Summary
The objective of this study is to evaluate the antilisterial effect of Pediococcus pentosaceus T1, which was isolated from kimchi, and to assess its potential for extending the shelf life of salmon and kimchi. Pediococcus pentosaceus T1 culture effectively inhibited proliferation of Listeria monocytogenes in a dose-dependent manner in a salmon-based medium. Antilisterial effect of the culture was stronger than that of nisin, an antibacterial peptide, as evidenced by lower minimum inhibitory concentration value (20 mg/mL) compared to nisin (over 20 mg/mL). P. pentosaceus T1 culture also effectively inhibited the growth of Listeria in salmon fillet. In particular, the culture (6 g per 100 mL) showed a stronger inhibitory effect than sodium hypochlorite (0.2 mg/mL), a disinfectant used in food processing. In kimchi fermentation, the treatment with P. pentosaceus T1 culture suppressed changes of acidity and pH during maturation. The inhibitory effect of the culture on kimchi lactic acid bacteria, which include Leuconostoc mesenteroides and Lactobacillus sakei, led to a drastic decrease in maturation rates of kimchi. Moreover, sensory test on kimchi treated with P. pentosaceus T1 showed that the culture improved overall acceptability of kimchi, which can be observed in higher scores of sourness, texture, off-flavour and mouthfeel compared with untreated kimchi. The results of this study suggest that kimchi-derived P. pentosaceus T1 could be a potential antilisterial agent in fish products as well as a starter to control overmaturation of kimchi.
Key words: Pediococcus pentosaceus T1, kimchi, salmon fillets, antilisterial activity, antibacterial activity
Introduction
Lactic acid bacteria (LAB), highly beneficial microorganisms for humans, have been used for a long time in fermented products such as fermented milk, sausages and kimchi (1, 2). They are usually Gram-positive, catalase-negative, and non-spore-forming bacteria (1). They are classified into various genera including Lactobacillus, Leuconostoc, Streptococcus, Lactococcus and Pediococcus. The properties of LAB are numerous: the enhancement of food preservation and flavour by their metabolites, antimicrobial effect against harmful bacteria, and supply of nutrients. The biological effects of LAB on human health have been studied in various research areas (1). These effects include activation of immunity, anticancer activity, reduction of cholesterol level and liver protection (3–6). A recent study has shown the suppressive effect on allergy such as atopic dermatitis by lactic acid bacteria via cell line and animal studies (7).
Kimchi is a traditional fermented vegetable dish in Korea, which has centuries long historical records of consumption (1). Its fermentation is a spontaneous process that is initiated by various microorganisms originally present in the raw materials for kimchi production (1). The microorganisms in kimchi include approx. 200 species of bacteria and several yeasts, which are involved in a series of fermentation stages. Various LAB species, among which Pediococcus spp., have been isolated from kimchi, and their different technological characteristics have been studied (8, 9). This strain is known to be used in the American-style fermented meat and vegetables as a main starter culture (10). It produces bacteriocin called pediocin, which usually possesses antilisterial activity. Recent studies have shown that pediocins or pediococcus cultures inhibit Listeria monocytogenes in fermented sausages or salami (11, 12). L. monocytogenes, a major human pathogen, is a bacterium causing listeriosis, a serious bacterial disease (13). Elderly people, newborns, and pregnant women, who have weakened immune systems, are susceptible to this disease, which is accompanied by sepsis and meningitis with high mortality rate (14).
Safe preservation of food is one of the critical issues in food industry. Traditionally, control of temperature such as by heating or refrigeration has usually been used for food preservation. However, these treatments can have high cost and cause the change of the components of food products, which results in the loss of food nutrients and changes in flavour, recognized as unnatural by consumers. In addition, the occurrence of psychrophilic pathogens does not guarantee safety in food preservation based on low temperature. Synthetic preservatives are used as an alternative way for food preservation, but they can be unfavourable for human health (15). Therefore, in recent years, biopreservation using biomaterials has received attention as a way of food preservation, with a trend demanding fresh and natural products. LAB are one of the good sources for biopreservation.
One of the beneficial properties of LAB is the production of antimicrobial substances like bacteriocin (13), which are used for biopreservation. Bacteriocin such as nisin is admitted as a GRAS (Generally Recognized As Safe) (16), and many European countries use nisin as a food preservative in commercial food products including canned food, mayonese and cheese (17).
In our previous study, we isolated a strain from kimchi and identified it by ribosomal DNA sequence analysis as the antilisterial strain Pediococcus pentosaceus T1 (18). In this study, we examine the antilisterial effect of P. pentosaceus T1 culture in fish products like salmon fillet, and its effect on maturation and quality of kimchi.
Materials and Methods
Isolation and identification of the LAB that produce antibacterial agents
Commercial kimchi was obtained from a store (Seoul, Korea), the samples were unwrapped, transferred to stomacher filter bags and mixed with sterile phosphate buffer (0.625 mM, pH=7.2). The samples were homogenized with a BagMixer® 400 VW (Interscience, Saint- -Nom-la-Bretčche, France) at 300 × g for 5 min, then serially diluted and plated onto de Man, Rogosa and Sharpe (MRS) agar (BD Difco, Detroit, MI, USA), followed by incubation under anaerobic conditions using the GasPakTM system (GENbox anaerobic indicator, bioMérieux S.A, Marcy l’Etoile, France) at 37–42 °C for 48 h. Colonies were Gram stained and tested for catalase. Gram-positive and catalase-negative bacilli or coccobacilli were selected (18). For identification of LAB that produce antibacterial agents, rDNA PCR analysis was performed. Genomic DNA was extracted using DNeasy tissue kit (Qiagen, Hiden, Germany), and PCR reaction for the amplification of 16S rDNA was performed using 20 pmol of universal bacterial primers: 27F (50-AGAGTTTGATCCTGGCTCA-30) and 1492R (50-GGTTACCTTGTTACGACTT-30) (19), and template DNA, 100 mM dNTP, 1 U of Taq DNA polymerase (Roche, Mannheim, Germany). After thermocycling amplification (18), agarose gel electrophoresis was performed to confirm PCR products. 16S rDNA from the gel was collected, purified using Solgent gel and PCR purification system (Solgent, Daejeon, South Korea), and then compared with 16S rDNA sequences of other strains using the BLAST programs in the National Center for Biotechnology Information database (Rockville Pike, Bethesda, MD, USA) and the EzTaxon server v. 2.1 (20) by Solgent. Phylogenetic analyses of the 16S rRNA gene sequences were conducted using Molecular Evolutionary Genetics Analysis (MEGA) software, v. 5 (21).
Antilisterial activity of LAB from kimchi
Antilisterial activity of the isolated LAB strains was tested using an agar well diffusion method, as described by de Carvalho et al. (22). One hundred and twenty five LAB were cultured overnight by inoculating 105 CFU/mL in tryptic soy broth (TSB; BD Difco). The agar well diffusion assay was performed by spreading Listeria monocytogenes cultures on tryptic soy agar (TSA) plates (BD Difco). Wells of 6.5 mm in diameter were punched in these plates, filled with 50 mL of cell-free culture supernatants of LAB and incubated at 35 °C for 24 h. Antilisterial activities were measured by examining the diameters of the inhibition zones around the wells. The inhibitory activities corresponding to the diameters of the inhibition zones were expressed in mm.
