Abstract
The control of cancer onset and progression is recognized to benefit from specific molecular targeting. MiRNAs are increasingly being implicated in prostate cancer, and the evidence suggests they are possible targets for molecular therapy and diagnosis. In cancer cells, growing attention has been dedicated to novel molecular mechanisms linking the epigenetic scenario to miRNA dysregulation. Currently, the rising evidence shows that nutritional and natural agents, the so-called nutraceuticals, could modulate miRNAs expression, and, as a consequence, might influence cellular responses in health or diseases conditions, including cancer. Among dietary components, plant-derived polyphenols are receiving wide interest, either for their anti-aging and anti-oxidant properties, or for their more general “cell-protective” effects. Above all, their role in preventing the occurrence/recurrence of cancer and, in particular, their potentiality in nutritional intervention for modulating the functions of miRNAs and the epigenetic mechanisms, is still under active debate.
This review is focused on the more recent highlights of the impact of miRNAs dysregulation on the onset and progression of prostate cancer, their interplay with epigenetic control and their modulation by natural agents.
Keywords: Prostate cancer, miRNAs, nutraceuticals, epigenomic, polyphenols, LNCaP, DU145, PC-3, DNMT, HDAC 1
1. Introduction
Prostate adenocarcinoma (PCa) is the most common form of solid cancer in males in the Western countries, and its incidence is expected to increase due to the increase in life expectancy. The related mortality of PCa is mainly due to cancer progression to androgen-independent forms, for which limited therapeutically interventions are available. To ameliorate the clinical management of PCa, urgent tasks are the identification of new specific molecular markers able to anticipate the diagnosis/prognosis and of new molecular targets to improve the effectiveness of therapeutic interventions, especially for androgen-independent forms [1, 2]. After years of studies on identification of novel microRNAs (miRNAs or miR) and their specific targets, new results support the enormous potential of miRNA-based therapeutics [1, 2].
MiRNAs are the most abundant non-coding regulatory RNA molecules in species ranging from human to viruses. They are encoded in the genomes and are engaged in the control of a large variety of physiologic processes, as development, differentiation, neuronal asymmetry, metabolism, stem cell biology, proliferation and programmed cell death [2, 3]. The mature miRNAs, usually 20-25 nucleotides in length, become part of an “RNA-induced silencing complex”, where they anneal the 3′-UTR of their target mRNAs. However binding at 5’-UTR or at the coding region is also possible. The binding at 3’-UTR of their targets occurs through an incomplete matching that commonly involves nucleotides 2–8 in the 5′ end of the miRNA (the so-called “seed region”), that leads to
the repression, or rarely to activation, of the translation of the target mRNA [4, 5].
Increasing published observations indicate that miRNAs can alternate suppressing and activating effects on cellular functions, depending on cellular conditions [4, 5]. Different cancer types are found to display different de-regulated miRNA profiles, which are therefore considered as a specific cancer signature. Today, the clinical relevance of miRNAs as putative biomarkers and targets for therapy in cancer and, more recently, in PCa is increasing [2].
Noteworthy, miRNA role in epigenetic modulation is one of the most interesting novelties in cancer studies [6-8]. Growing attention is dedicated to the molecular mechanisms linking the epigenetic scenario of cancer development to miRNA dysregulation. Indeed, epigenetic changes can lead to improper expression of miRNA genes by mechanisms like DNA methylation or histone acetylation [6-8].
On the other hand, miRNAs-dependent gene regulation, is itself part of the epigenetic control. In tumor cells, miRNAs can also control the levels of DNA methylation and histone acetylation, causing epigenetic dysregulation, which in turn can lead to aberrant mRNA transcription. In this context, still unclear is the interplay among miRNAs, DNA methylations and histone acetylations in a given pathological situation [6-8]. Growing evidences are showing the potentiality of bioactive food components, or nutraceuticals, in modulating the epigenetic control [8]. The so-called ”epigenetic-diet” may influence critical physiological processes, such as inflammation, mitochondrial metabolism, cell apoptosis and/or cell proliferation, and it may therefore counteract some degenerative diseases, including cancer. The benefits of the “epigenetic-diet” on prostate cancer are not completely clarified [8].
This review briefly overviews the miRNAs known to play a role in prostate cancer and resumes the more relevant and recent evidences on the effect of nutritional molecules on miRNAs and their interplay with key epigenetic mechanisms.
2. MIRNAS in prostate cancer
Recent micro-array studies on PCa tissue samples or PCa cell lines, demonstrate that miRNAs are frequently de-regulated in PCa, and implicated in disease progression [2, 9-11]. In PCa, miRNAs regulate apoptosis and cell cycle, acting either as oncomiRs or as tumor suppressors [2, 11, 12]. Several miRNAs have been shown to be involved either in proliferation or in apoptosis control, via their connections with apoptosis transcription factors and some components of the extrinsic death signalling pathway [13, 14]. Due to their association with progression of PCa disease, some miRNAs have been proposed as possible biomarkers of advanced stage and higher Gleason score cancers [15, 16] or as potential targets for PCa therapy [2]. The de-regulation of the expression of some miRNAs has also been related to tumor progression and poor recurrence-free survival in PCa patients, demonstrating that they promote signaling pathways involved in angiogenesis, cell growth and cell migration [16]. New studies are assigning a functional role in PCa onset also to those miRNAs, which have been generally recognized to take part in tumorigenesis and tumor progression in other cancers. Specifically, the tumor suppressive miRNA-218, miRNA-143/145 cluster, miRNA-29s and miRNA-203, which act on inhibition of cancer cell invasion and migration, have been recently found to be under-expressed in human PCa tissue specimens, and in PC-3, DU145 and LNCaP cell lines [11, 17-19]. Consistently with recent observations [20-22], a possible involvement of miRNA-663 and miR-744 in prostate cancer onset [20] may also be speculated. The expression levels of miRNA-663 and miR-744 are reduced in human breast cancer and inversely related to those of the eukaryotic translation elongation factor 1A2 (eEF1A2) [21]. In addition, exogenous over-expression of miR-663 has been found to attenuate in vitro and in vivo cell growth and invasion of pancreatic cancer cells, by targeting the 3’-UTR of eEF1A2 messenger [22].
The eEF1A proteins (eEF1A2 and eEF1A1), belonging to G-protein superfamily, are recognized to have a role in many tumors [23, 24]. The eEF1A1 is widely expressed among cells, while eEF1A2 is found in specialized adult tissues as skeletal muscle, heart and nervous system. Beyond their role in translation, eEF1A1 and eEF1A2 are involved in the regulation of several physiological processes by their non-canonical functions, which include actin binding and modulation of cytoskeleton, cell death and participation in signalling pathways, including cytokine and TGF-beta signalling [23-26]. The eEF1A1 expression has been found to be de- regulated in some tumors [21, 22, 24, 26], while the activation of eEF1A2 expression is recognized to play a role in tumorigenesis and progression of many cancers [20]. The over-expression of eEF1A proteins has been recently found in PCa cells, and the eEF1A2 expression has been suggested to mark prostate tumor onset and progression [20]. Thus, we believe that the miR-744/miR-663/eEF1A2 axis could also take part in human PCa progression.
MiRNAs can also contribute to regulate the stemness properties of PCa cells and epithelial- mesenchymal transition (EMT), a crucial step towards the metastatic spreading [27]. Cancer Stem Cells (CSCs) are often considered to be the ‘seed’ of a tumor and be responsible for multidrug-resistance, tumor recurrence and metastasis. Differential expression of miRNAs, can promote the EMT, where epithelial cells express markers and phenotype of mesenchymal cells, which are recognized to favor the generation of CSCs [27]. Notably, in PC-3 cells, the involvement of miRNAs in the modulation of EMT, E-cadherin and stemness has been also underlined [13, 28]. Some of the evidence reporting miRNA involvement in PCa cancer onset and progression are listed in Table 1 [11, 14-17, 29-63].
Table 1. Dysregulated miRNAs in PCa.
