastocystis sp. ST2 in the river water and conductivity, turbidity, chemical oxygen demand, total chlorine, sulfate and faecal coliforms (p < 0.05). This shows that this particular subtype is able to survive at more polluted water. However, Blastocystis sp. ST1 and ST3 are able to survive in any condition of the water, whether less or more polluted water. Both Blastocystis sp. ST1 and ST3 are not affected by physicochemical changes of the water. Among the three subtypes detected in the river water samples, only Blastocystis sp. ST3 is able to sustain and grow in the water after many months regardless of the environmental conditions.
Conclusion
The river water samples used by the aboriginal community were highly contaminated with organic materials and faeces of humans and animals. The occurrence of Blastocystis sp. in the water in this study hopes to raise awareness on the importance of the consumption of treated or boiled water and the need to improve sewage disposal system in the community. Seasonal variation plays a role in the occurrence and survival of Blastocystis sp. subtypes in the water samples. Among all the subtypes present in the water samples, Blastocystis sp. ST3 is suggested to be the most resistant and robust subtype and able to survive harsh environmental conditions since it was found in the river and other water sources in both seasons. The presence of Blastocystis sp. ST2 in the river water samples were significantly correlated with certain physicochemical parameters during wet season and this brings to the suggestion that Blastocystis sp. ST2 survives in more polluted and contaminated water. Blastocystis sp. ST1 and ST3 occurrence are not affected by any physicochemical changes of the water. Since faecal coliforms were detected at all sampling points of the rivers, therefore the possible sources of Blastocystis sp. contaminations could therefore be from the faeces of humans or animals. Avoidance of Blastocystis sp. infection and spread to wider areas especially by waterborne route to other communities require a collaborative effort from different authorities and communities. Health education, treated and safe water supply and toilets were among the strategies to be applied in order to obliterate the chances of Blastocystis sp. transmissions to humans and animals from water sources.
Supplemental Information
Acknowledgments
We would like to thank all the participants from Kampung Terbol, Kampung Lubok Wong and Kampung Penderas for their participation and contribution in providing the water samples. We would also like to thank Dr Anisah Nordin for technical support. Our deepest gratitude to Ms. Noor Wanie Hasan and Ms Siti Nur Su’aidah Nasarudin for their assistance during the field work.
Funding Statement
This study was funded by the UKMMC Fundamental Research Grant (FF-2014-219) and UKM Publication Enhancement Grant (DLP-2014-013). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Samseh Abdullah Noradilah conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables.
Ii Li Lee and Tengku Shahrul Anuar conceived and designed the experiments, reviewed drafts of the paper.
Fatmah Md Salleh performed field and laboratory works.
Siti Nor Azreen Abdul Manap, Noor Shazleen Husnie Mohd Mohtar and Syed Muhamad Azrul performed field work.
Wan Omar Abdullah reviewed drafts of the paper.
Norhayati Moktar conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Permission for water collection from the aboriginal dwellings were obtained from the Ministry of Rural and Regional Development Malaysia (reference number: JAKOA/PP.30.032Jld29(04).
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental Information 1.
References
- Abdullah et al. (2014).Abdullah NI, Zakaria R, Alagesh TN, Jaafar S, Ramli I, Alias NA, Husin S, Inus K, Lokman T, Nasa A. 4m-high waves to hit 3 states. http://www.nst.com.my/news/2015/09/4m-high-waves-hit-3-states. New Straits Times. 2014 [Google Scholar]
- Abdulsalam et al. (2012).Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Ahmed AH, Surin J, Mak JW. Drinking water is a significant predictor of Blastocystis infection among rural Malaysian primary schoolchildren. Parasitology. 2012;139(8):1014–1020. doi: 10.1017/S0031182012000340. [DOI] [PubMed] [Google Scholar]
- Abdulsalam (2013).Abdulsalam AM. Doctoral dissertation. 2013. Molecular epidemiology of Blastocystis isolated from Malaysia and Libya/Awatif Mohamed Abdulsalam. [Google Scholar]
- Alfellani et al. (2013).Alfellani MA, Jacob AS, Perea NO, Krecek RC, Taner-Mulla D, Verweij JJ, Levecke B, Tannich E, Clark G, Stensvold CR. Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology. 2013;140(08):966–971. doi: 10.1017/S0031182013000255. [DOI] [PubMed] [Google Scholar]
