Abstract
Public academic research sites, private institutions as well as small companies have made substantial contributions to the ongoing development of antidiabetic vanadium compounds. But why is this endeavor not echoed by the globally operating pharmaceutical companies, also known as “Big Pharma”? Intriguingly, today’s clinical practice is in great need to improve or replace insulin treatment against Diabetes Mellitus (DM). Insulin is the mainstay therapeutically and economically. So, why do those companies develop potential antidiabetic drug candidates without vanadium (vanadium-free)? We gathered information about physicochemical and pharmacological properties of known vanadium-containing antidiabetic compounds from the specialized literature, and converted the data into explanations (arguments, the “pros and cons”) about the underpinnings of antidiabetic vanadium. Some discoveries were embedded in chronological order while seminal reviews of the last decade about the Medicinal chemistry of vanadium and its history were also listed for further understanding. In particular, the concepts of so-called “noncomplexed or free” vanadium species (i.e. inorganic oxido-coordinated species) and “biogenic speciation” of antidiabetic vanadium complexes were found critical and subsequently documented in more details to answer the question.
Keywords: Antidiabetics, insulinomimetics, PTP1B, vanadium complexes, speciation, drug design, molecular modeling
1. INTRODUCTION
In 1922 a peptide hormone, insulin, was discovered in certain pancreas cells which regulated blood glucose levels [1, 2]. Decades later, insulin analogs became a theme of research as alternative treatments, giving rise to the observation that unlike insulin - which is not absorbed when applied orally [3] - certain vanadium containing inorganic or organic complexes constitute low-molecular mass substances [4]. They behave as phos-
phate analogs and permeate the plasma membrane and intestinal wall with relative ease [2]. In 1985, a seminal work ushered a new area of antidiabetic research treating rats with oral formulations of vanadium salts for their insulin-like effects [5].
Literature search revealed no articles describing research and development projects (R&D) by large transnational pharmaceutical companies aiming at vanadium-based antidiabetic oral drug candidates - in stark contrast to their endeavor to develop vanadium-free organic compounds cumulating in hundreds of patents worldwide. We became intrigued about what do they know better for not engaging in R&D in the field of antidiabetic vanadium drug candidates? Why do their R&D pipelines remain empty if drugs treating Diabetes Mellitus (DM) figure among the best-selling health products (“block buster” or “cash crop”)? Obviously, there is a lucrative market segment, hence other reasons than marketing should account for their reluctant conduct. Here, we present such arguments, which we collected from the current scientific literature together with specific concepts and the necessary background information to better understand the collected arguments which are summarized below.
1.1. Endogenous Vanadium
Constituting 0.015% of the earth's crust, vanadium is almost as abundant as zinc. It is omnipresent in the biosphere, another precondition for general availability for living organisms. Vanadium is the second most abundant transition element in seawater (45 nM), only below to molybdenum (100 nM) and more abundant than iron (0.02-1 nM) [6].
Vanadium is present in the human body tissues in smallest concentrations around 60 nM (see also nanomolar range in Table 1) [7]. Its daily intake comes from eating food, drinking water or industrially prepared nutrition supplements [2, 8, 9]. Its presence in the human body seems not to be essential - at least until now - and vanadium bearing coenzymes or enzymes have not been identified. Its presence seems more a matter of tolerance. Vanadate (H2VO4ˉ in oxidation state +5) geometrically resembles the ubiquitous biological messenger phosphate (H2PO4ˉ or HPO42ˉ in formal state +5). The charges and structural match may explain its physiological role in analogy to phosphate ions in biochemical reactions [10].
1.2. Relevant Antidiabetic Vanadium Properties
Vanadium ions form stable compounds in oxidation states +4 (IV) and +5 (V) in aqueous media at physiological pH of 7 - whereas in the human body coordinated species also coexist thanks to suited molecular chelators or ligands (Lig) leading to complexes of the general form VOLig or VO2Lig. Aside from vanadate (V) anions (H2VO4ˉ), also nanomolar traces of a very stable diatomic cation exist in the form of vanadyl (IV) VO2+ due to its precipitation as oxidovanadium(IV)-hydroxide. At lower pH and in the presence of organic chelators, however, (organically) complexed, non-oxido-vanadium (IV or V) complexes are favored over so-called “noncomplexed” (or “free”) species and gradually replace the latter [2, 4]. In this context, some authors refer to the terms “noncomplexed” or “free” when dealing with inorganic oxido-coordinated vanadium species. A stable vanadium ion coordination, which uses up to three functions (>C=O, N-OH, >NH), was described in the N-hydroxlylated carboxy-amide groups and known as hydroxamates (R-CONHOH) [4].
The redox potential for the H2VO4ˉ + 4H+ + eˉ = VO2+ + 3H2O reaction is -0.341 volt. Therefore, vanadate is well inside the voltage range where it can be reduced to vanadyl by cellular components like cysteines-containing peptides (glutathione) or proteins, ascorbate (vitamin C), NADH, or phenolic (quinone) compounds. Furthermore, oxido-vanadium cations are strong Lewis acids [11, 12].
Vanadium is special in at least two aspects: firstly, the tetrahedral vanadate anion is similar to the phosphate anion; vanadate can then interact with various physiological substrates that are otherwise functionalized by phosphate. On the other hand, vanadium, as transition metal, can easily expand its sphere of tetrahedral coordination, and switch between three oxidation states of V, IV and III in physiological environments.
Biogenic chelators are ligands like glutathione, citrate, ascorbate or lactate, and in particular, the high molecular mass blood components transferrin, albumin and immunoglobulin, which are biochemical components generated in the body, whereas ascorbate is not biosynthesized in the human body and known as vitamin C [13-16].
Pharmaceutically relevant vanadium is formed by oxidovanadium (IV) and (V) coordination complexes. It appears either as a cation (vanadyl) or anion (vanadate), e.g. vanadyl sulfate, vanadyl bis(acetyl-acetonate) [17], di-ammonium vanado-tartrate, sodium metavanadate (NaVO3) or vanadium acetate [18], a tetrahedral vanadate or an oxyanion of vanadium (V) [19, 20].
Vanadium solubility greatly varies according to the chemical composition of the complex: vanadates (V) are easily soluble, whereas vanadyl hydroxide (oxidovanadium (IV) hydroxide) is almost insoluble (sparingly soluble) and precipitates as VO(OH)2 [21].
1.3. Relevant Antidiabetic Effects of Inorganic Complexes and Organically Chelated Vanadium
Under physiological conditions, vanadium shows two stable oxidation states: IV and V. In strongly reducing conditions, the oxidation state III can also exist. In both more common states, vanadium complexes lower pathologic blood sugar levels. Because of their insulin-like activities, they are sometimes denominated as insulinomimetics, insulin-mimetics or insulin enhancers [2, 5, 22-36].
Antidiabetic vanadium salts act by separate pathways: vanadate (V) yield several beneficial effects concerning glucose and fat metabolism within the cells (cytosolic activity), while vanadyl salts (in form of vanadium IV) normalize glucose concentration in blood plasma by ameliorating the glucose uptake across cytoplasma membranes and inhibit lipolysis [2, 37].
Common bioligands of organically chelated vanadium compounds coordinate vanadium as their central atom through their O-, N- and S-functions like citrate, oxalate, nucleotides or ascorbic acid, as well as certain peptides [38, 39]. Comparing dose-effect relationships, insulinomimetic organo-vanadium complexes (2nd generation) were found superior to inorganic vanadium salts (1st generation) in both ways of in vitro and in vivo studies [34-36, 40-44]. For instance, BMOV (bis-(maltolato) oxido-vanadium (IV)) was three times more potent than “free” (that is uncomplexed) vanadyl sulfate when tested in the same in vivo bioassays [40, 41].
2. R&D WITH VANADIUM-CONTAINING DRUG CANDIDATES
Bibliographically recorded vanadium salts research began at the end of the 19th century with a report in French by Lyonnet, Martz and Martin (“L'emploi therapeutique des derivés du vanadium”) applying inorganic vanadate salts (H2VO4ˉ anion) [45]. At that time, patients suffering from different health problems were observed to find out any beneficial effect of oral vanadium preparations. Such inorganic salts marked the first generation of vanadium-based oral remedies and the 20th century medicine discovered their usefulness not only for Diabetes Mellitus (DM) but also for cardio-protection, or against cancer as well as microbial infections (virus, bacteria and parasites) [38, 46, 47]. The significant progress that has been made since that time has required an interdisciplinary research between chemists (synthesis and analytics), biochemists, pharmacologists (pharmacodynamics, pharmacokinetics) and experts in bio-pharmaceutics, medicinal chemistry and crystallography. Most of them are affiliated to public institutes or academic sites. Clinical studies (in humans) were reported from different groups [22-26]. The turn of the “2K” millenium has seen an organically chelated vanadium complex (BEOV, BMOV) entering the clinical stage - that means direct studies on patients again [21, 48, 49]. A vanadium complex with ethylmaltolate, BEOV for short, has advanced to phase II clinical trials carried out by a spin-off company (Akesis Pharmaceuticals in La Jolla, CA, USA) but drug development came to a standstill due to renal problems observed with some patients [20, 49]. Yet, as pointed out by Dieter Rehder, H. Sakurai and colleagues, so far no preparation has reached the status of an industrially commercialized drug [38, 50].
2.1. Pharmacodynamic Aspects: The Biological Targets of Vanadium-Containing Antidiabetic Compounds
The antidiabetic effects of vanadium are probably linked to the ability of its complexes to exchange ligands or chelators with the environment [21]. Structural requirements are reflected by either one or more unoccupied coordination sites, especially for weakly coordinating monodentate chelators [44]. Moreover, the change of oxidation state of bicationic vanadyl (IV) to vanadate (V) was reported in NADPH-dependent enzymatic redox reactions [51] and later reviewed [52]. Under oxidative stress conditions (reactive oxygen species) VO2+ is oxidized to H2VO4ˉ [21]. Moreover, the structural, electrostatic and chemical features of oxidovanadates (IV: O=V(OH)3ˉ V: O=V(OH)2(O)ˉ) resemble those of monoanionic phosphate (O=P(OH)2(O)ˉ). Of note, vanadate is reactive because it can undergo chemical reactions in solution, e.g. readily redox-convertible to vanadyl complexation by biogenic and reversible ligands. Yet, in biochemical pathways a sort of phosphate - vanadate antagonism could take place with vanadate substituting agonistic phosphate in all sorts of phosphate-regulated enzymatic reactions (phosphatases, kinases or phosphorylases) [21, 38, 52]. Concerning the molecular mechanism(s) of action, reports diverge and give rise to two controversial tenets.