| miRNA | Source | Levels | Effect | Refs. | ||||
|---|---|---|---|---|---|---|---|---|
| miR-574-3p | PC-3 and DU145 PCa tissue |
Reduced | -Related to advanced stage and higher Gleason score in PCa specimens. |
[15] | ||||
| miR-1296 | PCa Tissue | Down-regulated with respect to benign prostate hyperplasia | Not known | [16] | ||||
| miR-1247-5p | Castration-resistant PCa tissue samples and androgen-independent PC-3cells |
Up-regulated | Not known | [29] | ||||
| miR- 224 | PCa Tissue | Reduced | -Tumor progression and poor recurrence-free survival, in PCa patients. -Induction of the up-regulation of Apelin in PCa. |
[30] | ||||
| miRNA-218 miRNA143/145 cluster miRNA-29s miRNA-203 |
PCa Tissue PC-3, DU145 and LNCaP cell lines |
Down-regulated | -They are tumor suppressors. Their down-regulation induces cancer cell invasion and migration. | [11,14, 17-19] |
||||
| miR-133b | PC-3 cell line (and PCa tissue) |
Down-regulated | - Increased cell proliferation, reduced cell apoptosis and increased cell metabolic activity. -Higher recurrence incidence in PCa patients. | [13] | ||||
| miR-145 | PC-3 cells PCa specimen |
Down-regulated | -Aggressive phenotype and poor prognosis in prostate cancer | [14] | ||||
| miR-204 | LNCaP and 22Rv1 cell lines | Down-regulated | -Tumor suppressor inhibition in androgen-dependent LNCaP and 22Rv1 cell lines. |
[31] | ||||
| miR-204 | PC-3 and CL1 cells | Up-regulated | -OncomiR in the androgen-independent, PC-3and CL1 cells. | [31] | ||||
| miR-375 | Androgen-dependent cells | Up-regulated or down-regulated: depending on the cell phenotype | Dualistic role in PCa -Acts as oncomir which controls cell viability and death, depending on the cell phenotype: low levels of miR-375 in PC-3 cell line are associated with cell migration and invasiveness and reduced apoptosis; high expression in 22Rv1 cells is associated with high malignant phenotype. |
[32] | ||||
| miR-375 | Androgen-independent cells | Down-regulated | It is a tumor suppressor Not known |
[32] | ||||
| miR-100 | DU145 and PC-3 cells | Loss | -Increased cells migration, invasion, EMT colony and spheroid formation, and expression of stemness factors | [33] | ||||
| miR-34a | PCa tissue LNCaP-AI cells |
Decreased | -Akt proliferative cascade, migration and invasiveness, EMT modulation. |
[34] | ||||
| miR-548c-3p | Castrate-resistant PCa (CRPC) SCs cells |
Up-regulated | -Poor recurrence-free survival, biomarker for PCa progression. |
[35] | ||||
| miR-30 | PCa specimens | Down-regulated | -Increased Ets-related gene (ERG). | [36] | ||||
| miR-145 miR-143 |
PCa tissue | Reduced | -Bone metastasis in PCa patients -The expression levels of these miRNAs inversely correlate with either prostate specific antigen levels or the Gleason score. |
[11] | ||||
| miR-29b | PCa tissue PC-3 cells |
Down-regulated | EMT modulation. | [37] | ||||
| miR-155 | LNCaP, 22Rv1, PC-3 and DU-145 cells | Up-regulated | -Promotes cell proliferation by down-regulating annexin 7. | [38] | ||||
| miR-429 | IF11 and IA8 cell lines | Up-regulated | -OncomiR in PCa cells -Potential PCa marker. -Down-regulation of p27Kip1 expression and as a consequence induction of cell proliferation. |
[39] | ||||
| miR-126 miR-149 |
PC-3 | Up-regulated | -Modulates expression of syndecan-1, a protein promoting PCa cell growth and mediating the development of androgen-independent PCa. |
[40] | ||||
| miR-218 | PCa tissue specimens | Down-regulated | - It is a tumor suppressor. Its down-regulation leads to loss of inhibition of the tumor protein D52 (TPD52), an oncogene amplified and overexpressed in PCa. |
[[41] | ||||
| miRNA3195 miRNA374b |
PC-3 cells during hypoxia | Up-regulated by melatonin | -Increased expression of HIF- 1/2 and VEGF, and molecules involved in the angiogenesis. | [42] | ||||
| miR-221 miR-30d |
PCa tissues | Reduced | -It is a tumor suppressor. Its down-regulation promotes cell growth, as consequence of the reduced targeting on Bmi-1 polycomb- ring finger oncogene DNA. | [43] | ||||
| miR-200a miR-31 |
Primary PCa lesions |
Elevated | -A role in PCa tumorigenesis via Dicer regulation. -Promotes PCa onset rather than PCa progression. |
[44] | ||||
| miR-150 | PCa tissues | Up-regulated | -Tumor recurrence or metastasis and patient’s poor survival. | [45] | ||||
| miR-371 miR-34a |
PCa specimens and PC-3 and LNCaP cell lines | Low levels | -Complex disarrangement of AR signalling pathway which contributes to the PCa aggressiveness. | [46] | ||||
| miR-19a | CRPC tissues | Up-regulated | -Increased cell proliferation -Binds to the 3′-UTR of the tumor suppressor B-cell translocation gene 1 (BTG1), a member of a gene family of anti-proliferative proteins. |
[47] | ||||
| miRNA-302a | PCa tissue | Low levels | -Inversely associated with Gleason score | [48] | ||||
| miR-650 | PC-3 or DU145 cells PCa tissue |
Expressed | -Reduced CSR1 (tumor suppressor protein) expression levels by binding to 3’-UTR of CSR1 mRNA. -Inhibition of miR-650 expression in PC-3 or DU145-xenografted mice causes a marked decrease of tumor volume, metastatic rate, and mortality. |
[49] | ||||
| miR-187 | PC-3 cells PCa tissue |
Down-regulated | - Up-regulation of ALDH1A3 levels in PCa cells and PCa tissues -miR-187 expression is directly associated with Gleason score. |
[50] | ||||
| miR-340 | PCa tissues DU145 and BPH-1 cells |
Down-regulated | -Increased cell proliferation, migration and invasion in in vitro PCa cells. - MDM2 translation. -Destabilisation of p53 and consequent decreased of p21 protein and increased in the anti-apoptotic protein, BCL-2. |
[51] | ||||
| let-7a | PCa stem cells PCa tissue |
Down-regulated in PCa stem cells. Decreased in the recurrent PCa. |
-Increased proliferation of PCa cells by reducing cell apoptosis and increasing the expression of the insulin-like growth factor 1 receptor. | [52] | ||||
| miR-335 miR-543 |
Bone metastasis tissue samples | Down-regulated in bone metastasis compared with primary PCa. | -Increased expression levels of eNOS in PCa tissue. -Increased in vitro cell migration and invasion in PCa cells. |
[53] | ||||
| miR-32 | PCa cells | Up-regulated or overexpressed in PCa |
-Induces radio-resistance by targeting DAB2IP. | [54] | ||||
| miR-631 | PCa cell lines and -PCa tissue | Down-regulated | -In PCa cells: cell migration and invasion via up-regulation of ZAP70 expression. | [55] | ||||
| miR-223 | PC-3 and PC3M cells PCa tissue |
Down-regulated | -Enhance of ITGA3/ITGB1 signalling and contribute to cell migration and invasion in PCa cells. |
[56] | ||||
| miR-573 | PCa metastatic tissue |
Down-regulated | -Lower in PCa metastatic tissues than matched primary cancer. -Correlates with high Gleason score and relate to mortality of PCa patients. -Modulates EMT and metastasis of PCa cells. |
[57] | ||||
| miR-212 | PCa tissue | Down-regulated in PCa tissue Reduced circulating levels in PCa patients |
- Increased expression of SIRT1 -Reduced autophagy - Increased angiogenesis -Reduced cellular senescence. |
[58] | ||||
| miR-497 | PCa tissues | Down-regulated | - Increased IKK-β expression leading to cell proliferation, cell migration and invasion in PC3-AR cells. | [59] | ||||
| miR-7 | PCSCs cells | Down-regulated | - It is a tumor suppressor. -Lack of the inhibition of the stemness factor KLF4 expression. - Induction of prostate tumorigenesis both in vitro and in vivo (xenografts). |
[60] | ||||
| miR-26a or miR-26b |
PCa tissues | Down-regulated | -Enhance cancer cell invasion through direct regulation of LARP1. | [61] | ||||
| miR-22 miR-29a |
Primary tumor samples | Reduced | - Less abundant in the cancerous tissue compared with the benign counterpart. | [62] | ||||
| miR-135b | DU145, PC-3 cell lines | Down-regulated | -Increased proliferation. -Increased cell migration and invasion. |
[63] | ||||
List of abbreviations: PCa, prostate cancer; EMT, epithelial-mesenchymal transition; Akt, RAC-alpha serine/threonine-protein kinase; ERG, Ets-related gene; PSA, prostate specific antigen; p27Kip1, Cyclin-Dependent Kinase Inhibitor 1B; TPD52, tumor protein D52; VEGF, Vascular endothelial growth factor; HIF, Hypoxia Inducible Factor; AR, androgen receptor; BTG1, B-cell translocation gene 1; CSR1, Cellular stress response 1; ALDH1A3, Aldehyde dehydrogenase 1 family, member A3; MDM2, Mouse double minute 2 homolog; BCL-2, B-cell lymphoma 2; eNOS, Nitric oxide synthase;DAB2IP, DAB2 interacting protein; ZAP70, zeta-associated protein 70; ITGA3/ITGB1, integrin subunit alpha 3/integrin beta-1; SIRT1, sirtuin 1; KLF4, Kruppel-Like Factor 4;LARP1, La-related protein.
Finally, an intriguing facet of miRNAs is their selective excretion and extracellular transport into body fluids, thus functioning as signalling molecules in intercellular and inter-organ communication during various physiological and pathological processes [64]. Whereas the major source of these “circulating miRNAs” is cells undergoing necrosis and apoptosis, miRNAs are also passively and/or actively transported by lipoproteins, RNA-binding proteins (Argonaute2), microvesicles (MV), or exosomes [64-66]. These encapsulated miRNAs are highly stable, and can resist to unfavorable physiological conditions, thus sustaining physiological and pathological events such as tumor angiogenesis, invasion and metastasis [67]. The precise mechanism of release of miRNAs and their cell targeting remain largely unknown. However, it is widely accepted that circulating miRNAs can reach the cells of different organs and tissues in the body and control the expression of targets mRNAs [68]. In PCa, the tumor origin of some circulating miRNAs is suggested by the strict relation between their serum levels and the stage of PCa disease [69]. A brief summary of these evidences are reported in Table 2 [10, 66, 68, 70-72].
Table 2. Circulating miRNAs and their impact in PCa.
| miRNA | Levels | Role | Therapy Response | Refs. |
|---|---|---|---|---|
| miR16 miR-26a |
Increased in PCa patients | Not known | Decreased after radical prostatectomy | [71] |
| miR1290 miR-375 |
Increased in castration-resistant PCa |
Predicts the overall survival independently from PSA or from the failure time of androgen-deprivation therapy | Not known | [69] |
| miR-100 miR-125b miR143 miR-205 miR-296 |
High | Allows discriminating metastatic PCa patients from healthy individuals | Not known | [10] |
| miRNA-141 | Differential circulating profile in relation to different stage of PCa | Close to normal in the early stages of PCa but increase dramatically in advanced PCa patients, in parallel with prostate serum antigen levels |
Not known | [71] |
| miR-21 | Markedly higher in the early than in the advanced disease stage | Not known | Not known | [10, 72] |
| miR-26a miR-16 miR-195 |
Highly relatedwith surgical margin positivity | Not known | Not known | [71] |
| miR-195 miR- let7i |
Not determined | Correlates with Gleason score | Not known | [71] |
| miR26a miR21 miR-221 miR-151-3p miR-152 miR-205 miR-423-3p miR- 375 miR- let7i, miR-200b miR-195 miR-16 |
Not determined | Provides diagnostic or prognostic informations in patients with clinically localized PCa | Not known | [10] |
and DNMT3b) [80]. The intriguing miR-29 regulation of DNA methylation state has been described firstly in lung cancer, where miR-29 family was shown to be able to target DNA methylases or DNA demethylases [81]. In prostate cancer PC-3 and DU145 cell lines, down-regulation of miR-29s and in particular of miR-29b, has been found to affect focal adhesion pathway, stimulate MMP-2 expression and cell migration via laminin γ1 (LAMC1) gene targeting [82]. The recovery of miR-29b expression in PC-3 cells blocks migration and invasion [83] and EMT transition [37], hampering therefore PCa cells aggressiveness [37]. Notably, in DU145 and in TRAMP-C1 mice, the up-regulation of miR-29b, mediated by the activation of toll-like receptor 3 (TLR3), determines demethylation and restoration of gene expression of the tumor suppressor retinoic acid receptor beta. In formalin-fixed paraffin-embedded tissues of human primary PCa, the down-regulation of miRNA-29b, -29c, -148b, and -152 has also been confirmed [84].
