Abstract
Lung cancer is one of the most common malignancies in the world, and non–small cell lung cancer (NSCLC) is a major subtype of lung cancer. Overgrowth of tumor cells usually results from the intensive proliferation of cancer cells, but the mechanisms by which the proliferation of cancer cells are promoted are currently unclear. Thus, it is necessary to determine the vital factors involved in regulating the growth of NSCLC. The MTT assay, BrdU assay, western blots, and migration and invasion assays were used in our study. Here, we found that PKIB (cAMP-dependent protein kinase inhibitor-β), a novel molecular target, was up-regulated in NSCLC tissues compared with the normal tissues adjacent to the tumors. Moreover, overexpression of PKIB promoted cell proliferation and potentiated the invasion and migration in A549 cells, whereas knocking down PKIB gene expression inhibited the proliferation and attenuated the invasive behavior and metastasis in H1299 cells. However, all of these effects of PKIB on cell proliferation and metastasis were reduced by inhibiting the PI3K/Akt pathway. Our results indicate that PKIB promotes cell proliferation and tumorigenesis by activating the PI3K/Akt pathway in NSCLC, implying that this is an important underlying mechanism that affects the progression of NSCLC.
Keywords: cAMP-dependent protein kinase inhibitor-β, proliferation, non–small cell lung cancer, metastasis, invasion
Introduction
Lung cancer is one of the most prevalent malignant tumors and the leading cause of cancer-related death among men and women worldwide.1,2 Non–small cell lung cancer (NSCLC) is responsible for approximately 80% of lung cancer diagnoses. Currently, lung cancer therapeutic strategies include surgery, chemotherapy, radiotherapy, and recently established molecular targeted therapies, but the main challenge of targeted therapies is that only a small proportion of patients can benefit from the treatments.3,4 Moreover, the 5-year overall survival rate for patients with NSCLC has not been markedly improved by the current therapeutic strategies, which is only 16% for all stages of lung cancer.5 In recent years, although the discovery of targetable driver oncogenes, such as EGFR mutations, ALK fusions, and inhibition of hTERT overexpression, are major treatment strategies for patients with NSCLC,6–8 there is still an urgent need to understand the molecular mechanisms of lung cancer tumorigenesis and to identify new therapeutic targets to improve the treatment strategies for lung cancer patients.
PKIB (cAMP-dependent protein kinase inhibitor-β) is a member of the protein kinase inhibitors (PKIs), which are a class of proteins that can inhibit the activity of cAMP-dependent protein kinase (PKA). PKA is a complex composed of two regulatory subunits (R-subunits) and two catalytic subunits (C-subunits)9; the PKIs can bind to the C-subunits of PKA in the nucleus and then transport them to the cytoplasm to reform the inactive PKA complex with the R-subunits.10 To date, studies have found that PKIB has the ability to enhance the constitutive activity of the G-protein-coupled zinc receptor GPR39 and may play important roles in vascular endothelial cells.11,12 Furthermore, there are a few studies about the functions of PKIB in the tumor progression process. In breast cancers, PKIB expression is strongly correlated with the triple-negative breast cancer subtype, and overexpression of PKIB promotes tumor aggressiveness in prostate cancer.13,14 However, it is currently not known whether PKIB is involved in modulating the progression of NSCLC.
In the present study, we aim to clarify the roles of PKIB in the proliferation, migration, and invasion of NSCLC cells. We find that the expression of PKIB is significantly up-regulated in NSCLC tissues compared with the normal tissues adjacent to the tumors. Moreover, we have also demonstrated that PKIB serves as an important regulator of the cell proliferation and metastasis of NSCLC cells by activating the PI3K/Akt pathway. All of our results suggest that PKIB may be a novel potential therapeutic target for NSCLC.
Materials and methods
Materials
The antibodies against PCNA and beta-actin were purchased from Santa Cruz Biotechnology Inc., CA, USA, and the PKIB antibody was purchased from Abcam (Cambridge, MA, USA). The BrdU proliferation assay kit was purchased from Millipore Corporation (Billerica, MA). All other reagents were from common commercial sources.
