Abstract
Carotenoids, the carotenes and xanthophylls, are essential components in human nutrition. β, β-carotene-9′, 10′-oxygenase 2 (BCO2), also named as β, β-carotene-9′, 10'-dioxygenase 2 (BCDO2) catalyzes the asymmetrical cleavage of carotenoids, whereas β, β-carotene-15, 15′-monooxygenase (BCMO1) conducts the symmetrical cleavage of pro-vitamin A carotenoids into retinoid. Unlike BCMO1, BCO2 has a broader substrate specificity and has been considered an alternative way to produce vitamin A. In contrast to BCMO1, a cytoplasmic protein, BCO2 is located in the inner mitochondrial membrane. The difference in cellular compartmentalization may reflect the different substrate specificity and physiological functions with respect to BCMO1 and BCO2. The BCO2 gene mutations are proven to be associated with yellow color of skin and fat tissue and milk in livestock. Mutation in intron 2 of BCO2 gene is also supposed to be related to the expression of IL-18, a pro-inflammatory cytokine associated with obesity, cardiovascular diseases, and type 2 diabetes. Further, BCO2 is associated with the development of mitochondrial oxidative stress, macular degeneration, anemia, and hepatic steatosis. This review of the literature will mostly address recent updates regarding the role of BCO2 in carotenoid metabolism, and discuss the potential impacts of BCO2 protein and the mutations in mammalian diseases.
Keywords: β, β-carotene-9′, 10′-oxygenase 2, carotenoid, hepatic steatosis, interleukin 18, macular degeneration, mitochondrial stress
Introduction
Carotenoids are 40-carbon lipophilic pigments, which can be synthesized from plants, bacteria, and fungi.1 Carotenoids can be classified into two groups: the xanthophylls, which contain oxygenated groups, and the carotenes, which are only hydrocarbons without oxygenated groups and less polar than the xanthophylls.2,3 Carotenoids present several important functions in human physiology. First, carotenoids, such as zeaxanthin and lutein, function as antioxidants and filters of blue light. These macular pigments decrease chromatic aberration to protect the retina against light induced damage, which is initiated by the formation of reactive oxygen species (ROS) during a photosensitized reaction.4,5 Second, well-known provitamin A carotenoids such as β-carotene are the precursors of retinoid, which are important for eye function.1
For most of the world, provitamin A carotenoids are the major dietary source of vitamin A.6 Vitamin A deficiency leads to childhood blindness and increases morbidity in children, especially in developing countries.7 The amount of vitamin A produced from carotenoids is mainly based on two factors, the absorption and metabolism of carotenoid intake.8,9 The mode of vitamin A production has been suggested to be the symmetric oxidative cleavage of provitamin A carotenoids, such as β-carotene, α-carotene, and β-cryptoxanthin. The gene encoding this symmetric cleavage enzyme β, β-carotene-15, 15′-monooxygenase (BCMO1) was cloned from several species, including human, mouse, chicken, and Drosophila.10,11 However, recent biochemical studies showed that provitamin A carotenoids can also be catalyzed into apocarotenoids, which is different from retinoid. This eccentric cleavage, which was conducted by β, β-carotene-9′, 10′-oxygenase 2 (BCO2), also named as β, β-carotene-9′, 10′-dioxygenase 2 (BCDO2), has been considered as an alternative source of vitamin A production.12,13 Different from BCMO1, BCO2 can also metabolize non-provitamin A carotenoids including lutein and other xanthophylls.14,15 BCMO1 and BCO2 have different intracellular localizations as BCMO1 is located in the cytoplasm,11 while BCO2 is located in the inner mitochondrial membrane.16 The different cellular compartments may reflect the different substrate specificity and physiological functions with respect to BCMO1 and BCO2. Therefore, in this review, we will focus on the enzymatic activity of BCO2 in carotenoid metabolism, genetic characteristics and mutations of BCO2, and finally protein function of BCO2 in nutrition.
