Summary
Haloarchaea are found at very high concentrations in salt-conditioned environments, hence produce enzymes which are able to catalyze reactions under harsh conditions, typical of many industrial processes. In the present study, culture conditions for extracellular amylase production from Haloarchaea isolated from a solar saltern were optimized and the purified enzyme was characterized. Haloferax sp. HA10 showed maximum amylase production at 3 M NaCl, 37 °C, pH=7 and 1% starch content. Purified α-amylase was a calcium-dependent enzyme with an estimated molecular mass of about 66 kDa and many industrially useful properties. It was found to be stable in a broad range of pH (from 5 to 9) and NaCl concentrations (from 0.5 to 3.0 M), retaining 48% activity even at 4 M. The optimal temperature for Haloferax sp. HA10 amylase activity was 55 °C (99% activity), and 57% activity was retained at 80 °C, which dropped to 44% with the increase of temperature to 90 or 100 °C. It was able to sustain various surfactants and detergents. To the best of our knowledge the detergent-stable α-amylases from halophilic archaeon have not been reported yet.
Key words: halophiles, archaea, α-amylase, Haloferax sp., solar saltern
Introduction
Halophiles are extremophiles that live, grow and multiply in highly saline environments. Moderately halophilic bacteria are able to grow over a wide range of salt concentrations, from 0.4 to 3.5 M, with optimum growth at 0.5 to 2.0 M (1, 2), and constitute a very interesting group of microorganisms with a great potential for use in biotechnology. Since their enzymes are both salt- and thermotolerant, halophiles represent a good source of stable enzymes. Exoenzymes from these organisms with polymer-degrading ability at low water activity are of interest in many harsh industrial processes where concentrated salt solutions would inhibit enzymatic conversions. Starch--degrading amylolytic enzymes are one of the most important enzymes, constituting a class of industrial enzymes that cover approx. 25% of the world enzyme market (3). Halophilic amylase can tolerate high salt concentration, yet retains its activity at low concentrations of salt, hence it is very useful for industry (4). These amylases are used in bread and baking industries, starch liquefaction and saccharification, manufacturing of maltose, paper and detergent industries, textile designing, manufacturing of high molecular mass branched dextrins, direct fermentation of starch to ethanol, treatment of starch processing wastewater and analysis in medicinal and clinical chemistry (5).
Despite wide industrial importance of enzymes from archaea, there are few reports on characterization of halophilic α-amylase from Halomonas meridiana, a moderately halophilic archaeon (6). In the present paper, we describe α-amylase from an obligate extreme halophilic archaeon Haloferax sp. HA10, which has the ability to grow under extreme conditions of alkalinity, salinity and temperature. This organism, isolated from the solar saltern at Barmer, Rajasthan, India, requires high salt concentrations (mainly sodium chloride) for optimal growth as its metabolic machinery is adapted to extreme conditions of salt and pH in nature. Enzymes produced by this organism, such as the α-amylase studied here, function at salt concentrations at which other enzymes would lose their properties, a characteristic that distinguishes it from most of the other α-amylases from bacterial sources.
Materials and Methods
Sample collection
The samples were collected from solar salterns at Pachpadra, Barmer district of Rajasthan, India, located at latitude 25.9290° N and longitude 72.2466° E. These lakes are known for the production of very high quality salt, and their level of sodium chloride is 98%.
Spatial and temporal sampling of soil, sludge and water were done from salterns and their surroundings in 2009 and 2010 winter (December), summer (May) and rainy seasons (August). The samples were collected in sterile bottles or polyethylene bags and brought to the laboratory for isolation of microorganisms.
Isolation and screening of haloarchaeal strains
The isolation of haloarchaea from environmental samples was made on modified growth medium (MGM) by direct plating and dilution plate technique. The 23% MGM was prepared as follows (in g): peptone 5.0, yeast extract 1.0 and (in mL): distilled water 200 (pH=7.5), and concentrated salt water (30%) 767, containing (in g): NaCl 240, MgCl2·6H2O 30, MgSO4·7H2O 35, KCl 7, and 1 M Tris--HCl (pH=7.5), 5 mL and CaCl2·2H2O 5 mL from a 1 M sterile stock, distilled water 1000 mL (pH=7.5) and agar 1.5%. The screening for amylase-producing strains was done on starch agar plates and six were found positive. The plates were flooded with iodine solution (2% I2 and 0.2% KI). Haloarchaeal strain HA10 (Haloferax sp.) was selected for further studies on the basis of the ratio of halo zone/colony diameter.
Culture conditions
The amylase production by Haloferax sp. HA10 was studied under various physicochemical conditions and the parameters were as follows: NaCl concentration 1, 2, 3 and 4 M, temperature 30, 37, 42 and 50 °C, pH=5.0, 6.0, 7.0, 8.0, 9.0 and 10.0, starch 0, 0.5, 1.0, 1.5 and 2.0%, and CaCl2 0, 0.5, 1.0, 1.5 and 2.0%. Modified Sehgal and Gibbons complex (SGC) medium (7) was used for amylase production.