Cell line preparation and clinical samples
The A549 and H1299 cells, two human NSCLC cell lines, were obtained from the American Type Culture Collection (ATCC, Rockville, Maryland, USA). Both cell lines were routinely cultured in DMEM supplemented with 10% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 µg/mL) at 37℃ in an incubator with 95% air and 5% CO2. The tumor specimens used in this study were obtained from 30 NSCLC patients who underwent curative resection in the First Affiliated Hospital of Jiamusi University. Ethical approval was obtained and approved by the Ethics Committee of Jiamusi University, and written informed consent was obtained from each patient.
Generation of PKIB shRNA-expressing and PKIB-overexpressing lentiviruses
PKIB shRNA and a scrambled shRNA (used as a negative control) were designed and synthesized by Shanghai GenePharma Co., Ltd., Shanghai, China. We called the resulting lentiviruses containing the PKIB shRNA sequence or the negative control sequence PKIB shRNA and NC, respectively. The PKIB-overexpressing lentivirus was also purchased from Shanghai GenePharma Co., Ltd., Shanghai, China, and the PKIB-overexpressing lentivirus and blank vector were called PKIB and vector, respectively.
Assessment of cell viability
We used the MTT assay to assess cell viability. The A549 and H1299 cells were split into a 96-well plate (approximately 5000 cells per well), and the next day, the cells were subjected to growth arrest for 24 h before different treatments were added to the indicated groups described below. The groups were negative control and PKIB shRNA, or vector and PKIB overexpression. Then, after incubating cells at 37℃ for 3 days, a medium containing 5 g/L of 0.5% MTT (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide), which was a yellow mitochondrial dye and prepared in PBS, was added to the cells at 20 µL of MTT per well, and the cells were incubated at 37℃ for 4 h. The MTT reaction was terminated by adding DMSO to the medium and incubating the cells for 10 min at 37℃; then, the absorbance at 490 nm was read using a spectrophotometer. All experiments were done in triplicate and repeated thrice.
Bromodeoxyuridine incorporation
The A549 and H1299 cells were plated in 96-well plates at a density of 1 × 104 cells/well and then subjected to growth arrest for 24 h. After that, cells were cultured in DMEM containing 1% FBS with the indicated treatments. We measured the BrdU incorporation using a BrdU proliferation assay kit according to the manufacturer's protocol. Briefly, the cells were labeled with 10 µg/L of BrdU during the incubation, washed 3 times with a cold wash buffer, fixed, air-dried, and incubated with a mouse anti-BrdU monoclonal antibody (diluted 1:200) for 1 h at room temperature. The antibody was aspirated, and the cells were washed 3 times and then incubated with peroxidase goat anti-mouse IgG (1:2000) for 30 min at room temperature. The cells were washed 3 times, and 100 µl of substrate was added to each well and incubated for 30 min in the dark. Then, the absorbance was measured at dual-wave lengths of 450 to 540 nm.
Migration assay
Confluent cells were maintained in a growth factor-free medium for 24 h prior to the experiments. The cells were then harvested using trypsin, counted, centrifuged, and resuspended in DMEM containing 1% bovine serum albumin. Approximately 30,000 cells (500 µL) were then plated on the upper side of a membrane (24-well insert; pore size, 8 mm; Corning Costar), which was then seated in 750 mL of complete medium (10% FBS). After a 24-h incubation period, the non-migrating cells were mechanically removed with a cotton swab. Cells that had migrated through the membrane were stained with crystal violet. The cells in five representative microscopic fields (×100 magnifications) were counted and photographed.
Matrigel invasion assay
The Matrigel invasion assay was performed according to the manufacturer’s instructions. Each well was coated with fresh Matrigel (60 mg; BD Biosciences) before the invasion assay. Briefly, 50,000 cells were plated on the Matrigel-coated membrane in the top chamber (24-well insert; pore size, 8 mm; Corning Costar). The cells from indicated groups were added to the Transwell insert in 0.5 mL of medium containing 1% fetal bovine serum (FBS), which was seated in 750 mL of complete medium (10% FBS). After a 48-h incubation period, the non-invading cells were mechanically removed with a cotton swab. Cells that had crossed through the Matrigel assay were stained with crystal violet. The cells in five representative microscopic fields (×100 magnifications) were counted and photographed.