Culture conditions and preparation of crude supernatant
The composition of the culture medium was as follows (in %): sucrose 1.5 and fructose 1.5 (carbon source), soya peptone and yeast extract 1.5 (nitrogen source), K2HPO4 0.1, sodium acetate 0.1, tryptophan 0.05, cysteine 0.05, MgSO4 0.01, and MnSO4 0.005. A 5-litre laboratory scale fermentor (FMT ST-D, Fermentech, Cheongju, South Korea) was used for the growth of LAB under anaerobic conditions at 35 °C, with stirring at 100 × g for 20 h. The fermented culture was centrifuged at 8000 × g for 30 min, and the supernatant was autoclaved at 100 °C for 15 min to inactivate proteases. Organic acids in the culture were removed by ultrafiltration (molecular mass cut-off <3 kDa). The filter sludge was lyophilized for the study.
Listeria cultivation, salmon medium preparation, and antilisterial determination
Listeria monocytogenes KCCM 40307 was inoculated on TSA (BD Difco) corresponding to the cell number of 108 cells per mL. This Listeria solution was diluted to 105 cells per mL in 200 mL of TSB (BD Difco) containing Pediococcus pentosaceus T1 culture at mass per volume ratios of 1, 2, 3 and 4%, and incubated at 35 °C. The Listeria culture was harvested at 6, 9, 12, 15 and 18 h to count viable cell numbers on Listeria selective medium, an Oxford Medium Base (BD Difco) containing antimicrobial supplement (BD Difco). For antimicrobial activity of nisin (Sigma-Aldrich, St. Louis, MO, USA) and P. pentosaceus T1 culture, raw salmon (10 g) was ground under aseptic conditions, and added to TSB and phosphate buffer (0.625 mM, pH=7.2) (10 mL) to make a salmon-based medium. A volume of 100 µL of Listeria culture was added to the salmon-based medium followed by the addition of nisin and P. pentosaceus T1 culture with serial dilutions (20, 10, 5, 2.5, 1.25 and 0.625 mg/mL). The culture was incubated at 35 °C for 24 h, and spread onto Listeria selective medium. Minimal inhibitory concentration (MIC) was set where viable Listeria was not observed on the plate.
Antilisterial activity in raw salmon fillet
Frozen salmon was thawed, and sliced into fillets (200 g). Three fillets were used to examine the antilisterial activity of each P. pentosaceus T1 culture or sodium hypochlorite (ACL-60G, Namkang, Bucheon, South Korea) treatment. The fillets were inoculated with Listeria culture (106 CFU/mL), and then rested for 2 h at room temperature. Afterwards, they were dipped in sodium hypochlorite (0.2 mg/mL) or the P. pentosaceus T1 culture solution (6 g per 100 mL) for 10 min, or sprayed with sodium hypochlorite or the culture solution. The fillets were incubated in the refrigerator at 4 °C for 24 h. Listeria cells were taken from the fillets by grinding them and diluting with phosphate buffer (0.625 mM, pH=7.2), followed by spreading on the Listeria selective medium for counting the Listeria cells.
Antimicrobial activity on LAB from kimchi
Antimicrobial activity of the isolated P. pentosaceus T1 on LAB from kimchi was tested using an agar well diffusion method, as described by Jang et al. (18). Indicator strains, including 16 LAB strains, were cultured overnight by inoculating 105 CFU/mL in MRS medium (BD Difco). Sixteen strains of LAB were obtained from Korean Collection of Type Cultures (KCTC, Daejeon, Korea). The agar well diffusion assay was performed by spreading the LAB cultures on MRS agar plates (BD Difco). Wells of 6.5 mm in diameter were punched in these plates, filled with 50 µL of cell-free supernatants of P. pentosaceus T1, from which organic acid was removed, and incubated at 35 °C for 24 h. Antimicrobial activity was examined by measuring the diameters of inhibition zones around the wells. When the diameters of the clear zones were wider than 6.5 mm, the LAB were considered to be inhibited by P. pentosaceus T1. The inhibitory activity corresponding to the diameters of the inhibition zones was expressed in mm.
Preparation of kimchi
Kimchi was prepared in batches up to 500 kg at a kimchi factory (Our Home Co. Ltd, Seongnam, South Korea) using their production line. Chinese cabbage (Brassica campestris L. ssp. pekinensis Rupr.) was soaked in a solution of refined salt (80 g/L, Hanju Co, Ulsan, Korea) for 2 to 4 h, and washed 3 times with tap water. The washed Chinese cabbages were left to drain any excess water in a wicker container at 5 to 10 °C for 2 to 4 h. The salted Chinese cabbages were then mixed with the other kimchi ingredients including red pepper powder, radish, garlic, ginger, onion, sugar and fermented fish sauce. The final salt mass fraction of the kimchi was adjusted to 1.9 to 2.1% using refined salt. The filtered culture of Pediococcus pentosacesus T1 was inoculated into kimchi preparation (1%, 10 g/kg). As a control, the same kimchi recipe was used without the filtered culture of Pediococcus pentosacesus T1. The prepared kimchi was vacuum packed into 500-gram retort packages with polyethylene resin and incubated at 10 °C in a refrigerator (Daehan Science, Seoul, Korea) for 105 days.
Chemical analysis of kimchi
Ripened kimchi (500 g) was macerated using a hand blender (Hanil, Seoul, South Korea) for 2 min. The kimchi juice was centrifuged at 5000 ×g for 5 min, and the pH of the supernatant was tested with a pH meter (Mettler Toledo, Viroflay, France). The supernatant was then titrated with 0.1 M NaOH to pH=8.3 to determine the total titratable acidity (TTA), which was expressed as:
where V is the volume of 0.1 M NaOH (mL), f is the factor of 0.1 M NaOH solution, and k is the constant of organic acid equivalent to 1 mL of 0.1 M NaOH solution (in the case of lactic acid k=0.009).
Microbial analysis of kimchi
For the microbial analysis, kimchi samples were randomly selected and blended for 2 min. The juice samples were filtered with a sterile sieve (pore size: 0.15 mm, Chung Gye Sang Gong SA, Seoul, South Korea) and the aliquots of each filtrate were serially diluted with 0.1% peptone water and spread onto plate count agar (PCA; Merck, Darmstadt, Germany) for total microbial counts. The plates were counted after 2 to 3 days of incubation at 37 °C. Among the serially diluted plates, those with 30 to 300 CFU/mL were used for enumeration of the total microbial population in the kimchi samples.
Sensory analysis
Thirty-five panellists comprising 20- to 35-year-old housewives evaluated the acceptability of kimchi. Colour, sourness, sweetness, fizzy mouthfeel, mouldy flavour (off--flavour) and overall acceptability were scored using a 9-point hedonic scale: 1=very bad, 5=moderate and 9=very good.
Statistical analysis
Statistical analysis was performed using the SPSS-PC v. 11.0 software (SPSS, Chicago, IL, USA). Data were subjected to ANOVA, and the mean values were separated using Duncan’s multiple-range test, with significance at p<0.05. For the significance of the differences between the given samples and control group, Student’s t-test was used (p<0.05).