3. MIRNA and DNA methylation in PCA
In mammalian genome, DNA methylation occurs, nearly completely, on a cytosine residue preceding a guanine, namely CpG dinucleotides, or in regions having a high CpG repetition content, the so-called CpG islands (less than 1% in the genome). The CpG islands, which frequently reside in 5′ regulatory regions and in promoters of genes, are usually unmethylated. Gene expression can be negatively modulated by methylation of these CpG islands in response to some environmental and cellular stimuli [73].
Aberrant patterns of DNA methylation, in terms of hypermethylation or hypomethylation, are critically involved in tumorigenesis [74, 75]. Cancer cells are characterized by genomic instability associated with hypomethylation at CpG in intergenic regions and repetitive elements but also by a marked hypermethylation of CpG islands in tumor suppressor genes [74, 75]. Several studies have indicated that some miRNA genes reside near CpG islands, thus their expression can be regulated by epigenetic modifications at these sites. In cancer cells, the aberrant methylation of miRNA tumor suppressor genes contributes to the malignant transformation. Deregulation of several miRNA genes, has been associated with specific DNA methylation patterns, as shown in PCa progression [76-78].
Among them, it is worth of mention, the hypermethylation and down-regulation of tumor suppressors miRNA-205 and miRNA-31, which occur in WPE1-NB26 cells, a model of advanced-stage of PCa, but not in those representing a less aggressive PCa stage, such as the WPE1-NA22; this down-regulation appears to be crucial in determining chemo-resistance [79]. Functionally, in WPE1-NA22, miR-205 and miR-31 down-regulate two anti-apoptotic genes, BCL2L2 and E2F6, respectively, and promote the induction of chemotherapeutic apoptosis. In contrast, in the most aggressive cells WPE1- NB26, miR-205 and miR-31 down-regulation by hypermethylation, leads to the over-expression of BCL2L2 and E2F6 with the consequent increase in chemotherapy-resistance [79]. Notably, the treatment of the cells with an inhibitor of DNA methylation, the 5-aza-2'-deoxycytidine, induces miR-205 expression and down-regulation of anti-apoptotic Bcl-w protein, with the consequent reduction of apoptosis resistance to chemotherapies [79].
Members of miRNA-29 family (miR-29s), miR29a, miR29b, and miR29c, are commonly under-expressed in several cancers, including PCa, and induce global DNA hypomethylation likely by down-regulation of DNA methyltransferases (DNMT1, DNMT3a
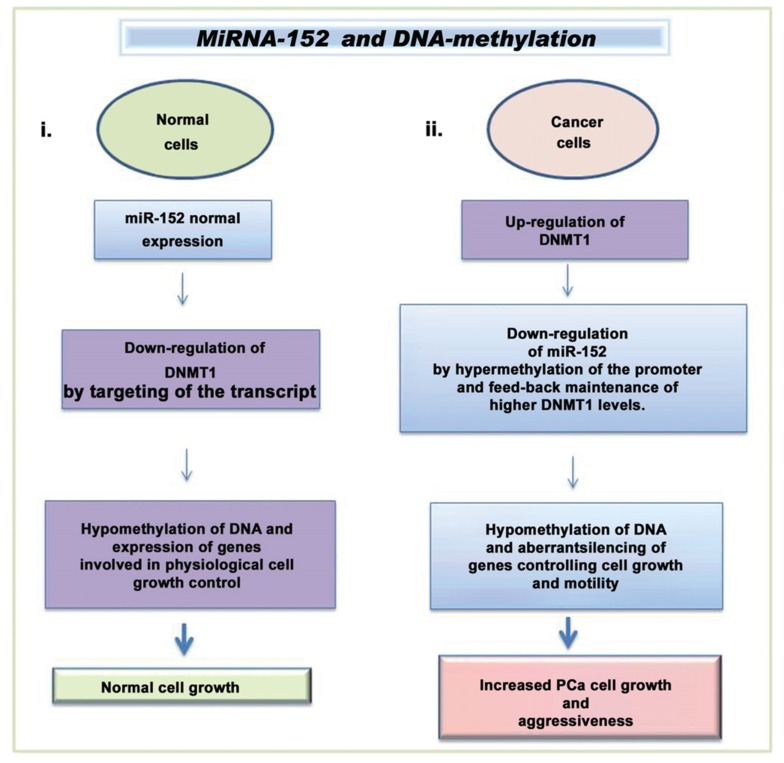
A mutual control between miRNAs and DNA-methylation, has been pointed out for miR-152 and DNA(cytosine-5)-methyltransferase 1 (DNMT1), in the PCa cell lines MDA-PCa-2b, PC-3 and LNCaP [85]. In normal cells (Fig. 1), miR-152 directly targets the 3′ UTR of the DNMT1 transcript, down-regulating DNMT1 expression and consequently lowering DNA methylation state. In contrast, in PCa cells, DNMT1 levels was found to be up-regulated and this relates to the increased methylation and down-regulation of miRNA-152 expression. This was found to be associated with PCa aggressiveness [85, 86]. In vitro experiments in the cell lines LNCaP, PC-3, and MDA-PCa-2b have evidenced that this “feedback loop” between miR-152 and DNMT1 is independent from androgen sensitivity, and arises during the progression to advanced PCa stage. Accordingly, study on a PCa patient cohort showed low miR-152 expression in the more advanced primary tumors, and in metastatic samples [85].
Fig. (1).
Methylation and miRNA dysregulation in PCa. (i.) In normal condition, miR-152 expression down-regulates DNMT1 and consequently controls the DNA methylation status, allowing the expression of genes involved in cell cycle check (ii.) In PCa cells (MDA-PCa-2b, PC-3 and LNCaP), DNMT1 expression down-regulates miRNA-152 by increasing its methylation state. This leads to the repression of genes involved in the control of cell growth, migration, and invasion [85, 86]. [List of abbreviations: PCa, prostate cancer; DNMT-1, DNA (cytosine-5)-methyltransferase-1].
Another interesting feedback loop has been demonstrated for miR-145 and DNMT3b in PC-3 cells: miR-145, which is up-regulated in PC-3, down-regulates DNMT3b expression by targeting the 3'- UTR of its mRNA; the down-regulation of DNMT3b, in turn, increases the expression of miR-145 because the CpG islands in its promoter become hypomethylated [87].
MiR-375 was recently identified as a new oncomiR significantly over-expressed in PCa primary tumors, where it was directly correlated with PCa aggressiveness: higher expression levels of miR-375 relates to higher Gleason score and regional lymphonode metastases [32]. It is worth of mention that miR-375 is considered a tumor suppressor in gastric, head and neck, pancreatic, and in hepatocellular cancers. In PCa cell lines, the main function of miR-375 seems to be related to the down-modulation of cyclin D2 (CCND2), a key protein in cell cycle regulation. Accordingly, low levels of CCND2 were found in primary PCa. Furthermore, in PCa cell lines the highest expression levels of miR-375 were observed in the androgen-dependent cells (22Rv1 and LNCaP) and the lowest in the androgen-independent ones (PC-3 and DU-145). The forced expression of the miR-375 in the androgen-independent cells leads to an increased apoptosis. In this respect, the authors [32] speculate that in the tumorigenesis of human PCa, the miR-375 rises and, at this stage, it works as an oncomiR; on the contrary, with the progression of PCa disease, the miR-375 levels decrease. This underlies the differential role of this miRNA among tumor onset and progression [32].
Finally, the dysregulation in protein of DNA mismatch repair (MMR) also frequently occurs in PCa and about 73% of PCa displays MMR gene mutations: a significant down-regulation of hMSH6, hMLH1, and hMSH2 genes was found in PCa tissues [88]. Among these, the hMLH1 transcript levels were seen to be inversely associated with the methylation status at CpG in PCa tissues. It was shown that oncomiRs hsa-miRNA-141, hsa-miRNA-155, and hsa-miRNA-21 are over-expressed and they interact with the 3’UTRs of hMHS6 and hMLH1 mRNAs, thus leading to a negative regulation of these transcripts. The more relevant oncomiR acting on hMLH1 and hMHS6 is resulted to be hsa-miR-155 [89].
4. MIRNA and Histone Deacetylases in PCA
The cross-interaction between histone deacetylases (HDACs) and miRNAs, represents an important epigenetic regulatory loop involved in the control of distinct cellular signalling pathways, whose dysregulation occurs at different stages of cancer progression [6].
In general, HDACs are considered transcriptional repressors, because they stabilize the condense structure of chromosome, making genes less accessible to transcription factors. HDACs can participate in the control of cell cycle, cell apoptosis, DNA damage and repair [6, 90-93]. In cancer, the aberrant expression of HDACs is implicated in tumorigenesis and influences the development of metastasis of hormone refractory cancer [94, 95]. For example, studies in human androgen sensitive PCa models (CWR22 mouse xenograft and LNCaP cell lines) have demonstrated a possible link between the observed nuclear accumulation of HDAC4 in human PCa specimens and the development of tumoral phenotype with androgen insensitivity [95]. The over-expression of both HDAC1 and HDAC2 was found to directly relate with Gleason score and proliferation rate in PCa tissues, showing highest expression levels in highest grade Gleason score samples [95]. HDAC2 may be also considered an independent prognostic factor since its over-expression has been detected in PCa patients with decreased disease-free survival [96].
