Abstract
This work mainly aimed to investigate the probable changes of aortic calcification by policosanol, omega-3 fatty acids in comparison with atorvastatin and subsequent progression of atherosclerosis in diabetic hyperlipemic rat model. Adult male albino rats of wistar strain (30) were divided into five groups (n = 6/group); one was fed normal diet and was used as a normal group, the other groups received alloxan, atherogenic diet (CCT – rat chow diet supplemented with 4% cholesterol, 1% cholic acid, and 0.5% thiouracil) and categorized as follows: the second group received no treatment and kept as control (diabetic hyperlipidemic control group (DHC)). The other groups received daily oral doses of atorvastatin, policosanol (10 mg/kg body weight) and ω-3 (50 mg/kg body weight), respectively, for eight weeks. Different biomarkers were used for the evaluation that included inflammatory (C reactive protein (CRP), tumor necrosis factor α (TNF-α)), oxidative stress (glutathione (GSH), malondialdehyde (MDA)) bone calcification markers (alkaline phosphatase (ALP), Vitamin D, parathyroid hormone (PTH)), lipogram pattern in addition to histochemical demonstration of calcium in the aorta. Diabetic hyperlipemic group demonstrated significant hyperglycemia, hyperlipidemia, and increased inflammation, oxidative stress, calcification, and finally atherogenesis progression. Treatment of diabetic hyperlipemic rats with, policosanol, omega-3 fatty acids (natural products) and atorvastatin for eight weeks significantly increased high-density lipoprotein cholesterol (HDL-C), Vitamin D, decreased aortic vacuoles number, and inhibited calcification process. Policosanol induced more remarkable reduction in the density and number of foam cells and improved the intimal lesions of the aorta as compared to atorvastatin. Drugs under study exerted hypoglycemic effect along with an inhibition of inflammation, oxidative stress, and calcium deposition with certain variations but policosanol effect was remarkable in comparison with other drugs.
Keywords: Diabetic hyperlipidemia, policosanol, omega-3 fatty acids, atorvastatin, inflammation, oxidative stress, aortic calcification
Introduction
It has been reported that inflammatory factors, such as cytokines and modified lipoproteins, play a significant role in atherosclerotic calcification, and many clinical studies attributed these factors to the progression of vascular calcification as indicated by the hyperlipidemia score.1
Oxidative stress, the subsequent free radical formation, and changes in serum Ca++ and phosphorus (P) levels can activate vascular smooth muscle cells (VSMCs) differentiation in the vascular wall. This may collectively lead to the loss of many calcification inhibitors, such as matrix Gla-protein, osteopontin, and Fetuin-A.2
A reported study, which used hyperlipidemic mice, has demonstrated that the promotion of calcification and cholesterol played a certain role in the precipitation of calcium crystals.3
Modified lipoproteins in a hyperlipidemic state can also induce calcification and osteogenic differentiation in vascular cells.4 The reverse was shown to be true in the osteoblasts of skeletal bones, a finding which may support the lipid hypothesis of osteoporosis.4
In addition to expressing osteopontin, bone morphogenetic protein (BMP-2) is also present in human atherosclerotic plaques.5
An in vitro study, which used calcifying vascular cells incubated with graded levels of TNF-α, showed a gradual increase in alkaline phosphatase and calcium deposition.6 This result indicates the important role of TNF-α considering that alkaline phosphatase is mainly derived from osteoblast cells.6
The link between atherosclerotic mineralization and inflammation has been recorded.7 Moreover, generation of reactive oxygen species (ROS) in atherosclerotic lesions and its role in vascular calcification has also been discussed.8
Statin drugs have been suggested for the treatment of vascular calcification.9
TNF-α, as an inducer of vascular calcification, showed significant inhibition in a dose-dependent manner under the influence of cerivastatin and atorvastatin.10 Similarly, inhibition of ALP expression was also observed.10
The hypolipidemic effects of policosanol (PC) were reported in experimental animals that received PC, demonstrating its efficacy as a natural antioxidant and a potential inhibitor of platelet aggregation, endothelial damage, and foam cell formation.11 Statins have the same mechanism as 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase), a reductase inhibitor and activator of low-density lipoprotein (LDL) receptor processing.12
Clinical studies conducted on healthy volunteers,13 hypercholesterolemic individuals,14 and diabetics with dyslipidemia15 have confirmed the efficacy and safety of statins and PC.
