Abstract
Staphylococcus aureus , a Gram-positive bacterium colonizing nares, skin, and the gastrointestinal tract, frequently invades the skin, soft tissues, and bloodstreams of humans. Even with surgical and antibiotic therapy, bloodstream infections are associated with significant mortality. The secretion of coagulases, proteins that associate with and activate the host hemostatic factor prothrombin, and the bacterial surface display of agglutinins, proteins that bind polymerized fibrin, are key virulence strategies for the pathogenesis of S. aureus bloodstream infections, which culminate in the establishment of abscess lesions. Pathogen-controlled processes, involving a wide spectrum of secreted factors, are responsible for the recruitment and destruction of immune cells, transforming abscess lesions into purulent exudate, with which staphylococci disseminate to produce new infectious lesions or to infect new hosts. Research on S. aureus bloodstream infections is a frontier for the characterization of protective vaccine antigens and the development of immune therapeutics aiming to prevent disease or improve outcomes.
Keywords: coagulation, agglutination, immune evasion, abscess formation, vaccine
INTRODUCTION
The Gram-positive microbe Staphylococcus aureus stably colonizes the nares, the skin, and/or the perineum of approximately one-third of the human population, whereas another third is colonized intermittently (1). S. aureus is also an invasive pathogen and frequent cause of skin and soft tissue as well as bloodstream infections (2). Once the pathogen enters the bloodstream, S. aureus replicates and disseminates to many different sites, causing severe disease manifestations such as sepsis, infective endocarditis, and deep-seated abscesses in virtually every organ tissue (2). S. aureus has evolved resistance mechanisms against antibiotics. Vancomycin, daptomycin, clindamycin, and linezolid are approved for the treatment of infections with antibiotic-resistant strains [MRSA (methicillin-resistant S. aureus)]; however, MRSA isolates develop resistance even against last resort therapeutics, and resistance is associated with therapeutic failure and increased mortality (2, 3). There is a clear medical need for vaccines and immune therapeutics that can address the public health crisis of MRSA infections. Although vaccines and antibody therapeutics targeting S. aureus capsular polysaccharides, surface proteins, and toxins are being developed, thus far such regimens have not reached the endpoints of clinical efficacy trials (4). This article summarizes what is known about the pathogenesis of S. aureus bloodstream infections—information that may be useful for the development of vaccines or immune therapeutics.
THE PATHOGEN
S. aureus
S. aureus was first isolated from the pus of surgical wounds by Alexander Ogston, who initially referred to the bacteria as micrococci (5). Their characteristic appearance—grape-like clusters (staphyle in Greek) of sphere-shaped bacteria—prompted Ogston to name the organisms staphylococci, which distinguishes them from chain-forming streptococci, also associated with surgical wound infections. Ogston further reported that injection of staphylococci into the subcutaneous tissue of experimental guinea pigs and mice produced abscess lesions (6).
Rosenbach differentiated staphylococci isolated from humans based on the pigmentation of their colonies, proposing the nomenclature Staphylococcus aureus and Staphylococcus albus for yellow and white colonies, respectively (7). The latter species is now designated Staphylococcus epidermidis. Staphyloxanthin, a membrane-bound carotenoid produced by S. aureus, is responsible for the yellow pigmentation of colonies. Pigment production scavenges reactive oxygen species and protects S. aureus from phagocytic killing (8). The ability to clot human and animal blood or plasma is a hallmark of S. aureus and is mediated by two secreted products, coagulase (Coa) and von Willebrand factor–binding protein (vWbp); S. epidermidis is Coa negative (9). Several other staphylococcal species are also classified as Coa positive, including S. delphini, S. intermedius, and S. pseudintermedius, species that have adapted to various hosts (mink, fox, pigeon, and dog) but do not colonize humans (10).
Clinical Burden of S. aureus Infections
In the United States, the annual burden of S. aureus diseases requiring medical treatment includes more than one million skin and soft tissue infections (SSTIs) with and without deep-seated abscess lesions; 490,000 hospitalizations; 93,000 cases of bacteremia; and 35,000 cases of sepsis and/or endocarditis (11). An annual mortality rate of more than 20,000 is attributed to S. aureus infection. Of particular concern are patients with recurrent SSTIs or bacteremia (12). Recurrent infection leads to invasive S. aureus disease and sepsis. Specific patient populations are at elevated risk of healthcare associated (HA) S. aureus infection: very low birth-weight neonates and patients with indwelling catheters, endotracheal intubation, medical implantation of foreign bodies, trauma, surgical procedures, hemodialysis, peritoneal dialysis, diabetes and who have undergone immunosuppressive or cancer therapy (12). Clinical use of antibiotics led to the selection of drug-resistant strains, designated MRSA for methicillin-resistant S. aureus. Acquisition of a specific penicillin-binding protein, PBP2a, that cannot be inhibited by penicillinase-resistant β-lactams (methicillin or oxacillin) is the molecular basis for broad-spectrum resistance against this class of compounds (13, 14). MRSA isolates account for >60% of clinical S. aureus infections in Japan, >50% in Italy and Portugal, and >30% in the United States (15). MRSA isolates are generally multidrug resistant and require treatment with vancomycin, clindamycin, linezolid, or daptomycin (3). The use of vancomycin as the antibiotic of last resort against MRSA has selected for the emergence of vancomycin-resistant S. aureus and is also responsible for the frequent occurrence of strains of with intermediate resistance (reviewed in 2).
Skin and Soft Tissue Infections
Breaches in skin barrier function occur following trauma and surgical procedures and favor entry of S. aureus into subcutaneous tissues. Nevertheless, S. aureus infection can occur at sites without apparent breaches, for example at hair follicles (folliculitis), as bullous or superficial lesions (impetigo), or as deep or confluent abscesses (furuncles or carbuncles, respectively) (2). All of these infections are referred to as SSTIs and manifest as purulent exudates draining from the infectious site. Approximately 20% of patients with SSTIs undergoing antibiotic therapy develop recurrent infections with the same strain (16). Thus, prior infection with S. aureus is not known to generate a protective immune response.
Studies in mice have revealed pathophysiological features associated with S. aureus SSTIs (for an extensive review, see 17). Within 24 h following subcutaneous inoculation of bacteria, a large inflammatory response is observed with indurated swelling at the site of infection. These lesions expand in size over 5–7 days and then resolve over the next 7–9 days (18). Dermonecrosis is often observed with subcutaneous abscess lesions, heals at a similar rate as the lesion resolves, and is caused by secreted products, α-hemolysin (α-toxin, Hla) (18), and phenol-soluble modulins (PSMs) (19). Some MRSA strains express the bacteriophage-encoded bicomponent Panton-Valentine leucocidin (PVL-S and PVL-FS), which binds to human complement receptors C5aR and C5L2 and mediates lysis of leukocytes (but not of murine immune cells) (20). PVL expression is thought to aggravate the pathology of S. aureus infections in humans (21). PSMs contribute to the pathogenesis of mouse skin infections and elicit proinflammatory responses by interacting with formyl peptide receptor 2 of immune cells and by activating, attracting, and lysing neutrophils (22). In contrast to immune-competent animals, leukopenic mice develop systemic infections with rapidly lethal outcomes following skin inoculation of S. aureus (23). In humans, leukopenia and hereditary defects in the NADPH oxidase or in the respiratory burst of myeloid cells are associated with increased susceptibility toward S. aureus SSTIs (24). Humans with abnormal T cell function (including HIV/AIDS patients), atopic dermatitis, and hyper-IgE syndrome display increased susceptibility toward S. aureus infection (25–27). Increased severity of skin infections has been observed in MyD88- and interleukin (IL)-1R-deficient mice, and to a lesser extent in Toll-like receptor (TLR)2-deficient mice. Compared with wild-type mice, MyD88-, IL-1R-, and TLR2-knockout animals develop larger skin lesions and elevated burden of staphylococci, which correlate with the decreased recruitment of neutrophils to the site of infection (28). Similar phenotypes were observed among mice lacking dendritic epidermal T cells or IL-17R (29). IL-17-producing T (TH17) lymphocytes play an important role in controlling S. aureus cutaneous infection by recruiting neutrophils to the site of infection. A growing body of evidence implicates TH17 cells as critically important for protection against S. aureus skin and lung infections; however, TH17/IL-17 responses may be less important for S. aureus infections in other tissues (30).
Bloodstream Infections
S. aureus is a leading cause of bacteremia, with an annual incidence of 4.3 to 38.2 per 100,000 person-years in the United States (31). The 30-day all-cause mortality of S. aureus bacteremia is 20% and has not changed since the 1990s (32). Based on its onset, bacteremia due to S. aureus can be classified into three categories: HA with hospital onset (33); HA with community onset (infection in an outpatient who has had recent, extensive contact with the health-care system); and community acquired (CA). In a prospective cohort study of 504 bloodstream infections at an academic center and two community hospitals in 2000, 35% were nosocomial, 37% were HA with community onset, and the remaining 28% were CA infections (34). MRSA predominated in nosocomial infections and in HA infections with community onset (61% and 52%, respectively); the MRSA burden was lower in patients with CA infections (14%).