A loopful of 48-hour-old culture plate was used for inoculation of 20 mL of SGC medium. The absorbance of the samples drawn from 36-hour grown Haloferax sp. HA10 culture was fixed at 0.1, 0.2, 0.3, 0.4 and 0.5 and used as inocula. The amount of inoculum showing maximum growth was used for further experiments on amylase production. The highest growth was recorded with inoculum prepared from 36-hour grown culture and absorbance fixed at 0.2. The samples were drawn at regular intervals for measurement of growth, expressed as turbidity at 600 nm, and amylase activity.
Amylase activity
Amylase activity was qualitatively estimated on starch agar plates in six haloarchaeal strains isolated from environmental samples. The plates were flooded with iodine solution (2% I2 and 0.2% KI). Haloarchaeal strain HA10 (Haloferax sp.) was selected for further studies on the basis of the ratio of halo zone/colony diameter.
Bradford assay was used for estimation of protein concentration (8). For quantitative determination of amylase activity, selected isolate Haloferax sp. HA10 was grown in modified SGC medium (7) for amylase production. The absorbance of approx. 36-hour-old culture was fixed at 0.2 and this solution was used as inoculum. The inoculated flasks were incubated at 37 °C on an incubator shaker operating at 4×g for 48 h. Cell-free supernatant was collected by centrifugation at 112×g for 20 min at 4 °C and used as a source of crude enzyme.
The amylase activity was determined following Bernfeld method (9). A volume of 2 mL of reaction mixture consisting of 1.0 mL of 1% soluble starch solution in buffer A (Tris-HCl 20 mM, NaCl 2.0 M and CaCl2 10 mM, pH=7.4) and 1.0 mL of crude enzyme was incubated for 15 min at 50 °C. A volume of 1 mL of 3,5-dinitrosalicylic acid reagent was then added in each test tube to stop the reaction. A control was prepared with 3,5-dinitrosalicylic acid added before the addition of enzyme. All tubes were placed in a boiling water bath for 10 min, cooled at room temperature, each diluted 5 times with water and vortexed. The amount of reducing sugar was determined spectrophotometrically at 540 nm. The amount of released reducing sugar was quantified using 3,5-dinitrosalicylic acid with maltose as standard. One unit of α-amylase activity was defined as the amount of enzyme that releases one µmol of reducing sugar as maltose per minute under assay conditions.
The amylase activity was quantitatively determined in all the experiments after 48 h of incubation, unless otherwise stated.
Partial purification of α-amylase
The protein was precipitated by chilled acetone. The purification process was initiated by drop-wise addition of two volumes of acetone (–20 °C) with constant stirring to the supernatant maintained at 4 °C in ice. The precipitate was collected and dissolved in appropriate amount of Tris-HCl buffer (pH=6.5) containing 2 M NaCl.
Characterization of purified α-amylase
The stability of purified α-amylase was characterized when incubated under various conditions of pH=2.0 to 10 at 4 °C for 24 h, salt mass fractions of 0, 3, 5, 10, 20 and 25% in buffer at pH=6.0 for 24 h, temperature of 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 and 100 °C for 30 min each; metal divalent cations, viz. Ca2+, Mg2+, Co2+, Mn2+, Zn2+ and Cu2+ at 5 mM concentration. Chelator, surfactants and detergents included in the study were: ethylenediaminetetraacetic acid (EDTA, 5 mM), sodium dodecyl sulphate (SDS, 0.1, 0.3 and 0.5%), 0.1% of Triton X-100 and Tween-80 each, and 0.1% of commercial detergents, viz. Rin and Surf Excel produced by Hindustan Unilever (Mumbai, India) and Tide (Procter & Gamble, Mumbai, India), added separately and incubated at 30 °C for 30 min. Activity in the absence of metal ions and effectors was taken as 100%. The residual activity was determined under standard assay conditions.
Molecular mass determination
The molecular mass of purified amylase was determined using standard protein markers by SDS-PAGE (10). A volume of 50 µL of the sample was mixed with 50 µL of loading dye and loaded into the wells. The gel was run at 50 V until the Bromophenol Blue front migrated into the resolving gel, then it was increased to 150 V until the tracking dye reached the bottom of the resolving gel. The gel was stained overnight in a solution of Coomassie Brillant Blue R-250 (0.1%) and destained for 5 h in destaining solution (40% methanol and 10% glacial acetic acid). The molecular mass marker used was a broad range marker (range 3.5–205.0 kDa, PMWB105975, Merck GeNeiTM, Mumbai, India).
Zymogram
For confirmation of the presence of amylase activity, in-situ staining was done. Native PAGE was run using the same composition as in the SDS-PAGE but without the SDS. Also, before the addition of ammonium persulphate, 0.2% soluble starch was incorporated into the separating gel. After electrophoresis, the gel was stained in a solution of iodine (I2 5 g/L and KI 50 g/L) for 30 min, and clear bands indicated the presence of amylase activity.
Statistical analysis
All experiments were done in triplicate and standard error was calculated at 5% level of significance.