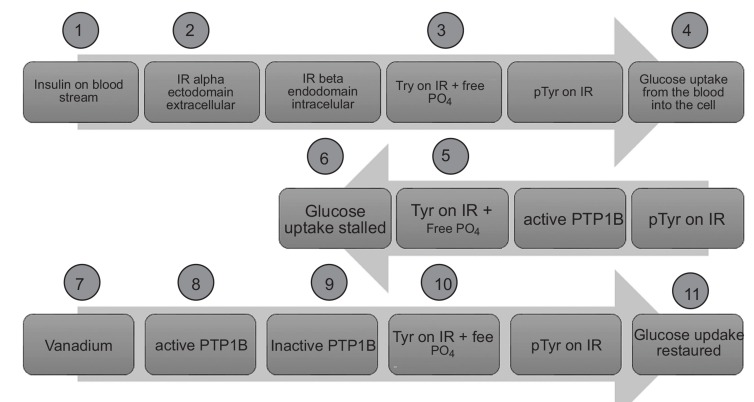
(1) On the one side, it is assumed that vanadate blocks certain protein tyrosine phosphatases (PTP, especially PTP-1B) which are active in an unbalanced way when insulin is missing (DM type 1) or insufficiently present and recognized (DM type 2) [51-54]. In vitro binding assays identified PTP-1B to be the target for the insulin(o)mimetic mechanism of action [55, 56]. By binding to cysteinate side chains at the active sites of PTP enzymes, vanadate anions act as antagonists blocking the access of agonistic phosphate groups (here: phosphorylated tyrosine residues) attached to substrate proteins (here: insulin receptor) to be dephosphorylated by PTP (here: PTP-1B). Subsequently, certain tyrosine residues of the intracellular beta subunit of the transmembrane insulin receptor (IR-ß) remain phosphorylated. The resulting phosphorylated state of the IR induced by vanadate is equivalent to an upstream signaling, which is triggered by insulin hormone upon its binding with the ectodomain of IR to phosphorylate IR-ß tyrosine residues. The glucose transporter for cellular glucose uptake is then activated in a multi-step process further downstream (see Fig. 1) [21]. The molecular mechanism of action is better understood for vanadium-free small organic inhibitors: The catalytic site of PTP-1B is reversibly blocked by such competitive ligands, almost all of which bind to its active state when certain residues (WPD loop) move closer to the catalytic site and thereupon closing it (active state = closed conformation) [54, 57]. Albeit, some molecules showed binding to the catalytically inactive (open) conformation. New drug design opportunities have arisen from the discovery of additional binding pockets or allosteric binding sites with more PTP1B-specific residues since the catalytic site is highly conserved among all PTPs [54, 58].
Fig. (1).
Schematic view concerning the insulin-like effect. Insulin triggers the blood glucose uptake into the cells (step 4) and marks the beginning of the cellular signaling cascade. The active form of PTP1B (steps 5 and 6) is a negative regulator to the physiological (normal) glucose uptake (steps 1 to 4). Pharmacological re-activation of the signal cascade (steps 7 to 10) is achieved by vanadate as a functional antagonist to agonistic phosphate groups. Adapted from [20]. IR = insulin receptor; pTyr = phosphorylated tyrosine residues of the cytosolic beta subunit of IR. Step 11 (insulin-like effect) resembles step 4 (effect by insulin).
(2) On the other hand, the role of PTP1B as the key player has not been accepted in general, leaving the question about the target biomolecule(s) for the insulin-like effects still unanswered [29, 37]. Other enzymes (mostly phosphatases and kinases) in the glucose uptake pathway have been considered as targets [37, 44, 59-63]. In the first biochemical step, the insulin hormone binds to the extracellular domain of the insulin receptor (IR) and activates the IR which is a membrane-spanning protein tyrosine kinase complex (PTK). In step 4 (in Fig. 1), the blood glucose uptake (by insulin responsive glucose transporter GLUT-4) into the tissue cells is triggered which describes the physiological (or normal) signaling protein network. Shechter et al. summarized the biochemical underpinnings of the insulin-mimetic effects of vanadium salts coining the two fundamental aspects: (1) enhancing the glucose utilization; and (2) its storing after entering the cells. Certain metabolic enzymes in key positions for incoming glucose utilization and storage are located in liver, muscle tissue or adipocytes. Some are blocked by vanadate (V) [2]. A cytosolic PTK can be activated by vanadate (V) in addition to another mechanism with similar metabolic effect: a cytosolic PTP can be inhibited by this metalooxide [2]. Generally, vanadate is more active in the cytosolic compartment, enhancing glucose and fat metabolism, while vanadyl (IV) acts on the membranes of the cell plasma facilitating the cell permeation of glucose and possibly inhibiting lipolysis [2]. In the literature, the glucose metabolism was found to be modulated at a site which is located further upstream to the phosphatidylinositol-3-kinase. Lipolysis was inhibited by a vanadate-dependent mechanism further downstream to the aforementioned kinase but not by insulin. Another vanadate-dependent inhibition was reported for the liver enzyme glucose-6-phosphatase and the hexose-6-phosphatase in muscles and adipose tissues of hyperglycemic diabetic rats, all of which led to the restoration of glucose-6-phosphate levels [2].
Of note, tenet (2) makes also sense since vanadate as a phosphate-replacing antagonist is not chemically substituted and cannot exercise (enzyme-independent) exclusive binding (specificity) to target PTP1B. Thanks to its similarity to phosphate (geometry, charge and volume), vanadate is recognized by phosphatases and kinases [64]. However, for being not exactly identical (hydrogen-bonding geometries, electronic mesomerism, redox, volume) it may possess different affinities as a binder in the enzyme network. It may stay longer at the active site of one or another enzyme, and thereby modulates the access for the endogenous (weaker) binder phosphate. With differential binding preferences for both enzyme families vanadate can be considered as a typical surrogate to phosphate. During eons of time, agonistic phosphate anions have been moved around in an evolutionary adapted network of kinases and phosphatases. Its binding strength has not been challenged in the absence of vanadium or in the presence of a negligible natural pool of intracellular vanadium. However, when vanadium supply increases, the cellular systems respond with a plethora of signaling changes.
2.2. Pharmacokinetics, ADMET Models and Cell Uptake
Dieter Rehder summarized the molecular absorption, distribution metabolism, excretion and toxicity processes (ADMET) of orally administered vanadium compounds [38, 63]. To this end, the molecular event of “speciation” has to be understood as the interchange of chelators (re-chelatization) with the extracellular and intracellular body media. Phosphate, oxalate, lactate or citrate anions as well as proteins, transferrin in particular, are potential chelators found everywhere in the body (ubiquitous, biogenic) [65]. Speciation may take place in the gastrointestinal tract, in the blood stream or within the cells. The original chelators are exchanged by new ligands and thereby new molecular properties originate that did not exist in the original compound [38].
Speciation might start in the mouth with saliva, and continue with the gastro-intestinal mucosa. Dieter Rehder wrote: “The main part of vanadium is thus converted into sparingly soluble VO(OH)2, most of which is excreted via the feces, minimizing or even excluding adverse effects that otherwise might be caused by non-physiologically high vanadium levels. Vanadate (V) is more easily resorbed than the soluble vanadyl species, and this can principally cause health problems” [38].
In more general terms, the phenomenon of vanadium speciation is a matter of complex stabilities of its synthetic chelators, biogenic ligands or functional carriers. It is crucial for understanding vanadium’s antidiabetic effects. Vanadium shows variable abilities to change oxidation states and complex geometries, or to exchange its ligands (chelators) with the environment so as to provide an appropriate carrier (furnished by so-called biogenic ligands) for cellular membrane passage. These ligands are found in the body and spontaneously coordinate with the central vanadium atom of the synthetic complexes [38, 66].
In the blood stream, vanadium (in form of vanadyl, VO2+) was reported to have high (binding) affinity to the serum proteins albumin, transferrin and immunoglobulin [38, 66-69]. A pharmacokinetic prerequisite of vanadium complexes to be absorbed and distributed states that vanadyl must be coordinated to appropriate ligands or chelators, for instance maltolate [66]. Specific membrane passages through phosphate and sulfate channels have been suggested for vanadate [21]. Recently, a more detailed look into the carrier-dependent transport through cellular membranes into the cytosol for BEOV was provided by the same review author [21]. BEOV’s maltolate ligand is partially replaced, while albumin and transferrin binding is established in the blood before cell uptake takes place by endocytosis or diffusion, or - in the case of uncomplexed vanadate (V) - via specific phosphate channels. Therefore, not complexed vanadate and the vanadate-transferrin complex enter the body cells, albeit- most probably -none of the intact BEOV moieties [63].
Extensive conversions by ligand displacements were reported for maltol ligand in BMOV by stronger binders, e.g. serum proteins or citrate and ascorbate ions, all of which modify the pharmacokinetic behavior during distribution, drug metabolism and also the final (pharmacodynamical) stage of the mechanism of action at the drug target site [70-72]. Speciation studies of BMOV, vanadyl picolinate, and vanadyl 6-methyl-picolinate were carried out and reported, concluding that 90% of vanadyl ions in circulation are likely to bind to citrate [73].
Insulin-mimetic vanadium compounds are considered mostly as oral drugs for the treatment of type 2 (non-insulin-dependent) diabetes. Therefore, the very first barrier is the gastrointestinal absorption, but very few reactivity studies of anti-diabetic vanadium compounds in gastrointestinal media have been performed. Recently, Levina et al. [73] applied the spectroscopic technique of XANES (X-ray absorption near edge structure) to the speciation derived by simulated gastrointestinal media of the compounds Na3VO4, [VO (OH2)5](SO4), [VO(maltolato)2(H2O)] and (NH4) [V (O)2(pyridine-2,5-dicarboxylate)]. Results showed that vanadyl sulphate and (NH4)[V(O)2(dipic)] were relatively stable in the gastric environment (pH ~ 2) in the absence of food, while the maltolate complex was mostly dissociated, and the sodium salt was converted to [H3V10O28]3-. XANES obtained from gastric digestion of these compounds in the presence of typical food components converged to that of a mixture of V(IV)-aqua, V(IV)-amino acid and V(+III)-aqua complexes. Formation of V(+III) may be important for further metabolism via Fe(+III) pathways, but the main absorption mechanisms appear to be associated with vanadate (poorly absorbed), VO2+ species via M2+ uptake mech-anisms, and passive diffusion of neutral species. These data confirm the role of such complexes as pro-drugs that release the active components on the interactions with biological media.
Strong evidence in the literature indicates that most of the vanadium in the serum is bound to holo-hTf (the iron-saturated protein human serum transferrin, whose main function is the transport of iron in the organism) rather than albumin or immunoglobulin (IgG) [74].
Sanna et al. showed that transport of vanadium compounds ([VO(maltolato)2 (H2O)], [VO(1,2-dim-ethyl-3-hydroxy-4(1H)-pyridinonato)2], and cis-[VO (picolinato)2(H2O)]) through blood and towards the cells proceeds by the formation of the corresponding cis- species cis-[VOLig2(H2O)] or cis-[VOLig2(OH)]ˉ, to later react by exchanging the equatorial coordinated H2O or OH- for one His-N localized on the surface of apo-hTF and holo-hTF, with the formation of the ternary complexes cis-VOLig2(apo-hTF) and cis-VOLig2(holo-hTF) [75]. In the case of the compound [VO(acetylacetonate)2], formation of a ternary complex with these proteins is not observed because this complex cannot be transformed to the corresponding cis- species and no equatorial coordination position is available for one histidine.
Further data reported by Sanna et al. suggest only the vanadium ternary species cis-VOLig2(holo-hTF) could be transported into the cell, while the mixed complexes formed by apo-hTF cannot be internalized by this route because apo-hTF is not recognized by the transferrin receptor [75]. Therefore, the formation of ternary holo-hTF species in the presence of iron bound at specific sites is another possible transport mechanism of insulin enhancing compounds, each with a specifically thermodynamic stability and activity. However, studies concerning the distribution of vanadium in biological fluids showed that a major proportion of vanadium is localized in red blood cells rather than serum [76]. In accord with this fact, Sanna et al. studied the interaction of the vanadium complexes cis-[VO (maltolato)2(H2O)] and [VO(1,2-dimethyl-3-hydroxy-4(1H)-pyridinonato)2] with haemoglobin (Hb) and observed once again that the cis-octahedral complexes bind Hb through an imidazole nitrogen of a superficial His residue in Hb [66]. The logarithmic complex formation constants of 19.6 and 25.8 were measured for the ternary complexes cis-[VO(ma)2(Hb)] and cis-[VO(dhp)2(Hb)], respectively. Also, here no ternary complex was observed with [VO(acetylacetonate)2], the square pyramidal geometry of which hinders equatorial coordination to protein donors in solution.
