Abstract
Uveitis is an important cause of vision loss worldwide due to its sight-threatening complications, especially cystoid macular edema, as well as choroidal neovascularization, macular ischemia, cataract, and glaucoma. Systemic corticosteroids are the mainstay of therapy for noninfectious posterior uveitis; however, various systemic side effects can occur. Intravitreal medication achieves a therapeutic level in the vitreous while minimizing systemic complications and is thus used as an exciting alternative. Corticosteroids, antivascular endothelial growth factors, immunomodulators such as methotrexate and sirolimus, and nonsteroidal anti-inflammatory drugs are currently available for intravitreal therapy. This article reviews the existing literature for efficacy and safety of these various options for intravitreal drug therapy for the management of noninfectious uveitis (mainly intermediate, posterior, and panuveitis).
Keywords: intravitreal therapy, noninfectious uveitis, posterior uveitis, intravitreal steroids, intravitreal methotrexate
Introduction
Uveitis is an important cause of vision loss worldwide and is the third leading cause of vision loss in developed countries.1,2 Uveitis is classified on the basis of the location of inflammation into anterior (iritis, iridocyclitis, and anterior cyclitis), intermediate (pars planitis, posterior cyclitis, and hyalitis), and posterior (focal, multifocal, or diffuse choroiditis, chorioretinitis, retinitis, and neuroretinitis). Panuveitis involves the inflammation of the anterior chamber, vitreous, retina, and choroid. Anterior uveitis is the most commonly encountered entity, and posterior uveitis constitutes 15%–22% of all cases of uveitis. Posterior uveitis is the most difficult to treat due to challenges encountered in delivering efficacious levels of therapeutic agents and can lead to visual morbidity.3
The goals of therapy in noninfectious uveitis (NIU) are to control inflammation, minimize recurrences, and prevent the occurrence of sight-threatening complications secondary to the disease or the therapy itself. The sight-threatening complications of chronic NIU include cystoid macular edema (CME) and choroidal neovascularization (CNV), with CME being the most common.4
Currently, systemic immunomodulation with oral corticosteroids is the mainstay of treatment to control the inflammation. Systemic steroid sparing immunomodulators such as antimetabolites (methotrexate, azathioprine, and mycophenolate mofetil) and calcineurin inhibitors (cyclosporine and tacrolimus), among others, are often included in the treatment plan.5
Although oral corticosteroids and immunomodulatory therapy are able to effectively control inflammation in the eyes, a number of systemic and ocular side effects are associated with their prolonged usage, which present a significant challenge in treating NIU.6 Additionally, topical corticosteroids may not reach the intermediate and posterior portions of the eye in therapeutic concentrations due to poor penetration to the posterior segment of the eye.7 With intrasvitreal corticosteroids, the drug is able to effectively reach the target area with the benefit of avoiding systemic side effects. In unilateral uveitis, intravitreal agents can be considered a safe and effective alternative to systemic immunosuppression. However, intravitreal steroids are commonly associated with raised intraocular pressure (IOP) and cataract formation, apart from the risks related to the intravitreal procedure itself such as endophthalmitis. Therefore, the use of alternate drugs for intravitreal therapy targeting different inflammatory pathways is being continuously explored.
This article reviews the current forms of intravitreal drug therapy for the treatment of NIU, and a summary of various forms of intravitreal therapy is provided in Tables 1–4.
Table 1.
Studies on intravitreal triamcinolone (demographics)
| Study | Period of study | Study design | Study duration | Number of participants/eyes | Demographics
|
|
|---|---|---|---|---|---|---|
| Age (years) |
Sex (female) |
|||||
| Kok et al8 | – | Retrospective noncomparative (nonrandomized, uncontrolled) interventional case series | Mean 8.0 months (range, 3–51 months) | 65 eyes of 54 patients | 44±15 (range, 14–76) | – |
| Park et al9 | July 2005 to February 2011 | Retrospective consecutive case series | Follow-up >24 months | 49 eyes of 49 patients | 38.6±9.8 (range, 20–68) | 38.80% |
| Tuncer et al10 | November 2002 to April 2006 | Retrospective consecutive case series | Mean follow-up 28 months (range, 9–50 months) | 18 eyes of 15 patients | 24.7±6.0 (range, 17–36) | 27% |
| Sallam et al11 | – | Retrospective consecutive case series | Follow-up ≥3 months | 41 eyes of 35 patients | – | – |
Notes: Data presented as mean ± SD. “–”, data not available.
Table 2.
Studies on intravitreal triamcinolone (clinical features)
| Study | Clinical features of participants
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of study eye | Details | Laterality of condition | Duration of uveitis | Presence of other ocular conditions | Previous uveitis treatment | Presence of systemic conditions | Mean baseline VA (logMAR) | Other baseline values | |
| Kok et al8 | Uveitic CME with inadequate response to oral CS ± orbital floor CS injections | – | – | Duration of CME: mean 27.7 months (range, 5–70 months) | 43% clear lens, 26% cataract, 29% pseudophakic | – | – | 0.65 | – |
| Park et al9 | Behçet’s disease, uveitis unresponsive or intolerant to systemic medications | 81.6% panuveitis, 18.4% posterior, 62.5% have angiographic CME | 0% bilateral | 55.3±38.9 months (range, 3–120 months) | 22.4% with known glaucoma or history of IOP elevation, 28.6%; clear lens, 30.6%; cataract, 40.8%; pseudophakic | 67.3% on oral prednisolone of >10 mg/day, 79.6% on immunosuppressants | – | 0.89±0.70 | Mean number of acute attacks during the year before the study: 1.93±0.85 (range, 1–4) |
| Tuncer et al10 | Severe panuveitis attacks secondary to Behçet’s disease. Unresponsive or intolerant to systemic medications | – | 87% bilateral | 22.5 months (range, 2–60 months) | – | 87% on systemic medications at study entry | 47% cushingoid, 71% of these patients had other systemic adverse effects | – | – |
| Sallam et al11 | CME proven on optical coherence tomography or fluorescein angiography | – | – | – | – | 54% treated with systemic therapy | – | – | – |
Note: “–” data not available.
Abbreviations: CME, cystoid macular edema; CS, corticosteroids; VA, visual acuity; IOP, intraocular pressure; logMAR, logarithm of Minimal Angle of Resolution.
Table 3.
Studies on intravitreal triamcinolone (outcomes)
| Study | Number of participants/eyes | Intervention
|
Numbers excluding those lost to follow-up/dropout | Outcomes measured
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVTA | Systemic CS | Immunosuppression | BCVA | ME | Uveitis activity/vitreous haze score | Mean time to first recurrence of uveitis | Uveitis recurrence rate | Reinjections | Others | |||
| Kok et al8 | 65 eyes of 54 patients | 4 mg/0.1 L | ± | ± | Nil | 0.39 (P<0.005). Mean improvement in VA only statistically significant in those ≤60. Best BCVA at 4 weeks. No change in 16.9% of eyes | – | – | – | – | 12% | 54.5% eyes could reduce or stop systemic medications |
| Park et al9 | 49 eyes of 49 patients | 4 mg/0.1 mL | ± | ± | – | 3 months: 0.59±0.55, 6 months: 0.60±0.58, 12 months: 0.70±0.65, 18 months: 0.62±0.60, 24 months: 0.64±0.72, Final visits: 0.68±0.79 (all P<0.001). BCVA improvement rate of ≥3 lines from baseline: 40.8% at 6 months, 42.9% at 12 months, 38.8% at 24 months | 85% either completely or partially resolved after 6 months | 6 months: 87% patients vitreous haze completely resolved after median period of 49 days (range, 6–152 days) postinjection | Median 210 days post-IVTA injection (74–900 days) | 60% recurrence before 12 months postinjection | 30.6% had repeated injections in 24 months (80% one repeat, 20% two repeats) (no difference in BCVA change with and without repeated injections) | 49% of patients could reduce or stop systemic medications at 24 months |
| Tuncer et al10 | 18 eyes of 15 patients | 4 mg/0.1 mL | ✓ (doses tapered per clinician discretion) | ✓ | – | Mean increase until first month: 0.61±0.33 (range, 0.1–1.1). 22.2% had further improvement after 1 month. 55.5% maintained improved VA until end of follow-up | Resolved after 1 month | Mean period of 25.4±11.3 days to resolution of intraocular inflammation | Mean 10 months (range, 10–28 months) | 22% of eyes | 0 | Retinal vasculitis resolved after 1 month. Doses of systemic medications could be stopped or reduced |
| Sallam et al11 | 41 eyes of 35 patients | At least two injections of 4 mg/0.1 mL | ± (doses tapered per clinician discretion) | ± | – | Each injection led to statistically significant improvement in BCVA (P<0.01). Efficacy of repeated injections was similar | After first injection: 88% resolved in mean of 5 weeks (range, 1–14 weeks). After second injection: 76% improved | – | After first injection: mean of 7 months (range, 2–23 months). After second injection: recurred at mean of 5 months (range, 1–13 months) | After first: 100% recurrence of ME. After second: 81% recurrence of ME | 57% had three injections, 29% >3 injections | 31% of patients could reduce or stop systemic medication |
Notes: Data presented as mean ± SD. “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: BCVA, best-corrected visual acuity; CS, corticosteroids; IVTA, intravitreal triamcinolone acetonide; ME, macular edema; VA, visual acuity.
Table 4.
Studies on intravitreal triamcinolone (adverse events)
| Study | Number of participants/eyes | Intervention
|
Adverse events
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ocular
|
Systemic | ||||||||
| IVTA | Systemic CS | Immunosuppression | Cataracts | Raised IOP
|
Others | ||||
| >10 mmHg/requiring medications | Requiring surgery | ||||||||
| Kok et al8 | 65 eyes of 54 patients | 4 mg/0.1 mL | ± | ± | 14% of previously clear lens developed PSC. 12% of eyes with preexisting cataract had increased opacity | 43% | None | None | None |
| Park et al9 | 49 eyes of 49 patients | 4 mg/0.1 mL | ± | ± | 62% of phakic eyes had surgery | 39.5% of eyes with no known raised IOP | 3% of eyes with no known raised IOP | None | None |
| Tuncer et al10 | 18 eyes of 15 patients | 4 mg/0.1 mL | ✓ (doses tapered per clinician discretion) | ✓ | 55.50% | 66.6% IOP elevation >21 mmHg detected mean 29.6 days (range, 7–66 days) | None | None | None |
| Sallam et al11 | 41 eyes of 35 patients | At least two injections of 4 mg/0.1 mL | ± (doses tapered per clinician discretion) | ± | 100% by fifth IVTA injection | 46% (magnitude of IOP change did not increase with repeat injections) | None | One eye from total of 118 IVTA injections: sterile endophthalmitis | None |
Notes: ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: IOP, intraocular pressure; IVTA, intravitreal triamcinolone acetonide; PSC, posterior subcapsular cataract; CS, corticosteroids.
Methods
In this study, English literature in PubMed, MEDLINE, and Cochrane databases was searched. The search included randomized trials and observational studies, comprised of prospective and retrospective cohort studies, case series, and case–control studies that evaluated the use of intravitreal therapy in the treatment of NIU. It also included preclinical studies for drugs, which have not undergone clinical trials. Studies with a sample size of <15 or pediatric population or animal studies for which human studies were present were excluded. The search was conducted with the following terminology: (((“Uveitis/therapy” [Mesh] OR “Uveitis, Intermediate/therapy” [Mesh]) OR “Uveitis, Posterior/therapy” [Mesh]) OR “Uveitis, Anterior/therapy” [Mesh]) AND (“Intravitreal Injections” [Mesh] OR “Drug implants” [Mesh]). This yielded a total of 201 papers from PubMed. A search of “Uveitis” and (“therapy” or “treatment”) and (“intravitreal injections” or “drug implants”) on Cochrane yielded 49 trials. References obtained from these articles were hand-searched to identify relevant literature (Figure 1).
Figure 1.
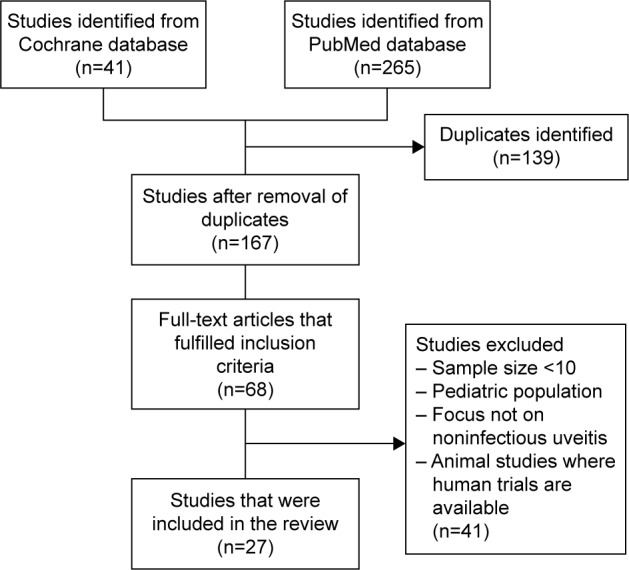
Literature review.
Intravitreal agents for noninfectious posterior uveitis
Intravitreal corticosteroids
Currently, there are various methods to deliver corticosteroids to the vitreous and retina: intravitreal triamcinolone acetonide (IVTA) (Triesence® [Alcon, Ft Worth, TX, USA] and Trivaris® [Allergan, Riverside, CA, USA], which are approved by the US Food and Drug Administration [FDA] for intraocular use, and off-label Kenalog® 40 [Bristol-Myers Squibb, Princeton, NJ, USA]), as well as intraocular drug implants: 0.7 mg dexamethasone implant (Ozurdex®; Allergan Inc., Irvine, CA, USA), 0.59 mg fluocinolone acetonide implant (FAi) (Retisert®; Bausch & Lomb Inc., Rochester, NY, USA), and 0.019 mg FAi (ILUVIEN®; Alimera Sciences Limited, Aldershot, UK).