The present study was undertaken to investigate the effects of PC and omega-3 fatty acids (ω-3) (natural products) on hyperlipidemia, oxidative stress, inflammatory degree, and aortic calcification patterns in a diabetic hyperlipidemic rat model and to compare these effects to the effects of the hypolipidemic drug atorvastatin (synthetic product).
Laboratory animals
Male albino rats (Wistar strain), weighing 150 ± 10 g (10 to 12 weeks old) were used. Rats had free access to water and a standard chow diet throughout the experimental period. The experimental design and animal handling were approved by the Ethical committee of the faculty of pharmacy at Zagazig University (NO p23/12/2014).
Experimental procedure
After one week of acclimation, rats were randomly assigned to five groups (six rats/group). The first group, referred to as the normal group (NG), received the standard chew diet. The other four groups received freshly prepared alloxan (125 mg/kg) dissolved in saline and delivered subcutaneously.16,17 Six hours later, the drinking water was supplemented with 10% glucose for 24 h to avoid hypoglycemic shock.18
A blood sample was collected from each rat to test for blood glucose levels. Rats with a serum glucose level ≥ 150 mg/dl were considered diabetic and fed the CCT diet (normal chow diet supplemented with 4% cholesterol, 1% cholic acid and 0.5% thiouracil in the drinking water) for two weeks.19
Three groups of rats received atorvastatin (10 mg/kg), PC (10 mg/kg), or ω-3 fatty acids (50 mg/kg) for eight weeks along with the CCT diet. Rats in the fourth group received no treatment (only CCT diet) and were treated as a control group.
Because these drugs are insoluble in water, fresh suspensions were prepared using gum acacia as a suspending agent prior to the oral intake.
At the end of the experiment, blood was withdrawn from fasted rats for serum preparation. The serum glucose and lipid profile were tested immediately. The remaining serum was stored at −20℃ to determine the levels of CRP, TNF-α, Vitamin D, PTH, ALP, MDH, and GSH.
After the rats were sacrificed, the whole aorta from each rat was removed, rinsed with cold normal saline, dried with filter paper, and kept in 10% formalin–saline at 4℃ for at least one week (primary fixation). Then, the specimens were dehydrated with a series of ascending grade ethanol from 75% to 100% and immediately processed by the paraffin technique. Sections of 5 µm thickness were cut, processed on slides, and stained with hematoxylin and eosin20 for histological microscopic analyses. Another part of the aorta was stained with alizarin red S for the histochemical demonstration of calcium deposition in the aorta.21 The sections were deparaffinized and hydrated with 70% alcohol, rinsed rapidly with distilled water and immersed in Alizarin red S solution for 5 min. The excess dye was removed, and the sections were treated with 20 drops of acetone followed by 20 drops of acetone–xylene and were then cleared in xylene. Calcium forms an alizarin red S-calcium complex through a chelation process and appears as orange–red deposits.
Chemical agents
Alloxan monohydrate (98%) was purchased from Acros-Organics Chemical Co. (USA). Cholesterol and cholic acid were purchased from Alpha Chemical Co. (India). 2-Thiouracil was purchased from Riedel-De Haen Ag Seelze-Hannover (Germany). Atorvastatin was kindly supplied by the Medical Union of Pharmaceuticals (MUP, Egypt). Fish oil (ω-3) fatty acids were purchased from Arab Co. for Gelatin and Pharmaceutical Products (Egypt). The alizarin red stain was supplied by the Oxford Lab, Mumbai, India.
Laboratory analysis
TC and HDL-c levels were determined colorimetrically using kits supplied by Spinreact Co., Spain. TAG levels were determined colorimetrically using the kit provided by Biomed Co., Egypt. LDL-C levels were calculated using the Friedewald formula. CRP levels were determined by a rat ELISA kit supplied by BD Biosciences Co., Germany.
TNF-α levels were determined by a rat immunoassay kit provided by R&D Systems, USA. MDA and GSH levels were determined by kits supplied by BioDiagnostics Co., Egypt. Alp levels were determined by a kit supplied by Vitro Medical Co., Germany. Vitamin D was determined by an ELISA kit supplied by Bio Vendor Research Co., Germany. PTH levels were determined by a rat ELISA kit provided by Cusabio Biotech Co., China.
