Abstract
The renin-angiotensin-aldosterone system (RAAS) is more complex than it was originally regarded. According to the current subject knowledge, there are two main axes of the RAAS: (1) angiotensin-converting enzyme (ACE)-angiotensin II-AT1 receptor axis and (2) ACE2-angiotensin-(1-7)-Mas receptor axis. The activation of the first axis leads to deleterious effects, including vasoconstriction, endothelial dysfunction, thrombosis, inflammation, and fibrosis; therefore, blocking the components of this axis is a highly rational and commonly used therapeutic procedure. The ACE2-Ang-(1-7)-Mas receptor axis has a different role, since it often opposes the effects induced by the classical ACE-Ang II-AT1 axis. Once the positive effects of the ACE2-Ang-(1-7)-Mas axis were discovered, the alternative ways of pharmacotherapy activating this axis of RAAS appeared. This article briefly describes new molecules affecting the RAAS, namely: recombinant human ACE2, ACE2 activators, angiotensin-(1-7) peptide and non-peptide analogs, aldosterone synthase inhibitors, and the third and fourth generation of mineralocorticoid receptor antagonists. The results of the experimental and clinical studies are encouraging, which leads us to believe that these new molecules can support the treatment of cardiovascular diseases as well as cardiometabolic disorders.
Keywords: Angiotensin converting enzyme 2, angiotensin-(1-7), Mas receptor, finerenone, mineralocorticoid receptor coregulators, aldosterone synthase inhibitors
Introduction
The renin-angiotensin-aldosterone system (RAAS) regulates blood pressure and fluid and electrolyte balance under physiological conditions. However, it plays also a role in pathological processes leading to cardiovascular and other disorders, e.g. hypertension, coronary artery disease, heart failure, and kidney diseases. The drugs blocking the RAAS, e.g. angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin II receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs), are the mainstays of current pharmacotherapy for cardiovascular diseases (CVD).1,2 Moreover, some data suggest the pleiotropic effects of RAAS blockers, since beneficial clinical effects of RAAS inhibition in atherosclerosis, atrial fibrillation, post-ischemic stroke state, pulmonary hypertension, diabetic vasculopathy, Alzheimer’s disease, or tumor angiogenesis were observed.3–5 Thus, it seems that in the near future, pharmacotherapy with RAAS-affecting agents could be extended to new indications. On the other hand, standard therapeutic procedure in CVD based on ACE-Is, ARBs, and MRAs appears to be insufficient sometimes, since it blocks only certain elements of the RAAS, while other pathways are still unaffected. This may lead to a phenomenon called ‘RAAS escape’, which may attenuate the clinical benefit of RAAS blockade. ‘RAAS escape’ was observed during either ACE-Is or ARBs treatment, when renin and angiotensin I (Ang I) accumulation overcame the ability of drugs to effectively suppress RAAS.6 Moreover, there are also ACE-independent pathways of Ang II formation. The reactivated Ang II promotes aldosterone secretion. ‘Aldosterone escape’ occurs during long-term ARBs therapy, as well as due to increased serum potassium levels or angiotensin II receptor type 2 (AT2)-dependent mechanism.7 Furthermore, the broad use of drugs blocking the RAAS has been limited by significant incidence of side effects, e.g. hyperkalemia, which forces research for new drugs expressing adequate efficacy while avoiding the adverse effects.8 Taking all these factors into account, no wonder scientists worldwide try to understand the functions of the RAAS better and to discover new drugs modulating it, as well as expand their indications. Indeed, the new molecules affecting the RAAS, namely: recombinant human ACE2 (rhACE2), ACE2 activators, angiotensin-(1-7) peptide and non-peptide analogs, aldosterone synthase inhibitors (ASIs) and the third and fourth generation of MRAs, have been described recently. Some of these new compounds are in clinical trials already. The question arises, whether the new agents modulating the RAAS will be new drugs in the future. The results of the experimental and clinical studies are encouraging, which leads us to believe that these new molecules can support the treatment of CVD.
Two axes of RAAS and their functions
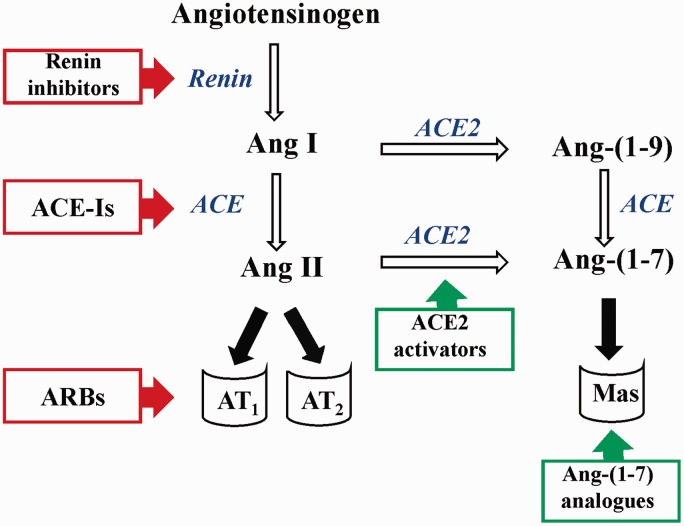
According to the current subject knowledge, there are two main axes of the RAAS: (1) ACE-angiotensin II-AT1 receptor axis and (2) ACE2-angiotensin-(1-7)-Mas receptor axis (Figure 1).9,10 Activation of the ACE-Ang II-AT1 causes deleterious effects, including vasoconstriction, endothelial dysfunction, thrombosis, inflammation, and fibrosis.11 ACE2-Ang-(1-7)-Mas receptor axis has a different role, since it often opposes the effects induced by the classical ACE-Ang II-AT1 axis.12 It has been suggested that the activity of the RAAS and the actions of RAAS blockers depend on the balance between these two axes. It was demonstrated that therapy with ACE-Is and ARBs prevented the decrease in ACE2 expression in myocardial infarcted rats and increased plasma Ang-(1-7) levels.13–15 This could be evidence that these drugs are effective not only due to the inhibition of Ang II effects, but also due to the activation of the ACE2-Ang-(1-7)-Mas axis of the RAAS. Since, the positive effects of the ACE2-Ang-(1-7)-Mas axis were discovered, the alternative ways of pharmacotherapy activating this axis of RAAS appeared. ACE2-Ang-(1-7)-Mas signaling, called ‘vasoprotective axis’ as well, has the potential to be considered a novel therapeutic approach to counterbalance the ACE-Ang II-AT1 axis as a novel approach targeting RAAS.10 Indeed, there are researched exogenous Ang-(1-7) analogs and ACE2 activators, which may be effective in the treatment of CVD, prevention and treatment of diabetic vasculopathy or metabolic syndrome.
Figure 1.
Targets of molecules affecting the RAAS. In red frames—drugs blocking the ACE-AngII-AT1 axis, in green—molecules activating the ACE2-Ang-(1-7)-Mas axis. Ang: angiotensin, ACE: angiotensin I converting enzyme, ACE-Is: angiotensin I converting enzyme inhibitors, ACE2: angiotensin I converting enzyme type 2, ARBs: angiotensin II receptor type 1 blockers, AT1: angiotensin II receptor type 1, AT2: angiotensin II receptor type 2, Mas: Mas receptor. (A color version of this figure is available in the online journal.)