Carotenoid metabolism by BCMO1 and BCO2
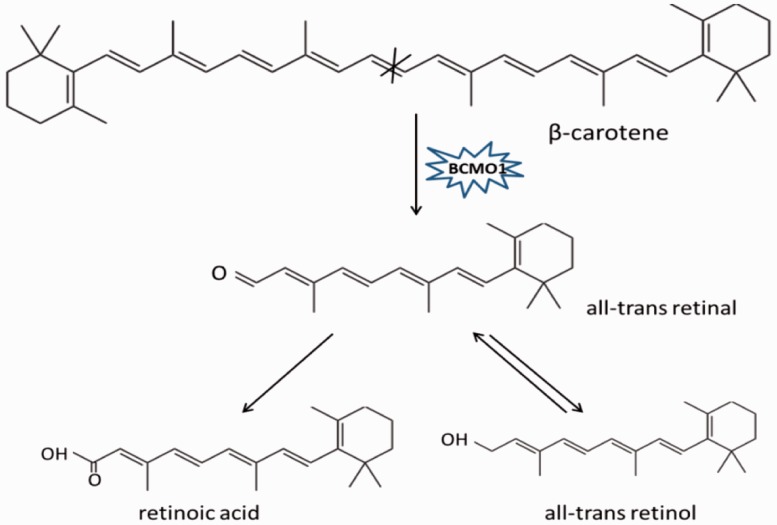
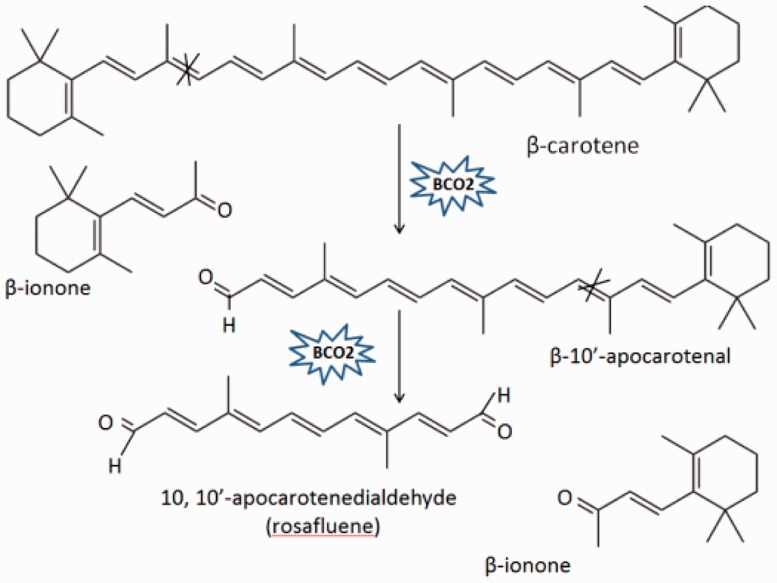
Carotenoids are natural fat-soluble pigments synthesized in plants, bacteria, and fungi, which play important roles in animal and plant physiology. For carotenoid metabolism, the existence of carotenoid cleavage enzymes was discussed as early as 1965, although the first cleavage enzyme VP14 was not identified until 1997.17 In animals, there are two carotenoid cleavage enzymes that have been identified. The first is BCMO1, which catalyzes the centric cleavage at 15, 15′ double bond of β-carotene, forming two molecules of all-trans retinal. This all-trans retinal can be irreversibly oxidized into retinoic acid or reversibly reduced into retinol (Figure 1).10,11 The second enzyme is BCO2, which participates in eccentric cleavage of β-carotene at 9′, 10′ and/or 9, 10 double bond, forming apocarotenoid, which is different from retinoid, including β-apo-10′-carotenal and β-ionone (Figure 2). Although BCMO1 functions as a major enzyme in vitamin A production, BCO2 is considered to be an alternative pathway for forming vitamin A.18,19 Both BCMO1 and BCO2 are family members of nonheme iron oxygenases, which are important in many physiological processes in both animals and plants.20 Besides BCMO1 and BCO2, the retinal pigment epithelium protein of 65 kDa (RPE65) was also included in this nonheme iron oxygenase family, which is crucial for vision as well.21,22
Figure 1.
Enzyme reaction mechanisms of BCMO1. BCMO1 catalyzes carotenoids by cleaving double bond at position 15, 15′ of carotenoids. BCMO1, β-carotene-15, 15′-monooxygenase. (A color version of this figure is available in the online journal.)
Figure 2.
Enzyme reaction mechanisms of BCO2. BCO2, which is located on the inner membrane of mitochondria, catalyzes carotenoids by cleaving double bond at position 9, 10 and 9′, 10′ of carotenoids. BCO2, β,β-carotene-9′, 10′-oxygenase 2. (A color version of this figure is available in the online journal.)
In a recombinant human BCMO1 study, BCMO1 was found to cleave pro-vitamin A carotenoids, which contain at least one nonsubstituted β-ionone ring, such as α-carotene, β-carotene, or β-cryptoxanthin, but fails to catalyze the cleavage of non-provitamin A carotenoids, such as zeaxanthin, lutein, or lycopene.11 BCMO1 was found to be present in the intestinal mucosa, where it facilitates the cleavage of a majority of the carotenoids. BCMO1 was also present in the classical steroidogenic cells, which are sensitive to vitamin A deficiency.23 BCMO1 was therefore proposed to be present in extra-intestinal tissues as a backup system for the local synthesis of vitamin A when intake was decreased.24 In mice, BCMO1 was found to play key roles in retinoid production.25 In humans, a heterozygotic mutation in BCMO1 gene led to the increased plasma levels of β-carotene and decreased plasma concentrations of retinoid.26
The expression and activity of BCO2 has been verified in humans,23 cattle,27 sheep,9 and chicken.28 BCO2 is highly expressed in hepatocytes, which are important for uptake and processing of retinol.29 Except for β-carotene, lutein, lycopene, β-cryptoxanthin, and zeaxanthin are all specific substrates for BCO2. For instance, BCO2 could cleave the acyclic carotenoid, lycopene. However, only the 13-cis- and 5-cis-isomers of lycopene could be cleaved by BCO2.30 BCO2 was also found to cleave lutein at both 9′, 10′ and 9, 10 double bond, leading to the formation of β-ionone, β-apo-10′-carotenal and apo-10, 10′- carotenedialdehyde.31 Interestingly, the production of β-apo-10′-carotenal was significantly affected by the concentration of ferrous iron. When the concentration of ferrous iron decreased from 78% to 67%, the formation of β-apo-10′-carotenal was significantly decreased, showing that iron was a necessary cofactor for this enzymatic cleavage.19 Therefore, these studies demonstrate that BCO2 displays a much broader substrate specificity for carotenoid metabolism as compared to BCMO1.16,32,33
BCO2 genetics