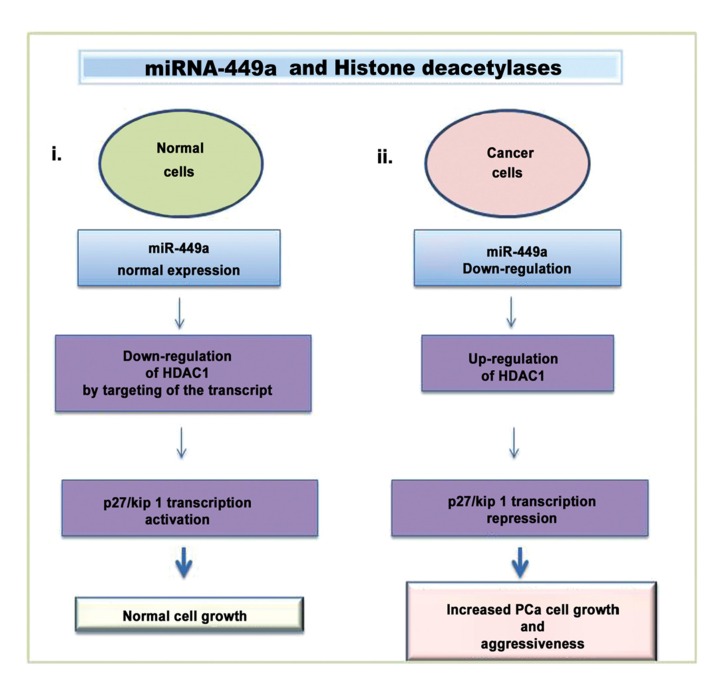
MiRNA and HDAC cross-interaction has been documented both in human PCa tissues and in PCa cell lines. In PC-3 cells the reduced expressions of miR-101, miR-449a, and miR-17-5p leads to the up-regulation of their targets (EZH2 protein, HDAC-1 and p300/CBP-associated factor, respectively) with consequent stimulation of cell growth and PCa progression) [76, 90]. MiR-449a has been found to target HDAC1 and the levels of miR-449a are frequently reduced in PCa tissues and in PC-3 cells and this is related to PCa cell growth increase. In fact, the forced expression of miRNA-449a in PC-3 has been found to induce the expression of the cell-cycle inhibitory protein p27 via the down-regulation of HDAC-1 expression, thus eliciting senescent-like phenotype and cell growth arrest [90] (Fig. 2).
Fig. (2).
Deacetylation and miRNA dysregulation in PCa. (i.) In normal cells, miR-449a is involved to regulate cell growth by repressing the expression of HDAC-1 that allows the expression of p27/kip 1 (ii.) in PCa cells (PC-3), miR-449a gene is down-regulated by HDAC1, which represses its expression. The loss of miR-449a expression maintains HDAC-1 expression activated; this leads to p27/kip1 silencing that promotes cancer cell viability and growth [90]. [List of abbreviations: PCa, prostate cancer; HDAC, Histone deacethylase].
MiRNA-34b controls gene transcription by down-regulating DNMT and HDAC expression; in particular it is involved in the regulation of cell viability and growth by targeting Akt pathway genes [97]. Once Akt expression is inhibited, the downstream GSK3β protein cannot be activated by phosphorylation with the consequent inhibition of cell growth. In PCa tissues, a significant low expression of miR-34b was found in medium and high Gleason grade samples compared to normal tissue or benign hyperplasia. Moreover, the low levels of miR-34b were related to short overall and recurrence-free survival of patients. In PCa tissues and PCa cell lines, the down-regulation of miR-34b was proved to derive from hypermethylation of CpG islands in the upstream region of the gene. Ectopic expression of miR-34b in PC-3 and LNCaP cell lines reduced the expression levels of both DNMTs and HDACs and the intra-tumoral delivery of miR-34b in a mouse model, carrying subcutaneous xenografts of PC-3 cells, significantly reduced tumors volume. Finally, miR-34b also plays a role in controlling the EMT, lowering cell migration and invasion [97]. On these bases miR-34b has been suggested to be a putative tumor suppressor, useful to be developed as a prognostic biomarker to define recurrence of PCa [97].
5. MIRNAS environmental modulation: when Nutrigenomics meets Epigenomics
Lifestyles and diet can significantly influence gene expression by epigenetic mechanisms. Nowadays, increasing evidence suggests that miRNAs take part in the effects that functional foods and natural nutritional agents exert in health and diseases conditions, influencing numerous signaling pathways involved in cell migration, invasion, cell cycle, proliferation and apoptosis control [78, 98, 99].
Several natural nutritional agents, the so called nutraceuticals, derived from sources such as plant extracts and microbial products, have been demonstrated to provide many health benefits, by sustaining tissue homeostasis. For some nutraceuticals the biological action can be reduced or modified, once the compounds are fractionated or isolated or even digested or cooked. In some cases, the active compounds are still not identified. At present, one of the major concerns and limitations of using the active nutraceutical molecules in humans is their low bioavailability that often needs to be optimized for possible translational applications into the clinic [100].
Despite some controversies, some dietary components have been suggested to play a role in preventing cancer or in cancer recurrence. These dietary micronutrients include resveratrol of red grapes and purpleberries, sulforaphane and isothiocyanates present in cruciferous vegetables, epigallocatechin-3-gallate (EGCG) of green tea, lignans present in linseed, selenium, vitamin E, vitamin D, folate from green leafy vegetables, cinnamic acids derivatives present in coffee, grain cereals, plums and kiwi [78]. Of note, some micronutrients, such as indol-3-carbinol (I3C), diindolylmethane (DIM), phenylethyl isothiocyanate (PEITC), sulforaphane, curcumin, phenylhexyl isothiocyanate, retinoic acid, anacardic acid, nordihydroguaiaretic acid, lycopene, garcinol,cambinol, and some components of allium species, are considered to have beneficial effects on health by targeting the epigenome. In particular, genistein, resveratrol, curcumin, green tea, isothiocyanates and I3C, DIM, and sulforaphane have revealed significant epigenetic effects in PCa cells [78].
Among all these micronutrients, the cell-protective effects of dietary plant-derived polyphenols are widely receiving attention, for their anti-oxidant properties, anti-aging effect, and/or for their capability to prevent the occurrence of cancer. The main constituents of polyphenols are phenolic acids, benzoic acids, stilbenes and flavonoids. Several in vivo and in vitro studies on different cancer models have shown the anti-proliferative and pro-apoptotic functions of polyphenols [101]. Given these promising results, the efficacy of polyphenols in cancer therapies are under investigation in different clinical trials (http://www.clinicaltrials.gov/).
In many human cancers, including PCa, a variety of phyto- chemicals have been proposed to exert anti-cancer activity by anti-inflammatory action that involves the activation of non-steroidal anti-inflammatory drug (NSAID)-activated gene-1 (NAG-1) [102].
Here we will focus on some of the main pieces of evidence supporting the effects of nutraceuticals on epigenetic and on miRNA modulation in PCa as summarised in Table 3 (epigenetic and nutraceutical relationships) and Table 4 (miRNA modulation by nutraceuticals).
5.1. Genistein
In the past, great attention to genistein was given for its capability to either lower blood cholesterol levels, so reducing the risk of cardiovascular diseases, or attenuate post-menopausal hormonal disturbances [103]. Today, a rising interest is directed towards the properties of genistein in preventing or treating PCa.
Genistein is an isoflavon, naturally found in lupins, soy and fava beans. This micronutrient is regarded as a phytoestrogen, namely a molecule with structural similarity to 17ß-estradiol estrogen, and therefore capable to mimic hormone effects at cellular level [103]. At the molecular level, this micronutrient shows antioxidant properties and the capacity to inhibit the signalling pathways of NF-𝜅B and Akt and to antagonize estrogen- and androgen-mediated effects [104].
In randomized clinical trials of patients with localized PCa, genistein administration demonstrated beneficial effects, either reducing serum PSA level or tumor tissue volume [105]. Notably, genistein did not show cytotoxicity on normal prostate cells [106], and its cancer-protective effects were dose-dependent [107].
At the molecular level, genistein has demonstrated to modulate some of the epigenetic alterations typically found in prostate cancer cell lines [108]. In particular, in PC-3, DU-145, LNCaP cells, genistein up-regulates mRNA expression of some tumor suppressor genes, including glutathione S-tranferase P1 (GSTP1) and ephrin B2, through the demethylation of their promoter regions and produces the inhibition of pro-proliferating factors such as mouse double minute 2 homolog (MDM2), Akt and NF-kB [104, 109]. Genistein can induce cell death and reduce cell proliferation and epithelial to mesenchymal transition (EMT) in PCa cells (ARCaP-E/ARCaP-M cell lines), as well as increase the expression of histone acetyltransferase 1 (HAT1), affecting histone H3K9 acetylation in the promoter region of Wnt antagonist genes [110]. Furthermore, genistein showed increased efficacy in inducing apoptosis and inhibition of cell proliferation in PCa cell lines once administered together with the chemotherapic histone deacetylase inhibitor vorinostat [110].
Genistein has also been shown to either down-regulate or up-regulate miRNAs genes [99, 111].
In PCa cell lines, PC-3, LNCaP, DU145, and in tissue samples of PCa genistein is found to induce the loss of tumor suppressor miR-145, compared to normal cells or to normal tissues matched in tumor sample. This is likely due to methylation of the promoter of miR-145 gene. In PC-3 cells, genistein in combination with 5- aza-2-deoxycytidine and trichostatin A, an inhibitor of the class I and II mammalian histone deacetylases, was shown to up-regulate miR-145, with consequent decrease in tumor cell viability and increase of both apoptosis and cell cycle arrest [112].
Genistein has also been found to up-regulate the expression levels of both miRNA-29a and miRNA-1256 in LNCaP, VCaP, PC-3, C4–2B and ARCaPM PCa cell lines, by inducing demethylation of their already partially methylated promoters. The demethylations activate expression of these miRNAs, which in turn cause a repressive effect on the expression of tripartite motif containing 68 (TRIM68) and phosphoglycerate kinase 1 (PGK1) proteins [113]. Accordingly, reduced levels of miR-1256 and miR-29a were found in PCa tissue specimens. Thus, it appears that genistein anti-tumor action is driven by up-regulation of miRNA-29a and miRNA-1256, which are epigenetically-induced by demethylation of their promoters; this subsequently down-regulates the two related effectors proteins, TRIM68 and PGK1 [78, 113].
