Abstract
Background
The rice receptor kinase XA21 confers robust resistance to the bacterial pathogen Xanthomonas oryzaepv. oryzae(Xoo). We previously reported that XA21 is cleaved in transgenic plants overexpressing XA21 with a GFP tag (Ubi-XA21-GFP) and that the released C-terminal domain is localized to the nucleus. XA21 carries a predicted nuclear localization sequence (NLS) that directs the C-terminal domain to the nucleus in transient assays, whereas alanine substitutions in the NLS disrupt the nuclear localization.
Methods
To determine if the predicted NLS is required for XA21-mediated immunity in planta, we generated transgenic plants overexpressing an XA21 variant carrying the NLS with the same alanine substitutions (Ubi-XA21nls-GFP).
Results
Ubi-XA21nls-GFP plants displayed slightly longer lesion lengths, higher Xoobacterial populations after inoculation and lower levels of reactive oxygen species production compared with the Ubi-XA21-GFP control plants. However, the Ubi-XA21nls-GFP plants express lower levels of protein than that observed in Ubi-XA21-GFP.
Discussion
These results demonstrate that the predicted NLS is not required for XA21-mediated immunity.
Keywords: Nuclear localization, Bacterial blight disease, Xanthomonas oryzae pv. oryzae, XA21-mediated immunity
Introduction
Pattern recognition receptors (PRRs) recognize conserved microbial signatures, activating immune responses (Ronald & Beutler, 2010; Schwessinger & Ronald, 2012). The rice PRR XA21 confers robust resistance to diverse strains of Xanthomonas oryzae pv. oryzae (Xoo), the causal agent of blight disease in rice (Khush, Bacalangco & Ogawa, 1990; Song et al., 1995). XA21 recognizes a tyrosine-sulfated protein called RaxX (required for activation of XA21-mediated immunity X) derived from Xoo. Tyrosine sulfation is required for RaxX’s activity. In rice plants expressing XA21, sulfated RaxX activates XA21-mediated immune responses, including production of reactive oxygen species (ROS), ethylene, and induction of defense gene expression (Pruitt et al., 2015).
We previously reported that transgenic plants overexpressing XA21 produce XA21 cleavage products (Chen et al., 2010a; Chen et al., 2010b; Park et al., 2010; Park et al., 2008; Park & Ronald, 2012; Wang et al., 2006). For example, a 110-kDa amino-terminal XA21 cleavage product was detected in Ubi-Myc-XA21 transgenic plants and a 70-kDa carboxy-terminal cleavage product was observed in Ubi-XA21-CFP and Ubi-XA21-GFP plants (Park & Ronald, 2012). A predicted nuclear localization sequence (NLS) located between the XA21 transmembrane and juxtamembrane domain was identified. In rice protoplasts, the predicted NLS is able to direct transiently expressed GFP-tagged XA21 C-terminal domain to the nucleus. In contrast, a construct carrying alanine substitutions in the predicted NLS fails to direct the nuclear localization of XA21 C-terminal domain (Park & Ronald, 2012).
To assess the biological relevance of the predicted XA21 nuclear localization sequence in planta, we generated transgenic plants expressing an XA21 variant with alanine substitutions in the predicted NLS (XA21nls). The Ubi-XA21nls-GFP transgenic lines displayed partial resistance to Xoo infection, as reflected in slightly longer lesion lengths and higher Xoo bacterial populations compared with Ubi-Xa21-GFP control plants. A tyrosine-sulfated 21-amino acid derivative of RaxX (RaxX21-sY) triggered ROS production in Ubi-XA21nls-GFP plants. The expressed protein levels of XA21nls-GFP in Ubi-XA21nls-GFP lines were lower than those observed in Ubi-XA21-GFP plants. These results suggest that lower levels of expressed protein account for the slightly longer lesions observed in Ubi-XA21nls-GFP transgenic plants and indicate that the predicted NLS is not critical for XA21-mediated immunity.