Isolation and characterization of PC
Sugarcane peels were collected from a local juice center in Egypt. The wax was extracted from the peels, and additional purification and characterization of PC were carried out as previously described.22,23
Statistical analysis
Data values were expressed as M ± SD. Differences between groups were assessed using one-way analysis of variance. Tukey–Kramer test was performed for inter-group comparisons (GraphPad). Pearson’s correlation coefficient was used to determine correlations.
Significant results at p values ≤ 0.001, ≤ 0.01, and ≤ 0.05 are represented with symbols in the tables and figures.
Results
Effects of the treatments on serum glucose and oxidative stress markers
For rats treated with alloxan, a significant increase (p < 0.001) in the serum glucose level was observed compared to the NG rats. Treatment of DHC rats with PC, ω-3 fatty acids, and atorvastatin for eight weeks induced a remarkable decrease in the serum glucose levels (Figure 1). Oxidative stress, expressed as MDA, significantly increased along with the GSH reduction in the DHC group compared to the NG group (p < 0.001). Additionally, PC treatment increased GSH (p < 0.001). PC and ω-3 attenuated the serum MDA content (p < 0.001, p < 0.05) compared to the DHC group. ATOR treatment induced similar effects (Table 1).
Figure 1.
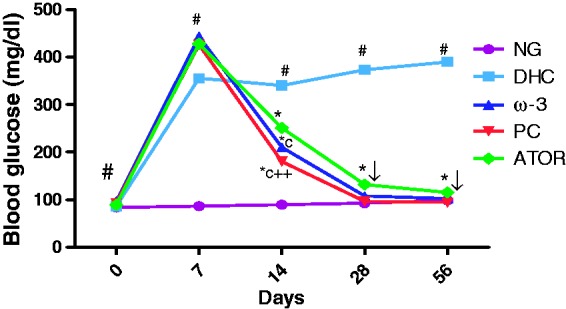
Effect of policosanol on blood glucose level. (#p < 0.001) NG vs DHC, (*p < 0.001) DHC vs. treated groups, (cp < 0.001) ATOR vs ω-3, PC, (++p < 0.001) ω-3 vs PC. (A color version of this figure is available in the online journal.)
Table 1.
Effect Of PC, ω-3 fatty acids and ATOR administration on serum lipogram pattern and oxidative stress markers of DH rats
| Parameters | NG | DHC | ATOR | ω3 | PC |
|---|---|---|---|---|---|
| TC (mg/dl) | 89.0 ± 2.8 | 242.3 ± 28.3♯ | 101.16 ± 1.9* | 92.1 ± 4.5* | 77.47 ± 0.6*a |
| TAG (mg/dl) | 71.3 ± 0.84 | 120.59 ± 0.77♯ | 72.95 ± 1* | 70.18 ± 1.7*b | 63.08 ± 0.66*c ++ |
| HDL-C (mg/dl) | 42.8 ± 0.72 | 31.8 ± 0.86♯ | 36 ± 0.94* | 35.13 ± 0.7* | 38.8 ± 0.93*c++ |
| LDL-C (mg/dl) | 31.88 ± 3.3 | 186.4 ± 28.3♯ | 50.5 ± 2.4* | 42.9 ± 4.5* | 26.02 ± 1.3*a |
| MDA (nmol/ml) | 5.01 ± 0.65 | 17.6 ± 1.7♯ | 3.3 ± 0.41* | 5.4 ± 0.66*a | 3.35 ± 0.93*+ |
| GSH (mmol/l) | 0.32 ± 0.037 | 0.15 ± 0.049♯ | 0.24 ± 0.007* | 0.25 ± 0.005* | 0.27 ± 0.02* |
NG: normal groups; DHC: diabetic hyperlipidemic rats receive no drug treatment; ATOR: atorvastatin, PC: policosanol; ω-3: Omega-3.
#p < 0.001: NG vs. DHC; *p < 0.001: DHC vs. treated groups; ap < 0.05, bp < 0.01, cp < 0.001: ATOR vs. ω-3, PC; +p < 0.05, ++p < 0.001: ω-3 vs. PC.
Effects of PC, ω-3 fatty acids, and ATOR on serum lipoproteins
The DHC group demonstrated a significant increase (p < 0.001) of serum TC, LDL-C, and TAG along with a decrease of HDL-C compared to NG group. Treatment of diabetic hyperlipemic (DH) rats with both PC and ω-3 fatty acids for eight weeks induced a significant decrease in serum levels of total LDL-C and TAG along with an increase of HDL-C (p < 0.001). PC showed more pronounced effects on these factors compared to the ATOR group. PC, ω-3, and ATOR significantly increased HDL-C (p < 0.001) and decreased LDL-C (p < 0.001, p < 0.05) compared to the DH rats (Table 1).