Western blotting
The methodology has been described in previous reports.15 Briefly, the cells from six wells were sonicated in an RIPS buffer, homogenized, and the debris was removed by centrifugation at 12,000 g for 10 min at 4℃. The samples containing 50 µg of protein were electrophoresed on polyacrylamide SDS gels and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 3% BSA, incubated with primary antibodies, and subsequently incubated with an alkaline phosphatase-conjugated secondary antibody. The membranes were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Tiangen Biotech Co. Ltd., Beijing, China). The blots were also stained with an anti-beta-actin antibody as an internal control for the amounts of the target proteins.
Real-time PCR
Real-time PCR (qPCR) was performed with an Applied Biosystems 7300 Fast Real-Time PCR system. Each 20 µL reaction contained 1 × SYBR® Premix Ex Taq™ II, 10 μM forward and reverse primers, 0.4 µL of ROX reference dye, and 2 µL of cDNAs. The ABI 7300 Sequence Detector (PerkinElmer Applied Biosystems) was programmed for the following PCR conditions: 95℃ for 30 s, 40 cycles of 95℃ for 5 s, and 60℃ for 31 s, followed by a routine melting curve analysis. Relative quantitation (RQ) of target gene expression was performed using the 2–ΔΔCT method.16 The first step in the RQ analysis is to normalize the expression level of the target gene to β-actin (ΔCt). The second step is to compare the differences between the normalized target gene expression levels between different samples (ΔΔCt). Each experiment was repeated 2–3 times using three to four samples.
Statistical analysis
The results were expressed as the mean values ± SEM. Statistical analysis was performed with the Student's t-test or one-way ANOVA followed by Dunnett's test, as appropriate, and a value of less than 0.05 (P < 0.05) was used for statistical significance.
Results
PKIB expression is increased in the NSCLC tissues compared with normal tissues adjacent to the tumors
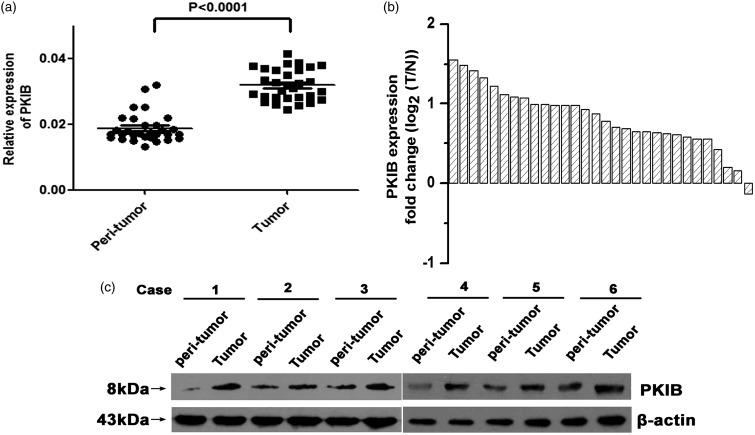
To determine whether PKIB participated in regulating the development of NSCLC, we applied real-time PCR and western blot assays to determine the expression levels of the PKIB mRNA and protein in 30 paired tumor tissues as well as their corresponding non-tumor lung tissues. Our results showed that PKIB expression was significantly up-regulated in the NSCLC tissues compared with the normal tissues adjacent to the tumors (Figure 1(a) and (b), P < 0.05). Similar results were acquired when we detected the levels of the PKIB protein (Figure 1(c)). The result indicates that PKIB is likely to be positively associated with the development of NSCLC.
Figure 1.