The most prominent risk factor for invasive S. aureus infection and bacteremia is prosthetic devices, including central venous catheters, surgically implanted materials, and orthopedic prostheses (35). These devices serve as a direct conduit into the intravascular space, allowing S. aureus access to the bloodstream. Furthermore, intravenous drug use and underlying medical comorbidities such as diabetes, immunosuppressive therapy, and malignancy predispose individuals to S. aureus bacteremia (36).
STAPHYLOCOCCAL DEFENSES AGAINST COMPLEMENT AND PHAGOCYTES
S. aureus has evolved an arsenal of factors to evade innate immunity. Neutrophils represent the most abundant polymorphonuclear leukocyte population in blood and are a crucial defense against S. aureus (37). The pathogen, in turn, targets all stages of neutrophil recruitment as well as neutrophil effector functions. Staphylococci keep neutrophils from migrating toward the site of infection by impeding chemotaxis via the secretion of staphylococcal superantigen-like 5 and 10 (SSL5 and SSL10); chemotaxis inhibitory protein of S. aureus (CHIPS); and its homologs, formyl peptide receptor-like inhibitory proteins (FLIPr and FLIPr-like) (38–40). Further, SSL5 prevents neutrophils from rolling on endothelial cells, a crucial step in their recruitment process (41). This interference is aided by staphylococcal secretion of extracellular adherence protein, which blocks adherence of neutrophil ligands to cognate endothelial adhesion receptors (42). Interestingly, S. aureus can also stimulate neutrophil chemotaxis via the secretion of formylated PSMs (43).
Neutrophil phagocytosis and microbial clearance are facilitated when target microbes are opsonized by components of the complement system and/or immunoglobulins (Igs) (44). Complement can rapidly recognize and opsonize bacteria or directly kill bacteria via the formation of membrane attack complexes (45). Recognition of nonself and activation of the complement cascade occur by any one of three independent pathways; however, S. aureus diminishes or delays opsonization by targeting a universal step of complement activation: factor C3 and its activation complexes, also designated C3 convertases (44). S. aureus blocks C3 convertases via staphylococcal complement inhibitor (SCIN) or the SCIN-B and SCIN-C homologs or with extracellular fibrinogen binding protein and its homolog, extracellular complement binding protein (46). S. aureus secretes aureolysin, a metalloprotease that cleaves and inactivates C3 (47), and recruits complement inhibitory factors H and I via surface-anchored proteins SdrE and clumping factor A (ClfA), another pathway promoting C3 cleavage (48). Staphylococci target complement factor C5 via the secretion of SSL7 (49). To prevent opsonization by Ig, S. aureus evolved Ig-binding factors, such as staphylococcal protein A (SpA), staphylococcal binder of Ig, and SSL10; each of these factors blocks the Fcγ effector domain of opsonizing antibodies (50–52). Staphylococcal binder of Ig also targets C3 via factor H (52). Staphylokinase (Sak) generates enzymatically active plasmin that cleaves fibrin, Ig, and C3 deposited on the bacterial surface (53). Staphylococci have several means to generate a protective coat composed of polysaccharide capsule or fibrin; such coatings impede phagocytic uptake of staphylococci by neutrophils or macrophages (54, 55) (Figure 1).
Figure 1.
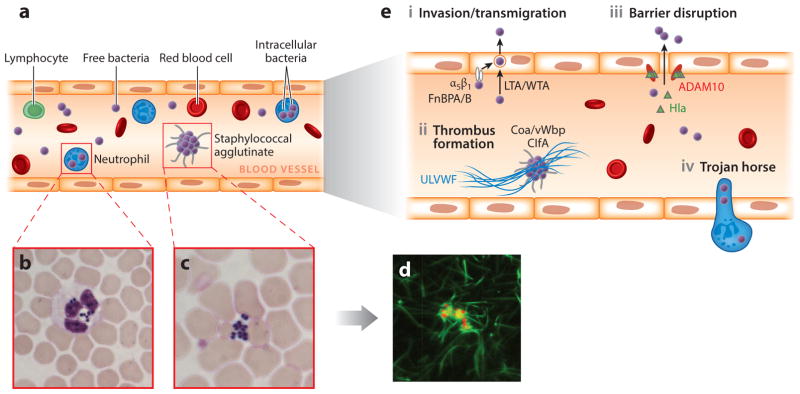
Survival of Staphylococcus aureus in the bloodstream and dissemination into host tissues. (a) Following intravenous inoculation of mice with ~107 colony-forming units (CFU), S. aureus survives in the bloodstream and disseminates from the vasculature into organ tissues. Bacteria from the initial inoculum cannot be detected in the bloodstream within 24 h. (b–c) Microscopy of Giemsa-stained blood samples from mice infected for 2 h reveals staphylococci that are (b) associated with neutrophils or (c) agglutinated outside of immune cells. Staphylococci phagocytosed by neutrophils deploy several virulence strategies that ensure their survival (see text for details). (d ) Extracellular S. aureus cocci expressing mCherry (red fluorescence) interact with fibrin ( green fluorescence) to generate large bacterial agglutinates covered with a shield of fibrin cables, thereby preventing phagocytosis. The image shows S. aureus mixed for 5 min with anticoagulated human plasma that has been supplemented with 5% Alexa488-conjugated human fibrinogen. Staphylococcal agglutination requires Coa/vWbp (coagulases) and prothrombin as well as ClfA (agglutinin) and fibrinogen. Agglutinins are S. aureus surface proteins that bind coagulase-derived fibrin cables to produce large aggregates of bacteria covered with fibrin. (e) Models for S. aureus exit from the bloodstream. (i ) FnBPA and FnBPB (staphylococcal surface proteins) bind fibronectin and interact with integrin α5β1 on the surface of the vascular endothelium, thereby triggering invasion of cells and transmigration. Wall teichoic acid (WTA) and lipoteichoic acid (LTA) of S. aureus, polymers in the bacterial envelope, have also been proposed to promote staphylococcal invasion of host cells. (ii ) Staphylococci induce formation of fibrin thrombi via Coa/vWbp- and ClfA-mediated agglutination and bind to von Willebrand factor (vWF) on endothelial surfaces, generating Ultra Large vWF (ULVWF) polymers. (iii ) Staphylococci secrete Hla, a toxin that binds to the ADAM10 receptor to disrupt the physiological barrier functions of the vascular endothelium. (iv) The Trojan horse model, whereby neutrophils with intracellular S. aureus extravasate to deliver bacteria into host tissues.
Neutrophils kill bacteria via phagocytosis, generating reactive oxygen and reactive nitrogen species and releasing antibacterial enzymes or peptide defensins from granules (37). Alternatively, neutrophils may release NETs (neutrophil extracellular traps), which are composed of neutrophil DNA, histones, antimicrobial peptides, and neutrophil proteases (56). S. aureus evades reactive oxygen species via staphyloxanthin, its yellow pigment and antioxidant, catalase, and other detoxifying enzymes that convert hydrogen peroxide to oxygen and water (57, 58). Reactive nitrogen species are detoxified by flavohemoglobin, a potent scavenger of host NO• (59). S. aureus metabolically adapts to circumvent the metabolic consequences of nitrosative stress, using lactate dehydrogenase to maintain redox hemostasis (60). Staphylococci fortify their cell wall envelope against muralytic enzymes and antimicrobial peptides using chemical modifications of peptidoglycan and synthesis of secondary cell wall polymers, including WTA and LTA (61, 62). Secreted factors such as Sak and aureolysin inactivate antimicrobial peptides by direct binding or proteolytic cleavage (63, 64). Finally, S. aureus secretes nuclease to degrade NETs, not only to escape DNA entrapment and peptide defensins, but also to generate deoxyadenosine, which triggers caspase-3-mediated death of immune cells (65, 66). Generation of deoxyadenosine and associated immune cell killing depend on staphylococcal adenosine synthase A, a surface protein that also accepts adenine nucleotides as substrates for the synthesis of adenosine and immunosuppressive signaling via adenosine receptors (66, 67).
Beyond provisions for escape from innate immunity to ensure staphylococcal survival in host tissues, S. aureus secretes toxins to disrupt epithelial or endothelial surfaces and to trigger lysis of immune cells (20). The most prominent cytolytic toxin is Hla, a pore-forming toxin with the capacity of lysing epithelial, endothelial as well as immune cells (69). Hla-mediated insults occur in a manner correlated with tissue-specific expression of ADAM10 (A-disintegrin and metalloprotease 10), the host receptor for Hla (69). Following Hla binding to ADAM10, the toxin assembles into a heptameric pore structure (70), causing cellular injury and activating signaling cascades that perturb the integrity of epithelial and endothelial surfaces (71). S. aureus strains produce three or more leukocidins, bi-component pore-forming toxins, where HlgAB, HlgCB, and LukAB (also known as LukGH) represent the core genome encoded virulence factors (72). Some S. aureus strains express one or two additional bi-component pore forming toxins, PVL and LukED (also known as LukMF) (72). Bi-component pore-forming toxins associate with specific receptors on myeloid cells, including complement and chemokine receptors or CD11b integrin (72). PSMs, small peptides with amino-terminal formyl groups that are secreted via a signal peptide independent mechanism (73), target eukaryotic cells, including immune cells, in a receptor-independent process and trigger their lysis (19); these toxic attributes depend on the unique charge distribution of individual PSMs (74).