IVTA injection
IVTA is able to effectively deliver corticosteroids to the vitreous and retina while avoiding the side effects associated with systemic therapy. Studies on IVTA have mainly evaluated its effect on uveitic CME as well as Behçet’s disease. Tables 1–4 provide the summary of studies regarding IVTA.
In a retrospective noncomparative interventional case series of 65 eyes, Kok et al reported the effects of 4 mg/0.1 mL IVTA on uveitic CME in the short term.8 It was found that best-corrected visual acuity (BCVA) improved at a mean of 4 weeks with the improvement being greater in younger patients as well as in those who had CME for a shorter period of time. About 54.5% of eyes were able to have their systemic medications reduced or stopped during the study duration with the mean follow-up time being 8 months. The main adverse ocular event observed was raised IOP; 43.1% of patients experienced a raise in IOP >10 mmHg but none required surgery, and 14.3% of patients with clear lens developed cataracts, whereas 11.8% of patients with preexisting cataracts experienced exacerbation during the mean follow-up period of 17.1 months. Eyes with a shorter mean follow-up period of 7 months did not show any lens changes. This is most possibly due to the likelihood of increased injections in the eyes with a longer follow-up period. Limitations of this study would be that it was a non-randomized and uncontrolled study with variable follow-up periods.
In another retrospective case series of 49 eyes with Behçet’s disease with a standardized follow-up period of at least 24 months, Park et al reported that 4 mg/0.1 mL of IVTA improved the BCVA in these eyes, which had been previously unresponsive or intolerant to systemic medications.9 After a median of 49 days, inflammation was under control as evident by the absence of vitreous haze (VH) in 87% of eyes. However, 60% of these eyes relapsed before 12 months post-IVTA, and the mean time for uveitis recurrence was 210 days. With repeated injections, there was no statistically significant difference in the BCVA change in eyes with single versus multiple injections. Systemic medications were reduced or stopped in 49% of patients after 24 months. Side effects reported include cataract formation that was observed in 62% of phakic eyes after repeated injections as well as raised IOP in eyes with and without preexisting raised IOP. The effect of repeated IVTA injections on IOP was not evaluated in this study, as eyes with a significant raise in IOP following the initial injection did not receive a repeated injection.
Tuncer et al also performed a retrospective case series of 18 eyes with panuveitis secondary to Behçet’s disease, which did not respond or were intolerant to systemic medications.10 The authors reported that there was an increase in mean BCVA following the injection. Resolution of intraocular inflammation was also achieved after a mean of 25.4 days. Retinitis, vasculitis, as well as macular edema were resolved at the end of 1 month. However, recurrence of uveitis occurred at a period of 10–18 months. Similar to the previous studies, the dose for systemic corticosteroids was tapered down at 1–5 months, resulting in the improvement in cushingoid features. Ocular adverse events of cataracts and raised IOP were also observed.
Given that the studies have shown that repeated IVTA injections are likely to be required in the treatment of NIU due to its short duration of action, there have been concerns regarding the effects and safety of repeated IVTA injections. Sallam et al performed a retrospective consecutive case series of 41 uveitic eyes with CME which received at least two IVTA injections.11 There was a statistically significant improvement in BCVA following each injection with no evidence of reducing efficacy with repeated injections. The majority of eyes had raised IOP, but there was no increase in the degree of change in IOP with each repeated injection. However, repeated IVTA injections were associated with increased cataract formation in all phakic patients (100%). Importantly, patients were followed up for only 3 months after their last IVTA injections so the variable follow-up time may have affected the results, possibly resulting in an under-representation of ocular adverse events.
In summary, based on the literature review, it is found that IVTA can achieve improved visual acuity and inflammation control acutely but that repeated injections are needed to maintain the effects. It is also important to look out for the associated ocular adverse events such as cataract formation, which are more prominent with repeated injections, as well as increased IOP. Therefore, IVTA can be useful in NIU where patients are intolerant or nonresponsive to systemic medications and is also advisable in unilateral disease. Typically in bilateral patients, systemic immunosuppression is considered by most uveitis specialists.
Corticosteroid implants
The corticosteroid implants are able to maintain a sustained release of steroids over a prolonged period of time. This therefore decreases the need for repeated administration, such as in IVTA injections. Various implants have different properties, which are elaborated in the following subsections.
0.59 mg Fai
The 0.59 mg FAi (Retisert®; Bausch & Lomb Inc.) is an FDA-approved nonbiodegradable implant that is designed to maintain a sustained release of drug for ~30 months.12 Tables 5–8 provide the summary of the studies regarding this implant.
Table 5.
Studies on fluocinolone acetonide implants (demographics)
| Study | Period of study | Study design | Study duration | Number of participants/eyes | Demographics
|
||
|---|---|---|---|---|---|---|---|
| Age (years) | Sex (female) | Ethnicity | |||||
| Multicenter Uveitis Steroid Treatment (MUST) Trial1,13 | – | Prospective, randomized comparative effectiveness trial cohort | 24 months 54 months |
255 (479 eyes with uveitis) | 46.3±15.0 | 75% | 56% white, 13% Hispanic or Latino, 26% black, 5% others |
| Callanan et al16 | 2000–2005 | Randomized, historically controlled trial | 3 years | 110 Fellow eye | 44.7±17.0 (range, 7.0–84.0) | 74% | 68% white, 17% African–American, 8% Asians, 4% Hispanic, 3% others |
| Pavesio et al15 | 2002–2005 | Randomized, controlled, phase 2b/3, open-label, multicenter superiority trial | 2 years | 140 eyes (more severe eye as study eye) | 40.36±14.363 (range, 12.2–74.7) | 48.50% | 90.9% white, 6.1% Hispanic, 3% others |
| 43.12±13.48 (range, 17.5–70) | 67.6% (the only variable where difference is statistically significant) | 86.5% white, 1.4% black, 5.4% Hispanic, 6.7% others | |||||
| Jaffe18 | March 2004 to July 2007 | Prospective, interventional trial | Mean follow-up post second implant: 17 months (range, 9–36 months) | 17 eyes of 14 patients | 50 (median: 46.5, range, 25–63) | 93% | 72% white, 28% black |
| Bollinger et al17 | June 2001 to March 2009 | Retrospective clinical case series | Median follow-up post-implant: 36 months (range, 6–60 months) | 47 eyes of 35 patients | 48.5±13.3 (range, 17–77) | 74% | 94% Caucasian, 6% African–American |
Table 6.
Studies on fluocinolone acetonide implant (clinical features)
| Study | Clinical features of participants
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of study eye | Details | Laterality | Duration of uveitis | Presence of other ocular conditions | Previous uveitis treatment | Presence of systemic conditions | Mean baseline VA (logMAR) | Mean baseline vitreous haze | Mean baseline CRT/CFT/CMT (μm) | Other baseline values | |
| Multicenter Uveitis Steroid Treatment (MUST) Trial1,13 | Active or recently active (≤60 days) NIU ≥1 eye with indications for systemic corticosteroids | 38% intermediate uveitis, 62% posterior uveitis or panuveitis, 41% ME in implant arm 39% ME in systemic arm |
89% bilateral | 6.1±7.2 years | 61% clear lens, 39% cataract, 41% aphakic/pseudophakic | – | 3% Behçet’s disease, 10% sarcoidosis, 2% multiple sclerosis, 2% juvenile idiopathic arthritis | 61.4±26.4 | 31% zero, 45% 1+, 17% 2+, 4% 3+, 1% 4+ | 268.0±185.0 | Eyes in implant arm had poorer visual field sensitivity than those in systemic group |
| Callanan et al16 | NIPU with controlled inflammation at the time of implantation | History of recurrent NIPU for ≥1 years | 76% bilateral | – | – | 62% systemic and 38% local | – | 0.54±0.35 0.32±0.42 |
– | – | Baseline uveitis recurrence rate: 62% Baseline uveitis recurrence rate: 30% |
| Pavesio et al15 | NIPU | – | – | – | – | – | – | – | – | – | – |
| Jaffe18 | NIPU | 35% idiopathic panuveitis, 23.5% sarcoidosis, 12% panuveitis associated with Crohn disease, 6% panuveitis associated with Behçet’s syndrome, 6% multiple sclerosis-associated uveitis | – | – | – | – | – | First implant: 1.3 (median, 1.3). Second implant: 0.68 (median, 0.51); (P=0.002) | – | Second implant, mean CFT: 466 (median, 330) | – |
| Bollinger et al17 | NIPU | – | – | – | – | – | – | At 1 year: 0.66±0.64, 2 years: 0.58±0.50, 3 years: 0.34±0.39 | – | – | – |
Note: “–” data not available.
Abbreviations: CFT, central foveal thickness; CMT, central macular thickness; CRT, central retinal thickness; ME, macular edema; NIPU, noninfectious posterior uveitis; NIU, noninfectious uveitis; VA, visual acuity; logMAR, logarithm of Minimal Angle of Resolution.
Table 7.
Studies on fluocinolone acetonide implants (outcomes)
| Study | Number of participants/eyes | Intervention
|
Number excluding those lost to follow-up/dropout | Outcomes measured
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAi | Systemic CS | Immunosuppression | BCVA | Visual field MD | Mean CRT/CMT/CFT (μm) | ME | Uveitis activity/vitreous haze score | Mean time to first recurrence of uveitis | Uveitis recurrence rate | Reimplantation | Others | ||||
| Multicenter Uveitis Steroid Treatment (MUST) Trial1,13 | 255 (479 eyes with uveitis) | 129 | ✓ | – | – | 122 | Mean improvement at 6 months: 5.9 letters, 12 months: 4.6 letters, 24 months: 6 letters | Remained similar to baseline throughout 48 months of follow-up in both arms | – | 6 months: 20% in implant vs 34% in systemic arm, (P<0.001 difference in change statistically significant between groups); 24 months: 22% in implant vs 30% in systemic arm (P=0.071) | Inflammation control 24 months: 88% implant arm vs 71% systemic arm (P=0.001) | – | – | 2.45% of eyes required reimplantation within 24 months | – |
| 126 | – | ✓ | In 86% | 118 | 6 months: 2.0 letters, 12 months: 3.3 letters, 24 months: 3.2 letters. No statistically significant difference between the arms | – | |||||||||
| 129 | ✓ | – | – | 110 | No statistically significant differences between arms. Mean improvement 54 months: 2.4 letters in implant arm vs 3.1 letters in systemic arm |
36 months: improved in systemic arm, stabilized in implant arm; 48 months: ~20% in each arm | Implant arm better in inflammation control at all time points assessed (P<0.016), but systemic arm also had substantial improvement | At 54 months: 87% of eyes ≥1 implant, 8% had two implants, 2% had three implants | – | ||||||
| 126 | – | ✓ | In 86% | 103 | – | ||||||||||
| Callanan et al16 | 110 | ✓ | ± | ± | 98 | 1 year: 0.56±0.44 (P=0.75), 2 years: 0.40±0.37 (P<0.01), 3 years: 0.48±0.41 (P=0.18) | Reduction in MD at 3 years: −1.42 dB (P=0.05 compared to baseline) | – | Reduction in CME 1 year: 86% eyes; 3 years: 73% eyes | – | No recurrences until 1,000 days after implantation | 1 year: 4%, 2 years: 10%, 3 years: 20% (P=0.01) | – | – | |
| Fellow eye | – | – | – | – | 1 year: 0.39±0.49 (P<0.01), 2 years: 0.39±0.49 (P<0.01), 3 years: 0.42±0.51 (P<0.01) | Reduction at 3 years: −1.05 dB (P=0.05 compared to baseline) | Reduction in CME 1 year: 28% eyes; 3 years: 28% eyes | – | 1 year: 44%, 2 years: 52%, 3 years: 59% (P<0.01) | ||||||
| Pavesio et al15 | 140 (more severe eye as study eye) | 66 | Yes | ± | ± | 61 | Mean VA in systemic group consistent, implant group deteriorated at 0,15, 18 months. At 2 years: VA stabilized in 71.2% implanted arm and 73% systemic arm; 17.2% implanted arm vs 14.3% systemic arm improved by ≥3 lines (P=0.66) | No statistically significant difference between groups. Mean change from baseline at 24 months <1 dB | – | Higher rate of CME improvement. Reduction in CME 2 years: 86.5% eyes | Mean vitreous haze severity of implanted arm < systemic arm (P<0.01) | 6.4±7.0 months | 18.20% | – | – |
| 74 | – | ✓ (monotherapy CS ≥0.2 mg/kg daily) |
± | 71 | Reduction in CME at 2 years: 74.4% eyes | 7.1±7.2 months (between treatment arms: P=0.07) | 63.5% (between treatment arms: P=0.01) | – | – | ||||||
| Jaffe18 | 17 eyes of 14 patients | ✓ | – | – | – | 52 weeks post-second implant mean BCVA: 0.60 (median, 0.35) (P=0.04 compared with the VA at the time of first implant) | – | 4 weeks post-second implant: 293 (median, 200) (P=0.0004), 52 weeks post second implant: 154 (median, 159) (P=0.02) | – | – | Mean time from first implant to first uveitis recurrence: 38 months | No recurrences after second implant at 52 weeks | Mean time of first recurrence of inflammation to reimplantation: 8 months (median, 5 months; range, 2–26 months) | Adjunctive CS use decreased significantly | |
| Bollinger et al17 | 47 eyes of 35 patients | ✓ (25.5% had multiple implants) | – | – | – | 1 year: 0.39±0.53 (P=0.03), 2 years: 0.28±0.36 (P=0.01), 3 years: 0.34±0.39 (P=0.04) | – | – | – | – | – | – | – | – | |
Notes: “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: BCVA, best-corrected visual acuity; CFT, central foveal thickness; CS, corticosteroids; CME, cystoid macular edema; CMT, central macular thickness; CRT, central retinal thickness; FAi, fluocinolone acetonide implant; MD, mean deviation; ME, macular edema; VA, visual acuity.
Table 8.
