Abstract
BACKGROUND
Double-hit lymphomas (DHL) are collectively defined as B cell non-Hodgkin lymphomas harboring rearrangements of MYC as well as BCL2 and/or BCL6. The impact of specific oncogene rearrangements on outcomes of DHL patients treated with immunochemotherapy has not been previously described.
METHODS
We identified cases in which metaphase karyotyping or fluorescence in situ hybridization for MYC as well as both BCL2 and BCL6 rearrangements were performed. Cohorts were defined by the presence (+) or absence (−) of rearrangements: MYC+/BCL2+/BCL6− (BCL2-DHL), MYC+/BCL2−/BCL6+ (BCL6-DHL) and MYC+/BCL2+/BCL6+ (THL).
RESULTS
117 cases were included in this analysis (n=76 BCL2-DHL, n=16 BCL6-DHL, n=25 THL). As compared to patients with BCL2-DHL, those with BCL6-DHL were more likely to be classified as non-germinal center cell of origin, present with extranodal disease and appeared to achieve higher rates of complete response despite receiving intensive induction therapy less frequently. However, BCL6-DHL patients experienced a shorter median overall survival if achieving initial complete response as compared to BCL2-DHL patients. THL patients experienced survival outcomes similar to BCL2-DHL patients.
CONCLUSIONS
Recognition of the specific oncogene rearrangements in DHL cases may be of prognostic value and potentially guide future therapeutic strategies for patients with this disease.
Keywords: gene rearrangement, MYC, BCL2, BCL6, non-Hodgkin lymphoma
Condensed abstract
This is the first large comparative analysis of Double-hit lymphomas by patterns of oncogene rearrangement.
Patterns of oncogene rearrangement may be predictive of Double-hit lymphoma patient outcome as well as response to targeted therapies.
Introduction
Double-hit lymphoma (DHL) is most commonly defined as a B cell non-Hodgkin lymphoma (B-NHL) with rearrangements of MYC as well as BCL2, BCL6 or rarely other oncogenes.1–3 While DHL patients are frequently analyzed as a single cohort, a few published reports have specifically described patients with MYC and BCL2 rearrangements,4, 5 MYC and BCL6 rearrangements,6, 7 or MYC, BCL2 and BCL6 rearrangements (so-called triple-hit lymphoma)8 individually, or distinguished these DHL subtypes within a larger case series.9 However, the inconsistency of uniform testing for all three gene rearrangements as well as the lack of clear reporting of outcomes based on treatment received limit the current understanding of potential differences in DHL based on genetic profile. Accordingly, we performed a comprehensive comparative analysis of DHL patients by genetic subtype.
Methods
From our previously-described database of DHL patients (Blood 2014 124:2354-61), we identified cases that underwent metaphase karyotyping or fluorescence in situ hybridization for MYC as well as both BCL2 and BCL6 rearrangements. Cohorts were defined by the presence (+) or absence (−) of rearrangements: MYC+/BCL2+/BCL6− (BCL2-DHL), MYC+/BCL2−/BCL6+ (BCL6-DHL) and MYC+/BCL2+/BCL6+ (THL). The histologic classification of these cases was either diffuse large B cell lymphoma (DLBCL) or B cell lymphoma unclassifiable with features intermediate between DLBCL and Burkitt lymphoma (BCLU)/Burkitt-like lymphoma (BLL). Cell of origin classification was determined via immunohistochemical staining (IHC) as per Hans algorithm.10 Therapy was given at the discretion of the treating physician. Overall survival was calculated from the date of diagnosis to the date of death or last documented follow-up. Categorical variables were analyzed by Fisher’s exact test. Survival times were analyzed by logistic regression and depicted by Kaplan Meier survival plots. Univariate and multivariate analyses were performed using Cox proportional-hazards regression, and variables with a two-sided P<0.05 on univariate analysis were considered statistically significant and included in multivariate analysis. All statistical analyses were performed with Stata version 13 (StataCorp, College Station, TX).
Results
Out of 311 DHL cases in our database, 117 underwent testing for MYC, BCL2 and BCL6 rearrangements (BCL2-DHL n=76, BCL6-DHL n=16, THL n=25) and were included in this analysis. Baseline characteristics are described in Table 1. Extranodal disease was more frequently reported in BLC6-DHL (88%) as compared to BCL2-DHL (58%) or THL (48%) patients (P=0.04 for both), and germinal center (GCB) cell of origin was more common in BCL2-DHL (92%) as compared to BCL6-DHL (56%) patients (P=0.001). Other baseline clinicopathologic features, including histologic classification, % Ki67 expression and International Prognostic Index (IPI) score did not differ significantly between genetic subtype group.