Results and Discussion
Isolation of antibacterial LAB from kimchi
One hundred and twenty five strains of LAB were isolated based on Gram-positive staining and catalase reaction. Antilisterial activity of the LAB was examined using well diffusion assay. Twenty LAB strains (SN2, SN3, SN5, SN8, SN10, SN12, SN14, SN19, SN22, SN43, SN46, SN47, SN48, SN52, SN54, SN59, SN65, SN66, SN68 and SN101) were shown to have an inhibitory effect on L. monocytogenes. Among them, the strain SN43 demonstrated the strongest antilisterial activity by showing the inhibition zone of over 6.5 mm in agar well diffusion assay (Table 1). We had identified SN43 strain as Pediococcus pentosaceus T1 via rDNA PCR analysis in the previous study (18). Therefore, we used the culture of P. pentosaceus T1 for subsequent experiments.
Table 1. Antilisterial activity of LAB from kimchi.
| Strain no. |
Inhibition zone |
Strain no. | Inhibition zone | Strain no. | Inhibition zone | Strain no. | Inhibition zone |
|---|---|---|---|---|---|---|---|
| SN01 | – | SN32 | – | SN63 | – | SN94 | – |
| SN02 | + | SN33 | – | SN64 | – | SN95 | – |
| SN03 | ++ | SN34 | – | SN65 | + | SN96 | – |
| SN04 | – | SN35 | – | SN66 | + | SN97 | – |
| SN05 | + | SN36 | – | SN67 | – | SN98 | – |
| SN06 | – | SN37 | – | SN68 | + | SN99 | – |
| SN07 | – | SN38 | – | SN69 | – | SN100 | – |
| SN08 | + | SN39 | – | SN70 | – | SN101 | + |
| SN09 | – | SN40 | – | SN71 | – | SN102 | – |
| SN10 | ++ | SN41 | – | SN72 | – | SN103 | – |
| SN11 | – | SN42 | – | SN73 | – | SN104 | – |
| SN12 | + | SN43 (T1) | +++ | SN74 | – | SN105 | – |
| SN13 | – | SN44 | – | SN75 | – | SN106 | – |
| SN14 | + | SN45 | – | SN76 | – | SN107 | – |
| SN15 | – | SN46 | + | SN77 | – | SN108 | – |
| SN16 | – | SN47 | ++ | SN78 | – | SN109 | – |
| SN17 | – | SN48 | + | SN79 | – | SN110 | – |
| SN18 | – | SN49 | – | SN80 | – | SN111 | – |
| SN19 | + | SN50 | – | SN81 | – | SN112 | – |
| SN20 | – | SN51 | – | SN82 | – | SN113 | – |
| SN21 | – | SN52 | ++ | SN83 | – | SN114 | – |
| SN22 | + | SN53 | – | SN84 | – | SN115 | – |
| SN23 | – | SN54 | + | SN85 | – | SN116 | – |
| SN24 | – | SN55 | – | SN86 | – | SN117 | – |
| SN25 | – | SN56 | – | SN87 | – | SN118 | – |
| SN26 | – | SN57 | – | SN88 | – | SN119 | – |
| SN27 | – | SN58 | – | SN89 | – | SN120 | – |
| SN28 | – | SN59 | ++ | SN90 | – | SN121 | – |
| SN29 | – | SN60 | – | SN91 | – | SN122 | – |
| SN30 | – | SN61 | – | SN92 | – | SN123 | – |
| SN31 | – | SN62 | – | SN93 | – | SN124/125 | – |
–=no inhibition zone, +=radius of inhibition zone <3 mm, ++=radius of inhibition zone 4 to 6 mm, +++=radius of inhibition zone >6 mm, T1=Pediococcus pentosaceus T1
Effect of P. pentosaceus T1 culture on L. monocytogenes proliferation
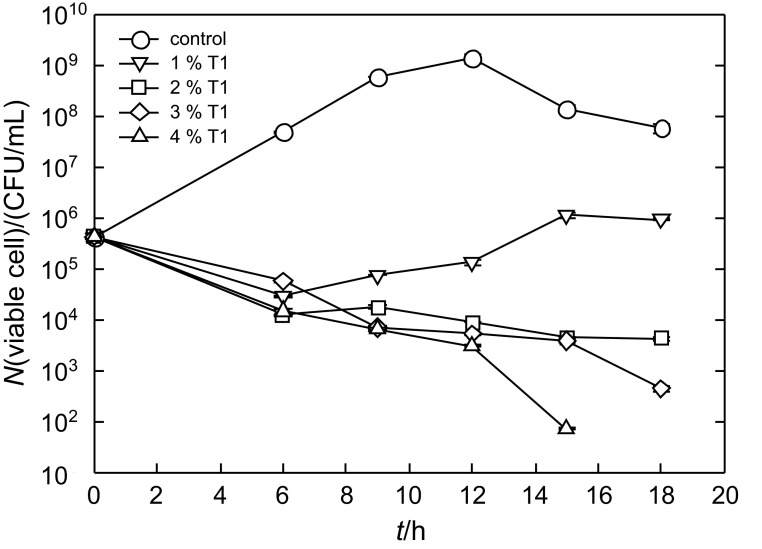
The inhibitory effect of the P. pentosaceus T1 culture against Listeria at different doses was determined. Listeria monocytogenes growth greatly increased until 12 h in the control group, reaching 1.3·109 CFU/mL, after which it gradually decreased to 5.8·107 CFU/mL (Fig. 1). In contrast, the treatment with the culture significantly inhibited cell proliferation of Listeria at all tested concentrations. After 6 h, the number of Listeria monocytogenes cells in all samples treated with the culture was 104 to 105 CFU/mL. The culture containing 1% P. pentosaceus T1 showed a small increase of the number of Listeria monocytogenes cells after 6 h (Fig. 1). However, the number of Listeria monocytogenes cells at the other mass per volume ratios of the culture (2, 3 and 4%) continually decreased after 6 h. In particular, 4% of the culture caused a dramatic decrease after 18 h, with less than 102 CFU/mL of Listeria cells. Thus, the treatment with P. pentosaceus T1 culture showed an effective inhibitory effect on Listeria monocytogenes in a dose-dependent manner. Our results indicate that the substances produced by P. pentosaceus T1 have an ability to inhibit Listeria monocytogenes.
Fig. 1.