So far no crystal structure of BMOV bound to PTP-1B has been reported (PDB last visited August 8th, 2015). Intriguingly, in 2003 a report presents the binding of a bipyramidal trigonal (BPT) vanadium oxide to the active site of PTP-1B, although BMOV was incubated with the enzyme at the beginning of the crystallization [77]. Thus, apparently BMOV constitutes a sort of prodrug (with an inorganic oxidovanadium complex) as its bioactive form [39].
3. PRODRUG VERSUS ACTIVE AGENT
The speciation phenomenon in the body implies the answer to the question whether organic vanadium complexes constitute inactive prodrugs (precursors of the active agent). Thanks to the basic research about vanadium speciation, the chemistry of vanadium-chelating organic ligands became much better understood [39, 50, 63]. We also tried to shed some theoretical light on this issue by a molecular modeling study about BMOV and found out that - in theory - an intact form (cis-BMOV but not trans-BMOV) could also dock into the active site of PTP1B [78].
Subsequently, complex design was no longer carried out under the assumption of stable complexes [79]. Since they are transformed by bio-ligands to some extent in competitive re-chelating reactions under steady state or sink conditions with impact on all stages of the ADME processes, the synthetic (original) vanadium complexes were now understood to become precursors to the active form(s) that hit the target receptors in the cells [63]. As a direct consequence, biopharmaceutical research focused on vanadium’s pharmacokinetic behavior and its speciation was also studied.
A paradigm change took place toward finding ligands with new characteristics: (a) better tolerated, less toxic, once set free by re-chelatization in the body; (b) with enhanced cellular uptake, preferably by active carrier systems through cell membranes, in either complexed or uncomplexed form; and (c) with the capability of converting already present intracellular vanadium into an insulinomimetically active species [2].
For instance, an appropriate chelator was found in the amino acid hydroxamate complexes, quite superior to inorganic oxidovanadium salts concerning glucose uptake (in vitro) and blood glucose lowering effects (in vivo) [41, 80]. It was suggested that, in order to explain their improved potency, hydroxamates exploit an amino acid carrier-mediated membrane passage because they constitute a derivative of natural glutamic acid. Once inside the cells, vanadium (bound to either the original ligands or fragments depending on which one passes the membranes) would join the intracellular vanadium reservoir by re-chelating into active insulinomimetic species [80]. Another proof of the prodrug concept was the experimental finding that organically chelated vanadium complexes (BMOV, BEOV) were (up to 3 times) more potent insulin enhancers than vanadyl sulfate in in vitro or in vivo tests what could be ascribed to changes in the bioavailability [20, 21, 35, 40, 42, 48, 63].
The new prodrug concept implies the dissociation of the vanadium complexes before they reach the target biomolecules (see Fig. 2). It has guided research to find appropriate ligands to improve pharmacokinetics, for example the dipicolinato-oxidovanadium(V) ([VO2 dipic]-) [81-83]. Since then, more speciation experiments were carried out under varying conditions (pH, free oxygen, more hydrophilic (aqueous) or hydrophobic, environments (membranes) to observe the complex stabilities and changing compositions [84, 85]. Intriguingly, a crystal structure presented a trigonal-bipyramidal geometry where the central atom vanadium is coordinated to four oxygen atoms of its own (VO4) in addition to another from serine 215 at the active site of PTP-1B, although BMOV was incubated with the enzyme for crystallogenesis [77].
Fig. (2).
Schematic view of a theoretically thinkable activation mechanism of a vanadium-based insulinomimetic drug. The idea was adopted from [37] and [122]. It is assumed that the PTP1B enzyme is the key target to treat diabetes effectively. This negative regulator of the glucose cell uptake can be inactivated by vanadate (V) which is strongly attached to the sulfide (deprotonated sulfhydryl) head group of the sidechain of cystein 215 at the catalytic site. In consequence, agonistic phosphate cannot access the same site because vanadate is the stronger competitive binder. Now, that PTP1B has been deactivated, the enzyme is kept in the phosphorylated state, thereby maintaining alive the signal transduction and glucose continues entering the cells as if more insulin was working through the insulin receptors. This is the clinically observed insulin-mimetic or enhancing effect. Vanadyl compounds BEOV or BMOV are converted into vanadate anions through speciation, a sort of prodrug conversion [122].
4. ARE VANADIUM COMPLEXES DRUGGABLE?
Why the transnational pharmaceutical industry is not active in the field of antidiabetic R&D leaving almost entire discovery to laboratories of public or private institutions and academic sites? Vanadium-containing organic compounds are not druggable then [86]? Vanadium-containing compounds modulate signaling pathways or nuclear transcription factors. For instance, bivanadate − also known as pyrovanadate − modulates the metabolic pathway by directly binding to a DNA primer, thereby modifying the read-out of the nuclear transcription factor complex [21, 86-88]. Therapeutic applications have been identified in the treatment of diabetes or cancer [89]. Vanadium toxicity is also subject to an ongoing debate, especially the question whether daily therapeutic doses of future vanadium drugs on the market would be small enough to avoid acute and long-term toxicity [90]? Moreover, not much is known about its effects on the immune system, inflammatory reactions or oxidative stress, especially if long term administration is required. Imtiaz et al. discussed the role of vanadium in the light of previous work that can help in interdisciplinary studies to evaluate the ecological significance of vanadium toxicity [91]. Other authors have already noticed that the fear of unexpected disadvantages have discouraged the clinical application of vanadium compounds for the treatment of diabetes [92]. As a direct consequence, research work on the biological role of vanadium has gained much importance in the recent years regarding its toxic, mutagenic and genotoxic effects [93]. Recently, vanadium pentoxide was classified by the IARC [94] as a possible human carcinogen: air-borne V2O5 concentration of 0.05 mg/m3 was set as work place protection limit for inhalation. For such reasons and others, some authors have considered vanadium as a weak mutagen [95]. Certain pharmacokinetic characteristics give rise to further concerns: (1) the poor absorption of inorganic vanadium salts in the drinking water [5, 96]; (2) the binding to blood serum proteins in circulation [97, 98]; (3) the uncontrolled variations in oxidation states during the body passage [99-101]; and finally (4) the variable vanadium clearance in dependency to uncontrolled speciation [102, 103]. In a more systematic way, possible reasons for the aforementioned reprobation are outlined in the following table (see Table 1).
Table 1.
List of favorable/unfavourable arguments for the development of vanadium-containing oral antidiabetics.
| Keywords | Comments | Ref. |
|---|---|---|
| Extensive chemical reactivity’s in body fluids | Vanadium shows a highly complex solution chemistry including changes of oxidation states, coordination number and geometries. | [20, 96-106] |
| Complex stability versus speciation | Speciation is a sort of biotransformation due to complex instabilities with subsequent chelator - bioligand interchange with the environment. Strikingly complex speciation pathways were described for the gastrointestinal tract, the blood stream as well as the pulmonary tract. | [38, 106-114] |
| Pharmacokinetics, metabolic properties | Analytical experiments have to study the in vivo behavior of the vanadium moieties in the body, considering that the original vanadium drug is converted into variable “metabolites” with changing composition by speciation. | [conclusions drawn by the authors] |
| Critical interactions with body components with key functions for living cells | Permeation of erythrocytes and binding to haemoglobin either by free species (VO2+) or the intact synthetic complex. In the blood serum plasma protein binding occurs with transferrin and to a lesser extent to albumin and immunoglobulin. | [38, 63, 115, 116] |
| Missing target specificities | Due to the high amino acid sequence conservation (homology) between target PTP1B and other PTPs, certain phosphatases are also targeted. In addition, the active form of the vanadium drugs which ultimately binds to PTP1B or others is still under debate. To complicate matter, reduction of vanadate to vanadyl in the cytoplasm gives rise to even more enzyme interferences. Conversely, administered vanadyl is the source supplying minute amounts of vanadate (IV) even without redox reactions. | [2, 21, 117] |
| Missing pathway selectivities | Phosphatases and kinases belong to two vast families of proteins, which are switched in large metabolic pathways, sometimes in key positions, sometimes with “sidewalks”. That said a range of (off-target) effects can be expected. Among desired and undesired side effects the following were reported: Leptin receptor, ATPase, PPARs agonist, AKT stimulation, AMPK activation, or the indirect stimulation of insulin secretion etc. | [20, 56, 117-122] |
| Defined structure-activity relationships | Due to the structural similarity natural phosphate anions must compete with oxidovanadate (V) which is a stronger binder. But vanadate occupies the active site much longer than phosphate what coined the more practical term of “irreversible binding” which leads to an effective enzyme blocking against weaker binding phosphates. Both, vanadate (V) and phosphate, coordinate into trigonal-bipyramidal complexes with five ligands. The penta-coordination at vanadium is a very stable geometry, while the fifth (covalent) bond is a quite instable transition state complex at the active sites for phosphate-dependent enzymes. The similarity to phosphate explains how vanadate irreversibly inhibits many phosphate-dependent enzymes, not only insulin-related protein tyrosine phosphatases, but also kinases, among others. |
[123-127] |
| Interindividual variability in animal tests and patients | Variable bioavailability and pharmacological response between tested patients for vanadyl sulfate under oral doses of 1mM per day. Also in diabetic rats, oral bioavailability was very low and severe side effects observed under treatment with inorganic vanadium salts. As a direct result organic V complexes were proposed. | [26, 84, 128-133] |
| Uncontrolled daily intake | Food additives or nutritional supplements, water and air account for the daily supply of vanadium but most of it is eliminated due to poor oral absorption. For instance, peroxidovanadates show no oral bioavailability at all. The risk of adverse health effects (threshold) starts at intake levels over 10 mg per kg body weight for a person. Normal exposure to these natural sources is below that threshold. The process of body detoxification was described concerning chemical and biochemical aspects. | [21, 60, 134-137] |
| Ambient concentration | Drinking water and food together supply an average between 0.01 mg and 2 mg per day. Drinking (sea) water has a concentration of 10 (45) nM, respectively. The food contents ranges from 1 to 30 µg per kg food. Vanadium oxides are found in lower concentrations in the air of rural areas with roughly 1 ng per cubic meter up to 100 ng per cubic meter in cities. | [21, 38, 47, 121, 137] |
| Accumulation risk and chronic intoxication risk | An adult person stores roughly 1mg vanadium. Blood levels oscillate around 45 nM. Phosphate in bones (apatite: Ca5(PO4)3OH) is replaced for vanadate. Its half-life amounts to 30 days (residence time 5 days). Vanadium accumulates more in bones, followed by kidneys, then liver. It is feared that therapeutic doses overlap with lower toxic concentrations of 0.2 to 3 mM. For instance, vanadyl sulfate and ammonium metavanadate were administered in animal tests in doses between 0.1 to 0.7 mM per kg and day and 2 mmol (100 mg) per day. Acute lethal doses were measured as LD50 with 0.15 mM per kg body weight for sodium metavanadate. Later it was reported that thanks to the poor bioavailability (low absorption combined with high excretion rate in humans) toxic and therapeutic doses do not overlap. | [21, 102-109, 113, 138-144] |
| High vanadium exposure shows effects on the immune system. | [81] | |
| Reactive oxygen species (ROS) were identified as problematic intermediates for vanadium metabolism. | [21] | |
| Mutagenesis risk | RNA or DNA-related problems were also reported, e.g. binding to DNA primer during DNA polymerase activity. Certain vanadium complexes may act by DNA intercalation (with a potential benefit in antineoplastic, antitumor therapy). | [110] |
Decavanadate ligand complexes also showed insulinomimetic activity but the polymers probably become instable which would ascribe the activity not to decavanadate since it remains stable at concentrations over 10 µM, but disintegrates to “normal” (monomeric) vanadium species [145, 146]. Under in vivo conditions, decavanadate can still be active at nanomolar concentrations in certain cell compartments or protein associations [147, 148]. Recent advances in the elucidation of the signal transduction pathway of PTP´s have revealed that another form of activity control resides inside the cell which is generated by reactive oxygen species. ROS are known to be involved in metabolic cascades that lead to cellular apoptosis, but could also influence insulin-like activities [149]
The question, whether the antidiabetic effect is triggered by a vanadium-blocked PTP1B alone, or indirectly through an interwoven signaling pathway between phosphates and kinases, can be evidenced by looking at the literature about observed PTP1B complexes with non-vanadium ligands. Blocking PTP1B by such ligands also provoked antidiabetic effects on experimental studies [150].
