Summary
Proteins from vegetable and cereal sources are an excellent alternative to substitute animal-based counterparts because of their reduced cost, abundant supply and good nutritional value. The objective of this investigation is to study a set of vegetable and cereal proteins in terms of physicochemical and functional properties. Twenty protein sources were studied: five soya bean flour samples, one pea flour and fourteen newly developed blends of soya bean and maize germ (five concentrates and nine hydrolysates). The physicochemical characterization included pH (5.63 to 7.57), electrical conductivity (1.32 to 4.32 mS/cm), protein content (20.78 to 94.24% on dry mass basis), free amino nitrogen (0.54 to 2.87 mg/g) and urease activity (0.08 to 2.20). The functional properties showed interesting differences among proteins: water absorption index ranged from 0.41 to 18.52, the highest being of soya and maize concentrates. Nitrogen and water solubility ranged from 10.14 to 74.89% and from 20.42 to 95.65%, respectively. Fat absorption and emulsification activity indices ranged from 2.59 to 4.72 and from 3936.6 to 52 399.2 m2/g respectively, the highest being of pea flour. Foam activity (66.7 to 475.0%) of the soya and maize hydrolysates was the best. Correlation analyses showed that hydrolysis affected solubility-related parameters whereas fat-associated indices were inversely correlated with water-linked parameters. Foam properties were better of proteins treated with low heat, which also had high urease activity. Physicochemical and functional characterization of the soya and maize protein concentrates and hydrolysates allowed the identification of differences regarding other vegetable and cereal protein sources such as pea or soya bean.
Key words: vegetable proteins, cereal proteins, functional properties, physicochemical parameters, soya and maize concentrates, pea flour
Introduction
Currently there is a rising interest in protein isolation for their subsequent use as food ingredient. Sixty percent of Americans take into consideration protein content in food or beverages when making a buying decision (1). Of the three macronutrients (carbohydrates, proteins and fats), proteins are the most appealing for consumers concerned about their health. Nearly half of adults perceive proteins as ingredients that increase energy levels, support overall good health and improve muscle tone. These macronutrients are also considered important in diets aimed to complete a weight management program. Despite the awareness of protein importance in a balanced diet, nearly 25% of adults believe that they cannot consume as much proteins as they would like because of the cost (2). The protein industry is segmented into animal (gelatin, egg white, casein and whey) or vegetable, of which soya bean is the only source of worldwide relevance. The former has the advantage of being of high nutritional quality, but with higher cost than the vegetable counterparts and frequently the supply is irregular and unreliable. The latter in turn are cheaper, abundant and with a good nutritional value, mainly when combined, therefore making them a good option as food ingredients.
Vegetable proteins, as food ingredients, should perform specific functions within formulations such as to provide or enhance texture, gelling, emulsifying or foaming characteristics, among others. The best way to test the role of high-protein ingredients in food is in a practical scenario, unfortunately this is not always possible and therefore laboratory procedures for protein characterization are of utmost importance (3). Functional tests are required to evaluate and predict how proteins may behave in specific systems, offering a pre-evaluation of the best application (4). The physicochemical and functional characterization thus should be clear before the use of proteins as food ingredients (5).
Currently there is a lot of information regarding functional properties of proteins starting with the overwhelming general data about soya and oilseed-derived materials (4, 6, 7). Several authors have compared the physicochemical and functional properties of buckwheat protein, soya protein isolate and casein (5, 6, 8). The functional characteristics of pseudocereals as quinoa and amaranth have also been reported in literature (5, 9, 10). Regarding cereals and other oilseeds, some authors have made comparisons among the protein functional properties of rice cultivars, peanut flour and peanut protein concentrate as indicators of their potential use in food industry (11, 12). Other high-protein crops, such as pulses, have been explored and characterized: marama bean (13), cowpea (14), pea, lentil, navy bean and chickpea (15). Despite the high quantity of information about specific crops and high protein materials, the characterization of specific and novel proteins is required to determine their physicochemical and functional characteristics. These data are valuable for the development of future protein sources through innovation and research, especially of new, less expensive materials capable of giving a well-balanced food in terms of health and sensorial characteristics. The aim of this work is to characterize the physicochemical and functional properties of a set of vegetable and cereal proteins (proposal of commercial and novel mixed materials from soya bean, pea and maize germ concentrates and hydrolysates) and also to explore their correlations in order to understand better the characteristics of proteins aimed to be used as food ingredients.
Materials and Methods
Materials
The analyzed samples were identified as: pea flour (TECSA, Monterrey, Mexico), soya bean flour national (SBFN; Food Proteins Corporation, Mexico City, Mexico), soya bean flour 120 (SBF120; Productos Industriales Gaf, Mexico City, Mexico), soya bean flour 200/20 (SBF200/20; Food Proteins Corporation), soya bean flour Nutrisoy (SBFNutri; ADM, Chicago, IL, USA), soya bean flour Ragasa (SBFRagasa; Ragasa, Monterrey, Mexico), concentrates of soya and maize (01 to 05) and hydrolysates of soya and maize (01 to 09). The number at the end of each code represents the sequence in which each protein was generated. All used materials were defatted. The mixtures of soya and maize proteins were obtained using a standard procedure of alkali extraction followed by acid precipitation (16). Briefly, the pH of a finely ground mixture of defatted soya flour and defatted maize germ (proportion 5:1 using 10 parts of water) was adjusted to pH=10 with 50% NaOH. Contents were mixed for 30 min at 50 °C before separation of bagasse using an industrial centrifuge (Model SA14, GEA Westfalia, Oelde, Germany) operated at 15 L/min and 5500×g. The supernatant was then collected and the pH adjusted to 4.5 with 3 M HCl. The curd was separated using the centrifuge operated at the previously described conditions. The resulting product was washed with an equal volume of water, separated by centrifugation and then the pH was adjusted to 7.0 (with 50% NaOH). The resulting material was dried using an industrial spray dryer designed by Nutrigrains (Monterrey, Mexico) with air inlet and outlet temperatures of 195 and 80 °C, respectively, and atomization pressure of 1726 N/cm2. For hydrolyzed proteins, enzymatic hydrolysis was performed before spray drying (Neutrase®, 0.25% of total solids in the curd, 30 min at 40 °C). The spray-dried samples were stored at room temperature in a dry and ventilated place.