STAPHYLOCOCCAL INTERFERENCE WITH HEMOSTASIS
S. aureus clots human or animal blood even in the presence of coagulation inhibitors such as warfarin, heparin, hirudin or calcium chelators (75). Staphylococcal coagulation is activated by two secreted proteins, Coa and vWbp, that bind prothrombin i.e., factor II of the blood coagulation pathway to generate enzymatically active staphylothrombin complexes (76, 77) (Figure 2a). Under physiological conditions, prothrombin activation requires cleavage by the prothrombinase complex composed of factors Va/Xa of the extrinsic blood coagulation pathway, also known as tissue factor pathway. Vascular damage initiates the tissue factor pathway and leads to the rapid and highly localized activation of a series of serine proteases that culminate with the accumulation of factors Va/Xa (78) (Figure 2a). Both thrombin and staphylothrombin complexes cleave the fibrinopeptides A and B off the fibrinogen α- and β-chains to generate fibrin (76, 77, 79). Fibrinogen is a soluble, 340-kDa dimer of trimers (α-, β-, and γ-chains), crosslinked by disulfides (79). When stripped of its fibrinopeptides, fibrinogen self-assembles into polymer cables to form a fibrin clot (79) that is further stabilized by factor XIIIa, a transglutaminase that crosslinks glutamine residues on one fibrin molecule to lysine residues of another. Factor XIIIa is itself the thrombin-cleaved product of the factor XIII zymogen (A2B2) (80).
Figure 2.
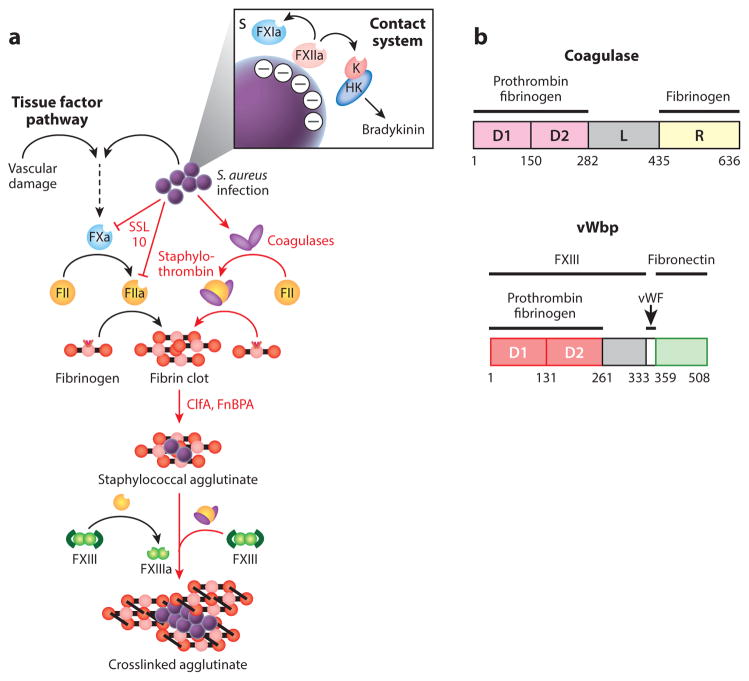
Agglutination and Staphylococcus aureus interference with host hemostasis. (a) Vascular damage and tissue factor or contact activation and bradykinin are pathways for hemostasis—a cascade of proteolytic activation for the zymogens of clotting factors culminating in the conversion of prothrombin (FII) to thrombin (FIIa), which cleaves fibrinopeptides A and B from fibrinogen, thereby triggering self-assembly of fibrin cables either to stem blood loss from injured vasculature or to immobilize pathogens that have activated the contact system. S. aureus bypasses the host hemostasis pathways to activate FII/prothrombin via the secretion of coagulases (Coa and vWbp), thus yielding staphylothrombin and, via nonproteolytic activation of fibrin-stabilizing factor (FXIII), the transglutaminase responsible for crosslinking fibrin cables. ClfA, an agglutinin of the surface of S. aureus, captures staphylothrombin-derived fibrin cables, thereby agglutinating staphylococci into large aggregates of bacteria enclosed by a fibrin shield. (b) A diagram illustrating the primary translational products of Coa and vWbp from S. aureus strain Newman; the catalytic D1 and D2 domains are shown in light and dark red boxes, and the R domain of coagulase with repeats is shown in yellow. The function of the linker domain (L) is not known. The number of repeats in the R region of coagulase varies between strains. The binding sites for prothrombin, fibrinogen, FXIII, and fibronectin are delineated. Abbreviation: vWF, von Willebrand factor.
Coa and vWbp are mosaic proteins made up of N-terminal D1 and D2 domains (D1–D2) that enable the association with and activation of prothrombin, whereas the C-terminal domains of the two molecules are distinct (81–83). A linker (L) domain connects the D1–D2 of Coa with the C-terminal repeat (R) region, composed of tandem repeats of a 27-residue peptide that bind fibrinogen (76) (Figure 2b). The C-terminal domain of vWbp includes a binding site for von Willebrand factor (vWF) (84) (Figure 2b). The vWbp-vWF interaction was identified via phage-display for recombinant vWbp (84); its biological significance remains unclear. The C-terminal part of vWbp recruits and activates factor XIII in a nonproteolytic manner and also binds to fibronectin (85) (Figure 2b). The crystal structure of the enzymatically active Coa·prothrombin complex revealed binding of the Coa D1–D2 to prothrombin and insertion of the Ile1-Val2 N terminus of Coa into the Ile16 pocket of prothrombin, inducing a functional active site in the zymogen through conformational change (76). Although vWbp·prothrombin complexes display slower enzyme kinetics than Coa·prothrombin, vWbp activates prothrombin by a similar mechanism (86). Staphylothrombins cleave the A and B fibrinopeptides of fibrinogen but do not cut any of the other substrates of thrombin. Thus, S. aureus, via the secretion of Coa and vWbp, usurps the zymogen of one clotting factor (prothrombin) to modify the coagulation cascade of human blood, thereby causing exuberant, uncontrolled polymerization of fibrin without activation of other clotting and inflammatory factors.
Staphylococcal coagulation itself is part of a reaction known as agglutination, i.e., the formation of a network of staphylococci with fibrin, which can be visualized by inoculating S. aureus into plasma and observing the assembly of large aggregates of bacteria (87). A related phenomenon is observed when staphylococci are mixed with purified fibrinogen, which causes smaller clumps of bacteria; however, bona fide agglutination requires the host factors prothrombin and fibrinogen (88) (Figure 1). In addition to Coa- and vWbp-mediated prothrombin activation, staphylococcal agglutination is dependent on agglutinins, sortase-anchored surface proteins such as ClfA or fibronectin-binding protein A (FnBPA), which bind to the D-domain of fibrin (87) (Figure 2a). ClfA and FnBPA bind to the C terminus of the fibrinogen γ-chain, capping staphylothrombin-polymerized fibrin cables and tethering these cables to the bacterial surface (87, 89). ClfA was initially identified as the staphylococcal factor required for bacterial clumping with soluble fibrinogen (90, 91). Recent analyses of the molecular mechanism of agglutination revealed the concerted action of Coa, vWbp, and ClfA in the pathogenesis of bloodstream infection (87). In contrast to mutants lacking any one gene (coa, vwb, or clfA), which displayed reductions in virulence, a strain with all three genes deleted was avirulent in a mouse model of bloodstream infection, suggesting a synergistic mode of action between staphylococcal factors for hemostatic interference (87). Of note, ClfA and other S. aureus surface proteins with C-terminal serine-aspartate (SD) repeats are N-acetylglucosaminylated, posttranslational modifications that contribute to S. aureus agglutination in animal or human blood (92). The molecular mechanism whereby N-acetylglucosaminylation of SD repeat proteins contributes to staphylococcal agglutination is not known.
The product of agglutination endows S. aureus with a fibrin shield, effectively protecting bacteria from uptake by phagocytes. Of note, the coagulases fulfill nonredundant roles in establishing the protective fibrin barrier: vWbp has been shown to recruit factor XIII through its interaction with prothrombin and fibrinogen, leading to the fortification of the nascent fibrin clot with covalent crosslinks (93) (Figure 2a). Coa, by contrast, has the unique ability to associate with a molar excess of fibrinogen via its C-terminal repeat (R) domain, comprising up to twelve tandem repeats of a conserved 27-amino-acid peptide (94). This interaction allows Coa to localize at the cell surface of S. aureus by binding to fibrinogen retained by cell wall anchored proteins such as ClfA and favors formation of a staphylothrombin-generated fibrin shield in the immediate vicinity of the staphylococci (L. Thomer, unpublished). These findings explain earlier observations of cell-bound (Coa) and cell-free coagulases (vWbp) (95).