Materials & Methods
Rice growth condition and leaf treatment with elicitors
Oryza sativa ssp. japonica cultivar Kitaake and a transgenic line expressing XA21 (Park & Ronald, 2012), Ubi-XA21-GFP (5B-5-4-3-2-1; the transgene driven by the maize ubiquitin promoter), were used in this study. The Kitaake genetic background lacks XA21 and is fully susceptible to the Xoo strain used in this study. Rice seeds were surfaced-sterilized with 15% bleach, rinsed with water, and soaked in water for one week. Well-germinated seedlings were transplanted into a soil mixture (80% sand, 20% peat from Redi-Gro, Sacramento, CA, USA) in an environmentally-controlled greenhouse.
Vector construction and rice transformation
The XA21nls-GFP (KRTKK678-682AATAA) construct was generated using site-directed mutagenesis. The DNA sequence containing alanine substitutions were amplified from an XA21-GFP/pENTR vector (Park & Ronald, 2012) using two overlapping primers (XA21nls-F and XA21nls-R, Table S1). The PCR product was digested with DpnI at 37 °C overnight and transformed into Escherichia coli competent cells, followed by kanamycin selection. Plasmids carrying the mutations were confirmed by standard Sanger sequencing. XA21nls-GFP was subcloned into Ubi-pCAMBIA-1300, which carries the maize ubiquitin promoter to drive expression of the transgene (Chern et al., 2005) using Gateway LR Clonase (Invitrogen, Carlsbad, CA, USA). The resulting Ubi-XA21nls-GFP construct was used to transform rice Kitaake calli by Agrobacterium-mediated method in Plant Transformation Facility at UC Davis (Hiei et al., 1994). The presence of the transgene was confirmed by PCR using gene-specific primers (Ubi-pro and XA21-seq2 in Table S1).
Bacterial infection assays
Xanthomonas oryzae pv. oryzae(Xoo) Philippines race 6 PXO99A (referred to as PXO99) was used in this study. Xoo was cultured on peptone sucrose agar plates supplemented with 20 mg L−1 cephalexin for two days, then washed off and re-suspended in sterilized water. The concentration of bacterial suspension was adjusted to an OD600 of 0.5 (approximately 1 × 108 cfu/mL) for inoculation.
Before inoculation, 5-week-old greenhouse-grown rice plants were transferred to a walk-in growth chamber (14-h-light/10-h-dark photo-period, 28°C/24 °C, 80%/85% humidity) to acclimate the chamber conditions for one week. Plants were inoculated using the scissor clipping method (Song et al., 1995). For each line, 8-12 plants were inoculated and in each plant two fully-expanded leaves from 3-6 tillers were clipped using scissors with the Xoo inoculum. The lesion lengths were measured 14 days post-inoculation. In planta bacterial growth was assessed as described previously (Bahar et al., 2014). Briefly, inoculated leaves were collected at indicated time points, cut into 5-mm pieces and incubated in 10 mL sterile water with shaking at 28 °C for 1 h. The suspension was diluted accordingly and spread out on PSA plates with 20 mg L−1 cephalexin. The bacterial colonies were counted after a two-day culture at 28 °C.
Protein extraction and western blot assays
Protein extraction from rice leaves and western blot assays were performed as previously described (Park & Ronald, 2012). Briefly, total protein was extracted from 100 mg of rice leaf tissue in 200 µL of pre-chilled extraction buffer (0.15 M NaCl, 0.01 M sodium phosphate buffer pH 7.2, 2 mM EDTA, 1% Triton X-100, 10 mM DTT, 20 mM NaF, 1 mM PMSF, 1% Sigma protease cocktail) and separated in an 8% SDS-polyacrylamide gel. A mouse anti-GFP antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the primary antibody for detection of GFP-tagged XA21 and XA21nls.
ROS assays
ROS assays were performed as previously described (Pruitt et al., 2015). Fully-expanded leaves were harvested from 4-week-old hydroponically grown rice plants, cut into 2-mm2 pieces and floated on water overnight. Leaf pieces were treated with water, 1 µM nonsulfated 21-amino acid synthetic RaxX peptides (RaxX21-Y) or tyrosine sulfated RaxX21 peptides (RaxX21-sY). For each treatment, four biological replicates were included and in each replicate two leaf pieces were used. Chemiluminescence was recorded every 30 s for 3 h in a high-sensitivity TriStar plate reader (Berthold, Germany).