The downsides of vanadium antidiabetic therapy (see Table 1) become a matter of balancing benefits and inseparable risks due to nonspecificities with a plethora of undesired effects all of which would be detrimental to its practical use in daily medication for diabetes patients not willing to accept large(r) lists of side effects. For being literally unreplaceable, however, other drugs with severe off-target effects, such as antiinflammatory steroids, are part of many established therapy plans world-wide [151]. In addition to future claims of patients, industrially prepared vanadium intake would add to unavoidable supply from natural sources and others which cannot be controlled such as: food additives, nutritional supplements, life style or doping. That, too, would counteract the safety needs of an exclusive pharmacotherapy. Besides, there is the unpromising perspective of a quite debatable juridical status with rather confusing definitions and unclear transitions between nutritional supplements and drugs which would demand spending time and money for regulatory affairs and lobbying policies. In the markets in the USA for instance - but not in Europe - vanadyl sulfate salts (VO2+SO42-) circulate legally in commercial offers as nutritional supplement labeled as “Vanadyl Fuel”, to endorse the consumer’s ideas and needs seeking performance enhancements during physical exercises and sport competitions.
In contrast and according to Table 1, a valuable asset awaits being exploited - admitting the prodrug function of vanadium complexes - new chelation strategies could improve absorption, distribution or better tolerated mediation thanks to lower dose ranges below toxicity.
Evaluating Table 1, it is not far-fetched to conclude that a very important shortcoming for vanadium-based antidiabetic drug development is that which remains without any remedy: the experimentally gained evidence that many - if not all - organically chelated vanadium compounds do not act as such, but rather in a non-complexed “free” form. In order to become “free vanadium” species, they dissociate into organic chelators and inorganic oxidovanadium cations (vanadyl, IV) or anions (vanadate, V). Both remain stable under normal body (physiological) conditions. They would function as inactive precursors (prodrugs) or delivery vehicles (molecular drug transporters) to a common active form, namely the “free vanadium” species, the true drug. This in turn implies a rare molecular mechanism of action, such that - prior to showing any antidiabetic effect - the synthetic vanadium-containing compounds are biotransformed into “free vanadium” species upon dissociation of their organic binders (metal ion chelators). This activation would then take place in a prodrug-like fashion either in extra- or intracellular spaces or in both. Finally, we suggest reviews for recommended reading (see Table 1 in [86] and the present Table 2).
With the new insight gained about the fate of vanadium in the living body, new ligands could be better chosen for their properties like lipophilicity (diffusion, aqueous solubility, membrane permeation) or complex stabilities (re-chelating bio-ligands) - all of which influence pharmaco-kinetic endpoints like absorption (active or passive), distribution (plasma protein bind-
ing) or body clearance of vanadium. The success of such “ligand profiling” could turn out in clinically attested higher antidiabetic potencies.
At best, we can suggest that vanadium would become useful as a sort of orally administered co-medication to insulin therapy. A thoughtful article already expressed uncertainty whether an entire lack of insulin would preclude the clinical effectiveness of such drugs [37]. Decades ago, critical voices could already be heard about vanadium that it should be considered “… indeed an ultra-trace metal with an elusive biological function” [152], or that it possessed too “many possible regulatory roles in the body” [105].
The current status of biological chemistry of vanadium and its foreseeable development in future years will focus on four main areas: 1) the detection of enzymes, the catalysis of which is governed by vanadium; 2) the insight into vanadium-based enzyme mechanisms; 3) the synthesis of compounds with catalytic activity; and 4) design, synthesis and biological testing of organic complexes for the treatment of diabetes and other diseases such as cell cancer.
Narrowing the focus on the state of the art antidiabetic lines of development, current efforts concentrate on: the apparent contradictory behavior of antidiabetic vanadium: 1) it is a question of doses [86]; 2) but also complex equilibrium [113] or speciation [114]. For this latter case, chelation research can be carried out with a dual purpose: increased lipophilicity to enhance gastrointestinal absorption, or protein adduct formation facilitated by a more pronounced stability than for uncomplexed “free” vanadium (note: “free” of organic chelators or ligands).
In the recent years, substantial progress has been made in elucidating the mechanisms of catalysis of vanadium. Moreover, phosphatases were characterized by x-ray crystallography, showing how inhibitors (with or w/o vanadium) occupy the active sites (visit PDB database at www.pdb.org). Recent studies using tau (τ) analysis support the claim that vanadium is best described as a trigonal bipyramid (TBPY-5); hence a geometry in the transition state ideal for the hydrolysis of phosphate ester substrate [113].
In 1980, Shechter and Karlish published their seminal work about the insulin-like activity (insulin-mimetics) of vanadate [59]. Then in the late 90’s of the last century, the discovery of the human protein-tyrosine phosphatase (PTP-1B) enzyme as a promising biomolecular target against diabetes [153] ushered a new era of antidiabetes research with vanadium-containing or vanadium-free PTP1B inhibitors. In 2004, a review described the PTP enzymes grouped into four classes, their substrates, structures, functions, biochemical signaling and regulation pathways, and their impact in certain human diseases [154]. At that time, so-called knock-out experiments revealed that living organisms - here mice without ptp1b genes - did not gain body weight (leptin target) and increased insulin sensitivity (PTP1B target). Both targets were found promising to find molecular modulators against adiposity and diabetes [153].
While the academic research sites, universities and public institutes have been proactive developing both types of PTP1B inhibitors, the globally operating pharmaceutical companies did not mingle with vanadium drug research at all. In sight of the lucrative drug markets for diabetes patients, many international pharmaceutical companies started intensive drug research programs for vanadium-free and nonpeptidic PTP1B inhibitors [150]. The big pharmaceutical companies, however, suddenly withdrew their PTP1B research activities leaving behind hundreds of patents of PTP1B inhibitors. The decision is clearly related to two unfavorable discoveries: (i) the primary amino acid sequences of PTP1B and other phosphatases are highly conserved, especially in their active sites and binding regions [155] all of which hamper the development of (more) target-selective or specific drugs against PTP1B; and (ii) knocking out T-cell protein tyrosine phosphatase (TCPTP) in mice led to very short life expectation of such mice. “Big Pharma” apparently had decided to withdraw from PTP1B antidiabetic research because of the potential risk of severe off-target (or side) effects which cannot be “dissociated” from the strong binders to PTP1B [156]. They would also bind strongly to other members of the phosphatase protein family.
Why the vanadium drug development has not been an opportunity for the Pharmaceutical global players to continue antidiabetic research targeting PTP1B? Did “free” vanadium species make antidiabetic research by “Big Pharma” vanadium-free? We did not find an official industry statement for not testing vanadium drug candidates, but the following articles most probably come close to which kind of uncertainties bothered the Medicinal chemists in charge of the in-house R&D programs: vanadium modulates not only the desired signaling pathways, more time and money have to be invested to follow all nonspecific cellular effects. Long-term toxicity and tissue accumulation issues have to be addressed because diabetes requires a life-long drug administration against this chronic disease. Vanadium may increase oxidative stress or pro-inflam-matory reactions [123]. Oxovanadates are anions and they could potentially bind to positively charged protein surfaces altering structure and function [147]. The drug or prodrug conversion into vanadate complicates the interpretation of bioassays, e.g. micromolar oxidovanadates can affect the enzyme function of phosphohydrolases in cell tests, whereas other concerns also include decavanadate, vanadate monomers versus oligomer formation, or simply the vanadyl cations [148].
CONCLUSION
Great progress has been made and research groups detected drug candidates with potential benefits to combat health problems, for instance diabetes mellitus, myocardial infarcts, tissue cancer or tropical infections (e.g. amoebiasis, leishmaniasis or Chagas’ disease). Vanadate was found to be associated with cardio-protective effects, while vanadium’s usefulness against virus (HIV) or mycobacteria (tuberculosis) has been detected. Without academic and small business initiatives all that would otherwise remain unknown, and thereby unexploitable in future pharmacotherapies.
Yet, in a more critical view, developing new organic ligand variations of vanadium-containing antidiabetic drugs could merely have an impact on pharmacokinetics - and since all variations would end up in bioliganded oxidovanadium (IV) or oxidovanadium (V) species no means would exist to profile vanadium drugs to bind to PTP1B as their specific target without binding to other PTPs.
Reaching that point - apparently very soon - the “big” transnational pharmaceutical industries had to conclude that vanadium compounds can never replace insulin therapy, and therefore vanadium can be dismissed as a drug-like option for R&D. They also expected a larger list of undesired side-effects that would become non-dissociable due to the high sequence similarity (over 70%) between target enzyme PTP1B and other members of the PTP family.
Thanks to the aforementioned academic endeavor and initiatives of rather “small” pharmaceutical industries, the situation about the principle mechanisms about the biogenic speciations has been elucidated. The challenge of developing vanadium-based antidiabetic oral drugs now focuses on the finding of organic ligands or chelators with enhanced properties: (i) improve target selectivity to block PTP1B, vanadium complex stabilities and ligand exchanges; (ii) facilitate membrane passages and tissue distributions, biotransformation, (iii) identify in vivo metabolites and (iv) favor excretion over body accumulation. Biochemical tests in concert with biomolecular and crystallographic studies should be a good combination to observe the structure-related molecular behavior of future vanadium (IV) and vanadium (V) drug candidates and hopefully one day find the ultimate compound for diabetes patients with no or few side effects.
Table 2.