The hemostatic system is responsible for preventing the dissemination of microbial invaders (96). Microbe-specific activation of the coagulation cascade is mediated by the intrinsic pathway, also known as contact system, and is activated by negatively charged structures present on the surface of bacteria (44) (Figure 2a). Such structures are recognized by factor XII, which subsequently auto-activates into factor XIIa (97). Factor XIIa, in turn, converts prekallikrein and factor XI into their active proteolytic forms, kallikrein and FXIa, respectively (97). FXIa converges with the extrinsic coagulation cascade and ultimately leads to fibrin clot formation, while activated plasma kallikrein cleaves the nonenzymatic cofactor high-molecular-weight kininogen and releases the proinflammatory peptide bradykinin (97). As recently reviewed by Berends et al. (44), the antibacterial effects of coagulation are manifold; first and foremost, bacteria become immobilized inside a fibrin meshwork, preventing their spread into surrounding tissues. Bradykinin, by contrast, has direct antimicrobial activity, functions as a chemoattractant, and induces vascular leakage, thereby recruiting additional immune cells (44). Peptides derived from other coagulation factors, such as thrombin or fibrinogen, have also been shown to be antibacterial, whereas fibrinopeptides A and B are potent chemoattractants (44). The relevance of this innate defense mechanism has been demonstrated for Streptococcus pyogenes, the causative agent of human pharyngitis and skin infections (98, 99).
Whereas the activation of the coagulation cascade represents a universal host defense against bacterial infections, S. aureus has evolved unique virulence strategies that usurp the hemostatic system for pathogen survival and replication in infected tissues. By circumventing both branches of the physiological blood coagulation cascade and activating prothrombin in a nonproteolytic manner that restricts the substrate specificity of the resulting staphylothrombin complex to fibrinogen, S. aureus avoids the vast majority of proinflammatory signaling described above. In fact, the bacterium secretes SSL10 to inhibit host blood coagulation by binding to the γ-carboxyglutamic acid domain of prothrombin and factor Xa (100) (Figure 2a). SSL10 only weakly interferes with staphylothrombin activity, indicating that its main function is to dampen a potential activation of the physiological coagulation cascade via the contact pathway while allowing staphylococcal coagulation to occur (100).
STAPHYLOCOCCAL INTERFERENCE WITH FIBRINOLYSIS
Fibrinolysis is the reversal of coagulation, as fibrin clots are being degraded by proteases and injured tissues are being repaired (101). A key factor for the initiation of fibrinolysis is plasminogen, a plasma zymogen. During repair, plasminogen is converted to enzymatically active plasmin by host plasminogen activators, urokinase or tissue type plasminogen activator (101). Similar to the coagulation cascade, fibrinolysis can be viewed as a cascade of protease activation and inhibitory events that are integrated with coagulation and inflammation as well as wound healing and angiogenesis. S. aureus secretes Sak, which binds plasmin and modifies its active site for catalytic activity (102). The Sak-plasmin complex is highly fibrin-specific and subject to rapid inhibition by the endogenous plasmin inhibitor α-2-antiplasmin unless it is bound to fibrin (103). Sak·plasminogen activity is therefore restricted to areas of fibrin deposition. In contrast to coa, vwb and clfA, which belong to the core genome of S. aureus, sak, the structural gene for Sak, is located in the immune evasion cluster of hlb-converting phages along with scn and chp (104).
Interestingly, sak expression is inversely correlated with a lethal outcome of S. aureus bacteremia, as sak-deficient isolates are four times more likely to cause death among patients (105). The high frequency of Sak production in nasal isolates suggests that Sak expression may be an adaptive mechanism of S. aureus colonization of its human host (105). Analyses on the contributions of sak toward S. aureus colonization and pathogenesis are confounded by the fact that Sak is selective for human plasminogen but inactive for plasminogen from many other vertebrates (105, 106). When the contribution of Sak to systemic infection was interrogated with transgenic mice expressing mouse and human plasminogen, sak expressing S. aureus strains displayed reduced virulence as evidenced by reduced mortality of infected animals and lower bacterial burden in infected organ tissues (107).
Given the importance of staphylothrombin-mediated fibrin deposition for bloodstream infection and formation of abscess lesions in organ tissues (vide infra) and the seemingly contradictory activities of pro- and anticoagulant virulence factors, Sak may play a role in disease manifestations such as SSTIs rather than bloodstream infections. In support of this hypothesis, plasminogen activation by Sak was shown to enhance local spreading of S. aureus in a mouse skin infection model (108). Of note, despite enhanced virulence in the skin, Sak expression did not lead to systemic dissemination of bacteria, suggesting that the virulence mechanism of Sak may be restricted to the dermal microenvironment (108).
STAPHYLOCOCCAL EXIT FROM THE BLOODSTREAM
In order to disseminate to organ tissues and seed abscess lesions, S. aureus needs to leave the vasculature. Most adhesion factors identified to date are members of the MSCRAMM family and rely on bridging molecules present in plasma or released by endothelial cells for their adhesive properties (109). Most prominent in this context are FnBPA and FnBPB, which were shown to significantly contribute to adherence of S. aureus to endothelial cells in vitro and in vivo (110, 111) (Figure 1e). Their role in escape from the bloodstream is particularly intriguing because they have also been implicated in staphylococcal invasion of host cells. The fibronectin-binding repeats of FnBPs engage multiple fibronectin molecules, which, in turn, are recognized by the α5β1 integrin on the endothelial surface to trigger bacterial uptake (112). Although it is unclear how staphylococci exit endothelial cells on their basal face, the contribution of FnBPA-mediated invasion to bloodstream infection was demonstrated in a murine model (113). Of note, staphylococcal strains expressing fnbA alleles with fewer fibronectin-binding repeats were observed to be defective in endothelial cell invasion and subsequent systemic spread, yet these strains promoted S. aureus survival in blood as well as immune signaling (113).
Teichoic acids, anionic glycopolymers found in the cell envelope of many different Gram-positive bacteria, have been implicated as nonproteinaceous adhesion and endothelial invasion factors. WTA was found to contribute to S. aureus attachment to endothelial cells in vitro, leading to reduced dissemination into renal tissues in a rabbit model of infective endocarditis (61). LTA, on the other hand, despite not contributing to adhesion to endothelial cells, affected invasion of endothelial cells and dissemination into brain tissue (114). The receptors for WTA or LTA on endothelial cells and the molecular mechanisms for uptake of S. aureus into these cells are, however, not known (Figure 1e).
vWF, a large glycoprotein stored in Weibel-Palade bodies of endothelial cells, is released in multimeric form into the bloodstream following vascular injury. vWF binds FVIII, collagen and platelets, forming a complex with platelet glycoprotein to quench blood loss, effectively coordinating platelet deposition and fibrin formation at sites of vascular injury (115). vWF has been implicated in S. aureus adhesion to vascular endothelia under shear stress (116), and secreted vWbp has been implicated as a bridging molecule for this event (117). vWbp is secreted into the extracellular milieu and not associated with bacterial surfaces (9); thus, it is not clear how vWbp and vWF may promote S. aureus adhesion to the vasculature. Alternatively, vWbp·prothrombin derived fibrin fibers may promote endothelial adherence by inducing microthrombi with staphylococci embedded with fibrin shields (117). This model highlights a possible route for S. aureus to invade organ tissues: blocking blood flow in the microvasculature via staphylococcal agglutination (Figure 1e). This mechanistic model may be correlated with the clinical phenomenon of disseminated intravascular coagulation, as occurs during S. aureus sepsis in humans and in a pig model for bloodstream infection (118).
Another important route for staphylococcal exit from the vasculature may be the disruption of endothelial barriers. In addition to its pore-forming activity, Hla binds to the receptor, ADAM10, and upregulates its metalloprotease activity (119). By cleaving vascular endothelial cadherin, which engages in homotypic intercellular interactions, ADAM10 compromises endothelial barrier function and promotes vascular leakage (119) (Figure 1e). Although this has not yet been experimentally demonstrated, S. aureus may exploit the increased vascular permeability for entry into host tissues.
As reported for several other bacterial pathogens, S. aureus may rely on professional phagocytes for extravasation and dissemination (Figure 1e). Considering its arsenal of immune evasion factors providing for intracellular survival, S. aureus can reside in the intracellular vacuoles of monocytes or macrophages for several days or enter the cytoplasm of immune cells (120). Intracellular survival was shown to require several virulence factors, including SpA, SrtA, and aureolysin, and may provide opportunities for staphylococcal travels from the bloodstream into organ tissues (120). To study the possibility that phagocytes may act as Trojan horses for the travels of staphylococci, mouse neutrophils were isolated from the peritoneal cavity of mice infected with S. aureus and transferred into naïve mice: infected neutrophils were indeed sufficient to establish abscess lesion (121).