Results and Discussion
Amylase production
All the six haloarchaeal strains isolated showed good amylase activity on starch agar plate (Fig. 1). They were identified by 16S rDNA sequencing as Haloferax sp. HA1, Haloferax sp. HA3, Halobacterium sp. HA4, Haloferax sp. HA8, Haloferax sp. HA9 and Haloferax sp. HA10. The ratio of halo zone/colony diameter was ≥1 in all six strains (Table 1). As per qualitative and quantitative determination of amylase activity, Halobacterium sp. HA4 (colony index, CI=2.3±0.02 and amylase activity 5.6 U/mL) and Haloferax sp. HA10 (CI=2.0±0.02 and amylase activity 4.2 U/mL) were found to be excellent producers of amylase, followed by Haloferax sp. HA3, HA8, HA9 and HA1. The partial sequence of Haloferax sp. HA10 has been submitted to GenBank (National Center for Biological Information, Bethesda, MD, USA) with the accession number HM368369 (unpublished data).
Fig. 1.
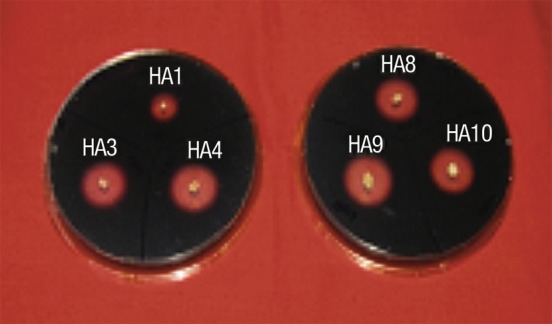
Qualitative estimation of amylase produced by six haloarchaeal strains on starch agar plates
Table 1. Colony index and amylase activity of six haloarchaeal strains.
| Isolate | Colony index | Amylase activity U/mL |
|---|---|---|
| Haloferax HA1 | 1.31±0.02 | 1.4 |
| Haloferax HA3 | 1.54±0.01 | 3.1 |
| Halobacterium HA4 | 2.32±0.02 | 5.6 |
| Haloferax HA8 | 1.24±0.01 | 2.8 |
| Haloferax HA9 | 1.83±0.04 | 2.4 |
| Haloferax HA10 | 2.03±0.02 | 4.2 |
Optimization of culture conditions
For further studies, Haloferax sp. HA10 was selected over other isolates as it was stable, showed good activity and grew fast. The most characteristic feature of halophilic proteins is their ability to thrive under highly saline conditions. Fig. 2 shows amylase at various NaCl concentrations, with maximum zone production at 3 M NaCl.
Fig. 2.
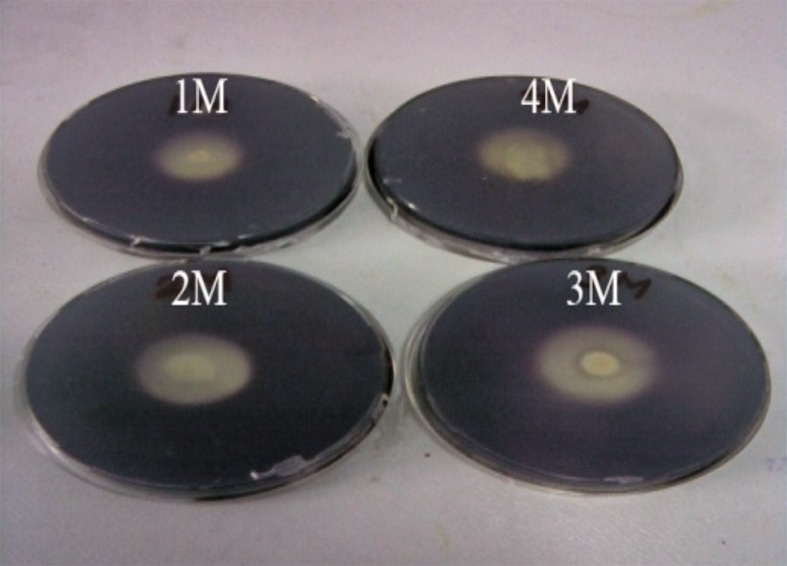
Amylase activity of Haloferax sp. HA10 at different NaCl concentrations
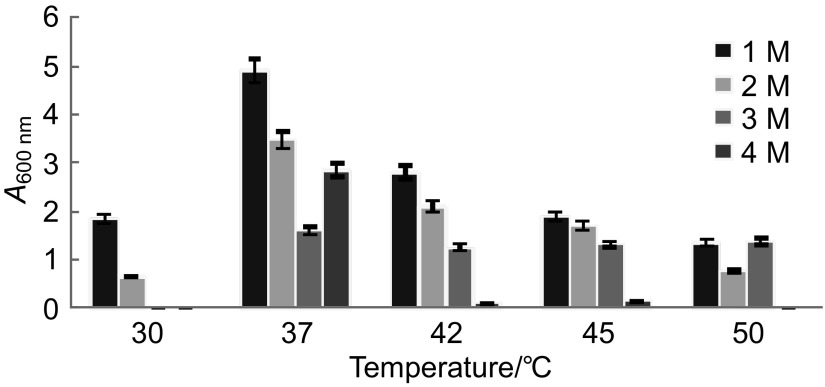
Under shaking conditions, the optimum salt concentration for growth and amylase production was 1 and 3 M NaCl, respectively, and 37 °C was optimum temperature for both growth and amylase production (3.2 U/mL of enzyme) (Figs. 3 and 4). Maximum α-amylase production at 3 M NaCl by Haloferax sp. HA10 indicates good adaptation as well as stability of enzymes under highly saline conditions. Hydrated salt ions are associated with acidic residues on the protein surface in such a way that the salt concentration of the solvation layer is higher than that of the bulk solvent. It was suggested that this characteristic allows halophilic proteins to remain stable and soluble at high salt concentrations (11).