Studies on fluocinolone acetonide implants (adverse events)
| Study | Intervention
|
Adverse events
|
Reasons for removal of implants (if any) | Other comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ocular
|
Systemic | |||||||||
| FAi | Systemic CS | Immunosuppression | Cataracts | Raised IOP
|
Others | |||||
| >10 mmHg/requiring medications | Requiring surgery | |||||||||
| Multicenter Uveitis Steroid Treatment (MUST) Trial1,13 | ✓ | – | – | 91% cataract, 80% required surgery | 61% transient elevated IOP needed medication, 17% required treatment for glaucoma | – | Transient vitreous hemorrhage, 16% implant arm vs 4% systemic. Low risk of hypotony, retinal detachment | 0.36/person-year infections requiring prescription | – | – |
| – | ✓ | In 86% | 45% cataract, 31% required surgery | 4% glaucoma | – | 0.60/person-year (P=0.034) without significant long-term consequences noted | ||||
| ✓ | – | – | – | – | – | – | ||||
| – | ✓ | In 86% | – | – | – | |||||
| Callanan et al16 | ✓ | ± | ± | 3 years postimplantation: 93%* | 3 years: 78% implanted eyes* | 40%* | Conjunctival hyperemia, conjunctival hemorrhage, blurred vision, reduced VA, incidence of hypotony, retinal detachments, 1% endophthalmitis | – | Uncontrolled elevated IOP, suspected depletion of medication, spontaneous dissociation of implant from its anchoring strut (prior to improved adhesive process), spontaneous expulsion of implant, lysis of the anchoring suture, endophthalmitis, necrotizing scleritis, hypotony | |
| – | – | – | 3 years post-implantation: 20% | 3 years: 36% (P<0.01 between groups) | 2% (P<0.1 between groups) | Eye pain, vitreous floaters, blurred vision, reduced VA | – | – | – | |
| Pavesio et al15 | ✓ | ± | ± | 87.8% phakic eyes require surgery | 62.10% | 21.20% | 4.5% endophthalmitis, 1.5% retinal detachment, 19.7% hypotony | – | 8 eyes explanted: hypotony, elevated IOP, scleral thinning, implant extrusion, postoperative complications | – |
| – | ✓ (monotherapy CS ≥0.2 mg/kg daily) | ± | 19.3% phakic eyes require cataract surgery (P<0.01) | 20.3% (P<0.01) | 2.7% (P<0.01) | 2.7% retinal detachment,1.4% hypotony (hypotony difference statistically significant P=0.0003) | 25.70% | – | – | |
| Jaffe18 | ✓ | – | – | NA (all patients either pseudophakic or aphakic at time of reimplantation) | 23.5% needed medications (proportion similar to that before second implantation) | 6% | 6% traction retinal detachment (patients had low IOP before reimplantation) | – | – | |
| Bollinger et al17 | ✓ (25.5% had multiple implants) | – | – | – | – | 45% for glaucoma (36.8% had multiple implants but 90% of those with multiple implants had operation after first implant) | – | – | – | Mean time after implant to IOP-lowering surgery: 14.0±9.5 months |
Notes:
Results from both dose groups in study (0.59 mg and 2.1 mg). “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: CS, corticosteroids; FAi, fluocinolone acetonide implant; NA, not applicable; IOP, intraocular pressure: VA, visual acuity.
The Multicenter Uveitis Steroid Treatment (MUST) trial is the largest randomized comparative trial to date regarding the efficacy, safety, and impact on quality of life of the FAi in comparison with systemic immunosuppression.13 About 479 uveitic eyes of 255 patients were observed over a period of 24 months. Both interventions resulted in improved BCVA with a larger absolute increase in mean BCVA in eyes treated with the FAi at all the time points. However, the difference was not statistically significant. Intraocular inflammation control was also achieved in most eyes by 9 months in each intervention. However, the implant achieved an increased frequency and rate of control compared with the systemic immunosuppression. The FAi was able to achieve resolution of macular edema in significantly more eyes than systemic treatment at 6 months, but this difference was not maintained at 24 months. Regarding adverse effects, patients treated with the implant were four times more likely to have an increased IOP, absolute IOP of >35 mmHg and increased need for medications and surgery to lower the IOP while 17% of eyes developed glaucoma. Friedman et al identified associations between raised IOP and black race, and uveitis activity and use of the implant.14 Cataracts developed in almost all the phakic eyes at the end of 24 months. As for systemic side effects, patients on systemic therapy had higher risk of a systemic infection requiring medications, but there was no significant increase in the risk of hospitalization. Vision-related quality of life was superior in patients with FAi at 6 months, but this advantage narrowed by the end of 24 months with minimal difference between the two.
In the 36-month follow-up to the original MUST trial, the FAi and systemic immunosuppression were similarly efficacious in improving the visual outcomes of the patients.1 However, there was no significant improvement of the mean BCVA at 54 months as compared to the baseline in either treatment arms. Lastly, macular edema was noted to improve significantly with the use of FAi in the first 6 months. However, with longer follow-up, the improvement in macular edema in both treatment arms was equal. The persistence of macular edema can potentially cause irreversible damage to the macula. The implant therapy may have an advantage in this area, as it is able to resolve macular edema to a greater extent initially. However, since there were no statistically significant differences in BCVA, the advantage conferred is unlikely to be significant. Interestingly, only 10% of the uveitic eyes received two or more implants in this entire 54-month trial even though the estimated duration of action of FAi is 2.5–3 years. Long-term studies are required to investigate whether this was due to the implant working for an extended duration or whether it is because the implant resulted in extended remission of uveitis.
In a randomized controlled phase 2b/3 open-label multicenter superiority trial by Pavesio et al15 comparing the effects of FAi to the standard care (systemic steroids and/or immunosuppressive agents) with regard to time to first recurrence of uveitis, it was found that the uveitis recurrence number and the median time to recurrence were significantly lower with the use of FAi. However, there was no statistical difference in the BCVA improvement in both treatment arms at 24 months, consistent with the findings in the MUST trial.15 Nevertheless, the findings of CME seemed to be inconsistent with the MUST trial. In this study, there was a statistically significant higher proportion of subjects treated with FAi with the reduction in CME. This difference could be attributed to the difference in the method of measurement of macular edema; MUST trial used the optical coherence tomography, whereas the trial by Pavesio et al15 measured the area of CME using fluorescein angiography. As expected, a higher proportion of eyes with FAi developed cataracts and increased IOP at the end of the trial. There was also a higher incidence of hypotony in implanted eyes.
Callanan et al reported the results of a 3-year multicenter, randomized historically controlled trial of 0.59 mg FAi in 110 patients.16 In this study, the FAi resulted in improved BCVA and significantly reduced uveitis recurrence. The use of the implant was associated with reduced dose of systemic medications. However, ocular adverse events, mainly increased IOP and cataract formation, were observed. There was also an increased incidence of hypotony in the implanted eyes as compared to the fellow eyes while retinal detachment occurred in 4% of the implanted eyes.
Bollinger et al evaluated the effect of FAi on IOP in a retrospective study of 47 eyes.17 They reported that glaucoma surgery was required for 45% of the patients over the 8-year study period. Interestingly, there was no increase in the need for another IOP-lowering surgery following reimplantation in patients with previous IOP surgery secondary to raised IOP from the first implant. However, patients who experienced the need for glaucoma surgery after the first implant would be unlikely to choose reimplantation causing a bias in this observation. Furthermore, this was a retrospective study, which has inherent biases.
Jaffe18 performed a prospective interventional trial, as a continuum from the study by Callanan et al.16 Reimplantation of FAi was effective in sustaining the control of intraocular inflammation and stabilization of BCVA of the eye in 17 eyes of 14 patients. None of the eyes developed recurrence of inflammation in the 52-week period after reimplantation. However, one patient developed recurrent iridocyclitis 34 months after the second implant and was treated with prednisolone and replacement of the second implant. It was possible to place the second implant at the original implant site, and no intraoperative complications were observed with the reimplantation. With regard to ocular adverse events as a result of the second implantation, the proportion of patients requiring IOP-lowering medications was similar to the proportion before reimplantation. Two patients had IOP >35 mmHg, but this was postulated to be due to noncompliance with IOP lowering medications. The risk of cataract formation after repeated implantations could not be evaluated, as all patients were either pseudophakic or aphakic at the time of reimplantation.
From the results of the studies, it is found that FAi does not seem to confer a substantial advantage in the improvement of BCVA but is advantageous in intraocular inflammation control. The use of the implant also allows for reduction in systemic medications. However, in patients with bilateral disease, the cost of bilateral FAi was greater than that of systemic corticosteroids.19 Therefore, given that the FAi has minimal advantage in visual outcomes and avoidance of systemic side effects from systemic corticosteroids, with additional ocular adverse events such as raised IOP and cataract development coupled with increased cost for bilateral disease, alternate forms of treatment such as newer implants or systemic agents may be preferable as a first-line treatment in patients with bilateral NIU.
Dexamethasone implant
The 0.7 mg dexamethasone implant (Ozurdex®; Allergan Inc) is an FDA-approved biodegradable dexamethasone implant. The implantation of the dexamethasone implant can be performed as an outpatient procedure, and it maintains sustained release for up to 6 months.2 Tables 9–12 provide the summary of studies regarding dexamethasone implant.
Table 9.
Studies on dexamethasone implants (demographics)
| Study | Period of study | Study design | Study duration | Number of participants/eyes | No of eyes | Demographics
|
||
|---|---|---|---|---|---|---|---|---|
| Age, years (SD) |
Sex (female) |
Ethnicity | ||||||
| Lowder et al20 | 2006–2009 | Prospective, randomized | 26 weeks | 229 (right eye as study eye) | 77 | 44 (14.8) | 59.70% | 61% white, 10% black, 23% Asian, 3% Hispanic, 3% others |
| 76 | 46 (13.6) | 63% | 61% white, 13% black, 16% Asian, 1% Hispanic, 9% others | |||||
| 76 | 44 (15.0) | 67% | 60.5% white, 12% black, 20% Asian, 3% Hispanic, 4.5% others | |||||
| Khurana and Porco21 | 2011–2012 | Retrospective, noncomparative consecutive interventional case series | Follow-up for ≥3 months | 18 eyes (13 patients) | 48, range, 27–72 | 77% | 62% white, 15% Latino, 23% Asian | |
| Arcinue et al25 | March 2005 to June 2011 | Retrospective comparative case series | Follow-up ≥6 months, ≤2 years | 27 eyes of 25 patients | 11 16 |
57.7 (12.1) 55.1 (10.6) |
81.80% 75% |
– – |
| Lam et al22 | 2010–2012 | Retrospective cohort study | ≥3 months of follow-up after initial dexamethasone implant | 23 eyes | 49.8 (16.7), range, 11–79 | 50% | 10% black, 85% white, 5% others | |
| Tomkins-Netzer et al23 | 2008–2013 | Retrospective, observational case series | Mean follow-up was 17.3±1.8 months after first implant | 38 eyes of 27 patients treated with 61 implants | 14 eyes with single implant 24 eyes with multiple implants (36.9% 2 implants, 18.4% 3 implants, 5.2% 4 implants, 2.6% 6 implants) | 48 (2.2) | 42.10% | – |
Note: “–” data not available.
Abbreviation: SD, standard deviation.
Table 10.
Studies on dexamethasone implants (clinical features)
| Study | Clinical features of participants
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of study eye | Details | Duration of uveitis (SD) | Presence of other ocular conditions | Previous uveitis treatment/at study entry | Presence of systemic conditions | Mean baseline VA (logMAR) | Mean baseline vitreous haze | Mean baseline CRT/CFT/CMT (μm) | Other baseline values | |
| Lowder et al20 | NIU | 81% intermediate, 19% posterior | 50.5 (54.2) | 81% phakic, 32% cataract in phakic lens | 26% on systemic medication | – | 58±15.2 | 2.06±0.55 | CMT: 344.0±141.6 | – |
| 43.9 (48.9) | 67% phakic, 63% cataract in phakic lens | 29% on systemic medication | – | 57±17.2 | 2.12±0.50 | CMT: 338.9±162.4 | – | |||
| 61.2 (62.5) | 72% phakic, 49% cataract in phakic lens | 24% on systemic medication | – | 63±15.2 | 2.01±0.54 | CMT: 324.6±145.5 | – | |||
| Khurana and Porco21 | Persistent, noninfectious uveitic CME | 39% intermediate uveitis, 22% birdshot chorioretinitis, 22% sarcoidosis, and 17% others | Median duration of CME: 16.5 months (range, 4–39 months) | 55% phakic | 78% eyes ≥1 therapy for uveitic CME. 72% not on any therapy. 28% eyes on systemic medication | – | 50% 10/30–10/50, 39% 10/60–10/80, 11% 10/100–10/150 | 56% score of 0, 33% score of 1, 11% score of 2 | Median CRT: 453 (range, 314–778) | – |
| Arcinue et al25 | NIU | 0.59 mg FAi | – | 36.4% glaucoma | 18% on systemic medications | – | – | – | CRT: 379.2±124.3 | – |
| 0.7 mg FAi panuveitis | – | 56.3% glaucoma | 56% on systemic medications | – | – | – | CRT: 340.3±141.0 | – | ||
| Lam et al22 | NIU with ME | – | <3 months of ME: 8.7%, ≥3–12 months: 30.4%, ≥12 months: 56.5%, unknown duration: 4.3% | 17.4% previous glaucoma surgery, 47.8% phakic, 52.2% pseudophakic | IVTA: 65.2%, sub-Tenon’s triamcinolone acetonide: 43.5%, some on systemic medications | 26% hypertension | 0.71±0.07 | – | CRT: 517.2±40.3 (range, 285–872) | – |
| Tomkins-Netzer et al23 | NIU | 23.69% intermediate uveitis, 76.31% posterior uveitis plus panuveitis, 92.1% CME, 7.81% vitritis | Mean: 90.95±11.06 months | 55.26% phakic | 74% on systemic prednisolone, 70% on second-line agents | – | 0.47±0.05 | 57.89% score 0, 41.22% score +0.5 to +2 | CRT: 453.29±33.57 | Mean IOP: 13.87 (0.43) mmHg, 7 steroid responders |
Note: “–” data not available.