STAPHYLOCOCCAL ABSCESS FORMATION AND PATHOGENESIS
S. aureus is endowed with several mechanisms to resist and escape killing in the bloodstream of infected hosts, and the pathological product of S. aureus invasion is the formation of deep-seated abscess lesions. Herein simply referred to as abscesses, these lesions differ from superficial skin lesions (17). In principle, abscess formation is considered a default response to biological (infectious), chemical and physical trauma, whereby damaged tissues are encapsulated, liquefied, drained and eventually repaired. A notable feature of staphylococcal abscess lesions is the massive infiltration of immune cells, responding to cellular destruction and proinflammatory signals, and resulting in the liquefaction of tissue into purulent exudates that are eventually drained. Unlike S. aureus, however, entry of S. epidermidis or other nonpathogenic or opportunistic pathogens does not elicit abscess lesions and purulent exudate in humans or experimental animals. Further, mutations in virulence genes diminish the ability of S. aureus to establish purulent abscess lesions in experimental animals (122). When studied in mice, histopathological analyses of S. aureus lesions in renal tissues revealed a pathogen-driven, developmental process, supporting staphylococcal replication and dissemination (122).
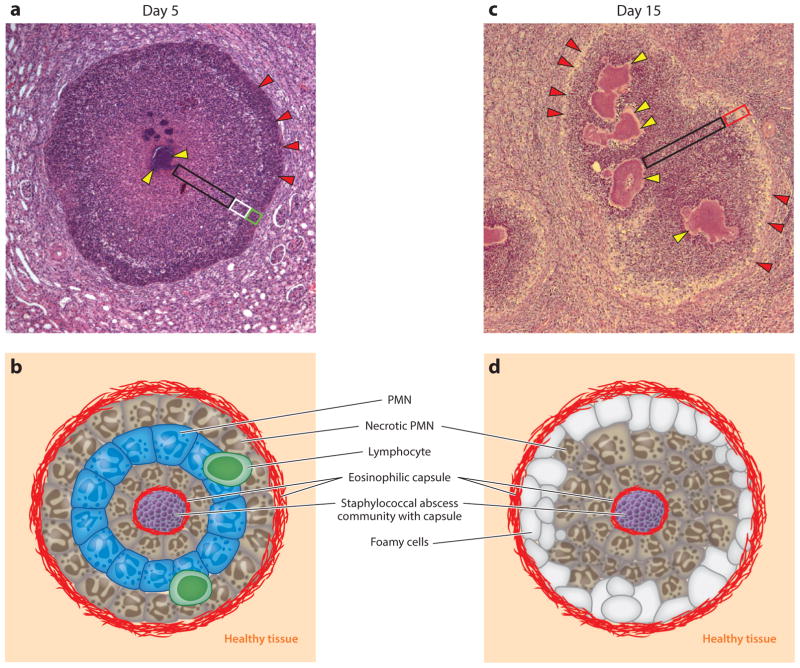
Earlier work described four stages of staphylococcal abscesses formation (123). Following entry into the bloodstream, S. aureus blocks innate defenses of its host and enters organ tissues. During this first stage (stage I), a characteristic drop in the staphylococcal load is observed in the bloodstream within hours of inoculation and this drop is accompanied by an increased burden of S. aureus in many different organ tissues (122). Staphylococcal invasion of host tissues, in turn, triggers tissue infiltration with large numbers of immune cells, predominantly neutrophils and macrophages (stage II). As early as two days post infection, these immune cell infiltrates are readily visualized in hematoxylin-eosin stained thin-sectioned tissue (122). During stage II, staphylococci in infected tissues display no discernable organization and appear to reside mostly within neutrophils (122). Within the next 48–72 h, the morphological features of S. aureus abscesses are profoundly altered, with staphylococcal abscess communities at the center of the lesion, enclosed by fibrin deposits, and surrounded by concentric layers of immune cells (Figure 3). Eosinophilic deposits, likely made up of fibrin, demarcate stage III lesions from healthy, uninfected tissues. During stage III, immune cell infiltrates are initially made up of neutrophils, arranged in concentric layers of apoptotic, healthy, and apoptotic appearance with other immune cells, for example macrophages and lymphocytes, at the peripheries of lesions (66, 122) (Figure 3a). Staphylococcal abscesses display multiple morphological changes over time. By day 15 post infection, SACs have expanded; most immune cells appear necrotic, with cellular detritus accumulating; and foam cells (macrophages) are positioned at the periphery (Figure 3b). Eventually, T and B lymphocytes as well as macrophages infiltrate lesions, which are slowly pushed toward organ surfaces and eventually rupture, releasing purulent exudate and staphylococci for renewed entry into the bloodstream or dissemination to new hosts (stage IV) (122).
Figure 3.
Staphylococcus aureus abscess lesions in murine renal tissue. (a) Microscopy image of hematoxylin-eosin-stained, thin-sectioned renal tissue 5 days following intravenous inoculation with S. aureus. Bacteria are organized into staphylococcal abscess communities (SACs) ( yellow arrowheads) at the center of the lesion. Depending on sectioning, SACs may appear fragmented; however, bacterial communities remain connected and are enclosed by eosinophilic deposits of fibrin and surrounded by zones of necrotic neutrophils (black box), healthy-appearing neutrophils (white box), and necrotic immune cells ( green box). Abscess lesions are demarcated from renal tissues by another layer of eosinophilic deposits (red arrowheads), through which immune cells enter the lesion. (b) Schematic illustrating the histopathology features of a 5-day-old S. aureus abscess lesion. (c) Microscopy image of hematoxylin-eosin-stained, thin-sectioned renal tissue 15 days following intravenous inoculation with S. aureus. The 15-day-old lesion differs from the 5-day-old lesion in that SACs have expanded and the layering of healthy and necrotic immune cells has been supplanted by diffuse infiltrates of immune cells, cellular detritus, liquefaction (black box), and foam cells positioned at the periphery of the lesion (red box). SACs remain enclosed by fibrin deposits ( yellow arrowheads). An outer eosinophilic layer (red arrowheads) demarcates the lesion from large numbers of immune cells and from the cellular detritus that has replaced renal tissue architecture in the vicinity of the abscess lesion. (d ) Schematic illustrating the histopathology features of a 15-day-old S. aureus abscess lesion. Abbreviation: PMN, polymorphonuclear leukocyte.
S. aureus lacking sortase A (ΔsrtA) cannot establish abscess lesions following bloodstream infection of mice, and the ΔsrtA mutant cannot persist in infected tissues over a period of 15 days (122). S. aureus mutants with defects in individual surface protein genes retain their ability to generate abscess lesions, indicating that the ΔsrtA phenotype is caused by the cumulative defect for all sortase-anchored surface proteins. Nonetheless, lesions derived from variants with mutations in clfA, clfB, isdC or sdrC were characterized by reduced staphylococcal load in infected tissues, whereas sdrD, isdA, isdB or spa mutants exhibited reduced numbers of abscess lesions (122) (Table 1). These data suggest that the heme-scavenging proteins IsdA, IsdB, and IsdC promote staphylococcal replication in infected tissues, likely providing iron from host hemoglobin and haptoglobin to support the nutritional requirements of S. aureus. The role of SdrD in the pathogenesis of S. aureus bloodstream infection and abscess formation is not known. A member of the MSCRAMM and SD repeat families of surface proteins, SdrD mediates staphylococcal adherence to desquamated epithelial cells (124). It seems plausible that SdrD may exert additional ligand activities supporting S. aureus abscess formation during stages II–III (Table 1). SpA binds Igs via their Fcγ domain, which inhibits opsonization and phagocytosis of bacteria (125). In fact, S. aureus expressing spaKK, a SpA variant that no longer binds to the Fcγ domain of Igs, is more readily phagocytosed and killed in the bloodstream of infected mice resulting in reduced numbers of abscess lesions (125). SpA B cell superantigen activity and escape from humoral immune responses is based on its ability to crosslink the Fab heavy chains of VH3 clan B cell receptors (126); this activity is, however, not required for S. aureus abscess formation in mice (125).
Table 1.