Angiotensin-(1-7)
Angiotensin-(1-7) is the element of the RAAS arousing big interest due to its opposite to Ang II action.16,17 The presence of Ang-(1-7) has been confirmed in the heart, blood vessels, kidneys, and liver.18 Ang-(1-7) is formed mainly through the removal of the C-terminal phenylalanine from Ang II by the action of ACE2. Ang-(1-7) can be also produced from Ang-(1-9) by ACE and neutral-endopeptidase. Ang-(1-7) exerts its action through stimulation of the specific G-protein coupled Mas receptor. Receptor Mas stimulation leads, among others, to increased phosphorylation of endothelial nitric oxide synthase (eNOS) and increased nitric oxide (NO) release. In addition, it augments prostacycline synthesis and suppresses the release of norepinephrine and thus Ang-(1-7) is considered a vasodilating and antiarrhythmogenic factor.12,19 The effects of Ang-(1-7) in diabetes and its cardiovascular disorders are a new research area, although there are already some data confirming the positive impact of this peptide on glucose metabolism and its role in the prevention of the hyperglycemia-induced disorders. It was demonstrated that Mas-knockout mice presented changes in glucose and lipid metabolism which ended up with a condition resembling metabolic syndrome, while chronic elevation of Ang-(1-7) levels in transgenic rats leads to better glucose tolerance and insulin sensitivity, a decrease in the plasma triglyceride and cholesterol levels and a reduction in adipose tissue mass.20,21 The possible mechanism of Ang-(1-7) action in glucose metabolism may be related to the modulation of blood flow and inhibition of fibrosis and therefore glucagon and insulin release.22 The protective role of Ang-(1-7) in the cardiovascular disorders of diabetes was also observed. It was demonstrated that Ang-(1-7) attenuates diabetic cardiomyopathy in rats because of vasodilatory, antiproliferative, and antifibrotic properties.23–26 Furthermore, the cardioprotective effect of this peptide was also related to a decrease in dyslipidemia. However, the therapeutic use of Ang-(1-7) is limited due to its unfavorable pharmacokinetic properties.27 Thus, new strategies (e.g. the use of cyclodextrins, liposomal delivery systems, modifications of the peptide—cyclic form) are sought to make clinical application of Ang-(1-7) possible.28
Non-peptide Ang-(1-7) analogs
The most widely studied so far Ang-(1-7) analog is AVE 0991, which is a non-peptide, orally active and physiologically well tolerated imidazole derivative.29 Despite the fact that the first studies on AVE 0991 come from the last decade, there are only few publications demonstrating the pharmacodynamics and pharmacokinetics of this agent.
In 2002, the first in vitro study of AVE 0991 took place.30 It was demonstrated that novel compound caused a subsequent increase in NO and low concomitant production of O2 in bovine aortic endothelial cells. AVE 0991 caused approximately five times higher release of bioactive NO compared with Ang-(1-7). Moreover, it was demonstrated that the effects of AVE 0991 were not completely abolished by inhibition of NOS or blockade of AT1 and AT2 receptors.30 The beneficial effects of AVE 0991 were confirmed in various experimental models of CVD and diabetes (Table 1).31–40 Despite the promising results of experimental studies, the development of AVE 0991 has been stopped for unknown strategic reasons.
Table 1.
New agents modulating RAAS in the experimental studies
| New agents in RAAS | Beneficial effects observed in animal models | Target diseases—potential clinical implication | Refs. |
|---|---|---|---|
| Non-peptide Ang-(1-7) analogs (AVE 0991) | • Decrease in infarcted area in rats with myocardial infarction • Inhibition of atherogenesis in apoE-knockout mice • Reduction of endothelial dysfunction in salt-fed rats • Anti-hypertensive effect in 2K1C and DOCA hypertensive rats • Improvement in hemodynamics and renal protection in streptozotocin-induced diabetic rats | • Myocardial ischemia • Heart failure • Atherosclerosis • Hypertension • Diabetes | 31–40 |
| Peptide Ang-(1-7) analogs (CGEN-856S) | • Decrease in blood pressure in SHR rats • Decrease in infarcted area and cardiac remodelling in rats with hypertrophy and myocardial infarction | • Hypertension • Myocardial ischemia • Arrhythmias | 41–42 |
| rhACE2 | • Reduced inflammation, renal dysfunction, and glomerulus injury in apoE-knockout mice • Reduced hypertrophy, diastolic dysfunction, and myocardial fibrosis in mice with hypertrophy and diastolic dysfunction | • Atherosclerotic renal injury • Kidney diseases • Heart failure | 60–62 |
| ACE2 activators (Xanthenon, DIZE) | • Decrease in blood pressure in SHR rats • Reduction in interstitial fibrosis in rats with pulmonary hypertension • Reduction in thrombus weight and area in rat venous thrombosis • Improvement in the autonomic and cardiac dysfunction in streptozotocin-induced diabetic rats | • Hypertension • Diabetes with cardiovascular autonomic dysfunction | 64–68 |
| MRAs (Finerenone) | • Improved mortality and nephroprotection in SHR stroke-prone rats • Cardiorenal protection in rats with diastolic heart failure | • Hypertension • Heart failure | 102–104 |
| ASIs (FAD286) | • Reduced cardiac hypertrophy, oxidative stress and improved hemodynamics and endothelial function in rats with post-myocardial infarction heart failure • Reduced lesion area and inflammation in apoE-knockout mice • Reduced renal inflammation, albuminuria in streptozotocin-induced diabetic rats • Lowered blood pressure in hypertensive salt-fed rats • Reduced neovascularization and retinopathy in rat model of retinopathy | • Congestive heart failure • Atherosclerosis • Diabetic nephropathy • Hypertension • Retinal neovascularisation | 110–121 |
Peptide Ang-(1-7) analogs
Peptide Ang-(1-7) analogs, natural ligands able to stimulate G-protein coupled receptors (Mas among others), were discovered during the human proteome analysis. As a result, two peptides, CGEN-856 and CGEN-857, were examined (amino acid sequence FLGYCIYLNRKRRGDPAFKRRLRD and SMCHRWSRAVLFPAAHRP, respectively). What is the most important is that the compounds have no significant homology to Ang-(1-7) or to known G-protein coupled receptor ligands. These peptides have several chemical structures, but CGEN-856S (a monomer in which cysteine was substituted with serine) displays the highest affinity for the Mas receptor confirmed in experimental in vitro and in vivo models. CGEN-856S displays high, like AVE 0991, affinity for the Mas receptor.41 The favorable effects of CGEN-856S in the cardiovascular system were confirmed in animal models of CVD (Table 1).41,42
Ang-(1-7) analogs in clinical trials
A major limitation of Ang-(1-7) use is that this molecule is a peptide with a short plasma half-life and is rapidly degraded in the gastrointestinal tract when given orally. Although, some attempts to make Mas stimulation suitable for clinical use of orally active derivatives of Ang-(1-7) are being made. Some of the Ang-(1-7) analogs entered the clinical studies, including NorLeu3-Ang-(1-7) which is currently studied as DSC127 for topic treatment of diabetic foot ulcers (DFU) (Table 2).43,44 DFU patients are being recruited into phase III clinical trials for DSC127 (NCT01830348 and NCT01849965).45 One pharmaceutical company aims to initiate clinical trials with another Ang-(1-7) analog—TXA127 in patients with Duchenne muscular dystrophy or congenital muscular dystrophy in early 2016. So far, the positive effects of TXA127 in muscle dystrophy, including reduction in muscle fibrosis, increases in muscle strength as well as normalization of cardiac dysfunction, were confirmed in experimental models.46–48
Table 2.