PKIB was up-regulated in NSCLC tissues. (a) Expression of the PKIB mRNA in the NSCLC tissues of 30 patients and the normal tissues adjacent to the tumors. (b) The fold change (log2 (T/N)) was used to present the expression of the PKIB mRNA in the 30 paired NSCLC samples. (c) Expression of the PKIB protein in six patients with NSCLC and their corresponding normal tissues adjacent to the tumors. All values are denoted as the means ± SEM (*P < 0.05)
Overexpression of PKIB promotes NSCLC cell proliferation
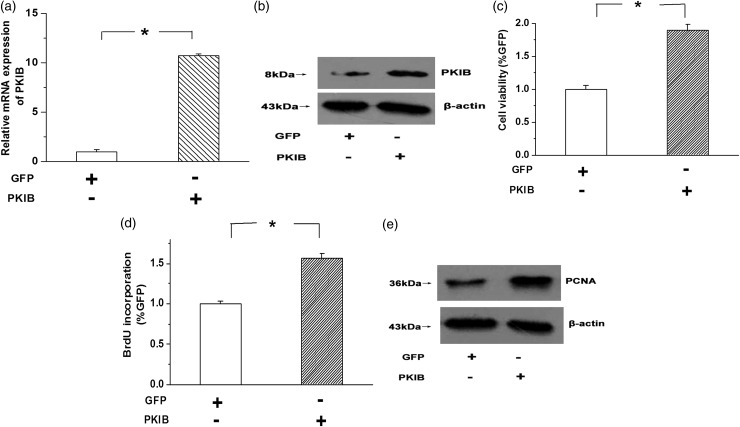
To confirm the role of PKIB in the growth of NSCLC cells, we stably overexpressed PKIB in A549 cells. As shown in Figure 2(a) and (b), real-time PCR and western blot assays were used to evaluate the efficiency of overexpression. The results showed that the PKIB mRNA and protein levels were greatly increased in the PKIB-transfected A549 cells compared with the vehicle group (Figure 2(a) and (b), n = 3, P < 0.05). MTT was used to determine the effects of PKIB on cell viability, and we found that cell viability was significantly increased by PKIB overexpression in A549 cells (Figure 2(c), n = 3, P < 0.05). Moreover, the BrdU assay and expression of the PCNA (proliferating cell nuclear antigen) protein were used as indicators of cell proliferation. The results showed that overexpression of PKIB enhanced BrdU incorporation and induced the expression of the PCNA protein in A549 cells (Figure 2(d) and (e), n = 3, P < 0.05). These results indicate that overexpression of PKIB promotes cell proliferation in NSCLC cells.
Figure 2.
(a,b) The expression of the PKIB mRNA and protein was examined by real-time PCR and western blotting, respectively. The results showed that the expression of the PKIB mRNA and protein was significantly increased in the PKIB-transfected cells transfected compared with the vector group. (c) Overexpression of PKIB increased the viability of the A549 cells, as determined by the MTT assay. (d) The results of the BrdU assay implied that BrdU incorporation was enhanced by PKIB overexpression. (e) PKIB overexpression induced PCNA expression in A549 cells. All values are denoted as the means ± SEM from three or more independent batches of cells (*P < 0.05)
Knocking down the expression of PKIB inhibits NSCLC cell proliferation
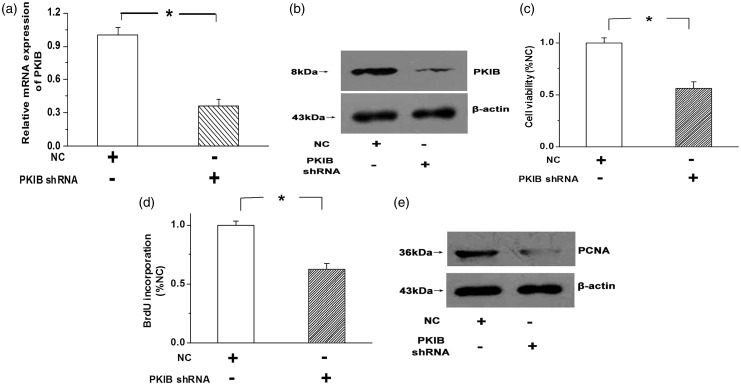
Meanwhile, the expression of PKIB was knocked down by PKIB shRNA in H1299 cells. As shown in Figure 3(a) and (b), the expression of the PKIB mRNA and protein was significantly decreased by PKIB shRNA in H1299 cells compared with the NC (negative control) group (Figure 3(a) and (b), n = 3, P < 0.05). The MTT results showed that reducing PKIB expression with PKIB shRNA decreased the viability of the H1299 cells (Figure 3(c), n = 3, P < 0.05). Moreover, BrdU incorporation and expression of the PCNA protein were suppressed by knocking down PKIB expression (Figure 3(d) and (e), n = 3, P < 0.05). The results indicate that NSCLC cell proliferation is attenuated by reducing PKIB expression.