Abbreviations: CFT, central foveal thickness; CME, cystoid macular edema; CMT, central macular thickness; CRT, central retinal thickness; FAi, fluconinolone acetnoide implant; IOP, intraocular pressure; IVTA, intravitreal triamcinolone acetonide; logMAR, logarithm of Minimal Angle of Resolution; ME, macular edema; NIU, noninfectious uveitis; VA, visual acuity.
Table 11.
Studies on dexamethasone implants (outcomes)
| Study | Number of participants/eyes | Intervention
|
Numbers excluding those lost to follow-up/dropout | Outcomes measured
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DEX implant | Systemic CS (unless otherwise stated) | Immunosuppression | BCVA | Mean CRT/CFT/CMT (μm) | ME | Uveitis activity/vitreous haze score | Mean time to first recurrence of uveitis | Uveitis recurrence rate | Reimplantation | Others | ||||
| Lowder et al20 | 229 (right eye as study eye) | 77 | 0.7 mg | ± | ± | 73 | Mean improvement in BCVA: Dex > sham groups. Statistically significant at all time points for 0.7 mg. Dex implant 2–6 times more eyes with 15-letter improvement from baseline compared with sham group | Week 8 and 26: statistically significant lower CMT compared to baseline (P≤0.004). Mean decrease from baseline > sham at week 8 but not week 26 | – | 47% score of 0 at week 8 | – | – | – | – |
| 76 | 0.35 mg | ± | ± | 73 | – | 36% score of 0 at week 8 | – | – | – | – | ||||
| 76 | Sham procedure | ± | ± | 71 | – | – | 12% score of 0 at week 8 | – | – | – | – | |||
| Khurana and Porco21 | 18 eyes of 13 patients | ✓ | ± | ± | – | At 3 months, mean BCVA improved by +2.1 lines (P=0.01) | – | No CME detected in 89% of eyes at 1 month and 72% at 3 months | Score 0 at all months | Median time to recurrence of CME: 201±62 days | Recurrence of CME: 65% at 6 months, 70% at 12 months | 56% ≥2 implants. Median time to retreatment: 300±71 days | – | |
| Arcinue et al25 | 27 eyes of 25 patients | 11 | 0.7 mg | ± | ± | – | No significant differences in the BCVA improvement between the two arms | 1 month: 278.3±43.8, 6 months: 314.3±72.6, 12 months: 341.8±139.3 (P=0.1254) | – | Rate of improvement: 24/1,000 person-months | – | 0.5/100 person-months | 45% two implants. Median survival time for second implant: 13 months | – |
| 16 | 0.59 mg FAi | ± | ± | – | 1 month: 298.1±125.8, 6 months: 276.6±125.8, 12 months: 248.6±48.4 (P=0.163) | Rate of improvement: 47/1,000 person-months | 1.7/100 person-months. 3.16 times more at risk of recurrence (P=0.41) | 12.5% two implants. Median survival time for second implant: 28 months | ||||||
| Lam et al22 | 23 | 0.7 mg | ± | ± | – | 0.76±0.08 (81% gaining one or more lines of vision) | Peak improvement in CRT was 274.3±42.3 (66.7% had reduction in central retinal thickness and improved vision) | – | – | – | – | – | – | |
| Tomkins-Netzer et al23 | 38 eyes of 27 patients treated with 61 implants | 14 eyes with single implant 24 eyes with multiple implants (36.9% 2 implants, 18.4% 3 implants, 5.2% 4 implants, 2.6% 6 implants) | 0.7 mg | ± | ± | – | 2 months: 0.27±0.07, 6 months: 0.43±0.12 Second implant has similar effect as first implant within 1 month. Long-term accumulative effect: continued improvement in BCVA |
CRT at 1 month: Change of −263±44 (P=0.003), 6 months: −127±52 (P=0.01), stable until 12 months Second implant similar effect as first. Long-term accumulative effect: significant improvement and stabilization of CRT |
50% eyes persistent ME – |
93% score of 0 – |
Median time 6 months (range, 2–42 months) Second implantation: median time 6 months (range, 1–12 months) |
69% Second implant: 48% |
– | – |
Notes: Data presented as ± SD. “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: BCVA, best-corrected visual acuity; CFT, central foveal thickness; CME, cystoid macular edema; CMT, central macular thickness; CRT, central retinal thickness; CS, corticosteroids; Dex, dexamethasone; FAi, fluocinolone acetonide implant; MD, mean deviation; ME, macular edema.
Table 12.
Studies on dexamethasone implants (adverse effects)
| Study | Number of participants/eyes | No of eyes | Intervention
|
Adverse events
|
Reasons for removal of implants (if any) | Other comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ocular
|
Systemic | |||||||||||
| DEX implant | Systemic CS (unless otherwise stated) | Immunosuppression | Cataracts | Raised IOP
|
Others | |||||||
| >10 mmHg/requiring medications | Requiring surgery | |||||||||||
| Lowder et al20 | 229 (right eye as study eye) | 77 | 0.7 mg | ± | ± | 15% | 23% requiring medication, 7.1% IOP >25 mmHg | None | None | Conjunctival hemorrhage, ocular discomfort, eye pain, iridocyclitis. 1 case of suspected endophthalmitis or uveitis flare in 0.7 mg implant group. 4 retinal detachments | – | – |
| 76 | 0.35 mg | ± | ± | 12% | 8.7% >25 mmHg | None | 1% | – | – | |||
| 76 | Sham procedure | ± | ± | 7% | 4.2% >25 mmHg | None | None | – | – | |||
| Khurana and Porco21 | 18 eyes (13 patients) | ✓ | ± | ± | None | 10% had increased IOP | None | 11% eyes had ≥1 episode of IOP >25 mmHg within first 3 months, all effectively managed with topical medications | None | – | ||
| Arcinue et al25 | 27 eyes of 25 patients | 11 | 0.7 mg | ± | ± | 50% | None | None | None | 1 implant migration into the anterior chamber, 1 intralenticular location of the Ozurdex implant, possible endophthalmitis | – | – |
| 16 | – | 0.59 mg FAi | ± | 100% | 44% | None | 1 postoperative hypotony, cyclodialysis cleft, choroidal effusion, and hypotony | – | – | |||
| Lam et al22 | 23 | 0.7 mg | ± | ± | 5% phakic eyes developed cataract. 45.5% cataract surgery |
22.7% >10 mmHg increase, 8.7% require topical eye drops | None | None | 5% retinal detachment | – | 1 eye with uveitis was switched to FAi as a longer-acting intraocular steroid was deemed needed | |
| Tomkins-Netzer et al23 | 38 eyes of 27 patients treated with 61 implants | 14 eyes with single implant 24 eyes with multiple implants (36.9% Two implants, 18.4% Three implants, 5.2% 4 implants, 2.6% 6 implants) |
0.7 mg | ± | ± | First implantation: 5% phakic eyes. Repeat implantation: 5% phakic eyes |
First implantation: 7.9% increased IOP of >21 mmHg after 2 months. Second implantation: 17.9% increased IOP of >25 mmHg | None | None | After first implant: 1 eye with implant migration | – | – |
Notes: “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: DEX, dexamethasone; FAi, fluocinolone acetonide implant; IOP, intraocular pressure; CS, corticosteroids.
The HURON trial, a multicenter randomized controlled trial reported by Lowder et al evaluated the effect of 0.7 mg dexamethasone implant in 77 eyes over a period of 26 weeks in improving VH as the primary outcome.20 There was a statistically significant improvement in BCVA in eyes implanted with 0.7 mg dexamethasone compared with the controls. The implant also proved its ability to control ocular inflammation as 47% of eyes achieved a VH score of 0 by the end of 8 weeks. A significant decrease in central macular thickness (CMT) from baseline was observed. Improvement in VH and BCVA were noted up to 26 weeks; however, 22% of patients required rescue medications. Of note, there was no statistically significant difference in the proportion of patients requiring rescue medications as compared to the control. As for adverse events, ≤23% patients with 0.7 mg dexamethasone implant required IOP-lowering medications. Cataract was observed in 15% of the phakic eyes treated with the implant compared with 7% of eyes in the control group, and only one eye required surgery. However, this difference was not statistically significant. Limitations of this study include a shorter follow-up period (6 months), and adverse effects such as cataract formation would not have been detected fully. Furthermore, the trial had no information regarding the efficacy of repeated implantation of 0.7 mg dexamethasone.
In a retrospective case series of 18 eyes, Khurana and Porco investigated the effect of 0.7 g dexamethasone implant on persistent uveitic CME.21 BCVA improved in this retrospective study with a complete resolution of CME at 1 month. However, CME recurred at a median time interval of 201 days. Adverse events noted in the study included an increase in IOP in 11% of eyes (>25 mmHg). However, IOP was controlled in all patients with medical therapy. The results from this study are largely consistent with another retrospective study by Lam et al, which studied 23 eyes with uveitic macular edema.22 BCVA also improved with the reduction of the central retinal thickness (CRT). About 22% of uveitic eyes had an increase in IOP of >10 mmHg, but were all under control with medications. However, the incidence of cataract surgery seems to be higher in this study at 43.6% of phakic eyes. This could be due to the fact that there was variable follow-up time and lack of baseline lens opacity and that most patients have had other types of treatment such as IVTA administered prior to this trial. However, there is still an inconsistency as the study by Khurana and Porco21 also consisted of patients with previous treatment using IVTA and other drugs; therefore, the difference could be because both the studies did not manage to measure the lens opacity at baseline, since they were retrospective studies.
A retrospective study by Tomkins-Netzer et al looked into the clinical question of the effect of repeated dexamethasone implants in the treatment of NIU in 38 eyes.23 The study reported that BCVA and CRT improved within 1–2 months after each implantation, and the effect was sustained for about 6 months. Repeated implantations showed similar efficacy and resulted in a cumulative effect that allowed for continuous improvement of BCVA and CRT of the eyes. Following the first implantation of dexamethasone, systemic or local immunosuppressive therapy could be reduced or terminated in 87% of eyes. Cataract development was minimal in this study, in only 5% of phakic eyes after the first and third implantations. There were seven cases of increased IOP of >25 mmHg, three eyes after the first implantation and four eyes after the second. However, all were treated with medications with none requiring surgery. There was one case of migration of the implant into the anterior chamber.
A retrospective study of 20 eyes with intraocular inflammation, mostly secondary to NIU, implanted with bilateral dexamethasone implants by Ryder et al revealed that the bilateral implants appeared to be well-tolerated with no patients developing cataracts during their follow-up period.24 However, similar to patients with unilateral dexamethasone implants, there was an elevation of IOP with 18.2% of eyes, requiring medications. Large-scale studies are required to establish the safety profile of bilateral dexamethasone implants.
In summary, based on the literature review, the studies showed that the dexamethasone implant improves BCVA and CMT as well as CME with a lower incidence of cataract formation and raised IOP compared with the FAi among patients with NIU. Repeated implants seem to work with the same efficacy with minimal additional side effects, and bilateral implants appear to be well-tolerated as well. However, common limitations in these trials except the HURON trial were that they were all retrospective studies with relatively smaller sample size. The HURON trial did not examine the long-term effects of the dexamethasone implant as well as the effect of repeated implantations.
Since both the FAi and dexamethasone implants are long-acting and avoid the systemic side effects of oral corticosteroids, Arcinue et al performed a retrospective study to compare the safety and efficacy of the two, which may help to arrive at a conclusion.25 The main outcome evaluated in this study was the recurrence rate of uveitis following implantation. The FAi and dexamethasone implants showed relatively similar efficacy since there were no statistically significant differences with regard to their effect on BCVA and inflammation control. Recurrence rates were higher in the FAi group, but the difference was not statistically significant. The investigator postulated that this could be due to the increased severity of uveitis in the eyes implanted with FAi. Furthermore, it was more likely for a patient to have had a reimplantation of the dexamethasone implant given its designated functioning duration of 6 months, therefore decreasing the recurrence rate. Expanding on that point, as the duration of action of the Ozurdex is significantly shorter, it was five times more likely for eyes with dexamethasone implants to require a second implant. Expectedly, FA-implanted eyes had a statistically higher rate of requiring IOP-lowering medications or surgeries, and 4.7-fold increased risk in cataract formation was noted with FAis. Similar to other trials, this study had several limitations including retrospective nature, small sample size, and variable follow-up period. Therefore, the choice between the two depends on the patient’s individual circumstances.
0.019 mg Fai
The 0.019 mg FAi (ILUVIEN®; Alimera Sciences Limited) was recently FDA-approved for the treatment of diabetic macular edema. The effect lasts for up to 36 months.26 With its lower dosage than 0.59 mg FAi, the corticosteroid side effects are thought to be reduced with this implant. The phase III clinical trial for FAi in NIU is currently ongoing.27
Intravitreal antivascular endothelial growth factor: bevacizumab and ranibizumab
The vascular endothelial growth factor (VEGF) has been found to be a vital component in the pathogenesis of CME and CNV. Inhibition of VEGF with the anti-VEGF is therefore able to impair the angiogenic effects. It has been widely used in the treatment of CNV secondary to age-related macular degeneration and has also been used in other ocular vasoproliferative conditions such as diabetic retinopathy. Since CNV is also a well-known sight-threatening complication of NIU, various studies have evaluated the efficacy and safety of intravitreal anti-VEGF in the treatment of CNV and CME secondary to NIU.28–30 Some studies have also studied the use of intravitreal anti-VEGF in the treatment of CME.31–33 Data from seven studies were gathered. All were retrospective case studies with the exception of one randomized controlled trial. Tables 13–16 provide the summary of these studies.
Table 13.