Table 1.
Baseline characteristics
| Baseline characteristics | BCL2-DHL n=76 (%) |
BCL6-DHL n=16 (%) |
THL n=25 (%) |
P BCL2-DHL vs. BCL6-DHL |
P BCL2-DHL vs. THL |
P BCL6-DHL vs. THL |
|---|---|---|---|---|---|---|
| Age | 1.00 | 0.49 | 0.53 | |||
| <60 years | 35 (46) | 7 (44) | 14 (56) | |||
| ≥60 years | 41 (54) | 9 (56) | 11 (44) | |||
| Ki67 | 0.41 | 0.16 | 0.75 | |||
| <90% | 37 (49) | 6 (38) | 8 (32) | |||
| ≥90% | 36 (47) | 10 (62) | 17 (68) | |||
| Unknown | 3 (4) | 0 | 0 | |||
| Histologic classification | 0.42 | 0.82 | 0.75 | |||
| DLBCL | 32 (42) | 9 (56) | 12 (48) | |||
| BCLU/BLL | 41 (54) | 7 (44) | 13 (52) | |||
| Other | 3 (4) | 0 | 0 | |||
| Cell of Origin | 0.001 | 0.35 | 0.14 | |||
| GCB | 60 (79) | 9 (56) | 14 (56) | |||
| non-GCB | 5 (7) | 7 (44) | 3 (12) | |||
| Unknown | 11 (14) | 0 | 8 (32) | |||
| IPI | 0.53 | 1.00 | 0.49 | |||
| <3 | 25 (33) | 6 (38) | 8 (32) | |||
| ≥3 | 48 (63) | 7 (44) | 17 (68) | |||
| Unknown | 3 (4) | 3 (18) | 0 | |||
| Extanodal disease | 0.04 | 0.49 | 0.02 | |||
| No | 32 (42) | 2 (13) | 13 (52) | |||
| Yes | 44 (58) | 14 (87) | 12 (48) | |||
| LDH | 0.07 | 0.001 | 0.51 | |||
| Normal | 12 (16) | 6 (38) | 13 (52) | |||
| Elevated | 60 (79) | 9 (56) | 11 (44) | |||
| Unknown | 4 (5) | 1 (6) | 1 (4) | |||
| Stage | 0.49 | 0.56 | 1.00 | |||
| <3 | 13 (17) | 4 (25) | 6 (24) | |||
| ≥3 | 63 (83) | 12 (75) | 19 (76) | |||
| Bone marrow lymphoma | 1.00 | 1.00 | 1.00 | |||
| No | 40 (53) | 8 (50) | 13 (52) | |||
| Yes | 32 (42) | 7 (44) | 10 (40) | |||
| Unknown | 4 (5) | 1 (6) | 2 (8) | |||
| CNS disease | 0.34 | 0.66 | 1.00 | |||
| No | 49 (64) | 9 (56) | 14 (92) | |||
| Yes | 5 (7) | 2 (13) | 2 (8) | |||
| Unknown | 22 (29) | 5 (31) | 0 |
Abbreviations: DLBCL, diffuse large B cell lymphoma; BCLU, B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; BLL, Burkitt-like lymphoma; GCB, germinal center B; IPI, International Prognostic Index; LDH, lactate dehydrogenase; CNS, central nervous system
rituximab-EPOCH, rituximab-hyperCVAD, rituximab-CODOX-M/IVAC
Treatment received and outcomes are described in Table 2. Receipt of intensive induction therapy, defined as rituximab-EPOCH, rituximab-hyperCVAD or rituximab-CODOX-M/IVAC, was similar between BCL2-DHL, BCL6-DHL and THL patients (55%, 44% and 68%, respectively) as was receipt of any central nervous system prophylaxis (61%, 44% and 60%, respectively). A trend towards a higher rate of complete response (CR) was seen in BCL6-DHL patients (75%) as compared to BCL2-DHL patients (54%, P=0.17) and THL patients (46%, P=0.10) although these difference were not statistically significant. A similar non-statistically significant trend was found for patients receiving intensive induction therapy, as those with BCL6-DHL also experienced a higher CR rate (86%) as compared to those with BCL2-DHL (55%, P=0.22) and THL (63%, P=0.37). Of patients in first CR (CR1), 14/39 (36%) BCL2-DHL, 2/12 (17%) BCL6-DHL and 6/11 (55%) THL patients underwent consolidative stem cell transplantation (SCT). All of these patients received an autologous SCT except for two patients with BCL2-DHL and two patients with THL who received an allogeneic SCT. The relapse rate was similar for all BCL2-DHL, BCL6-DHL and THL patients (42%, 50% and 23%, respectively) as well as those receiving intensive induction therapy (36%, 33% and 25%, respectively).