Determination of physicochemical parameters
For all samples, moisture (AOAC method 934.06) (17), crude protein (AOAC Method 984.13-1994) (18), reducing sugars (RS) (19) and free α-amino nitrogen (FAN; AOAC method 945.30-1945) (20) contents were calculated as well as pH and electrical conductivity (EC; potentiometer model 250, Hanna Instruments, Padova, Italy).
Functional properties
The water absorption (WAI) and water solubility (WSI) indices were determined using 1 g of sample placed in 15 mL of distilled water according to Cheftel et al. (21). The nitrogen solubility index (NSI) was assayed using 0.5 g of sample dispersed in 50 mL of 0.1 M sodium chloride (pH=7.0) (21). Nitrogen was determined with micro-Kjeldahl method in total and soluble fractions (AOAC Official Method 984.13-1994; 18). Fat absorption index (FAI) was determined based on a previously reported method (22). The turbidimetric procedure (23) was used for determining emulsifying activity index (EAI) in all samples, whereas emulsion stability (ES) was calculated according to Haque and Kito (24). Regarding functional properties related to protein and air interaction, foaming characteristics were evaluated: foaming activity (FA), foam stability (FS) and foam density (FD) in 3% (by mass) protein dispersions in water (24). Urease activity (UA) was determined as a change in pH according to AOCS Method Ba 9–58 (25) and heat coagulation capacity (HCC) with the technique proposed by Regenstein and Regenstein (26).
Statistical analysis
All determinations were performed in triplicate and data were analyzed with ANOVA (Minitab Statistical Software v. 16, Minitab Inc., State College, PA, USA). Mean values were compared with Tukey’s test (α=0.05). Pearson’s correlations, linear regression and principal component analysis (PCA) were determined with the use of the same statistical software.
Results and Discussion
Physicochemical characterization of vegetable and cereal proteins
Table 1 shows the physicochemical properties of the array of analyzed vegetable and cereal proteins. The pH, an important parameter associated with protein solubility, ranged from 6.42 to 7.57, except for the soya and maize concentrates 01 and 02 with values of 5.63 and 5.89, respectively. At lower or higher values than the isoelectric point, the electrostatic repulsion increases and consequently the solubility of proteins improves. This parameter is also related to the water absorption capacity of the material: ionized amino acid groups bind more water than non-ionized ones. Lowering the pH below 4 changes the carboxyl groups into non-ionized forms, thus reducing water-binding properties of the protein.
Table 1. Physical and chemical characterization of vegetable and cereal proteins.
| Sample | pH | EC/(mS/cm) | w(moisture)/% | w(protein)/% | w(RS)/(mg/g) | w(FAN)/(mg/g) | UA |
|---|---|---|---|---|---|---|---|
| Pea flour | (6.42±0.12)h | (1.35±0.01)kl | (10.54±0.46)ab | (20.78±0.35)j | (136.65±2.11)Ş | (0.72±0.04)gh | (0.17±0.03)kl |
| Soya bean flour national (SBFN) | (6.63±0.01)fg | (2.59±0.01)ef | (10.73±0.02)a | (50.74±2.54)h | (6.07±0.39)kl | (0.54±0.02)h | (0.13±0.01)lm |
| Soya bean flour 120 (SBF120) | (6.63±0.00)fg | (2.64±0.04)e | (7.94±0.12)d | (49.61±1.98)hi | (5.26±0.35)l | (0.59±0.01)h | (0.15±0.01)kl |
| Soya bean flour 200/20 (SBF200/20) | (6.74±0.01)ef | (2.79±0.03)d | (4.96±0.33)f | (54.20±0.15)h | (5.32±0.30)kl | (0.60±0.02)h | (0.21±0.01)k |
| Soya bean flour Nutrisoy (SBFNutri) | (6.60±0.01)g | (2.90±0.05)d | (3.97±0.07)hi | (49.63±0.59)hi | (6.19±0.40)kl | (0.57±0.01)h | (0.44±0.01)i |
| Soya bean flour Ragasa (SBFRagasa) | (6.79±0.02)e | (2.26±0.03)g | (3.58±0.07)i | (45.38±0.12)i | (85.09±0.68)b | (1.40±0.03)f | (2.20±0.03)a |
| Soya and maize concentrate 01 | (5.63±0.01)j | (2.82±0.01)d | (10.09±0.31)b | (69.93±1.73)de | (24.83±0.14)d | (0.65±0.04)gh | (0.08±0.01)m |
| Soya and maize concentrate 02 | (5.89±0.01)i | (2.47±0.05)f | (6.08±0.37)e | (68.38±1.26)def | (35.68±0.70)c | (0.90±0.02)g | (2.07±0.02)b |
| Soya and maize concentrate 03 | (7.54±0.01)a | (3.64±0.02)c | (5.71±0.10)e | (62.62±0.44)g | (19.46±0.43)gh | (1.58±0.06)f | (0.27±0.01)j |
| Soya and maize concentrate 04 | (7.57±0.04)a | (3.54±0.06)c | (4.98±0.04)f | (71.69±1.11)d | (21.07±0.49)efg | (2.11±0.12)de | (0.14±0.02)l |
| Soya and maize concentrate 05 | (6.57±0.06)g | (3.62±0.12)c | (4.11±0.03)hi | (67.13±0.24)defg | (7.26±0.17)k | (1.88±0.04)e | (0.40±0.01)i |
| Soya and maize hydrolysate 01 | (6.60±0.00)g | (4.00±0.06)b | (5.04±0.02)f | (68.38±1.41)def | (5.99±0.07)kl | (2.25±0.07)de | (0.94±0.02)g |
| Soya and maize hydrolysate 02 | (6.43±0.06)h | (4.32±0.04)a | (4.07±0.19)hi | (65.29±1.67)efg | (5.01±0.08)l | (2.68±0.07)ab | (1.14±0.02)f |
| Soya and maize hydrolysate 03 | (7.14±0.01)b | (4.14±0.02)b | (2.18±0.14)j | (64.69±0.10)fg | (16.17±0.79)i | (1.53±0.02)f | (0.31±0.01)j |
| Soya and maize hydrolysate 04 | (6.57±0.01)g | (1.32±0.06)l | (8.31±0.06)d | (94.24±1.61)a | (11.52±0.01)j | (2.79±0.08)a | (1.93±0.02)c |
| Soya and maize hydrolysate 05 | (6.76±0.01)e | (1.47±0.05)jk | (4.70±0.12)fg | (77.37±0.81)c | (11.97±0.25)j | (2.87±0.01)a | (2.10±0.02)b |
| Soya and maize hydrolysate 06 | (7.08±0.01)bc | (1.57±0.02)ij | (4.36±0.07)gh | (78.44±3.76)c | (21.97±0.27)ef | (2.39±0.06)cd | (1.85±0.01)d |
| Soya and maize hydrolysate 07 | (7.05±0.01)bcd | (1.76±0.04)h | (3.79±0.18)hi | (76.53±2.41)c | (18.66±0.49)h | (2.36±0.10)bc | (1.95±0.01)c |
| Soya and maize hydrolysate 08 | (6.94±0.07)d | (1.52±0.03)ij | (7.93±0.07)d | (91.16±0.39)ab | (20.37±0.76)fgh | (2.61±0.08)ab | (1.69±0.01)e |
| Soya and maize hydrolysate 09 | (6.98±0.06)cd | (1.64±0.03)hi | (9.03±0.07)c | (87.96±1.03)b | (22.42±0.20)e | (2.52±0.17)bc | (0.81±0.04)h |
Mean values are the average of at least three replicates±standard deviation. Mean values with different letter(s) in superscript within columns are statistically different (p<0.05). EC=electrical conductivity, RS=reducing sugars, FAN=free amino nitrogen, UA=urease activity
Electrical conductivity (EC) of all samples was between 4.32 and 1.32 mS/cm (soya and maize hydrolysates 02 and 04). This parameter is a measure of the ability of material to conduct electrical current and is affected by the content of protein, fat and minerals, among others. Foods with electrolytes such as salts, acids, certain gums and thickeners contain charged groups that have a notable effect on the EC. Protein charge and amino acid composition also affect this property. Foods such as apples, strawberries and potatoes have EC of 0.7, 1.9 and 0.4 mS/cm at 25 °C, respectively (27).