Figure 3.
Real-time PCR (a) and western blotting (b) were applied to confirm the efficiency of the PKIB shRNA infection in H1299 cells. PKIB shRNA significantly decreased the expression of the PKIB mRNA and protein. (c,d) Cell viability (c) and BrdU incorporation (d) were decreased after the expression of PKIB was knocked down with PKIB shRNA. (e) PCNA expression was down-regulated in H1299 cells after PKIB inhibition. “NC” means negative control, and all values are denoted as the means ± S.E.M. from at least three separate experiments (n = 3, *P < 0.05)
PKIB overexpression promotes the invasion-metastasis cascade of NSCLC cells
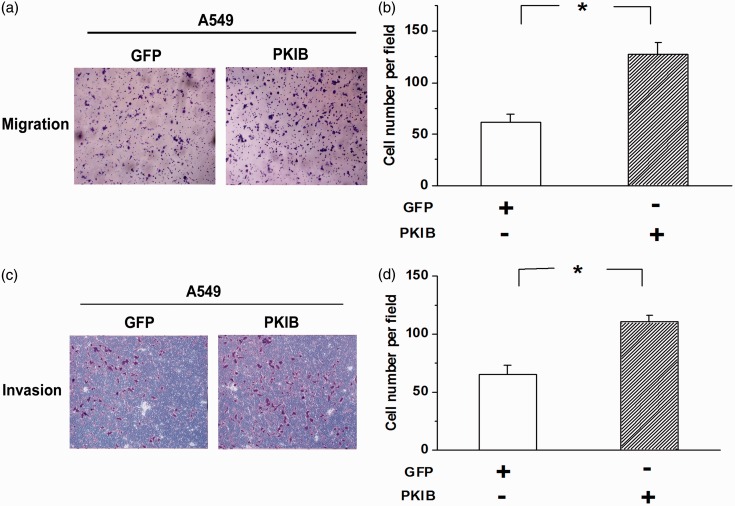
The invasion-metastasis cascade, which disseminates cancer cells to distant tissues, is believed to be the major reason for patient death. Therefore, we examined the effects of PKIB on the invasion and migration of NSCLC cells to emphasize the role of PKIB in the progression of NSCLC. As shown in Figure 4(a) and (b), the number of migrating A549 cells was increased by PKIB overexpression. Moreover, PKIB overexpression increased cell invasion (Figure 4(c) and (d), n = 3, P < 0.05). The results indicate that the metastasis of NSCLC cells is potentiated by overexpression of PKIB.
Figure 4.
PKIB overexpression promotes the migration and invasion of NSCLC cells. (a) Transwell experiments showed that A549 cell migration was obviously potentiated by PKIB overexpression. (b) Quantitative analysis of the number of migrating cells among the groups. (c) Overexpression of PKIB enhanced the invasion of A549 cells. (d) Quantitative analysis of the number of invading cells among the groups. All values are denoted as the means ± S.E.M. from three independent batches of cells (n = 3, *P < 0.05). (A color version of this figure is available in the online journal.)