Effect of P. pentosaceus T1 culture (T1) on Listeria monocytogenes proliferation. L. monocytogenes was grown in the presence or absence of various mass per volume ratios of the culture (powder) for 18 h. Listeria cultures harvested at 6, 9, 12, 15 and 18 h were spread onto Listeria monocytogenes selective medium to count the viable cells. Data are expressed as mean values± standard deviation (S.D.) (N=3)
Previously, we had isolated P. pentosaceus T1 as an antilisterial LAB from kimchi (18). In general, Pediococcus spp. have been known to exhibit antilisterial activity like Lactobacillus spp. (23). Recent studies have reported antilisterial activity of Pediococcus spp. from various sources (23, 24). Pediococcus spp. were also known to inhibit other pathogens such as Escherichia coli and Staphylococcus aureus (25). A major antimicrobial substance from Pediococcus spp. has been found to be a bacteriocin called pediocin, which is classified into Class II (24). Its molecular size is less than 5 kDa containing 36–48 residues (25). However, our series of analyses including chromatography showed that P. pentosaceus T1-derived antilisterial material was proteinous substance with a molecular size of 23 kDa (18). In addition, liquid chromatography-mass spectrometry showed that P. pentosaceus T1-derived antimicrobial substance contained LysM domain (18), which is known to hydrolyze peptidoglycan, a cell wall component (26). Therefore, the active substance from our sample could be considered as a novel antilisterial substance, which is different from pediocin. LAB have been known to produce organic acids to inhibit other microbes, and these acids are possibly major antilisterial substances found in this work. However, we removed organic acids from the culture by ultrafiltration (cut off <3 kDa) to exclude this potential from our investigation. Lactic and acetic acids, at concentrations of 19.9 and 2.6 g/L respectively, before ultrafiltration were completely removed after ultrafiltration from the culture.
Antilisterial effects of P. pentosaceus T1 culture and nisin in raw salmon medium
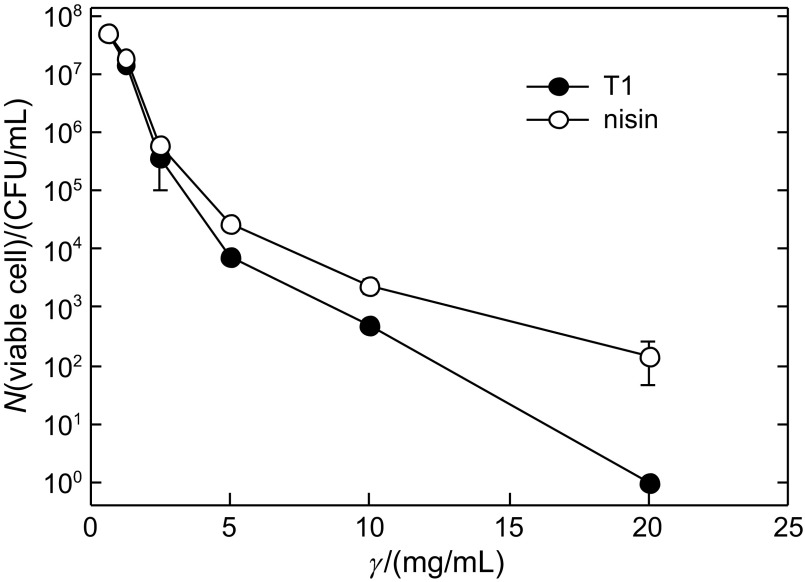
We compared the antilisterial effect of our sample (P. pentosaceus T1 culture) with that of nisin, a known bacteriocin with antilisterial activity. For this experiment, the raw salmon medium was inoculated with Listeria monocytogenes culture. Our culture and nisin were tested on Listeria in salmon in various doses to get minimum inhibitory concentrations (MIC). The number of Listeria cells in raw salmon medium significantly decreased with both treatments in a dose-dependent manner. Based on our data, inhibitory effect of the culture on Listeria proliferation was shown to be stronger than that of nisin (Fig. 2), showing a more decreased number of Listeria cells at most of the treatment concentrations. MIC value of the culture was 20 mg/mL, while that of nisin was over 20 mg/mL. This result shows that P. pentosaceus T1 produces stronger antilisterial substances than nisin. Considering that our culture sample was not completely purified, it is believed that real antilisterial activity of P. pentosaceus T1-derived active substance is underestimated. However, this result might be different under different conditions because optimal conditions for antimicrobial activity of nisin can be different from those of our samples. Generally, nisin is known to be more active in acidic pH, which is related to its cell membrane permeation (27–29). In contrast, another study reported that nisin was rather less sensitive to foodborne pathogens such as Staphylococcus aureus and Listeria monocytogenes in the acidic pH (pH=4.5–5) (30). Our test of antilisterial activity of nisin and P. pentosaceus T1 culture was performed under the optimal growth conditions for L. monoctogenes (pH=7.2 and 35 °C) to count viable Listeria cells in the selective medium. Since our previous study had shown that the culture had broader spectrum of pH and temperature for maximal antilisterial activity (31), it could be more favourable as an antilisterial agent in food products. However, the application of antimicrobial materials on food is more complex due to various factors such as salt concentration and temperature, which affect the antimicrobial activity (30). Therefore, further detailed analysis of antilisterial effects of both samples will be performed in the next study.
Fig. 2.
Antilisterial effects of P. pentosaceus T1 culture (T1) and nisin, which were added to L. monocytogenes-inoculated salmon medium at serially diluted concentrations to test the antilisterial effects for 24 h at 35 °C. Data are expressed as mean values± S.D. (N=3)
Antilisterial effect of P. pentosaceus T1 culture on salmon fillets inoculated with Listeria monocytogenes
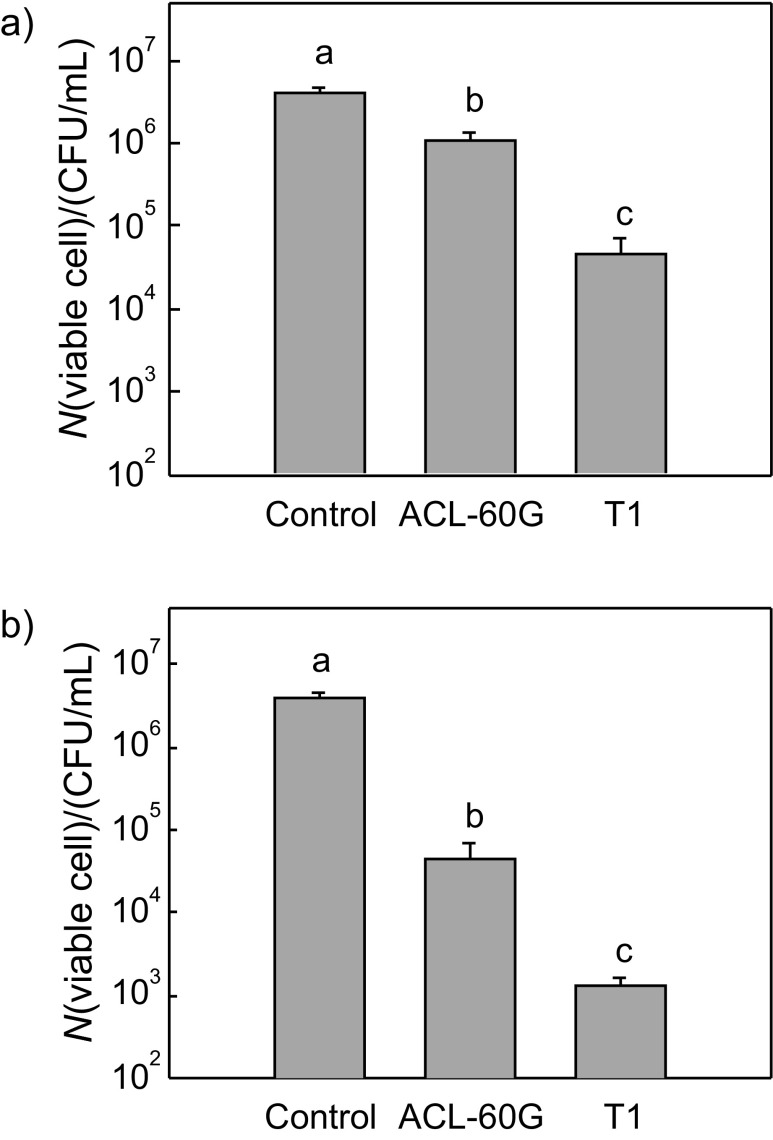
Fish is highly susceptible to contamination with food pathogens like L. monocytogenes, causing a serious food- -derived infection globally (32). We tested the antilisterial effect of our culture sample on a fish product contaminated with Listeria. Listeria-inoculated salmon fillets were dipped and sprayed with our samples and sodium hypochlorite. After incubation for 24 h at 4 °C, significant decreases in the bacterial cell numbers were observed in sample-treated groups (Fig. 3). Treatment with sodium hypochlorite, a disinfectant normally used in fish product processing, also showed a significant reduction of Listeria cells. Interestingly, our culture caused a dramatic decrease in the number of Listeria cells after the treatment (Fig. 3). The culture showed a much stronger inhibitory effect on Listeria growth compared with sodium hypochlorite (0.2 mg/mL, ACL-60G), which served as a positive control. However, we cannot directly compare the inhibitory effects of P. pentosaceus T1 culture and ACL-60G disinfectant because the concentrations of the two samples were different in the treatments, where 0.2 mg/mL of ACL-60G is maximum allowed criterion in food processing. Nevertheless, P. pentosaceus T1 culture (6 g per 100 mL) clearly has an inhibitory effect on Listeria growth in salmon product. Therefore, P. pentosaceus T1 culture could be used as an inhibitor of Listeria contamination in fish products. This result is correlated with the data derived from the experiment performed in raw salmon medium (Fig. 2). Our data suggest an applicable potential of P. pentosaceus T1 in raw fish product processing in food industry.