In the entire genome of Drosophila, only BCMO1 gene is found, which is encoded by the ninaB gene. However, in vertebrates, two other genes besides BCMO1, BCO2 and RPE65, are found. Based on the deduced amino acid sequence, BCMO1, BCO2, and RPE65 share approximately 40% overall sequence identity.34 Additionally, there are four histidine residues within the amino acid sequence, conserved in their positions along with several highly conserved regions which can be viewed as protein family motifs.35 According to functional assays in vitro, two tyrosine residues, Y326 and Y235, are reported to be important for the activity of mouse BCMO1. Y235 is completely conserved in BCMO1, BCO2, and RPE65. However, Y326 is well conserved in all BCMO1 homologues and changed to tryptophan and glutamine in all RPE65 and most BCO2, respectively.36 In humans, BCO2 genomically maps to chromosomal position 11q23.1. So far 19 different splice variants of human BCO2 have been identified, 9 of which are protein coding (http://www.ensemble.org). Among all of the 11 identified exonic SNPs in the open reading frame of human BCO2, only rs17113607 and rs10891338 have minor allele frequencies over 5%, according to HapMap (http://hapmap.ncbi.nlm.nih.gov). This indicates that splice variants could be important in introducing large inter-individual differences in BCO2 activity. For example, the intronic SNP rs2115753 at the locus of BCO2 was correlated with IL-18 concentration.37
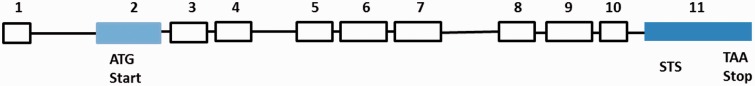
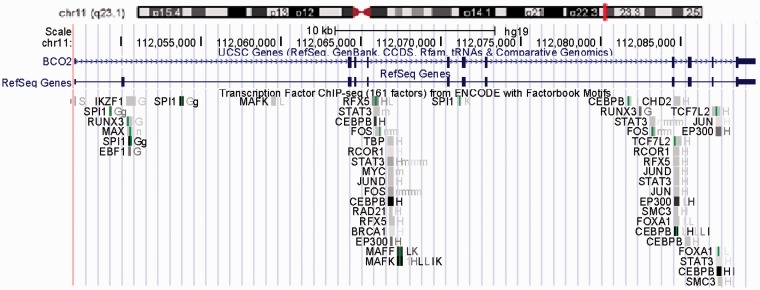
The overall human BCO2 gene spans 11 exons across 42.5 kilobases (kb) on chromosomal 11q23.1 (Figure 3). The coding sequence is 1424 bases (Accession Number is NM_001256400). According to the genetic analysis of the 5′ UTR domain of BCO2, several regulatory transcription factor binding sites are found in the BCO2 gene promoter section, including TATA-binding protein (TBP), activator protein family (AP), and signal transducer and activator of transcription family (STAT) family (Figure 4). AP is a family of transcription factors, composed of proteins including c-Jun, c-Fos, Jun dimerization partner (JDP), and activating transcription factor (ATF). Regulation of the human BCO2 gene expression by AP is found in response to a variety of stimuli, including growth factors, cytokines, stress, and viral and bacterial infections. AP also participates in several cellular processes, such as proliferation, differentiation, and apoptosis.38,39 These characteristics in the human BCO2 gene promotor region indicate that the human BCO2 gene may be a stress responsive gene and participates in several important cellular processes.
Figure 3.
Human BCO2 gene (NM_001256400) structure. Exon-intron structure drawn based on approximate scale. Exon 2 (light blue) contains the translation start codon. Exon 11 (dark blue) contains the stop code and sequence tagged site (STS). (A color version of this figure is available in the online journal.)
Figure 4.
Regulatory transcription factor binding sites in the human BCO2 gene promoter. The human BCO2 gene is on chromosomal 11q23.1. Scale bar at top indicates 10 kb. Assessed from http://genome.ucsc.edu/. (A color version of this figure is available in the online journal.)
Compared with the mouse BCO2 gene, in exon 3, there is an insertion of GGT AAA GCT GCA in the human BCO2 gene. This insertion DNA sequence is a coding sequence, which codes an amino acid sequence in human BCO2 protein. In this way, the major difference between human BCO2 enzyme and mouse BCO2 enzyme is the existence of 4 amino acid residues GKAA in human BCO2. This specific insertion of GKAA is unique to primates and is absent in sheep, rats, mice, cows, and ferrets according to the NCBI protein database search. This GKAA sequence is also absent in mouse and human RPE65, which can cleave all-trans-retinyl esters.40
The effects of BCO2 gene mutation in animals
The deficiency of BCO2 was found to be associated with carotenoids accumulating in the adipose tissues, such as subcutaneous adipose tissue,41 which leads to occurrence of yellow fat in sheep,42,43 cow,27 and chicken.28 In the yellow fat experimental sheep, the BCO2 coding region was sequenced and the nonsense mutation of BCO2 was found in nucleotide position 196 (c.196C > T). This nonsense mutation introduced a stop codon in position 66 of the amino acid, while the full-length protein BCO2 in sheep contains 575 amino acids. It was hypothesized that the mutant in the BCO2 gene forms a nonfunctional enzyme.44
In cows, BCO2 mutation was associated with the yellow color of the adipose tissue and milk, which was mainly caused by deposition of carotenoids.27 Association analysis showed that there were significant differences among cattle with different BCO2 genotypes in concentration of β-carotene and color of subcutaneous fat. Compared with genotypes of BCO2 AA, GA, and GG, the animals with AA had a higher concentration of β-carotene and more yellow fat than those animals with GG or GA genotype. The A allele which is in bovine BCO2 exon 3 is a nonsense mutation. As the full length of BCO2 protein in cattle is 530 amino acids, the change in this allele results in a different polypeptide with presumably a loss in BCO2 enzyme function.27 The data showed that there is a strong link between this BCO2 SNP and fat color in cattle.