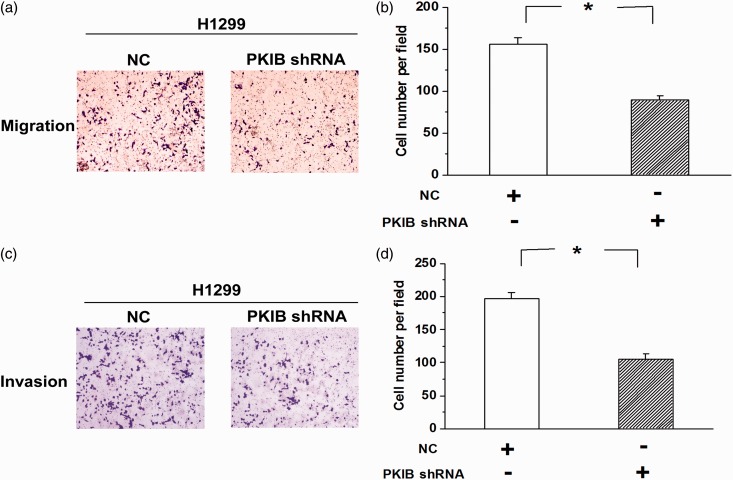
Metastasis of NSCLC cells is attenuated by knocking down PKIB expression
Meanwhile, we examined the effects of repressing PKIB expression on the invasion and migration of H1299 cells. We found that knocking down PKIB expression mitigated the migration of H1299 cells (Figure 5(a) and (b), n = 3, P < 0.05). Moreover, the invasion experiments showed that the number of invasive H1299 cells was greatly decreased after the expression of PKIB was reduced (Figure 5(c) and (d), n = 3, P < 0.05). These results indicate that PKIB plays a pro-tumorigenic role in the development of NSCLC.
Figure 5.
NSCLC cell metastasis was mitigated by the knockdown of PKIB. (a) Representative pictures of transwell experiments showing that the decrease in PKIB expression reduced the migration of H1299 cells. (b) Quantitative analysis of the number of migrating cells among the groups. (c) The invasive behavior was greatly attenuated after the expression of PKIB was repressed. (d) Quantitative analysis of the number of invading cells among the groups. All values are denoted as the means ± S.E.M. from three independent batches of cells (n = 3, *P < 0.05). (A color version of this figure is available in the online journal.)
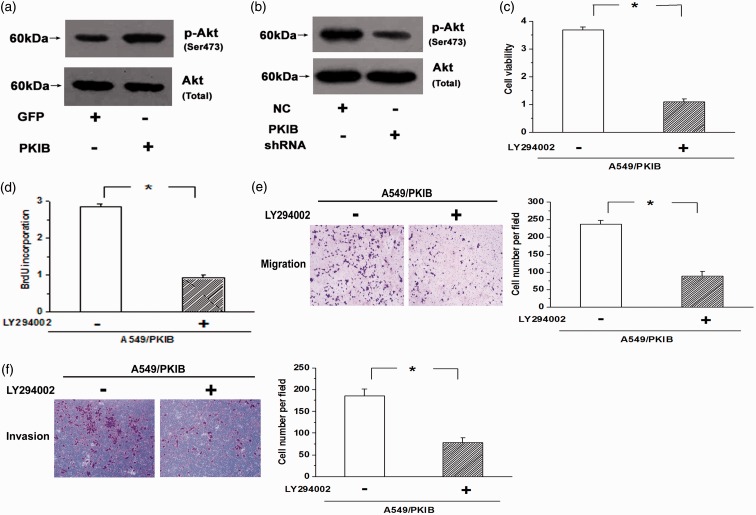
The effects of PKIB on cell proliferation are mediated by the PI3K/Akt pathway
It is well known that the PI3K/Akt pathway, which is often activated in the progression of some cancers, including NSCLC, is one of the most classical pro-survival pathways. Therefore, we next examined whether PKIB was correlated with the activation of Akt. Our results showed that overexpression of PKIB increased the levels of Akt phosphorylation at Ser473 in A549, and knockdown of PKIB decreased the levels of Akt phosphorylation; however, the total Akt level was not significantly affected by PKIB (Figure 6(a) and (b), n = 3, P < 0.05). Moreover, LY294002, an inhibitor of the PI3K/Akt pathway, attenuated the cell proliferation elicited by PKIB overexpression (Figure 6(c) and (d), n = 3, P < 0.05). In addition, cell migration and invasion were reversed by LY294002 in the PKIB-overexpressing A549 cells (Figure 6(e) and (f), P < 0.05). The results indicate that PKIB promotes cell proliferation and metastasis by activating the PI3K/Akt pathway in NSCLC cells.
Figure 6.