Fig. 3.
Antilisterial effect of P. pentosaceus T1 culture (T1, 6 g per 100 mL) on salmon fillets inoculated with Listeria monocytogenes. Salmon fillets were inoculated with Listeria and: a) dipped, or b) sprayed with disinfectant ACL-60G or P. pentosaceus T1 solution. Treated salmon fillets were incubated for 24 h at 4 °C, swabbed with cotton and diluted with phosphate buffer. The dilutions were spread onto Listeria selective medium to count the cells. Data show mean values±S.D. (N=3). Different letters indicate significant differences (p<0.05). γ(ACL-60G)=0.2 mg/L
Effects of P. pentosaceus T1 culture on acidity and pH changes in kimchi during fermentation
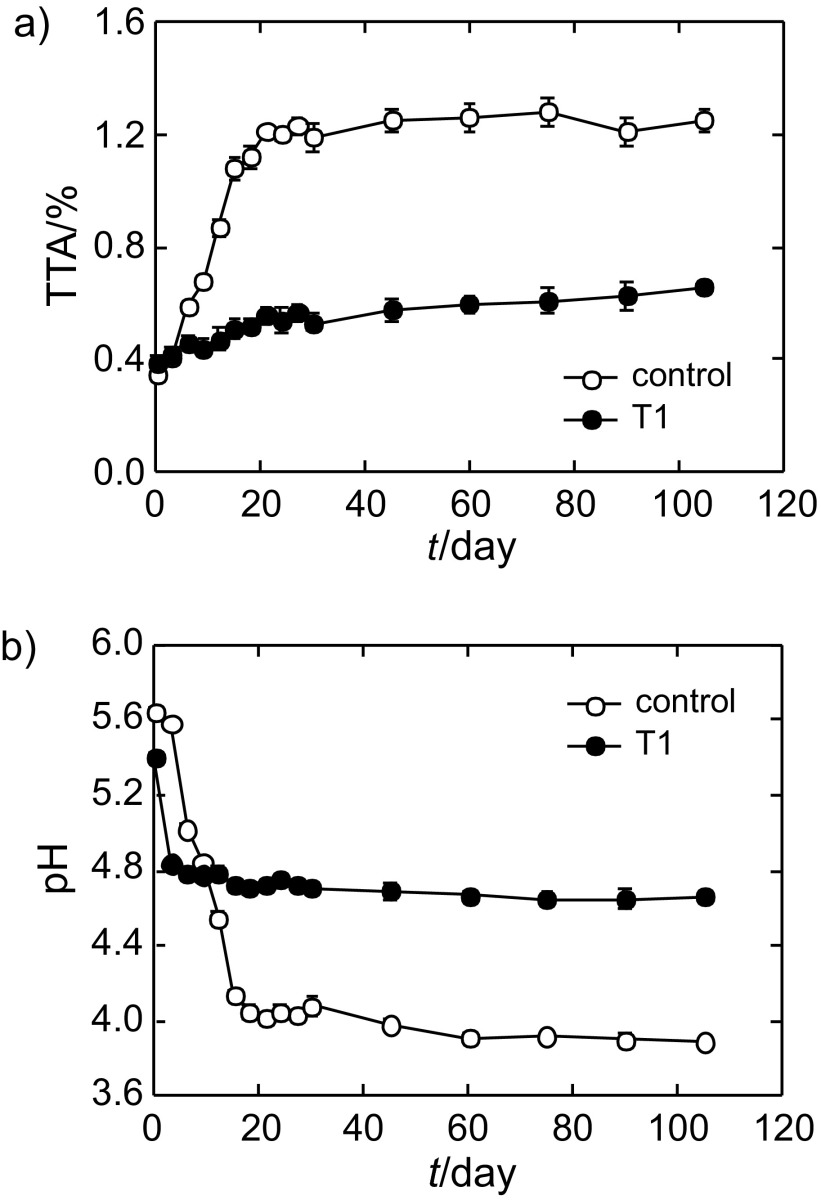
To examine the effect of P. pentosaceus T1 culture on the maturation of kimchi, we determined the changes in the pH values and titratable acidity of kimchi preparations during fermentation at 10 °C for 105 days (Fig. 4). When the kimchi was prepared (0 day fermentation), the total acidity was 0.40–0.42%, and pH values were 5.4–5.6. The acidity of the fermented kimchi without the culture treatment (control) increased faster than that of the culture-treated kimchi, reaching 1.21% (pH=4.02) within 21 days (Fig. 4), after which it remained stable until the 105th day (1.28%, pH=3.89). The acidity of the kimchi treated with the culture increased very slowly during fermentation, and reached up to 0.66% at pH=4.66 in 105 days (Fig. 4). Fast increase of acidity during fermentation in control kimchi resulted in rapid decrease of pH value, while the culture treatment deferred these changes. According to our data, normal kimchi acidity reached 1% at around day 15, but the culture-treated kimchi never reached that acidity during entire fermentation (Fig. 4a).
Fig. 4.
