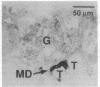
Abstract
Tubular-fluid reabsorption by specialized cells of the nephron at the junction of the ascending limb of the loop of Henle and the distal convoluted tubule, termed the macula densa, releases compounds causing vasoconstriction of the adjacent afferent arteriole. Activation of this tubuloglomerular feedback response reduces glomerular capillary pressure of the nephron and, hence, the glomerular filtration rate. The tubuloglomerular feedback response functions in a negative-feedback mode to relate glomerular capillary pressure to tubular-fluid delivery and reabsorption. This system has been implicated in renal autoregulation, renin release, and longterm body fluid and blood-pressure homeostasis. Here we report that arginine-derived nitric oxide, generated in the macula densa, is an additional intercellular signaling molecule that is released during tubular-fluid reabsorption and counters the vasoconstriction of the afferent arteriole. Antibody to rat cerebellar constitutive nitric oxide synthase stained rat macula densa cells specifically. Microperfusion of the macula densa segment of single nephrons with N omega-methyl-L-arginine (an inhibitor of nitric oxide synthase) or with pyocyanin (a lipid-soluble inhibitor of endothelium-derived relaxation factor) showed that generation of nitric oxide can vasodilate the afferent arteriole and increase glomerular capillary pressure; this effect was blocked by drugs that prevent tubular-fluid reabsorption. We conclude that nitric oxide synthase in macula densa cells is activated by tubular-fluid reabsorption and mediates a vasodilating component to the tubuloglomerular feedback response. These findings imply a role for arginine-derived nitric oxide in body fluid-volume and blood-pressure homeostasis, in addition to its established roles in modulation of vascular tone by the endothelium and in neurotransmission.
Full text
PDF

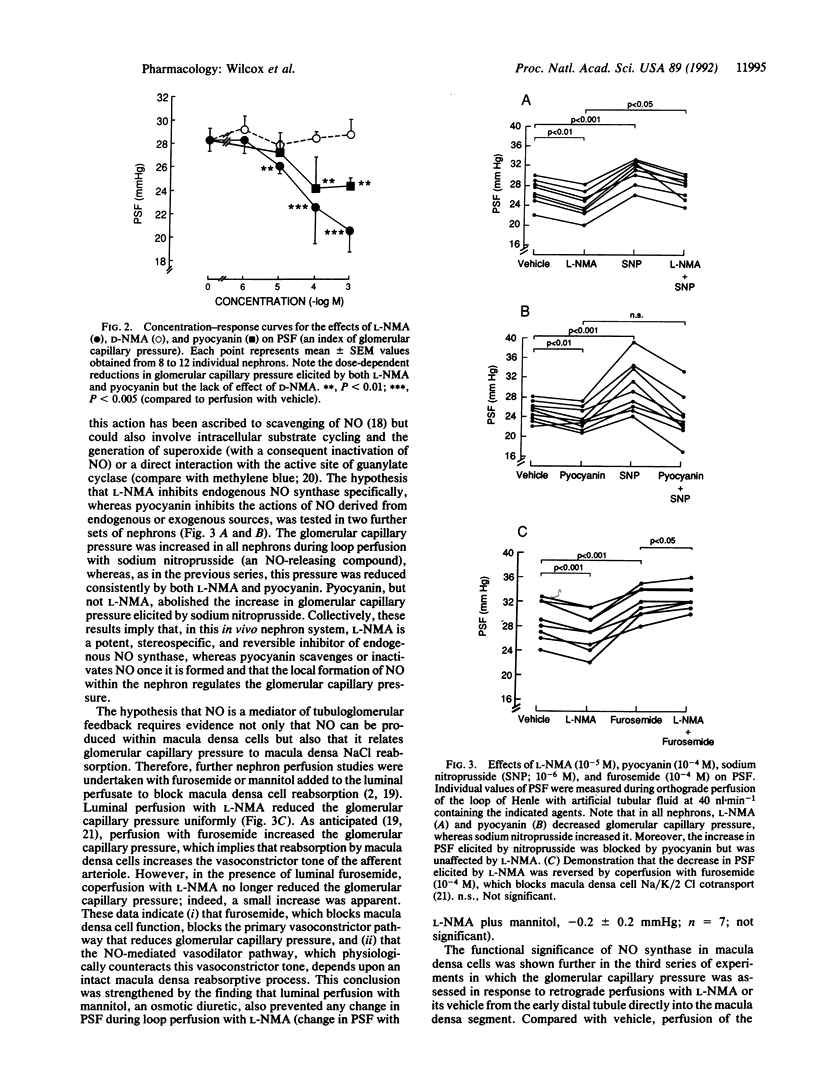
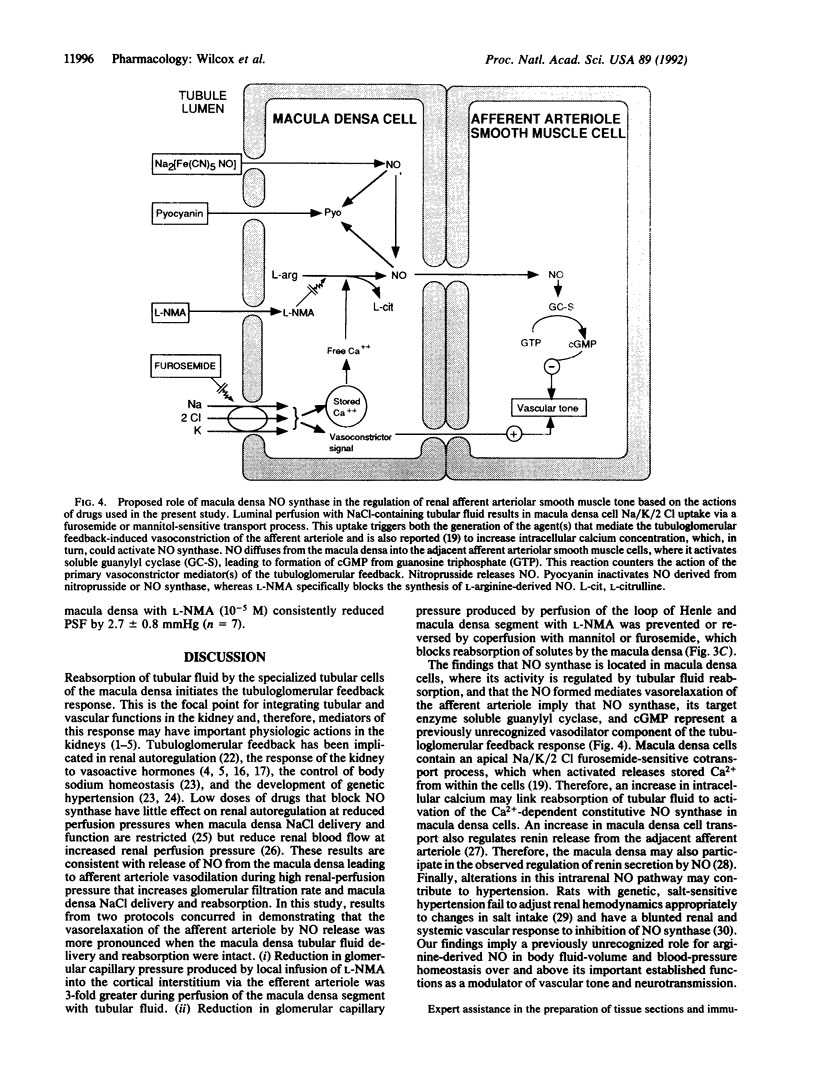

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Chen P. Y., Sanders P. W. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991 Nov;88(5):1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels F. H., Arendshorst W. J., Roberds R. G. Tubuloglomerular feedback and autoregulation in spontaneously hypertensive rats. Am J Physiol. 1990 Jun;258(6 Pt 2):F1479–F1489. doi: 10.1152/ajprenal.1990.258.6.F1479. [DOI] [PubMed] [Google Scholar]