Studies on intravitreal vascular endothelial growth factors (demographics)
| Study | Period of study | Study design | Study duration | Number of participants/eyes | Demographics
|
|||
|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex (female) | Ethnicity | ||||||
| Rouvas et al30 | – | Retrospective, noncomparative, interventional, and observational case series | 70.4±24 weeks (17.6 months; range, 11–29 months) | 16 eyes of 15 consecutive patients | 46±9 (range, 22–56) | 87% | – | |
| Julián et al29 | 2007–2008 | Retrospective case series | Median follow-up: 17.6 months (range, 8–25 months) | 15 eyes of 15 patients | Median: 41.93 | 40% | – | |
| Mansour et al35 | – | Retrospective multicenter consecutive case series | Follow-up between 6 and 24 months | 99 eyes of 96 patients | 40.4±17.0 | 64% | 81.2% white, 9.7% Asian, 8.2% Hispanic, and 0.9% black | |
| Mansour et al34 | – | Retrospective, multicenter, consecutive case series | Follow-up period of 3 months | 84 eyes of 79 patients | 40.0 (range, 8–85) | 62% | 66.7% white | |
| Bae et al36 | May 2006 to August 2008 | Retrospective case series | Follow-up: 22.3±7.8 weeks (range, 14–37 weeks) | 21 eyes | 10 eyes | 54.8±16.6 (18–77) | 60% | – |
| 11 eyes | 44.5±12.5 (21–65) | 45% | ||||||
| Rahimi et al32 | – | Randomized clinical trial | Mean follow-up: 25.3 weeks | 60 eyes of 55 patients | 31 eyes | 23.2±11.7 | 54% | – |
| 29 eyes | 23±10.9 (no significant difference) | 52% | ||||||
| Mansour et al28 | 2008–2010 | Retrospective multicenter consecutive case series | 3 years | 81 eyes | 43.6 (range, 11–78) | 67% | 64.2% Caucasians, 23.4% Asians, 9.9% Hispanics, 2.5% African–Americans | |
Note: “–” data not available.
Table 14.
Studies on intravitreal vascular endothelial growth factors (clinical features)
| Study | Clinical features of participants
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of study eye | Details | Laterality of condition | Duration of uveitis | Presence of other ocular conditions | Previous uveitis treatment | Presence of systemic conditions | Mean baseline VA (logMAR) | Mean baseline vitreous haze | Mean baseline CRT/CFT/CMT (μm) | Other baseline values | |
| Rouvas et al30 | NIU with CNV and no active inflammation | 25% toxoplasmosis, 12.5% serpiginous choroidopathy, 31.25% punctate inner choroidopathy, 18.75% multifocal choroiditis, 12.5% scleroderma. 68.75% subfoveal CNV, 18.75% juxtafoveal CNV, 12.5% extrafoveal CNV | – | – | – | Treated with topical and systemic CS, sub-Tenon’s steroid injections, and systemic cyclosporine where appropriate | – | 0.9±0.4 | – | CFT: 285±20 | – |
| Julián et al29 | NIU with CNV and no active inflammation | 47% multifocal choroiditis and panuveitis, 13% ampiginous choroiditis, 40% remaining serpiginous choroiditis, sympathetic ophthalmia, Vogt–Koyanagi–Harada syndrome, punctuate inner choroidopathy, tuberculosis and idiopathic inflammation. 87% subfoveal CNV and 13% peripapillary CNV | – | – | – | Mean time under treatment: 30 months for systemic immunosuppression, 44 months for CS | – | 0.53 | – | CFT: 239.06±47.68 | – |
| Mansour et al35 | Eyes with inflammatory ocular neovascularization. 28% with active inflammation. Resistant to CS ± immunosuppression | 23% punctate inner choroidopathy, 19% multifocal choroiditis with panuveitis, 13% ocular histoplasmosis, 12% idiopathic, 9% serpiginous choroiditis, 6% Vogt–Koyanagi–Harada disease, 5% ocular toxoplasmosis, 4% Eales disease, 2% sarcoidosis, 2% sympathetic ophthalmia, 2% tuberculosis, 1% acute placoid pigment epitheliopathy, and 1% birdshot choroiditis. CNV mean 1.3 disc diameters (range, 0.25–5). 49% subfoveal, 38% juxtafoveal, 6% peripapillary, 6% NVD/NVE | 3% bilateral | – | – | – | – | 0.65±0.44 | – | CFT: 338±87 | – |
| Mansour et al34 | Eyes with inflammatory ocular neovascularization. 27.4% with active inflammation. Resistant to CS ± immunosuppression | 17.9% multifocal choroiditis with panuveitis; 17.9% punctate inner choroidopathy; 15.5% ocular histoplasmosis; 11.9% idiopathic uveitis; 6% Vogt–Koyanagi–Harada, 6% serpiginous choroiditis, 6% retinal vasculitis; 4.8% Eales disease; 3.6% pars planitis, 3.6% ocular toxoplasmosis; 2.4% tuberculosis, 2.4% sarcoidosis; 1.2% birdshot choroiditis. 40.5% juxtafoveal CNV, 40.5% subfoveal CNV, 9.5% peripapillary CNV, 13.1% NVD/NVE | 7% bilateral | 30.6 months (range, 1–240 months) at study entry | – | 17% systemic immunosuppressive agents, 49% oral CS, 10% sub-Tenon’s CS, 13% intravitreal CS | – | 0.68 | – | CFT: 346 | CNV size: mean 1.3 disc diameters (range, 0.25–4 disc diameters) |
| Bae et al36 | NIU with CME >3 months despite conventional treatment | 50% eyes with Behçet’s disease | 40% bilateral | – | No glaucoma or other macular abnormalities | No previous treatment for CME | No hypertension or diabetes mellitus | 0.73±0.41 | – | CFT: 537±214 | – |
| 55% eyes with Behçet’s disease | 27% bilateral | – | 0.73±0.33 | CFT: 594±151 | |||||||
| Rahimi et al32 | CME refractory to conventional topical medication | 40% intermediate uveitis, 25% pars planitis, 12% idiopathic anterior uveitis, 10% Behçet’s disease, 7% idiopathic posterior uveitis, 3% Vogt–Koyanagi–Harada syndrome, 3% idiopathic panuveitis and vasculitis | – | – | Nil | – | – | 0.47±0.18 | Mean vitreous reaction grade: 2.00 | CMT: 309.87±52.43 | Mean grade for anterior chamber reaction: 0.7 |
| 0.48±0.22 | Mean vitreous reaction grade: 1.24 | CMT: 295.62±33.19 | Mean grade for anterior chamber reaction: 0.9 | ||||||||
| Mansour et al28 | Inflammatory ocular neovascularization refractory to standard therapy. 16% of eyes with active uveitis | 29.6% punctate inner choroidopathy, 14.8% multifocal choroiditis with panuveitis, 23.5% ocular histoplasmosis, 12.3% serpiginous choroiditis, 4.9% Vogt–Koyanagi–Harada syndrome, 6.2% ocular toxoplasmosis, and 3.8% vasculitis. 61.7% subfoveal CNV, 32.1% juxtafoveal, 9.9% peripapillary, 6.2% NVD/NVE | 0% bilateral | – | – | 38.6% on oral CS; 4.9% sub-Tenon’s CS, 11.1% intraocular CS, 21% immunosuppressive agents | – | 0.70±0.43 | – | CFT: 322.5±101.8 | CNV size: 1.19±0.79 disc diameters |
Note: “–”, data not available.
Abbreviations: CFT, central foveal thickness; CME, cystoid macular edema; CMT, central macular thickness; CNV, choroidal neovascularization; CRT, central retinal thickness; CS, corticosteroids; logMAR, logarithm of Minimal Angle of Resolution; ME, macular edema; NIU, noninfectious uveitis; NVD, neovascularization of disc; NVE, neovascularization elsewhere; VA, visual acuity.
Table 15.
Studies on intravitreal vascular endothelial growth factors (outcomes)
| Study | Number of participants/eyes | Intervention
|
Outcomes measured
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-VEGF injection | Systemic CS (unless otherwise stated) | Immunosuppression | BCVA | Mean CRT/CFT/CMT (μm) | Uveitis activity/vitreous haze score | Mean time to first recurrence of uveitis | Uveitis recurrence rate | Reinjections | Others | |||
| Roukas et al30 | 16 eyes of 15 consecutive patients | 0.5 mg ranibizumab | – | – | End of follow-up: 0.6±0.4 (P=0.0001). Improved in 88%, stable in 12.5% | CFT: 233±21 | – | Nil | No CNV recurrence | Mean: 2.3 injections | CNV regressed in all | |
| Julián et al29 | 15 eyes from 15 patients | 1.25 mg bevacizumab | ✓ | 60% received treatment | 1 month postinjection: 0.29. 80% of eyes improved, 20% worsened. Statistically significant positive difference between initial BCVA and 4 months BCVA but not at 8, 12, 16 months | CFT: 195.2 in 87% after 1 month, 13% worsened | – | Nil | Nil | 80% >1 injection. Mean 4.25 (2–8), frequency: 1 every 12.97 weeks | – | |
| Mansour et al35 | 99 eyes of 96 patients | 33.3% 2.5 mg bevacizumab, 66.6% 1.25 mg bevacizumab | ± | ± | 6 months: 0.43±0.41 (P=0.000), 12 months: 0.40±0.37 (P=0.000), 18 months: 0.37±0.41 (P=0.001), 24 months: 0.32±0.32 (P=0.013) | CFT: 6 months 257±102 (P=0.000), 12 months 264±81 (P=0.000), 18 months 258±77 (P=0.003), 24 months of 254±78 (P=0.022) | – | – | Mean 2.3 injections | – | ||
| Mansour et al34 | 84 eyes of 79 patients | 45% 2.5 mg bevacizumab, 55% 1.25 mg bevacizumab | ± | ± | 0.44 (P<0.001), BCVA worsened in 10.7% | CFT: 252 (P<0.001) | – | – | – | – | For CNV: 43.2% complete regression, 36.5% partial regression, 6.8% no response, 13.5% not evaluated. For NVD or NVE: 63.6% complete regression of new vessels, 36.4% partial regression | |
| Bae et al36 | 21 eyes | 10 eyes | 1.25 mg intravitreal bevacizumab | ± | ± | Best improvement at 4 weeks of 0.26±0.22. BCVA worsened at 12 weeks but still improved from baseline (P<0.001). Significantly better improvement of BCVA in Behçet’s uveitis than in non-Behçet’s uveitis (P=0.045) |
CFT: best at 4 weeks: 293±234 mm, 45.4% reduction. Worsened with time | – | – | – | – | Median period of effect: 16 weeks |
| 11 eyes | 4 mg IVTA | ± | ± | Best improvement at 4 weeks of 0.35±0.19. No statistically significant difference between BCVA change in eyes treated with bevacizumab and IVTA | CFT: best at 4 weeks: 230±99 mm, 61.3% reduction. Worsened with time | Median period of effect: 30 weeks | ||||||
| Rahimi et al32 | 60 eyes of 55 patients | 31 eyes | 1.25 mg bevacizumab | ± | ± (baseline) | 1 month: 0.14±0.08 (P<0.001), 3 months: 0.06±0.06 (P<0.001), 6 months: 0.03±0.04 (P<0.001) | CMT: 1 month: 254.54±30.15 (P<0.001), 3 months: 233.90±12.56 (P<0.001), 6 months: 221.06±12.13 (P<0.001) | At 6 months, anterior chamber reaction grade: 0.15, vitreous reaction grade: 0.52 | – | – | – | – |
| 29 eyes | 4 mg IVTA | ± | ± (baseline) | 1 month: 0.15±0.08 (P<0.001), 3 months: 0.07±0.06 (P<0.001), 6 months: 0.03±0.04 (P<0.001). No statistically significant difference in the two groups at all time points | CMT: 1 month: 251.75±30.41 (P<0.001), 3 months: 218.13±29.00 (P<0.001), 6 months: 199.27±27.64 (P<0.001). Intergroup difference is statistically significant at 3 and 6 months | At 6 months, anterior chamber reaction grade: 0.1, vitreous reaction grade: 0.55. Intergroup difference is not statistically significant | ||||||
| Mansour et al28 | 81 eyes | 72.8% 2.5 mg bevacizumab, 27.2% 1.25 mg bevacizumab | ± | ± | 3 years: 0.43±0.43, mean difference of 0.27±0.46 (P<0.001) | 3 years: 224.5±62.5, mean difference of 97.9±85.8 (P<0.001) | – | – | – | Median: 3 injections | – | |
Notes: “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: BCVA, best-corrected visual acuity; CFT, central foveal thickness; CMT, central macular thickness; CNV, choroidal neovascularization; CRT, central retinal thickness; CS, corticosteroids; IVTA, intravitreal triamcinolone acetonide; MD, mean deviation; ME, macular edema; NIU, noninfectious uveitis; NVD, neovascularization of disc; NVE, neovascularization elsewhere; VEGF, vascular endothelial growth factors.
Table 16.
Studies on intravitreal vascular endothelial growth factors (adverse effects)
| Study | Number of participants/eyes | Intervention
|
Adverse events
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-VEGF Injection | Systemic CS (unless otherwise stated) | Immunosuppression | Ocular
|
Systemic | ||||||
| Cataracts | Raised IOP
|
Others | ||||||||
| >10 mmHg/requiring medications | Requiring surgery | |||||||||
| Rouvas et al30 | 16 eyes of 15 consecutive patients | 0.5 mg ranibizumab | – | – | None | None | None | Retinal pigment epithelial atrophy surrounding the regressed CNV was developed in 11 of the 16 eyes (68.8%) | None | |
| Julián et al29 | 15 eyes from 15 patients | 1.25 mg bevacizumab | ✓ | 9 | None | None | None | Retinal atrophy in 13% | None | |
| Mansour et al35 | 99 eyes of 96 patients | 33.3% 2.5 mg bevacizumab, 66.6% 1.25 mg bevacizumab | ± | ± | None | 1% mild ocular hypertension (not quantified) | None | 3% submacular fibrosis and 1% submacular hemorrhage | None | |
| Mansour et al34 | 84 eyes of 79 patients | 45% 2.5 mg bevacizumab, 55% 1.25 mg bevacizumab | ± | ± | None | None | None | 1% macular hemorrhage after injection | None | |
| Bae et al36 | 21 eyes | 10 eyes | 1.25 mg intravitreal bevacizumab | ± | ± | None | 10% of eyes had increase in IOP >5 mmHg above baseline | None | None | None |
| 11 eyes | 4 mg IVTA | ± | ± | 36% | 9% | |||||
| Rahimi et al32 | 60 eyes of 55 patients | 31 eyes | 1.25 mg bevacizumab | ± | ± (baseline) | – | Mean of maximum increase in IOP: 17.77±2.15 mmHg | None | – | None |
| 29 eyes | – | 4 mg IVTA ± (baseline CS) | ± (baseline) | Mean of maximum increase in IOP: 20.00±1.89 mmHg (significantly higher than bevacizumab) | None | |||||
| Mansour et al28 | 81 eyes | 72.8% 2.5 mg bevacizumab, 27.2% 1.25 mg bevacizumab | ± | ± | – | – | – | 6.2% submacular fibrosis, 1.2% eye retinal pigment epithelial tear, 1.2% eye macular ischemia in the context of vasculitis | None | |
Notes: “–”, data not available; ±, treatment was or was not administered based on physician’s discretion; ✓, treatment administered.