In addition, in vitro studies in PC-3 and DU145 cells indicate that genistein may directly modulate also the expression of miR-1260b, miR-222, miR-221, miRNA-151, miR-1296 and miR-574-3p [99, 111, 114, 115]. The miR-1260b is an oncomiR, highly expressed in PCa tissues, wherein it inhibits apoptosis and promotes cell growth and invasion. In PCa cells, treatment with genistein led to a decrease of cell proliferation, migration, and invasion, via a mechanism involving a genistein-mediated down-regulation of miR1260b by methylation or histone modifications at promoter region [111]. Computer algorithms followed by in vitro validation
analysis, revealed the secreted frizzled-related protein 1 (sFRP1) and Smad4 genes as the potential targets of genistein induction of miR-1260b-mediated signalling in PCa cells [99, 111]. MiR-222, miR-221 and miRNA-151, are other important genistein-targeted oncomiRs [114, 115]. These miRNAs are over-expressed in PC-3 cells. Genistein down-regulation of miR-222 and miR-221 in PC-3 cell line reduces cell colony formation and proliferation, whereas the down-regulation of miRNA-151 expression, reduces cell invasion, migration, but not proliferation [99, 114, 115]. In PC-3 and DU145 cells, genistein has been found to up-regulate, both miR-1296 and miR-574-3p. The increased levels of these miRNAs induce both cell death by apoptosis and inhibition of migration and invasion of cancer cells [99].
In PC-3 and LNCaP cells, genistein was seen to cause a marked decrease in MCM2 gene expression and an up-regulation of miR-1296. The gene family of minichromosome maintenance (MCM) plays a crucial role for DNA replication and many components are frequently up-regulated in a variety of cancers. In PC-3 cells, the forced overexpression of miR-1296 was proved to decrease MCM2 expression and, consistently, miR-1296 inhibition was found to preserve MCM2 expression. Notably, up-regulation of MCM2 was found in the luminal epithelial cells of malignant glands in PCa tissue specimens where miR-1296 resulted down-regulated. Therefore, in PCa cells, genistein can up-regulate miR-1296, which in turn decreases MCM2 expression, thus lowering PCa cell proliferation [16].
Aplysia Ras Homology member I (ARHI), is a human, maternally imprinted tumor suppressor gene that encodes a 26-kDa small G protein with 60% homology to both Rap and Ras. The expression of ARHI is found to be lost or down-regulated by methylation in many cancers, including prostate cancer cell lines (PC-3 and DU145) and in PCa tissues. In PC-3 cells the up-regulated miR-221 and miR-222, down-modulate ARHI by targeting the 3’-UTR of the transcript. In this cell line, genistein was able to increase ARHI levels by reducing the levels of both miR-221 and miR-222 [114].
The miR-151 is recognized to have two mature forms, namely miR-151a-5p and miR-151a-3p. MiR-151a-5p has been defined as an oncomiR, showing a high expression in hormone refractory PC-3 and DU145 cell lines [115]. MiR-151a-5p can potentially target many genes, such as castor zinc finger 1, X-linked interleukin-1 receptor accessory protein-like 1, SRY-related HMG-box transcription factor (SOX)- 17, NEDD4 binding protein 1, and Rho GDP-dissociation inhibitor 1, which are recognized as tumor suppressors. Genistein was found to down-modulate miR-151-5p levels in PCa cells, reducing cell motility and invasiveness, but not cell proliferation. Interestingly, miR-151-5p levels were also found considerably increased in PCa tissues compared to benign ones [115].
5.2. Resveratrol
Resveratrol (3,4’,5-trihydroxystilbene) is a natural phytoalexin with anti-oxidant and anti-inflammatory effects, present in the skin of grapes and in several red fruits such as grapes, berries, plums, and peanuts. Recently, resveratrol (RS) has received great attention not so much for its potential health benefits, namely cell-protection from inflammation and from oxidative stress-induced damage, but rather for its effects on cell proliferation, differentiation and apoptosis [116]. At a glance, the evidence shows the potentiality of RS to inhibit the in vitro growth of various human cancers. To date, the molecular mechanism by which RS exerts antitumor activity is not yet fully elucidated, although it is suggested that RS may be involved in the destabilization of mitochondria membrane potential with consequent disruption of mitochondrial functions [117].
In PCa cells, the anti-cancer effects of RS appear to be related to its capability of inhibiting ERK-1/2 and Akt proteins, and to suppress both estrogen and insulin growth factor-1 (IGF-1) receptor levels; this in turn leads to increased apoptosis and reduction of cell proliferation and metastasis [116, 118]. Similarly to genistein, epigenetic data demonstrate that RS can reduce promoter methylation state in some genes, including miRNA genes. In particular, microarray analysis in LNCaP and DU145 cells has revealed that RS either reduces or increases the levels of many miRNAs associated to prostate tumor [119]. The oncogenic miRNAs that are down-regulated by RS include: miR-17-92 cluster (miR-17, miR-18, miR-20a, miR-20b, miR-92b), miR-106a and miR-106b, miR-7,miR-1260, miR-1267, and let-7c; while those up-regulated include several tumor suppressor, as miR-654-5p, miR-150, miR-149, and miR-152 (a brief summary of these evidences is reported in the reference 119). Notably, the main oncomiRs, negatively modulated by RS, commonly inhibit the expression of phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a tumour suppressor that is down-regulated in several types of cancer [119].
RS reduces the expression of miR-21 in the androgen-receptor negative and highly aggressive human PC-3M-MM2 cell line [118]. This effect is related to a significant reduction of tumour cell viability, migration and invasiveness. In normal cells miR-21 is commonly down-regulated. Only in high aggressive PC-3M-MM2 cells, miR-21 is up-regulated and this seems to be dependent on Akt cellular levels. In PC-3M-MM2 cells, miR-21 suppresses PDCD4 (programmed cell death 4-neoplastic transformation inhibitor) and Maspin (mammary serine protease inhibitor, belonging to serpin protein superfamily) tumour suppressors and this contributes to increase cell growth and aggressiveness [118]. In PC-3M-MM2 cells, the administration of RS has been found to diminish miR-21 and pAkt levels, and to restore the expression levels of PDCD4 and Maspin tumour suppressor activities, thereby reducing cell proliferation. These in vitro findings have also been confirmed in an in vivo model of PCa: in immunodeficient mice, xenografted with PCa cells, the oral administration of RS has resulted in inhibition of both PCa growth and metastatic lesions in the lung [118].
5.3. Curcumin
Curcumin (diferuloylmethane) has obtained high interest in the last years for its anticancer activities against various tumors, such as breast, cervical, oral, gastric, melanoma, pancreatic, colon, and prostrate [99]. This polyphenol is the principal flavonoid of the Curcuma longa’s root, widely used for a long time as a drug against many diseases in traditional alternative medicines. Curcumin (CUR) is described as “one of the most powerful and promising chemopreventive and anticancer agents” [120, 121]. CUR shows numerous pharmacological activities, including antioxidant, anti-inflammatory and antimicrobial properties. It has been recognized to inhibit cell proliferation, invasion, migration, angiogenesis, and inflammation and to promote cell cycle arrest and apoptosis in several cancer cells [122, 123]. A number of studies pointed out the effects of CUR on epigenetic mechanisms, demonstrating that it can act either on both HDAC and DNMT1 activities or on miRNA expression. Specifically, CUR shows opposite effects depending on the class of HDAC enzymes modulated. For example, it works as an HDAC inhibitor by targeting HDAC-1,3 and 8, or as a HDAC activator by targeting HDAC-2 [124-126].
CUR is recognized to be an inhibitor of DNMT and thus in many cancers it plays a role as hypomethylating agent. Moreover, CUR has been demonstrated to be a modulator of several types of miRNAs (i.e.miR-15a, miR-26, miR-21, miR-16, miR-146, miR-22, miR-101, miR-200, and let-7, miR-203) [127]. However, the effects of CUR on DNA methylation status or on miRNAs modulations in PCA are still largely unknown [127]. In murine prostate cancer TRAMP-C1 cells, CUR can elicit demethylation of the promoter of Nrf2 gene, a master regulator of detoxifying/antioxidant enzymes. The demethylation of Nrf2 leads to the re-expression of Nrf2 and NAD(P)H dehydrogenase, and quinine-1(NQO-1), key players in the anti-oxidant and stress responses [125]. Thus, the anti-cancer effect of CUR in PCa cells is suggested to derive, at least in part, by the restoration of the antioxidant activity upon specific gene activation [125, 128]. In hypoxic microenvironment, which is critically linked to the development and aggressiveness of several solid tumors, the treatment of PCa cells (PC-3 and LNCaP) with a curcumin-derived synthetic analogue Difluorinated-Curcumin(CDF), revealed an anti-tumoral effect, by interfering with hypoxia-mediated pathways and inhibiting VEGF and IL-6 expressions [129]. The tumor cells treated with CDF also show a decrease in cell migration under hypoxic condition. Moreover, in hypoxic conditions, CDF has been proved to inhibit cell survival, clonogenicity, migration, invasion, angiogenesis and cancer stem cell renewal capacity of PCa cell lines, all events mediated by the reduction of the levels of oncogenic miR- 21 and miR-210 [129].
The EF24 (diphenyl difluoroketone) is an analogue of CUR that has shown anti-neoplastic properties in colon and gastric cancers. EF24 has proved to cause cell death by apoptosis in DU145 cells, through the inhibition of NF-κB signalling pathway. EF24 also reduces the levels of oncogenic miR-21 leading to the increase of its targets, the tumor suppressors PTEN and PDCD4. In mice with xenograft of PCa cells, EF24 has been found to suppress the growth of tumors, thus showing its potential for the in vivo treatment of PCa [130].
Recently, nanoparticles of poly (lactic-co-glycolic acid)-CUR (PLGA-CUR NPs), were found to efficiently drive CUR into PCa cell lines with respect to free CUR and thus to more efficiently inhibit cell growth and colony formation in PCa cells. PLGA-CUR NPs are able to cause an important reduction of the oncogenic miR-21 levels and an increase of miR-205 expression. The latter is frequently down-regulated in PCa. A further improvement of PLGA-CUR NPs performance was achieved by conjugation of the particles with prostate-specific membrane antigen (PSMA) antibody, leading to significant increase in tumor targeting, as proved in PCa-xenografted mice [131].