Effects of PC, ω-3 fatty acids, and ATOR on serum inflammatory markers
TNF-α and CRP were significantly increased in DH rats compared to the NG rats (p < 0.001). PC, ω-3, and ATOR individually induced a significant decrease in the serum TNF-α and CRP levels (p < 0.001; Table 2).
Table 2.
Effect of PC, ω-3 fatty acids and ATOR administration on inflammation, and calcification markers Of DH rats
| Parameters | NG | DHC | ATOR | ω3 | PC |
|---|---|---|---|---|---|
| TNF-α (pg/ml) | 31 ± 1.9 | 120.4 ± 3.8♯ | 78.3 ± 1.5* | 62.9 ± 0.4*c | 49.83 ± 0.7*c++ |
| CRP (mg/l) | 0.3 ± 0.03 | 3.1 ± 0.14♯ | 0.65 ± 0.04* | 0.5 ± 0.07* | 0.3750 ± 0.05* |
| ALP (U/l) | 125 ± 2.78 | 377.8 ± 47.7♯ | 150.7 ± 0.44* | 162.8 ± 0.4* | 140.3 ± 0.36* |
| VIT.D (ng/ml) | 46.23 ± 0.84 | 12.77 ± 1.5♯ | 28.3 ± 0.7* | 22.7 ± 1.6*c | 36.08 ± 1.9*c+ + |
| PTH (pg/ml) | 22.92 ± 2.2 | 74.92 ± 1.4♯ | 37 ± 1.09* | 30.18 ± 0.9*c | 25.62 ± 0.9*c++ |
All data are presented as the mean ± SD, n = 6.
#p < 0.001: NG vs. DHC; *p < 0.001: DHC vs. treated groups; cp < 0.001: ATOR vs. ω-3, PC;++p < 0.001: ω-3 vs. PC.
Effects of PC, ω-3 fatty acids, and ATOR on serum PTH, ALP, and Vitamin D levels
Treatment with PC, ω-3 fatty acids, and ATOR for eight weeks caused a significant increase in PTH and ALP serum levels (p < 0.001) and a decrease in Vitamin D serum levels in DH rats, compared to the NG rats (p < 0.001). PC and ω-3 treatments for eight weeks resulted in decreased PTH and ALP levels (p < 0.001) and increased Vitamin D levels(p < 0.001). These effects were more remarkable than those that were observed after ATOR treatment (Table 2).
Histopathological results
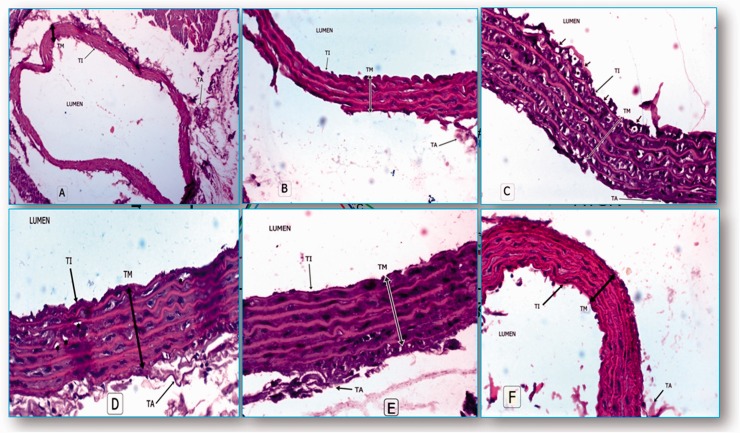
Hematoxylin and eosin staining of the aorta of normal rats illustrated irregular tunica intimae (TI), tunica media (TM) with elastic fibers and tunica adventitia (TA) with red blood cells in the lumen (Figure 2a and 2b). The aorta of DHC rats demonstrated a separation of the endothelial cells, denoting an intimal injury. Neointimal lesions showed more foam cells (Figure 2c). The aorta of ATOR-treated rats showed a reduction in the density and the number of foam cells, reflecting an improvement of the intimal lesions (Figure 2d). The aorta of ω-3-treated rats exhibited a greater reduction in the density and number of foam cells (Figure 2f). The aorta of PC-treated rats showed a marked improvement of the intimal lesions, approaching the normal pattern (Figure 2e).