The EC of soya and maize concentrates ranged from 2.47 to 3.64 mS/cm, comparable to the results reported by Režek Jambrak et al. (28) of 3.28 mS/cm of a soya protein concentrate. The soya and maize protein hydrolysates 02 and 04 showed the lowest and the highest conductivity of 1.32 and 4.32 mS/cm. Therefore, neither the protein content nor the higher degree of hydrolysis influenced the high EC of the protein, which depended on the charge density of the protein and configuration acquired after a particular process (thermal or enzymatic hydrolysis). EC is important for the development of foods or beverages because of its influence on solubility, emulsifying and foaming activities, and consequently on the interaction with other ingredients and the protein stability in a given food system. EC is also important because it determines the heating rate and effectiveness of novel food processes, such as ohmic heating and pulsed electric-based operations (dehydration, extraction, pasteurization, etc.) (29).
Table 1 shows that the protein content and the degree of hydrolysis affect the EC significantly (p<0.05). The pea flour and soya and maize protein hydrolysate 04 contained the lowest and the highest protein fractions of 20.78 and 94.24% (on dry mass basis), respectively. The concentrates and some soya and maize hydrolysates contained approx. 70% protein, similar to soya bean concentrates available on the market. The free amino nitrogen (FAN) content, which determines free amino acids or small peptides and therefore the degree of protein hydrolysis, solubility and water absorption capacity, ranged between 0.54 and 2.87 mg/g. As expected, the hydrolyzed proteins (treated with protease) had a higher FAN content (>2.0 mg/g), except for soya and maize hydrolysate 03, which contained 1.52 mg/g, similar to SBFRagasa and soya and maize protein concentrate 03. FAN values between 12 and 27% in bean pods, 5 and 12% in spinach and 34 and 56% in potato tubers have been reported (30). The percentage of FAN in total nitrogen ranged between 0.6 and 2.3% (Table 1), and these values were below those reported by Eppendorfer and Bille (30) in vegetable protein products. The differences, besides the availability of proteins and amino acids, could be associated with the method used for FAN determination.
The reducing sugar (RS) assay is highly relevant because the amounts of sugars relate with the stability or retention of protein functionality during storage (3). Foods can deteriorate during storage due to both enzymatic and Maillard-type reactions of primary amino groups with RS (31). Determination of reducing sugars by dinitrosalicylic acid method is thus a good index for characterization of high-protein materials (Table 1). Pea flour had the highest content of RS of 136.65 mg/g, followed by SBFRagasa with 85.09 mg/g. The lowest content of RS was measured in SBF120, SBF200/20 and soya and maize hydrolysate 02 with only 5 mg of glucose reducing equivalents per gram.
The urease activity (UA) indicates the intensity of heat treatment during processing of protein meals. A value of 0.3 or less suggests that the protein source retains slight urease activity but has received sufficient heat treatment for the inactivation of antibiological factors. A product with a pH increase of 0.02 or less during urease activity test (25) was surely overheated, yielding thus a material with diminished functional properties. All UA results shown in Table 1 were between 0.08 and 2.20 (soya and maize protein concentrate 01 and SBFRagasa, respectively), indicating that these protein sources received high and low thermal treatments and thus contained low and high residual enzymatic activity, respectively. In the specific case of pea flour, its UA was similar to that of SBF120, SBF200/20 and soya and maize protein concentrate 04. Values are also similar to the ones reported by Valencia et al. (32), who compared UA activity of pea protein vs. soya bean protein concentrates.
Functional analysis of vegetable and cereal proteins
Functional characterization of the array of analyzed proteins is summarized in Table 2. The water absorption index (WAI) is one of the most important parameters to take into consideration for product development, particularly for dairy products and foods exposed to thermal treatments such as baking and thermoplastic extrusion (33). WAI is defined as the water absorbed per gram of tested material and it is regularly used as synonymous with water holding, water binding or water retention capacity (34). WAI values were between 0.41 and 18.52 (Table 2). The protein concentrates exhibited higher WAI values compared to the other vegetable or cereal protein sources (average of 8), followed by the soya bean and pea flour (4.31 to 5.38 and 4.97, respectively) and the hydrolysates (around 1.0). Despite the direct relationship between water holding capacity and protein concentration (7), the higher protein concentration of hydrolysates did not improve the WAI compared with the other samples. This is influenced by protein structure and composition. According to Barbut (34) water can be divided in two general types according to its relationship with the protein molecule: absorbed and retained. The first is the water bound to the protein molecule and therefore no longer available for its use as solvent, whereas the second is trapped within the protein matrix. The first kind depends mainly on the amino acids and pH of the system, and the second is more dependent on the same relationship among protein molecules. Because of the type of water absorption procedure used herein, the second type of water is the one that varied the most among samples (Table 2), which is mainly due to protein structure organization. On the other hand, the smaller protein molecules that form hydrolysates reduce the interaction among molecules, yielding structures that do not hold water. Nitrogen solubility index (NSI) is another parameter related to the hygroscopic properties of proteins: it is a measurement of the protein dispersability in a NaCl solution. The NSI values of all the analyzed proteins were between 10.14 (of the SBFNutri) and 74.89 (soya and maize protein hydrolysate 05). These values were similar to the amounts reported for commercial high--protein soya bean products which ranged from 10 to 90% (33). NSI is generally related to the extent of heating or protein denaturation, and is also important because it affects the solubility of proteins at different ionic strengths. It offers a more realistic approach to the performance of the protein in foods (since these are complex ionic systems). As expected, the hydrolysates showed the highest NSI because these proteins were hydrolyzed with protease beforehand, which according to Kinsella and Melachouris (3) markedly improves nitrogen solubility.
