Abstract
Enrofloxacin (ENX) has been widely used in the prevention and control of bacterial diseases in sturgeon aquaculture due to its characteristics of wide antibacterial spectrum, strong antibacterial activity, less toxicity and fewer side effects, rapid action, extensive in vivo distribution, and little cross-resistance with other antibiotics. However, the spinal abnormality was found in Acipenser baerii soon after ENX administration, which resulted an “S”-shaped curvature of the spine and retarded fish growth. It was still not clear whether ENX could cause spinal abnormality in sturgeons by now. The aim of this work was to determine the accumulation rule and toxicity of ENX to A. baerii when used at a high dose and/or unusually long durations. Here, ENX was orally given to A. baerii for 3–5 d continuously at the dosage of 0, 20, 40, and 80 mg/kg once daily, respectively. The accumulation of ENX in blood, liver, kidney, and cartilage was detected after withdrawal, and the tissues were made into sections for morphological examination. The results showed that the levels of ENX increased in the four tissues with the increase of dose and duration, and the ENX level in serum was far lower than that in other tissues. At 240 h, ENX levels in the four tissues decreased significantly. The histology indicated that the liver, kidney, and cartilage began to show structural damages at 5 d after withdrawal of 40 mg/kg ENX. The damage was aggravated at 3–5 d after withdrawal of 80 mg/kg ENX. At 240 h, the damaged tissues showed signs of recovery. These results suggested that ENX should be no more than 40 mg/kg and that exposure time should not be greater than 5 d to prevent liver, kidney, and cartilage damage. More attention should be paid to the impact of ENX on the occurrence and development of chondrocytes in juvenile A. baerii and the potential damage to the cartilage.
Keywords: Enrofloxacin, Acipenser baerii, rule of accumulation, toxicity
Introduction
Enrofloxacin (ENX) is the third-generation fluoroquinolone with the molecular formula of C19H22FN3O3. It was first invented by Bayer Corporation and introduced to the market in 1987. The Food and Drug Administration approved ENX for use as a specialized antibacterial drug in livestock and poultry in October 1996.1 Possessing a wide antibacterial spectrum, strong antibacterial activity, less toxicity and fewer side effects, rapid action, extensive in vivo distribution, and little cross-resistance with other antibiotics,2 ENX is gaining popularity in the control and treatment of aquaculture diseases.3–5
Sturgeons (genus Acipenser) are one of the earliest vertebrate communities to evolve and are now included in the Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). Providing valuable materials for the study in the origin and evolution of species,6 sturgeons are a transitional species between cartilaginous fish and bony fish,7 and thus have a high ecological value. Acipenser baerii belongs to family Acipenseridae, order Acipenseriformes, subclass Chondrostei, and class Actinopterygii. After its first introduction into China in 1996, A. baerii has become a leading species of China’s sturgeon breeding industry due to its fast growth, strong adaptability, easy domestication, and the high quality of its caviar.8 Aquatic bacteria are highly sensitive to ENX,9 which is commonly used in the control and treatment of bacterial diseases in sturgeons. However, ENX can cause spinal abnormality in some aquacultured fish, and the resulting “S”-shaped curvature of the spine retards growth. It is not yet certain whether ENX is teratogenic when used at a high dose and/or unusually long durations. Moreover, there are few studies based on the cartilage sections of sturgeons10 or reports on the cartilage damage and repair mechanism.
We performed oral gavage of ENX at different doses and for different durations in A. baerii and measured the drug’s accumulation in liver, kidney, and cartilage tissues. The rule of accumulation and toxicity of ENX in A. baerii was studied, thereby providing reference for the reasonable use of ENX in the control and prevention of diseases in sturgeons.
Materials and methods
Experimental sturgeons and reagents
Healthy, non-immunized A. baerii (150 ± 8.47 g) were purchased from Technological and Engineering Center of Sturgeon’s Reproduction, Chinese Academy of Fishery Sciences. The experiment was carried out in a fish tank with automatic cycling, and the temperature was maintained at 22 ± 0.5℃. Oxygen was continuously supplied throughout the experiment.
ENX standard (content 99.5%, batch number Cl5648000) was purchased from China Institute of Veterinary Drugs Control; ENX powder (content 98%, batch number: 20080418) was purchased from Henan Huijie Kechuang Animal Pharmacy Co., Ltd; acetonitrile and normal hexane (Merck, Germany) were chromatographically pure, and all other reagents were analytically pure (China).