Effect of P. pentosaceus T1 culture (T1) on the acidity and pH changes in kimchi during fermentation. P. pentosaceus T1 (1%) was added to kimchi preparation, and the changes of: a) total titratable acidity (TTA), and b) pH were measured during fermentation. Data are expressed as mean values±S.D. (N=3)
Sensory quality of kimchi depends on the duration of maturation, which means that its optimal quality is maintained only for a certain period of time. Today, kimchi is commercially produced and sold via distribution networks. The supply of fresh and tasty kimchi is one of the important challenges for producers. During fermentation of kimchi, prolonged maturation allows proliferation of other putrefying or spoilage bacteria as well as deterioration of quality, which increases the acidity above 1% (33). Thus, acidity is used as a direct indicator of prolonged maturation (33). Usually, the acidity of kimchi in early fermentation stage is known to be in the range of 0.4–0.6%, after which it increases over 1% at around day 30 (34). The acidity of kimchi in our work reached 1% in 15 days, earlier than in the study by Shin et al. (34), suggesting faster maturation of the kimchi in our study. This result could be due to the differences in compositions of ingredients, which can affect LAB proliferation. Several studies have shown that optimal acidity is 0.5–0.75%, and kimchi with an acidity level over 1% is recognized as unacceptable for consumption (34, 35). Our results show that the treatment with the culture suppresses the increase of kimchi acidity during fermentation, maintaining it at 0.6%. Normally fermented kimchi has an optimal acidity for maturity only for a short period of time (within 20 days), while culture-treated kimchi maintains optimal acidity for a longer time (105 days) (Fig. 4). Our data indicate that culture treatment could play an important role in controlling the acidity of kimchi with optimal maturity, which is desirable for distribution and storage of the product, and meets the commercial demands. Our findings suggest that the shelf life of kimchi could be properly extended using the P. pentosaceus T1 as a starter culture under optimal maturation conditions.
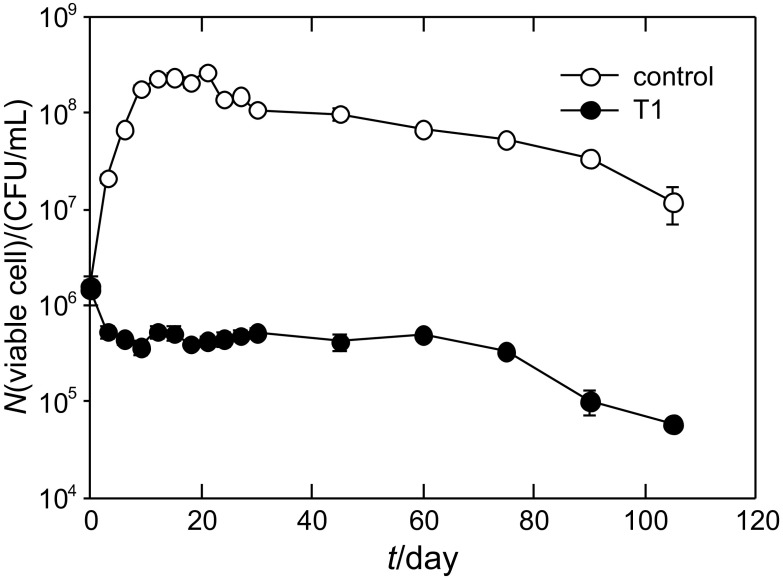
Effect of P. pentosaceus T1 culture on total viable cell number in kimchi during fermentation
Total viable cell number in two kimchi sample groups (control and culture-treated group) was determined during fermentation (Fig. 5). The initial total cell number was around 1.0–1.6·106 CFU/mL in control and culture-treated kimchi. Similar numbers of cells in the two samples show a clear difference after 3 days of fermentation; cell number of control kimchi significantly increased to 2.1·107 CFU/mL, while the cell number of culture-treated kimchi decreased to 5.4·105 CFU/mL. The increase of the cell number in control kimchi is correlated with the increase of acidity during early fermentation period. In control kimchi, cells continued to increase up to 2.7·108 CFU/mL and gradually decreased to 107 CFU/mL in 105 days, but the culture-treated kimchi had a stable cell number in the range of 2 to 5·105 CFU/mL during the investigated fermentation period (Fig. 5). This result shows that the culture treatment inhibited cell proliferation during fermentation. The suppression of cell number occurred at an early stage of fermentation of samples treated with P. pentosaceus T1 culture, indicating that the culture could inhibit the growth of other LAB, which is responsible for overmaturation of kimchi, by producing antimicrobial substances including LysM domain. According to our results, P. pentosaceus T1 culture treatment could control the number of total cells, which can affect kimchi maturity or fermentation quality.
Fig. 5.
Effect of P. pentosaceus T1 culture (T1, 6 g per 100 mL) on total viable cell number in kimchi during fermentation. Kimchi samples were blended to prepare the juice. The juice samples were filtered, diluted, and spread onto plate count agar. The plate agar was counted after 2 to 3 days of incubation at 25 °C. Data are expressed as mean values±S.D. (N=3)
Growth inhibition of indicator LAB by P. pentosaceus T1 culture
The above data show that P. pentosaceus T1 culture suppressed the total cell viability in kimchi during fermentation. We then determined which LAB were inhibited by P. pentosaceus T1. Sixteen strains of LAB including Leuconostoc spp., Lactobacillus spp. and Weisella spp. were tested against P. pentosaceus T1. Well diffusion assay showed that the wells treated with P. pentosaceus T1 had significant inhibition zones, over 12 mm, only against Leuoconostoc mesenteroides and Lactobacillus sakei (Fig. 6), suggesting that P. pentosaceus T1 has a strong antimicrobial activity on these two bacteria (Table 2). P. pentosaceus T1 also inhibited the other tested strains, although the inhibitory effects on them weakened compared to those on Leu. mesenteroides and L. sakei (Table 2). Leu. mesenteroides and L. sakei are the major LAB in kimchi, which are responsible for its maturation during fermentation (1). Leu. mesenteroides is known to be a predominant strain in the early or middle stages of kimchi fermentation, and L. sakei is one of the predominant strains in the late stage of fermentation (36, 37). Moreover, the prolonged predominance of L. sakei can result in an excessively acidic taste and soft texture of kimchi (38). Thus, our P. pentosaceus T1 is thought to control the change of microflora during fermentation of kimchi by inhibiting various LAB including Leu. mesenteroids and L. sakei. Our result indicated that the inhibition of LAB by P. pentosaceus T1 could prevent prolonged maturation, maintaining proper fermentation level which could be induced by other non-inhibited LAB. In addition, it would be interesting to analyze the changes of overall LAB microflora in the presence or absence of P. pentosaceus T1 during kimchi fermentation in the next study.
Fig. 6.