Suggested reading to further increase the understanding about the chances and odds to develop vanadium-containing antidiabetic drugs.
| Review title | Comments | Ref. |
|---|---|---|
| Historic perspective and recent developments on the insulin-like actions of vanadium; toward developing vanadium-based drugs for diabetes | To further the reader’s insight about the biochemical aspects of the insulin signaling cascade, metabolic effects and molecular mechanisms of vanadium actions. | [2] |
| Are Vanadium Compounds Drugable? Structures and Effects of Antidiabetic Vanadium Compounds: A Critical Review | At the time of writing, many details were still under debate and the present bibliographic study answers the question raised. (Of note, typing error in the original title’s word “druggable”.) | [86] |
| Vanadium in diabetes: 100 years from Phase 0 to Phase I | A chronological synopsis of the mayor cornerstones in antidiabetic vanadium research spanning the time of over one hundred years. | [20] |
| An evidence-based systematic review of vanadium by the Natural Standard Research Collaboration | The review evaluates the problems with vanadium concerning efficacy, physiological interactions and safety issues. | [112] |
| The future of/for vanadium | The review study covers a wide range of natural sites and life forms which contain vanadium or bio-transformed molecular components bearing it. | [21] |
| Recent Advances in PTP1B Inhibitor Development for the Treatment of Type 2 Diabetes and Obesity (book chapter 6) | The electronic book provides an excellent synopsis about 15 new chemical classes of antidiabetic agents under development. Albeit, book chapter 6 about PTP1B target dedicates merely one single phrase to vanadium complexes: “Vanadium compounds, which are potent inhibitors of PTPs including PTP1B, also displayed insulin mimetic or enhancing effects”. | [150] |
| Thirty years through vanadium chemistry | The important scientific landmarks (events) are reported to form a historical description about the discoveries made concerning biochemical functions, pathways and cellular reactions, along with the discoveries of new structures, their studies as well as the role of speciation for vanadium-containing compounds in aqueous solution and in biological fluids among other issues relevant for the Medicinal Chemist. | [123] |
AcknowledgEments
We are beholden to Prof. Dr. Dieter Rehder, University of Hamburg, Germany for personal invitation and scientific discussion thereafter in addition to the revision of an advanced version of our manuscript during summer 2015.
LIST OF ABBREVIATIONS AND GLOSSARY
- BEOV
= Bis(ethylmaltolato)oxido-vanadium(IV); an ethylmaltol ana-logue of BMOV
- Big pharma
= Top global pharmaceutical compa-nies
- Biogenic
= In vivo speciation
- BMOV
= Bis(maltolato)oxidovanadium(IV); see also BEOV
- cyclic tetravanadate
= V4O12 4ˉ
- Decavanadate
= V10O28 6ˉ
- Divanadate
= H2V2O7 2ˉ (pyrovanadate), a di-phosphate analogue
- DM
= Diabetes mellitus
- Endogenous vanadium
= Intracellularly located vanadium from exogeneous sources, “endog-enous” here means no biosynthesis applied
- Free
= Free of organic chelators, also de-scribed as “non-complexed” vana-dium species, at least not com-plexed by organic chelators, e.g. H2VO4ˉ or VO(OH)(H2O)4+
- IR
= Insulin receptor
- Kinases
= Enzymes that transfer the phos-phate group to a specific substrate
- Metavanadate
= VO3ˉ; does not exist in aqueous media. The species actually present in aqueous media is orthovanadate H2VO4ˉ
- Metavanadate salt
= Sodium metavanadate (NaVO3) etc.
- Peroxooxidovanadates
= Species with the nucleous V=O(O2)n
- Phosphatases
= Dephosphorylation of substrates, mostly tyrosine residues of other proteins
- PTP
= Protein tyrosine phosphatase(s); activated PTP is a negative regula-tor against glucose cell uptake; blocking PTP is a positive regula-tion which enhances the glucose cell uptake as if insuline was bound to the IR
- R&D
= Research and development of drug candidates
- ROS
= Reactive oxygen species
- Superoxide radical
= O2ˉ
- Tf
= Transferrin; an iron transporting globular protein in the blood stream
- Vanadate
= Anions like H2VO4ˉ or [VO4]3− ; oxidovanadium with an oxidation state +5 and stable under natural conditions or in cellular life forms
- Vanadinite
= A water-insoluble mineral: Pb5[VO4]3Cl [21]
- Vanadium pentoxide
= Known as an orange-colored solid salt: “vanadia” or “navajoitite” in the form of the mineral V2O5x3H2O
- Vanadyl
= Cations like VO2+ in the form of a blue-colored salt with [VO(H2O)5]2+; oxidovanadium with the oxidation state +4 under natural conditions or in cellular life forms, e.g. vanadyl sultate (VO2+ SO42ˉ)
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Banting F.G., Best C.H., Collip J.B., Campbell W.R., Fletcher A.A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 1922;12(3):141–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Shechter Y., Goldwaser I., Mironchik M., Fridkin M., Gefel D. Historic perspective and recent developments on the insulin-like actions of vanadium; toward developing vanadium-based drugs for diabetes. Coord. Chem. Rev. 2003;237:3–11. [Google Scholar]
- 3.Hirsová D., Koldovský O. On the question of the absorption of insulin from the gastrointestinal tract during postnatal development. Physiol. Bohemoslov. 1969;18(3):281–284. [PubMed] [Google Scholar]
- 4.Alí K., Fatima N., Maqsood Z.T., Kazmi S.A. Complexation of Vanadium(IV) with Hydroxamate. J. Iran. Chem. Soc. 2004;1(1):65–70. [Google Scholar]
- 5.Heyliger C.E., Tahiliani A.G., McNeill J.H. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985;227(4693):1474–1477. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- 6.Guevara García J.A. Una Semblanza de la Química Bioinorgánica del Vanadio. Educ. Quim. 1996;7(4):185–189. [Google Scholar]
- 7.Sitprija V., Ong S.E. Vanadium and Metabolic Problems. In: Nriagu J.O., editor. Vanadium in the Environment, Part 2: Health Effects. New York: John Wiley & Sons; 1998. p. 91. [Google Scholar]
- 8.Simons T.J. Vanadatea new tool for biologists. Nature. 1979;281(5730):337–338. doi: 10.1038/281337a0. [DOI] [PubMed] [Google Scholar]
- 9.Macara I.G. Vanadium — an element in search of a role. Science. 1980;5(4):92–94. [Google Scholar]
- 10.Brichard S.M., Henquin J.C. The role of vanadium in the management of diabetes. Trends Pharmacol. Sci. 1995;16(8):265–270. doi: 10.1016/s0165-6147(00)89043-4. [DOI] [PubMed] [Google Scholar]
- 11.Rehder D. Biological and medicinal aspects of vanadium. Inorg. Chem. Commun. 2003;6:604–617. [Google Scholar]
- 12.Soriano-Agueda L.A., Ortega-Moo C., Garza J., Guevara-García J.A., Vargas R. Formation of reactive oxygen species by vanadium complexes. Comput. Theor. Chem. 2016;1077:99–105. [Google Scholar]
- 13.Nechay B.R., Nanninga L.B., Nechay P.S. Vanadyl (IV) and vanadate (V) binding to selected endogenous phosphate, carboxyl, and amino ligands; calculations of cellular vanadium species distribution. Arch. Biochem. Biophys. 1986;251(1):128–138. doi: 10.1016/0003-9861(86)90059-7. [DOI] [PubMed] [Google Scholar]
- 14.Tracey A.S., Gresser M.J., Parkinson K.M. Vanadium (V) oxyanions. Interactions of vanadate with oxalate, lactate, and glycerate. Inorg. Chem. 1987;26:629–638. [Google Scholar]
- 15.Tsiani E., Fantus I.G. Vanadium compounds biological actions and potential as pharmacological agents. Trends Endocrinol. Metab. 1997;8(2):51–58. doi: 10.1016/s1043-2760(96)00262-7. [DOI] [PubMed] [Google Scholar]
- 16.Buglyó P., Kiss T., Kiss E., Sanna D., Garribba E., Micera G. Interaction between the low molecular mass components of blood serum and the VO(IV)-DHP system (DHP = 1,2-dimethyl-3-hydroxy-4(1H)-pyridinone). J. Chem. Soc., Dalton Trans. 2002:2275–2282. [Google Scholar]
- 17.Dodge R.R., Templeton D., Zalkin A. Crystal structure of vanadyl bisacetylacetonate. Geometry of vanadium in five fold coordination. J. Chem. Phys. 1961;35:55–67. [Google Scholar]
- 18.Bernheim F., Bernheim M.L. The action of vanadium on the oxidation of phospholipids by certain tissues. J. Biol. Chem. 1939;127:353–360. [Google Scholar]
- 19.Carrington A., Symons M.C. Structure and reactivity of the oxyanions of transition metals. Chem. Rev. 1963;63(5):443–460. [Google Scholar]
- 20.Thompson K.H., Orvig C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006;100(12):1925–1935. doi: 10.1016/j.jinorgbio.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Rehder D. The future of/for vanadium. Dalton Trans. 2013;42(33):11749–11761. doi: 10.1039/c3dt50457c. [DOI] [PubMed] [Google Scholar]
- 22.Cohen N., Halberstam M., Shlimovich P., Chang C.J., Shamoon H., Rossetti L. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J. Clin. Invest. 1995;95(6):2501–2509. doi: 10.1172/JCI117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldfine A.B., Simonson D.C., Folli F., Patti M.E., Kahn C.R. In vivo and in vitro studies of vanadate in human and rodent diabetes mellitus. Mol. Cell. Biochem. 1995;153(1-2):217–231. doi: 10.1007/BF01075941. [DOI] [PubMed] [Google Scholar]
- 24.Halberstam M., Cohen N., Shlimovich P., Rossetti L., Shamoon H. Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes. 1996;45(5):659–666. doi: 10.2337/diab.45.5.659. [DOI] [PubMed] [Google Scholar]
- 25.Boden G., Chen X., Ruiz J., van Rossum G.D., Turco S. Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1996;45(9):1130–1135. doi: 10.1016/s0026-0495(96)90013-x. [DOI] [PubMed] [Google Scholar]
- 26.Cusi K., Cukier S., DeFronzo R.A., Torres M., Puchulu F.M., Redondo J.C. Vanadyl sulfate improves hepatic and muscle insulin sensitivity in type 2 diabetes. J. Clin. Endocrinol. Metab. 2001;86(3):1410–1417. doi: 10.1210/jcem.86.3.7337. [DOI] [PubMed] [Google Scholar]
- 27.Meyerovitch J., Farfel Z., Sack J., Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J. Biol. Chem. 1987;262(14):6658–6662. [PubMed] [Google Scholar]