- Ambu (2015).Ambu S. Floods–the consequence of human intrusion into nature. International e-Journal of Science, Medicine and Education. 2015;9(1):1–2. [Google Scholar]
- Anuar et al. (2013).Anuar TS, Ghani MKA, Azreen SN, Salleh FM, Moktar N. Blastocystis infection in Malaysia: evidence of waterborne and human-to-human transmissions among the Proto-Malay, Negrito and Senoi tribes of Aborigines. Parasites & Vectors. 2013;6:40. doi: 10.1186/1756-3305-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharuddin et al. (2015).Baharuddin KA, Wahab SFA, Ab Rahman NHN, Mohamad NAN, Kamauzaman THT, Noh AYM, Majod MRA. The record-setting flood of 2014 in kelantan: challenges and recommendations from an emergency medicine perspective and why the medical campus stood dry. The Malaysian Journal of Medical Sciences. 2015;22(2):1–7. [PMC free article] [PubMed] [Google Scholar]
- Clark et al. (2013).Clark CG, Van der Giezen M, Alfellani MA, Stensvold CR. Recent developments in Blastocystis research. Advances in Parasitology. 2013;82:1–32. doi: 10.1016/B978-0-12-407706-5.00001-0. [DOI] [PubMed] [Google Scholar]
- Das et al. (2016).Das R, Khalil S, Mirdha BR, Makharia GK, Dattagupta S, Chaudhry R. Molecular characterization and subtyping of blastocystis species in irritable bowel syndrome patients from North India. PLoS ONE. 2016;11(1):e2541. doi: 10.1371/journal.pone.0147055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Marquez et al. (2009).Dominguez-Marquez MV, Guna R, Munoz C, Gomez-Munoz MT, Borras R. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain) Parasitology Research. 2009;105(4):949–955. doi: 10.1007/s00436-009-1485-y. [DOI] [PubMed] [Google Scholar]
- Ithoi et al. (2011).Ithoi I, Jali A, Wah MJ, Wan Yusoff WS, Rohela M. Occurrence of Blastocystis in water of two rivers from recreational areas in Malaysia. Journal of Parasitology Research. 2011;2011:123916. doi: 10.1155/2011/123916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagals (2006).Jagals P. Does improved access to water supply by rural households enhance the concept of safe water at the point of use? A case study from deep rural South Africa. Water Science and Technology. 2006;54(3):9–16. doi: 10.2166/wst.2006.441. [DOI] [PubMed] [Google Scholar]
- Jones Ii et al. (2008).Jones Ii MS, Ganac RD, Hiser G, Hudson NR, Le A, Whipps CM. Detection of blastocystis from stool samples using real-time PCR. Parasitology Research. 2008;103(3):551–557. doi: 10.1007/s00436-008-1006-4. [DOI] [PubMed] [Google Scholar]
- Karanis, Kourenti & Smith (2007).Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. Journal of Water and Health. 2007;5(1):1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- Malaysian Meterological Department (MetMalaysia), Ministry of Science, Technology Innovation (2015).Malaysian Meterological Department (MetMalaysia), Ministry of Science, Technology Innovation General Climate of Malaysia. 2015. http://www.met.gov.my/web/metmalaysia/education/climate/generalclimateofmalaysia http://www.met.gov.my/web/metmalaysia/education/climate/generalclimateofmalaysia
- Lee et al. (2012).Lee LI, Chye TT, Karmacharya BM, Govind SK. Blastocystis sp.: waterborne zoonotic organism, a possibility? Parasites & Vectors. 2012;5:130. doi: 10.1186/1756-3305-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelayoova et al. (2004).Leelayoova S, Rangsin R, Taamasri P, Naaglor T, Thathaisong U, Mungthin M. Evidence of waterborne transmission of Blastocystis hominis. The American Journal of Tropical Medicine and Hygiene. 2004;70(6):658–662. [PubMed] [Google Scholar]
- Li et al. (2007).Li LH, Zhou XN, Du ZW, Wang XZ, Wang LB, Jiang JY, Yoshikawa H, Steinmann P, Utzinger J, Wu Z, Chen JX, Chen SH, Zhang L. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitology International. 2007;56(4):281–286. doi: 10.1016/j.parint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Moosavi et al. (2012).Moosavi A, Haghighi A, Mojarad EN, Zayeri F, Alebouyeh M, Khazan H, Kazemi B, Zali MR. Genetic variability of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Iran. Parasitology Research. 2012;111(6):2311–2315. doi: 10.1007/s00436-012-3085-5. [DOI] [PubMed] [Google Scholar]