Table 2. Functional properties of vegetable and cereal proteins.
| Sample | WAI | NSI/% | WSI/% | FAI | EAI/(m2/g) | ES1/% | ES2/% | FA/% | FS/% | FD/% | HCC/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pea flour | (4.97±0.04)cd | (18.13±0.71)g | (20.42±0.87)i | (4.72±0.15)a | (52399±2306)a | (72.73±2.26)abc | (73.69±0.31)ab | (75.00±0.00)k | (0.00±0.00)g | (57.14±0.00)a | (66.35±2.08)i |
| Soya bean flour national (SBFN) | (5.38±0.18)c | (14.84±0.80)gh | (23.22±1.42)i | (3.16±0.11)cdef | (8713±146)ghij | (73.24±0.15)ab | (73.33±0.00)ab | (108.33±0.00)ij | (50.67±7.51)f | (35.00±0.00)b | (84.05±1.10)d |
| Soya bean flour 120 (SBF120) |
(4.92±0.26)cde | (13.42±0.54)hi | (29.05±0.10)gh | (3.01±0.02)efgh | (16747±822)e | (71.19±1.61)bc | (71.81±1.32)ab | (275.00±0.00)gh | (0.00±0.00)g | (24.23±0.48)e | (75.89±0.47)gh |
| Soya bean flour 200/20 (SBF200/20) |
(4.49±0.15)def | (14.07±0.61)hi | (25.07±0.29)hi | (2.89±0.06)fghi | (25157±2084)cd | (75.71±0.00)a | (77.62±1.65)a | (261.90±0.00)h | (77.16±0.81)cde | (27.25±0.00)d | (78.72±1.35)ef |
| Soya bean flour Nutrisoy (SBFNutri) | (4.45±0.13)ef | (10.14±0.76)i | (25.47±1.68)hi | (2.77±0.09)ghi | (51300±3325)a | (73.33±0.00)ab | (73.33±0.00)ab | (288.00±0.00)gh | (0.00±0.00)g | (20.77±0.88)fg | (77.76±0.39)fg |
| Soya bean flour Ragasa (SBFRagasa) |
(4.31±0.10)f | (13.66±0.74)hi | (47.00±1.79)d | (3.10±0.03)cdef | (29293±1575)b | (69.23±0.00)c | (57.14±0.00)d | (375.00±0.00)e | (89.40±0.00)ab | (20.00±0.00)gh | (95.16±0.29)a |
| Soya and maize concentrate 01 | (18.52±0.52)a | (10.70±0.58)i | (39.19±5.41)e | (2.74±0.03)hi | (10687±680)fgh | (73.41±2.00)ab | (76.92±0.00)a | (376.19±0.00)de | (74.05±0.10)de | (20.84±0.00)fg | (84.95±0.20)cd |
| Soya and maize concentrate 02 | (3.60±0.02)g | (15.38±0.57)gh | (30.00±0.59)gh | (3.07±0.07)defg | (8381±262)hij | (72.60±0.00)abc | (73.33±0.00)ab | (397.58±39.92)cde | (93.20±9.95)a | (19.42±1.10)gh | (93.39±0.41)a |
| Soya and maize concentrate 03 | (8.35±0.16)b | (33.84±0.55)f | (33.69±1.37)fg | (3.33±0.11)bcde | (22234±1506)d | (74.71±2.8)ab | (63.57±1.01)cd | (84.55±0.00)jk | (0.00±0.00)g | (29.45±0.43)c | (75.55±0.53)gh |
| Soya and maize concentrate 04 | (8.04±0.25)b | (27.61±0.96)f | (37.88±0.66)ef | (2.59±0.10)i | (17491±1188)e | (72.85±1.37)abc | (68.57±8.92)bc | (66.67±0.00)k | (84.72±1.27)abc | (33.33±0.00)b | (73.87±0.31)h |
| Soya and maize concentrate 05 | (8.34±0.08)b | (36.06±1.46)e | (37.82±0.21)ef | (2.76±0.09)ghi | (10147±123)fgh | (73.33±0.00)ab | (73.33±0.00)ab | (113.00±0.00)i | (73.77±1.68)de | (24.20±2.30)e | (95.50±0.22)a |
| Soya and maize hydrolysate 01 | (3.74±0.11)g | (33.09±2.12)e | (39.22±2.13)e | (3.35±0.13)bdc | (12414±145)fg | (73.17±0.27)ab | (73.65±0.55)ab | (298.61±2.41)g | (79.72±0.43)cd | (25.09±0.15)e | (87.65±0.16)b |
| Soya and maize hydrolysate 02 | (3.59±0.06)g | (33.65±2.54)e | (32.71±2.32)fg | (2.88±0.09)fghi | (27282±1822)bc | (73.97±0.36)ab | (75.34±0.00)ab | (328.33±2.89)f | (80.67±0.22)bcd | (22.22±0.00)f | (86.97±0.08)bc |
| Soya and maize hydrolysate 03 | (4.82±0.04)de | (34.80±1.14)e | (37.58±1.04)ef | (3.40±0.07)bc | (14055±999)ef | (72.84±1.60)abc | (73.33±0.00)ab | (300.00±0.00)g | (68.17±0.29)e | (24.62±0.00)e | (77.62±1.59)fg |
| Soya and maize hydrolysate 04 | (1.05±0.04)i | (40.45±1.55)d | (84.80±1.45)bc | (3.02±0.06)efgh | (5426±435)jk | (74.68±1.80)ab | (78.10±0.82)a | (475.00±0.00)a | (82.78±0.19)bcd | (16.67±0.00)i | (94.11±0.40)a |
| Soya and maize hydrolysate 05 | (1.83±0.02)h | (74.89±0.90)a | (81.39±0.89)c | (3.31±0.07)bcde | (3936±75)k | (73.61±0.00)ab | (75.24±0.82)ab | (423.33±2.89)bc | (83.42±3.24)bc | (18.24±0.10)hi | (93.95±0.37)a |
| Soya and maize hydrolysate 06 | (1.13±0.08)i | (45.37±1.68)c | (88.84±1.04)b | (3.20±0.21)bcdef | (8880±466)ghij | (73.33±1.65)ab | (73.33±1.65)ab | (375.00±0.00)e | (79.60±1.44)cd | (20.00±0.00)gh | (80.69±0.55)e |
| Soya and maize hydrolysate 07 | (1.03±0.06)j | (48.27±2.69)c | (88.63±1.37)b | (3.05±0.00)defgh | (9495±98)ghi | (75.71±0.00)a | (71.43±0.00)ab | (375.00±0.00)e | (79.00±1.00)cd | (20.00±0.00)gh | (84.00±1.52)d |
| Soya and maize hydrolysate 08 | (1.12±0.01)i | (56.55±0.82)b | (87.26±0.32)b | (3.51±0.20)b | (6053±13)ijk | (73.66±0.57)ab | (74.32±0.00)ab | (428.33±2.89)b | (80.73±0.31)bcd | (18.18±0.00)hi | (93.14±0.23)a |
| Soya and maize hydrolysate 09 | (0.41±0.02)j | (55.20±0.53)b | (95.65±0.36)a | (2.89±0.09)fghi | (9346±292)ghij | (73.38±0.82)ab | (74.74±2.86)ab | (403.81±0.00)bcd | (80.67±0.10)bcd | (19.09±0.00)gh | (85.26±0.21)bcd |
Mean values are the average of at least three replicates±standard deviation. Mean values with different letter(s) in superscript within columns are statistically different (p<0.05). WAI=water absorption index, NSI=nitrogen solubility index, WSI=water solubility index, FAI=fat absorption index, EAI=emulsifying activity index, ES1 and ES2=emulsion stability at 24 and 48 h respectively, FA=foaming activity, FS=foam stability, FD=foam density, HCC=heat coagulation capacity