5.4. Green Tea
The anti-tumoral properties and efficacy of green tea and its extracts have been widely investigated in several preclinical studies. Green tea leaves, extracts, polyphenols and catechins mixtures, as well as individual isolated catechins, have been demonstrated to have in vitro anti-tumoral effects on PCa cell lines and chemopreventive efficacy in PCa patients [99]. In cell cultures, the green tea molecules regulate cell proliferation, apoptosis, cell invasion, angiogenesis and metastasis acting either on miRNAs or on signal transduction pathways. Epigallocatechin-3-gallate (EGCG), the main polyphenol of green tea, is a direct antagonist of androgen action, able to inhibit gene transcriptions regulated by androgen receptor (AR) [132, 133]. EGCG replaces and antagonizes the functions of androgens, by interacting with the ligand-binding domain of AR [133]. EGCG inhibition of AR signalling severely compromises cell growth both in hormone-dependent PCa cells (LNCaP) and in hormone-refractory ones (22Rv1 and C4-2). This appears an interesting adjuvant tool for the treatment of advanced PCa, as evidenced in a PCa mouse model with hormone-refractory xenograft, where the administration of EGCG lowered AR expression and inhibition of AR nuclear translocation [132].
EGCG has been shown to act on miRNAs regulated by AR in PCa. This is the case of the tumor suppressor miRNA-330, known to induce apoptosis, and of the oncomir miRNA-21, known to sustain the growth of hormone-dependent and independent cells. In PCa cells, EGCG up-regulates miR-330 and down-modulates miR-21 [132]. In this respect, it is worth of note that several other miRNAs have been found to be controlled by AR, such as miR-130a, miR-203 and miR-205, miR-141, miR-222, miR-221, miR-34 and miR-21. Thus, it is conceivable that part of the effects of EGCG on PCa, both in vivo and in vitro, could derive from the modulation of these miRNAs [134-136].
It is worth of mentioning that miRNAs are also involved in the modulation of AR transcriptional activity, by targeting AR co-activator factors (e.g., miR-17-5p targeting of p300/CBP-associated factor) or partner molecules, e.g. miR-141 targeting the orphan receptor small heterodimer partner (Shp) [137-139]. Such miRNAs (e.g., miRNA-616) promote the androgen-independent growth of PCa cells, inducing therefore a more aggressive tumor phenotype [140]. Specifically, the over-expression of miR-125bor miR-21 promotes the more aggressive castration-resistant PCa [141, 142]. In LNCaP-androgen dependent PCa cells, EGCG down-regulates AR acetylation, induces AR translocation from the cytoplasm to nucleus and inhibits histone acetyltransferase activity, which leads to cancer cell growth inhibition [133].
It is worthwhile to cite that in the malignant neuroblastomas cells [143], EGCG treatment decreases the expression of the oncomiRs (miR-92, miR-93, and miR-106b) and increases the expression of the tumor suppressor miRs (miR-7-1, miR-34a, and miR-99a) and, as a consequence, is capable to induce either the extrinsic or the intrinsic pathways of apoptosis [143]. Some of these miRNAs (miR-106b, miR-93 and miR-34a) have been seen to be up-regulated (miR-106b and miR-93) or down-regulated (miR-34a) also in PCa tissues [46, 144]. Thus, it could be speculated that EGCG can display effects on the control of PCa activating the expression of these miRNAs.
5.5. Indole-3-Carbinol (I3C) and 3,3’- Diindolylmethane (DIM)
Both I3C and DIM are potent molecules able to inhibit PCa cell growth with mechanisms mediated by alterations in multiple signalling pathways [145]. I3C is a natural glucosinolate found in the Brassica vegetables, such as cabbage, broccoli, cauliflower, kale, radish, turnip, and Brussels sprouts, while DIM is an I3C by-product produced when I3C undergoes condensation reactions during digestion. Conversion of I3C into DIM is facilitated by cooking, making DIM the dominant active dietary indole obtained from crucifers [146]. DIM modulates the metabolism of estrogens and acts as an anti-androgen molecule capable to reduce PSA expression, without exhibiting androgen receptor-agonist activity. Some studies have reported cell growth reduction in both androgen-independent PC-3 and androgen-sensitive LNCaP cells, following DIM administration [147]. Other potential mechanisms of action of DIM in PCa include the induction of apoptosis by increasing the levels of BCL2-Associated X Protein (BAX), and by decreasing those of Bcl-2 and BCLXL; the inactivation of Akt and NF-kB is also involved [147]. A revision of published data on cancers supports the potential benefit of DIM supplement for the treatment of castrate-resistant non-metastatic PCa and High Grade Prostatic Intraepithelial Neoplasia [148].
A direct effect of DIM on miRNAs expression was described in particular for the novel formulation of DIM molecule, the BioResponse 3,3’- Diindolylmethane or the so called BR-DIM, or B-DIM. BR-DIM causes up-regulation of miRNAs typically lost in PCa cells, such as miR-200b and miR-200c and let-7 miRNA family members [149]. The histone methyltransferase EZH2 is involved in cancer cell proliferation and progression by silencing genes taking part in cell survival and growth control. Different studies show that the expression of let-7 family, especially let-7a, let-7b, let-7c and let-7d, is very high in human normal prostate but is suppressed in PCa tissues and this is particularly evident in high Gleason grade specimens [149]. In normal tissue, let-7 down-regulates the expression of EZH2, while in PCa cells the consequent decreased level of let-7 leads to the significant increase in EZH2 expression. The most extensive information is available for the effect of BR-DIM on let-7 miRNA family. In a phase II clinical trial, PCa patients were treated with BR-DIM before undergoing prostatectomy. The treatment up-regulated let-7 miRNAs, that in turn, mediated the down-regulation of EZH2, a protein known to contribute to PCa aggressiveness and recurrence, thus suggesting that BR-DIM could prevent cancer progression and recurrence [149]. In PCa, this effect on EZH2 may be also piloted by other miRNAs. For instance, analysis of PCa tissue specimens and PCa cell lines showed a reduction of expression of miR-101 in cancer progression paralleled by an increase in EZH2 expression [150].
5.6. Isothiocyanates
It is widely reported that the constant consumption of cruciferous vegetables is protective against various types of human cancers, including PCa. Cruciferous vegetables, such as cabbage and broccoli, are rich of glucosinolates, which are molecules containing sulfur [99]. During digestion, glucosinolates are degraded into isothiocyanate (ITC), which represents the bioactive form of glucosinolates. Numerous epidemiologic studies suggest that ITC is able to induce anti-carcinogenic effects in several types of cancer [151]. In general ITC suppresses cancer cell proliferation by epigenetic control, involving either the inhibition of DNA methylation, acting on DNMT1, or the decrease of histone acetylation, through depletion of HDAC3. This in turn restores the expression of genes regulating either cell cycle arrest or cell proliferation [151, 152].
Among the several type of glucosinolate-derived isothiocyanate, the glucosinolate-derived phenethyl isothiocyanate (PEITC) shows promising antitumorigenic effects in PCa, based on the epigenetic control of AR signalling and on the up-regulation of the miRNA-17 that targets the transcript of p300/CBP-associated factor (PCAF) [153]. PEITC is obtained from the hydrolysis of glucosinolate gluconasturtiin that is found in crucifers and particularly occurs in high amounts in watercress, from which it is extracted by the enzyme myrosinase when the vegetable tissues are crushed or masticated [152, 153]. In PCa, androgen-responsive LNCaP cell line, PEITC can markedly inhibit dihydrotestosterone (DHT)-stimulated AR transcriptional activity and subsequently reduce LNCaP cell growth [153]. Its mechanism of action involves the inhibition of histone deacetylases and the transactivation of the prostate tumor suppressor miRNA-17 gene. In tumour cells, mR-17 expression is suppressed leading to the overexpression of the p300/CBP-associated factor (PCAF), also known as K(lysine) acetyltransferase 2B, a co-activator in AR signalling and key mediator in epigenetic control, whose action is based on up-regulation of histone acetyltransferase activity [139]. In response to PEITC, miR-17 is up-regulated and can repress PCAF to consequently restore the normal histone deacetylase activity [153]. The details of the mechanisms underlying miR-17 regulation via transcriptional or epigenetic factors, however, are still under investigation. Finally, it is worthwhile to mention that also the isothiocyanate –‘Sulforaphane’ has received a wide attention, since it induces a potent anti-proliferative effect in PCa cells, by epigenetic mechanisms involving the inhibition of HDAC enzymes, or decreasing methylation state of the cyclin D2 promoter [154].
Concluding remarks
The incidence of Prostate cancer (PCa) is expected to rise in the next years. Due to the poor curative intervention options for the aggressive forms, the identification of novel therapeutic targets is of utmost importance for clinicians. MiRNAs constitute a class of gene modulators that is frequently de-regulated in human cancer. In this respect, miRNAs represent an interesting frontier in the research for fighting PCa, as they are implicated in cancer cell growth and apoptosis control, in angiogenesis, in invasion and metastasis and finally they contribute to the stemness properties of cancer. Recently, in PCa research, growing attention has emerged on the mechanism of epigenetic dysregulation, and the list of miRNAs epigenetically modulated by environmental factors such as nutraceuticals is increasing, thus anticipating the possibility to identify novel tools for the control of PCa.