- Nithyamathi, Chandramathi & Kumar (2016).Nithyamathi K, Chandramathi S, Kumar S. Predominance of Blastocystis sp. infection among school children in Peninsular Malaysia. PLoS ONE. 2016;11:e2541. doi: 10.1371/journal.pone.0136709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özyurt et al. (2008).Özyurt M, Kurt Ö, Mølbak K, Nielsen HV, Haznedaroglu T, Stensvold CR. Molecular epidemiology of Blastocystis infections in Turkey. Parasitology International. 2008;57(3):300–306. doi: 10.1016/j.parint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Pandey et al. (2015).Pandey PK, Verma P, Marathe N, Shetty S, Bavdekar A, Patole MS, Stensvold CR, Shouche YS. Prevalence and subtype analysis of blastocystis in healthy Indian Individuals. Infection, Genetics and Evolution. 2015;31:296–299. doi: 10.1016/j.meegid.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Ramírez et al. (2014).Ramírez JD, Sánchez LV, Bautista DC, Corredor AF, Flórez AC, Stensvold CR. Blastocystis subtypes detected in humans and animals from Colombia. Infection, Genetics and Evolution. 2014;22:223–228. doi: 10.1016/j.meegid.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Scicluna, Tawari & Clark (2006).Scicluna SM, Tawari B, Clark CG. DNA barcoding of Blastocystis. Protist. 2006;157(1):77–85. doi: 10.1016/j.protis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Souppart et al. (2010).Souppart L, Moussa H, Cian A, Sanciu G, Poirier P, El Alaoui H, Delbac F, Boorom K, Delhaes L, Dei-Cas E, Viscogliosi E. Subtype analysis of Blastocystis isolates from symptomatic patients in Egypt. Parasitology Research. 2010;106(2):505–511. doi: 10.1007/s00436-009-1693-5. [DOI] [PubMed] [Google Scholar]
- Stensvold (2013).Stensvold CR. blastocystis: genetic diversity and molecular methods for diagnosis and epidemiology. Tropical Parasitology. 2013;3(1):26–33. doi: 10.4103/2229-5070.113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold, Alfellani & Clark (2012).Stensvold CR, Alfellani M, Clark CG. Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infection, Genetics and Evolution. 2012;12:263–273. doi: 10.1016/j.meegid.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Stensvold et al. (2009).Stensvold CR, Alfellani MA, Nørskov-Lauritsen S, Prip K, Victory EL, Maddox C, Nielsen HV, Clark CG. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. International Journal for Parasitology. 2009;39(4):473–479. doi: 10.1016/j.ijpara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Suresh, Smith & Tan (2005).Suresh K, Smith HV, Tan TC. Viable Blastocystis cysts in Scottish and Malaysian sewage samples. Applied and Environmental Microbiology. 2005;71(9):5619–5620. doi: 10.1128/AEM.71.9.5619-5620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh, Tan & Illi (2009).Suresh KG, Tan TC, Illi F. Blastocystis in water—need for screening? Water Practice Technology. 2009;4:1–21. [Google Scholar]
- Tan, Ong & Suresh (2009).Tan TC, Ong SC, Suresh KG. Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitology Research. 2009;105(5):1283–1286. doi: 10.1007/s00436-009-1551-5. [DOI] [PubMed] [Google Scholar]
- Tan, Suresh & Smith (2008).Tan TC, Suresh KG, Smith HV. Phenotypic and genotypic characterisation of Blastocystis hominis isolates implicates subtype 3 as a subtype with pathogenic potential. Parasitology Research. 2008;104(1):85–93. doi: 10.1007/s00436-008-1163-5. [DOI] [PubMed] [Google Scholar]
- Wei (2014).Wei SL. Fenomena “New Moon”, cetus banjir luar biasa. http://www.sinarharian.com.my/semasa/fenomena-full-moon-cetus-banjir-luar-biasa-1.344222. Sinar Harian. 2014 [Google Scholar]
- Whelan & Willis (2007).Whelan JJ, Willis K. Problems with provision: barriers to drinking water quality and public health in rural Tasmania, Australia. Rural Remote Health. 2007;7(3):627. [PubMed] [Google Scholar]
- World Health Organization (2008).World Health Organization . Guidelines for drinking-water quality. 3rd edition, incorporating first and second addenda. Geneva: World Health Organization; 2008. [PubMed] [Google Scholar]
- World Health Organization (2011).World Health Organization . World health organization guidelines for drinking-water quality (WHO GDWQ) 4th edition. Malta: Gutenberg; 2011. Microbial fact sheets; pp. 271–273. [Google Scholar]
- Yakoob et al. (2010).Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R. Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitology Research. 2010;106:1033–1038. doi: 10.1007/s00436-010-1761-x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa et al. (2004).Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IKM, Hossain MB. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitology Research. 2004;92(1):22–29. doi: 10.1007/s00436-003-0995-2. [DOI] [PubMed] [Google Scholar]
- Zaki, Zaman & Khan (1996).Zaki M, Zaman V, Khan A. Resistance of Blastocystis hominis Cysts to Chlorine. Journal of Pakistan Medical Association. 1996;46(8):178–179. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental Information 1.