PKIB promotes the proliferation and metastasis of NSCLC cells by activating the PI3K/Akt pathway. (a) Overexpression of PKIB increased the levels of Akt phosphorylation at Ser473, but the total Akt levels were not significantly affected by PKIB. (b) Knockdown of PKIB decreased the level of Akt phosphorylation. (c,d) The increased cell viability and BrdU incorporation elicited by PKIB overexpression were attenuated by blocking the PI3K/Akt pathway. (e,f) Inhibition of the PI3K/Akt pathway with LY294002 resulted in decreased migration and invasion of the PKIB-overexpressing A549 cells. (A color version of this figure is available in the online journal.)
Discussion
Because tumor progression usually results from the overgrowth and metastasis of tumor cells, it is necessary to determine the critical molecules that participate in regulating the growth and invasion-migration cascade of cancer cells. In our study, we found that PKIB is up-regulated in NSCLC tissues compared with the normal tissues adjacent to the tumors. Moreover, PKIB overexpression promotes cell proliferation and the invasion-migration cascade of NSCLC cells, and knockdown of PKIB expression inhibits cell proliferation and metastasis. The effects of PKIB on cell proliferation and metastasis are attenuated by blocking the PI3K/Akt pathway. We provide new evidence that the up-regulated PKIB expression in the NSCLC tissues facilitates the proliferation and metastasis of NSCLC cells by activating the PI3K/Akt pathway.
Accumulating evidence has indicated that the major reason for tumor occurrence is the excessive growth of tumor cells, and conditions that either promote tumor cell proliferation or inhibit tumor cell apoptosis can lead to the overgrowth of tumor cells.17,18 Thus, it is important to explore the critical factors involved in regulating the growth of cancer cells, which may provide novel therapeutic targets and improve the treatment efficiency. It has been reported that PKA activates a number of ligand-receptor signaling pathways and controls cell growth and differentiation, and PKIB is described as an inhibitor of PKA.9 Moreover, previous research indicates that PKIB plays a positive role in promoting the aggressiveness of prostate cancer.14 In our study, we demonstrated that the expression of PKIB was significantly increased in the NSCLC tissues compared with the normal tissues adjacent to the tumors. Furthermore, PKIB overexpression promoted A549 cell proliferation, and knocking down the expression of the PKIB gene inhibited the growth and proliferation of H1299 cells. These results indicate that PKIB participates in regulating the progression of NSCLC by driving tumor growth.
The invasion of surrounding tissues by malignant tumors and the dissemination of cancer cells to distant tissues are accomplished by the invasion-metastasis cascade, which is regarded as the major reason for patient death and makes the treatment of cancers extremely difficult.19,20 To emphasize the pro-tumorigenic role of PKIB in NSCLC, we also examined the effects of PKIB on the invasion and migration of NSCLC cells. Our results showed that the invasion and migration of A549 cells were enhanced by overexpression of PKIB, and silencing PKIB expression attenuated the invasive behavior in H1299 cells, implying an important role for PKIB in the progression and development of NSCLC.
The PI3K/Akt pathway is one of the most classical pro-survival pathways, and it plays an important role in regulating cell growth and metastasis.21 Previous studies have shown that the development of some cancers is closely correlated with the hyper-activation of Akt.22 To illustrate the mechanism by which PKIB promotes the proliferation and metastasis of NSCLC cells, we examined whether there was an association between PKIB and the PI3K/Akt pathway. Our results showed that PKIB positively regulated the activation of Akt, and the cell proliferation enhanced by PKIB overexpression was obviously mitigated by LY294002. Moreover, the migration and invasion of the PKIB-overexpressing A549 cells were decreased by blocking the PI3K/Akt pathway. The results indicate that PKIB promotes the proliferation and metastasis of NSCLC cells by activating the PI3K/Akt pathway.
In conclusion, it has been demonstrated that PKIB, which is up-regulated in the NSCLC tissues, promotes the proliferation and metastasis of NSCLC cells by activating the PI3K/Akt pathway. These findings may help to elucidate a potential mechanism for modulating the progression of NSCLC and thus may provide a new potential therapeutic target for NSCLC.
Acknowledgments
This work was supported by grants from the Heilongjiang province department of education Science and technology research fund project (No. 12541814), the Natural Science Foundation of Heilongjiang Province (No. H201373), and Jiamusi University Youth Foundation (No. q2013-024). The excellent assistance of all the nurses from Department of Thoracic Surgery made this work possible.