Table 2.
Treatment received and outcomes
| BCL2-DHL | BCL6-DHL | THL |
P BCL2-DHL vs. BCL6-DHL |
P BCL2-DHL vs. THL |
P BCL6-DHL vs. THL |
|
|---|---|---|---|---|---|---|
| Receipt of IIT | 55% | 44% | 65% | 0.42 | 0.35 | 0.19 |
| Receipt of CNS prophylaxis | 61% | 44% | 60% | 0.27 | 1.00 | 0.35 |
| Complete response (CR1) | 54% | 75% | 46% | 0.17 | 0.49 | 0.10 |
| If receiving IIT | 55% | 86% | 63% | 0.22 | 0.77 | 0.37 |
| Stem cell transplant (SCT) in CR1 | 36% | 17% | 55% | 0.30 | 0.31 | 0.09 |
| Primary refractory disease | 32% | 13% | 33% | 0.14 | 1.00 | 0.25 |
| If receiving IIT | 25% | 14% | 14% | 1.00 | 0.71 | 1.00 |
| Relapse (if responding) | 42% | 50% | 23% | 0.75 | 0.33 | 0.23 |
| If receiving IIT | 36% | 33% | 25% | 0.67 | 0.29 | 1.00 |
| Median overall survival (months) | 34.8 | 14.5 | 17.2 | 0.89 | 0.69 | 0.90 |
| If receiving IIT | 37.5 | 12.1 | NYR | 0.67 | 0.91 | 0.84 |
| If achieving CR1 | NYR | 14.5 | NYR | 0.02 | 0.61 | 0.10 |
| If relapsing | 22.2 | 11.5 | 9.7 | 0.15 | 0.43 | 0.82 |
Abbreviations: IIT, intensive induction therapy (rituximab-EPOCH, rituximab-hyperCVAD, rituximab-CODOX-M/IVAC); NYR, not yet reached; CNS, central nervous system
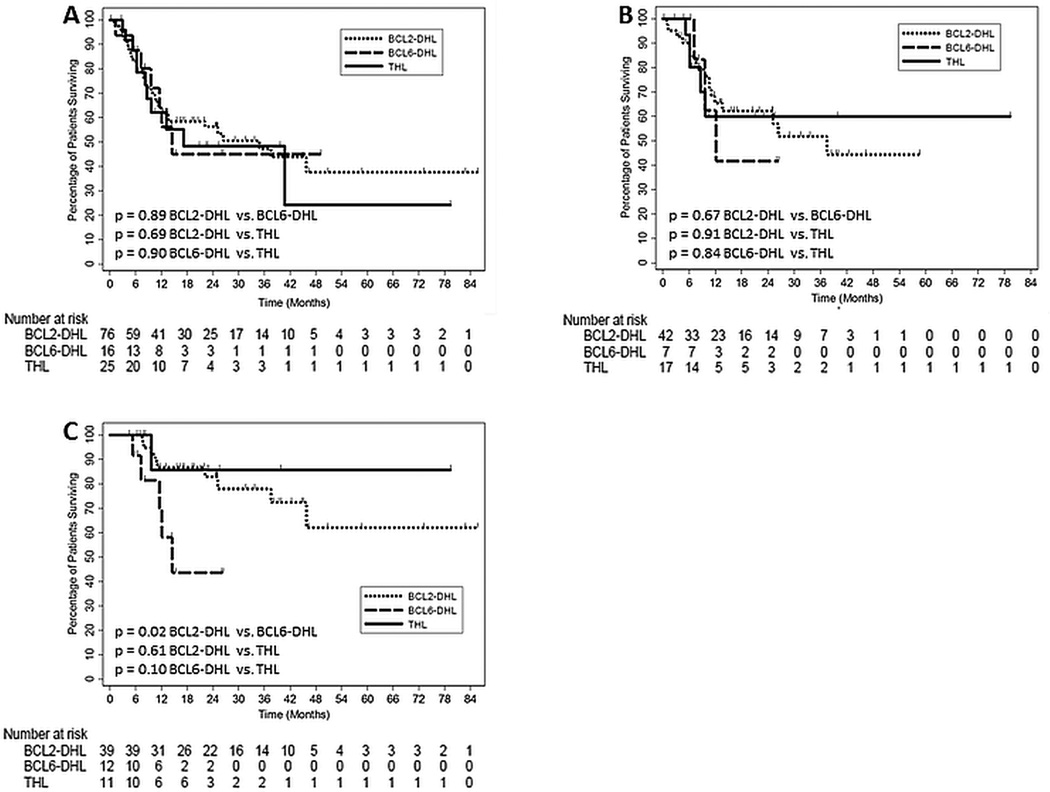
Overall survival (OS) is shown in Figure 1. With a median follow-up of 24.5 months, the median OS for BCL2-DHL, BCL6-DHL and THL patients was 34.8, 14.5 and 17.2 months (Figure 1A), and if receiving intensive induction therapy, 37.5, 12.1 months and not yet reached, respectively (Figure 1B). For patients achieving CR1, the median OS was 14.5 months for BCL6-DHL patients and not yet reached for BCL2-DHL and THL patients (Figure 1C). BCL2-DHL patients experienced significantly longer median OS than BCL6-DHL patients if achieving CR1 (P=0.02). Valid comparisons of survival outcomes based on receipt of SCT in CR1 could not be made across genetic subtypes due to small sample size.
Figure 1. Comparison of overall survival.
Kaplan Meier plots depicting overall survival based on Double-hit lymphoma genetic subtype for (A) all patients, (B) patients receiving intensive induction therapy and (C) patients achieving first complete remission.