Results
Generation of Ubi-XA21nls-GFP transgenic plants
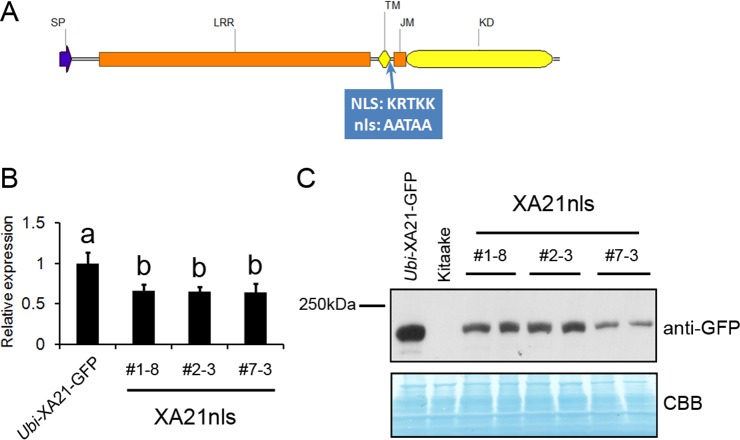
The predicted NLS in XA21 is a basic amino acid-rich sequence localized between the transmembrane and juxtamembrane domains (Park & Ronald, 2012). An XA21 variant with alanine substitutions in the predicted NLS (XA21nls; Fig. 1A) was generated using site-directed mutagenesis and introduced into the rice Kitaake cultivar. Five independent Ubi-XA21nls-GFP lines were obtained, and three lines that expressed detectable full-length XA21nls-GFP protein were selected for further analysis. We observed reduced gene and protein expression levels of XA21-GFP in these transgenic lines (#1–8, #2–3 and #7–3) as compared with those in the Ubi-XA21-GFP control plants (Figs. 1B and 1C).
Figure 1. Ubi-XA21nls-GFP transgenic plants have reduced transcript and protein levels.
(A) Domain organization of XA21 receptor (SP, signal peptide; LRR, leucine rich repeat; TM, transmembrane domain; JM, juxtamembrane domain; KD, kinase domain). The predicted nuclear localization sequence (NLS: KRTKK, amino acids 678-682) highlighted in blue, is mutated to AATAA in Ubi-XA21nls-GFP. (B) Relative expression levels of XA21 in Ubi-XA21-GFP and T2 progeny derived from three independent Ubi-XA21nls-GFP T1 lines (XA21nls #1–8, #2–3 and #7–3) determined by qRT-PCR and normalized to ubiquitin reference gene. The XA21 expression level in Ubi-XA21-GFP was set to 1. Bars represent mean ± SD of three technical replicates. Different letters indicate significant differences between the groups (Tukey’s honestly significant difference test, α < 0.05). The experiment was repeated three times with similar results. (C) Western blot analysis of 70 µg total protein extracts from 5-wk Ubi-XA21-GFP, Kitaake and two individual T2 plants derived from three Ubi-XA21nls-GFP T1 lines. The full-length XA21-GFP was detected with an anti-GFP antibody. Equal loading of total proteins was confirmed by Coomassie blue staining (CBB).
The T1 progeny of Ubi-XA21nls-GFP plants display resistance to Xoo
To determine whether Ubi-XA21nls-GFP confers resistance to Xoo, the T1 progeny of three Ubi-XA21nls-GFP T0 lines (#1, #2 and #7) were assessed for resistance to Xoo. Genotyping revealed that all three Ubi-XA21nls-GFP lines segregated for the transgene in the T1 generation (Fig. S1). The ratio of the transgene to null in T1 segregants in line #1, #2 and #7 were 9:6, 2:2 and 8:10 respectively, suggesting the presence of single T-DNA insertions.