Secreted virulence factors and their possible contributions to abscess formation and pathology
| Secreted virulence factors involved in survival in the bloodstream | |||
|---|---|---|---|
| Name | Gene | Proposed functiona | Virulence defect associated with loss of geneb |
| Adenosine synthase | adsA | Synthesis of immune suppressors Ado, dAdo | Decreased survival in blood |
| Nuclease | nuc | Degradation of NETs | Decreased survival in blood |
| Clumping factor A | clfA | Agglutination | Increased phagocytic uptake |
| Coagulases |
coa vwb |
Agglutination | Increased phagocytic uptake |
| Protein A (SpA) | spa | Binding of the Fcγ domain of Igs | Increased opsonization and phagocytosis of bacteria |
| Leukocidins |
hlgAB hlgCB lukAB |
Lysis of leukocytes | Unknown Unknown Decreased survival in blood |
| Fibronectin-binding repeat protein A, B |
fnBPA fnBPB |
Recognition of fibronectin-FnBR complexes by α5β1 integrin; fibrinogen binding | Unknown |
| Staphylococcal complement inhibitor (SCIN), SCIN-B, SCIN-C |
scin scinB scinC |
Blockage of C3 convertases | Unknown |
| Extracellular complement–binding protein | ecb | Blockage of C3 convertases | Unknown |
| Aureolysin | aur | Degradation of antimicrobial peptides | Unknown |
| Secreted virulence factors required to breach the bloodstream | |||
| α-Hemolysin | hla | Disruption of epithelial or endothelial surfaces | Reduced bacterial loads in organs |
| Serine Aspartic Repeat protein C, D |
sdrC sdrD |
Members of the MSCRAMM, ligands unknown | Reduced bacterial loads in organs |
| Secreted factors required for bacterial replication and persistence in abscesses | |||
| Iron surface determinant A, B, C |
isdA isdB isdC |
Heme iron scavenging | Reduced bacterial loads |
| Coagulases |
coa vwb |
Coagulation, agglutination | Reduced bacterial loads and numbers of abscesses |
| Protein A (SpA) | spa | B cell superantigen activity that prevents humoral immune response | Reduced bacterial loads and numbers of abscesses in organs |
| Lipoprotein diacyl-glyceride transferase | lgt | Lipidation of lipoproteins | Hypervirulence, abscesses devoid of immune cells due to loss of TLR2 ligands |
| Extracellular adherence protein | eap | Blockade of neutrophil adherence to endothelia | Reduced bacterial loads in organs |
| Extracellular fibrinogen–binding protein | efb | Blockade of C3 convertase; fibrinogen binding | Reduced bacterial loads in organs |
| Phenol soluble modulins α3 | psmα3 | Generation of inflammatory signals for the recruitment of immune cells | Increased bacterial loads in organs |
| Staphylococcal superantigen-like 5, 10 |
ssl5 ssl10 |
Impediment of neutrophil chemotaxis | Unknown |
| Chemotaxis inhibitory protein | chips | Impediment of neutrophil chemotaxis | Unknown |
| Formyl peptide receptor-like inhibitory proteins |
flipr flipr-like |
Impediment of neutrophil chemotaxis | Unknown |
The proposed function is derived from in vitro biochemical experimentation.
Virulence defect was tested as survival of bacteria in whole blood or enumeration of bacterial loads in animal organ tissues following infection.
Secreted Coa is found in the immediate proximity of staphylococcal abscess communities and their surrounding fibrin deposits, whereas vWbp is localized throughout and in the periphery of abscess lesions (77) (Table 1). In an effort to dissect the relative contribution of Coa and vWbp to fibrin deposition even further, staphylococcal abscess formation was reconstituted in a three-dimensional collagen matrix supplemented with host fibrinogen and prothrombin (55). Formation of two concentric fibrin structures was observed, with Coa being involved in formation of the inner fibrin capsule and vWbp giving rise to an outer fibrin meshwork (55). Using deletion mutants, both fibrin layers were shown to protect the central staphylococcal community against attacking neutrophils (55). In addition to helping staphylococci escape phagocytic clearance in the bloodstream, the two coagulases, likely in tandem with ClfA, also contribute to abscess development by concerting the formation of the distinct layers described above.
Immune cells, in particular neutrophils, are an integral part of abscess lesions. This begs the question of how staphylococci strike a balance between blocking their own clearance from these phagocytes and recruiting cells to develop abscess lesions for their dissemination to new sites and or new hosts. It seems unlikely that the dramatic infiltration of immune cells at stage II of abscess formation can be explained by massive bacterial replication because colonies of staphylococci are not discernable early during infection (122). Instead, S. aureus seems to actively recruit immune cells through the release of proinflammatory molecules perceived by innate immune sensors such as TLRs. This conjecture is supported by the observation that S. aureus mutants lacking lipoprotein diacyl-glyceride transferase (Lgt), which catalyzes diacyl-glycerol modification of lipoprotein precursors, are defective in the recruitment of immune cells (127). Although lgt mutants can replicate in host tissues, mutant colonies are not surrounded by neutrophils and do not form typical abscess lesions (127). More recently, it was found that the clonal complex 30 MRSA lineage, which is associated with HA bacteremia and high occurrence of metastatic abscesses, expresses an allelic variant of PSMα3 that shows reduced cytolytic and chemotactic potential toward human neutrophils (128). In a mouse model of renal abscess formation, infection with this PSMα3 allele was correlated with increased bacterial load and increased numbers of infectious lesions in renal tissues (128) (Table 1). As described above, PSMs are recognized by formyl peptide receptors to elicit proinflammatory signals for the recruitment of immune cells to staphylococcal abscess lesions (128). Whereas increased recruitment of immune cells may precipitate staphylococcal elimination, diminished recruitment, for example due to secretion of an altered PSM, may provide staphylococci with the means for escape from innate defenses and persistence in host tissues. Although not tested experimentally, the products of SSL5, SSL10, CHIPS, and FLIPr, by impeding chemotaxis of neutrophils, may also contribute to the maturation and pathology of abscess lesions (Table 1).
PERSPECTIVES
Vaccines that protect humans against bacterial pathogens elicit antibody responses to either neutralize a key virulence factor, for example Corynebacterium diphtheriae diphtheria toxin (129), or induce opsonophagocytic killing of the invading pathogen, typically by binding to bacterial capsular polysaccharides (130, 131). The latter are currently used to establish protective immunity against Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumonia (132). Applying similar approaches to S. aureus vaccine development has thus far not proved successful, as neither antibodies against staphylococcal polysaccharide (133) nor antibodies against secreted virulence factors could protect humans against staphylococcal disease (134, 135). Such failures can be explained by the multifactorial, parallel strategies that S. aureus deploys toward disease establishment and by the wide spectrum of the pathogen’s immune evasive attributes that block opsonophagocytic killing. In other words, S. aureus secretes so many virulence factors that formylation of subunit vaccines would have to involve dozens of antigens and does not seem feasible. Further, opsonophagocytosis of staphylococci is blocked by the microbe’s agglutination pathway, by complement evasion factors and by staphylococcal protein A, each presenting formidable obstacles toward antibody-induced clearance of S. aureus. Thus, the development of S. aureus vaccines is indeed a daunting task. Recent work has explored new vaccine strategies by targeting the immune-evasive attributes of the pathogen (136–138). Although it is not possible to predict success for such endeavors, the approaches are certainly innovative and may stimulate parallel work on vaccines for other pathogens with expertise at evading human immune responses, including Mycobacterium tuberculosis.
Acknowledgments
We thank Hwan Keun Kim for help with histopathology samples and Wenqi Yu for assistance with fluorescence microscopy experiments. This work was supported by grants AI038897 (O.S.), AI052474 (O.S.), and AI110937 (D.M.) from the National Institute of Allergy and Infectious Diseases and Grant 14PRE19910021 (L.T.) from the American Heart Association.
Footnotes
DISCLOSURE STATEMENT
O.S. and D.M. are named inventors of patent applications related to the development of Staphylococcus aureus vaccines.