BCO2 gene mutation affects IL-18 levels in humans
IL-18, found in Kupffer cells and macrophages, is an important pro-inflammatory cytokine which plays key roles in innate and acquired immunities. It has been shown that the plasma concentration of IL-18 is associated with different diseases, including atherosclerosis, cardiovascular diseases, and type 2 diabetes.37,45,46 In humans, variants at the BCO2 locus were associated with IL-18 levels, but not carotenoid levels in either plasma or macula.37 A two-stage genome-wide association study among women of the Women’s Genome Health Study (WGHS) and European ancestry from the Nurses’ Health Study (NHS) was performed to test this association between BCO2 and IL-18. In the stage of discovery, seven SNPs located at the IL18-BCO2 locus were significantly related to concentrations of IL-18. According to these combined analyses, SNPs rs2115763, rs7106524, and rs1834481 were found to show significant association between the BCO2 and IL-18 genes. SNP rs2115763 was found to be the strongest among these three SNPs. Further selection analysis showed that SNPs rs1834481 and rs2115763 were independently associated with IL-18 levels. Variation of 2.9% plasma levels of IL-18 could be explained together by these two SNPs. SNP rs2115763, which presents the strongest association, is located in intron two of the BCO2 gene and also in the same location of the IL-18 gene.37 It is reported that the IL-18 level was significantly lower in the BCO2 knockout mice.30 However, the underlying mechanism between the IL-18 level and SNP rs2115763 in the BCO2 gene is unknown. SNP rs2115763 might regulate transcription of the IL-18 gene directly, as the BCO2 gene is upstream of the IL-18 gene. Another possibility is that SNP rs2115763 is an indicator of IL-18 gene functional mutation, affecting plasma levels of IL-18.45,46
The function of BCO2 in nutrition
Genetic studies in sheep, cows, and chickens revealed that BCO2 gene mutations could alter the homeostasis of carotenoid metabolism. This was also confirmed in a mouse model. In accordance with the subcellular location of BCO2 (in the inner mitochondrial membrane), carotenoids were found to be accumulated in mitochondria and to impair respiration.16 In hepatic mitochondria, accumulated carotenoids lead to mitochondrial dysfunction, such as reducing ADP-dependent respiration rates. This dysfunction was associated with cell signaling pathways related to oxidative stress and diseases.14 Therefore, BCO2 functions as a key regulator of preventing the adverse effect caused by excess carotenoids. However, we are still at the early stage of understanding the importance of BCO2 in health and disease. The advanced knowledge of BCO2 function might help us to better understand the biochemical, developmental, and physiological roles of carotenoids.
Function of BCO2 on eyes
Carotenoids, such as lutein and zeaxanthin, accumulated in the retina and circulation system, were supposed to be associated with common eye diseases, such as diabetic retinopathy, age-related macular degeneration (AMD) and so on.9,47,48 Researchers tested various genes related to zeaxanthin and lutein status for association with AMD in the Age-Related Eye Disease Study (CAREDS). Four hundred twenty-four SNPs from 24 candidate genes were genotyped in 1663 of 2005 CAREDS participants. After adjusting for ancestry and age, 24 variants from five genes (BCMO1, BCO2, NPCL1L1, ABCG8, and FADS2) were related to AMD independently. SNP rs2250417 in BCO2 is one of the strongest in statistical significance for carotenoid-candidate genes association with AMD.49 The two minor alleles for SNP rs2250417 in BCO2 accounted for increasing risk by almost 50% for AMD.49 Carotenoids with a hydroxylated β-ionone ring including cryptoxanthin and lutein and zeaxanthin are substrates for BCO2, which is located in the mitochondria. As discussed above, mutations of BCO2 might cause an accumulation of zeaxanthin and lutein in the skin of livestock to form yellow fat. However, in this CAREDS, the variants of BCO2 were not related to concentrations of lutein and zeaxanthin in the macular pigment and blood. These findings suggested that BCO2 mutations had tissue specific influences. Moreover, the demonstrated function of BCO2 in inhibiting oxidative stress indicated that BCO2 could be associated with the lower damage and oxidative stress to mitochondria in retinal pigment epithelium.50 Variants of BCO2 might also be associated with AMD through mechanisms involving an impact on the activity of the enzymes involved in inflammation and lipid homeostasis.51,52 One of these common variants, rs2115763 in BCO2, is weakly linked with rs2250417, which was also reported to be related to AMD in this study.34,49
Knock down of BCO2 causes anemia and apoptosis of blood cells
To further understand the importance of BCO2 in development, antisense morpholino oligonucleotides were used to knock down the BCO2 gene in zebrafish.53 No defects in growth pattern or obvious malformations of organ systems, such as eyes and brain, were observed in BCO2 knock down morphants.54 However, the red blood cells in BCO2 knock down morphants were no longer red and the blood filling rate of the heart was also reduced in BCO2 knock down morphants compared with wild type groups. All these results showed that knock down of BCO2 caused anemia. Although the primitive erythropoiesis was not affected in BCO2 knock down morphants, the expression of a later erythrocyte differentiation marker, embryonic α-globin (hbae3), was increased. During the same time, fragmented nuclei were also presented in erythrocytes of BCO2 knock down morphants, indicating the occurrence of apoptosis of red blood cells.55,56 It was also reported that the differentiating blood cells were more sensitive to apoptosis when BCO2 was deficient in zebrafish. Thus, BCO2 is associated with early development of red blood cells in zeabrafish.54