The positive segregants in line #1, #2 and #7 (closed columns in Fig. S1) displayed short lesion lengths, while the null segregants (open columns in Fig. S1) displayed lesions as long as the Kitaake control plants. These results demonstrate that the T1 progeny derived from the three Ubi-XA21nls-GFP lines displayed similar or slightly longer lesions than the Ubi-XA21-GFP control.
Ubi-XA21nls-GFP plants display partial resistance to Xoo
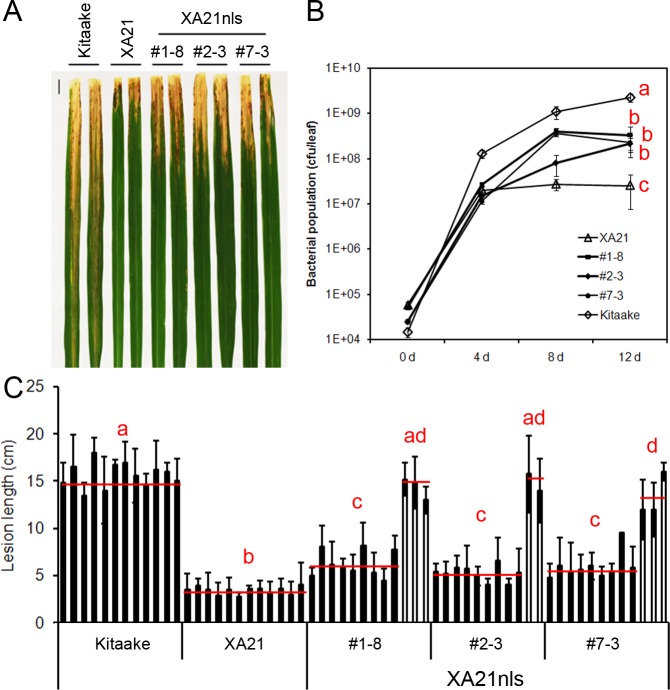
To validate the observation that the Ubi-XA21-GFP plants maintain resistance to Xoo, the bacterial infection assays were repeated on the T2 progeny derived from three Ubi-XA21nls-GFP transgenic T1 lines (#1–8, #2–3 and #7–3). Five-week-old Kitaake, Ubi-XA21-GFP and Ubi-XA21nls-GFP plants were inoculated with PXO99. Fourteen days after inoculation, Kitaake plants developed long water-soaked lesions typical of the disease (15.6 ± 2.5 cm), whereas Ubi-XA21-GFP developed very short lesions (3.4 ± 1.2 cm) (Figs. 2A and 2C). The Ubi-XA21nls-GFP plants segregated for Xoo resistance. The null segregants in three Ubi-XA21nls-GFP lines displayed long lesions, similar to the susceptible Kitaake plants. In contrast, the positive segregants from these lines displayed relatively short lesions, 6.3 ± 2.1 cm in #1–8, 5.3 ± 1.8 cm in #2–3 and 5.6 ± 1.9 cm in #7–3 (Fig. 2C and Fig. S2). The lesion lengths in the Ubi-XA21nls-GFP lines were significantly longer than Ubi-XA21-GFP but shorter than Kitaake (Fig. 2C). A second inoculation experiment #2 was performed with additional T2 progeny from the Ubi-XA21nls-GFP T1 lines. The slightly longer lesion phenotype also co-segregated with the Ubi-XA21nls-GFP transgene (Fig. S2), which is consistent with the results in the first experiment (Fig. 2).
Figure 2. T2 generation of Ubi-XA21nls-GFP plants display partial resistance to Xoo.