- Dilley J. R., Arendshorst W. J. Enhanced tubuloglomerular feedback activity in rats developing spontaneous hypertension. Am J Physiol. 1984 Oct;247(4 Pt 2):F672–F679. doi: 10.1152/ajprenal.1984.247.4.F672. [DOI] [PubMed] [Google Scholar]
- Fink G. D., Takeshita A., Mark A. L., Brody M. J. Determinants of renal vascular resistance in the Dahl strain of genetically hypertensive rat. Hypertension. 1980 May-Jun;2(3):274–280. doi: 10.1161/01.hyp.2.3.274. [DOI] [PubMed] [Google Scholar]
- Folger W. H., Lawson D., Wilcox C. S., Mehta J. L. Response of rat thoracic aortic rings to thromboxane mimetic U-46,619: roles of endothelium-derived relaxing factor and thromboxane A2 release. J Pharmacol Exp Ther. 1991 Aug;258(2):669–675. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Ishii K., Kerwin J. F., Jr, Murad F. N omega-nitro-L-arginine: a potent inhibitor of the L-arginine-dependent soluble guanylate cyclase activation pathway in LLC-PK1 cells. Can J Physiol Pharmacol. 1990 Jun;68(6):749–751. doi: 10.1139/y90-114. [DOI] [PubMed] [Google Scholar]
- Lapointe J. Y., Bell P. D., Hurst A. M., Cardinal J. Basolateral ionic permeabilities of macula densa cells. Am J Physiol. 1991 Jun;260(6 Pt 2):F856–F860. doi: 10.1152/ajprenal.1991.260.6.F856. [DOI] [PubMed] [Google Scholar]
- Majid D. S., Navar L. G. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol. 1992 Jan;262(1 Pt 2):F40–F46. doi: 10.1152/ajprenal.1992.262.1.F40. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Brock T. A., Ballermann B. J. Glomerular endothelial cells respond to calcium-mobilizing agonists with release of EDRF. Am J Physiol. 1990 May;258(5 Pt 2):F1295–F1303. doi: 10.1152/ajprenal.1990.258.5.F1295. [DOI] [PubMed] [Google Scholar]
- Mitchell K. D., Navar L. G. Enhanced tubuloglomerular feedback during peritubular infusions of angiotensins I and II. Am J Physiol. 1988 Sep;255(3 Pt 2):F383–F390. doi: 10.1152/ajprenal.1988.255.3.F383. [DOI] [PubMed] [Google Scholar]
- Mitchell K. D., Navar L. G. Superficial nephron responses to peritubular capillary infusions of angiotensins I and II. Am J Physiol. 1987 May;252(5 Pt 2):F818–F824. doi: 10.1152/ajprenal.1987.252.5.F818. [DOI] [PubMed] [Google Scholar]