BCO2 is related to carotenoid-induced oxidative stress
Lobo GP and colleagues showed that the protection of cells against apoptosis induced by carotenoids is strictly dependent on BCO2 enzymatic function.54 After transfecting with the murine BCO2 gene, transfected cells could transiently express BCO2. Compared to control cells, the mitochondrial membrane was more polarized in transfected cells. Intrinsic apoptotic markers were assessed in these transfected cells after treatment with carotenoid. Activated caspase-3, which is a mediator of intrinsic apoptosis, was not changed in the transfected cells compared to control cells. Conversely, knock down of BCO2 in cells caused increased ROS production when cells were treated with carotenoids. More importantly, these BCO2 knock down cells showed an initiation of apoptosis after incubation with carotenoid for 2 hr.54,57 Thus, BCO2 expression is inversely associated with cellular oxidative stress.14
BCO2 is important in prevention of hepatic steatosis
A high fat diet induces hepatic steatosis in mice through a possible mechanism of increasing ROS levels and suppressing mitophagy and mitochondrial biogenesis. The hepatic concentration of zeaxanthin and lutein and the expression of BCO2 in hepatic mitochondria were lowered in mice fed with a high fat diet. These results suggested that BCO2 might be a stress responsive gene in mice.58 BCO2 knockout mice also showed lipid accumulation in the liver.14 However, elevated expression of BCO2 attenuated the hepatic steatosis. Knockout of BCO2 might cause mitochondrial dysfunction in the liver, which leads to the impaired fatty acid oxidation and increased hepatic cell susceptibility to oxidative stress.59–61 All these results may suggest that the BCO2 protein is important in prevention of hepatic steatosis.
BCO2 status affects dietary Lycopene’s impacts on hepatic nuclear receptor-, stress- and metabolism-related gene expression
Lycopene, mainly found in dietary tomato, is one of the most abundant carotenoids in human plasma. Recently, lycopene has attracted more attention because of its impact on a decreased risk of certain chronic diseases, including cancers and cardiovascular diseases.62 Therefore, large amounts of work have been expended to study the biological properties of lycopene. Within the past few years, apo-lycopenoids, which are the major derivatives of lycopene cleaved by BCO2, were found to present several biological benefits.63 The lycopene induced effects on nuclear receptor expression was affected by the BCO2 presence. For instance, the tomato diet-induced up-regulation of androgen receptor (Ar) was dependent on the presence of BCO2. Increased hepatic expression of Ar has been linked to lower risk of hepatocellular carcinoma,64 indicating a role of BCO2-mediated expression of hepatic Ar by dietary tomato. Lycopene-induced down-regulation of seven nuclear receptors was also dependent on BCO2 status. The impacted nuclear receptors and coactivators play diverse roles, including mitochondrial metabolism (Ppargc1b),65 carcinogenesis (Nr3c1, Esrra),66 liver metabolic regulation (Esrra),67 inflammation and immunity (Nr3c1),68 gene transcription silencing by histone deacetylation (Hdac3), heme-mediated transcription repression (Nr1d2)69 and mediation of steroid receptor-driven gene transcription (Ncoa2 and Ncoa4). The effect of lycopene diet on metabolism- and stress-related genes was also related to BCO2. However, the pattern is not consistent with the nuclear receptor-related genes.30,70
Summary and future prospective
Xiangdong Wang, Johannes von Lintig, etc. have demonstrated that BCO2 plays an important role in the metabolism of nonprovitamin A carotenoids, such as lycopene, lutein, and zeaxanthin.18,19 BCO2 catalyzes these nonprovitamin A carotenoids through asymmetric cleavage at 9, 10 and/or 9′, 10′ double bond and metabolites.18,19 Genetic evidence suggests that BCO2 mutation is associated with yellow skin, fat tissue, and milk in livestock. Human genetic study shows that mutation in intron 2 of BCO2 gene may be related to the IL-18, a pro-inflammatory cytokine associated with various types of human diseases, including obesity, cardiovascular diseases, and type 2 diabetes.37 Furthermore, BCO2 may play important roles in the development of mitochondrial oxidative stress,14 macular degeneration,49 anemia,54 and hepatic steatosis.71
Carotenoids, such as zeaxanthin, lutein and their metabolites, meso-zeaxanthin, are macular pigments of human retina, which are known to function as antioxidant and light-screening players in nature.72–74 The chemical and spatial accumulation of high levels of xanthophylls in the retina is a specific feature of primates in contrast to other mammals, but the biochemical mechanism for this unique feature is unclear.5,75,76 To better understand the underlying mechanism, it is hypothesized that BCO2 is not expressed, or inactive in primates’ retina. However, studies have already shown that BCO2 is expressed in both human and mouse retina. Therefore, the enzyme activity of human BCO2 was investigated by cell-based carotenoid cleavage assay. According to this study, BCO2 was shown to be an inactive carotenoid cleavage enzyme. Another study showed that xanthophylls carotenoids are highly uptaken by human ARPE-19 cells, although no cleavage products of xanthophyll were found in these cells.77 Moreover, no eccentric cleavage products were found when human subjects were given labeled supplements of β-carotene.78,79 No association between human BCO2 SNPs and optical density of macular pigment was reported, which also implies that human BCO2 cannot catalyze macular pigments. Moreover, human BCO2 was demonstrated to be unable to bind with appropriate carotenoid substrates in the active site. This might be another reason that human BCO2 is inactive in the human retina. However, Johannes von Lintig and colleagues provided evidence that the enzymatic function of BCO2 is conserved in primates. Therefore, it is still controversial about the enzymatic activity of BCO2 in humans.40,80