New agents modulating RAAS in the clinical studies
| New agents in RAAS | Clinical study |
Results | Mechanism of action | Ref. | |
|---|---|---|---|---|---|
| Phase/Acronim | Patients | ||||
| Ang-(1-7) analogs (DSC127) | Phase II clinical study | Patients with chronic, noninfected, neuropathic, or neuroischemic plantar Wagner Grade 1 or 2 foot ulcers | • Safety and efficacy of DSC127 in accelerating the healing of diabetic foot ulcers | • Induction of progenitor proliferation • Accelerated vascularisation | 43 |
| rhACE2 | Phase I clinical study | Healthy volunteers | • Determined pharmacokinetics, pharmacodynamics, safety, and tolerability • Lack of cardiovascular effects despite of marked changes in angiotensin system peptide concentrations | • The presence of effective compensatory mechanisms in healthy volunteers is suggested | 63 |
| MRAs (Finerenone) | Phase IIa clinical study (ARTS) | Patients with heart failure associated with a reduced left ventricular ejection fraction and chronic kidney disease | • Determined pharmacokinetics, pharmacodynamics, safety, tolerability, and optimal dose range for further studies • Less hyperkalemia and renal dysfunction compared with spironolactone | • Greater selectivity than spironolactone and stronger mineralocorticoid receptor binding affinity than eplerenone | 105 |
| Phase IIb clinical study (ARTS-DN) | Patients with type 2 diabetes and clinical diagnosis of diabetic nephropathy | • Well tolerated with good safety profile • Future long-term clinical studies examining the effects of finerenone on the progression of renal disease as well as on CV morbidity and mortality in patients with DKD are needed | 106 | ||
| Phase IIb clinical study (ARTS-HF) | Patients with worsening chronic heart failure and reduced left ventricular ejection fraction and at high risk of hyperkalemia and worsening renal dysfunction | • Investigated the safety and potential efficacy finerenone in a high-risk population of patients • Assessed the effects of finerenone on a composite clinical endpoint | 107 | ||
| ASIs (LCI699) | Phase II clinical study | Patients with primary aldosteronism | • Decreased plasma aldosterone concentration • Corrected the hypokalemia and mildly decreased blood pressure | • Effectively and safely inhibited aldosterone synthase (CYP11B2) | 123 |
| Phase II clinical study | Patients with primary hypertension | • Lowered clinic and ambulatory blood pressure | • Effectively and safely inhibited aldosterone synthase (CYP11B2) | 124 | |
| A multicenter, proof-of-concept study | Patients with Cushing's disease | • Efficacious and well tolerated in patients with Cushing's disease • Normalized urinary cortisol • Decreased blood pressure | • Inhibited cortisol synthesis (CYP11B1) | 125 | |
Angiotensin converting enzyme 2
A monocarboxypeptidase, angiotensin-converting enzyme 2 (ACE2) is 42% homolog to ACE1 and is expressed in the heart, kidney, testis, endothelium of coronary, intrarenal vessels, and renal tubular epithelium.12 ACE2 shows a 400-fold higher affinity to Ang II than to Ang I. ACE2 produces vasodilator peptides Ang-(1-7) from Ang II (Figure 1). ACE-Is increase angiotensin-(1-9) and Ang-(1-7) levels, which is probably related with the enhanced activation of ACE2.12 Moreover, ACE2 is a target for severe acute respiratory syndrome coronavirus (SARS)-CoV. During infection with this virus the expression of ACE2 is decreased, which probably contributes significantly to the development of pulmonary insufficiency.49 ACE2 is activated in the heart ventricles of primary pulmonary hypertension patients, which suggests that ACE2 could be a cardioprotective enzyme.50 Indeed, the results of experimental studies support this thesis. Studies in rats overexpressing ACE2 showed a reduction in blood pressure and an improvement in endothelial function.51 It has been also demonstrated that lack of the ACE2 gene leads to an increase in adhesion molecules and proinflammatory cytokine expression, which augments vascular inflammation and atherogenesis in ApoE knockout mice.52 Moreover, the benefits of ACE2 in experimental models of diabetes were demonstrated. Infection with the adenovirus containing human ACE2 gene resulted in improved fasting glycemia and glucose tolerance, an increase in pancreatic β cells proliferation and limitation of their apoptosis in diabetic mice.53 Moreover, it was demonstrated that ACE2 plays a protective role in diabetic nephropathy in experimental animals.54,55 According to the latest reports, changes in ACE2 gene expression were observed during clinical studies in type 1 and type 2 diabetic patients.56,57 There was a positive correlation between the ACE/ACE2 ratio and such variables as blood pressure, fasting glycemia, creatinine levels, and urine protein.
The presented data proves a potential ACE2 role in the prevention of CVD and organ damage provoked by sustained hyperglycemia, thus the search for new molecules and methods of modulating ACE2 activation is required.
Recombinant human ACE2
One of the possibilities activating the ACE2-Ang-(1-7)-Mas axis is the use of rhACE2. It was demonstrated that treatment of Ang II-infused wild-type mice with rhACE2 blunted the hypertrophic response and expression of hypertrophy markers and reduced Ang II-induced superoxide production and Ang II-mediated myocardial fibrosis.58 These effects were associated with reduced plasma and myocardial Ang II and increased plasma Ang-(1-7) levels. Importantly, rhACE2 partially prevented the development of dilated cardiomyopathy in pressure-overloaded wild-type mice.58 These data prove that ACE2 is an important negative regulator of Ang II-induced heart disease and can suppress adverse myocardial remodeling. The beneficial effects of rhACE2 were also demonstrated in experimental models of diabetic kidney injury in association with a reduction in blood pressure and a decrease in oxidative stress.59 Moreover, blocking Ang-(1-7) action prevents the beneficial effects of rhACE2 leading to systolic dysfunction.60 These results highlight a key cardioprotective role of Ang-(1-7) and potential therapeutic strategy for CVD (Table 1).61,62 Actually, rhACE2 was successfully taken through a phase I trial and was well tolerated by healthy volunteers. Although, despite marked changes in angiotensin peptide concentrations, cardiovascular effects were lacking, suggesting the presence of some compensatory mechanisms in healthy volunteers (Table 2).63
ACE2 activators
The second way to increase ACE2 activity, and therefore Ang-(1-7) synthesis, is to use agents modulating ACE2 gene expression. In 2008, two ACE2 activators were discovered: xanthenon (XNT) and resorcinolnaphthalein. In vitro studies showed that these two compounds in a dose-dependent manner enhanced ACE2 activity by approximately two-fold from control levels.64 However, due to the results of a solubility study only XNT was researched in vivo. XNT is significantly more soluble than resorcinolnaphthalein, thus it was commonly used in in vivo studies. The protective cardiovascular effects of XNT were confirmed in various animal models of CVD and diabetes (Table 1).64–68
Recently, an antitrypanosomal drug, diminazene aceturate (DIZE), was shown to exert an “off-target” effect of enhancing the activity of ACE2 in vivo. The potential benefits of DIZE in the therapy of hypertension and its complications were demonstrated in different animal models (Table 1).69–73 The protective effects of DIZE were associated with the activation of the vasoprotective axis of the lung RAAS, decreased inflammatory cytokines, improved pulmonary vasoreactivity, and enhanced cardiac function.69 A recent report demonstrated that the mechanism of DIZE’s antihypertensive action involves Mas receptor activation and the NO-dependent pathway.70 Moreover, it was shown that treatment with DIZE improved hypercholesterolemia-induced corpus cavernosum injury, suggesting ACE2 as a potential target for treating erectile dysfunction.72
The cardioprotective properties of ACE2 activators could mean future use of these compounds in the prevention of cardiac insufficiency or diabetes complications, including hemostasis disturbances. These results, with the reduction of lipogenesis markers, open a new perspective for metabolic disorder pharmacotherapy. At the moment, the effects of ACE2 activators were evaluated only in preclinical studies.
Aldosterone
Aldosterone, the final product of the RAAS, plays a crucial role in the pathophysiology of the cardiovascular system.74 Aldosterone contributes to endothelial dysfunction, fibrinolytic disorders, inflammation, oxidative stress, fibrosis, hypertrophy, and arrhythmias leading to progression of CVD.75–78
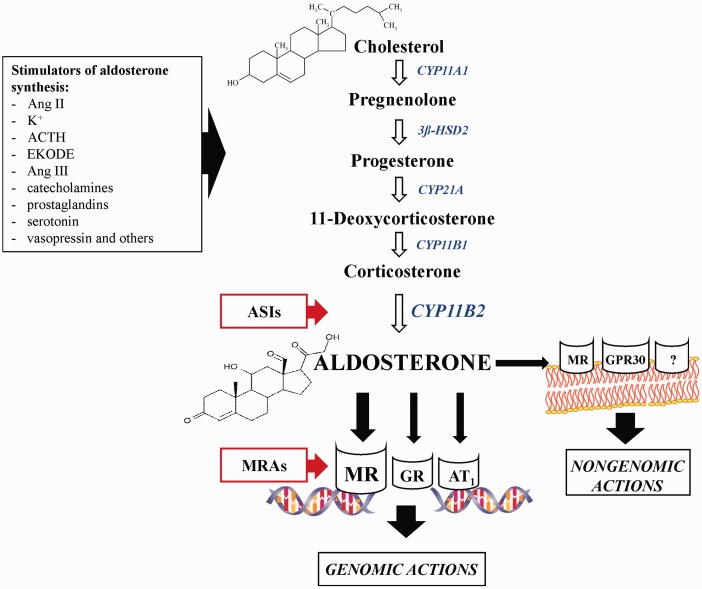
The blockade of aldosterone action has been demonstrated to be an extremely beneficial therapy in CVD. Clinical trials with spironolactone and eplerenone, steroidal MRAs, investigated the potential role of aldosterone and MRAs in a variety of CVD. These trials are a result of clinical interest in the significant function of aldosterone in the cardiovascular system, which became evident after publication of the outcomes of two clinical trials: Randomized Aldactone Evaluation Study (RALES) and Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS).79,80 Moreover, numerous animal studies have shown that MR blockade reduces cardiovascular, renovascular, and cardiometabolic disorders associated with obesity and diabetes.81–83 Moreover, the prothrombotic effect of aldosterone was showed in experimental models of thrombosis.84–87 It was demonstrated that the hormone enhances venous thrombosis in normotensive rats in the mechanism involving primary hemostasis, fibrinolysis, NO, and oxidative stress-dependent pathways.88 Furthermore, the MR blockade was not sufficient to reverse aldosterone effects in hemostasis. The other receptors, e.g. glucocorticoid receptor (GR) and AT1, were also involved in the prothrombotic action of aldosterone.89,90 These results show that the aldosterone action is more complex and involves not only MR activation as it was previously thought (Figure 2).