Abbreviations: CNV, choroidal neovascularization; CS, corticosteroids; IOP, intraocular pressure; IVTA, intravitreal triamcinolone acetonide; VEGF, vascular endothelial growth factors.
In a retrospective multicenter case study of 84 eyes receiving either 1.25 or 2.5 mg of intravitreal bevacizumab (IVB), Mansour et al reported that IVB resulted in significant visual improvement of 2.5 lines as well as decrease in CRT in a short term.34 However, BVCA worsened in 10.7% of the eyes, but no possible reason was discussed. Macular hemorrhage occurred in one eye, but no other systemic or ocular adverse events occurred. As this was a multicenter retrospective study, the researchers were unmasked and the given doses of IVB were inconsistent.
Another retrospective study by Mansour et al focused on the long-term effects of IVB on 99 uveitic eyes with CNV refractory to systemic treatment and reported that IVB resulted in long-term significant improvement in mean BCVA and CRT with an average of 3.6 injections up to the follow-up period of 24 months.35 The angiographic regression pattern correlated with the primary disease, and complete regression was associated with younger age. However, this correlation was not found with regard to the location of CNV or the concomitant intake of immunosuppressive therapy. Ocular adverse events were observed in this study: submacular fibrosis in three eyes, submacular hemorrhage in one eye, and mild ocular hypertension in another. Yet another retrospective study of 81 eyes by Mansour et al34 showed the improvement of BCVA and CRT after the use of IVB with a median of three injections in 3 years. Adverse events observed were submacular fibrosis, retinal pigment epithelial tear, and macular ischemia in the context of vasculitis.
Also focusing on evaluating the long-term effects of IVB, a retrospective case series of 15 uveitic eyes with CNV refractory to systemic therapy over 17.6 months by Julián et al29 reported that 1.25 mg/0.05 mL IVB resulted in a statistically significant improvement of BCVA and CRT in most of the eyes after the first month and at the fourth month. However, this effect was transient as BCVA and CRT in the later months did not show statistically significant difference. Notably, BCVA and CRT also worsened in a few eyes. Most of the eyes had more than one injection given with the mean number being 4.25 at a frequency of one every 12.97 weeks. No adverse ocular or systemic side effects were observed in this retrospective study. Larger-scale studies were recommended to evaluate the correlation between the number of injections and the subgroups of uveitis. The discrepancy in the findings of this study and Mansour et al34 with regard to the long-term effect of IVB could be due to the fact that the sample size is different and that the doses of IVB given were inconsistent between the two. Furthermore, their inclusion criteria differed slightly with Mansour et al’s study including uveitic eyes with active inflammation.
The use of another anti-VEGF agent, ranibizumab in the treatment of inflammatory CNV was studied by Rouvas et al.30 In this retrospective study of 16 eyes over a mean of 17.6 months, most patients had a significant improvement in BCVA with no patients showing deterioration following an injection of 0.5 mg ranibizumab.30 There was also a significant decrease in CRT. Although all eyes demonstrated regression of CNV, 68.8% of eyes developed retinal pigment epithelial atrophy in the surrounding of the regressed CNV.
Several comparative studies were performed to study the efficacy and safety of IVB in comparison with IVTA in the treatment of uveitic macular edema. In a retrospective comparative study, Bae et al reported that both 1.25 mg IVB and 4 mg IVTA resulted in an improvement in BCVA and CRT, which peaked in week 4 but deteriorated thereafter.36 The improvement in BCVA was greater in IVT, but the difference did not reach statistical significance. Of note, IVB resulted in a significantly larger gain in BCVA in Behçet’s uveitis as compared with non-Behçet’s uveitis; however, the exact details were not provided in the study. The median period of effect of the IVB was 16 weeks as compared with 30 weeks for IVTA. With regard to side effects, an increase in IOP >5 mmHg was observed five times more frequently in eyes treated with IVTA. However, this was a retrospective comparative study that had a small sample size and short duration of study.
Rahimi et al also compared 1.25 mg IVB and 4 mg IVTA on their effect on uveitic CME that was not responding to topical corticosteroids in a randomized comparative trial.32 Both IVB and IVTA resulted in improvements in BCVA that peaked at 6 months with no statistically significant difference between the two. Both the drugs also resulted in a statistically significant decrease in CRT; however, IVTA was significantly better than IVB in this aspect. Regarding adverse effects, IVTA resulted in statistically significantly greater rise in IOP as opposed to IVB, which had minimal effect on IOP.
The results of these studies demonstrated that intravitreal anti-VEGF agents, in particular bevacizumab, resulted in improvement in BCVA and CRT. However, the effects tend to be short-lasting with a need for repeated injections. With regard to adverse events, submacular fibrosis appears to be related to the use of IVB. Side effects commonly seen in intravitreal corticosteroids were not evident with IVB. However, there was variation in the medication dosages in these studies. Furthermore, common limitations in these studies were that most of the participants were still on systemic therapy during the course of the study, and most of these studies were retrospective. Therefore, long-term and larger-scale randomized controlled trials are needed to establish the efficacy and duration of action as well as safety and side effect profile of intravitreal anti-VEGF agents in the treatment of NIU.
Intravitreal methotrexate
In NIU, methotrexate is usually used for systemic immunosuppression. It is an anti-metabolite that is commonly used for the treatment of rheumatoid arthritis and cancer. Intravitreal methotrexate was first introduced for the treatment of intraocular lymphoma. Taylor et al investigated the use of intravitreal methotrexate in a pilot prospective interventional case series study and in a multicenter retrospective case series study.37,38 Tables 17–19 provide the summary of studies regarding intravitreal methotrexate.
Table 17.
Studies on intravitreal methotrexate (demographics and clinical features)
| Study | Period of study | Study design | Study duration | Number of participants/eyes | Demographics
|
Clinical features of participants
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
Sex (female) |
Diagnosis of study eye | Details | Laterality of condition | Duration of uveitis | Presence of other ocular conditions | Previous uveitis treatment | Mean baseline VA (logMAR) | Mean baseline vitreous haze | Mean baseline CRT/CFT/CMT (μm) | |||||
| Taylor et al37 | – | Prospective, consecutive, interventional case series | Follow-up of 6 months in 80% | 15 eyes of 15 patients | 50 (range, 25–68) | 47% | Active NIU ± CME, all steroid responders | 27% anterior uveitis with long-standing CME, 53% intermediate uveitis with active vitritis and CME, 20% panuveitis with vitritis and CME | 100% unilateral | Median duration of CME in current disease episode: 6 months (range, 1–54 months) | 20% vitrectomized eyes, 67% pseudophakic | 47% on systemic medication at study entry. 27% eyes had previous IVTA injection | 1.06±0.12 | 1.40±0.16 | 425±57 |
| Taylor et al38 | – | Multicenter, retrospective interventional case series | Mean follow-up: 11.2 months (range, 3–28 months) | 38 eyes of 30 patients | Median: 46 (range, 20–73) | 53% | NIU | 18% chronic anterior uveitis with CME, 42% intermediate uveitis or pars planitis, 39% posterior uveitis or panuveitis | – | – | – | 47% on systemic medication at time of study entry | 0.60 (range, 0.10–1.30) | – | 436±33 (range, 227–1,173) |
Note: “–”, data not available.
Abbreviations: CME, cystoid macular edema; CFT, central foveal thickness; CMT, central macular thickness; CRT, central retinal thickness; IVTA, intravitreal triamcinolone acetonide; logMAR, logarithm of Minimal Angle of Resolution; NIU, noninfectious uveitis; VA, visual acuity.
Table 18.
Studies on intravitreal methotrexate (outcomes)
| Study | Number of participants/eyes | Intervention
|
Numbers excluding those lost to follow-up/dropout | Outcomes measured
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravitreal methotrexate | Systemic CS | Immunosuppression | BCVA | Mean CRT/CFT/CMT (μm) | ME | Uveitis activity/vitreous haze score | Mean time to first recurrence of uveitis | Uveitis recurrence rate | Reinjections | Others | |||
| Taylor et al37 | 15 eyes of 15 patients | 400 g in 0.1 mL | ± (same as baseline) | ± | 12 (3 lost at different times for different reasons) | 1 week: 0.82±0.13, 1 month: 0.73±0.12, 3 months: 0.63±0.11, 6 months: 0.59±0.09 (P<0.01) | 2 months: 299±55 P=0.01), 4 months: 291±53 (P=0.01), 6 months: 275±51 (P=0.01) | – | 1 month: 0.70±0.23 (P=0.07), 3 months: 0.50±0.17 (P=0.05), 6 months: 0.25±0.18 (P=0.01) | Median 4.0 months (range, 1–4 months) | – | 27% had repeat injections after relapse. All gained median of 17 letters (range, 6–23 letters) by 2 months after reinjection | |
| Taylor et al38 | 38 eyes of 30 patients | 400 g in 0.1 mL | ± (same as baseline) | ± | – | 0.48 (range, 0.00–1.30) (P=0.000) | 363±25 (range, 150–826) (P=0.001) | – | – | 21% eyes relapsed at median 3 months (range, 1–17 months). 58% of eyes in extended period of remission: Kaplan–Meier estimate is 17 months to recurrence | – | – | 57% reduced dose of systemic medication. 20% still require at final follow-up |
Notes: “–”, data not available; ±, treatment was or was not administered based on physician’s discretion.
Abbreviations: BCVA, best-corrected visual acuity; CME, cystoid macular edema; CFT, central foveal thickness; CMT, central macular thickness; CRT, central retinal thickness; CS, corticosteroids; ME, macular edema.
Table 19.
Studies on intravitreal methotrexate (adverse effects)
| Study | Number of participants/eyes | Intervention
|
Adverse events
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Intravitreal methotrexate | Systemic CS | Immunosuppression | Ocular
|
Systemic | |||||
| Cataracts | Raised IOP
|
Others | |||||||
| >10 mmHg/requiring medications | Requiring surgery | ||||||||
| Taylor et al37 | 15 eyes of 15 patients | 400 g in 0.1 mL | ± (same as baseline) | ± | 6.7% (thought unlikely to be due to methotrexate) | None | None | 6.7% corneal epitheliopathy | |
| Taylor et al38 | 38 eyes of 30 patients | 400 g in 0.1 mL | ± (same as baseline) | ± | None | None | 3% | None | None |
Note: ±, treatment was or was not administered based on physician’s discretion.
Abbreviations: IOP, intraocular pressure; CS, corticosteroids.
In the pilot study of 15 eyes in 15 patients, Taylor et al reported that intravitreal methotrexate resulted in an improvement in BCVA and ocular inflammation as well as CRT with significant effects seen within 1 week except in two patients.37 Systemic medications were also reduced in patients who responded. A relapse occurred in 30% of patients at a median time of 4 months. A repeat injection in these patients showed improvement within 2 months. Importantly, there were no instances of raised IOP following intravitreal methotrexate even though all patients had raised IOP secondary to corticosteroids (steroid responders). Corneal epitheliopathy occurred in one pseudophakic patient while opacification of lens occurred in another patient (although it was postulated to be unrelated to the methotrexate injection).
The multicenter retrospective case series study consisted of 38 uveitic eyes.38 In this study, 79% of eyes responded to the intravitreal methotrexate with improved visual acuity, ocular inflammation, and macular thickness, which was consistent with the pilot study, and 27% of the eyes responded to intravitreal methotrexate relapsed after a median period of 3 months. However, a larger proportion of eyes entered an extended period of remission with no relapses throughout the period of follow-up. Taylor et al38 estimate the time of relapse for these eyes to be 17 months on the basis of the Kaplan–Meier estimate. Similar to the pilot study, 57% of patients, who were on systemic therapy, were able to reduce their doses. Regarding adverse effects, only one eye had an increased IOP of >25 mmHg, which was controlled with medications. No other ocular or systemic adverse events were recorded. However, short-term adverse events might not have been recorded, and this is a limitation of the retrospective study.
Intravitreal methotrexate appears to be a promising alternative to IVTA in unilateral diseases especially in phakic, steroid responders due to lower risk of increasing IOP and cataract formation. The extended remission effect by methotrexate in some patients should be explored in future studies. Thus far, studies have shown that intravitreal methotrexate alone may not be adequate to achieve remission in various uveitic entities and may be used as an adjunct with other forms of therapy. It is imperative to monitor the development of adverse events such as corneal decompensation, which may require treatment with topical folinic acid. Larger-scale and randomized controlled trials are definitely required for the establishment of efficacy and safety profile of intravitreal methotrexate. Contraindications to systemic methotrexate should also be observed.
Intravitreal sirolimus
Sirolimus, previously known as rapamycin, is one of the latest drugs in the spotlight for intravitreal treatment of NIU. It is a macrolide antibiotic, which is a potent immunosuppressant, and has antiproliferative properties. Tables 20–23 provide the summary of studies regarding intravitreal sirolimus.
Table 20.