We are still at the early stage of understanding the importance of BCO2 in health and disease. As BCO2 is located in the inner mitochondrial membrane,16 BCO2 might impact on normal mitochondrial function through targeting the ETC/OXPHOS, including inhibiting the activity of different complexes, increasing the proton leak or decreasing the ATP production efficiency. As discussed above, knockout of BCO2 in the liver could increase the production of ROS.14 Recently, it has been proposed that mitochondria could prevent the ROS production through forming supercomplexes.81 There is accumulating evidence demonstrating that mitochondrial respiratory chain complexes associated with formation of different supermolecules, known as supercomplexes.82,83 Recent evidence supports the function of these supercomplexes, including: (1) the formation of mitochondrial respiratory chain supercomplexes could minimize the production of ROS which is generated from complex I,81 (2) the mitochondrial respiratory chain supercomplexes may intergrate the cytochrome c and coenzyme Q to induce a kinetic advantage via facilitating efficient transportation of electrons through substrate channeling,84 (3) mammalian complex I is assembled in mitochondrial respiratory chain supercomplexes, which help to stabilize the complex I.85,86 It would therefore be interesting to investigate the association between BCO2 and the formation of mitochondrial respiratory chain supercomplexes. This might provide an explanation for why BCO2 knockout could cause increased ROS production.
Acknowledgments
This work was supported by the grants from the USDA National Institute of Food and Agriculture 2015-67018-23176, USDA NIFA Hatch Project OKL02992, National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103648, and Oklahoma State University start-up project (to DL). This paper was presented in part at the Experimental Biology, May 2015, Boston, MA, USA.
Authors’ contributions
All authors participated in the writing, review, and editing of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.von Lintig J. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr 2012; 96: 1234S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demmig-Adams B, Adams WW. Antioxidants in photosynthesis and human nutrition. Science 2002; 298: 2149–53. [DOI] [PubMed] [Google Scholar]
- 3.Nagao A. Absorption and metabolism of dietary carotenoids. Biofactors 2011; 37: 83–7. [DOI] [PubMed] [Google Scholar]
- 4.Barker FM, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci 2011; 52: 3934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 2003; 23: 171–201. [DOI] [PubMed] [Google Scholar]
- 6.Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. Beta-carotene is an important vitamin A source for humans. J Nutr 2010; 140: 2268S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 2012; 96: 1204S–6S. [DOI] [PubMed] [Google Scholar]
- 8.Amengual J, Widjaja-Adhi MA, Rodriguez-Santiago S, Hessel S, Golczak M, Palczewski K, von Lintig J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem 2013; 288: 34081–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 2010; 30: 35–56. [DOI] [PubMed] [Google Scholar]
- 10.Lietz G, Lange J, Rimbach G. Molecular and dietary regulation of beta,beta-carotene 15,15′-monooxygenase 1 (BCMO1). Arch Biochem Biophys 2010; 502: 8–16. [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15’-monooxygenase. J Biol Chem 2002; 277: 23942–8. [DOI] [PubMed] [Google Scholar]
- 12.Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J Lipid Res 2013; 54: 1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW, Jr, Harrison EH. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J Biol Chem 2012; 287: 15886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 2011; 25: 948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9’,10’-monooxygenase. Arch Biochem Biophys 2011; 506: 109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palczewski G, Amengual J, Hoppel CL, von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J 2014; 28: 4457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997; 276: 1872–4. [DOI] [PubMed] [Google Scholar]
- 18.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001; 276: 14110–6. [DOI] [PubMed] [Google Scholar]
- 19.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9’,10’-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006; 281: 19327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moise AR, von Lintig J, Palczewski K. Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids. Trends Plant Sci 2005; 10: 178–86. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Conley SM, Naash MI. RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet 2009; 30: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 1998; 20: 344–51. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist A, Andersson S. Cell type-specific expression of beta-carotene 15,15’-mono-oxygenase in human tissues. J Histochem Cytochem 2004; 52: 491–9. [DOI] [PubMed] [Google Scholar]
- 24.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9’,10’-monooxygenase in human tissues. J Histochem Cytochem 2005; 53: 1403–12. [DOI] [PubMed] [Google Scholar]