Figure 2.
Aldosterone synthesis and targets of hormone blockers. ACTH: adrenocorticotropic hormone, Ang II: angiotensin II, Ang III: angiotensin III, ASIs: aldosterone synthase inhibitors, AT1: angiotensin II receptor type 1, CYP11B2: aldosterone synthase, EKODE: oxidized derivative of linoleic acid, GPR30: estrogen receptor, GR: glucocorticoid receptor, K+: potassium ions, MR: mineralocorticoid receptor, MRAs: mineralocorticoid receptor antagonists, ‘?’: non-identified cell-membrane receptor. (A color version of this figure is available in the online journal.)
However, the molecular mechanism of aldosterone action is not completely understood. The effects of aldosterone are mediated via classic nuclear receptors (genomic actions of aldosterone) and cell-membrane receptors (non-genomic actions of aldosterone) with alternative pathways (activation of protein kinases or secondary messenger signaling cascades).91,92 It was well documented that aldosterone in supraphysiological concentrations can also act via GR.93 Recently, it has been demonstrated that another important receptor aldosterone acts on is G protein coupled estrogen receptor (GPR30). GPR30 activation plays an important role in aldosterone-mediated regulation of endothelial cell growth and in aldosterone's endothelial-mediated regulation of vasoreactivity.94
This multiple mechanism of aldosterone action points to the need of a search for new strategies of aldosterone blockade.
New aldosterone blockers
To date, only two steroidal MRAs have been clinically used. Spironolactone represents first generation of nonselective MRAs, while eplerenone corresponds to the second generation with significantly improved selectivity for MR over other steroid receptors.95 The IC50 of eplerenone for MR (990 nmol/L) is over 10-fold less than for androgen, progesterone, and estrogen receptors.96]. Despite the irrefutable beneficial effects of spironolactone and eplerenone confirmed in patients with heart failure and kidney disease, the use of MRAs is limited by the risk of hyperkalemia, especially in patients with renal disorders.79,80,97 In fact, hyperkalemia was reported in up to 36% among elderly heart failure outpatients. Hence, the risk of hyperkalemia was the strongest stimulus for further research with third generation of MRAs, which are nonsteroidal, more cardioselective thus exerting less renal side effects.98 Few pharmaceutical companies have nonsteroidal MRAs in clinical development. However, no clinical data have been published so far for MT-3995, SC-3150, LY2623091, and PF-03882845. Although, there are some data available from a phase II trial for finerenone (developmental code name BAY 94-8862), showing safety and efficacy in patients with heart failure and chronic kidney diseases.99
Finerenone—third generation of MRAs
Finerenone is a dihydropyridine derivative without L-type Ca2+ channel activity and with less relative affinity to other steroid receptors than currently available MRAs.99 Finerenone has unique pharmacodynamics as a consequence of different molecular properties. Similar to spironolactone and eplerenone, finerenone competitively antagonizes the MR, although it shows more natriuretic effects since it exerts a 3–10-fold higher potency and efficacy with IC50 of 18 nmol/L with exceptional selectivity versus the GR, androgen and progesterone receptors (>500-fold).99–101 Finerenone shows cardiac and renal protection, which was confirmed in preclinical studies in rats (Table 1).102–104 Furthermore, the end-organ protective activity were more pronounced in finerenone-treated rats compared to eplerenone-treated animals.102 These positive outcomes from preclinical studies were further confirmed in trials (Table 2). The safety and tolerability of finerenone was studied during the Mineralocorticoid-Receptor Antagonist Tolerability Study (ARTS) in patients with heart failure and mild/moderate chronic kidney disease.105 Treatment with finerenone resulted in less hyperkalemia and slower renal dysfunction compared with spironolactone, whereas the other cardiac and renal parameters were at least similar. Further clinical studies with finerenone in patients with worsening chronic systolic heart failure and type 2 diabetes and/or chronic kidney disease (ARTS-HF, ARTS-DN) showed positive outcomes as well.106,107 However, the long-term effects of finerenone will be investigated in a phase III study for the treatment of chronic heart failure.
Fourth generation of MRAs
While MR blocking in the cardiovascular tissues is particularly sought after, the fourth generation of MRAs, presenting high tissue selectivity (cardiovascular over renal effects) and a renal-sparing profile (combined Na+ excretion with a mild K+ retention), is now postulated. This tissue selectivity can be achieved by improving the physiochemical properties of MRAs that alter their tissue distribution or by the interaction of novel MRAs with “coregulator molecules”. There is evidence that coregulators, a heterogeneous group of nonreceptor proteins, are required to influence nuclear receptor-mediated transactivation of target genes.108 It is expected that the interaction of novel MRAs with certain coregulators may allow the modulation of MR activity and selectivity. Thus, rather selective MR modulators, than MR blockers per se, may be a key factor in proper MR antagonism. Understanding the nature of MR-coregulator interactions may be a stimulator for a rational design of the fourth generation of MRAs.
Aldosterone synthase inhibitors
Bearing in mind that the harmful effects of aldosterone are not fully abolished by the MR blockade, since alternative receptors (GR, GPR30, AT1), as well as nongenomic mechanisms are involved in the hormone action, the question arises whether blocking at the level of aldosterone synthesis would be more beneficial in this case (Figure 2).
The key enzyme in aldosterone production is aldosterone synthase (CYP11B2). CYP11B2 is predominantly expressed in the adrenal gland but is also expressed in the cardiovascular system or brain.109–111 Lack of optimal effectiveness in aldosterone receptor blockade initiated some research on ASIs, like FAD286 or LCI699.112
FAD286
FAD286, the R-enantiomer of fadrozole, was initially developed as an aromatase (CYP19A1) inhibitor and used as a drug to treat breast cancer.111 There were also demonstrated potential benefits of FAD286 in the therapy of cardiovascular disorders in different experimental models of CVD and diabetes (Table 1).110,113–121 Some effects were similar to the effects of MRAs, proving that aldosterone plays a key role in the pathogenesis of CVD. Considering that aldosterone may also act through the MR-independent pathways, ASIs seem to be an excellent supplement of classic MRAs therapy in the prevention of cardiac insufficiency. The results of experimental studies are promising, which allow us to believe that inhibition of aldosterone synthesis can support treatment of CVD.