The water solubility index (WSI) of the proteins is the most important functional property because it affects other functional characteristics such as EAI, FA and HCC. WSI depends on the protein ability to interact with water. The WSI of soya bean flour and soya and maize concentrates was around 35%, while that of the hydrolysates (02 and 09) ranged from 32.71 to 95.65%. Arrese et al. (6) reported the WSI values of soya proteins of 36.3 to 83.6% and according to Tomotake et al. (5), WSI of a buckwheat isolate at similar pH value as used in our study was around 50%, followed by the soya protein isolate, with values below 20% and that of peanut flour of 30% (12). Therefore, the values of WSI obtained in some analyzed samples (Table 2) are higher than those reported for similar products.
Fat absorption index is the ability of the vegetable and cereal proteins to physically bind fat by capillary attraction. This is a parameter of paramount importance in food development, because fats act as flavour retainer and also increase the mouth feel of the foods. The FAI of pea, soya bean flour and soya and maize concentrates and hydrolysates ranged from 2.59 to 4.72. Meng and Ma (35) reported a value of FAI of a commercial soya protein of 1.52. FAI variation may be due to the different surface hydrophobicities of vegetable proteins, because the absorption of fat has been attributed to physical entrapment within the protein and non-covalent bonds such as hydrophobic, electrostatic and hydrogen bonding, the forces involved in lipid-protein interactions (36). Another property related to the hydrophobicity is the emulsifying activity index (EAI), i.e. the ability of a protein to form and stabilize the emulsion by creating electrostatic repulsion on oil droplet surface. The emulsion stability index (ESI) reflects the ability of the proteins to form and maintain a stable emulsion over a period of time by preventing flocculation and coalescence of the oil globules (9). The soya and maize protein hydrolysate 05 had the lowest EAI of 3936.62 m2/g, while the pea flour had the highest, 52 399 m2/g. These results may be due to the high content of non-protein solids in pea flour, favourable for emulsion. In fact, the good performance of pea protein as egg replacer in mayonnaise-like products has received wide coverage in the media (2). In general, the differences in protein emulsifying activity may be related to their solubility and conformational stability. This property is widely utilized in totally or partially emulsified foods, such as mayonnaise, cream, sauces, desserts, comminuted meat products and some beverages. Moreover, the emulsions of pea, soya bean flour and soya and maize concentrates and hydrolysates were all stable for 24 to 48 h, i.e. they all have similar capacity to stabilize an emulsion. The high stability of the emulsions of vegetable and cereal proteins is due to their conformation. They are globular protein structures that reduce surface tension and form more rigid interfacial films.
Similar to emulsion characteristics, another related property with two-phase interaction is foaming capacity or activity (FA). Foam can be defined as a two-phase system where air cells are separated by a continuous liquid layer, and foam stability (FS) is the capacity of a protein to reduce the surface tension by forming strong interfacial membranes via protein-protein interactions at the air-water interface. FA values of pea flour and soya and maize hydrolysate 04 were 75 and 475%, respectively (Table 2). The high content of nonprotein solids of the pea flour may have increased the surface tension of the dispersion, reducing significantly the FA. The FS of pea flour was one of the lowest, with a value of zero, and the highest of soya and maize concentrate 02 (93.20%). The foam density (FD) was similar in all samples. Protein foams can provide unique textures (as in meringue and nougat) that are associated with many foods such as angel and pound cakes, ice cream and confectionary products.
Heat coagulation capacity (HCC) was also determined and results are shown in Table 2. Results ranged from 66.35 to 95.50% for pea flour and soya and maize concentrate 05, respectively. Coagulation is the capacity of the protein to form a clot or a semisolid mass after an initial denaturation (driven by different factors, such as heat). It involves the rupture of hydrogen bonds within peptide chains, and when an advanced state is reached, denaturation becomes irreversible. According to Kinsella (7), in soya proteins an initial heating above 60 °C is necessary to induce dissociation of quaternary globulins. This thermal treatment causes unfolding of polypeptides of the protein subunits with an increase in viscosity. Upon cooling, the unfolded polypeptides reassociate via hydrophobic associations, hydrogen bonding, ionic interactions and possibly some disulphide linkages, forming a gel. HCC is thus an important property in food applications such as processed meat, sausages and cheese.