LITERATURE CITED
- 1.van Belkum A, Melles DC, Nouwen J, van Leeuwen WB, van Wamel W, et al. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect Genet Evol. 2009;9:32–47. doi: 10.1016/j.meegid.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 2.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Bayer AS, Cosgrove SE, Daum RS, Fridkin SK, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 4.Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:16. doi: 10.3389/fcimb.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogston A. Report upon micro-organisms in surgical diseases. Br Med J. 1881;1:369–75. doi: 10.1136/bmj.1.1054.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogston A. Micrococcus poisoning. J Anat Physiol. 1882;17:24–58. [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbach FJ. Mikroorganismen bei den Wund-Infections-Krankheiten des Menchen. Wiesbaden, Germany: Bergmann; 1884. [Google Scholar]
- 8.Clauditz A, Resch A, Wieland KP, Peschel A, Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74:4950–53. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLOS Pathog. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guardabassi L, Schmidt KR, Petersen TS, Espinosa-Gongora C, Moodley A, et al. Mustelidae are natural hosts of Staphylococcus delphini group A. Vet Microbiol. 2012;159:351–53. doi: 10.1016/j.vetmic.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 12.Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–48. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 13.Hartman BJ, Tomasz A. Low affinity penicillin binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–16. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubukata K, Nonoguchi R, Matsuhashi M, Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989;171:2882–85. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32:S114–32. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 16.Sreeramoju P, Porbandarwalla NS, Arango J, Latham K, Dent DL, et al. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg. 2011;201:216–20. doi: 10.1016/j.amjsurg.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518–27. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, et al. Targeting of α-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–58. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1418–20. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 20.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13:584–94. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. PNAS. 2010;107:5587–92. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–73. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn BL, Onunkwo CC, Watts CJ, Sohnle PG. Systemic dissemination and cutaneous damage in a mouse model of staphylococcal skin infections. Microb Pathog. 2009;47:16–23. doi: 10.1016/j.micpath.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–84. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 25.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 26.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–76. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–57. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, et al. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395–405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank KM, Zhou T, Moreno-Vinasco L, Hollett B, Garcia JG, Bubeck Wardenburg J. Host response signature to Staphylococcus aureus α-hemolysin implicates pulmonary TH17 response. Infect Immun. 2012;80:3161–69. doi: 10.1128/IAI.00191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland TL, Arnold C, Fowler VG., Jr Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312:1330–41. doi: 10.1001/jama.2014.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev. 2012;25:362–86. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klevens RM, Edwards JR, Gaynes RP. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–30. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 34.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–97. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 35.Jensen AG, Wachmann CH, Poulsen KB, Espersen F, Scheibel J, et al. Risk factors for hospital-acquired Staphylococcus aureus bacteremia. Arch Intern Med. 1999;159:1437–44. doi: 10.1001/archinte.159.13.1437. [DOI] [PubMed] [Google Scholar]
- 36.Musher DM, Lamm N, Darouiche RO, Young EJ, Hamill RJ, Landon GC. The current spectrum of Staphylococcus aureus infection in a tertiary care hospital. Medicine. 1994;73:186–208. doi: 10.1097/00005792-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol. 2013;67:629–50. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 38.Bestebroer J, van Kessel KP, Azouagh H, Walenkamp AM, Boer IG, et al. Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood. 2009;113:328–37. doi: 10.1182/blood-2008-04-153882. [DOI] [PubMed] [Google Scholar]
- 39.de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, van Wamel WJ, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–95. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prat C, Bestebroer J, de Haas CJ, van Strijp JA, van Kessel KP. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J Immunol. 2006;177:8017–26. doi: 10.4049/jimmunol.177.11.8017. [DOI] [PubMed] [Google Scholar]
- 41.Bestebroer J, Poppelier MJ, Ulfman LH, Lenting PJ, Denis CV, et al. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood. 2007;109:2936–43. doi: 10.1182/blood-2006-06-015461. [DOI] [PubMed] [Google Scholar]
- 42.Chavakis T, Hussain M, Kanse SM, Peters G, Bretzel RG, et al. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med. 2002;8:687–93. doi: 10.1038/nm728. [DOI] [PubMed] [Google Scholar]
- 43.Forsman H, Christenson K, Bylund J, Dahlgren C. Receptor-dependent and -independent immunomodulatory effects of phenol-soluble modulin peptides from Staphylococcus aureus on human neutrophils are abrogated through peptide inactivation by reactive oxygen species. Infect Immun. 2012;80:1987–95. doi: 10.1128/IAI.05906-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berends ET, Kuipers A, Ravesloot MM, Urbanus RT, Rooijakkers SH. Bacteria under stress by complement and coagulation. FEMS Microbiol Rev. 2014;38:1146–71. doi: 10.1111/1574-6976.12080. [DOI] [PubMed] [Google Scholar]
- 45.Müller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–47. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 46.Jongerius I, Kohl J, Pandey MK, Ruyken M, van Kessel KP, et al. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med. 2007;204:2461–71. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laarman AJ, Ruyken M, Malone CL, van Strijp JA, Horswill AR, Rooijakkers SH. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol. 2011;186:6445–53. doi: 10.4049/jimmunol.1002948. [DOI] [PubMed] [Google Scholar]
- 48.Hair PS, Echague CG, Sholl AM, Watkins JA, Geoghegan JA, et al. Clumping factor A interaction with complement factor I increases C3b cleavage on the bacterial surface of Staphylococcus aureus and decreases complement-mediated phagocytosis. Infect Immun. 2010;78:1717–27. doi: 10.1128/IAI.01065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bestebroer J, Aerts PC, Rooijakkers SH, Pandey MK, Kohl J, et al. Functional basis for complement evasion by staphylococcal superantigen-like 7. Cell Microbiol. 2010;12:1506–16. doi: 10.1111/j.1462-5822.2010.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itoh S, Hamada E, Kamoshida G, Takeshita K, Oku T, Tsuji T. Staphylococcal superantigen-like protein 5 inhibits matrix metalloproteinase 9 from human neutrophils. Infect Immun. 2010;78:3298–305. doi: 10.1128/IAI.00178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forsgren A, Quie PG. Effects of staphylococcal protein A on heat labile opsonins. J Immunol. 1974;112:1177–80. [PubMed] [Google Scholar]
- 52.Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun. 2011;79:3801–9. doi: 10.1128/IAI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rooijakkers SH, van Wamel WJ, Ruyken M, van Kessel KP, van Strijp JA. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005;7:476–84. doi: 10.1016/j.micinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 54.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–34. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guggenberger C, Wolz C, Morrissey JA, Heesemann J. Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLOS Pathog. 2012;8:e1002434. doi: 10.1371/journal.ppat.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–35. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 57.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–15. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandell GL. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal–leukocyte interaction. J Clin Investig. 1975;55:561–66. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson AR, Dunman PM, Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol. 2006;61:927–39. doi: 10.1111/j.1365-2958.2006.05290.x. [DOI] [PubMed] [Google Scholar]
- 60.Richardson AR, Libby SJ, Fang FC. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science. 2008;319:1672–76. doi: 10.1126/science.1155207. [DOI] [PubMed] [Google Scholar]
- 61.Weidenmaier C, Peschel A, Kempf VA, Lucindo N, Yeaman MR, Bayer AS. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect Immun. 2005;73:8033–38. doi: 10.1128/IAI.73.12.8033-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herbert S, Bera A, Nerz C, Kraus D, Peschel A, et al. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLOS Pathog. 2007;3:e102. doi: 10.1371/journal.ppat.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172:1169–76. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 64.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wojcik K, Puklo M, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus--derived proteinases. Antimicrob Agents Chemother. 2004;48:4673–79. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–86. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus conversion of neutrophil extracellular traps into deoxyadenosine promotes immune cell death. Science. 2013;342:863–66. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206:2417–27. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin-mediated cellular injury. PNAS. 2010;107:13473–78. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–66. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 71.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–14. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alonzo F, 3rd, Torres VJ. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, et al. Essential Staphylococcus aureus toxin export system. Nat Med. 2013;19:364–67. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol. 2013;11:667–73. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Much H. Über eine Vorstufe des Fibrinfermentes in Kulturen von Staphylokokkus aureus. Biochem Z. 1908;14:143–55. [Google Scholar]
- 76.Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–39. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 77.Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLOS Pathog. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams RL, Bird RJ. Review article: coagulation cascade and therapeutics update: relevance to nephrology. Part 1: overview of coagulation, thrombophilias and history of anticoagulants. Nephrology. 2009;14:462–70. doi: 10.1111/j.1440-1797.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 79.Doolittle RF. Structural basis of the fibrinogen-fibrin transformation: contributions from X-ray crystallography. Blood Rev. 2003;17:33–41. doi: 10.1016/s0268-960x(02)00060-7. [DOI] [PubMed] [Google Scholar]