LCI699
Following the experimental studies with FAD286, LCI699 was synthesized, based on the chemical structure of FAD286, as the first orally active ASI for human use (Table 2).122 LCI699 is a potent inhibitor of CYP11B2, but it also inhibits CYP11B1, the enzyme that catalyses the final step of cortisol synthesis. The results of phase II studies showed that in patients with primary hyperaldosteronism characterized by severe hypertension and hypokalemia LCI699 induced a reversible and dose-dependent 70–80% decrease in plasma and urinary aldosterone concentrations with a massive accumulation of the aldosterone precursor, deoxycorticosterone, in the plasma, confirming the inhibition of the product of the CYP11B2 gene. Treatment with LCI699 caused correction of hypokalemia and a mild decrease in blood pressure.123 The efficacy of LCI699 for lowering BP was investigated in patients with essential hypertension. The antihypertensive effect of 1 mg of LCI699 was similar to that of eplerenone at a dose of 50 mg.124
However, the effects of LCI699 on the glucocorticoid axis limit the use of higher doses because of the loss of selectivity for CYP11B2.122 These effects on the glucocorticoid axis may not be a problem in the case of Cushing disease patients. In fact, preliminary results from a multicenter, proof-of-concept study are that patients with Cushing disease achieve normal urinary cortisol with LCI699.125 Another LCI699 trial goal was to evaluate the effect of LCI699 on cortisol response to adrenocorticotropic hormone stimulation in patients with essential hypertension in order to find the maximally tolerated dose in this patient population, which was estimated to be 1.30 mg once daily. The treatment was well tolerated with no serious adverse effects.126 In a trial comparing LCI699 to eplerenone in 14 patients with primary aldosteronism, the effects on blood pressure and plasma potassium and renin concentrations of four weeks of eplerenone treatment were more significant than those of four weeks of LCI699 treatment, with the opposite drugs effect on plasma aldosterone concentrations.127
Other ASIs
The new inhibitors of CYP11B2 are already existing drugs that according to some researchers could be used either in the treatment of hyperaldosteronism-related diseases or as precursors to achieve safer and selective new ASIs.128 Moreover, several dihydropyridine Ca2+ channel blockers block T-type channel as well, which brings upon the inhibition of aldosterone synthesis in vitro. The dihydropiridine structure might be the base for the development of novel molecules that dually (a) block aldosterone synthase and MR for more potent aldosterone antagonism and (b) inhibit the L-type Ca2+ channel for more pronounced antihypertensive effects.129
Combined treatment—another approach to RAAS blocking
The another approach to effective treatment of CVD is the usage of new combinations of agents modulating the RAAS. There are many clinical studies (RESOLVD, CHARM, ALOFT) showing the efficacy of dual RAAS blockade based on combination of various doses of well-known ACE-Is, ARBs, and direct renin inhibitor. Unfortunately, several clinical trials (ONTARGET, ALTITUDE and VA NEPHRON-D) in patients with hypertension, heart failure, and chronic kidney disease with proteinuria have demonstrated no beneficial effects of dual versus single RAAS blockade, but a higher incidence of adverse events.130 Some new combined agents affecting RAAS occurred recently. According to the latest network meta-analysis of Xie et al., ARNI, a novel dual-acting angiotensin receptor-neprilysin inhibitor has the highest probability of being the most effective therapy for heart failure and reduced ejection fraction compared to ACE-Is and/or ARBs.131
Conclusions and perspectives
The efficacy of classic RAAS affecting drugs in CVD is widely known, but previously it was not assumed these effects could also be related with the activation of other regulatory elements of RAAS. Understanding the mechanism of new molecules’ action in the RAAS allows the introduction of alternative therapies and thus elimination of the adverse effects of already used drugs. The emergence of these novel drugs may not only help to improve the effectiveness of treatment of CVD, but it may further significantly broaden the therapeutic potential of the RAAS. The results of basic experiments and clinical studies are encouraging, which leads us to believe that new molecules can support treatment of CVD and could be helpful in the management of cardiometabolic disorders.
More detailed information about the results of experimental studies with the usage of new agents affecting RAAS are enclosed in supplementary files (Supplementary Tables 1–3).
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
This work was supported by the research project NN405 627938 of the Polish Committee for Scientific Research and research projects No N/ST/MN/16/001/2226 and N/ST/ZB/16/002/2226 of the Medical University of Bialystok.
Author contributions
All authors contributed to the writing of this review article and have read and approved the final manuscript. The overall layout and content, preparation of figures and writing of the manuscript was carried out by AG-P. The survey of the literature regarding experimental studies with the use of agents modulating RAAS and summary tables were performed by PS and PK. The survey of the literature regarding clinical studies and part of writing was performed by KK and MW-Z. The part of the writing and revision of the manuscript was carried out by EC.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet 2007; 369: 1208–19. [DOI] [PubMed] [Google Scholar]
- 2.Ma TK, Kam KK, Yan BP, Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol 2010; 160: 1273–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrek L, Thor PJ. Future potential indications for pharmacotherapy using renin-angiotensin-aldosterone system inhibitory agents. Adv Clin Exp Med 2010; 19: 389–98. [Google Scholar]
- 4.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010; 10: 745–59. [DOI] [PubMed] [Google Scholar]
- 5.Parviz Y, Iqbal J, Pitt B, Adlam D, Al-Mohammad A, Zannad F. Emerging cardiovascular indications of mineralocorticoid receptor antagonists. Trends Endocrinol Metab 2015; 26: 201–11. [DOI] [PubMed] [Google Scholar]
- 6.Lakkis J, Lu WX, Weir MR. RAAS escape: a real clinical entity that may be important in the progression of cardiovascular and renal disease. Curr Hypertens Rep 2003; 5: 408–17. [DOI] [PubMed] [Google Scholar]
- 7.Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution? Expert Opin Pharmacother 2007; 8: 529–35. [DOI] [PubMed] [Google Scholar]
- 8.Desai A. Hyperkalemia associated with inhibitors of the renin-angiotensin-aldosterone system: balancing risk and benefit. Circulation 2008; 14: 1609–11. [DOI] [PubMed] [Google Scholar]
- 9.Moon JY. Recent update of renin-angiotensin-aldosterone system in the pathogenesis of hypertension. Electrolyte Blood Press 2013; 11: 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira AJ, Santos RA, Bradford CN, Mecca A, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the Ras in cardiovascular diseases. Hypertension 2010; 55: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenalrenin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–87. [DOI] [PubMed] [Google Scholar]
- 12.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000; 87: e1–e9. [DOI] [PubMed] [Google Scholar]
- 13.Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, Roman M, Ramirez C, Copaja M, Diaz-Araya G, Castro P, Lavandero S. Enalapril attenuates downregulation of angiotensin converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 2006; 48: 572–8. [DOI] [PubMed] [Google Scholar]
- 14.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarct by blockade of angiotensin II receptors. Hypertension 2004; 43: 970–6. [DOI] [PubMed] [Google Scholar]
- 15.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension 1998; 31: 699–705. [DOI] [PubMed] [Google Scholar]