Authors' contributions
PD and LZ conceived and designed the experiments; PD and DZ performed the experiments and analyzed the results; ZC and PD revised the manuscript; PD, GZ, and LZ prepared the submission together.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 2011; 61: 91–112. [DOI] [PubMed] [Google Scholar]
- 3.Gower A, Wang Y, Giaccone G. Oncogenic drivers, targeted therapies, and acquired resistance in non-small-cell lung cancer. J Mol Med (Berl) 2014; 92: 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013; 13: 714–26. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O'Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385–94. [DOI] [PubMed] [Google Scholar]
- 8.Eldholm V, Haugen A, Zienolddiny S. CTCF mediates the TERT enhancer-promoter interactions in lung cancer cells: identification of a novel enhancer region involved in the regulation of TERT gene. Int J Cancer 2014; 134: 2305–13. [DOI] [PubMed] [Google Scholar]
- 9.Olsen SR, Uhler MD. Inhibition of protein kinase-A by overexpression of the cloned human protein kinase inhibitor. Mol Endocrinol 1991; 5: 1246–56. [DOI] [PubMed] [Google Scholar]
- 10.Taskén K, Skålhegg BS, Taskén KA, Solberg R, Knutsen HK, Levy FO, Sandberg M, Orstavik S, Larsen T, Johansen AK, Vang T, Schrader HP, Reinton NT, Torgersen KM, Hansson V, Jahnsen T. Structure, function and regulation of human cAMP-dependent protein kinases. Adv Second Messenger Phosphoprotein Res 1997; 31: 191–204. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs Z, Schacht T, Herrmann AK, Albrecht P, Lefkimmiatis K, Methner A. Protein kinase inhibitor β enhances the constitutive activity of G-protein-coupled zinc receptor GPR39. Biochem J 2014; 462: 125–32. [DOI] [PubMed] [Google Scholar]
- 12.Lum H, Hao Z, Gayle D, Kumar P, Patterson CE, Uhler MD. Vascular endothelial cells express isoforms of protein kinase A inhibitor. Am J Physiol Cell Physiol 2002; 282: C59–66. [DOI] [PubMed] [Google Scholar]
- 13.Dabanaka K, Chung S, Nakagawa H, Nakamura Y, Okabayashi T, Sugimoto T, Hanazaki K, Furihata M. PKIB expression strongly correlated with phosphorylated Akt expression in breast cancers and also with triple-negative breast cancer subtype. Med Mol Morphol 2012; 45: 229–33. [DOI] [PubMed] [Google Scholar]
- 14.Chung S, Furihata M, Tamura K, Uemura M, Daigo Y, Nasu Y, Miki T, Shuin T, Fujioka T, Nakamura Y, Nakagawa H. Overexpressing PKIB in prostate cancer promotes its aggressiveness by linking between PKA and Akt pathways. Oncogene 2009; 28: 2849–59. [DOI] [PubMed] [Google Scholar]
- 15.Wang SJ, Gao Y, Chen H, Kong R, Jiang HC, Pan SH, Xue DB, Bai XW, Sun B. Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett 2010; 293: 99–108. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 17.Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med (Berl) 2007; 85: 1301–7. [DOI] [PubMed] [Google Scholar]
- 18.Kim KR, Moon HE, Kim KW. Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J Mol Med (Berl) 2002; 80: 703–14. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Zhang L, Zhang J, Liu M, Wei L, Shen T, Ma C, Wang Y, Chen Y, Zhu D. 15-lipoxygenase-1/15-hydroxyeicosatetraenoic acid promotes hepatocellular cancer cells growth through protein kinase B and heat shock protein 90 complex activation. Int J Biochem Cell Biol 2013; 45: 1031–41. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev 2007; 26: 319–31. [DOI] [PubMed] [Google Scholar]
- 21.Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong SW, Li RL, Zhao MY, Zhen Y, Yu XL, Chen YY, Luo RC, Li R, Li LB, Deng XJ, Fang WY, Liu Z, Song X. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol 2015; 8: 22–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gan W, Liu P, Wei W. Akt promotes tumorigenesis in part through modulating genomic instability via phosphorylating XLF. Nucleus 2015; 6: 261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]