Figure 2.
((a) and (b)) Photomicrograph of normal aorta, (a) (H&E × 150), (b) (H&E × 200). (c) Aorta of DHC rats illustrated large lipid vacuoles or foam cells (*stars) in the cells of tunica intimae (TI), tunica media (TM) with ulceration of the intimal endothelial cells and loose tunica adventitia (TA) (H&E × 200). (d) Aorta of ATOR-treated rats (H&E × 200). (f) Aorta of ω-3 treated-rats (H&E × 200). (e) Aorta of PC-treated rats (H & E × 200). (A color version of this figure is available in the online journal.)
Discussion
The observed decrease in total cholesterol is mainly attributed to an inhibition of HMG-CoA reductase activity and to the induction of AMP kinase phosphorylation. The latter may lead to further inhibition of HMG-CoA reductase activity24 and the inactivation of acetyl CoA carboxylase,25 leading to an inhibition of fatty acid biosynthesis.26
Several studies have discussed that hypercholesterolemic rabbits, following the oral administration of PC (50 mg/kg) for 30 days, demonstrated a significant decrease of hepatic sterol content and an improvement in LDL-C clearance.27 Similar findings were also observed during the incubation of human lung fibroblasts with PC for 48 h,28 where the incorporation of H3 water and C14 uptake (C14-acetate) showed a significant decrease.
Clinical studies have been conducted using different patient categories, e.g. Type 11 hypercholesterolemic, hypertensive, and T2 DM, and daily administration of 5–20 mg PC resulted in a significant reduction in LDL-c levels.29
These findings may collectively support the PC mechanism as an effective hypolipidemic agent.
Marked side effects, such as hepatotoxicity and myopathy,30 have been observed following the long-term use of various hypolipidemic drugs, such as statins, nicotinic acid fibrates, and ion–exchange resin.
The opposite was true during PC intake (10 mg/day) compared to Lovastatin intake (20 mg/day) for 12 weeks where PC demonstrated safety, tolerability, and marked improvement in the HDL-C level.15
The clinical use of PC in combination with ω-3 fatty acids for hypercholesterolemic patients also demonstrated marked improvements in their lipid profiles.31
The present study has shown the antioxidant properties of PC represented by a significant increase in GSH and a decrease in MDA.
Previous studies indicated that PC administration in a dose range between 100 and 250 mg/kg daily for four weeks resulted in a partial prevention of rat microsomal lipid peroxidation.32
An in vitro study using copper-induced lipoprotein peroxidation has reported similar results.32
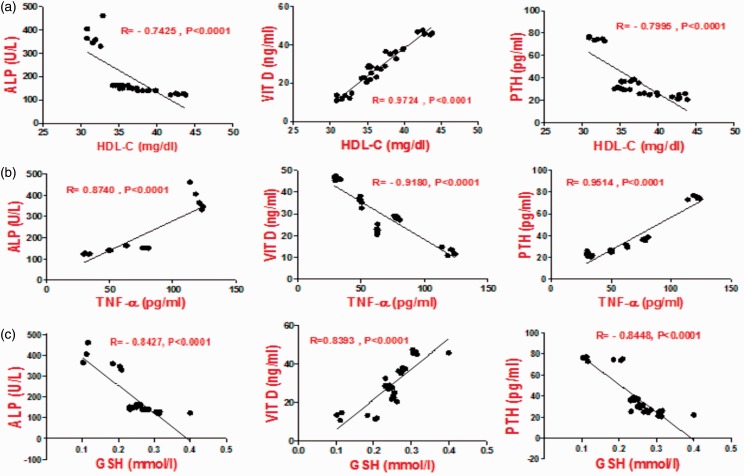
Inflammatory markers, such as TNF-α and CRP, also showed a significant decrease, which was more remarkable after PC treatment compared to treatment with atorvastatin and ω-3 fatty acids (Figure 3).
Figure 3.
Correlation between HDL-C, TNF-α, GSH with calcification markers (ALP, VIT.D, PTH). (A color version of this figure is available in the online journal.)