- 16.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol 2014; 11: 413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bader M. ACE2, angiotensin-(1–7), and Mas: the other side of the coin. Pflugers Arch 2013; 465: 79–85. [DOI] [PubMed] [Google Scholar]
- 18.Santos RA, Frézard F, Ferreira AJ. Angiotensin-(1-7): blood, heart, and blood vessels. Curr Med Chem Cardiovasc Hematol Agents 2005; 3: 383–91. [DOI] [PubMed] [Google Scholar]
- 19.Oudit GY, Penninger JM. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep 2011; 8: 176–83. [DOI] [PubMed] [Google Scholar]
- 20.Santos SH, Fernandes LR, Mario EG, Ferreira AV, Pôrto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 2008; 57: 340–7. [DOI] [PubMed] [Google Scholar]
- 21.Santos SH, Braga JF, Mario EG, Pôrto LC, Rodrigues-Machado Mda G, Murari A, Botion LM, Alenina N, Bader M, Santos RA. Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler Thromb Vasc Biol 2010; 30: 953–61. [DOI] [PubMed] [Google Scholar]
- 22.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol 2009; 302: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. Angiotensin-(1-7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 2007; 292: 666–72. [DOI] [PubMed] [Google Scholar]
- 24.Singh K, Singh T, Sharma PL. Beneficial effects of angiotensin (1-7) in diabetic rats with cardiomyopathy. Ther Adv Cardiovasc Dis 2011; 5: 159–67. [DOI] [PubMed] [Google Scholar]
- 25.Akhtar S, Yousif MH, Dhaunsi GS, Chandrasekhar B, Al-Farsi O, Benter IF. Angiotensin-(1-7) inhibits epidermal growth factor receptor transactivation via a Mas receptor-dependent pathway. Br J Pharmacol 2012; 165: 1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol 2010; 638: 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iusuf D, Henning RH, van Gilst WH, Roks AJ. Angiotensin-(1-7): pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol 2008; 585: 303–12. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira AJ, Murça TM, Fraga-Silva RA, Castro CH, Raizada MK, Santos RA. New cardiovascular and pulmonary therapeutic strategies based on the angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor axis. Int J Hypertens 2012; 2012: 147825–147825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos RA, Ferreira AJ. Pharmacological effects of AVE 0991, a nonpeptide angiotensin-(1–7) receptor agonist. Cardiovasc Drug Rev 2006; 24: 239–46. [DOI] [PubMed] [Google Scholar]
- 30.Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension 2002; 40: 847–52. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira AJ, Jacoby BA, Araújo CA, Macedo FA, Silva GA, Almeida AP, Caliari MV, Santos RA. The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol 2007; 292: 1113–19. [DOI] [PubMed] [Google Scholar]
- 32.Ebermann L, Spillmann F, Sidiropoulos M, Escher F, Heringer-Walther S, Schultheiss HP, Tschöpe C, Walther T. The angiotensin-(1-7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol 2008; 590: 276–80. [DOI] [PubMed] [Google Scholar]
- 33.Toton-Zuranska J, Gajda M, Pyka-Fosciak G, Kus K, Pawlowska M, Niepsuj A, Wolkow P, Olszanecki R, Jawien J, Korbut R. AVE 0991-angiotensin-(1-7) receptor agonist, inhibits atherogenesis in apoE-knockout mice. J Physiol Pharmacol 2010; 61: 181–3. [PubMed] [Google Scholar]
- 34.Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1-7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol 2011; 301: 1341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng WT, Chen WY, Leng XY, Tang LL, Sun XT, Li CL, Dai G. Impairment of cardiac function and remodeling induced by myocardial infarction in rats are attenuated by the nonpeptide angiotensin-(1-7) analog AVE 0991. Cardiovasc Ther 2012; 30: 152–61. [DOI] [PubMed] [Google Scholar]
- 36.Barroso LC, Silveira KD, Lima CX, Borges V, Bader M, Rachid M, Santos RA, Souza DG, Simões E, Silva AC, Teixeira MM. Renoprotective effects of AVE0991, a nonpeptide mas receptor agonist, in experimental acute renal injury. Int J Hypertens 2012; 2012: 808726–808726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh K, Sharma K, Singh M, Sharma PL. Possible mechanism of the cardio-renal protective effects of AVE-0991, a non-peptide Mas-receptor agonist, in diabetic rats. J Renin Angiotensin Aldosterone Syst 2012; 13: 334–40. [DOI] [PubMed] [Google Scholar]
- 38.Jawien J, Toton-Zuranska J, Gajda M, Niepsuj A, Gebska A, Kus K, Suski M, Pyka-Fosciak G, Nowak B, Guzik TJ, Marcinkiewicz J, Olszanecki R, Korbut R. Angiotensin-(1-7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol 2012; 63: 77–85. [PubMed] [Google Scholar]
- 39.Singh Y, Singh K, Sharma PL. Effect of combination of renin inhibitor and Mas-receptor agonist in DOCA-salt-induced hypertension in rats. Mol Cell Biochem 2013; 373: 189–94. [DOI] [PubMed] [Google Scholar]
- 40.Cunha TM, Lima WG, Silva ME, Souza Santos RA, Campagnole-Santos MJ, Alzamora AC. The nonpeptide ANG-(1-7) mimic AVE 0991 attenuates cardiac remodeling and improves baroreflex sensitivity in renovascular hypertensive rats. Life Sci 2013; 92: 266–75. [DOI] [PubMed] [Google Scholar]
- 41.Savergnini SQ, Beiman M, Lautner RQ, de Paula-Carvalho V, Allahdadi K, Pessoa DC, Costa-Fraga FP, Fraga-Silva RA, Cojocaru G, Cohen Y, Bader M, de Almeida AP, Rotman G, Santos RA. al., Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension 2010;56:112–20. [DOI] [PubMed]
- 42.Savergnini SQ, Ianzer D, Carvalho MB, Ferreira AJ, Silva GA, Marques FD, Peluso AA, Beiman M, Cojocaru G, Cohen Y, Almeida AP, Rotman G, Santos RA. The novel Mas agonist, CGEN-856S, attenuates isoproterenol-induced cardiac remodeling and myocardial infarction injury in rats. PLoS One 2013; 8: e57757–e57757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balingit PP, Armstrong DG, Reyzelman AM, Bolton L, Verco SJ, Rodgers KE, Nigh KA, diZerega GS. NorLeu3-A(1-7) stimulation of diabetic foot ulcer healing: results of a randomized, parallel-group, double-blind, placebo-controlled phase 2 clinical trial. Wound Repair Regen 2012; 20: 482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bader M, Santos RA, Unger T, Steckelings UM. New therapeutic pathways in the RAS. J Renin Angiotensin Aldosterone Syst 2012; 13: 505–8. [DOI] [PubMed] [Google Scholar]
- 45.Rodgers KE, Bolton LL, Verco S, diZerega GS. NorLeu(3)-angiotensin (1-7) [DSC127] as a therapy for the healing of diabetic foot ulcers. Adv Wound Care 2015; 4: 339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-β signalling. Hum Mol Genet 2014; 23: 1237–49. [DOI] [PubMed] [Google Scholar]
- 47.Sabharwal R, Cicha MZ, Sinisterra RD, De Sousa FB, Santos RA, Chapleau MW. Chronic oral administration of Ang-(1-7) improves skeletal muscle, autonomic and locomotor phenotypes in muscular dystrophy. Clin Sci (Lond) 2014; 127: 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riquelme C, Acuna MJ, Torrejon J, Rebolledo D, Cabrera D, Santos RA, Brandan E. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS One 2014; 9: e93449–e93449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation 2003; 108: 1707–12. [DOI] [PubMed] [Google Scholar]
- 51.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RA, Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 2008; 52: 967–73. [DOI] [PubMed] [Google Scholar]
- 52.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 2010; 107: 888–97. [DOI] [PubMed] [Google Scholar]
- 53.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes 2010; 59: 2540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon JY, Jeong KH, Lee SH, Lee TW, Ihm CG, Lim SJ. Renal ACE and ACE2 expression in early diabetic rats. Nephron Exp Nephrol 2008; 110: 8–16. [DOI] [PubMed] [Google Scholar]
- 55.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 2007; 171: 438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH. FinnDiane Study Group. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 2012; 30: 375–83. [DOI] [PubMed] [Google Scholar]
- 57.Mizuir S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 2008; 51: 613–23. [DOI] [PubMed] [Google Scholar]
- 58.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 2010; 122: 717–28. [DOI] [PubMed] [Google Scholar]