- 28.Gil J., Miralpeix M., Carreras J., Bartrons R. Insulin-like effects of vanadate on glucokinase activity and fructose 2,6-bisphosphate levels in the liver of diabetic rats. J. Biol. Chem. 1988;263(4):1868–1871. [PubMed] [Google Scholar]
- 29.Venkatesan N., Avidan A., Davidson M.B. Antidiabetic action of vanadyl in rats independent of in vivo insulin-receptor kinase activity. Diabetes. 1991;40(4):492–498. doi: 10.2337/diab.40.4.492. [DOI] [PubMed] [Google Scholar]
- 30.Rossetti L., Lauglin M.R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J. Clin. Invest. 1989;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossetti L., Smith D., Shulman G.I., Papachristou D., DeFronzo R.A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J. Clin. Invest. 1987;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brichard S.M., Lederer J., Henquin J.C. The insulin-like properties of vanadium: a curiosity or a perspective for the treatment of diabetes? Diabete Metab. 1991;17(5):435–440. [PubMed] [Google Scholar]
- 33.Brichard S.M., Assimacopoulos-Jeannet F., Jeanrenaud B. Vanadate treatment markedly increases glucose utilization in muscle of insulin-resistant fa/fa rats without modifying glucose transporter expression. Endocrinology. 1992;131(1):311–317. doi: 10.1210/endo.131.1.1612011. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H., Nakai M., Komazawa K., Sakurai H. A new orally active insulin-mimetic vanadyl complex: bis(pyrrolidine-N-carbodithioato)oxovanadium (IV). J. Med. Chem. 1994;37(7):876–877. doi: 10.1021/jm00033a002. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai H., Fujii K., Watanabe H., Tamura H. Orally active and long-term acting insulin-mimetic vanadyl complex:bis(picolinato)oxovanadium (IV). Biochem. Biophys. Res. Commun. 1995;214(3):1095–1101. doi: 10.1006/bbrc.1995.2398. [DOI] [PubMed] [Google Scholar]
- 36.Thompson K.H., McNeill J.H., Orvig C. Vanadium compounds as insulin mimics. Chem. Rev. 1999;99(9):2561–2572. doi: 10.1021/cr980427c. [DOI] [PubMed] [Google Scholar]
- 37.Kiss T., Jakusch T., Hollender D., Dörnyei Á., Enyedy É.A., Pessoa J.C., Sakurai H., Sanz-Medel A. Biospeciation of antidiabetic VO(IV) complexes. Coord. Chem. Rev. 2008;252:1153–1162. [Google Scholar]
- 38.Rehder D. The role of vanadium in biology. Metallomics. 2015;7(5):730–742. doi: 10.1039/c4mt00304g. [DOI] [PubMed] [Google Scholar]
- 39.Thompson K.H., Orvig C. Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans. 2006;(6):761–764. doi: 10.1039/b513476e. [DOI] [PubMed] [Google Scholar]
- 40.McNeill J.H., Yuen V.G., Hoveyda H.R., Orvig C. Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J. Med. Chem. 1992;35(8):1489–1491. doi: 10.1021/jm00086a020. [DOI] [PubMed] [Google Scholar]
- 41.Goldwaser I., Qian S., Gershonov E., Fridkin M., Shechter Y. Organic vanadium chelators potentiate vanadium-evoked glucose metabolism in vitro and in vivo: establishing criteria for optimal chelators. Mol. Pharmacol. 2000;58(4):738–746. doi: 10.1124/mol.58.4.738. [DOI] [PubMed] [Google Scholar]
- 42.Shechter Y., Shisheva A., Lazar R., Libman J., Shanzer A. Hydrophobic carriers of vanadyl ions augment the insulinomimetic actions of vanadyl ions in rat adipocytes. Biochemistry. 1992;31(7):2063–2068. doi: 10.1021/bi00122a024. [DOI] [PubMed] [Google Scholar]
- 43.Willsky G.R., Goldfine A.B., Kostyniak P.J., McNeill J.H., Yang L.Q., Khan H.R., Crans D.C. Effect of vanadium(IV) compounds in the treatment of diabetes: in vivo and in vitro studies with vanadyl sulfate and bis(maltolato)oxovandium(IV). J. Inorg. Biochem. 2001;85(1):33–42. doi: 10.1016/s0162-0134(00)00226-9. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson J., Degerman E., Haukka M., Lisensky G.C., Garribba E., Yoshikawa Y., Sakurai H., Enyedy E.A., Kiss T., Esbak H., Rehder D., Nordlander E. Bis- and tris(pyridyl)amine-oxidovanadium complexes: characteristics and insulin-mimetic potential. Dalton Trans. 2009;14(38):7902–7911. doi: 10.1039/b903456k. [DOI] [PubMed] [Google Scholar]
- 45.Lyonnet B., Martz X., Martin E. L'emploi therapeutique des derivés du vanadium. Presse Med. 1899;32:191–192. [Google Scholar]
- 46.Rehder D. 2013. [Google Scholar]
- 47.Rehder D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012;4(14):1823–1837. doi: 10.4155/fmc.12.103. [DOI] [PubMed] [Google Scholar]
- 48.Thompson K.H., Orvig C. Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans. 2006;6(6):761–764. doi: 10.1039/b513476e. [DOI] [PubMed] [Google Scholar]
- 49.Thompson K.H., Lichter J., LeBel C., Scaife M.C., McNeill J.H., Orvig C. Vanadium treatment of type 2 diabetes: a view to the future. J. Inorg. Biochem. 2009;103(4):554–558. doi: 10.1016/j.jinorgbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa Y., Sakurai H., Crans D.C., Micera G., Garribba E. Structural and redox requirements for the action of anti-diabetic vanadium compounds. Dalton Trans. 2014;43(19):6965–6972. doi: 10.1039/c3dt52895b. [DOI] [PubMed] [Google Scholar]
- 51.Elberg G., Li J., Shechter Y. Vanadium activates or inhibits receptor and non-receptor protein tyrosine kinases in cell-free experiments, depending on its oxidation state. Possible role of endogenous vanadium in controlling cellular protein tyrosine kinase activity. J. Biol. Chem. 1994;269(13):9521–9527. [PubMed] [Google Scholar]
- 52.Shechter Y., Li J., Meyerovitch J., Gefel D., Bruck R., Elberg G., Miller D.S., Shisheva A. Insulin-like actions of vanadate are mediated in an insulin-receptor-independent manner via non-receptor protein tyrosine kinases and protein phosphotyrosine phosphatases. Mol. Cell. Biochem. 1995;153(1-2):39–47. doi: 10.1007/BF01075917. [DOI] [PubMed] [Google Scholar]
- 53.Cam M.C., Brownsey R.W., McNeill J.H. Mechanisms of vanadium action: insulin-mimetic or insulin-enhancing agent? Can. J. Physiol. Pharmacol. 2000;78(10):829–847. [PubMed] [Google Scholar]
- 54.Ajmani S., Karanam S., Kulkarni S.A. Rationalizing protein-ligand interactions for PTP1B inhibitors using computational methods. Chem. Biol. Drug Des. 2009;74(6):582–595. doi: 10.1111/j.1747-0285.2009.00894.x. [DOI] [PubMed] [Google Scholar]
- 55.Alqahtani A., Hamid K., Kam A., Wong K.H., Abdelhak Z., Razmovski-Naumovski V., Chan K., Li K.M., Groundwater P.W., Li G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013;20(7):908–931. [PubMed] [Google Scholar]
- 56.Ramírez-Espinosa J.J., Rios M.Y., Paoli P., Flores-Morales V., Camici G., de la Rosa-Lugo V., Hidalgo-Figueroa S., Navarrete-Vázquez G., Estrada-Soto S. Synthesis of oleanolic acid derivatives: In vitro, in vivo and in silico studies for PTP-1B inhibition. Eur. J. Med. Chem. 2014;87:316–327. doi: 10.1016/j.ejmech.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 57.Scapin G., Patel S., Patel V., Kennedy B., Asante-Appiah E. The structure of apo protein-tyrosine phosphatase 1B C215S mutant: more than just an S > O change. Protein Sci. 2001;10(8):1596–1605. doi: 10.1110/ps.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiesmann C., Barr K.J., Kung J., Zhu J., Erlanson D.A., Shen W., Fahr B.J., Zhong M., Taylor L., Randal M., McDowell R.S., Hansen S.K. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. Biol. 2004;11(8):730–737. doi: 10.1038/nsmb803. [DOI] [PubMed] [Google Scholar]
- 59.Shechter Y., Karlish S.J. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature. 1980;284(5756):556–558. doi: 10.1038/284556a0. [DOI] [PubMed] [Google Scholar]
- 60.Posner B.I., Faure R., Burgess J.W., Bevan A.P., Lachance D., Zhang-Sun G., Fantus I.G., Ng J.B., Hall D.A., Lum B.S., et al. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J. Biol. Chem. 1994;269(6):4596–4604. [PubMed] [Google Scholar]
- 61.Goldwaser I., Gefel D., Gershonov E., Fridkin M., Shechter Y. Insulin-like effects of vanadium: basic and clinical implications. J. Inorg. Biochem. 2000;80(1-2):21–25. doi: 10.1016/s0162-0134(00)00035-0. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava A.K., Mehdi M.Z. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabet. Med. 2005;22(1):2–13. doi: 10.1111/j.1464-5491.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 63.Rehder D. Bioinorganic Vanadium Chemistry. John Wiley & Sons; 2008. p. 224. [Google Scholar]
- 64.Kuznetsov V.I., Alexandrova A.N., Hengge A.C. Metavanadate at the active site of the phosphatase VHZ. J. Am. Chem. Soc. 2012;134(35):14298–14301. doi: 10.1021/ja305579h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiss E., Kawabe K., Tamura A., Jakusch T., Sakurai H., Kiss T. Chemical speciation of insulinomimetic VO(IV) complexes of pyridine-N-oxide derivatives: binary and ternary systems. J. Inorg. Biochem. 2003;95(2-3):69–76. doi: 10.1016/s0162-0134(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 66.Sanna D., Serra M., Micera G., Garribba E. Interaction of antidiabetic vanadium compounds with hemoglobin and red blood cells and their distribution between plasma and erythrocytes. Inorg. Chem. 2014;53(3):1449–1464. doi: 10.1021/ic402366x. [DOI] [PubMed] [Google Scholar]
- 67.Chasteen N.D., Francavilla J. An electron paramagnetic resonance study of vanadyl(IV)-serum albumin complexes. J. Phys. Chem. 1976;80(8):867–871. [Google Scholar]
- 68.Chasteen N.D., Lord E.M., Thompson H.J., Grady J.K. Vanadium complexes of transferrin and ferritin in the rat. Biochim. Biophys. Acta. 1986;884(1):84–92. doi: 10.1016/0304-4165(86)90230-8. [DOI] [PubMed] [Google Scholar]
- 69.Yasui H., Kunori Y., Sakurai H. Specific binding of vanadyl ion (VO2+) with thiolate of the cysteine-34 residue in serum albumin, demonstrated by CD spectroscopy and kinetic property. Chem. Lett. 2003;32:1032–1033. [Google Scholar]
- 70.Orvig C., Abrams M.J. Medicinal inorganic chemistry: introduction. Chem. Rev. 1999;99(9):2201–2204. doi: 10.1021/cr980419w. [DOI] [PubMed] [Google Scholar]
- 71.Song B., Aebischer N., Orvig C. Reduction of [VO2(ma)2]- and [VO2(ema)2]- by ascorbic acid and glutathione: kinetic studies of pro-drugs for the enhancement of insulin action. Inorg. Chem. 2002;41(6):1357–1364. doi: 10.1021/ic0111684. [DOI] [PubMed] [Google Scholar]