- 25.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 2007; 282: 33553–61. [DOI] [PubMed] [Google Scholar]
- 26.Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15’-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 2007; 137: 2346–50. [DOI] [PubMed] [Google Scholar]
- 27.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, van der Does Y, Macgibbon AH, Spelman RJ, Lehnert K, Snell RG. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 2009; 182: 923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson J, Larson G, Gunnarsson U, Bed’hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, Jensen P, Andersson L. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet 2008; 4: e1000010–e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, Blaner WS. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys 2010; 504: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan HL, Moran NE, Cichon MJ, Riedl KM, Schwartz SJ, Erdman JW, Jr, Pearl DK, Thomas-Ahner JM, Clinton SK. beta-Carotene-9’,10’-oxygenase status modulates the impact of dietary tomato and lycopene on hepatic nuclear receptor-, stress-, and metabolism-related gene expression in mice. J Nutr 2014; 144: 431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landrum JT, Chatfield DC, Mebel AM, Alvarez-Calderon F, Fernandez MV. The conformation of end-groups is one determinant of carotenoid topology suitable for high fidelity molecular recognition: a study of beta- and epsilon-end-groups. Arch Biochem Biophys 2010; 493: 169–74. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann J, Bona-Lovasz J, Beuttler H, Altenbuchner J. In vivo and in vitro studies on the carotenoid cleavage oxygenases from Sphingopyxis alaskensis RB2256 and Plesiocystis pacifica SIR-1 revealed their substrate specificities and non-retinal-forming cleavage activities. FEBS J 2012; 279: 3911–24. [DOI] [PubMed] [Google Scholar]
- 33.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15’-dioxygenase. J Biol Chem 2001; 276: 6560–5. [DOI] [PubMed] [Google Scholar]
- 34.Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Importance of beta,beta-carotene 15,15’-monooxygenase 1 (BCMO1) and beta,beta-carotene 9’,10’-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res 2012; 56: 241–50. [DOI] [PubMed] [Google Scholar]
- 35.Poliakov E, Gentleman S, Cunningham FX, Jr, Miller-Ihli NJ, Redmond TM. Key role of conserved histidines in recombinant mouse beta-carotene 15,15’-monooxygenase-1 activity. J Biol Chem 2005; 280: 29217–23. [DOI] [PubMed] [Google Scholar]
- 36.Poliakov E, Gentleman S, Chander P, Cunningham FX, Jr, Grigorenko BL, Nemuhin AV, Redmond TM. Biochemical evidence for the tyrosine involvement in cationic intermediate stabilization in mouse beta-carotene 15, 15’-monooxygenase. BMC Biochem 2009; 10: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ, Hu FB, Qi L. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol 2010; 30: 885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 2002; 4: E131–6. [DOI] [PubMed] [Google Scholar]
- 39.Steinmuller L, Cibelli G, Moll JR, Vinson C, Thiel G. Regulation and composition of activator protein 1 (AP-1) transcription factors controlling collagenase and c-Jun promoter activities. Biochem J 2001; 360: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babino D, Palczewski G, Widjaja-Adhi MA, Kiser PD, Golczak M, von Lintig J. Characterization of the Role of β-Carotene 9,10-Dioxygenase in Macular Pigment Metabolism. J Biol Chem 2015;290:24844–57. [DOI] [PMC free article] [PubMed]
- 41.Kloer DP, Schulz GE. Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci 2006; 63: 2291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt H, Kurtzer R, Eisenreich W, Schwab W. The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J Biol Chem 2006; 281: 9845–51. [DOI] [PubMed] [Google Scholar]
- 43.Vage DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet 2010; 11: 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill F. Xanthophyll pigmentation in sheep fat. Nature 1962; 194: 865–6. [DOI] [PubMed] [Google Scholar]
- 45.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, Rafferty I, Lauretani F, Murray A, Gibbs JR, Paolisso G, Rafiq S, Simon-Sanchez J, Lango H, Scholz S, Weedon MN, Arepalli S, Rice N, Washecka N, Hurst A, Britton A, Henley W, van de Leemput J, Li R, Newman AB, Tranah G, Harris T, Panicker V, Dayan C, Bennett A, McCarthy MI, Ruokonen A, Jarvelin MR, Guralnik J, Bandinelli S, Frayling TM, Singleton A, Ferrucci L. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet 2008; 4: e1000072–e1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson SR, McCaskie PA, Beilby JP, Hun J, Jennens M, Chapman C, Thompson P, Humphries SE. IL18 haplotypes are associated with serum IL-18 concentrations in a population-based study and a cohort of individuals with premature coronary heart disease. Clin Chem 2007; 53: 2078–85. [DOI] [PubMed] [Google Scholar]
- 47.von Lintig J. Metabolism of carotenoids and retinoids related to vision. J Biol Chem 2012; 287: 1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurnham DI. Macular zeaxanthins and lutein – a review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr Res Rev 2007; 20: 163–79. [DOI] [PubMed] [Google Scholar]
- 49.Meyers KJ, Mares JA, Igo RP, Jr, Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, Blodi B, Gehrs K, Tinker L, Wallace R, Robinson J, LeBlanc ES, Sarto G, Bernstein PS, SanGiovanni JP, Iyengar SK. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Invest Ophthalmol Vis Sci 2014; 55: 587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005; 26: 459–516. [DOI] [PubMed] [Google Scholar]