Fig. 3.
Effect of temperature and salt concentration on the growth of Haloferax sp. HA10 after 48 h
Fig. 4.
Amylase production by Haloferax sp. HA10 at different temperatures and NaCl concentrations after 48 h
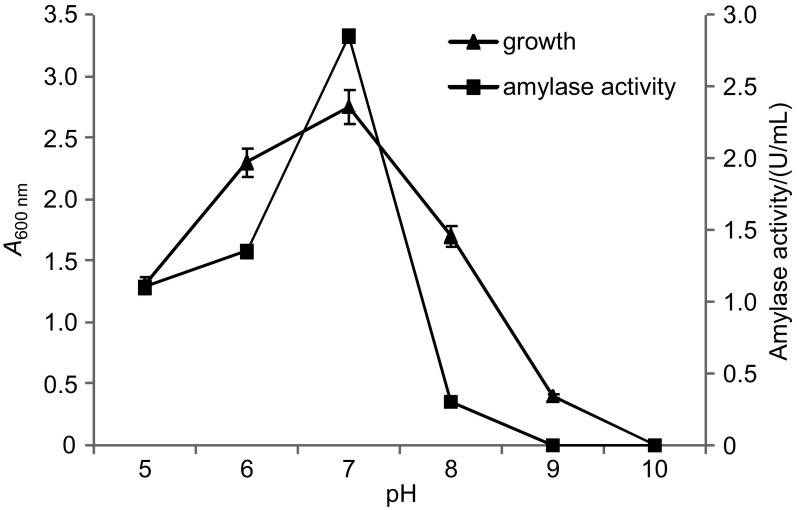
The pH=7.0 was most conducive for growth and amylase production, which sharply decreased either at pH=6.0 or 8.0, and there was absolutely no activity at pH=9.0 (Fig. 5). Rodriguez-Valera et al. (12) also reported pH=7.2 to be the usual pH of culture medium for growing Haloferax mediterranei. Our isolate showed maximum amylase production at pH=7.0 and 37 °C, which confirms the findings of Coronado et al. (6) in Halomonas meridian.
Fig. 5.
Growth of and amylase production by Haloferax sp. HA10 at different pH values
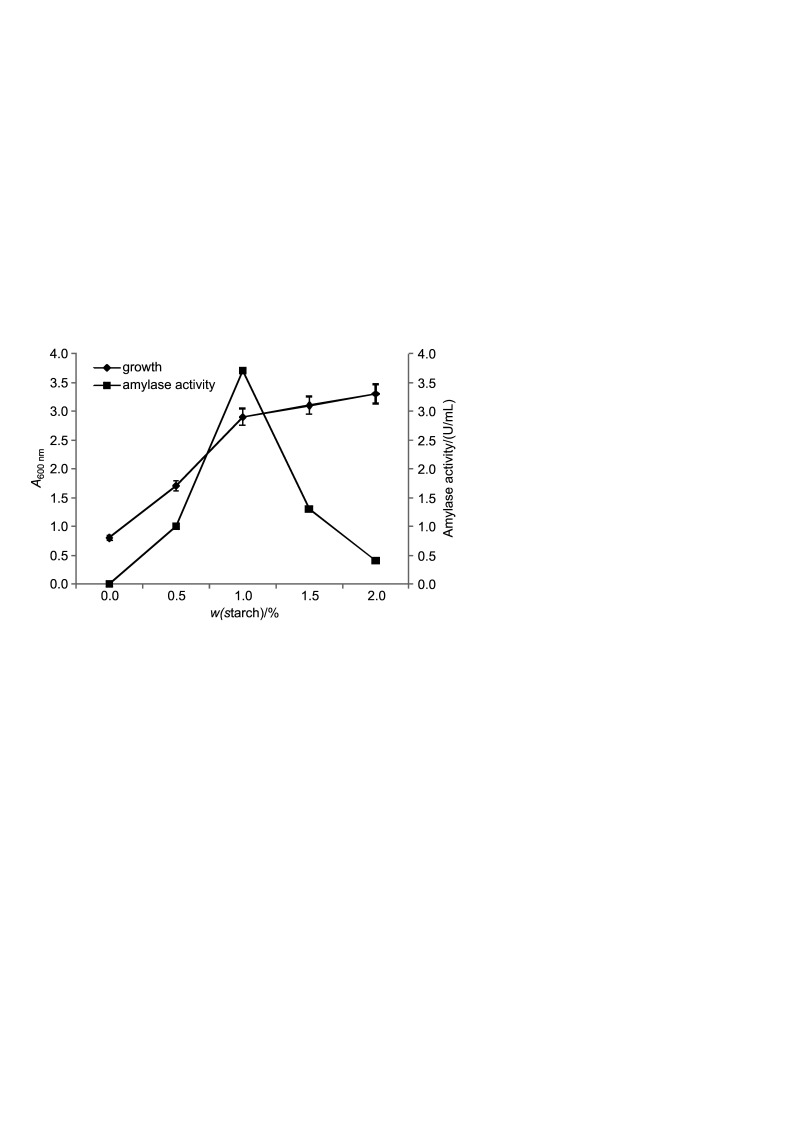
Haloferax sp. HA10 showed maximum amylase activity in broth at 1% starch (Fig. 6), which decreased with increasing starch mass fraction. No amylase was produced in the medium in the absence of starch, indicating inducible nature of the enzyme. Likewise, Swain et al. (13) demonstrated that 1% starch was optimum for extracellular α-amylase production by Bacillus subtilis, and the enzyme production decreased gradually with increasing starch mass fraction. Kiran and Chandra (14) also reported inducible nature of enzyme in Bacillus sp. and 1% starch as optimal mass fraction.