- 59.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 2010; 59: 529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel VB, Takawale A, Ramprasath T, Das SK, Basu R, Grant MB, Hall DA, Kassiri Z, Oudit GY. Antagonism of angiotensin 1-7 prevents the therapeutic effects of recombinant human ACE2. J Mol Med (Berl) 2015; 93: 1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin HY, Chen LJ, Zhang ZZ, Xu YL, Song B, Xu R, Oudit GY, Gao PJ, Zhu DL, Zhong JC. Deletion of angiotensin-converting enzyme 2 exacerbates renal inflammation and injury in apolipoprotein E-deficient mice through modulation of the nephrin and TNF-alpha-TNFRSF1A signaling. J Transl Med 2015; 13: 255–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Wang SJ, Han ZH, Li YQ, Xue JH, Gao DF, Wu XS, Wang CX. PI3K/AKT signaling pathway plays a role in enhancement of eNOS activity by recombinant human angiotensin converting enzyme 2 in human umbilical vein endothelial cells. Int J Clin Exp Pathol 2014; 7: 8112–17. [PMC free article] [PubMed] [Google Scholar]
- 63.Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J, Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet 2013; 52: 783–92. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 2008; 51: 1312–17. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179: 1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fraga-Silva RA, Sorg BS, Wankhede M, Dedeugd C, Jun JY, Baker MB, Li Y, Castellano RK, Katovich MJ, Raizada MK, Ferreira AJ. ACE2 activation promotes antithrombotic activity. Mol Med 2010; 16: 210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferreira AJ, Shenoy V, Qi Y, Fraga-Silva RA, Santos RA, Katovich MJ, Raizada MK. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp Physiol 2011; 96: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murca TM, Almeida TC, Raizada MK, Ferreira AJ. Chronic activation of endogenous angiotensin-converting enzyme 2 protects diabetic rats from cardiovascular autonomic dysfunction. Exp Physiol 2012; 97: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shenoy V, Gjymishka A, Jarajapu YP, Qi Y, Afzal A, Rigatto K, Ferreira AJ, Fraga-Silva RA, Kearns P, Douglas JY, Agarwal D, Mubarak KK, Bradford C, Kennedy WR, Jun JY, Rathinasabapathy A, Bruce E, Gupta D, Cardounel AJ, Mocco J, Patel JM, Francis J, Grant MB, Katovich MJ, Raizada MK. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med 2013; 187: 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Maria ML, Araujo LD, Fraga-Silva RA, Pereira LA, Ribeiro HJ, Menezes GB, Shenoy V, Raizada MK, Ferreira AJ. Anti-hypertensive effects of diminazene aceturate: an angiotensin-converting enzyme 2 activator in rats. Protein Pept Lett 2016; 23: 9–16. [DOI] [PubMed] [Google Scholar]
- 71.Malek M, Nematbakhsh M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats. Adv Prev Med 2014; 2015: 740647–740647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraga-Silva RA, Costa-Fraga FP, Montecucco F, Sturny M, Faye Y, Mach F, Pelli G, Shenoy V, da Silva RF, Raizada MK, Santos RA, Stergiopulos N. Diminazene protects corpus cavernosum against hypercholesterolemia-induced injury. J Sex Med 2015; 12: 289–302. [DOI] [PubMed] [Google Scholar]
- 73.de Macedo SM, Guimarares TA, Andrade JM, Guimaraes AL, Batista de Paula AM, Ferreira AJ, Sousa Santos SH. Angiotensin converting enzyme 2 activator (DIZE) modulates metabolic profiles in mice, decreasing lipogenesis. Protein Pept Lett 2015; 22: 332–40. [DOI] [PubMed] [Google Scholar]
- 74.Patel BM, Mehta AA. Aldosterone and angiotensin: role in diabetes and cardiovascular diseases. Eur J Pharmacol 2012; 697: 1–12. [DOI] [PubMed] [Google Scholar]
- 75.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci 2002; 103: 425–31. [DOI] [PubMed] [Google Scholar]
- 76.Brown NJ, Nakamura S, Ma L, Nakamura I, Donnert E, Freeman M, Vaughan DE, Fogo AB. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 2000; 58: 1219–27. [DOI] [PubMed] [Google Scholar]
- 77.Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction with the rennin-angiotensin system. Am J Hypertens 2004; 17: 597–603. [PubMed] [Google Scholar]
- 78.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart. Role of oxidative stress. Am J Pathol 2002; 161: 1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. NEJM 1999; 341: 709–17. [DOI] [PubMed] [Google Scholar]
- 80.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. NEJM 2003; 348: 1309–21. [DOI] [PubMed] [Google Scholar]
- 81.Homma T, Fujisawa M, Arai K, Ishii M, Sada T, Ikeda M. Spironolactone, but not eplerenone, impairs glucose tolerance in a rat model of metabolic syndrome. J Vet Med Sci 2012; 74: 1015–22. [DOI] [PubMed] [Google Scholar]
- 82.Ahn JH, Hong HC, Cho MJ, Kim YJ, Choi HY, Eun CR, Yang SJ, Yoo HJ, Kim HY, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Kim NH. Effect of eplerenone, a selective aldosterone blocker, on the development of diabetic nephropathy in type 2 diabetic rats. Diabetes Metab J 2012; 36: 128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramírez E, Klett-Mingo M, Ares-Carrasco S, Picatoste B, Ferrarini A, Rupérez FJ, Caro-Vadillo A, Barbas C, Egido J, Tuñón J, Lorenzo Ó. Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc Diabetol 2013; 12: 172–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bodary PF, Sambaziotis C, Wickenheiser KJ, Rajagopalan S, Pitt B, Eitzman DT. Aldosterone promotes thrombosis formation after arterial injury in mice. Arterioscler Thromb Vasc Biol 2006; 26: 233–233. [DOI] [PubMed] [Google Scholar]
- 85.Chander PN, Rocha R, Ranaudo J, Singh G, Zuckerman A, Stier CT., Jr Aldosterone plays a pivotal role in the pathogenesis of thrombotic microangiopathy in SHRSP. J Am Soc Nephrol 2003; 14: 1990–7. [DOI] [PubMed] [Google Scholar]
- 86.Stankiewicz A, Gromotowicz A, Szemraj J, Wojewodzka-Zelezniakowicz M, Skrzypkowski P, Chabielska E. Acute aldosterone infusion enhances thrombosis development in normotensive rats. Thromb Haemost 2007; 98: 697–9. [PubMed] [Google Scholar]
- 87.Zakrzeska A, Gromotowicz-Popławska A, Szemraj J, Szoka P, Kisiel W, Purta T, Kasacka I, Chabielska E. Eplerenone reduces arterial thrombosis in diabetic rats. J Renin Angiotensin Aldosterone Syst 2015; 16: 1085–94. [DOI] [PubMed] [Google Scholar]
- 88.Gromotowicz A, Szemraj J, Stankiewicz A, Zakrzeska A, Mantur M, Jaroszewicz E, Rogowski F, Chabielska E. Study of the mechanisms of aldosterone prothrombotic effect in rats. J Renin Angiotensin Aldosterone Syst 2011; 12: 430–9. [DOI] [PubMed] [Google Scholar]
- 89.Szoka P, Zakrzeska A, Bycul U, Kisiel W, Kasacka I, Chabielska E. Adrenal gland-dependent process of arterial thrombus formation in diabetic rats. Thrombosis Res 2012; 130: 136–136. [Google Scholar]
- 90.Gromotowicz-Poplawska A, Stankiewicz A, Kramkowski K, Gradzka A, Wojewodzka-Zelezniakowicz M, Dzieciol J, Szemraj J, Chabielska E. The acute prothrombotic effect of aldosterone in rats is partially mediated via angiotensin II receptor type 1. Thromb Res 2016; 138: 114–20. [DOI] [PubMed] [Google Scholar]
- 91.Dooley R, Harvey BJ, Thomas W. Non-genomic actions of aldosterone: from receptors and signals to membrane targets. Mol Cell Endocrinol 2012; 350: 223–34. [DOI] [PubMed] [Google Scholar]
- 92.Krug AW, Pojoga LH, Williams GH, Adler GK. Cell membrane-associated mineralocorticoid receptors? New evidence. Hypertension 2011; 57: 1019–25. [DOI] [PubMed] [Google Scholar]
- 93.Rossier MF, Python M, Maturana AD. Contribution of mineralocorticoid and glucocorticoid receptors to the chronotropic and hypertrophic actions of aldosterone in neonatal rat ventricular myocytes. Endocrinology 2010; 151: 2777–87. [DOI] [PubMed] [Google Scholar]
- 94.Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol 2013; 304: C532–40. [DOI] [PubMed] [Google Scholar]
- 95.Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev 2005; 10: 23–9. [DOI] [PubMed] [Google Scholar]
- 96.Ravis WR, Reid S, Sica DA, Tolbert DS. Pharmacokinetics of eplerenone after single and multiple dosing in subjects with and without renal impairment. J Clin Pharmacol 2005; 45: 810–21. [DOI] [PubMed] [Google Scholar]