Correlation analysis between physicochemical and functional properties of vegetable and cereal proteins
Results of correlation analyses between functional properties and physicochemical parameters are summarized in Table 3. Protein content was positively correlated with the FAN (R=0.75), NSI (R=0.69), WSI (R=0.78), FA (R=0.59) and FS (R=0.62), because higher protein mass fraction was observed in the hydrolyzed samples (last nine rows in Tables 1 and 2). On the other hand, protein mass fraction correlated negatively with RS (R=–0.56), EAI (R= –0.77) and FD (R=–0.72), which means that a higher protein content lowered EAI and FD, despite the fact that high-protein materials are good emulsifiers (37). This result is reinforced by the fact that EAI showed an inverse relationship with WSI. Protein content was also positively correlated with FS and FA, and as expected, inversely with FD.
Table 3. Pearson’s correlation coefficients between physicochemical parameters and functional properties of vegetable and cereal proteins.
| Parameter | pH | EC | Moisture | P | RS | FAN | UA | WAI | NSI | WSI | FAI | EAI | ES1 | ES2 | FA | FS | FD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| EC | 0.08 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| Moisture | –0.38 | –0.44 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| P | 0.18 | –0.18 | –0.09 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| RS | –0.13 | –0.37 | 0.26 | –0.56 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| FAN | 0.39 | –0.17 | –0.28 | 0.75 | –0.23 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| UA | –0.04 | –0.50 | –0.28 | 0.47 | 0.07 | 0.58 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| WAI | –0.35 | 0.43 | 0.22 | –0.26 | 0.03 | –0.50 | –0.60 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| NSI | 0.41 | –0.33 | –0.18 | 0.69 | –0.23 | 0.87 | 0.50 | –0.52 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| WSI | 0.28 | –0.62 | –0.02 | 0.78 | –0.14 | 0.77 | 0.65 | –0.53 | 0.81 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| FAI | 0.002 | –0.33 | 0.30 | –0.42 | 0.70 | –0.08 | 0.02 | –0.21 | 0.09 | –0.06 | - - - | - - - | - - - | - - - | - - - | - - - | - - - |
| EAI | –0.04 | 0.12 | –0.04 | –0.77 | 0.53 | –0.48 | –0.36 | 0.10 | –0.53 | –0.56 | 0.33 | - - - | - - - | - - - | - - - | - - - | - - - |
| ES1 | 0.12 | –0.02 | –0.02 | 0.31 | –0.35 | 0.22 | –0.05 | –0.08 | 0.28 | 0.20 | –0.08 | –0.15 | - - - | - - - | - - - | - - - | - - - |
| ES2 | –0.40 | –0.13 | 0.29 | 0.29 | –0.34 | 0.08 | –0.12 | –0.06 | 0.18 | 0.13 | –0.02 | –0.21 | 0.40 | - - - | - - - | - - - | - - - |
| FA | –0.29 | –0.43 | –0.10 | 0.59 | –0.19 | 0.42 | 0.73 | –0.39 | 0.39 | 0.65 | –0.18 | –0.40 | 0.02 | 0.24 | - - - | - - - | - - - |
| FS | –0.08 | –0.06 | –0.26 | 0.62 | –0.21 | 0.54 | 0.56 | –0.18 | 0.38 | 0.47 | –0.36 | –0.63 | 0.06 | 0.13 | 0.52 | - - - | - - - |
| FD | –0.06 | 0.04 | 0.38 | –0.72 | 0.62 | –0.43 | –0.56 | 0.21 | –0.36 | –0.54 | 0.64 | 0.55 | –0.05 | –0.07 | –0.78 | –0.52 | - - - |
| HCC | –0.30 | –0.15 | –0.14 | 0.54 | –0.26 | 0.45 | 0.67 | –0.20 | 0.36 | 0.42 | –0.32 | –0.57 | –0.07 | 0.03 | 0.59 | 0.67 | –0.69 |
EC=electrical conductivity, P=protein, RS=reducing sugars, FAN=free amino nitrogen, UA=urease activity, WAI=water absorption index, NSI=nitrogen solubility index, WSI=water solubility index, FAI=fat absorption index, EAI=emulsifying activity index, ES1 and ES2=emulsion stability at 24 and 48 h respectively, FA=foaming activity, FS=foam stability, FD=foam density, HCC=heat coagulation capacity
Regarding water-related properties, WSI correlated positively with protein content (R=0.78), FAN (R=0.77), UA (R=0.65), NSI (R=0.81) and FA (R=0.65), and negatively with EC (R=–0.62) and EAI (R=–0.56) as previously stated. WSI had a good correlation coefficient with FA, perhaps because of the reduced size of the protein molecules that favoured protein-water interaction, which also increased the water-air interface. NSI also correlated positively with protein and FAN content (R=0.69 and 0.87). The positive and highly significant relationships among WSI and NSI with FAN clearly indicate that the degree of hydrolysis of a protein promotes solubility. Proteolysis enhances the protein-water interactions, because as the molecular mass decreases, it simplifies the secondary structure, increases the number of ionizable groups and exposes the hydrophobic groups, changing the physicochemical interactions of the protein with the medium (38). WAI showed an inverse relationship with UA. Since the latter is an indicator of thermal treatment or heating index, it would be expected that the denaturation of high-protein materials affected its water absorption capacity. The other important group of functional properties is the fat-associated indices (FAI and EAI). EAI was correlated with foam capacity and, as described previously, inversely with protein. The EAI had positive correlation with FD (R=0.55) and negative with FS (R=–0.63) and HCC (R=–0.57). These correlations make reference to the balance between hydrophilic and hydrophobic groups exposed on the surface of vegetable and cereal proteins.
FA and FS were, on the other hand, positively correlated with UA, with correlation values of R=0.73 and 0.56, respectively. These correlations can be associated with the heating used during the extraction process and not directly with the residual enzyme activity. FA and FS could then be associated with the degree of denaturation of the protein structure.
HCC, the only functionality evaluated for protein- -protein interaction showed a good correlation coefficient with UA (R=0.67), which meant that high UA increased HCC values. Therefore, proteins with lower denaturation due to lower exposure to heat treatments were more prone to coagulation.