Experimental design and sample collection
Eighty A. baerii were randomly divided into eight groups of 10 fish. According to the Updated Handbook of Fishery Medicine,1 control, low-dose, medium-dose, and high-dose groups were set up, and continuous oral gavage was performed at the dose of 0, 20, 40, and 80 mg/kg, respectively. The groups treated for 3 d were designated as groups A, B, C, and D, and those treated for 5 d were designated as group E, F, G, and H, respectively. Group A and Group E were control groups. The blood was sampled using a syringe from the tail vein, and the liver, kidney, and cartilage tissues were simultaneously sampled at 24 h and 240 h after discontinuation, respectively. The blood samples were subjected to centrifugation at 4000 rpm for 10 min at 4℃, and the supernatant was collected and analyzed.
Conditions of high performance liquid chromotography and sample treatment
Agilent 1100 Series high performance liquid chromotography (HPLC) System (Agilent, USA) was used. The contents of ENX in different tissues were determined based on the method established by Si et al.10
Tissue section preparation
Three individuals were selected randomly in each group, and the liver, kidney, and cartilage tissues were harvested. The tissues were fixed in Bouin’s solution for 24 h and then transferred to 70% alcohol. The specimens were subjected to dehydration, clearing, paraffin embedding, and serial sectioning to the thickness of 5 µm. After HE staining, the specimens were imaged with a CCD camera connected to a Leica 4000B microscope.
Results and analysis
Behavioral changes
Groups A, B, C, E, and F showed no abnormal swimming, feeding, or body color. However, the individuals in groups D and H showed black body color, slow swimming, and aggregation at the bottom of the fish tank 3 d after continuous administration. No animals died during this experiment.
Accumulation of ENX in the tissues
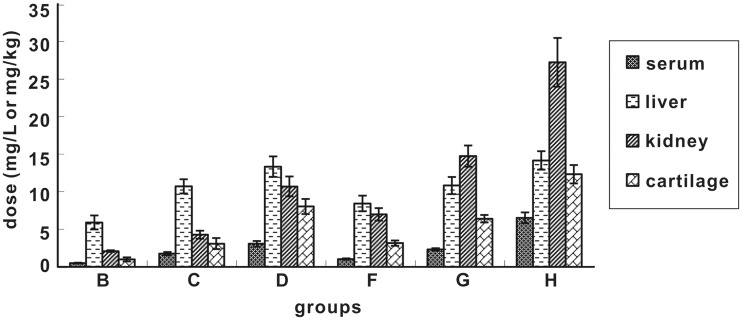
The serum, liver, kidney, and cartilage tissues were pre-treated following Si’s method10 and then analyzed with HPLC. No ENX was detected in any of the four tissues in the control group. The detection results of each group are shown in the figure below.
As seen from Figure 1, at 24 h after discontinuation, ENX level in the four tissues increased with the increase of dose and duration. The accumulation in the liver, kidney, and cartilage was obviously higher than that in the serum. In groups B, C, D, and F, the drug level in the liver was higher than that in the kidney, while in groups G and H, the drug level in the kidney was higher than that in the liver. Under the same dose and duration (B/F group, C/G group, and D/H group), the ENX accumulation in the serum, liver, and cartilage did not differ significantly. However, the accumulation in the kidney was significantly higher after five consecutive administrations than that after three consecutive administrations.
Figure 1.
Accumulation of ENX in different tissues at 24 h after drug discontinuation
Two-factor (dose and duration) analysis of variance showed that dose and duration had a significant impact on ENX accumulation in the cartilage, with P value of 0.040 and 0.041, respectively. However, the effect of two factors to ENX accumulation was apparently greater in the kidney, with P value of 0.0029 and 0.0038 (P < 0.01), respectively.