- 97.Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7: 51–8. [DOI] [PubMed] [Google Scholar]
- 98.Liu LC, Schutte E, Gansevoort RT, van der Meer P, Voors AA. Finerenone: third-generation mineralocorticoid receptor antagonist for the treatment of heart failure and diabetic kidney disease. Expert Opin Investig Drugs 2015; 24: 1123–35. [DOI] [PubMed] [Google Scholar]
- 99.Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Pérez S, Heckroth H, Nitsche A, Ergüden JK, Gielen-Haertwig H, Schlemmer KH, Mittendorf J, Paulsen H, Platzek J, Kolkhof P. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem 2012; 7: 1385–403. [DOI] [PubMed] [Google Scholar]
- 100.Lentini S, Heinig R, Kimmeskamp-Kirschbaum N, Wensing G. Pharmacokinetics, safety and tolerability of the novel, selective mineralocorticoid receptor antagonist finerenone—results from first-in-man and relative bioavailability studies. Fundam Clin Pharmacol 2016;30:172–84. [DOI] [PubMed]
- 101.Bramlage P, Swift SL, Thoenes M, Minguet J, Ferrero C, Schmieder RE. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail 2016; 18: 28–37. [DOI] [PubMed] [Google Scholar]
- 102.Kolkhof P, Kretschmer A, Baerfacker L, Hartmann E, Schaefer S. Improved survival and nephroprotection in hypertensive rats by BAY 94-8862, a novel non-steroidal mineralocorticoid receptor antagonist. Eur Heart J 2012; 33(Suppl): 978–9. [Google Scholar]
- 103.Delbeck M, Kretschmer A, Kast R, Baerfacker L, Hartmann E, Schaefer S, Kolkhof P. Cardiorenal protection by BAY 94-8862, a novel mineralocorticoid receptor antagonist in a preclinical model of hypertension and diastolic heart failure. Eur Heart J 2012; 33(Suppl): 772–3. [Google Scholar]
- 104.Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, Eitner F, Albrecht-Küpper B, Schäfer S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 2014; 64: 69–78. [DOI] [PubMed] [Google Scholar]
- 105.Pitt B, Filippatos G, Gheorghiade M, Kober L, Krum H, Ponikowski P, Nowack C, Kolkhof P, Kim SY, Zannad F. Rationale and design of ARTS: a randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail 2012; 14: 668–75. [DOI] [PubMed] [Google Scholar]
- 106.Ruilope LM, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Ferreira AC, Pieper A, Kimmeskamp-Kirschbaum N, Bakris GL. Rationale, design, and baseline characteristics of ARTS-DN: a randomized study to assess the safety and efficacy of finerenone in patients with type 2 diabetes mellitus and a clinical diagnosis of diabetic nephropathy. Am J Nephrol 2014; 40: 572–81. [DOI] [PubMed] [Google Scholar]
- 107.Pitt B, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Nowack C, Kim SY, Pieper A, Kimmeskamp-Kirschbaum N, Filippatos G. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail 2015; 17: 224–32. [DOI] [PubMed] [Google Scholar]
- 108.Fuller PJ. Novel interactions of the mineralocorticoid receptor. Mol Cell Endocrinol 2015; 408: 33–7. [DOI] [PubMed] [Google Scholar]
- 109.Takeda Y. Vascular synthesis of aldosterone: role in hypertension. Mol Cell Endocrinol 2004; 217: 75–9. [DOI] [PubMed] [Google Scholar]
- 110.Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation 2005; 111: 3087–94. [DOI] [PubMed] [Google Scholar]
- 111.Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, Park HW. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol Endocrinol 2013; 27: 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bramlage P, Turgonyi E, Montalescot G. Aldosterone blockade: current research and future trends. Eur Heart J 2011; 13: B46–50. [Google Scholar]
- 113.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol 2008; 93: 817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang BS, White RA, Ahmad M, Jeng AY, Leenen FH. Central infusion of aldosterone synthase inhibitor prevents sympathetic hyperactivity and hypertension by central Na+ in Wistar rats. Am J Physiol Regul Integr Comp Physiol 2008; 295: R166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mulder P, Mellin V, Favre J, Vercauteren M, Remy-Jouet I, Monteil C, Richard V, Renet S, Henry JP, Jeng AY, Webb RL, Thuillez C. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur Heart J 2008; 29: 2171–9. [DOI] [PubMed] [Google Scholar]
- 116.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int 2009; 75: 936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang BS, White RA, Jeng AY, Leenen FH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2009; 296: R994–R1000. [DOI] [PubMed] [Google Scholar]
- 118.Huang BS, White RA, Ahmad M, Tan J, Jeng AY, Leenen FH. Central infusion of aldosterone synthase inhibitor attenuates left ventricular dysfunction and remodelling in rats after myocardial infarction. Cardiovasc Res 2009; 81: 574–81. [DOI] [PubMed] [Google Scholar]
- 119.Gomez-Sanchez EP, Gomez-Sanchez CM, Plonczynski M, Gomez-Sanchez CE. Aldosterone synthesis in the brain contributes to Dahl salt-sensitive rat hypertension. Exp Physiol 2010; 95: 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gamliel-Lazarovich A, Gantman A, Coleman R, Jeng AY, Kaplan M, Keidar S. FAD286, an aldosterone synthase inhibitor, reduced atherosclerosis and inflammation in apolipoprotein E-deficient mice. J Hypertens 2010; 28: 1900–7. [DOI] [PubMed] [Google Scholar]
- 121.Deliyanti D, Miller AG, Tan G, Binger KJ, Samson AL, Wilkinson-Berka JL. Neovascularization is attenuated with aldosterone synthase inhibition in rats with retinopathy. Hypertension 2012; 59: 607–13. [DOI] [PubMed] [Google Scholar]
- 122.Azizi M, Amar L, Menard J. Aldosterone synthase inhibition in humans. Nephrol Dial Transplant 2013; 28: 36–43. [DOI] [PubMed] [Google Scholar]
- 123.Amar L, Azizi M, Menard J, Peyrard S, Watson C, Plouin PF. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension 2010; 56: 831–8. [DOI] [PubMed] [Google Scholar]
- 124.Calhoun DA, White WB, Krum H, Guo W, Bermann G, Trapani A, Lefkowitz MP, Ménard J. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation 2011; 124: 1945–55. [DOI] [PubMed] [Google Scholar]
- 125.Bertagna X, Pivonello R, Fleseriu M, Zhang Y, Robinson P, Taylor A, Watson CE, Maldonado M, Hamrahian AH, Boscaro M, Biller B. LCI699, a potent 11β-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing’s disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab 2014; 99: 1375–83. [DOI] [PubMed] [Google Scholar]
- 126.Andersen K, Hartman D, Peppard T, Hermann D, Van Ess P, Lefkowitz M, Trapani A. The effects of aldosterone synthase inhibition on aldosterone and cortisol in patients with hypertension: a phase II, randomized, double-blind, placebo-controlled, multicenter study. J Clin Hypertens 2012; 14: 580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amar L, Azizi M, Menard J, Peyrard S, Plouin PF. Sequential comparison of aldosterone synthase inhibition and mineralocorticoid blockade in patients with primary aldosteronism. J Hypertens 2013; 31: 624–9. [DOI] [PubMed] [Google Scholar]
- 128.Hakki T, Hübel K, Waldmann H, Bernhardt R. The development of a whole-cell based medium throughput screening system for the discovery of human aldosterone synthase (CYP11B2) inhibitors: old drugs disclose new applications for the therapy of congestive heart failure, myocardial fibrosis and hypertension. J Steroid Biochem Mol Biol 2011; 125: 120–8. [DOI] [PubMed] [Google Scholar]
- 129.Unger T, Paulis L, Sica DA. Therapeutic perspectives in hypertension: novel means for renin–angiotensin–aldosterone system modulation and emerging device-based approaches. Eur Heart J 2011; 32: 2739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chrysant SG, Chrysant GS. Dual renin-angiotensin-aldosterone blockade: promises and pitfalls. Curr Hypertens Rep 2015; 17: 511–511. [DOI] [PubMed] [Google Scholar]
- 131.Xie W, Zheng F, Song X, Zhong B, Yan L. Renin-angiotensin-aldosterone system blockers for heart failure with reduced ejection fraction or left ventricular dysfunction: network meta-analysis. Int J Cardiol 2016; 205: 65–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.