- 72.Kiss T., Kiss E., Garribba E., Sakurai H. Speciation of insulin-mimetic VO(IV)-containing drugs in blood serum. J. Inorg. Biochem. 2000;80(1-2):65–73. doi: 10.1016/s0162-0134(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 73.Levina A., McLeod A.I., Kremer L.E., Aitken J.B., Glover C.J., Johannessen B., Lay P.A. Reactivity-activity relationships of oral anti-diabetic vanadium complexes in gastrointestinal media: an X-ray absorption spectroscopic study. Metallomics. 2014;6(10):1880–1888. doi: 10.1039/c4mt00146j. [DOI] [PubMed] [Google Scholar]
- 74.Pessoa J.C., Tomaz I. Transport of therapeutic vanadium and ruthenium complexes by blood plasma components. Curr. Med. Chem. 2010;17(31):3701–3738. doi: 10.2174/092986710793213742. [DOI] [PubMed] [Google Scholar]
- 75.Sanna D., Micera G., Garribba E. Interaction of insulin-enhancing vanadium compounds with human serum holo-transferrin. Inorg. Chem. 2013;52(20):11975–11985. doi: 10.1021/ic401716x. [DOI] [PubMed] [Google Scholar]
- 76.Willsky G.R., Halvorsen K., Godzala M.E., III, Chi L.H., Most M.J., Kaszynski P., Crans D.C., Goldfine A.B., Kostyniak P.J. Coordination chemistry may explain pharmacokinetics and clinical response of vanadyl sulfate in type 2 diabetic patients. Metallomics. 2013;5(11):1491–1502. doi: 10.1039/c3mt00162h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters K.G., Davis M.G., Howard B.W., Pokross M., Rastogi V., Diven C., Greis K.D., Eby-Wilkens E., Maier M., Evdokimov A., Soper S., Genbauffe F. Mechanism of insulin sensitization by BMOV (bis maltolato oxo vanadium); unliganded vanadium (VO4) as the active component. J. Inorg. Biochem. 2003;96(2-3):321–330. doi: 10.1016/s0162-0134(03)00236-8. [DOI] [PubMed] [Google Scholar]
- 78.Scior T., Mack H.G., García J.A., Koch W. Antidiabetic Bis-Maltolato-OxoVanadium(IV): conversion of inactive trans- to bioactive cis-BMOV for possible binding to target PTP-1B. Drug Des. Devel. Ther. 2009;2:221–231. [PMC free article] [PubMed] [Google Scholar]
- 79.Scior T., Guevara-García J.A., Melendez F.J., Abdallah H.H., Do Q-T., Bernard P. Chimeric design, synthesis, and biological assays of a new nonpeptide insulin-mimetic vanadium compound to inhibit protein tyrosine phosphatase 1B. Drug Des. Devel. Ther. 2010;4:231–242. [PMC free article] [PubMed] [Google Scholar]
- 80.Goldwaser I., Li J., Gershonov E., Armoni M., Karnieli E., Fridkin M., Shechter Y. L-Glutamic acid gamma-monohydroxamate. A potentiator of vanadium-evoked glucose metabolism in vitro and in vivo. J. Biol. Chem. 1999;274(37):26617–26624. doi: 10.1074/jbc.274.37.26617. [DOI] [PubMed] [Google Scholar]
- 81.Crans D.C., Trujillo A.M., Bonetti S., Rithner C.D., Baruah B., Levinger N.E. Penetration of negatively charged lipid interfaces by the doubly deprotonated dipicolinate. J. Org. Chem. 2008;73(24):9633–9640. doi: 10.1021/jo801707y. [DOI] [PubMed] [Google Scholar]
- 82.Crans D.C., Rithner C.D., Baruah B., Gourley B.L., Levinger N.E. Molecular probe location in reverse micelles determined by NMR dipolar interactions. J. Am. Chem. Soc. 2006;128(13):4437–4445. doi: 10.1021/ja0583721. [DOI] [PubMed] [Google Scholar]
- 83.Crans D.C., Trujillo A.M., Pharazyn P.S., Cohen M.D. How environment affects drug activity: Localization, compartmentalization and reactions of a vanadium insulin-enhancing compound, dipicolinatooxovanadium(V). Coord. Chem. Rev. 2011;255(19-20):2178–2192. [Google Scholar]
- 84.Crans D.C. Chemistry and insulin-like properties of vanadium(IV) and vanadium(V) compounds. J. Inorg. Biochem. 2000;80(1-2):123–131. doi: 10.1016/s0162-0134(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 85.Crans D.C., Yang L.T., Jakusch T., Kiss T. Aqueous chemistry of ammonium (Dipicolinato)oxovanadate(V): the first organic vanadium(V) insulin mimetic compound. Inorg. Chem. 2000;39(20):4409–4416. [Google Scholar]
- 86.Scior T., Guevara-García A., Bernard P., Do Q-T., Domeyer D., Laufer S. Are vanadium compounds drugable? Structures and effects of antidiabetic vanadium compounds: a critical review. Mini Rev. Med. Chem. 2005;5(11):995–1008. doi: 10.2174/138955705774575264. [DOI] [PubMed] [Google Scholar]
- 87.Akabayov B., Kulczyk A.W., Akabayov S.R., Theile C., McLaughlin L.W., Beauchamp B., van Oijen A.M., Richardson C.C. Pyrovanadolysis, a pyrophosphorolysis-like reaction mediated by pyrovanadate, Mn2+, and DNA polymerase of bacteriophage T7. J. Biol. Chem. 2011;286(33):29146–29157. doi: 10.1074/jbc.M111.250944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortoul T.I., González-Villalva A., Piñón-Zarate G., Rodríguez-Lara V., Montaño L.F., Saldivar-Osorio L. Ultrastructural megakaryocyte modifications after vanadium inhalation in spleen and bone marrow. J. Electron Microsc. (Tokyo) 2009;58(6):375–380. doi: 10.1093/jmicro/dfp031. [DOI] [PubMed] [Google Scholar]
- 89.Kioseoglou E., Petanidis S., Gabriel C., Salifoglou A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015;301-302:87–105. [Google Scholar]
- 90.McNeill J.H. Vanadium and diabetes. What about vanadium toxicity?: A reply. Mol. Cell. Biochem. 2000;208(1-2):167–168. doi: 10.1023/a:1007019129085. [DOI] [PubMed] [Google Scholar]
- 91.Imtiaz M., Rizwan M.S., Xiong S., Li H., Ashraf M., Shahzad S.M., Shahzad M., Rizwan M., Tu S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015;80:79–88. doi: 10.1016/j.envint.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 92.Costa Pessoa J., Garribba E., Santos M.F., Santos-Silva T. Vanadium and proteins: Uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015;301-302:49–86. [Google Scholar]
- 93.Rodríguez-Mercado J.J., Mateos-Nava R.A., Altamirano-Lozano M.A. DNA damage induction in human cells exposed to vanadium oxides in vitro. Toxicol. In Vitro. 2011;25(8):1996–2002. doi: 10.1016/j.tiv.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 94.IARC International Agency for Research on Cancer. 2006.
- 95.Rodríguez-Mercado J.J., Altamirano-Lozano M.A. Vanadio: Contaminación, Metabolismo y Genotoxicidad. Rev. Int. Contam. Ambie. 2006;22(4):173–189. [Google Scholar]
- 96.Nielsen F.H. Possible Future Implications of Nickel, Arsenic, Silicon, Vanadium, and Other Trace Elements in Human Nutrition. In: Prasad A.S., Liss A.R., editors. Clinical, Biochemical, and Nutritional Aspects of Trace Elements. New York: John Wiley & Sons; 1982. pp. 379–404. [Google Scholar]
- 97.Sabbioni E., Marafante E., Amantini L., Ubertalli L., Birattari C. Similarity in metabolic patterns of different chemical species of vanadium in the rat. Bioinorg. Chem. 1978;8(6):503–515. doi: 10.1016/0006-3061(78)80004-0. [DOI] [PubMed] [Google Scholar]
- 98.Harris W.R., Carrano C.J. Binding of vanadate to human serum transferrin. J. Inorg. Biochem. 1984;22(3):201–218. doi: 10.1016/0162-0134(84)80029-x. [DOI] [PubMed] [Google Scholar]
- 99.Nechay B.R. Mechanisms of action of vanadium. Annu. Rev. Pharmacol. Toxicol. 1984;24:501–524. doi: 10.1146/annurev.pa.24.040184.002441. [DOI] [PubMed] [Google Scholar]
- 100.Harris W.R., Friedman S.B., Silberman D. Behavior of vanadate and vanadyl ion in canine blood. J. Inorg. Biochem. 1984;20(2):157–169. doi: 10.1016/0162-0134(84)80015-x. [DOI] [PubMed] [Google Scholar]
- 101.Chasteen N.D. 1983. The Biochemistry of Vanadium. [Google Scholar]
- 102.Hopkins L.L., Jr, Tilton B.E. Metabolism of trace amounts of vanadium 48 in rat organs and liver subcellular particles. Am. J. Physiol. 1966;211(1):169–172. doi: 10.1152/ajplegacy.1966.211.1.169. [DOI] [PubMed] [Google Scholar]
- 103.Parker R.D., Sharma R.P. Accumulation and depletion of vanadium in selected tissues of rats treated with vanadyl sulfate and sodium orthovanadate. J. Environ. Pathol. Toxicol. 1978;2(2):235–245. [PubMed] [Google Scholar]
- 104.Rubinson K.A. Concerning the form of biochemically active vanadium. Proc. R. Soc. Lond. B Biol. Sci. 1981;212(1186):65–84. doi: 10.1098/rspb.1981.0025. [DOI] [PubMed] [Google Scholar]
- 105.Ramasarma T., Crane F.L. Does vanadium play a role in cellular regulation? Curr. Top. Cell. Regul. 1981;20:247–301. doi: 10.1016/b978-0-12-152820-1.50011-0. [DOI] [PubMed] [Google Scholar]
- 106.Byrne A.R., Kosta L. Vanadium in foods and in human body fluids and tissues. Sci. Total Environ. 1978;10(1):17–30. doi: 10.1016/0048-9697(78)90046-3. [DOI] [PubMed] [Google Scholar]
- 107.Chasteen N.D., Grady J.K., Holloway C.E. Characterization of the binding, kinetics and redox stability of vanadium (IV) and vanadium (V) protein complexes in serum. Inorg. Chem. 1986;25:2754–2760. [Google Scholar]
- 108.Patterson B.W., Hansard S.L., II, Ammerman C.B., Henry P.R., Zech L.A., Fisher W.R. Kinetic model of whole-body vanadium metabolism: studies in sheep. Am. J. Physiol. 1986;251(2 Pt 2):R325–R332. doi: 10.1152/ajpregu.1986.251.2.R325. [DOI] [PubMed] [Google Scholar]
- 109.Bogden J.D., Higashino H., Lavenhar M.A., Bauman J.W., Jr, Kemp F.W., Aviv A. Balance and tissue distribution of vanadium after short-term ingestion of vanadate. J. Nutr. 1982;112(12):2279–2285. doi: 10.1093/jn/112.12.2279. [DOI] [PubMed] [Google Scholar]
- 110.Akabayov B., Kulczyk A.W., Akabayov S.R., Theile C., McLaughlin L.W., Beauchamp B., van Oijen A.M., Richardson C.C. Pyrovanadolysis, a pyrophosphorolysis-like reaction mediated by pyrovanadate, Mn2+, and DNA polymerase of bacteriophage T7. J. Biol. Chem. 2011;286(33):29146–29157. doi: 10.1074/jbc.M111.250944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boyd D.W., Kustin K. Vanadium: a versatile biochemical effector with an elusive biological function. Adv. Inorg. Biochem. 1984;6:311–365. [PubMed] [Google Scholar]
- 112.Ulbricht C., Chao W., Costa D., Culwell S., Eichelsdoerfer P., Flanagan K., Guilford J., Higdon E.R., Isaac R., Mintzer M., Rusie E., Serrano J.M., Windsor R.C., Woods J., Zhou S. An evidence-based systematic review of vanadium by the Natural Standard Research Collaboration. J. Diet. Suppl. 2012;9(3):223–251. doi: 10.3109/19390211.2012.709365. [DOI] [PubMed] [Google Scholar]