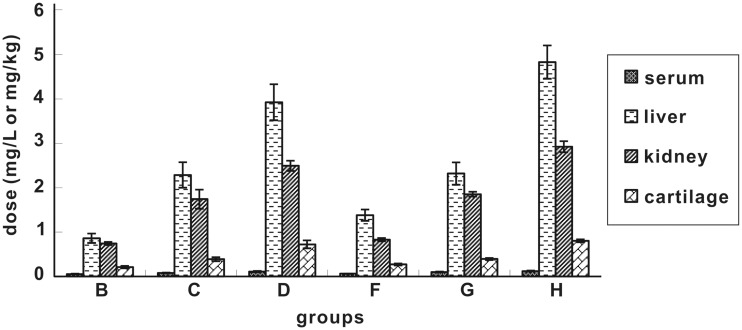
As seen from Figure 2, ENX level in all four tissues showed a tendency of liver> kidney>cartilage>serum at 240 h after discontinuation. Under the same dose and duration (B/F group, C/G group, and D/H group), the ENX level in four tissues did not show significant differences at 240 h after discontinuation. However, the differences in ENX level were significant under different doses but the same duration at 240 h after discontinuation.
Figure 2.
Accumulation of ENX in different tissues at 240 h after drug discontinuation
Morphological observations
Liver
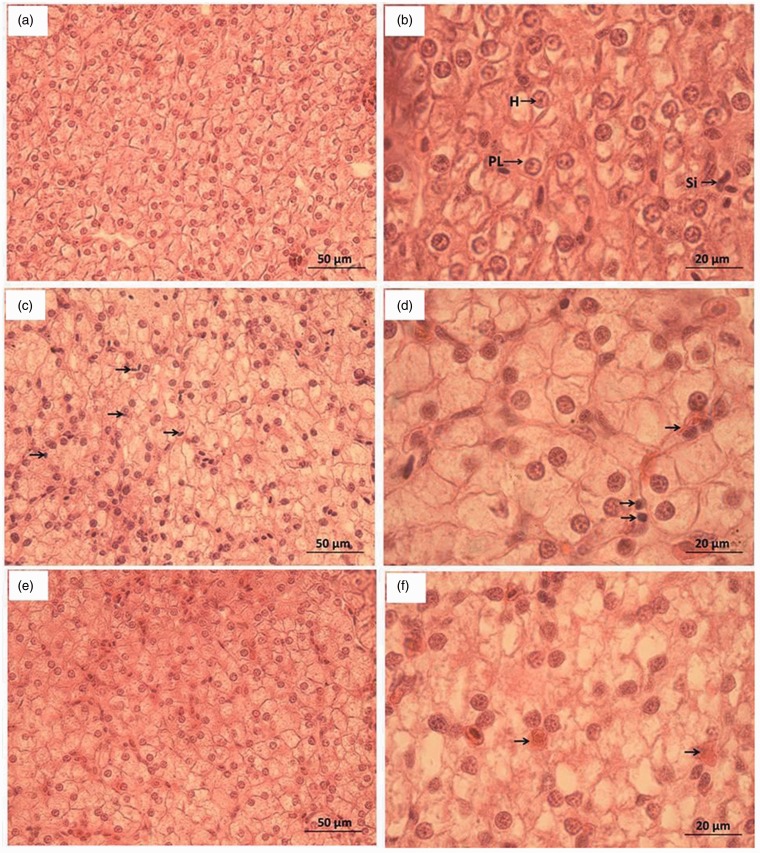
The liver tissue sections were prepared and observed as shown in Figure 3. In the control group (Figure 3(a) and (b)), the liver cells were compact and had a clear structure, intact hepatic cord and hepatic sinusoid. The liver cells were arranged regularly, with nearly round nuclei and abundant cytoplasm. At 24 h after discontinuation, the morphology of liver cells in groups B, C, F, and G was similar to that of the control group. In groups D and H, infiltration by blood cells was observed (Figure 3(c)). Some cells were atrophic and apoptotic at high magnification (Figure 3(d)). At 240 h, the damaged liver cells in groups D and H showed signs of recovery (Figure 3(e) and (f)). The liver cells were uniformly distributed with abundant cytoplasm. Pyknosis and apoptosis were not seen, and the infiltrating blood cells were dissolved and absorbed.
Figure 3.
Morphological observation of liver tissue sections H: Liver cells; PL: Liver cell plate; Si: Sinusoid. (a) Control group (×400), (b) Control group (×1000), (c) Groups D and H at 24 h after discontinuation (×400), (d) Groups D and H at 24 h after discontinuation (×1000), (e) Groups D and H at 240 h after discontinuation (×400); and (f) Groups D and H at 240 h after discontinuation (×1000). (A color version of this figure is available in the online journal.)