Fig. 6.
Growth of and amylase production by Haloferax sp. HA10 at different mass fractions of starch
For the production and stability of α-amylase calcium ions are often necessary. Supplementing the medium with calcium chloride increased both growth and α-amylase production, which decreased proportionally with the increase of calcium ion mass fraction (Fig. 7). Optimal mass fraction was 0.5% CaCl2 (4.7 U per mL of enzyme). Amylases are calcium metalloenzymes, thus CaCl2 acts as an activator for amylase production, as also observed by Prakash et al. (15) in Chromohalobacter sp. A decrease in α-amylase production by Streptomyces sp. was also reported by Kar and Ray (16).
Fig. 7.
Growth of and amylase production by Haloferax sp. HA10 at different mass fractions of CaCl2
The effect of pH on purified amylase
Fig. 8 shows that purified amylase from Haloferax sp. HA10 was stable in broad range of pH, i.e. from 5 to 9, the optimal being pH=6 (97%). The stability of amylase in alkaline pH may be accounted for alkaline properties of Pachpadra salt lake region from where the organism was isolated. These values are comparable with an amylase from alkaliphilic Bacillus sp., which was stable in a range of pH=6 to 10 (17). Similar to our strain, α-amylase of halophilic archaeon, Haloarcula hispanica, showed maximum activity at pH=6.5, which dropped more than 65% at acidic pH of around 5 (18). The high stability of the enzyme is a remarkable feature, which can find numerous industrial applications (19). Nielsen and Borchert (20) have emphasized the use of α-amylases with high stability in washing environments, usually in detergent industries.
Fig. 8.
Relative activity of purified amylase from Haloferax sp. HA10 after incubation in a buffer at various pH values for 24 h
The effect of temperature on purified amylase
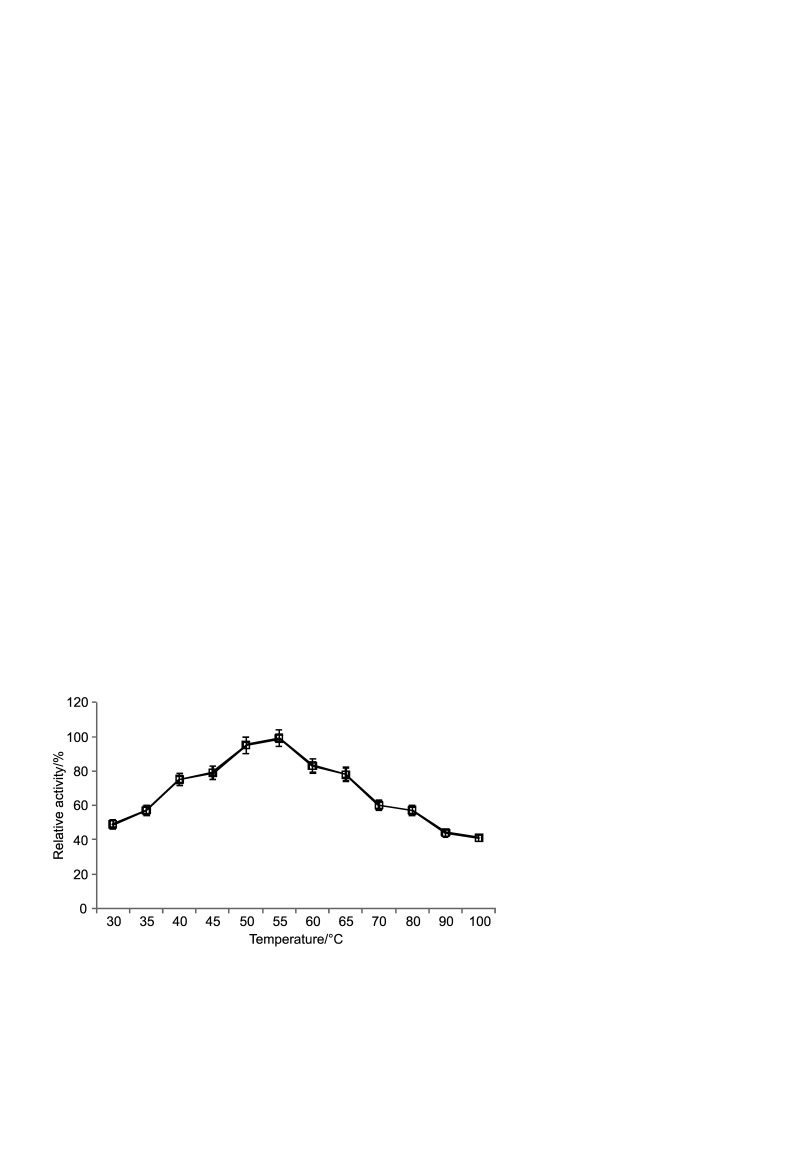
The effect of temperature on the stability of purified enzyme is shown in Fig. 9. The optimal temperature for Haloferax sp. HA10 amylase activity was 55 °C (99% activity) and 57% activity was retained at 80 °C, which dropped between 41 and 44% as the temperature was increased to 90 or 100 °C. Chen et al. (21) also reported highest amylase activity between 50 and 60 °C in a halophilic archaebacterium belonging to the species Haloferax mediterranei R4. This high optimal temperature may be a result of an adaptive response of archaea to high temperatures. Since solar salterns are exposed to intense sunlight, the enzymes of halophilic organisms have to endure high temperatures in their natural environments. This thermophilic nature has been reported not only for amylase but also for other halophilic enzymes in several organisms (22–25).