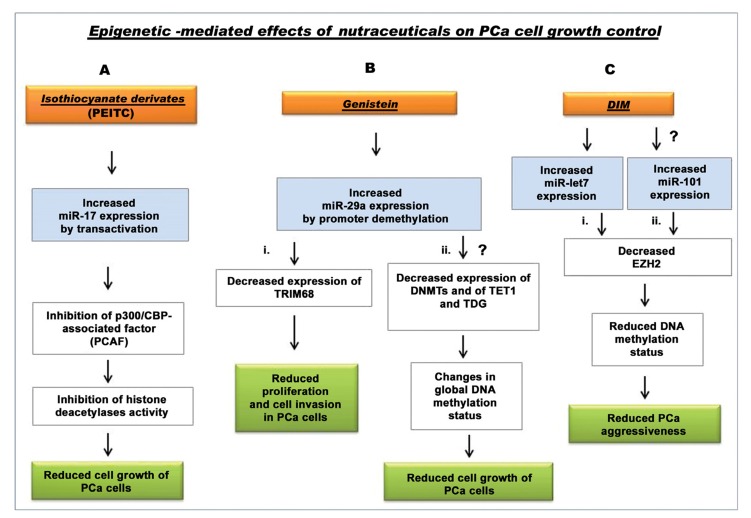
In general, the role of some nutraceuticals in preventing PCa is supported by many studies. Recently, their ability to modulate miRNAs expression and epigenetic status of DNA has been also evidenced. This has been well demonstrated for PEIC and genistein (Fig. 3, A and Bi), but, new relationships are expected. For example, from the literature data one significant indication regards miR-29a, whose up-regulation by genistein in PCa cells has been demonstrated. MiRNA29a is known to contribute to the modulation of DNA methylation status by lowering DNA methyltransferase and factors involved in DNA demethylation (TET1 and TDG) [81]. It is conceivable that genistein might also contribute to inhibit PCa cancer cell growth by changing the global DNA-methylation status through the increase in the levels of this miRNA (Fig. 3Bii). In PCa the down-regulation of let-7 promotes cancer aggressiveness by increasing the levels of EZH2. BR-DIM treatment can restore let-7 expression, both in vitro and in vivo, and reduce the levels of EZH2, thus inhibiting cancer growth [149] (Fig. 3,Ci). However, in PCa cells also the down-regulation of miRNA-101 has been found to be paralleled by increased levels of EZH2 [150.155]. At present there are no available data about a DIM-induced epigenetic modulation through miRNA-101 targeting, but it might be that DIM, or its formulated derivatives, are able to decrease the expression of EZH2 also by increasing the levels of miR-101 (Fig. 3,Cii), similarly to that demonstrated for let-7 family.
Fig. (3).
Scheme of known and potential nutraceuticals targeting of miRNAs for epigenetic control of PCa. (A) The glucosinolate-derived phenethyl isothiocyanate (PEITC) may stimulate transactivation of the prostate tumor suppressor miR-17gene in LNCaP cell line. Induction of miR-17 expression inhibits PCAF factor which, in turn, inhibits HDAC activity and stimulate LNCaP cell growth [137, 153, 154]. The mechanisms of miR-17 regulation via transcriptional or epigenetic factors are still to be fully defined. (B) (i.) Genistein induces the demethylation of promoters in miRNA-29a and causes its up-regulation that leads to decrease expression of tripartite motif containing 68 (TRIM68), with a consequent inhibition of cell proliferation, migration and invasion in LNCaP, VCaP, PC-3, C4–2B and ARCaPM PCa cells. (ii.) Potential mechanism of inhibition of PCa cell growth induced by genistein involving the down-modulation of DNA-methylation status, through miR-29a up-regulation which involves DMNTs, TET and TDG inhibition. (C) (i) DIM derivatives (BR-DIM), decrease the expression of EZH2, by increasing the levels of let-7, thus contributing to cancer progression inhibition [149]. (ii) DIM could act on the epigenetic control of cancer by up-regulation of miR-101 and inhibition of EZH2 expression, thus leading to control in PCa cell growth and aggressiveness [155]. [List of abbreviations: PCa, prostate cancer; PEITC, glucosinolate-derived phenethyl isothiocyanate; p300/CBP-associated factor (PCAF), p300 and CREB binding protein (CBP) associated factor; DNMT, DNA (cytosine-5-)-methyltransferase; TRIM68, tripartite motif containing 68; TET1, methylcytosine dioxygenase 1; TDG, thymine DNA glycosylase; I3C, Indole-3-Carbinol; DIM, 3,3’- Diindolylmethane; EZH2, Enhancer of Zeste homologue 2 protein].
A note is needed on the effect of RS: although in cell culture RS has shown to be able to control cancer growth, this has not yet been confirmed in rodent and human studies, including PCa. In general, rather than an anti-tumor molecule, RS seems to be able to protect cells against oxidative damages, thus playing a major role in cancer prevention [156].
In conclusion, the understanding of nutraceuticals role in PCa, by the interplay between epigenetic modifications and miRNA modulation, can raise the chances for improved therapeutic interventions. However, controversy remains about their benefit that may be linked to the anti-oxidant activities of many of these phytochemicals. Recent evidences suggest that anti-oxidants can sustain cancer cell viability and survival by maintaining a reduced oxidative status that appears to be related to p53 inactivation [157, 158]. In this respect, it is worth to mention the results of a randomized double-blind placebo controlled phase I-II study in men with precancerous prostatic lesions (multifocal high grade prostatic intraepithelial neoplasia–mHGPIN-and/or atypical small acinar proliferation–ASAP-), wherein the cohorts daily received lycopene (35 mg), selenium (55 µg), and Green Tea Catechins (600 mg), or placebo for 6 months. The treated cohort showed higher incidence of PCa at re-biopsy with respect the placebo group. Interestingly, at re-biopsy analysis compared with placebo group, PCa tissues of the group with the supplementation had a stronger up-regulation of many miRNAs involved in cancer progression, and a down-modulation of miRNA tumor suppressors [159].
Another problem in the use of nutraceuticals for anti-cancer treatments in humans is their activity upon enzymatic modifications. For example, tea catechins are subjected to extensive biotransformations via methylation, sulfation, and glucuronidation reactions, which may enrich or reduce the quality of the target tissue response, thus, representing a foremost problem to the translation of research findings into clinical practice [100]. In this respect, an in vitro study showed that LNCaP cells are capable to methylate EGCG to 4”- O-methyl EGCG. This EGCG methylated form is less effective in inhibiting cell growth or in inducing apoptosis in LNCaP cells, compared to the unmethylated form [160]. In addition, a follow-up study, done to assess the accumulation of tea catechin in prostate tissue in subjects scheduled for prostatectomy, confirmed that both EGCG and its less active methylated form (4”-O-methyl–EGCG) are accumulated in prostate cell [160]. Likely, 4”-O-methyl EGCG levels measured in the prostate tissue derive from EGCG methylation occurring in the PCa tissue. Therefore, the EGCG methylation status may reduce the beneficial effects in cancer prevention and it is interesting to mention that EGCG methylation may be related to the polymorphism of catechol- O-methyltransferase, thus introducing the variability of EGCG effects based on genetic polymorphisms of population [160]. So, in the near future it might be expected that the nutritional interventions in PCa cancer will move not only towards improving the findings of the potential epigenetic regulation by nutraceuticals, but also to the comprehension of the in vivo balance between epigenetic/anti-oxidant activities and of the variability of the biological effects, by metabolic modifications, of phytochemicals. [Table 3 (161-167)].
Author contributions
Bruna Scaggiante has conceptualized the manuscript. Alessandra Bosutti wrote the first draft of the manuscript, amended all drafts and worked on the final draft. Bruna Scaggiante has extensively edited the manuscript, wrote the concluding remarks and reviewed the final manuscript. Fabrizio Zanconati, Barbara Dapas and Gabriele Grassi contributed in reviewing with important comments, valuable insights and additions. Sabina Passamonti critically revised the manuscript. All authors discussed the revisions and approved the final manuscript.
Table 3. Nutraceuticals and Epigenetic: some of the main evidence in prostate cancer cells.
| Nutraceuticals | Cellular Effects | Epigenetic Effects | Refs. | ||
|---|---|---|---|---|---|
| GENISTEIN | -Inhibition of PCa cell proliferation and invasion. -Decreased risk of PCa. -Reduced PSA. -Reduced tumor volume. -Antioxidant properties. -Inhibitory effect on NF- 𝜅B and Akt signalling pathways -Antagonizes estrogen and androgen-mediated signalling. -Suppresses the expression of the androgen receptor. -Inhibition of cell motility and invasiveness via miR-151a-5p modulation (PC-3 cell line). -Decreases the expression level of the minichromosome maintenance MCM2 gene (PC-3 cell line). -Up-regulation of Aplysia Ras Homology member I levels by down regulating miR-221 and miR-222 levels (PC-3 cell line). -Repressive effects on tripartite motif containing 68 (TRIM68) and phosphoglycerate kinase 1 (PGK1) protein expression (LNCaP, VCaP, PC-3, C4–2B and ARCaPM cell lines). |
-Demethylation or histone modification of miR genes (in PC-3, LNCaP, VCaP, C4-2B, ARCaPM and DU145 cell lines). -Inhibition of DMNT-1. -Up-regulation of mRNA expression of some tumor suppressor genes including GSTP1 and EPH through DNA demethylation and modulation of MDM2, Akt and NF-kB activity (in PC-3, LNCaP, DU145 cell lines). -Changes in histone H3K9 acetylation in the promoter region of Wnt antagonist genes (in ARCaP-E/ARCaP-M cell lines). -Up-regulation of miR-145 (PC-3 cell line). -Promoter demethylation of miR-29a and miR-1256 (in LNCaP, VCaP, PC-3, C4-2B and ARCaPM cell lines). -DNA methylation or histone modifications on miR-1260b promoter (in PC-3 and DU145 cell lines). |
[16, 99, 104, 105, 109,110, 111,112, 113,115, 117,161] |
||
| RESVERATROL | -Decreases the risk of PCa. - Suppresses the expression of the androgen receptor. -Down-regulates the expression of the metastasis-associated antigen 1 (MAT1) protein. - Inhibition of ERK-1/2 and Akt and IGF-1 (PC-3M-MM2 cell line). - Restoration of Sirt1- S6K function and autophagy control (PCa tissue). - Up-regulation of programmed cell death 4-neoplastic transformation inhibitor and Maspin tumor suppressors (PC-3M-MM2 cell lines). |