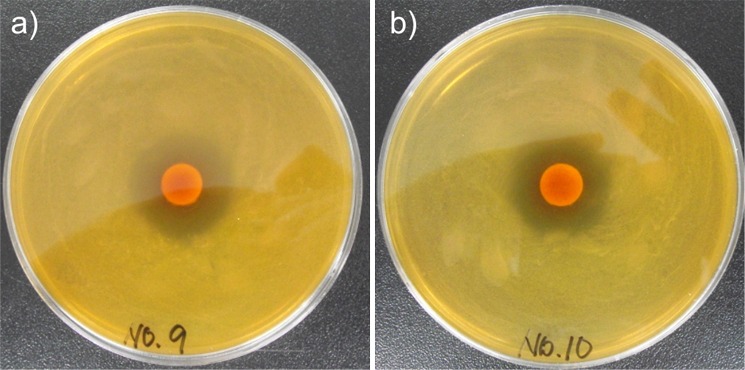
Growth inhibition of: a) Leu. mesenteroides, and b) L. sakei by P. pentosaceus T1. Sixteen LAB were cultured overnight by inoculating 105 CFU/mL in MRS broth. LAB cultures (100 µL) were spread on MRS agar plates. Well diffusion assay was performed with 50 µL of cell-free supernatants of P. pentosaceus T1. Data are representative of three independent experiments
Table 2. Antimicrobial activity of P. pentosaceus T1 against 16 indicator LAB strains.
| Indicator strain | Antimicrobial activity |
|---|---|
| Lactobacillus sakei KCTC 3603 | +++ |
| Lactobacillus plantarum KCTC 3108 | ++ |
| Lactobacillus paraplantarum KCTC 5045 | ++ |
| Lactobacillus pentosus DSM 20314 | ++ |
| Lactobacillus curvatus KCTC 3767 | ++ |
| Leuconostoc citreum KCTC 3526 | ++ |
| Leuconostoc carnosum KCTC 3525 | ++ |
| Leuconostoc gasicomitatum KCTC 3753 | ++ |
| Leuconostoc gelidum KCTC 3527 | ++ |
| Leuconostoc kimchii KCTC 2386 | ++ |
| Leuconostoc lactis KCTC 3528 | ++ |
| Leuconostoc mesenteroides KCTC 3505 | +++ |
| Leuconostoc inhae KCTC 3774 | + |
| Weisella cibaria KCTC 3746 | + |
| Weisella confusa KCTC 3499 | + |
| Weisella koreensis KCTC 3621 | + |
+=radius of inhibition zone <8 mm, ++=radius of inhibition zone 8 to 10 mm, +++=radius of inhibition zone >12 mm
Sensory evaluation of kimchi
We showed that our P. pentosaceus T1 culture could positively affect the kimchi quality by controlling the acidity and bacterial cell number. However, if the treatment with P. pentosaceus T1 culture negatively affected kimchi sensory properties, its beneficial effect such as antilisterial activity would be less meaningful. Therefore, we performed sensory evaluation of kimchi samples treated with P. pentosaceus T1 and the control. Sensory characteristics of kimchi include a proper combination of sour, sweet and salty tastes along with freshness, fizzy mouthfeel, and crunchy texture. Sensory properties of kimchi are shown in Table 3. Kimchi treated with P. pentosaceus T1 received a higher score on most of the items in sensory evaluation including overall acceptability compared with the control. Specifically, sourness, off-flavour and fizzy mouthfeel were greatly improved when P. pentosaceus T1 culture was added to the kimchi as a starter. Colour differences between the starter and nonstarter kimchi preparations were not significant, but the kimchi treated with P. pentosaceus T1 had a brighter appearance than the control. Interestingly, the culture-treated kimchi had a characteristic flavour. This result showed that P. pentosaceus T1 delayed maturation stage, maintaining optimal kimchi quality. Accordingly, our data indicate that the use of P. pentosaceus T1 as a starter in kimchi preparation could prolong optimal conditions of kimchi fermentation, maintaining optimal sensory properties during storage or distribution.
Table 3. Sensory evaluation of kimchi.
| Property | Kimchi+T1 | Control |
|---|---|---|
| Overall acceptability | (6.2±1.2)* | 4.0±1.5 |
| Colour | 5.6±0.1 | 5.0±0.8 |
| Sourness | (6.9±1.8)* | 2.7±1.4 |
| Sweetness | (5.8±0.6)* | 5.0±1.3 |
| Fizzy mouthfeel | (6.9±1.4)* | 3.2±1.1 |
| Mouldy flavour (off-flavour) | (6.0±1.1)* | 4.0±1.1 |
| Texture | (5.7±0.7)* | 4.6±1.3 |
*Significant differences between the values of the same tested property (p<0.05, independent samples t-test). Results are expressed as mean values of scores on a 9-point hedonic scale
Conclusion
In this study, we compared the antilisterial effect of Pediococcus pentosaceus T1 isolated from kimchi using salmon with a commercial bacteriocin (nisin) and a disinfectant (sodium hypochlorite). P. pentosaceus T1 was evaluated as a competitive antilisterial agent that showed a stronger inhibitory effect than nisin and the disinfectant. Current study also showed beneficial effects of P. pentosaceus T1 used as a starter culture on kimchi quality. P. pentosaceus T1 effectively controlled maturation of kimchi by suppressing lactic acid bacteria such as Leu. mesenteroides and L. sakei, which are responsible for kimchi maturation. Moreover, P. pentosaceus T1 culture improved organoleptic quality of kimchi, as shown by sensory evaluation. We suggest that P. pentosaceus T1 be used as an antilisterial agent in fish products as well as a starter to control the fermentation of kimchi.
References
- 1.Kanmani P, Satish Kumar R, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Probiotics and its functionally valuable products-a review. Crit Rev Food Sci Nutr. 2013;53:641–58. 10.1080/10408398.2011.553752 [DOI] [PubMed] [Google Scholar]
- 2.Park KY, Jeong JK, Lee YE, Daily JW, 3rd. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food. 2014;17:6–20. 10.1089/jmf.2013.3083 [DOI] [PubMed] [Google Scholar]
- 3.Kim JE, Kim JY, Lee KW, Lee HJ. Cancer chemopreventive effects of lactic acid bacteria. J Microbiol Biotechnol. 2007;17:1227–35. [PubMed] [Google Scholar]
- 4.Kim Y, Whang JY, Whang KY, Oh S, Kim SH. Characterization of the cholesterol-reducing activity in a cell-free supernatant of Lactobacillus acidophilus ATCC 43121. Biosci Biotechnol Biochem. 2008;72:1483–90. 10.1271/bbb.70802 [DOI] [PubMed] [Google Scholar]
- 5.Onilude A, Fagade O, Bello M, Fadahunsi I. Inhibition of aflatoxin-producing aspergilli by lactic acid bacteria isolates from indigenously fermented cereal gruels. Afr J Biotechnol. 2005;4:1404–8. [Google Scholar]
- 6.Ren C, Zhang Q, Wang G, Ai C, Hu M, Liu X, et al. Modulation of peanut-induced allergic immune responses by oral lactic acid bacteria-based vaccines in mice. Appl Microbiol Biotechnol. 2014;98:6353–64. 10.1007/s00253-014-5678-7 [DOI] [PubMed] [Google Scholar]
- 7.Hayashi A, Kimura M, Nakamura Y, Yasui H. Anti-atopic dermatitis effects and the mechanism of lactic acid bacteria isolated from Mongolian fermented milk. J Dairy Res. 2009;76:158–64. 10.1017/S0022029908003725 [DOI] [PubMed] [Google Scholar]
- 8.Rattanachaikunsopon P, Phumkhachorn P. Lactic acid bacteria: their antimicrobial compounds and their uses in food production. Ann Biol Res. 2010;1:218–28. [Google Scholar]
- 9.Shin MS, Han SK, Ryu JS, Kim KS, Lee WK. Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceus K23-2 isolated from kimchi. J Appl Microbiol. 2008;105:331–9. 10.1111/j.1365-2672.2008.03770.x [DOI] [PubMed] [Google Scholar]