Fig. 9.
Relative activity of purified amylase from Haloferax sp. HA10 after 30 min of incubation at various temperatures
Stability of amylase at different salt concentrations
The amylase of Haloferax sp. HA10 revealed substantial stability from 0.5 to 3.0 M NaCl, retaining 48% activity even at 4 M. Maximum activity was observed in the presence of 1 M NaCl (Fig. 10). Recently, an extracellular halophilic α-amylase from Nesterenkonia sp. strain F has shown maximum activity at 0.5 M NaCl and was active up to 4 M salt concentration (26). In contrast, amylase from Halobacillus sp. MA-2 showed maximum activity at 5% (0.85 M) NaCl (27). Madern et al. (28) reported that most of the halophilic enzymes studied were inactivated when the NaCl or KCl concentrations decreased to less than 2 M. However, α-amylase from Haloferax mediterranei was stable even at lower salt concentrations (19).
Fig. 10.
Relative activity of amylase from Haloferax sp. HA10 after 24 h of incubation in a buffer at pH=6.0 and different NaCl concentrations
The influence of metal ions on amylase activity
The effect of various metal ions on the activity of purified amylase was analyzed. The activity was enhanced by 15% in the presence of CaCl2, as compared to control. Bacillus spp. ANT-6 and TSCVKK also showed increased activity in the presence of Ca2+ ions (14, 29). Regarding other metal ions, Fe2+ (70%) and Mg2+ (46%) had negative effect on amylase activity, whereas Co2+, Zn2+, Cu2+ and Mn2+ inhibited the activity only mildly by 22, 18, 10 and 2%, respectively (Fig. 11). Thus, it can be deduced from the data that amylase from Haloferax is a calcium-dependent enzyme. Gupta et al. (30) have reported that most of the α-amylases are calcium-dependent metalloenzymes. In Haloferax sp. HA10, only Fe3+ had strong negative effect, which corroborated the results of Shafiei et al. (26).
Fig. 11.
Effect of metal ions (5 mM) on the activity of purified amylase from Haloferax sp. HA10
Amylase activity in the presence of EDTA, surfactants and detergents
In the presence of 5 mM EDTA 39% of enzyme activity was lost. As shown in Table 2, among the various tested surfactants, 54% of the activity was retained in the presence of 0.5% SDS, which decreased with increasing its concentration. In the presence of Tween 80 and Triton X-100, 97 and 52%, respectively, activity was retained. Among the commercial detergents, 84% activity was found in the presence of Surf Excel. The amylase was stable in the presence of detergents. The first report about a detergent-stable amylase from moderate halophilic bacteria was published by Kiran and Chandra (14). To the best of our knowledge, the detergent-stable α-amylases from halophilic archaeon have not been reported yet.
Table 2. Inhibition of amylase activity of Haloferax sp. HA10 by metal chelator, surfactants and detergents.
| Treatment | Inhibition/% |
|---|---|
| Metal chelator | |
| EDTA | 39.4±0.5 |
| Surfactant/detergent | |
| SDS | 54.4±0.5 |
| Tween 80 | 0.34±0.1 |
| Triton X-100 | 48.2±0.4 |
| Tide | 53.2±0.2 |
| Surf Excel | 16.1±1.5 |
| Rin | 49.3±0.4 |
Molecular mass determination and zymogram analysis
SDS-PAGE analysis of the enzyme revealed a single band which had a molecular mass of about 66 kDa (Fig. 12). The in-gel activity staining confirmed that it was an amylase enzyme (Fig. 13). Similar molecular masses of α-amylases obtained from Bifidobacterium adolescentis and halotolerant alkaliphilic Bacillus sp. AB68 were determined by Lee et al. (31). Haloferax mediterranei α-amylase appeared as a single band of 58 kDa (19). Most of the reports indicated that molecular mass was between 53 and 70 kDa; however, an alkalophilic Halobacterium sp. H371 amylase produced a single band of 27-kDa molecular mass (17, 32, 33).
Fig. 12.