(A) Leaves of Kitaake, Ubi-XA21-GFP (XA21), and T2 progeny derived from three independent Ubi-XA21nls-GFP T1 lines (#1–8, #2–3 and #7–3) 14 days post-inoculation (dpi). (B) Bacterial population in Kitaake, Ubi-XA21-GFP, and Ubi-XA21nls-GFP plants at 0, 4, 8 and 12 dpi determined by the number of colony-forming units (CFU) per inoculated leaf. Error bars represent standard deviation from at least four leaves. (C) Lesion lengths of Kitaake, Ubi-XA21-GFP, and Ubi-XA21nls-GFP plants at 14 dpi. The segregants (closed columns) and null segregants (open columns) in each Ubi-XA21nls-GFP line were separated into two groups for statistical analysis. Bars represent mean ± SD from about six leaves. The red horizontal line indicates the average lesion length of each genotype. Different letters in (B) and (C) indicate significant differences between the groups (Tukey’s honestly significant difference test, α < 0.05). This experiment was repeated a second time (Fig. S2) with similar results.
To assess Xoo growth in planta, the same plants from the first inoculation experiment were used for a bacterial growth assay. Inoculated leaves were collected 0, 4, 8 and 12 days post-inoculation (dpi). As shown in Fig. 2B, the bacterial population in Kitaake reached to 108 cfu/leaf at 4 dpi, whereas the populations in Ubi-XA21-GFP and Ubi-XA21nls-GFP plants reached over 107 cfu/leaf. At 8 dpi, the Xoo population grew in Ubi-XA21nls-GFP plants but remained unchanged in the Ubi-XA21-GFP control plants. At 12 dpi, the bacterial populations in three Ubi-XA21-GFP transgenic lines were significantly higher than the Ubi-XA21-GFP plants but lower than that in the Kitaake plants (Fig. 2B). The result of bacterial populations is consistent with the lesion lengths in Ubi-XA21nls-GFP plants, indicating that Ubi-XA21nls-GFP plants display partial resistance to Xoo as compared with the Ubi-XA21-GFP control.
RaxX21-sY triggers ROS production in Ubi-XA21nls-GFP plants
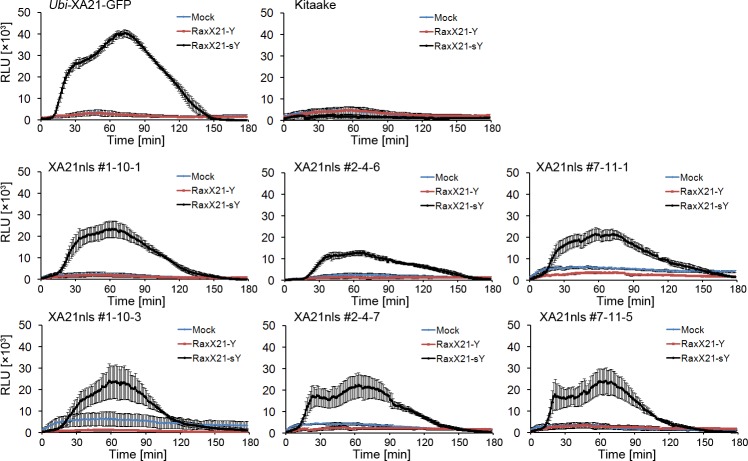
The production of ROS is a hallmark of XA21-mediated immune responses (Pruitt et al., 2015). We therefore carried out a ROS assay to test whether RaxX21-sY treatment leads to ROS production in Ubi-XA21nls-GFP plants. Detached leaves from four-week-old hydroponically-grown Ubi-XA21-GFP, Kitaake and Ubi-XA21nls-GFP plants were treated with water, 1 µM RaxX21-Y or RaxX21-sY. As shown in Fig. 3, RaxX21-sY triggered ROS production in Ubi-XA21-GFP plants but not in Kitaake. The ROS production peaked around 70 min and last for 150 min. The three Ubi-XA21nls-GFP lines displayed ROS production upon RaxX21-sY treatment, in a similar manner as the Ubi-XA21-GFP control plants. RaxX21-sY induced ROS production reached a lower level in three Ubi-XA21nls-GFP lines compared with that in Ubi-XA21-GFP plants. These results demonstrate that Ubi-XA21nls-GFP plants maintain the ROS response to RaxX21-sY.
Figure 3. Sulfated RaxX triggers ROS production in Ubi-XA21nls-GFP plants.