Univariate analysis of the following clinicopathologic and treatment-related factors was performed: age <60 vs. ≥60, %Ki67 expression <90% vs. ≥90%, DLBCL vs. BCLU/BLL histologic classification, germinal center vs. non-germinal center cell of origin, International Prognostic Index (IPI) score <3 vs. ≥3, presence vs. absence of extranodal disease, LDH normal vs. >upper limit of normal, stage <3 vs. ≥3, presence vs. absence of bone marrow lymphoma, R-CHOP vs. intensive induction therapy and receipt vs. non-receipt of CNS prophylaxis. IPI score ≥3 (HR 3.0, 95% CI 1.2–7.3, P=0.02), lactate dehydrogenase (LDH) > upper limit of normal (HR 7.6, 95% CI 1.0–55.3, P=0.046), stage ≥3 (HR 4.2, 95% CI 1.0–17.4, P=0.050) and bone marrow lymphoma (HR 2.6, 95% CI 1.3–5.3, P=0.01) for BCL2-DHL patients and IPI score ≥3 (HR 3.6, 95% CI 1.1–12.5, P=0.04) and bone marrow lymphoma (HR 10.4, 95% CI 1.3–83.5, P=0.03) for THL patients was found to be significantly associated with death. However, no factor remained statistically significant on multivariate analysis performed for either subtype. Additionally, risk-stratification by the DHL Prognostic Index did not demonstrate differences in survival outcomes for patients within any genetic subtype.
Discussion
Analysis of this largest reported series of BCL2-DHL, BCL6-DHL and THL patients by genetic subtype highlights two important findings. First, BCL6-DHL patients may have the poorest prognosis of all genetic subtypes of DHL. This is supported by an independent analysis of DHL cases by genetic subtype demonstrating a significantly shorter median overall survival for 13 BCL6-DHL as compared to 20 BCL2-DHL patients,11 as well as reports of a median OS of 9 months in 10 patients7 and only 1 out of 4 patients surviving >6 months6 in two cases series of BCL6-DHL patients. Interestingly, analysis of baseline characteristics did show a significantly higher proportion of BCL6-DHL cases to be of non-GCB cell of origin as compared to BCL2-DHL cases, and given the poor prognosis of non-GCB high-grade B-NHL, an unfavorable cell of origin may explain the survival outcome experienced by BCL6-DHL patients. However, univariate analysis did not demonstrate cell of origin to be predictive of overall survival in BCL6-DHL patients, although the relatively small sample size of BCL6-DHL patients should be acknowledged when interpreting this finding. As BCL6-DHL is underreported in the literature as compared to BCL2-DHL, and therefore potentially under-recognized as a poor prognosis entity in clinical practice, our results highlight the importance of analyzing diagnostic tissue specimens for BCL6 rearrangements in order to better inform treatment decisions.