- 51.Ford NA, Elsen AC, Erdman JW., Jr Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res 2013; 33: 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012; 249: 218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 2000; 26: 216–20. [DOI] [PubMed] [Google Scholar]
- 54.Lobo GP, Isken A, Hoff S, Babino D, von Lintig J. BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 2012; 139: 2966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood 2007; 109: 5208–14. [DOI] [PubMed] [Google Scholar]
- 56.Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 2006; 25: 963–75. [DOI] [PubMed] [Google Scholar]
- 57.Siems W, Sommerburg O, Schild L, Augustin W, Langhans CD, Wiswedel I. Beta-carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J 2002; 16: 1289–91. [DOI] [PubMed] [Google Scholar]
- 58.Lin D, He H, Ji H, Willis J, Willard L, Jiang Y, Medeiros DM, Wark L, Han J, Liu Y, Lu B. Wolfberries potentiate mitophagy and enhance mitochondrial biogenesis leading to prevention of hepatic steatosis in obese mice: the role of AMP-activated protein kinase alpha2 subunit. Mol Nutr Food Res 2014; 58: 1005–15. [DOI] [PubMed] [Google Scholar]
- 59.Arendt BM, Allard JP. Effect of atorvastatin, vitamin E and C on nonalcoholic fatty liver disease: is the combination required? Am J Gastroenterol 2011; 106: 78–80. [DOI] [PubMed] [Google Scholar]
- 60.Morinobu T, Tamai H. Beta-carotene 15,15’-dioxygenase activity in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo) 2000; 46: 263–5. [DOI] [PubMed] [Google Scholar]
- 61.Musso G, Gambino R, De Michieli F, Biroli G, Premoli A, Pagano G, Bo S, Durazzo M, Cassader M. Nitrosative stress predicts the presence and severity of nonalcoholic fatty liver at different stages of the development of insulin resistance and metabolic syndrome: possible role of vitamin A intake. Am J Clin Nutr 2007; 86: 661–71. [DOI] [PubMed] [Google Scholar]
- 62.Story EN, Kopec RE, Schwartz SJ, Harris GK. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 2010; 1: 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr 2012; 96: 1214S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 2014; 33: 3225–34. [DOI] [PubMed] [Google Scholar]
- 65.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006; 27: 728–35. [DOI] [PubMed] [Google Scholar]
- 66.Chang CY, Kazmin D, Jasper JS, Kunder R, Zuercher WJ, McDonnell DP. The metabolic regulator ERRalpha, a downstream target of HER2/IGF-1R, as a therapeutic target in breast cancer. Cancer Cell 2011; 20: 500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dufour CR, Levasseur MP, Pham NH, Eichner LJ, Wilson BJ, Charest-Marcotte A, Duguay D, Poirier-Heon JF, Cermakian N, Giguere V. Genomic convergence among ERRalpha, PROX1, and BMAL1 in the control of metabolic clock outputs. PLoS Genet 2011; 7: e1002143–e1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramamoorthy S, Cidlowski JA. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr Dev 2013; 24: 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burris TP. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol Endocrinol 2008; 22: 1509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ip BC, Liu C, Ausman LM, von Lintig J, Wang XD. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev Res (Phila) 2014; 7: 1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ip BC, Liu C, Lichtenstein AH, von Lintig J, Wang XD. Lycopene and apo-10’-lycopenoic acid have differential mechanisms of protection against hepatic steatosis in beta-carotene-9’,10’-oxygenase knockout male mice. J Nutr 2015; 145: 268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res 1985; 25: 1531–5. [DOI] [PubMed] [Google Scholar]
- 73.Bone RA, Landrum JT. Macular pigment in Henle fiber membranes: a model for Haidinger’s brushes. Vision Res 1984; 24: 103–8. [DOI] [PubMed] [Google Scholar]
- 74.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 1997; 38: 1802–11. [PubMed] [Google Scholar]
- 75.Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol 2006; 124: 1038–45. [DOI] [PubMed] [Google Scholar]
- 76.Beatty S, van Kuijk FJ, Chakravarthy U. Macular pigment and age-related macular degeneration: longitudinal data and better techniques of measurement are needed. Invest Ophthalmol Vis Sci 2008; 49: 843–5. [DOI] [PubMed] [Google Scholar]
- 77.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res 2008; 49: 1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krinsky NI, Wang XD, Tang G, Russell RM. Mechanism of carotenoid cleavage to retinoids. Ann N Y Acad Sci 1993; 691: 167–76. [DOI] [PubMed] [Google Scholar]
- 79.Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr 2002; 22: 483–504. [DOI] [PubMed] [Google Scholar]
- 80.Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS. Inactivity of human β, β-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci 2014;111:10173–8. [DOI] [PMC free article] [PubMed]
- 81.Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 2013; 19: 1469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chance B, Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature 1955; 176: 250–4. [DOI] [PubMed] [Google Scholar]
- 83.Cruciat CM, Brunner S, Baumann F, Neupert W, Stuart RA. The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J Biol Chem 2000; 275: 18093–8. [DOI] [PubMed] [Google Scholar]
- 84.Genova ML, Lenaz G. A critical appraisal of the role of respiratory supercomplexes in mitochondria. Biol Chem 2013; 394: 631–9. [DOI] [PubMed] [Google Scholar]
- 85.Genova ML, Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim Biophys Acta 2014; 1837: 427–43. [DOI] [PubMed] [Google Scholar]
- 86.Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A, Ugalde C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab 2012; 15: 324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]