ROS production was measured in leaves from 4-wk hydroponically-grown Kitaake, Ubi-XA21-GFP, and T2 transgenic plants derived from three independent Ubi-XA21nls-GFP transgenic T1 lines (#1–10, #2–4 and #7–11) treated with water, 1 µM nonsulfated or sulfated 21-amino acid RaxX peptide (Mock, RaxX21-Y and RaxX21-sY, respectively). Bars represent mean ± SE of four technical replicates. RLU: relative light units.
Discussion
We previously reported that XA21 is cleaved in transgenic plants overexpressing XA21 with a GFP tag (Ubi-XA21-GFP), that the released GFP tagged C-terminal domain is localized to the nucleus and that a predicted NLS directs this domain to the nucleus in transient assays (Park & Ronald, 2012). To investigate the biological relevance of these observations, here we used a genetic approach to assess the resistance of transgenic plants expressing XA21 with mutations in the predicted NLS (Ubi-XA21nls-GFP) to Xoo.
We observed that the three Ubi-XA21nls-GFP transgenic lines displayed slightly reduced resistance to Xoo, as illustrated by slightly longer lesion lengths and higher bacterial populations compared with the Ubi-XA21-GFP control plants (Fig. 2). Sulfated, but not nonsulfated, RaxX21 is able to trigger ROS production in the Ubi-XA21nls-GFP plants, to slightly lower levels than that observed in the Ubi-XA21-GFP control (Fig. 3). Considering the relatively lower XA21 transcript and protein levels of XA21 in Ubi-XA21nls-GFP plants as compared with the Ubi-XA21-GFP control plants (Figs. 1B and 1C), we hypothesize that the reduced level of XA21 protein accumulation is responsible for the slightly reduced resistance and reduced ROS levels observed in the Ubi-XA21nls-GFP plants. The differences in XA21 protein levels in Ubi-XA21-GFP vs. Ubi-XA21nls-GFP may be due to position effects of the transgene, as we and others have observed in previous studies.
Together, these results indicate that Ubi-XA21nls-GFP is able to respond to RaxX21-sY and confers resistance to Xoo, which suggest that the predicted NLS is not required for XA21-mediated immunity. This result conflicts with our previous report that disruption of XA21 nuclear localization in Ubi-XA21-GFP-NES caused enhanced susceptibility (Park & Ronald, 2012). One possible explanation for this discrepancy is that the addition of the NES to XA21 disrupts activity of XA21.
The biological role of the XA21 cleavage product remains unknown. The XA21 intracellular domain interacts with several proteins predicted to be nuclear localized, including the XB10/WRKY62 transcription factor (Seo et al., 2011). The nuclear localization of the XA21-GFP cleavage products in transient assays suggests a role in transcriptional regulation in the nucleus (Park & Ronald, 2012). However, here we demonstrate that nuclear localization of the XA21 intracellular domain is not critical for XA21-mediated immunity.
Supplemental Information
Lesion lengths and genotyping results of Kitaake, Ubi-XA21-GFP (XA21), and T1 progeny derived from three independent Ubi-XA21nls-GFP T0 lines (#1, #2 and #7). The inoculation experiments were carried out separately on 8/19/14 (upper panel) and 9/23/14 (lower panel). The genotyping results of Ubi-XA21nls-GFP plants were shown in the gel picture under the columns (segregants represented by closed columns and null segregants by open ones). Bars represent mean ± SD from about six leaves in each plant. The statistical analysis was performed using Tukey’s honestly significant difference test to compare the lesion lengths between groups. In the upper panel, Kitaake, null segregants from XA21nls line #1 and #2 are in group “a”, XA21 in “b”, #1 segregants in “c”, and #2 segregants in “bc”; in the lower panel, Kitaake is in group “a”, XA21in “b”, XA21nls#7 segregants in “b”, and null segregants in “c” (α < 0.05). The lesion lengths on the T1 progeny from Ubi-XA21nls-GFP line #7 were comparable with those on Ubi-XA21-GFP, due to the Ubi-XA21nls-GFP transgenic plants slightly stressed as reflected in browning at leaf tips.