Kidney
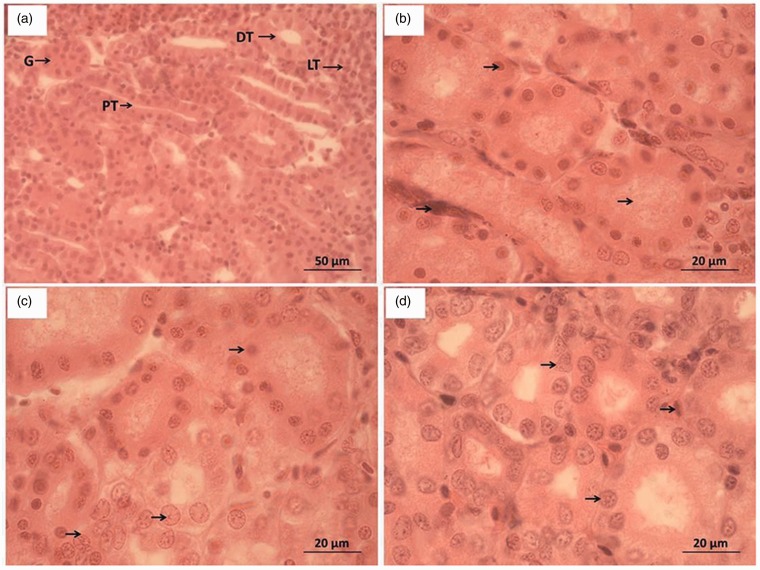
The kidney tissue sections were prepared and observed. As shown in Figure 4, the kidney cells had intact structure in the control group (Figure 4(a)), with clearly visualized renal tubules and compact cells of tubule wall. At 24 h after discontinuation, the morphological observation in groups B, C, F and G was similar to that of the control; for group D, the cells of tubule wall underwent pyknosis and deformation, showing sparse arrangement and lumens filled with particles (Figure 4(b)); for group H, the renal tubuli were indistinct, the cells of tubule wall underwent pyknosis, and the glomeruli were swollen. At 240 h, the damaged kidney cells in groups D and H (Figure 4(d)) showed signs of recovery, and the cells of tubule wall restored normal morphology. However, some blood cells and pyknotic kidney cells remained unabsorbed.
Figure 4.
Morphological observation of kidney tissue sections PT: Proximal convoluted tubules; DT: Distal convoluted tubules; LT: Lymphoid tissues; G: Glomeruli. (a) Control group (×400), (b) Group D at 24 h after discontinuation (×1000), (c) Group H at 24 h after discontinuation (×1000); and (d) Groups D and H at 240 h after discontinuation (×1000). (A color version of this figure is available in the online journal.)
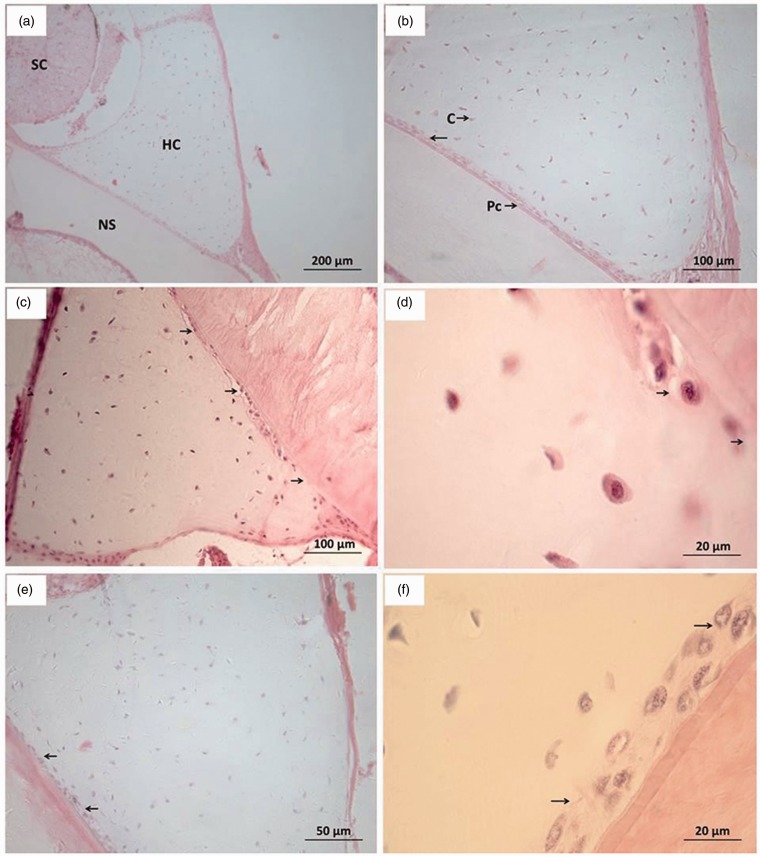
Cartilage
The cartilage tissue sections were prepared and observed as shown in Figure 5. In the control group (Figure 5(a)), intact spinal cord, notochordal sheath, and cartilage were seen. The chondrocytes of the perichondrium (Figure 5(b)) were mostly flat and in compact arrangement. The chondrocytes of the inner layer were larger and elliptical or falcate in shape (probably the result of lateral sectioning). The chondrocytes were located in the recesses, which were quite large. Each recess contained only one chondrocyte, and the cartilage matrix was uniform and abundant. At 24 h after discontinuation, the morphological observation of groups B, C, F and G was similar to that of the control; groups D and H had consistent morphological changes of the cartilage (Figure 5(c) and (d)). The flat cells in the inner layer of the perichondrium decreased, the cells were swollen and existed in a monolayer; some chondrocytes attached to the perichondrium disappeared. At 240 h, the damaged cartilage in groups D and H showed recovery. The flat cells in the inner layer of the perichondrium increased, and the cell layer was thickened, but there were still gaps left unfilled by cells.
Figure 5.