This decrease may reflect its anti-inflammatory property, which is in agreement with the previous reports.11 First, TNF-α can increase ALP and calcium deposition in the calcifying vascular cells,6 and second, it can regulate the osteogenic signal that is involved in aortic calcification.33 Specific atherosclerotic factors, such as Lpa, can also exert an important role in vascular calcification.34 Rabbit aortic smooth muscle cells incubated with Lpa in vivo showed significantly increased ALP and calcium accumulation.34
Endothelial dysfunction has previously been attributed in several studies to increased BMP expression, calcification, oxidative stress, and hypoxia.35,36
VSMCs incubation with either TNF-α or ox LDL also significantly increased BMP-2 expression,37 taking into consideration that inflammation and inflammatory cytokine formation, such as TNF-α, are mostly attributed to oxidative stress.38
There are two types of major calcium modulators that regulate hormones, and their receptors are known, namely PTH, Vitamin D,39 serum calcium (ionized), and the calcium-sensing receptor.40
The present study recorded a significant decrease in Vitamin D and an increase in the PTH levels of DHC rats. The administration of drugs under this study induced the opposite effect compared to the control group, and PC exerted remarkable effects (Figure 3).
For this reason, the PTH determination in this study may be beneficial for monitoring calcium disturbance in individuals suffering from kidney disease.41
Increased PTH activity may also lead to excessive H2O2 liberation from mononuclear cells and an intracellular increase of calcium.42
This may collectively lead to disturbed ATP output due to impairments of mitochondrial function, oxidative stress, ROS formation, inflammation and necrosis of cardiac tissues.42
The hypoglycemic effect of PC has mainly been attributed to an AMP-kinase activation that was similar to a metformin mechanism, which led to the activation of glucose uptake into the skeletal muscle, inhibition of hepatic gluconeogenesis, and finally decreased circulating lipids.43,44
Conclusions
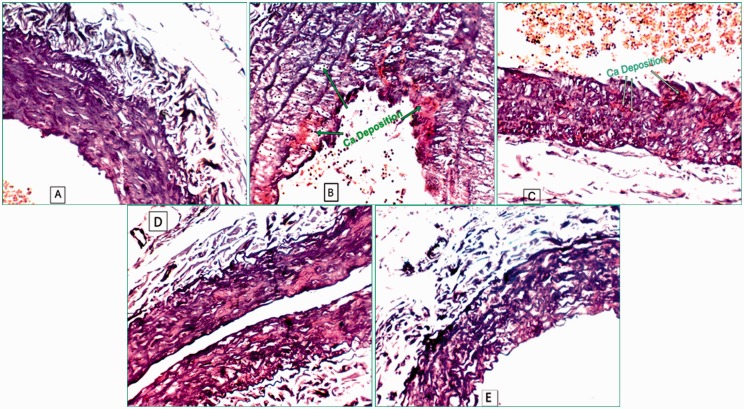
PC administration to diabetic hypercholesterolemic rats demonstrated hypolipemic, hypoglycemic, anti-oxidant, and anti-inflammatory effects along with remarkable inhibition of vascular calcification (Figure 4). Histopathological examination of the aorta revealed an improvement of intimal lesion as compared to ω-3 fatty acids and atorvastatin.
Figure 4.
Alizarin red staining of the aorta: (a) normal aorta of NG rats, (b) aorta of DH-rats, (c) aorta of ATOR-treated rats, (d) aorta of ω-3-treated rats and (e) aorta of PC-treated-rats. (A color version of this figure is available in the online journal.)
Acknowledgments
The authors acknowledge the effort of Dr HMK, Assistant Lecturer, Delta university for his assist in manuscript preparation. All individuals who made contributions to this study are included whether as authors or are acknowledged in the Authors’ contribution section. The study did not receive specific grants from any funding agency in the public, commercial or not-for profit sector.