Leison lengths of Kitaake, Ubi-XA21-GFP (XA21) and T2 progeny derived from three Ubi-XA21nls-GFP T1 lines (#1–4, #2–1 and #7–10) were measured 14 days post-inoculation. The genotyping results were shown in the gel pictures under the columns (segregants represented by closed columns and null segregants by open ones). Bars represent mean ± SD from about six leaves. The statistical analysis was performed using Tukey’s honestly significant difference test to compare the lesion lengths between groups. Kitaake is in group “a”, XA21 in “b”, Ubi-XA21nls-GFP line #1-4 segregants and null segregants in “d” and “ac”, line #2–1 segregants and null segregants in “d” and “c”, and line #7-10 segregants and null segregants in “e” and “ac”, respectively (α < 0.05).
Acknowledgments
We thank Rory N. Pruitt, Brittany N. Anderton and Anna Joe for discussion and critical reading of the manuscript.
Funding Statement
The work was supported by NIH GM59962 to Pamela C. Ronald. The work conducted by the Joint BioEnergy Institute was supported by the Office of Science, Office of Biological and Environmental Research, of the US Department of Energy under Contract No. DE-AC02-05CH11231. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Pamela C. Ronald is an Academic Editor for PeerJ.
Author Contributions
Tong Wei conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Tsung-Chi Chen conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Yuen Ting Ho performed the experiments.
Pamela C. Ronald conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate, collect or analyse any raw data or code.
References
- Bahar et al. (2014).Bahar O, Pruitt R, Luu DD, Schwessinger B, Daudi A, Liu F, Ruan R, Fontaine-Bodin L, Koebnik R, Ronald P. The Xanthomonas Ax21 protein is processed by the general secretory system and is secreted in association with outer membrane vesicles. PeerJ. 2014;2:e2507. doi: 10.7717/peerj.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2010a).Chen X, Chern M, Canlas PE, Jiang C, Ruan D, Cao P, Ronald PC. A conserved threonine residue in the juxtamembrane domain of the XA21 pattern recognition receptor is critical for kinase autophosphorylation and XA21-mediated immunity. Journal of Biological Chemistry. 2010a;285:10454–10463. doi: 10.1074/jbc.M109.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2010b).Chen X, Chern M, Canlas PE, Ruan D, Jiang C, Ronald PC. An ATPase promotes autophosphorylation of the pattern recognition receptor XA21 and inhibits XA21-mediated immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:8029–8034. doi: 10.1073/pnas.0912311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern et al. (2005).Chern M, Canlas PE, Fitzgerald HA, Ronald PC. Rice NRR, a negative regulator of disease resistance, interacts with Arabidopsis NPR1 and rice NH1. Plant Journal. 2005;43:623–635. doi: 10.1111/j.1365-313X.2005.02485.x. [DOI] [PubMed] [Google Scholar]
- Hiei et al. (1994).Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza-sativa L) mediated by Agrobacterium and sequence-analysis of the boundaries of the T-DNA. Plant Journal. 1994;6:271–282. doi: 10.1046/j.1365-313X.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Khush, Bacalangco & Ogawa (1990).Khush GS, Bacalangco E, Ogawa T. A new gene for resistance to bacterial blight from O. longistaminata. Rice Genetics Newsletter. 1990;7:121–122. [Google Scholar]