Morphological observation of cartilage tissue sections NS: Notochordal sheath; SC: Spinal cord; HS: Cartilage; PC: Perichondrium; C: Chondrocytes. (a) Control group (×100), (b) Control group (×200), (c) Groups D and H at 24 h after discontinuation (×200), (d) Groups D and H at 24 h after discontinuation (×1000), (e) Groups D and H at 240 h after discontinuation (×400); and (f) Groups D and H at 240 h after discontinuation (×1000). (A color version of this figure is available in the online journal.)
Discussion
Influence of different medication schemes on the ENX accumulation in different tissues of sturgeons
Types, dose, and route of drug administration, as well as temperature, influence drug utilization.11 Under low water temperature 10℃, ENX can maintain a high biological utilization rate.12 Juvenile Salmo salar fed with 10 mg/kg and 5 mg/kg ENX by gavage did not show significant differences in drug accumulation in different tissues.13 However, the biological utilization rate of 53% in Oncorhynchus mykiss after oral gavage of 5 mg/kg ENX at 15℃ was far higher than that (9%) in O. mykiss after oral gavage of 50 mg/kg ENX at 10℃.14 Intraperitoneal injection of ENX usually resulted in a fast absorption, shorter time interval to peak concentration and a higher concentration compared with oral gavage.15 The safe dose of ENX for sturgeons (159 mg/kg)16 is similar to that for Cyprinus carpio var. specularis (155 mg/L)17 and Carassius auratus gibelio (194.98 mg/kg)18 but is far higher than that for Poecilia reticulata (25 mg/L).19 These results may be explained by the difference in water temperature.
Taking 20 mg/kg as low dose according to the manual of fish medicine,1 the medium and high doses were set at 40 and 80 mg/kg, respectively. Different medication schemes of ENX were adopted with different combinations of dose (0, 20, 40, 80 mg/kg) and duration (3 d, 5 d). The ENX accumulation in serum, liver, kidney, and cartilage of A. baerii at 24 h and 240 h after oral gavage was detected. Liver>kidney>cartilage>serum in terms of ENX concentration in all other groups, except that the highest drug concentration was achieved in the kidney tissues in groups G and H at 24 h after discontinuation. The ENX concentrations in tissues were higher than that in the serum, consistent with the detection in hybrid sturgeons after oral gavage of ENX.20 ENX administered orally enters the blood via intestinal absorption and then goes to various tissues. ENX is mainly metabolized in liver21 and excreted after passing through the kidney. At 24 h after discontinuation, groups G and H had a higher drug concentration in the kidney than in the liver. This was probably because continuous administration at medium and high dose overburdened the kidney, leading to tissue damage and dysfunction. Drug discharge was affected, and the accumulation in the tissues subsequently increased.