- 80.Ware S, Donahue JP, Hawiger J, Anderson WF. Structure of the fibrinogen gamma-chain integrin binding and factor XIIIa cross-linking sites obtained through carrier protein driven crystallization. Protein Sci. 1999;8:2663–71. doi: 10.1110/ps.8.12.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panizzi P, Friedrich R, Fuentes-Prior P, Bode W, Bock PE. The staphylocoagulase family of zymogen activator and adhesion proteins. Cell Mol Life Sci. 2004;61:2793–98. doi: 10.1007/s00018-004-4285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panizzi P, Friedrich R, Fuentes-Prior P, Richter K, Bock PE, Bode W. Fibrinogen substrate recognition by staphylocoagulase·(pro)thrombin complexes. J Biol Chem. 2006;281:1179–87. doi: 10.1074/jbc.M507956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. PNAS. 2009;106:7786–91. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bjerketorp J, Nilsson M, Ljungh A, Flock JI, Jacobsson K, Frykberg L. A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology. 2002;148:2037–44. doi: 10.1099/00221287-148-7-2037. [DOI] [PubMed] [Google Scholar]
- 85.Thomer L, Schneewind O, Missiakas D. Multiple ligands of von Willebrand factor-binding protein (vWbp) promote Staphylococcus aureus clot formation in human plasma. J Biol Chem. 2013;288:28283–92. doi: 10.1074/jbc.M113.493122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. PNAS. 2009;106:7786–91. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLOS Pathog. 2011;7:20. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Birch-Hirschfeld L. Über die Agglutination von Staphylokokken durch Bestandteile des Säugetierblutplasmas. Klin Woschenschrift. 1934;13:331–33. [Google Scholar]
- 89.Ganesh VK, Rivera JJ, Smeds E, Ko Y-P, Bowden MG, et al. A structural model of the Staphylococcus aureus ClfA-fibrinogen interaction opens new avenues for the design of anti-staphylococcal therapeutics. PLOS Pathog. 2008;4:e1000226. doi: 10.1371/journal.ppat.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDevitt D, Francois P, Vaudaux P, Foster TJ. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–48. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 91.McDevitt D, Francois P, Vaudaux P, Foster TJ. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol Microbiol. 1995;16:895–907. doi: 10.1111/j.1365-2958.1995.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 92.Thomer L, Becker S, Emolo C, Quach A, Kim HK, et al. N-acetylglucosaminylation of serine-aspartate repeat proteins promotes Staphylococcus aureus bloodstream infection. J Biol Chem. 2014;289:3478–86. doi: 10.1074/jbc.M113.532655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomer L, Schneewind O, Missiakas D. Multiple ligands of von Willebrand factor-binding protein (vWbp) promote Staphylococcus aureus clot formation in human plasma. J Biol Chem. 2013;288:28283–92. doi: 10.1074/jbc.M113.493122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Panizzi P, Nahrendorf M, Figueiredo JL, Panizzi J, Marinelli B, et al. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat Med. 2011;17:1142–46. doi: 10.1038/nm.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duthie ES. Evidence for two forms of staphylococcal coagulase. J Gen Microbiol. 1954;10:427–36. doi: 10.1099/00221287-10-3-427. [DOI] [PubMed] [Google Scholar]
- 96.Levi M, Keller TT, van Gorp E, ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. doi: 10.1016/s0008-6363(02)00857-x. [DOI] [PubMed] [Google Scholar]
- 97.Frick IM, Bjorck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007;98:497–502. [PubMed] [Google Scholar]
- 98.Herwald H, Morgelin M, Dahlback B, Bjorck L. Interactions between surface proteins of Streptococcus pyogenes and coagulation factors modulate clotting of human plasma. J Thromb Haemost. 2003;1:284–91. doi: 10.1046/j.1538-7836.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 99.Loof TG, Morgelin M, Johansson L, Oehmcke S, Olin AI, et al. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood. 2011;18:2589–98. doi: 10.1182/blood-2011-02-337568. [DOI] [PubMed] [Google Scholar]
- 100.Itoh S, Yokoyama R, Kamoshida G, Fujiwara T, Okada H, et al. Staphylococcal superantigen-like protein 10 (SSL10) inhibits blood coagulation by binding to prothrombin and factor Xa via their γ-carboxyglutamic acid (Gla) domain. J Biol Chem. 2013;288:21569–80. doi: 10.1074/jbc.M113.451419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb Haemost. 2007;98:512–20. [PubMed] [Google Scholar]
- 102.Parry MA, Fernandez-Catalan C, Bergner A, Huber R, Hopfner KP, et al. The ternary microplasmin-staphylokinase-microplasmin complex is a proteinase-cofactor-substrate complex in action. Nat Struct Biol. 1998;5:917–23. doi: 10.1038/2359. [DOI] [PubMed] [Google Scholar]
- 103.Collen D. Staphylokinase: a potent, uniquely fibrin-selective thrombolytic agent. Nat Med. 1998;4:279–84. doi: 10.1038/nm0398-279. [DOI] [PubMed] [Google Scholar]
- 104.van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on β-hemolysin-converting bacteriophages. J Bacteriol. 2006;188:1310–15. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin T, Bokarewa M, McIntyre L, Tarkowski A, Corey GR, et al. Fatal outcome of bacteraemic patients caused by infection with staphylokinase-deficient Staphylococcus aureus strains. J Med Microbiol. 2003;52:919–23. doi: 10.1099/jmm.0.05145-0. [DOI] [PubMed] [Google Scholar]
- 106.Okada K, Ueshima S, Tanaka M, Fukao H, Matsuo O. Analysis of plasminogen activation by the plasmin-staphylokinase complex in plasma of α2-antiplasmin-deficient mice. Blood Coagul Fibrinolysis. 2000;11:645–55. doi: 10.1097/00001721-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 107.Kwiecinski J, Josefsson E, Mitchell J, Higgins J, Magnusson M, et al. Activation of plasminogen by staphylokinase reduces the severity of Staphylococcus aureus systemic infection. J Infect Dis. 2010;202:1041–49. doi: 10.1086/656140. [DOI] [PubMed] [Google Scholar]
- 108.Peetermans M, Vanassche T, Liesenborghs L, Claes J, Vande Velde G, et al. Plasminogen activation by staphylokinase enhances local spreading of S. aureus in skin infections. BMC Microbiol. 2014;14:310. doi: 10.1186/s12866-014-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peacock SJ, Foster TJ, Cameron BJ, Berendt AR. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology. 1999;145(Pt. 12):3477–86. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 111.Kerdudou S, Laschke MW, Sinha B, Preissner KT, Menger MD, Herrmann M. Fibronectin binding proteins contribute to the adherence of Staphylococcus aureus to intact endothelium in vivo. Thromb Haemost. 2006;96:183–89. [PubMed] [Google Scholar]
- 112.Sinha B, Francois PP, Nusse O, Foti M, Hartford OM, et al. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1999;1:101–17. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 113.Edwards AM, Potts JR, Josefsson E, Massey RC. Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLOS Pathog. 2010;6:e1000964. doi: 10.1371/journal.ppat.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheen TR, Ebrahimi CM, Hiemstra IH, Barlow SB, Peschel A, Doran KS. Penetration of the blood-brain barrier by Staphylococcus aureus: contribution of membrane-anchored lipoteichoic acid. J Mol Med. 2010;88:633–39. doi: 10.1007/s00109-010-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 116.Pappelbaum KI, Gorzelanny C, Grassle S, Suckau J, Laschke MW, et al. Ultralarge von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation. 2013;128:50–59. doi: 10.1161/CIRCULATIONAHA.113.002008. [DOI] [PubMed] [Google Scholar]
- 117.Claes J, Vanassche T, Peetermans M, Liesenborghs L, Vandenbriele C, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–76. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soerensen KE, Olsen HG, Skovgaard K, Wiinberg B, Nielsen OL, et al. Disseminated intravascular coagulation in a novel porcine model of severe Staphylococcus aureus sepsis fulfills human clinical criteria. J Comp Pathol. 2013;149:463–74. doi: 10.1016/j.jcpa.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus α-hemolysin. J Infect Dis. 2012;206:352–56. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, et al. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLOS ONE. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–22. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 122.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19:225–32. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Corrigan RM, Miajlovic H, Foster TJ. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009;9:22. doi: 10.1186/1471-2180-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falugi F, Kim HK, Missiakas DM, Schneewind O. The role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio. 2013;4:e00575–613. doi: 10.1128/mBio.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goodyear CS, Silverman GJ. Death by a B cell superantigen: in vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J Exp Med. 2003;197:1125–39. doi: 10.1084/jem.20020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bubeck Wardenburg J, Williams WA, Missiakas D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. PNAS. 2006;103:13831–36. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheung GY, Kretschmer D, Duong AC, Yeh AJ, Ho TV, et al. Production of an attenuated phenol-soluble modulin variant unique to the MRSA clonal complex 30 increases severity of bloodstream infection. PLOS Pathog. 2014;10:e1004298. doi: 10.1371/journal.ppat.1004298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta RK, Collier RJ, Rappuoli R, Siber GR. Differences in the immunogenicity of native and formalinized cross reacting material (CRM197) of diphtheria toxin in mice and guinea pigs and their implications on the development and control of diphtheria vaccine based on CRMs. Vaccine. 1997;15:1341–43. doi: 10.1016/s0264-410x(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 130.Heidelberger M, Avery OT. The soluble specific substances of pneumococcus. J Exp Med. 1923;38:73–79. doi: 10.1084/jem.38.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacLeod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–65. [PMC free article] [PubMed] [Google Scholar]
- 132.Robbins JB, Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990;161:821–32. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 133.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–96. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 134.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–78. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 135.Kernodle DS. Expectations regarding vaccines and immune therapies directed against Staphylococcus aureus α-hemolysin. J Infect Dis. 2011;203:1692–93. doi: 10.1093/infdis/jir141. [DOI] [PubMed] [Google Scholar]
- 136.Kim HK, Cheng AG, Kim H-Y, Missiakas DM, Schneewind O. Non-toxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections. J Exp Med. 2010;207:1863–70. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McAdow M, DeDent AC, Emolo C, Cheng AG, Kreiswirth BN, et al. Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect Immun. 2012;80:3389–98. doi: 10.1128/IAI.00562-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salgado-Pabón W, Schlievert PM. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol. 2014;12:585–91. doi: 10.1038/nrmicro3308. [DOI] [PubMed] [Google Scholar]