Second, in spite of the presence of a concurrent BCL6 rearrangement, THL patients may experience similar outcomes to BCL2-DHL patients. This finding is in agreement with findings reported in a case series of 14 patients with THL whose survival did not differ significantly from that of patients with c-MYC and BCL2 rearrangements.9 This clinical outcome may be supported by a pathologic analysis of follicular lymphoma cases which demonstrated that those with only a BCL2 rearrangement had similar morphologic characteristics to those with a concurrent BCL2 and BCL6 rearrangements, suggesting that the presence of BCL6 rearrangement may not affect the phenotype of follicular lymphomas also containing a BCL2 rearrangement.12 Our findings suggest that the additional BCL6 rearrangement seen in THL patients may not indicate a worse prognosis than that of BCL2-DHL patients, and clinicians should consider the use of similar therapies for both groups of patients.
We recognize the limitations of our analysis. First, treatment decisions, particularly the use of intensive induction therapy and SCT in CR1, were not standardized for patients across institutions. Furthermore, small cohort sizes, particularly in the BCL6-DHL and THL cohorts, may have prevented the identification of true differences in outcome. However, when compared to combined9 or individual series of BCL6-DHL6, 7 or THL8 patients, our study represents the largest comprehensive analysis of BCL2-DHL, BCL6-DHL and THL patients. In terms of cytogenetic assays, cutoffs for reporting of positive FISH results were not standardized across participating institutions; nevertheless, we believe that our reported cases of DHL are valid as tissue specimens were analyzed by expert cytogeneticists at large academic medical centers. Furthermore, we included DHL cases which were diagnosed in part by metaphase karyotype, which is not as sensitive as FISH for detection of specified gene rearrangements. However, DHL cases determined by metaphase karyotype have also been included in other large DHL series’,4, 9 and we believe that including these cases in our analysis is valid.
Additionally, our analysis did not address the significance of overexpression of MYC, BCL2 and BCL6 protein by IHC, a finding which may also carry a poor prognosis in patients with high-grade B-NHL. Increased expression of MYC and BCL2 protein by IHC (“double-expression”) has been associated with reduced survival in older13 but not younger14 patients with DLBCL receiving rituximab-CHOP who were enrolled in clinical trials of the German High-Grade Non-Hodgkin Lymphoma Study Group; however, a biologic rationale for this difference is unclear. Furthermore, DLBCL patients with double-expression were reported to have improved survival in one analysis15 but a similar survival in another analysis16 as compared to BCL2-DHL patients, demonstrating uncertainty of the prognostic significance of double-expression relative to BCL2-DHL. Finally, decreased expression of BCL6 in combination with increased expression of MYC has been associated with reduced survival in DLBCL patients,13 suggesting that decreased BCL6 expression by IHC may in fact predict for an unfavorable outcome. Given these reported findings, as well as a lack of standardized cutoffs used to define increased protein expression across studies, the significance of overexpression of MYC, BCL2 and BCL6 by IHC should be carefully considered by treating clinicians.
Classification of DHL by genetic subtype may not only be prognostic, but potentially predictive of response to emerging targeted therapies. Responses to the BH3 mimetic ABT-737 as well as the BCL2-specifc BH3 mimetic ABT-199 alone and in combination with cytotoxic chemotherapy have been reported in a transgenic mouse model harboring c-MYC and BCL2 rearrangements,17, 18 and patients with relapsed/refractory non-Hodgkin lymphoma achieved 62% overall response rate when treated with ABT-199 combined with immunochemotherapy.19 Although direct BCL6 inhibitors have only been studied in preclinical models,20 the heat-shock protein 90 inhibitor PU-H71 has demonstrated suppression of tumor growth in BCL6-dependent cell lines21 and is now being studied in patients with advanced lymphomas (NCT01393509).
In conclusion, oncogene rearrangement patterns may be prognostic for patients with DHL, and the importance of identifying specific oncogene rearrangements may become more clinically relevant in the near future with the emergence oncogene-specific targeted agents.
Acknowledgments
Grant Support: P30 008748
Funding: None
Footnotes
There are no financial disclosures, conflicts of interest, and/or acknowledgements for the authors
Contributor Information
Daniel J. Landsburg, Division of Hematology/Oncology, Hospital of the University of Pennsylvania, Philadelphia, PA.