ENX accumulation in serum, liver, kidney, and cartilage of A. baerii increased with the increasing dose and greater duration, but significant dose proportionality was not observed.22 However, we found that there was significant difference of the drug level in the kidney between Group G and Group C, Groups H and D at 24 h after discontinuation. Wang et al.23 also reported that florfenicol accumulation increased with the increase of dosage at the same time in blood while the increase amplitude decreased, which indicated the decreased utilization of drug to the increasing dosage in Tilapia. Two-factor analysis of variance indicated that dose and duration had a significant impact and highly significant effect on cartilage and kidney tissues. Combining with morphological observation, administrations for three times at high dose or for five times at medium and high dose both damaged the kidney and cartilage tissues.
Drug-induced damage and recovery in three tissues of A. baerii
With the increase of dose and duration, damages occurred to liver, kidney, and cartilage of A. baerii in groups treated with medium and high dose. The damaged tissues began to recover at 240 h after continuation.
Zhao et al.18 pointed out that ENX given at conventional dosages (20 mg/kg) would not cause liver damage in allogenetic Carassius auratus gibelio, which agreed with the observation of the present study in the low-dose group. Zhu et al. monitored two sensitive indicators of liver cell damage, glutamic-pyruvic transaminase and glutamic oxaloacetic transaminase activity. They found that at twice the conventional dose, ENX greatly affected the contents of these two enzymes in carp (Triploid crucian), causing liver damage.24 Zhou et al.25 also found that ENX injection at 150 mg/kg inhibited activity of phase I drug-metabolizing enzymes in the liver tissues of Pelodiscus sinensis. The present study also indicated that repeated administrations at medium and high dose caused certain damage to the liver tissues of A. baerii.
Continuous administrations at medium dose and high dose both caused damage to the kidney tissues at 24 h after discontinuation, which was probably related to the high ENX level in the kidney. As ENX was excreted, the ENX level in the tissues decreased dramatically, and the damaged tissues were recovered.
According to some reports, fluoroquinolone residues are mainly found in the skin and bones of fish26 causing considerable damage to articular cartilage and bones of young animals.27 The mechanism of damage induced by fluoroquinolones to cartilage is unclear. ENX may chelate Ca2+ and Mg2+, which would inhibit the synthesis and increases the decomposition of cartilage matrix components such as proteoglycans and collagen. This will result in damage to DNA structure, chondrocyte apoptosis, and active oxygen production.28 Wu et al. believed that the toxicity of fluoroquinolones to cartilage was related to the pharmacokinetic features of fish belonging to a specific species and age. The drug content in the target organ needs to be considered as well, and the drug content in cartilage should be detected as an important indicator of cartilage toxicity.29 We found that the ENX accumulation in the cartilage of A. baerii was always higher than the plasma concentration. Repeated administrations at medium dose (group H) and administration at high dose (groups D and G) all affected the occurrence and development of cartilage. As the drug accumulation decreased at 240 h after discontinuation, the tissues began to recover with more chondrocytes in the inner layer of perichondrium and thickening of the cell layer. Juvenile A. baerii weighing 150 g was studied in the present paper, and cartilage toxicity in various growth stages of A. baerii needs to be further investigated.
ENX undergoes de-ethylation in the liver, giving rise to ciprofloxacin. Ciprofloxacin acts in synergy with the unchanged drug ENX.30 However, ciprofloxacin causes damage to fetal articular cartilage31 and cartilage of young animals.32 Whether the toxicity of ciprofloxacin should be taken into account in the evaluation of cartilage toxicity of ENX remains to be discussed.
Suggestions about the use of ENX in sturgeon breeding
Continuous administrations of ENX at conventional dose (20 mg/kg) through oral gavage in A. baerii for three to five times did not cause significant damage to the tissues. However, administrations at medium and high dose (40 and 80 mg/kg) led to liver damage, which was recovered to a certain extent after drug discontinuation. Therefore, ENX should be given at conventional doses and not continuously in sturgeon breeding, according to the instructions. Long-term accumulation of drug residues can lead to retarded development or even malformation of the cartilage tissues. In order to avoid adverse impact on the cartilage development of juveniles, withdrawal should be strictly implemented.