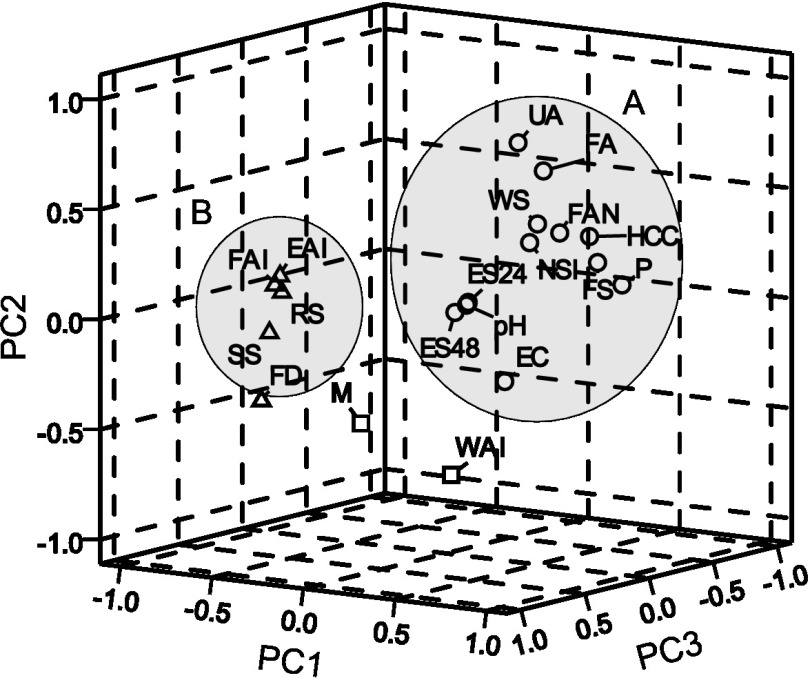
Principal component analysis
Principal component analysis (PCA) was used to visualize the correlation among the physicochemical and functional properties of the twenty vegetable proteins. A 3D graphic presentation of the first three components (PC1, PC2 and PC3) described 67.3% of the variance. The PCA showed two correlated clusters as shown in Fig. 1. The first group (A) is formed by pH, EC, UA, protein content (P), WSI, NSI, FAN, FA, FS, HCC and ES after 24 and 48 h, whereas the second group (B) includes FAI, EAI, RS, soluble solids (SS) and FD.
Fig. 1.
Graph of principal components (PC1, PC2 and PC3) in rotated space. Properties associated with solubility (o, A), solid content (∆, B) and intermediate (□). This plot describes 67.3% of the variance
Group A is characterized by association with the charging properties of the protein (pH, EC, UA and FAN) that influenced the protein-water interactions. Group B included properties related to solid content. Aluko et al. (39) reported a significant effect of the content of soluble solids on the emulsifying activity of coriander flour and protein concentrate. This is similar to the results discussed previously regarding the high emulsification capacity (52 399 m2/g) observed in pea flour mainly due to its high RS mass fraction (136.65 mg/g).
Conclusions
This research characterized and compared chemical and functional properties of some vegetable and cereal proteins including commercial and new protein concentrates and hydrolysates obtained from a mixture of soya bean and maize germ. Correlations were obtained between physicochemical and functional properties in order to acquire a better comprehension of vegetable and cereal proteins as food ingredients. Water-related properties, such as WSI and NSI, in the soya and maize hydrolysates were higher, thus making them good options for use as ingredients in beverages. WAI was better in soya and maize concentrates, indicating their best suitability as extenders for sausages and related products. Fat-related properties (mainly FAI and EAI) were better in the pea flour, making it a good emulsifier option for dressings and other high-fat formulations. FA and FS were on average better in the soya and maize hydrolysates, which also had the best air trapping or foaming properties. The degree of protein hydrolysis was positively correlated with solubility-related parameters. Fat-associated characteristics were inversely correlated with water-associated characteristics. Foam and coagulation properties were better in low-heat-treated materials, which had high UA. The PCA of pea flour and soya and maize concentrates and hydrolysates was linked within two groups, the first mainly associated with foam and coagulation properties and the second related to emulsification characteristics. This research characterized a set of vegetable and cereal proteins from a wide range of samples of raw materials and demonstrated relationships among their physicochemical and functional properties.
Acknowledgements
This research was supported by the Research Chair Funds 0020IDP001 from Research Centre for Protein Development (CIDPRO), Monterrey Institute of Technology, Monterrey, Mexico, and Cintya Soria´s posgraduate scholarship by National Board for Science and Technology (CONACyT) and Monterrey Institute of Technology.
References
- 1.International Food Information Council Foundation. Consumer attitudes toward food safety, nutrition and health. 2013 Food & health survey. Washington DC, USA; 2013. pp. 1–9. [Google Scholar]
- 2.Cheatham R. Protein: a plant-based look at this power macronutrient, special report. In: Food product design. Phoenix, AZ, USA: Virgo Publishing LLC; 2014. pp. 1–7. [Google Scholar]
- 3.Kinsella JE, Melachouris N. Functional properties of proteins in foods: a survey. Crit Rev Food Sci Nutr. 1976;7:219–80. 10.1080/10408397609527208 [DOI] [Google Scholar]
- 4.Moure A, Dominguez H, Parajo JC. Antioxidant properties of ultrafiltration recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2010;41:447–56. 10.1016/j.procbio.2005.07.014 [DOI] [Google Scholar]
- 5.Tomotake H, Shimaoka I, Kayashita J, Nakajoh M, Kato N. Physicochemical and functional properties of buckwheat protein product. J Agric Food Chem. 2002;50:2125–9. 10.1021/jf011248q [DOI] [PubMed] [Google Scholar]
- 6.Arrese EL, Sorgentini DA, Wagner JR, Ańón MC. Electrophoretic, solubility, and functional properties of commercial soy protein isolates. J Agric Food Chem. 1991;39:1029–32. 10.1021/jf00006a004 [DOI] [Google Scholar]
- 7.Kinsella JE. Functional properties of soy proteins. J Am Oil Chem Soc. 1979;56:242–58. 10.1007/BF02671468 [DOI] [Google Scholar]
- 8.Luo Y, Pan K, Zhong Q. Physical, chemical and biochemical properties of casein hydrolyzed by three proteases: partial characterizations. Food Chem. 2014;155:146–54. 10.1016/j.foodchem.2014.01.048 [DOI] [PubMed] [Google Scholar]
- 9.Shevkani K, Singh N, Rana JC, Kaur A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int J Food Sci Technol. 2014;49:541–50. 10.1111/ijfs.12335 [DOI] [Google Scholar]
- 10.Abugoch LE, Romero N, Tapia CA, Silva J, Rivera M. Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. J Agric Food Chem. 2008;56:4745–50. 10.1021/jf703689u [DOI] [PubMed] [Google Scholar]