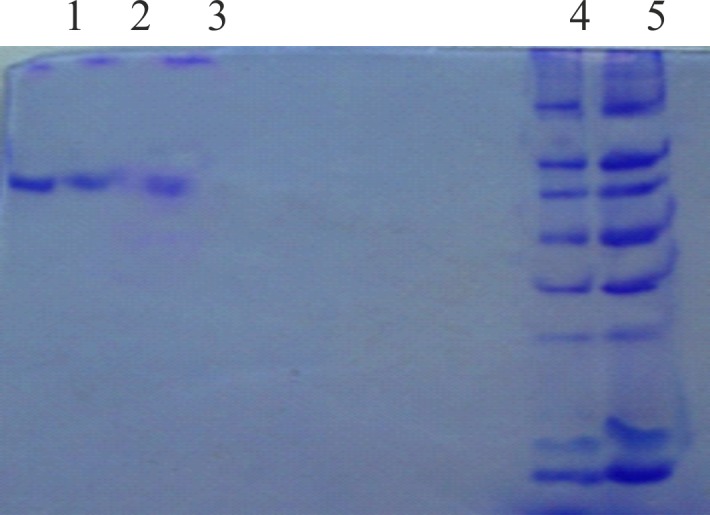
SDS-PAGE of purified amylase from Haloferax sp. HA10. Lanes 1, 2 and 3: samples; lanes 4 and 5: molecular mass marker in kDa
Fig. 13.
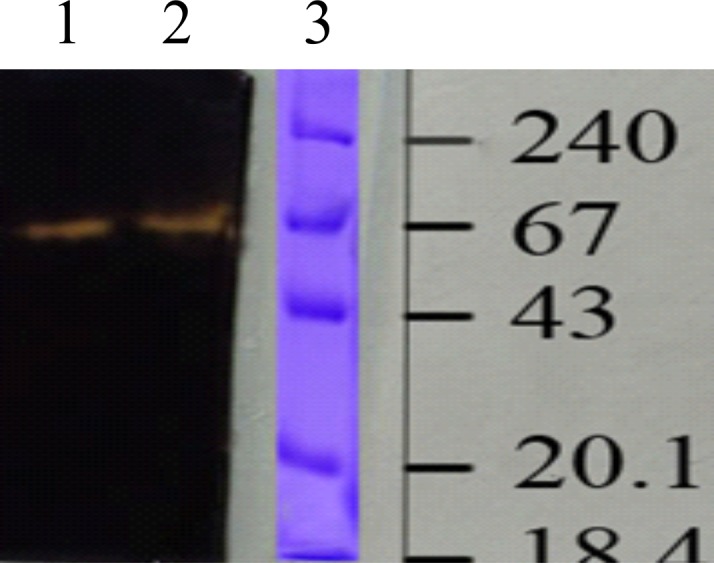
Zymogram of amylase from Haloferax sp. HA10 on native-PAGE. Lanes 1 and 2: samples; lane 3: molecular mass marker in kDa
Conclusion
The purified amylase from halophilic archaeon Haloferax sp. HA10 is active at salt concentrations at which other enzymes would lose their activity, can tolerate and retain activity at high temperature and pH, and is stable in the presence of various surfactants and detergents. These characteristics distinguish it from most of the other α-amylases obtained from common bacterial sources and make it suitable for industrial applications.
References
- 1.Ventosa A, Nieto JJ, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joo WA, Kim CW. Proteomics of halophilic archaea. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:237–50. 10.1016/j.jchromb.2004.10.041 [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan G, Krishnan C. Alpha-amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour Technol. 2008;99:3044–50. 10.1016/j.biortech.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 4.Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31:135–52. 10.1042/BA19990073 [DOI] [PubMed] [Google Scholar]
- 5.Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A. Alpha-amylase from microbial sources: an overview on recent developments. Food Technol Biotechnol. 2006;44:173–84. [Google Scholar]
- 6.Coronado M, Vargas C, Hofemeister J, Ventosa A, Nieto JJ. Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridian. FEMS Microbiol Lett. 2000;183:67–71. 10.1111/j.1574-6968.2000.tb08935.x [DOI] [PubMed] [Google Scholar]
- 7.Sehgal SN, Gibbons NE. Effect of metal ions on the growth of Halobacterium cutirubrum. Can J Microbiol. 1960;6:165–9. 10.1139/m60-018 [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 9.Bernfeld P. Amylases α- and β. Methods Enzymol. 1955;1:49–58. [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 11.Franzetti B, Schoehn G, Garcia D, Ruigrok RWH, Zaccai G. Characterization of the proteasome from the extremely halophilic archaeon Haloarcula marismortui. Archaea. 2002;1:53–61. 10.1155/2002/601719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Valera F, Ruiz-Berraquero F, Ramos-Camenzana A. Isolation of extremely halophilic bacteria able to grow in defined inorganic media with single carbon sources. J Gen Microbiol. 1980;119:535–8. 10.1099/00221287-119-2-535 [DOI] [Google Scholar]
- 13.Swain MR, Kar S, Padmaja G, Ray RC. Partial characterization and purification of extracellular α-amylase from Bacillus subtilis isolated from culturable cow dung microflora. Pol J Microbiol. 2006;55:289–96. [PubMed] [Google Scholar]
- 14.Kiran KK, Chandra TS. Production of surfactant and detergent-stable, halophilic, alkalitolerant alpha-amylase by moderately halophilic Bacillus sp. strain TSCVKK. Appl Microbiol Biotechnol. 2008;77:1023–31. 10.1007/s00253-007-1250-z [DOI] [PubMed] [Google Scholar]
- 15.Prakash B, Vidyasagar M, Madhukumar MS, Muralikrishna G, Sreeramulu K. Production, purification, and characterization of two extremely halotolerant, thermostable and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem. 2009;44:210–5. 10.1016/j.procbio.2008.10.013 [DOI] [Google Scholar]
- 16.Kar S, Ray RC. Statistical optimization of α-amylase production by Streptomyces erumpens MTCC7317 cells in calcium alginate beads using response surface methodology. Pol J Microbiol. 2008;57:49–57. [PubMed] [Google Scholar]