Animal cartilage is increasingly used in healthcare products due to its anti-cancer component chondroitin sulfate. Although livestock and sharks are the main sources of cartilage, cartilage production from livestock is low,33 and the capture of sharks is restricted by natural availability and seasonal reasons.34 Sturgeons are the preferred choice for the production of cartilage because of the high yield, its rich nutrients, and the ease of artificial breeding. A strict control of drug residues in the cartilage and an exact understanding of the metabolic and residue parameters in sturgeons are important for the healthy development of the sturgeon cartilage production industry. Some reports suggest that flowing water can accelerate the clearing of ENX residues in the sturgeon.35 Kidney-tonifying traditional Chinese medicine has the effect of improving the robustness and growth of bones and cartilage.36 Therefore, ENX application can be combined with the stimulus from flowing water and traditional Chinese medicine to accelerate drug metabolism, alleviate damage, promote chondrocyte differentiation and repair, and guarantee food safety.
Acknowledgment
This work was supported by grants from National “Twelfth Five-Year” Plan for Science & Technology (2012BAD25B10) and the Central-Level Non-profit Scientific Research Institutes Special Funds (HSY201311).
Authors’ contributions
DW and TL designed the experiments; DW and SL carried out experiments; DW analyzed experimental results. SL assisted with analysis of histology observation results. DW and SL wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yang XL, Lu CP, Zhan WB. Newly compiled fishery drugs manuals, Beijing: China Agriculture Press, 2005. [Google Scholar]
- 2.Dowling PM, Wilson RC, Tyler JW, Duran SH. Pharmacokinetics of ciprofloxacin in ponies. J Vet Pharmacol Therapeut 1995; 18: 7–12. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Jin S, Yu K. Pharmacodynamics effect of enrofloxacin on four aquatic pathogenic vibrio. Microbiol China 2011; 38: 1216–21. [Google Scholar]
- 4.Wang XD, Chen H, Sha S. Study on curative effect of enrofloxacin compound with pu powder to bacterial septicemia of Carassius Auratus Gibelio. J Nanjing Normal Univ (Natural Science Edition) 2011; 34: 86–8, 95. [Google Scholar]
- 5.Wang ZQ, Zhu L. Antibacterial activities of fluoroquinolones for animals against Aeromonas hydrophila and Aeromonas sobria. J Tradit Chin Vet Med 2005; 2: 34–6. [Google Scholar]
- 6.Song W, Song JK. Observations on morphology of post-embryonic development and histology of sensory organs in larval and juvenile Siberian sturgeon Acipenser baerii. J Fish Sci China 2012; 19: 790–8. [Google Scholar]
- 7.Chen XH. The status of Acipenseriformes biology and resource, Beijing: Ocean Press, 2007. [Google Scholar]
- 8.Deng ML, Geng Y, Liu D. Isolation identification and detection of virulence genes of Streptococcus iniae from Acipenser baerii. J Fish China 2015; 39: 127–35. [Google Scholar]
- 9.Li SW, Wang D, Liu HB. Molecular characterization of integrin-gene cassettes in multi-drug resistant Aeromonas hydrophila from fish. J Fish Sci China 2013; 20: 1015–22. [Google Scholar]
- 10.Si L, Chen C, Li SW. Pharmacokinetics of enrofloxacin in golden rainbow trout and Hucho taimen. Jiangsu Agr Sci 2011; 39: 390–2. [Google Scholar]
- 11.Kang T, Gheng B, Wang Q. Review on pharmacokinetic study of quinolones in aquatic animals. Chin Fish Qual Stand 2015; 5: 13–9. [Google Scholar]
- 12.Martinsen B, Horsberg TE. Comparative single-dose pharmacokinetics of four quinolones, oxolinic acid, flumequine, sarafloxacin, and enrofloxacin, in Atlantic salmon (Salmo salar) held in seawater at 10 degrees C. Antimicrob Agents Chemother 1995; 39: 1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffregen DA, Wooster GA, Bustos PS, Bowser PR, Babish JG. Multiple route and dose pharmacokinetics of enrofloxacin in juvenile Atlantic salmon. J Vet Pharmacol Therapeut 1997; 20: 111–23. [DOI] [PubMed] [Google Scholar]