- Park et al. (2010).Park C-J, Bart R, Chern M, Canlas PE, Bai W, Ronald PC. Overexpression of the endoplasmic reticulum chaperone BiP3 regulates XA21-mediated innate immunity in rice. PLoS ONE. 2010;5:e2507. doi: 10.1371/journal.pone.0009262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2008).Park C-J, Peng Y, Chen X, Dardick C, Ruan D, Bart R, Canlas PE, Ronald PC. Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS Biology. 2008;6:1910–1926. doi: 10.1371/journal.pbio.0060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park & Ronald (2012).Park CJ, Ronald PC. Cleavage and nuclear localization of the rice XA21 immune receptor. Nature Communications. 2012;3:920. doi: 10.1038/ncomms1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt et al. (2015).Pruitt RN, Schwessinger B, Joe A, Thomas N, Liu F, Albert M, Robinson MR, Chan LJG, Luu DD, Chen H, Bahar O, Daudi A, De Vleesschauwer D, Caddell D, Zhang W, Zhao X, Li X, Heazlewood JL, Ruan D, Majumder D, Chern M, Kalbacher H, Midha S, Patil PB, Sonti RV, Petzold CJ, Liu CC, Brodbelt JS, Felix G, Ronald PC. The rice immune receptor XA21 recognizes a tyrosine-sulfated protein from a Gram-negative bacterium. Science Advances. 2015;1:e2507. doi: 10.1126/sciadv.1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald & Beutler (2010).Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330:1061–1064. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- Schwessinger & Ronald (2012).Schwessinger B, Ronald PC. Plant innate immunity: perception of conserved microbial signatures. In: Merchant SS, editor. Annual review of plant biology. Vol. 63. 2012. pp. 451–482. [DOI] [PubMed] [Google Scholar]
- Seo et al. (2011).Seo YS, Chern M, Bartley LE, Han MH, Jung KH, Lee I, Walia H, Richter T, Xu X, Cao PJ, Bai W, Ramanan R, Amonpant F, Arul L, Canlas PE, Ruan R, Park CJ, Chen XW, Hwang S, Jeon JS, Ronald PC. Towards establishment of a rice stress response interactome. PLoS Genetics. 2011;7:e2507. doi: 10.1371/journal.pgen.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (1995).Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, Fauquet C, Ronald P. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2006).Wang Y-S, Pi L-Y, Chen X, Chakrabarty PK, Jiang J, De Leon AL, Liu G-Z, Li L, Benny U, Oard J, Ronald PC, Song W-Y. Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. The Plant Cell. 2006;18:3635–3646. doi: 10.1105/tpc.106.046730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lesion lengths and genotyping results of Kitaake, Ubi-XA21-GFP (XA21), and T1 progeny derived from three independent Ubi-XA21nls-GFP T0 lines (#1, #2 and #7). The inoculation experiments were carried out separately on 8/19/14 (upper panel) and 9/23/14 (lower panel). The genotyping results of Ubi-XA21nls-GFP plants were shown in the gel picture under the columns (segregants represented by closed columns and null segregants by open ones). Bars represent mean ± SD from about six leaves in each plant. The statistical analysis was performed using Tukey’s honestly significant difference test to compare the lesion lengths between groups. In the upper panel, Kitaake, null segregants from XA21nls line #1 and #2 are in group “a”, XA21 in “b”, #1 segregants in “c”, and #2 segregants in “bc”; in the lower panel, Kitaake is in group “a”, XA21in “b”, XA21nls#7 segregants in “b”, and null segregants in “c” (α < 0.05). The lesion lengths on the T1 progeny from Ubi-XA21nls-GFP line #7 were comparable with those on Ubi-XA21-GFP, due to the Ubi-XA21nls-GFP transgenic plants slightly stressed as reflected in browning at leaf tips.
Leison lengths of Kitaake, Ubi-XA21-GFP (XA21) and T2 progeny derived from three Ubi-XA21nls-GFP T1 lines (#1–4, #2–1 and #7–10) were measured 14 days post-inoculation. The genotyping results were shown in the gel pictures under the columns (segregants represented by closed columns and null segregants by open ones). Bars represent mean ± SD from about six leaves. The statistical analysis was performed using Tukey’s honestly significant difference test to compare the lesion lengths between groups. Kitaake is in group “a”, XA21 in “b”, Ubi-XA21nls-GFP line #1-4 segregants and null segregants in “d” and “ac”, line #2–1 segregants and null segregants in “d” and “c”, and line #7-10 segregants and null segregants in “e” and “ac”, respectively (α < 0.05).
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate, collect or analyse any raw data or code.