Adam M. Petrich, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL.
Jeremy S. Abramson, Center for Lymphoma, Massachusetts General Hospital Cancer Center, Boston, MA.
Aliyah R. Sohani, Department of Pathology, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Oliver Press, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA.
Ryan Cassaday, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA.
Julio C. Chavez, Department of Malignant Hematology, Moffitt Cancer Center, Tampa, FL.
Kevin Song, Leukemia/BMT Program of British Columbia, British Columbia Cancer Agency, Vancouver, BC, Canada.
Andrew D. Zelenetz, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY.
Mitul Gandhi, Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL and Virginia Cancer Specialists, Fairfax, VA.
Namrata Shah, Division of Hematology & Oncology, Medical College of Wisconsin, Milwaukee, WI.
Timothy S. Fenske, Division of Hematology & Oncology, Medical College of Wisconsin, Milwaukee, WI.
Jesse Jaso, Department of Pathology, MD Anderson Cancer Center, Houston, TX.
L. Jeffrey Medeiros, Department of Pathology, MD Anderson Cancer Center, Houston, TX.
David T. Yang, Department of Pathology and Laboratory Medicine, University of Wisconsin, Madison, WI.
Chadi Nabhan, Section of Hematology and Oncology, Department of Medicine, University of Chicago, Chicago, IL.
References
- 1.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH. Diagnosis of 'double hit' diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program. 2014;2014(1):90–99. doi: 10.1182/asheducation-2014.1.90. [DOI] [PubMed] [Google Scholar]
- 3.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124(15):2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 4.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114(11):2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25(1):145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 6.Turakhia SK, Hill BT, Dufresne SD, Nakashima MO, Cotta CV. Aggressive B-cell lymphomas with translocations involving BCL6 and MYC have distinct clinical-pathologic characteristics. Am J Clin Pathol. 2014;142(3):339–346. doi: 10.1309/AJCPBWVHTF7RRSA4. [DOI] [PubMed] [Google Scholar]
- 7.Pillai RK, Sathanoori M, Van Oss SB, Swerdlow SH. Double-hit B-cell lymphomas with BCL6 and MYC translocations are aggressive, frequently extranodal lymphomas distinct from BCL2 double-hit B-cell lymphomas. Am J Surg Pathol. 2013;37(3):323–332. doi: 10.1097/PAS.0b013e31826cebad. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Hu S, Lu X, Young KH, Medeiros LJ. Triple-hit B-cell Lymphoma With MYC, BCL2, and BCL6 Translocations/Rearrangements: Clinicopathologic Features of 11 Cases. Am J Surg Pathol. 2015 doi: 10.1097/PAS.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 9.Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 10.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 11.Aukema SM, Kreuz M, Kohler CW, et al. Biological characterization of adult MYC-translocation-positive mature B-cell lymphomas other than molecular Burkitt lymphoma. Haematologica. 2014;99(4):726–735. doi: 10.3324/haematol.2013.091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gollub W, Stassek B, Huckhagel T, et al. BCL6-translocations affect the phenotype of follicular lymphomas only in the absence of t(14;18)IgH/BCL2. Anticancer Res. 2009;29(11):4649–4655. [PubMed] [Google Scholar]
- 13.Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 14.Horn H, Ziepert M, Wartenberg M, et al. Different biological risk factors in young poor-prognosis and elderly patients with diffuse large B-cell lymphoma. Leukemia. 2015;29(7):1564–1570. doi: 10.1038/leu.2015.43. [DOI] [PubMed] [Google Scholar]
- 15.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, Doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 17.Mason KD, Vandenberg CJ, Scott CL, et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci U S A. 2008;105(46):17961–17966. doi: 10.1073/pnas.0809957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121(12):2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vos S, Flowers CR, Wang D, et al. The BCL-2 Inhibitor ABT-199 (GDC-0199) in Combination with Bendamustine and Rituximab in Patients with Relapsed or Refractory Non-Hodgkin’s Lymphoma. 2014 [Google Scholar]
- 20.Cerchietti LC, Ghetu AF, Zhu X, et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 2010;17(4):400–411. doi: 10.1016/j.ccr.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerchietti LC, Lopes EC, Yang SN, et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat Med. 2009;15(12):1369–1376. doi: 10.1038/nm.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]