- Persson A. E., Bianchi G., Boberg U. Tubuloglomerular feedback in hypertensive rats of the Milan strain. Acta Physiol Scand. 1985 Feb;123(2):139–146. doi: 10.1111/j.1748-1716.1985.tb07570.x. [DOI] [PubMed] [Google Scholar]
- Romero J. C., Lahera V., Salom M. G., Biondi M. L. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol. 1992 Mar;2(9):1371–1387. doi: 10.1681/ASN.V291371. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnermann J., Briggs J. P. Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol. 1989 Mar;256(3 Pt 2):F421–F429. doi: 10.1152/ajprenal.1989.256.3.F421. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., Schorer A. E., Raij L. Effects of endothelium-derived relaxing factor and nitric oxide on rat mesangial cells. Am J Physiol. 1990 Jan;258(1 Pt 2):F162–F167. doi: 10.1152/ajprenal.1990.258.1.F162. [DOI] [PubMed] [Google Scholar]
- Skøtt O., Briggs J. P. Direct demonstration of macula densa-mediated renin secretion. Science. 1987 Sep 25;237(4822):1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- THURAU K., SCHNERMANN J. DIE NATRIUMKONZENTRATION AN DEN MACULA DENSA-ZELLEN ALS REGULIERENDER FAKTOR FUER DAS GLOMERULUMFILTRAT (MIKROPUNKTIONSVERSUCHE) Klin Wochenschr. 1965 Apr 15;43:410–413. doi: 10.1007/BF01483845. [DOI] [PubMed] [Google Scholar]
- Vidal M. J., Romero J. C., Vanhoutte P. M. Endothelium-derived relaxing factor inhibits renin release. Eur J Pharmacol. 1988 May 10;149(3):401–402. doi: 10.1016/0014-2999(88)90679-6. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Loi R., Rendell N. B., Taylor G. W. Nitric oxide is inactivated by the bacterial pigment pyocyanin. Biochem J. 1990 Mar 15;266(3):921–923. [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Wilcox C. S. Modulating role for thromboxane in the tubuloglomerular feedback response in the rat. J Clin Invest. 1988 Jun;81(6):1843–1849. doi: 10.1172/JCI113529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Wilcox C. S. Potentiation of tubuloglomerular feedback in the rat by thromboxane mimetic. Role of macula densa. J Clin Invest. 1992 Jun;89(6):1857–1865. doi: 10.1172/JCI115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. S., Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974 Jun;53(6):1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]