- 10.Raccach M, Tilley HR. Thermal inactivation of the frozen thawed traditional meat starter culture, Pediococcus pentosaceus, in a meat model system. Meat Sci. 2006;72:751–6. 10.1016/j.meatsci.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 11.Albano H, Todorov SD, van Reenen CA, Hogg T, Dicks LM, Teixeira P. Characterization of two bacteriocins produced by Pediococcus acidilactici isolated from 'Alheira', a fermented sausage traditionally produced in Portugal. Int J Food Microbiol. 2007;116:239–47. 10.1016/j.ijfoodmicro.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 12.Mattila K, Saris P, Tyopponen S. Survival of Listeria monocytogenes on sliced cooked sausage after treatment with pediocin AcH. Int J Food Microbiol. 2003;89:281–6. 10.1016/S0168-1605(03)00299-X [DOI] [PubMed] [Google Scholar]
- 13.Popovic I, Heron B, Covacin C. Listeria: an Australian perspective (2001–2010). Foodborne Pathog Dis. 2014;11:425–32. 10.1089/fpd.2013.1697 [DOI] [PubMed] [Google Scholar]
- 14.Miranda-Bautista J, Padilla-Suarez C, Bouza E, Munoz P, Menchen L, Marin-Jimenez I. Listeria monocytogenes infection in inflammatory bowel disease patients: case series and review of the literature. Eur J Gastroenterol Hepatol. 2014;26:1247–52. 10.1097/MEG.0000000000000188 [DOI] [PubMed] [Google Scholar]
- 15.Leistner L. Basic aspects of food preservation by hurdle technology. Int J Food Microbiol. 2000;55:181–6. 10.1016/S0168-1605(00)00161-6 [DOI] [PubMed] [Google Scholar]
- 16.Snyder AB, Worobo RW. Chemical and genetic characterization of bacteriocins: antimicrobial peptides for food safety. J Sci Food Agric. 2014;94:28–44. 10.1002/jsfa.6293 [DOI] [PubMed] [Google Scholar]
- 17.Bali V, Panesar PS, Bera MB, Kennedy JF. Bacteriocins: Recent trends and potential applications. Crit Rev Food Sci Nutr. 2014. Forthcoming 10.1080/10408398.2012.729231 [DOI] [PubMed] [Google Scholar]
- 18.Jang S, Lee J, Jung U, Choi HS, Suh HJ. Identification of an anti-listerial domain from Pediococcus pentosaceus T1 derived from kimchi, a traditional fermented vegetable. Food Contr. 2014;43:42–8. 10.1016/j.foodcont.2014.02.040 [DOI] [Google Scholar]
- 19.Shim S, Lee J. Evaluation of lactic acid bacterial community in kimchi using terminal-restriction fragment length polymorphism analysis. Kor J Microbiol Biotechnol. 2008;36:247–59. [Google Scholar]
- 20.Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, et al. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57:2259–61. 10.1099/ijs.0.64915-0 [DOI] [PubMed] [Google Scholar]
- 21.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Carvalho AA, de Paula RA, Mantovani HC, de Moraes CA. Inhibition of Listeria monocytogenes by a lactic acid bacterium isolated from Italian salami. Food Microbiol. 2006;23:213–9. 10.1016/j.fm.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 23.Eijsink VG, Axelsson L, Diep DB, Havarstein LS, Holo H, Nes IF. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek. 2002;81:639–54. 10.1023/A:1020582211262 [DOI] [PubMed] [Google Scholar]
- 24.Beyatli Y, Gündüz U. Isolation and characterization of pediocin producing Pediococcus pentosaceus Pep1 from vacuum-packed sausages. Turk J Biol. 2001;25:133–43. [Google Scholar]
- 25.Kingcha Y, Tosukhowong A, Zendo T, Roytrakul S, Luxananil P, Chareonpornsook K, et al. Anti-listeria activity of Pediococcus pentosaceus BCC 3772 and application as starter culture for Nham, a traditional fermented pork sausage. Food Contr. 2012;25:190–6. http://dx.doi.org/doi:10.1016/j.foodcont.2011.10.005 10.1016/j.foodcont.2011.10.005 [DOI] [Google Scholar]
- 26.Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido) glycans. Mol Microbiol. 2008;68:838–47. 10.1111/j.1365-2958.2008.06211.x [DOI] [PubMed] [Google Scholar]
- 27.Adhikari MD, Das G, Ramesh A. Retention of nisin activity at elevated pH in an organic acid complex and gold nanoparticle composite. Chem Commun (Camb). 2012;48:8928–30. 10.1039/c2cc34653b [DOI] [PubMed] [Google Scholar]
- 28.Delves-Broughton J. Nisin as a food preservative. Food Aust. 2005;57:525–32. [DOI] [PubMed] [Google Scholar]
- 29.Islam MR, Nagao J, Zendo T, Sonomoto K. Antimicrobial mechanism of lantibiotics. Biochem Soc Trans. 2012;40:1528–33. 10.1042/BST20120190 [DOI] [PubMed] [Google Scholar]
- 30.Thomas LV, Wimpenny J. Investigation of the effect of combined variations in temperature, pH, and NaCl concentration on nisin inhibition of Listeria monocytogenes and Staphylococcus aureus. Appl Environ Microbiol. 1996;62:2006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papagianni M, Anastasiadou S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb Cell Fact. 2009;8:3–16. 10.1186/1475-2859-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimothe J, Nightingale KK, Gall K, Scott VN, Wiedmann M. Tracking of Listeria monocytogenes in smoked fish processing plants. J Food Prot. 2004;67:328–41. [DOI] [PubMed] [Google Scholar]
- 33.Park SH, Lee JH. The correlation of physico-chemical characteristics of kimchi with sourness and overall acceptability. Kor J Food Cookery Sci. 2005;21:103–9. [Google Scholar]
- 34.Shin JH, Kim R, Kang MJ, Kim GM. Quality and fermentation characteristics of garlic-added kimchi. Kor J Food Preserv. 2012;19:539–46. 10.11002/kjfp.2012.19.4.539 [DOI] [Google Scholar]
- 35.Kim J, Bang J, Beuchat LR, Kim H, Ryu JH. Controlled fermentation of kimchi using naturally occurring antimicrobial agents. Food Microbiol. 2012;32:20–31. 10.1016/j.fm.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Jung JY, Lee SH, Lee HJ, Seo HY, Park WS, Jeon CO. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int J Food Microbiol. 2012;153:378–87. 10.1016/j.ijfoodmicro.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 37.Kim M, Chun J. Bacterial community structure in kimchi, a Korean fermented vegetable food, as revealed by 16S rRNA gene analysis. Int J Food Microbiol. 2005;103:91–6. 10.1016/j.ijfoodmicro.2004.11.030 [DOI] [PubMed] [Google Scholar]
- 38.Lee H, Yoon H, Ji Y, Kim H, Park H, Lee J, et al. Functional properties of Lactobacillus strains isolated from kimchi. Int J Food Microbiol. 2011;145:155–61. 10.1016/j.ijfoodmicro.2010.12.003 [DOI] [PubMed] [Google Scholar]