- 17.Igarashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, Uemura T, et al. Enzymatic properties of a novel liquefying α-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl Environ Microbiol. 1998;64:3282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutcheon GW, Vasisht N, Bolhuis A. Characterization of highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles. 2005;9:487–95. 10.1007/s00792-005-0471-2 [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Pomares F, Bautista V, Ferrer J, Pire C, Marhuenda-Egea FC, Bonete MJ. Alpha-amylase activity from halophilic archaeon Haloferax mediterranei. Extremophiles. 2003;7:299–306. 10.1007/s00792-003-0327-6 [DOI] [PubMed] [Google Scholar]
- 20.Nielsen JE, Borchert TV. Protein engineering of bacterial α-amylases. Biochim Biophys Acta. 2000;1543:253–74. 10.1016/S0167-4838(00)00240-5 [DOI] [PubMed] [Google Scholar]
- 21.Chen WM, Chang JS, Chiu CH, Chang SC, Chen WC, Jiang CM. Caldimonas taiwanensis sp. nov., α-amylase producing bacterium isolated from a hot spring. Syst Appl Microbiol. 2005;28:415–20. 10.1016/j.syapm.2005.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Bonete MJ, Camacho ML, Cadenas E. Purification and some properties of NAD-dependent glutamate dehydrogenase from Halobacterium halobium. Int J Biochem. 1986;18:785–9. 10.1016/0020-711X(86)90054-6 [DOI] [Google Scholar]
- 23.Bonete MJ, Camacho ML, Cadenas E. A new glutamate dehydrogenase from Halobacterium halobium with different coenzyme specificity. Int J Biochem. 1987;19:1149–55. 10.1016/0020-711X(87)90096-6 [DOI] [Google Scholar]
- 24.Camacho ML, Brown RA, Bonete MJ, Danson MJ, Hough DW. Isocitrate dehydrogenases from Haloferax volcanii and Sulfolobus solfataricus: enzyme purification, characterization and N-terminal sequence. FEMS Microbiol Lett. 1995;134:85–90. 10.1111/j.1574-6968.1995.tb07919.x [DOI] [PubMed] [Google Scholar]
- 25.Marhuenda-Egea FC, Piera-Velazquez S, Cadenas C, Cadenas E. Stability of an extreme halophilic alkaline phosphatase from Halobacterium salinarium in non-conventional medium. J Biotechnol. 2001;87:255–61. 10.1016/S0168-1656(01)00250-4 [DOI] [PubMed] [Google Scholar]
- 26.Shafiei M, Ziaee AA, Amoozegar MA. Purification and characterization of an organic-solvent-tolerant halophilic α-amylase from the moderately halophilic Nesterenkonia sp. strain F. J Ind Microbiol Biotechnol. 2011;38:275–81. 10.1007/s10295-010-0770-1 [DOI] [PubMed] [Google Scholar]
- 27.Amoozegar MA, Malekzadeh F, Malik KA. Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J Microbiol Methods. 2003;52:353–9. 10.1016/S0167-7012(02)00191-4 [DOI] [PubMed] [Google Scholar]
- 28.Madern D, Ebel C, Zaccai G. Halophilic adaptation of enzymes. Extremophiles. 2000;4:91–8. 10.1007/s007920050142 [DOI] [PubMed] [Google Scholar]
- 29.Burhan A, Nisa U, Gökhan C, Ömer C, Ashabil A, Osman G. Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem. 2003;38:1397–403. 10.1016/S0032-9592(03)00037-2 [DOI] [Google Scholar]
- 30.Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial α-amylase: a biotechnological perspective. Process Biochem. 2003;38:1599–616. 10.1016/S0032-9592(03)00053-0 [DOI] [Google Scholar]
- 31.Lee SK, Kim YB, Ji GE. Purification of amylase secreted from Bifidobacterium adolescentis. J Appl Microbiol. 1997;83:267–72. 10.1046/j.1365-2672.1997.00145.x [DOI] [PubMed] [Google Scholar]
- 32.Egas MCV, Costa MS, Cowan DA, Pires EMV. Extracellular α-amylase from Thermus filiforms Ork A2: purification and biochemical characterization. Extremophiles. 1998;2:23–32. 10.1007/s007920050039 [DOI] [PubMed] [Google Scholar]
- 33.Hamilton LM, Kelly CT, Fogarty WM. Production and properties of the raw starch-degrading amylase of Bacillus sp. IMD. Process Biochem. 1999;35:27–31. 10.1016/S0032-9592(99)00028-X [DOI] [Google Scholar]