Studies on intravitreal sirolimus (demographics)
| Study | Period of study | Study design | Study duration | Number of participants
|
Demographics
|
|||
|---|---|---|---|---|---|---|---|---|
| Age (years) |
Sex (female) |
Ethnicity | ||||||
| Ibrahim et al39 | – | Prospective, randomized, open-label, interventional study | 12 months | 15 eyes | 20% cat 1 (active uveitis) 60% cat 2 (active uveitis) 20% cat 3 (inactive uveitis) |
45±19.8 | 40% | 73% Caucasian, 20% African–American, 7% others |
| Sepah et al40 | Ongoing | Randomized, phase 2, open-label study | 6 months | 25 eyes | 11 (440 µg injection every month) 14 (880 µg injection every month) |
40±18.53 53±14.09 |
55% 86% |
100% white 93% white, 7% Hispanic |
| Srivastava42 | Ongoing | Multicenter, randomized, double-masked phase III studies | 5 months | 347 eyes | ≥ 18 | 60% | – | |
Note: “–”, data not available.
Abbreviation: cat, category.
Table 21.
Studies on intravitreal sirolimus (clinical features)
| Study | Clinical features of participants
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis of study eye | Details | Presence of other ocular conditions | Previous uveitis treatment/at study entry | Presence of systemic conditions | Mean baseline VA (logMAR) | Mean baseline vitreous haze | Mean baseline CRT/CFT/CMT (μm) | Other baseline values | |
| Ibrahim et al39 | Active and inactive NIU | 33% intermediate, 60% posterior, 7% panuveitis. 7% birdshot choroidopathy, 7% punctate inner choroidopathy, 13% multifocal choroiditis, 7% Vogt–Koyanagi–Harada, 67% idiopathic | NA; specifically for eyes treated intravitreally | – | – | cat 1: 55±6.2, cat 2:66±16.8, cat 3:66±23.1 | – | 377±178 | 47% with ME, CMT:377±178 |
| Sepah et al40 | Active NIU | 18% intermediate, 64% posterior, 18% panuveitis | – | – | – | 27.9+15.1 | Both ≥ 1 + vitreous haze | CMT of subjects with ME: 63%, 467.42± 134.65 | – |
| 28.5% intermediate, 57.5% posterior, 14% panuveitis | 45.2±10.9 | ME: 42%, 375.33±88.63 | |||||||
| Srivastava42 | Active chronic NIPU | – | – | 20% on systemic CS at entry, tapered before injection administered | – | 0.4 | ≥1.5 | – | 33.3% with ME |
Abbreviations: cat, category; CFT, central foveal thickness; CME, cystoid macular edema; CMT, central macular thickness; CRT, central retinal thickness; CS, corticosteroids; logMAR, logarithm of Minimal Angle of Resolution; ME, macular edema; NA, not applicable; NIPU, noninfectious posterior uveitis; NIU, noninfectious uveitis; VA, visual acuity.
Table 22.
Studies on intravitreal sirolimus (outcomes)
| Study | Number of participants | Intervention
|
Numbers excluding those lost to follow-up/dropout | Outcomes measured
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravitreal sirolimus | Systemic CS | Immunosuppression | BCVA | Mean CRT/CFT/CMT (μm) | Uveitis activity/vitreous haze score | Reinjections | Others | ||||
| Ibrahim et al39 | 15 eyes | 20% cat 1 (active uveitis) | 352 µg | – | – | 14 | No statistically significant change from baseline | Patients without ME: CMT did not change *Patients with ME at baseline: mean change of 105 at month 6, 106 at month 12 (not statistically significant) Patient with ME at baseline: mean change of −30 at month 6 and −47 at month 12 (changes are not statistically significant) | 6 months: 40% showed reduction of ≥2 steps vitreous haze, 60% no change or reduction of one step. 12 months: 70% reduction of ≥2 steps, 0% with increase in vitreous haze (P<0.05, month 12) | 70 injections in 14 eyes | – |
| 60% cat 2 (active uveitis) | CS ≥10 mg/day | – | |||||||||
| 20% cat 3 (inactive uveitis) | ± CS <10 mg/day | ± | – | 6 months and 12 months: 88% no change or a reduction of one step of vitreous haze. At month 12: 12% showed worsening of one step (P>0.05) | |||||||
| Sepah et al40 | 25 eyes | 11 | 440 µg monthly | ± | ± | – | Mean change: +3.66 ETDRS letters | Mean change in CFT in those with ME: −89.42 | Decreased ≥1 step: 81.8%, ≥2 steps: 63.6% | ||
| 14 | 880 µg every 2 months | ± | ± | – | Mean change: –2.91 EDTRS letters | Mean change in CFT in those with ME: +81.5 | Decreased ≥1 step: 92.9% (no statistically significant difference in 2 groups, P=0.564), ≥2 steps: 50% (P=0.695) | ||||
| Srivastava42 | 347 eyes | 44 µg or 440 µg or 880 µg or 440 µg every 2 months | ± | ± | 95% | Baseline BCVA ≥20/40: little improvement, baseline <20/40: gained 5 letters (440 µg and 44 µg), baseline <20/100: 10.5 letters in 440 µg vs 4.5 in controls | Minimal change in those with ME at baseline | Vitreous haze score of 0: 22.8% in 440 µg, 16.4% in 880 µg, 10.3% in 44 µg. (P=0.025), vitreous haze score of 0 or 0.5+: 52.6% 440 µg, 35% 44 µg, 43.1% 880 µg (P=0.008) | – | Tapering systemic CS: 76.9% in 440 µg arm, 63.6% in 44 µg arm | |
Notes: “–”, data not available; ±, treatment was or was not administered based on physician’s discretion.
Abbreviations: BCVA, best-corrected visual acuity; cat; category; CFT, central foveal thickness; CMT, central macular thickness; CRT, central retinal thickness; CS, corticosteroids; ETDRS, Early Treatment Diabetic Retinopathy Study; ME, macular edema.
Table 23.
Studies on intravitreal sirolimus (adverse effects)
| Study | Number of participants | Intervention
|
Adverse events
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intravitreal sirolimus | Systemic CS | Immunosuppression | Ocular
|
Systemic | ||||||
| Cataracts | Raised IOP
|
Others | ||||||||
| >10 mmHg/requiring medications | Requiring surgery | |||||||||
| Ibrahim et al39 | 15 eyes | 20% cat 1 (active uveitis) | 352 µg | – | – | 14.30% | One case IOP >25 mmHg | None | Postulated to be unrelated to drug: vitreous floaters | None |
| 60% cat 2 (active uveitis) | CS ≥10 mg/day | – | ||||||||
| 20% cat 3 (inactive uveitis) | ± CS <10 mg/day | ± | ||||||||
| Sepah et al40 | 25 eyes | 11 | 440 µg monthly | ± | ± | – | – | – | – | – |
| 14 | 880 µg every 2 months | ± | ± | |||||||
| Srivastava42 | 347 eyes* | 44 µg or 440 µg or 880 µg or 440 µg every 2 months | ± | ± | Single cases | Single cases in 44 µg and 440 µg arms | One case of culture-negative endophthalmitis in 440 µg arm. Noninfectious endophthalmitis: 0.9% patients’ 440 µg, 3.4% patients in the 880 µg arm |
– | ||
Notes:
Data from both intravitreal and subconjunctival administration. “–”, data not available; ±, treatment was or was not administered based on physician’s discretion.
Abbreviations: CS, corticosteroids; IOP, intraocular pressure.
In 2003, the Sirolimus as a therapeutic Approach uVEitis (SAVE) study, a 12-month study of 30 patients, was initiated to evaluate the efficacy of intravitreal and subconjuctival sirolimus.39 The results of the 6-month interim analysis were reported by Nguyen et al.39 Sirolimus appeared to be well-tolerated in both the administration routes with improved inflammation and no ocular adverse events. Approximately 40% of eyes with active uveitis had improvement in inflammation with two or more steps difference in VH while 60% remained at baseline or had one-step improvement. However, some eyes did not have the potential to improve two steps, so the results may be skewed. Regarding its effects on visual acuity, improvement in BCVA was only seen in one-third of the patients with the rest maintaining stability and 20% had deterioration. This was attributed to the high baseline BCVA, with lower likelihood of improvement. Initial but nonsustained improvement in CRT was seen in some of the patients. Ibrahim et al39 reported the 1 year results of the SAVE study. A total number of 70 intravitreal sirolimus injections were administered to the 14 study eyes. Again, sirolimus, regardless of administration route, showed efficacy in reducing intraocular inflammation. At the end of 1 year, 70% of eyes with active uveitis showed a statistically significant reduction in two steps or more of VH. In patients with inactive uveitis, 88% of eyes showed no change or one-step decrease in VH, whereas 12% had a one-step increase. However, these changes in the eyes with inactive uveitis were not statistically significant. There was no statistically significant improvement in mean BCVA or change in CRT. Sirolimus appeared to be well-tolerated with repeated administration as well.
In 2015, the SAVE-2 study was initiated. This was a randomized comparative trial that compared, in 25 uveitic eyes, the effect of 440 µg administered monthly as opposed to 880 µg of intravitreal sirolimus administered every 2 months. The 6-month interim results as reported by Sepah et al were that low-dose sirolimus (440 µg) appeared to have an advantage in reducing uveitic macular edema.40 However, both the doses seemed to be equally efficacious in the reduction of VH. Results of the SAVE-2 study are awaited.
In addition, there are ongoing multicenter, randomized, double-masked Phase III studies (SAKURA study), which are investigating the efficacy and tolerability of three doses of intravitreal sirolimus: 44 µg, 440 µg, and 880 µg administered every 2 months in the management of NIU in 347 patients.41 Srivastava et al42 presented the data for the primary endpoint of this study: the percentage of eyes with a VH score of 0 at month 5. It was reported that 440 µg sirolimus was found to be significantly better than the other doses in achieving the primary endpoint, with 22.8% of eyes in the therapeutic arm achieving a VH score of 0 as compared with 10.3% in the 44 µg arm and 16.6% in the 880 µg arm. The 440 µg sirolimus was also significantly superior in achieving the secondary endpoint: VH score of 0 or 0.5+ was achieved in 52.6% of patients in the 440 µg arm as compared with 43.1% in the 880 µg arm and 35% in the 44 µg arm. BCVA was maintained overall in the first 5 months. Regarding the safety of sirolimus, there was one case of culture-negative endophthalmitis in the 440 µg arm and noninfectious endophthalmitis in 0.9% of patients in the 440 µg arm and 3.4% of patients in the 880 µg arm. There were also single cases of raised IOP, glaucoma, and cataract formation in the 44 µg and 440 µg arms.42
In summary, based on the published literature, sirolimus appears to be effective in controlling intraocular inflammation and is well-tolerated regardless of the administration route. However, no significant effects were shown in improving BCVA or CMT. The results of currently ongoing studies may help us to establish the efficacy and side effect profile of intravitreal sirolimus.
Intravitreal anti-tumor necrosis factor: infliximab
Infliximab, an anti-tumor necrosis factor agent, is a chimeric monoclonal antibody biologic drug usually used systemically for the treatment of autoimmune diseases. Administering infliximab intravitreally eliminates the systemic side effects of the drug, which is ideal. These side effects include congestive heart failure, reactivation of latent tuberculosis, and increased risk of infections. Intravenous infliximab is also contraindicated in patients such as those with a history of advanced congestive cardiac failure, active infections, or cancer.43
Limited trials are available for the use of intravitreal infliximab in NIU. To the best of our knowledge, there is only one study that fits our inclusion criteria. Markomichelakis et al performed a prospective, noncomparative interventional pilot study on the effect of intravitreal infliximab in the treatment of sight-threatening relapsing uveitis in Behçet’s disease of 15 eyes.44 The study observed the effects of 1 mg/0.05 mL of infliximab up to 30 days posttreatment. Significant improvement in BCVA was noted by day 7 and was sustained until day 30. A decrease in intraocular inflammation and improvement in retinal vasculitis was maintained until day 30. However, even though there was a decrease in mean CMT, persistent CME was noted in 80% of the eyes. There were no statistically significant differences between the results of those with and without baseline systemic immunosuppressants. No ocular or systemic side effects were observed during the course of 30 days; however, the study did not evaluate the possibility of retinal toxicity and autoantibodies that may have formed as a response to intravitreal infliximab. This potential immunogenic and retinotoxic effect was previously reported in a study on low-dose (0.05 mg) intravitreal administration of infliximab in eyes with age-related macular degeneration and CNV.45 Furthermore, as the follow-up was only for 30 days, the long-term effects of intravitreal infliximab and the effects of repeated injections are not known. The small sample size was also a limitation.
Markomichelakis et al also noted that based on a similar study performed by their group regarding the effect of intravenous administration of infliximab, the intravenous route seemed to have a significantly faster effect as compared with the intravitreal route.46 This was postulated to be due to the systemic nature of Behçet’s disease. Therefore, it was recommended that intravitreal injections be considered only when there are systemic side effects or contraindications to the intravenous route. Due to various factors related to intravitreal infliximab such as retinotoxicity, there has been less enthusiasm to pursue this agent using the intravitreal route for NIU. Large-scale and long-term studies are required to establish the safety and efficacy profile of these drugs. It is also important to recognize that drugs that are deemed tolerable via systemic administration may not be well-tolerated intravitreally.
Intravitreal nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs are commonly used systemically for their analgesic and also anti-inflammatory effects.47 Furthermore, the risks of cataract formation or increased IOP are not known. Therefore, the ability of the intravitreal injections to deliver the drug at potentially efficacious levels straight to the posterior segment without the side effects of lens opacification and increased IOP is a favorable prospect. However, to the best of our knowledge, there has been only one trial performed with regard to the use of intravitreal nonsteroidal anti-inflammatory drugs in NIU that fulfills our inclusion criteria.
A pilot randomized comparative clinical trial by Soheilian et al compared the efficacy and safety of 500 mg/0.1 mL of intravitreal diclofenac (IVD) as opposed to 2 mg of IVTA in the treatment of CME in 15 uveitic eyes.48 Both IVD and IVTA showed improvement in BCVA and CMT; however, the improvement in eyes treated with IVTA was statistically significant, whereas the improvement in eyes treated with IVD was not statistically significant. However, there were no statistically significant differences when comparing the mean BCVA and CMT values of the two. The only adverse effect observed in this study was cataract formation; 12.5% in IVD and 28.5% in IVT. This did not reach statistical significance. From this study, it appears that IVD is not as effective as IVTA in the treatment of uveitic CME. However, IVD may still have the potential of being an alternative to IVTA in steroid responders.