- 14.Bowser PR, Wooster GA, St Leger J, Babish JG. Pharmacokinetics of enrofloxacin in fingerling rainbow trout (Oncorhynchus mykiss). J Vet Pharmacol Therapeut 1992; 15: 62–71. [DOI] [PubMed] [Google Scholar]
- 15.Li CY, Li JC, Lu TY. Pharmacokinetic of enrofloxacin in carp following different ways of administration. J Jimei Univ (natural science) 2009; 13: 8–13. [Google Scholar]
- 16.Guo JJ, Yang H, Pan HY. Study on acute toxicity of enrofloxacin in sturgeon. Mod Agr Sci Technol 2010; 20: 311, 5–311, 5. [Google Scholar]
- 17.Liu YH, Zu XJ, Li GJ. The research of enrofloxacin acute toxicity study on fish. Jilin Water Resour 2012; 5: 46–7, 50. [Google Scholar]
- 18.Zhao L, Cao HP, Chen H. Acute toxicity of enrofloxacin to Carassius auratus gibelio and its effects on blood biochemical indexes. Chin J Zool 2013; 48: 446–50. [Google Scholar]
- 19.Fang YC, Qi Y, Li Y. Acute toxicity experience to guppy with ciprofloxacin HCL, enrofloxacin and norfloxacin. J Shenyang Univ (natural science) 2012; 24: 15–7. [Google Scholar]
- 20.Guo JJ, Pan HY, Yang H. Pharmacokinetics of enrofloxacin in sturgeon Acipenser schrenckii. J Dalian Ocean Univ 2011; 26: 362–6. [Google Scholar]
- 21.Wang XL, Yang XL, Lin M. Elimination of enrofloxacin and detection of metabolic enzyme activity in Micropterus salmoides hepatocytes. J Fish Sci China 2007; 14: 1004–9. [Google Scholar]
- 22.Yin Y, Chen C. Optimizing first-time-in-human trial design for studying dose proportionality. Drug Informat J 2001; 35: 1065–78. [Google Scholar]
- 23.Wang WL, Luo L, Jiang L. Blood concentration and histological toxicology of different dosages of florfenicol in GIFT Nile tilapia. J Shanghai Ocean Univ 2014; 23: 90–4. [Google Scholar]
- 24.Zhu XZ, Zheng YH, Tang HY. The effect of enrofloxacin on transaminase (GPT, GOT) activities of Triploid crucian carp. Freshwat Fish 2011; 41: 58–63. [Google Scholar]
- 25.Zhou F, Lin L, He F. Effects of enrofloxacin injection on the hepatic phase I and II enzyme activities of Chinese soft-shelled turtles Pelodiscus sinensis. Acta Agriculturae Zhejiangensis 2013; 25: 1228–33. [Google Scholar]
- 26.Steffenak I, Hormazabal V, Yndestad M. Reservoir of quinolone residues in fish. Food Addit Contam 1991; 8: 777–80. [DOI] [PubMed] [Google Scholar]
- 27.Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol Lett 2002; 127: 269–77. [DOI] [PubMed] [Google Scholar]
- 28.Maslanka T, Jaroszewski JJ, Chrostowska M. Pathogenesis of quinolone-induced arthropathy: a review of hypotheses. Pol J Vet Sci 2004; 7: 323–31. [PubMed] [Google Scholar]
- 29.Wu YK, Li QX, Xia ZP. Histopathological effect of norfloxacin on gill arch cartilages in common carp (Cyprius carpio). J Dalian Fish Univ 2006; 21: 383–6. [Google Scholar]
- 30.Zhou S, Hu LL, Fang WH. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in mud crab (Scylla paramamosain). J Fish China 2011; 35: 1182–90. [Google Scholar]
- 31.Lan H, Qu F, Jiang SC. Immunohistochemistry study of ciprofloxacin on fetal cartilage. Chin J Antibiot 2001; 26: 298–303. [Google Scholar]
- 32.Liu XY, Sun MQ, Hou J. Effect of quinolone in cartilage of immature rats. Chin J Antibiot 2000; 25: 457–61. [Google Scholar]
- 33.Lee A, Langer R. Shark cartilage contains inhibitors of tumor angiogenesis. Science 1983; 221: 1185–7. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Ye JD, Liu Y. Determination and evaluation of nutrients in sturgeon cartilage. Acta Nutrimenta Sinica 2006; 28: 187–8. [Google Scholar]
- 35.Song BL, Xu ZN, Wang ZQ. Effect of water velocity on behavior, antioxidant enzyme and residues of enrofloxacin-HCl in Siberian Sturgeon (Acipenser baerii). Acta Agriculturae Universitatis Jiangxiensis 2014; 36: 1109–14. [Google Scholar]
- 36.Guo J, Zhang QD. Advance in the research on the mechanism of tonifying kidney herbs that protect the articular cartilage. Chin Arch Tradit Chin Med 2010; 28: 2515–8. [Google Scholar]