Authors’ contribution
All authors participated in the design, the interpretation of the studies, the analysis of the data and reviewed of the manuscript MME, NZ, SIA, MME, SAS, MME conceived, designed the experiments and reviewed the manuscript. MME performed the experiments, contributed reagent, materials and analysis tools and wrote the manuscript. NZ analyzed the data. SIA and SAS separated and purified the PC.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Schmermund A, Baumgart D, Möhlenkamp S, Kriener P, Pump H, Grönemeyer D, Seibel R, Erbel R. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: an electron-beam CT study. Arterioscler Thromb Vasc Biol 2001; 21: 421–6. [DOI] [PubMed] [Google Scholar]
- 2.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nature Rev Cardiol 2010; 7: 528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarig S, Weiss TA, Katz I, Kahana F, Azoury R, Okon E, Kruth HS. Detection of cholesterol associated with calcium mineral using confocal fluorescence microscopy. Lab Investigat 1994; 71: 782–7. [PubMed] [Google Scholar]
- 4.Demer L, Tintut Y. The roles of lipid oxidation products and receptor activator of nuclear factor-κB signaling in atherosclerotic calcification. Circulat Res 2011; 108: 1482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boström K, Watson K, Horn S, Wortham C, Herman I, Demer L. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Investigat 1993; 91: 1800–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000; 102: 2636–42. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa E, Nahrendorf M, Figueiredo J-L, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007; 116: 2841–50. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification pathobiological mechanisms and clinical implications. Circulat Res 2006; 99: 1044–59. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Yu D, Nasir K, Mehrotra R, Chen L, Takasu J, Agrawal N, Liu ST, Blumenthal RS. Diabetes and progression of coronary calcium under the influence of statin therapy. Am Heart J 2005; 149: 695–700. [DOI] [PubMed] [Google Scholar]
- 10.Kizu A, Shioi A, Jono S, Koyama H, Okuno Y, Nishizawa Y. Statins inhibit in vitro calcification of human vascular smooth muscle cells induced by inflammatory mediators. J Cell Biochem 2004; 93: 1011–19. [DOI] [PubMed] [Google Scholar]
- 11.Varady KA, Wang Y, Jones PJ. Role of policosanols in the prevention and treatment of cardiovascular disease. Nutrition Rev 2003; 61: 376–83. [DOI] [PubMed] [Google Scholar]
- 12.Menendez R, Amor AM, Rodeiro I, Gonzalez RM, Gonzalez PC, Alfonso JL, Mas R. Policosanol modulates HMG-CoA reductase activity in cultured fibroblasts. Arch Med Res 2001; 32: 8–12. [DOI] [PubMed] [Google Scholar]
- 13.Hernández F, Illnait J, Más R, Castaño G, Fernández L, González M, Cordovi N, Fernández J. Effect of policosanol on serum lipids and lipoproteins in healthy volunteers. Curr Therapeut Res 1992; 51: 568–75. [Google Scholar]
- 14.Aneiros E, Más R, Calderon B, Illnait J, Fernández L, Castaño G, Fernández JC. Effect of policosanol in lowering cholesterol levels in patients with type II hypercholesterolemia. Curr Therapeut Res 1995; 56: 176–82. [Google Scholar]
- 15.Crespo N, Illnait J, Mas R, Fernandez L, Fernandez J, Castano G. Comparative study of the efficacy and tolerability of policosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int J Clin Pharmacol Res 1998; 19: 117–27. [PubMed] [Google Scholar]
- 16.Iqbal M, Kalsoom, Jafri SA. Effect of Punica granatum flowers extract on hypercholesterolemic and alloxan induced diabetic rats. Glob J Biotechnol Biochem 2011; 6: 83–86. [Google Scholar]
- 17.Ahmed MF, Kazim SM, Ghori SS, Mehjabeen SS, Ahmed SR, Ali SM, Ibrahim M. Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Int J Endocrinol 2010. 2010:841090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desco M-C, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, Sastre J, Viña J. Xanthine oxidase is involved in free radical production in type 1 diabetes protection by allopurinol. Diabetes 2002; 51: 1118–24. [DOI] [PubMed] [Google Scholar]
- 19.Sethi A, Parmar HS, Kumar A. The effect of aspirin on atherogenic diet-induced diabetes mellitus. Basic Clin Pharmacol Toxicol 2011; 108: 371–7. [DOI] [PubMed] [Google Scholar]
- 20.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols 2008; 2008: prot4986–prot4986. [DOI] [PubMed] [Google Scholar]
- 21.Carson WP, Peterson CJ. The role of litter in an old-field community: impact of litter quantity in different seasons on plant species richness and abundance. Oecologia 1990; 85: 8–13. [DOI] [PubMed] [Google Scholar]
- 22.Inarkar MB, Lele S. Extraction and characterization of sugarcane peel wax. ISRN Agron 2012; 2012: 340158–340158. [Google Scholar]
- 23.El-Said M and Amer M. Oils, fats, waxes and surfactants. Cairo, Egypt: Anglo–Egyptian Bookshop, 1965.