- 11.Pinciroli M, Vidal AA, Ańón MC, Martínez EN. Comparison between protein functional properties of two rice cultivars. LWT –. Food Sci Technol (Campinas). 2009;42:1605–10. 10.1016/j.lwt.2009.06.003 [DOI] [Google Scholar]
- 12.Yu J, Ahmedna M, Goktepe I. Peanut protein concentrate: production and functional properties as affected by processing. Food Chem. 2007;103:121–9. 10.1016/j.foodchem.2006.08.012 [DOI] [Google Scholar]
- 13.Maruatona GN, Duodu KG, Minnaar A. Physicochemical, nutritional and functional properties of marama bean flour. Food Chem. 2010;121:400–5. 10.1016/j.foodchem.2009.12.054 [DOI] [Google Scholar]
- 14.Khalid II, Elhardallou SB, Elkhalifa EA. Composition and functional properties of cowpea (Vigna ungiculata L. Walp) flour and protein isolates. Am J Food Technol. 2012;7:113–22. 10.3923/ajft.2012.113.122 [DOI] [Google Scholar]
- 15.Toews R, Wang N. Physicochemical and functional properties of protein concentrates from pulses. Food Res Int. 2013;52:445–51. 10.1016/j.foodres.2012.12.009 [DOI] [Google Scholar]
- 16.Riaz MN. Processing of soybeans into ingredients. In: M.N. Riaz, editor. Soy application in food. Boca Raton, FL, USA: CRC Press, Taylor and Francis; 2006. pp. 39–62. [Google Scholar]
- 17.Official Method AOAC. 934.06. Moisture in dried fruits. Rockville, MD, USA: AOAC International; 1996. [Google Scholar]
- 18.Official Method AOAC. 984.13–1994 (1996). Protein (crude) in animal feed and pet food. Rockville, MD, USA: AOAC International; 1996. [Google Scholar]
- 19.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–8. 10.1021/ac60147a030 [DOI] [Google Scholar]
- 20.Official Method AOAC. 945.30–1945. Characteristics of wort. Rockville, MD, USA: AOAC International; 1945. [Google Scholar]
- 21.Cheftel JC, Cuq JL, Lorient D. Functional properties of proteins. In: Food proteins. Zaragoza, Espańa: Acribia; 1989. pp. 49–106 (in Spanish). [Google Scholar]
- 22.Ahn HJ, Kim JH, Ng PKW. Functional and thermal properties of wheat, barley and soy flours and their blends treated with a microbial transglutaminase. J Food Sci. 2005;70:380–6. 10.1111/j.1365-2621.2005.tb11433.x [DOI] [Google Scholar]
- 23.Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–23. 10.1021/jf60217a041 [DOI] [Google Scholar]
- 24.Haque Z, Kito M. Lipophilization of αSl-casein. 2. Conformational and functional effects. J Agric Food Chem. 1983;31:1231–7. 10.1021/jf00120a022 [DOI] [Google Scholar]
- 25.Official Method Ba AOCS. 9-58. Urease activity. Champaign, IL, USA: American Oil Chemists´ Society; 1987. [Google Scholar]
- 26.Regenstein JM, Regenstein CE. Protein functionality for food scientists. In: Food protein chemistry: an introduction for food scientists. Gainesville, FL, USA: Academic Press; 1984. pp. 274–332. [Google Scholar]
- 27.Smith PG. Introduction to food process engineering. New York, NY, USA: Kluwer Academic/Plenum Publishing Corporation; 2011. pp. 510. [Google Scholar]
- 28.Režek Jambrak A, Lelas V, Mason TJ, Krešić G, Badanjak M. Physical properties of ultrasound treated soy proteins. J Food Eng. 2009;93:386–93. 10.1016/j.jfoodeng.2009.02.001 [DOI] [Google Scholar]
- 29.Rastogi NK. Opportunities and challenges in non-thermal processing of foods. In: M.L. Passos, C.P. Ribeiro, editors. Innovation in food engineering: new techniques and products. Boca Raton, FL, USA: CRC Press; 2010. pp. 3–58. [Google Scholar]
- 30.Eppendorfer WH, Bille SW. Free and total amino acid composition of edible parts of beans, kale, spinach, cauliflower and potatoes as influenced by nitrogen fertilization and phosphorus and potassium deficiency. J Sci Food Agric. 1996;71:449–58. [DOI] [Google Scholar]
- 31.Friedman M. Nutritional value of proteins from different food sources. A review. J Agric Food Chem. 1996;44:6–29. 10.1021/jf9400167 [DOI] [Google Scholar]
- 32.Valencia DG, Serrano MP, Centeno C, Lázaro R, Mateos GG. Pea protein as a substitute of soya bean protein in diets for young pigs: effects on productivity and digestive traits. Livest Sci. 2008;118:1–10. 10.1016/j.livsci.2008.01.018 [DOI] [Google Scholar]
- 33.Wolf WJ. Soybean proteins: their functional, chemical, and physical properties. J Agric Food Chem. 1970;18:969–76. 10.1021/jf60172a025 [DOI] [Google Scholar]
- 34.Barbut S. Determining water and fat holding. In: GM Hall, editor. Methods of testing protein functionality. London, UK: Blackie Academic and Professional; 1996. pp. 186–225. [Google Scholar]
- 35.Meng G, Ma CY. Characterization of globulin from Phaseolus angularis (red bean). Int J Food Sci Technol. 2002;37:687–95. 10.1046/j.1365-2621.2002.00601.x [DOI] [Google Scholar]
- 36.Kaushal P, Kumar V, Sharma HK. Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeonpea (Cajanus cajan) flour and their blends. LWT –. Food Sci Technol (Campinas). 2012;48:59–68. 10.1016/j.lwt.2012.02.028 [DOI] [Google Scholar]
- 37.Cabra V, Arreguín R, Farres A. Emulsifying properties of proteins. BSQM. 2008;2:80–9. [Google Scholar]
- 38.Tavano OL. Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal, B Enzym. 2013;90:1–11. 10.1016/j.molcatb.2013.01.011 [DOI] [Google Scholar]
- 39.Aluko RE, McIntosh T, Reaney M. Comparative study of the emulsifying and foaming properties of defatted coriander (Coriandrum sativum) seed flour and protein concentrate. Food Res Int. 2001;34:733–8. 10.1016/S0963-9969(01)00095-3 [DOI] [Google Scholar]