Emerging drug therapies
The use of gene therapy in the treatment of NIU is an exciting prospect. Intravitreal delivery of adeno-associated viral vectors coupled with genes can be used as anti-inflammatory proteins. Some of these agents are AAV-Tat-Nrf2mer, AAV2/2-tetON-vIL-10, AAV-CARD, and AAV-sGFP-TatM013. These agents have been shown to have anti-inflammatory effects in the eyes of mice.49–52 Therefore, this could be potentially useful in NIU given that inflammation is the primary pathology.
New-generation calcineurin inhibitor, voclosporin showed a potential reduction in the VH in 50% of patients along with the reduction in the oral prednisolone therapy. However, the phase III study did not show a significant difference between the placebo and disease groups.53,54 The possible reason could be due to the oral route of administration; therefore, it will be worthwhile to study the efficacy after local ocular administration. In line, there are several monoclonal antibodies such as secukinumab, gevokizumab, taclizumab, sarilumab, ESBA 105, rituximab, daclizumab, alemtuzumab, adalimumab, abatacept, etanercept, and rilonacept that are under various phase trials for treating uveitis.54
Another interesting prospect would be the advancement of drug delivery methods for the treatment of retinal diseases such as suprachoroidal drug delivery methods. Delivering drugs through the suprachoroidal space (such as triamcinolone acetonide) potentially allows for an increased amount of drugs to bypass the sclera and diffuse into the posterior segment without the risk that comes with intraocular injections.55
Conclusion
Intravitreal injections are an effective alternative to systemic medications as they are able to avoid systemic side effects but achieve a therapeutic dose in the vitreous. As covered in the review, there are a multitude of different drugs that can be used intravitreally for the treatment of NIU. However, it is difficult to compare the drugs with a lack of comparative studies. Furthermore, each drug appears to be advantageous in targeting certain sequelae or complications of NIU. Therefore, the use of the intravitreal drug should be largely customized to each individual patient with the calculation of risk/benefit ratio when deciding between various intravitreal, systemic, and local therapies. Ideally, we would have liked to evaluate the cost-effectiveness of each drug, as we believe that it is an important factor in the decision-making process.
Finally, systemic treatment still has an important role in treating NIU associated with systemic conditions such as sarcoidosis, autoimmune disease, and Behçet’s disease and also for most cases of bilateral, symmetric, disease.
Acknowledgments
Dr Rupesh Agrawal was sponsored by Clinician Scientist career scheme, National Healthcare Group, Singapore for clinician scientist career track.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Multicenter Uveitis Steroid Treatment Trial Research Group. Kempen JH, Altaweel MM, et al. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: fifty-four-month results of the multicenter uveitis steroid treatment (MUST) trial and follow-up study. Ophthalmology. 2015;122(10):1967–1975. doi: 10.1016/j.ophtha.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DJ. Intraocular implants for the treatment of autoimmune uveitis. J Funct Biomater. 2015;6(3):650–666. doi: 10.3390/jfb6030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner A, BenEzra D. Clinical patterns and associated conditions in chronic uveitis. Am J Ophthalmol. 1991;112(2):151–158. doi: 10.1016/s0002-9394(14)76694-2. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasadhika S, Rosenbaum JT. Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics. 2014;8:67–81. doi: 10.2147/BTT.S41477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2):131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 7.Morrison PW, Khutoryanskiy VV. Advances in ophthalmic drug delivery. Ther Deliv. 2014;5(12):1297–1315. doi: 10.4155/tde.14.75. [DOI] [PubMed] [Google Scholar]
- 8.Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005;112(11):1916.e1911–e1917. doi: 10.1016/j.ophtha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Park UC, Park JH, Yu HG. Long-term outcome of intravitreal triamcinolone acetonide injection for the treatment of uveitis attacks in Behcet disease. Ocul Immunol Inflamm. 2014;22(1):27–33. doi: 10.3109/09273948.2013.829109. [DOI] [PubMed] [Google Scholar]
- 10.Tuncer S, Yilmaz S, Urgancioglu M, Tugal-Tutkun I. Results of intravitreal triamcinolone acetonide (IVTA) injection for the treatment of panuveitis attacks in patients with Behcet disease. J Ocul Pharmacol Ther. 2007;23(4):395–401. doi: 10.1089/jop.2007.0015. [DOI] [PubMed] [Google Scholar]
- 11.Sallam A, Taylor SR, Habot-Wilner Z, et al. Repeat intravitreal triamcinolone acetonide injections in uveitic macular oedema. Acta Ophthalmol. 2012;90(4):e323–e325. doi: 10.1111/j.1755-3768.2011.02247.x. [DOI] [PubMed] [Google Scholar]
- 12.Retisert [Accessed April 6, 2016]. Available from: http://www.psivida.com/products-retisert.html.
- 13.Multicenter Uveitis Steroid Treatment Trial Research Group. Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118(10):1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman DS, Holbrook JT, Ansari H, et al. Risk of elevated intraocular pressure and glaucoma in patients with uveitis: results of the multicenter uveitis steroid treatment trial. Ophthalmology. 2013;120(8):1571–1579. doi: 10.1016/j.ophtha.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW, Fluocinolone Acetonide Study Group Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology. 2010;117(3):567–575. 575.e561. doi: 10.1016/j.ophtha.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 17.Bollinger K, Kim J, Lowder CY, Kaiser PK, Smith SD. Intraocular pressure outcome of patients with fluocinolone acetonide intravitreal implant for noninfectious uveitis. Ophthalmology. 2011;118(10):1927–1931. doi: 10.1016/j.ophtha.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe GJ. Reimplantation of a fluocinolone acetonide sustained drug delivery implant for chronic uveitis. Am J Ophthalmol. 2008;145(4):667–675. doi: 10.1016/j.ajo.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Multicenter Uveitis Steroid Treatment Trial Research Group. Sugar EA, Holbrook JT, et al. Cost-effectiveness of fluocinolone acetonide implant versus systemic therapy for noninfectious intermediate, posterior, and panuveitis. Ophthalmology. 2014;121(10):1855–1862. doi: 10.1016/j.ophtha.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowder C, Belfort R, Jr, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 21.Khurana RN, Porco TC. Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema. Retina. 2015;35(8):1640–1646. doi: 10.1097/IAE.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 22.Lam W, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–1268. doi: 10.2147/OPTH.S80500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomkins-Netzer O, Taylor SR, Bar A, et al. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121(8):1649–1654. doi: 10.1016/j.ophtha.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Ryder SJ, Iannetta D, Bhaleeya SD, Kiss S. Efficacy and tolerability of bilateral sustained-release dexamethasone intravitreal implants for the treatment of noninfectious posterior uveitis and macular edema secondary to retinal vein occlusion. Clin Ophthalmol. 2015;9:1109–1116. doi: 10.2147/OPTH.S84207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013;29(5):501–507. doi: 10.1089/jop.2012.0180. [DOI] [PubMed] [Google Scholar]
- 26.Sanford M. Fluocinolone acetonide intravitreal implant (Iluvien®) Drugs. 2013;73(2):187–193. doi: 10.1007/s40265-013-0010-x. [DOI] [PubMed] [Google Scholar]
- 27.Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol. 2014;8:1891–1911. doi: 10.2147/OPTH.S47778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour AM, Arevalo JF, Fardeau C, et al. Three-year visual and anatomic results of administrating intravitreal bevacizumab in inflammatory ocular neovascularization. Can J Ophthalmol. 2012;47(3):269–274. doi: 10.1016/j.jcjo.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Julián K, Terrada C, Fardeau C, et al. Intravitreal bevacizumab as first local treatment for uveitis-related choroidal neovascularization: long-term results. Acta Ophthalmol. 2011;89(2):179–184. doi: 10.1111/j.1755-3768.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 30.Rouvas A, Petrou P, Douvali M, et al. Intravitreal ranibizumab for the treatment of inflammatory choroidal neovascularization. Retina. 2011;31(5):871–879. doi: 10.1097/IAE.0b013e3182003ca8. [DOI] [PubMed] [Google Scholar]
- 31.Jeon S, Lee WK, Jung Y. Changes in the intraocular cytokine levels after intravitreal bevacizumab in uveitic macular edema. Ocul Immunol Inflamm. 2012;20(5):360–364. doi: 10.3109/09273948.2012.709576. [DOI] [PubMed] [Google Scholar]
- 32.Rahimi M, Shahrzad SS, Banifatemi M. Comparison of intravitreal injection of bevacizumab and triamcinolone acetonide in the treatment of uveitic macular edema. Iran J Immunol. 2012;9(2):136–144. [PubMed] [Google Scholar]
- 33.Reddy AK, Cabrera M, Yeh S, Davis JL, Albini TA. Optical coherence tomography-guided ranibizumab injection for cystoid macular edema in well-controlled uveitis: twelve-month outcomes. Retina. 2014;34(12):2431–2438. doi: 10.1097/IAE.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 34.Mansour AM, Mackensen F, Arevalo JF, et al. Intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol. 2008;146(3):410–416. doi: 10.1016/j.ajo.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Mansour AM, Arevalo JF, Ziemssen F, et al. Long-term visual outcomes of intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol. 2009;148(2):310–316.e2. doi: 10.1016/j.ajo.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Bae JH, Lee CS, Lee SC. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina. 2011;31(1):111–118. doi: 10.1097/IAE.0b013e3181e378af. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116(4):797–801. doi: 10.1016/j.ophtha.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SR, Banker A, Schlaen A, et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina. 2013;33(10):2149–2154. doi: 10.1097/IAE.0b013e31828ac07d. [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim MA, Sepah YJ, Watters A, et al. One-year outcomes of the SAVE study: sirolimus as a therapeutic approach for uveitis. Transl Vis Sci Technol. 2015;4(2):4. doi: 10.1167/tvst.4.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sepah YJ, Sadiq MA, Soliman MK, et al. Interim analyses of the SAVE-2 study: sirolimus as a therapeutic approach for uveitis: a phase 2, open-label, randomized study to assess the safety, tolerability, and bioactivity of two doses of intravitreal injection of sirolimus in patients with non-infectious uveitis. Invest Ophthalmol Vis Sci. 2015;56(7):5775. [Google Scholar]
- 41.Merrill P, Nguyen QD, Clark WL, et al. The SAKURA study, a phase iii, multicenter, randomized, double-masked, study of intravitreal injections of DE-109 for the treatment of active, noninfectious uveitis of the posterior segment: baseline ocular disease characteristics. Invest Ophthalmol Vis Sci. 2014;55(13):109. [Google Scholar]
- 42.Srivastava SK. Study assessing double-masked uveitis treatment (SAKURA) phase 3 trial; Paper presented at: American Academy of Ophthalmology 2014 Annual Meeting; October 17–21, 2014; Chicago, IL. [Google Scholar]
- 43.Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]
- 44.Markomichelakis N, Delicha E, Masselos S, Sfikakis PP. Intravitreal infliximab for sight-threatening relapsing uveitis in Behçet disease: a pilot study in 15 patients. Am J Ophthalmol. 2012;154(3):534–541.e1. doi: 10.1016/j.ajo.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Giganti MM. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30(1):71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- 46.Markomichelakis N, Delicha E, Masselos S, Fragiadaki K, Kaklamanis P, Sfikakis PP. A single infliximab infusion vs corticosteroids for acute panuveitis attacks in Behçet’s disease: a comparative 4-week study. Rheumatology. 2011;50(3):593–597. doi: 10.1093/rheumatology/keq366. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55(2):108–133. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Soheilian M, Eskandari A, Ramezani A, Rabbanikhah Z, Soheilian R. A pilot study of intravitreal diclofenac versus intravitreal triamcinolone for uveitic cystoid macular edema. Ocul Immunol Inflamm. 2013;21(2):124–129. doi: 10.3109/09273948.2012.745883. [DOI] [PubMed] [Google Scholar]
- 49.Smith JR, Verwaerde C, Rolling F, et al. Tetracycline-inducible viral interleukin-10 intraocular gene transfer, using adeno-associated virus in experimental autoimmune uveoretinitis. Hum Gene Therapy. 2005;16(9):1037–1046. doi: 10.1089/hum.2005.16.1037. [DOI] [PubMed] [Google Scholar]
- 50.Ildefonso CJ, Jaime H, Biswal MR, et al. Gene therapy with the caspase activation and recruitment domain reduces the ocular inflammatory response. Mol Ther. 2015;23(5):875–884. doi: 10.1038/mt.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ildefonso CJ, Jaime H, Brown EE, et al. Targeting the Nrf2 signaling pathway in the retina with a gene-delivered secretable and cell-penetrating peptideocular gene delivery of an Nrf2-derived peptide. Invest Ophthalmol Vis Sci. 2016;57(2):372–386. doi: 10.1167/iovs.15-17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ildefonso CJ, Jaime H, Rahman MM, et al. Gene delivery of a viral anti-inflammatory protein to combat ocular inflammation. Hum Gene Ther. 2015;26(1):59–68. doi: 10.1089/hum.2014.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anglade E, Aspeslet LJ, Weiss SL. A new agent for the treatment of noninfectious uveitis: rationale and design of three LUMINATE (Lux Uveitis Multicenter Investigation of a New Approach to Treatment) trials of steroid-sparing voclosporin. Clin Ophthalmol. 2008;2(4):693–702. doi: 10.2147/opth.s2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maya JR, Sadiq MA, Zapata LJ, et al. Emerging therapies for noninfectious uveitis: what may becoming to the clinics. J Ophthalmol. 2014;2014:310329. doi: 10.1155/2014/310329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moisseiev E, Loewenstein A, Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016;10:173–178. doi: 10.2147/OPTH.S89784. [DOI] [PMC free article] [PubMed] [Google Scholar]