- 113.Sánchez-Lombardo I., Alvarez S., McLauchlan C.C., Crans D.C. Evaluating transition state structures of vanadium-phosphatase protein complexes using shape analysis. J. Inorg. Biochem. 2015;147:153–164. doi: 10.1016/j.jinorgbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 114.Crans D.C., Woll K.A., Prusinskas K., Johnson M.D., Norkus E. Metal speciation in health and medicine represented by iron and vanadium. Inorg. Chem. 2013;52(21):12262–12275. doi: 10.1021/ic4007873. [DOI] [PubMed] [Google Scholar]
- 115.Liboiron B.D., Thompson K.H., Hanson G.R., Lam E., Aebischer N., Orvig C. New insights into the interactions of serum proteins with bis(maltolato)oxovanadium(IV): transport and biotransformation of insulin-enhancing vanadium pharmaceuticals. J. Am. Chem. Soc. 2005;127(14):5104–5115. doi: 10.1021/ja043944n. [DOI] [PubMed] [Google Scholar]
- 116.Carnelis R. R.; Versieck, J.; Mees, L.; Hoste, J.; Barbier, F. The ultratrace element vanadium in human serum. Biol. Trace Elem. Res. 1981;3:257–263. [Google Scholar]
- 117.Li J., Elberg G., Crans D.C., Shechter Y. Evidence for the distinct vanadyl(+4)-dependent activating system for manifesting insulin-like effects. Biochemistry. 1996;35(25):8314–8318. doi: 10.1021/bi960209i. [DOI] [PubMed] [Google Scholar]
- 118.Cantley L.C., Jr, Aisen P. The fate of cytoplasmic vanadium. Implications on (NA,K)-ATPase inhibition. J. Biol. Chem. 1979;254(6):1781–1784. [PubMed] [Google Scholar]
- 119.Gordon J.A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- 120.Willsky G.R., Goldfine A.B., Kostyniak P.J. In: Tracey Alan S., Crans Debbie C., editors. ACS Symposium Series; Washington, D.C.. 1998. pp. 278–296. [Google Scholar]
- 121.Baran E.J. Vanadium detoxification: chemical and biochemical aspects. Chem. Biodivers. 2008;5(8):1475–1484. doi: 10.1002/cbdv.200890136. [DOI] [PubMed] [Google Scholar]
- 122.Willsky G.R., White D.A., McCabe B.C. Metabolism of added orthovanadate to vanadyl and high-molecular-weight vanadates by Saccharomyces cerevisiae. J. Biol. Chem. 1984;259(21):13273–13281. [PubMed] [Google Scholar]
- 123.Costa Pessoa J. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015;147:4–24. doi: 10.1016/j.jinorgbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Costa Pessoa J., Etcheverry S., Gambino D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015;301-302:24–48. doi: 10.1016/j.ccr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crans D.C., Smee J.J., Gaidamauskas E., Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004;104(2):849–902. doi: 10.1021/cr020607t. [DOI] [PubMed] [Google Scholar]
- 126.Crans D.C., Tarlton M.L., McLauchlan C.C. Trigonal bipyramidal or square pyramidal coordination geometry? investigating the most potent geometry for vanadium phosphatase inhibitors. Eur. J. Inorg. Chem. 2014:4450–4468. [Google Scholar]
- 127.McLauchlana C.C., Peters B.J., Willsky G.R., Crans D.C. Vanadium-phosphatase complexes: Phosphatase inhibitors favor the trigonal bipyramidal transition state geometries. Coord. Chem. Rev. 2015;301-302:163–199. [Google Scholar]
- 128.Degani H., Gochin M., Karlish S.J., Shechter Y. Electron paramagnetic resonance studies and insulin-like effects of vanadium in rat adipocytes. Biochemistry. 1981;20(20):5795–5799. doi: 10.1021/bi00523a023. [DOI] [PubMed] [Google Scholar]
- 129.Goldfine A.B., Willsky G.R., Kahn C.R. Vanadium Salts in the Treatment of Human Diabetes Mellitus. In: Tracey Alan S., Crans Debbie C., editors. ACS Symposium Series; Washington, D.C.. 1998. pp. 353–368. [Google Scholar]
- 130.Thompson K.H., Orvig C. Vanadium compounds in the treatment of diabetes. Met. Ions Biol. Syst. 2004;41:221–252. [PubMed] [Google Scholar]
- 131.McNeill J.H., Yuen V.G., Dai S., Orvig C. Increased potency of vanadium using organic ligands. Mol. Cell. Biochem. 1995;153(1-2):175–180. doi: 10.1007/BF01075935. [DOI] [PubMed] [Google Scholar]
- 132.Reul B.A., Amin S.S., Buchet J-P., Ongemba L.N., Crans D.C., Brichard S.M. Effects of vanadium complexes with organic ligands on glucose metabolism: a comparison study in diabetic rats. Br. J. Pharmacol. 1999;126(2):467–477. doi: 10.1038/sj.bjp.0702311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li M., Ding W., Smee J.J., Baruah B., Willsky G.R., Crans D.C. Anti-diabetic effects of vanadium(III, IV, V)-chlorodipicolinate complexes in streptozotocin-induced diabetic rats. Biometals. 2009;22(6):895–905. doi: 10.1007/s10534-009-9241-4. [DOI] [PubMed] [Google Scholar]
- 134.Kadota S., Fantus I.G., Deragon G., Guyda H.J., Hersh B., Posner B.I. Peroxide(s) of vanadium: a novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem. Biophys. Res. Commun. 1987;147(1):259–266. doi: 10.1016/s0006-291x(87)80115-8. [DOI] [PubMed] [Google Scholar]
- 135.Fantus I.G., Kadota S., Deragon G., Foster B., Posner B.I. Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry. 1989;28(22):8864–8871. doi: 10.1021/bi00448a027. [DOI] [PubMed] [Google Scholar]
- 136.Crans D.C., Keramidas A.D., Hoover-Litty H., Anderson O.P., Miller M.M., Lemoine L.M., Pleasic-Williams S., Vandenberg M., Rossomando A.J., Sweet L.J. Synthesis, structure, and biological activity of a new insulinomimetic peroxovanadium compound: Bisperoxovanadium Imidazole Monoanion. J. Am. Chem. Soc. 1997;119(23):5447–5448. [Google Scholar]
- 137.Leuschner J., Haschke H., Sturm G. New Investigations on Acute Toxicities of Vanadium Oxides. Monatsh. Chem. 1994;125:623–646. [Google Scholar]
- 138.Stroop S.D., Helinek G., Greene H.L. More sensitive flameless atomic absorption analysis of vanadium in tissue and serum. Clin. Chem. 1982;28(1):79–82. [PubMed] [Google Scholar]
- 139.Bendayan M., Gingras D. Effect of vanadate administration on blood glucose and insulin levels as well as on the exocrine pancreatic function in streptozotocin-diabetic rats. Diabetologia. 1989;32(8):561–567. doi: 10.1007/BF00285328. [DOI] [PubMed] [Google Scholar]
- 140.Brichard S.M., Pottier A.M., Henquin J.C. Long term improvement of glucose homeostasis by vanadate in obese hyperinsulinemic fa/fa rats. Endocrinology. 1989;125(5):2510–2516. doi: 10.1210/endo-125-5-2510. [DOI] [PubMed] [Google Scholar]
- 141.Pederson R.A., Ramanadham S., Buchan A.M., McNeill J.H. Long-term effects of vanadyl treatment on streptozocin-induced diabetes in rats. Diabetes. 1989;38(11):1390–1395. doi: 10.2337/diab.38.11.1390. [DOI] [PubMed] [Google Scholar]
- 142.Brichard S.M., Bailey C.J., Henquin J-C. Marked improvement of glucose homeostasis in diabetic ob/ob mice given oral vanadate. Diabetes. 1990;39(11):1326–1332. doi: 10.2337/diab.39.11.1326. [DOI] [PubMed] [Google Scholar]
- 143.Thompson K.H., Leichter J., McNeill J.H. Studies of vanadyl sulfate as a glucose-lowering agent in STZ-diabetic rats. Biochem. Biophys. Res. Commun. 1993;197(3):1549–1555. doi: 10.1006/bbrc.1993.2654. [DOI] [PubMed] [Google Scholar]
- 144.Marliss E.B., Nakhooda A.F., Poussier P. Clinical forms and natural history of the diabetic syndrome and insulin and glucagon secretion in the BB rat. Metabolism. 1983;32(7) Suppl. 1:11–17. doi: 10.1016/s0026-0495(83)80005-5. [DOI] [PubMed] [Google Scholar]
- 145.Treviño S., Sánchez-Lara E., Sarmiento-Ortega V.E., Sánchez-Lombardo I., Flores-Hernández J.A., Pérez-Benítez A., Brambila-Colombres E., González-Vergara E. Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3 [V10O28]·8H2O using hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J. Inorg. Biochem. 2015;147:85–92. doi: 10.1016/j.jinorgbio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 146.Sánchez-Lombardo I., Sánchez-Lara E., Pérez-Benítez A., Mendoza Á., Bernès S., González-Vergara E. Synthesis of metforminium(2+) decavanadates - crystal structures and solid-state characterization. Eur. J. Inorg. Chem. 2014:4581–4588. [Google Scholar]
- 147.Aureliano M., Henao F., Tiago T., Duarte R.O., Moura J.J., Baruah B., Crans D.C. Sarcoplasmic reticulum calcium ATPase is inhibited by organic vanadium coordination compounds: pyridine-2,6-dicarboxylatodioxovanadium(V), BMOV, and an amavadine analogue. Inorg. Chem. 2008;47(13):5677–5684. doi: 10.1021/ic702405d. [DOI] [PubMed] [Google Scholar]
- 148.Aureliano M. Decavanadate contribution to vanadium biochemistry: In vitro and in vivo studies. Inorg. Chim. Acta. 2014;420:4–7. [Google Scholar]
- 149.Matsugo S., Kanamori K., Sugiyama H., Misu H., Takamura T. Physiological roles of peroxido-vanadium complexes: Leitmotif as their signal transduction pathway. J. Inorg. Biochem. 2015;147:93–98. doi: 10.1016/j.jinorgbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 150.Rongjun H., Li-Fan Z., Yantao H., Zhong-Yin Z. Recent Advances in PTP1B Inhibitor Development for the Treatment of Type 2 Diabetes and Obesity. In: Jones R.M., editor. New Therapeutic Strategies for Type 2 Diabetes. Small Molecule Approaches. RSC Publishing; 2012. pp. 142–176. [Google Scholar]
- 151.Scior T., Verhoff M., Gutierrez-Aztatzi I., Ammon H.P., Laufer S., Werz O. Interference of boswellic acids with the ligand binding domain of the glucocorticoid receptor. J. Chem. Inf. Model. 2014;54(3):978–986. doi: 10.1021/ci400666a. [DOI] [PubMed] [Google Scholar]
- 152.Boyd D.W., Kustin K. Vanadium: a versatile biochemical effector with an elusive biological function. Adv. Inorg. Biochem. 1984;6:311–365. [PubMed] [Google Scholar]
- 153.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A.L., Normandin D., Cheng A., Himms-Hagen J., Chan C.C., Ramachandran C., Gresser M.J., Tremblay M.L., Kennedy B.P. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 154.Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117(6):699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 155.Zhao S., Sedwick D., Wang Z. Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene. 2015;34(30):3885–3894. doi: 10.1038/onc.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Klebe. Wirkstoffdesign. 2nd ed. Heidelberg: Spektrum Akademischer Verlag; 2009. [Google Scholar]