- 24.Viola F, Oliaro S, Binello A, Cravotto G. Policosanol: updating and perspectives. Med J Nutrition Metabol 2008; 1: 77–83. [Google Scholar]
- 25.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett 1987; 223: 217–22. [DOI] [PubMed] [Google Scholar]
- 26.Singh DK, Li L, Porter TD. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J Pharmacol Exp Therapeut 2006; 318: 1020–6. [DOI] [PubMed] [Google Scholar]
- 27.Menéndez R, Arruzazabala L, Más R, RíO AD, Amor AM, GonzáLez RM, Carbajal D, Fraga V, Molina V, Illnait J. Cholesterol-lowering effect of policosanol on rabbits with hypercholesterolaemia induced by a wheat starch-casein diet. Br J Nutrition 1997; 77: 923–32. [DOI] [PubMed] [Google Scholar]
- 28.Menéndez R, Fernández L, Del Rio A. Policosanol inhibits cholesterol biosynthesis and enhances LDL processing in cultured human fibroblasts. Biol Res 1994; 27: 199–203. [PubMed] [Google Scholar]
- 29.Pons P, Rodríguez M, Más R, Illnait J, Fernández L, Robaina C, Fernández JC. One-year efficacy and safety of policosanol in patients with type II hypercholesterolemia. Curr Therapeut Res 1994; 55: 1084–92. [PubMed] [Google Scholar]
- 30.Bolego C, Baetta R, Bellosta S, Corsini A, Paoletti R. Safety considerations for statins. Curr Opin Lipidol 2002; 13: 637–44. [DOI] [PubMed] [Google Scholar]
- 31.Castano G, Fernandez L, Mas R, Illnait J, Gamez R, Mendoza S, Mesa M, Fernandez J. Effects of addition of policosanol to omega-3 fatty acid therapy on the lipid profile of patients with type II hypercholesterolaemia. Drugs R&D 2005; 6: 207–19. [DOI] [PubMed] [Google Scholar]
- 32.Fraga V, Menendez R, Amor AM, González RM, Jiménez S, Mas R. Effect of policosanol on in vitro and in vivo rat liver microsomal lipid peroxidation. Arch Med Res 1996; 28: 355–60. [PubMed] [Google Scholar]
- 33.Al-Aly Z, Shao S-J, Lai C-F, Huang E, Cai J, Behrmann A, Cheng S-L, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-α-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol 2007; 27: 2589–96. [DOI] [PubMed] [Google Scholar]
- 34.Sun H, Unoki H, Wang X, Liang J, Ichikawa T, Arai Y, Shiomi M, Marcovina SM, Watanabe T, Fan J. Lipoprotein (a) enhances advanced atherosclerosis and vascular calcification in WHHL transgenic rabbits expressing human apolipoprotein (a). J Biol Chem 2002; 277: 47486–92. [DOI] [PubMed] [Google Scholar]
- 35.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 2001; 31: 509–19. [DOI] [PubMed] [Google Scholar]
- 36.Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstruct Surg 2002; 109: 2384–97. [DOI] [PubMed] [Google Scholar]
- 37.Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun 2004; 320: 424–7. [DOI] [PubMed] [Google Scholar]
- 38.Delanaye P, Cavalier E. Vascular calcifications in dialysis patients. In Nephrology 2010;20.
- 39.Jurutka PW, Whitfield GK, Hsieh J-C, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord 2001; 2: 203–16. [DOI] [PubMed] [Google Scholar]
- 40.Brown E. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. In: Carafoli E, Brini M. (eds). Calcium signalling and disease, Dordrecht: Springer, 2008, pp. 139–67. [DOI] [PubMed] [Google Scholar]
- 41.Gruson D, Buglioni A, Burnett J., Jr PTH: Potential role in management of heart failure. Clin Chim Acta 2014; 433: 290–6. [DOI] [PubMed] [Google Scholar]
- 42.Tomaschitz A, Ritz E, Pieske B, Fahrleitner-Pammer A, Kienreich K, Horina JH, Drechsler C, März W, Ofner M, Pieber TR. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res 2012; 94: 10–19. [DOI] [PubMed] [Google Scholar]
- 43.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Investigat 2001; 108: 1167–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw RJ, Lamia KA, Vasquez D, Koo S-H, Bardeesy N, DePinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310: 1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]