-Modulation of Histone decetylation activity. -Activator of sirtuins and DNMT 3b inhibitor. -Up –and/or- down-regulation of miRNAs clusters (down-regulation of: miR-17, miR-18, miR-20a, miR-20b,miR-92b, miR-106a and miR-106b, miR-7, miR-1260, miR-1267, let-7c; up-regulation of: miR-654-5p, miR-150, miR-149, and miR-152, in DU145 and PC-3 cell lines). -Destabilization of MAT1/HDAC1 subunit with consequent p53 acetylation and apoptosis (in DU145 and LNCaP cell lines). |
[116,118, 119,120, 163,164] |
||
|
CURCUMIN AND CURCUMIN-DERIVED ANALOGUES (CDF AND EF24) |
-Antioxidant and anti-inflammatory activities, increases the glutathione level and inhibits oxidant and cytokine-induced NF-αB activation and cytokines release. -Inhibition of cell proliferation, invasion, migration, angiogenesis, and inflammation. -Induces cell cycle arrest and apoptosis. -Decreases nuclear β-catenin and TCF4 and hence inhibits β-catenin /TCF signalling. -Down-regulates AR signal transductions. -Down-regulation of Akt, NF-κB pathways. - Inhibition of VEGF and IL-6 expression in hypoxic microenvironment (PC-3 and LNCaP cell lines). |
-Modulation of HDAC1,3,8 and DNMT1 activities. -De-methylation of the Nrf2 gene (in TRAMP-C1 mice). -Modulation of miR-15a, miR-16, miR-21, miR-22, miR-26, miR-101, miR-146, miR-200, miR-203, and let-7 in many cancer type (with possible targeting in PCa cells). -EF24: reduces the expression level of the oncogenic miR-21, and enhances the levels of PTEN and PDCD4 (in DU145 cell line). |
[122,123, 124,125, 126,127, 128,129, 130,162] |
||
| GREEN TEA EXTRACTS (EGCG) | -Antagonizes androgen action. -Inhibition of prostate cancer development and of distant site metastasis. -Inhibition of vascular endothelial growth factor A, matrix metalloproteinase-2 and -9, and insulin growth factor (IGF)-1 signalling (in TRAMP mice). |
-Down-regulation of AR acetylation and induction of AR protein translocation from the cytoplasmic compartment to nucleus (in Xenograft mouse model of PCa; LNCaP cell line). -Anti-histone acetyltransferase activity (in LNCaP, 22Rv1 and C4-2 cell lines). -AR antagonism and consequent up-regulation of miR-330 and down-regulation of miR-21 (in Xenograft mouse model of PCa). - Decreased expression of the oncomiRs (miR-92, miR-93, and miR-106b) and increased expression of the tumor suppressor miRs (miR-7-1, miR-34a, and miR-99a) leading to induction of extrinsic and intrinsic pathways of apoptosis (in several cancer types, and possibly in PCa cells). |
[132,133, 143,165, 166,167] |
||
| I3C AND DIM | -Modulation of estrogen metabolism and down-regulation of PSA expression. -Inhibition of cell growth and induction of G1 cell-cycle arrest (in PC-3 cell line). - Induction of apoptosis by up-regulation of BAX, and down-regulation of BCL-2 and BCLXL, and inactivation of Akt and NF-kB (in LNCaP cell line). |
-Reduced expression of EZH2- histone methyltransferase, via modulation of let-7 targeting in EZH2 gene (PCa tissue). DIM: up-regulation of miR-200b, miR-200c and let-7 miRNA family members (cancer stem cells). |
[147-149] | ||
| ISOTHIOCYANATES AND SULFORAPHANE | -Inhibition of DHT-stimulated AR transcriptional activity -Anti-proliferative effects |
-Direct inhibition of DNA methylation, acting on DNMT1, or decreasing the acetylation of histones, by depletion of HDAC3 (in LNCaP cell line). PEITC: Modulation of miRNA targeting on p300/CBP-associated factor (PCAF). -Inhibition of dihydrotestosterone (DHT)-stimulated AR transcriptional activity. -Restoration of the normal histone deacetylase activity via miR-17 transactivation (in LNCaP cell line). -SULFORAPHANE: Demethylation of cyclin D2 promoter (in LNCaP cell line). |
[152,153, 154] |
||
List of abbreviations: PCa: Prostate cancer; PSA, Prostate specific antigen; DNMT-1, DNA (cytosine-5-)-methyltransferase-1; GSTP-1, Glutathione S-transferase Pi 1 gene; EPH2, Ephrin receptor subfamily of the protein-tyrosine kinase family; MDM2, Mouse double minute 2 homolog; NF-kB, Nuclear factor k-B; AKT, Protein kinase B (PKB); H3K9, Histone H3 lysine 9 methylation; MAT1, metastasis associated 1 protein; Maspin, mammary serine protease inhibitor, belonging to serpin protein superfamily; S6K, ribosomal protein S6 kinase; TCF4, Transcription factor 4; Nrf2, Nuclear factor erythroid 2 [NF-E2]-related factor 2; p300/CBP, p300 and CREB binding protein (CBP); PCAF, p300/CBP-associated factor or K(lysine) acetyltransferase 2B; IGF-1, Insulin growth factor-1; CDF, Difluorinated-Curcumin; EF24 (diphenyl difluoroketone); PTEN, Phosphatase and tensin homolog protein; PDCD4, Programmed cell death protein 4; AR, Androgen receptor; EGCG, Epigallocatechin-3-gallate; I3C, Indole-3-Carbinol; DIM, 3,3’- Diindolylmethane; BAX, BCL2-Associated X Protein; Bcl-2, B-cell lymphoma 2 protein; BCLXL, B-cell lymphoma-extra large protein; EZH2, Enhancer of zeste homolog 2; DHT, Diidrotestosterone; HDAC-3, Histone deacetylases-3; PEITC, Glucosinolate-derived phenethyl isothiocyanate.
Table 4. MiRNA modulation by nutraceuticals.
| Nutraceuticals |
Targeted
miRNAs (miR) |
MiRNA
Regulation |
Cellualr Effect of
miRNA-Targeting |
Refs. |
|---|---|---|---|---|
| GENISTEIN | -miR-145 | -Up | -Decreases tumour cell viability, increases apoptosis and cell cycle arrest (PC-3 cell line). | [112] |
| -miR-1256 -miR-29a |
-Up -Up |
-Epigenetic down-regulation of TRIM68 and PGK1 proteins, inhibition of prostate cancer cell proliferation and invasion (LNCaP, VCaP, PC-3, C4–2B and ARCaPM PCa cells). | [113] | |
| -miR-1296 | -Up | -Decreased minichromosome maintenance (MCM2) expression. | [16] | |
| -miR-222 -miR-221 |
-Down -Down |
-Up-regulation of Aplysia Ras Homology member I (ARHI) expression level. -Reduced cell colony formation and proliferation (PC-3 cell line). |
[114] [99] | |
| -miR-151 -miR-151-5p |
-Down -Down |
-Reduced cell motility and invasiveness, without effect on cell growth (PC-3 cell line). | [99, 115] | |
| -miR-574-3p | -Up | -Inhibition of cell proliferation, migration, and invasion, induction of apoptosis (PC-3 and DU145 cell lines). | [78, 99] | |
| -miR-1260b | -Down | -Inhibition of cell proliferation, migration, and invasion (PC-3 and DU145 cell lines). | [99, 111] | |
| RESVERATROL | -miR-654-5p | -Up | -AR inhibition and inhibition of cell proliferation (LNCaP, DU145 cell lines) |
[119] |
| -miR-17-92cluster (miR-17,miR-18, miR- 20a,miR-20b,miR-92b) -miR-106a, miR-106b (cluster) -miR-21 |
-Down | -Phosphatase and tensin homologue deleted on chromosome 10 (PTEN)-gene targeting (LNCaP and DU145 cell lines). -Up-regulation of PDCD4 and Maspin tumour suppressors. -Reduced PCa cell growth and aggressiveness. -Reduced cell viability, migration and invasiveness in PC-3M-MM2 cell line and mouse xenograft model ofPCa -Inhibition of the Akt/MicroRNA-21 pathway |
[118, 119] | |
| -miR-150, miR-149, and miR-152 | -Up | Not known | [119] | |
| -miR-744, miR-663 (?) | -Up (?) | -EEF1A2 targeting and reduced cell growth in PCa (?) | [21] | |
|
CURCUMIN; CURCUMIN-DERIVED SYNTHETIC ANALOGUE AND PLGA-CUR NPS (NANOPARTICLES) |
-miR-21 and miR-210 | -Down | -CFD (curcuma derived synthetic analogue): inhibition of cell survival, migration, invasion and angiogenesis, and the CSC self-renewal capacity of human PCa cells under hypoxic conditions. Inhibition of HIF-1α. |
[129] |
| -miR-21 | -Down | -EF24 (diphenyl difluoroketone): induces apoptosis inhibiting the NF-κB signalling pathway and enhancing PTEN and PDCD4 expression levels (DU-145 cell line). | [130] | |
| -miR-205 -miR-21 |
-Up -Down |
-PLGA-CURNPs nanoparticles: down-modulate nuclear β-catenin and AR expression and suppress STAT3 and AKT phosphorylation, leading to cell apoptosis, and reduced PCa tumor volume in xenograft mice model. | [131] | |
|
GREEN TEA EXTRACTS (EGCG) |
-miR-21 -miR-330 |
-Down -Up |
-Reduce PCa cell growth (xenograft mouse model of PCa). | [132] |
| -miR-92, miR-93, and miR-106b (in PCa ?). -miR-7-1, miR-34a, miR-99a (in PCa?). |
-Down -Up |
-Induction of extrinsic and intrinsic pathways of apoptosis. | [46, 143, 144] | |
| I3C AND DIM | -miR-200b; miR-200c; let-7 |
-Up | -Anti-proliferative action. (LNCaP, C4-2B and CWR22RV1 cell lines and in human PCa tissue). |
[149] |
|
ISOTHIOCYANATES AND SULFORAPHANE |
-miR-17 | -Up | -PEITC (glucosinolate-derived phenethylisothiocyanate): decreased expression of p300/CBP-associated factor. Anti-proliferative effects (LNCaP cell line). | [137, 154] |
The table reports some of the main evidences on the effects of nutraceuticals on miRNA modulation in prostate cancer cells and some potential proposed modulations (?).
List of abbreviations: PCa, prostate cancer; PLGA-CUR NPs, poly (lactic-co-glycolic acid)- CUR nanoparticles; STAT3, Signal transducer and activator of transcription 3; TRIM68, Tripartite motif-containing protein 68; PGK1, Phosphoglycerate kinase 1; PTEN, Phosphatase and tensin homolog protein; PDCD4, Programmed cell death protein 4; Maspin (mammary serine protease inhibitor, belonging to serpin protein superfamily); EEF1A2, Eukaryotic translation elongation factor-2; CSC, Cancer stem cell; HIF-1α, Hypoxia-inducible factor 1-alpha; EGCG, Epigallocatechin-3-gallate; I3C, Indole-3-Carbinol; DIM, 3,3’- Diindolylmethane; p300/CBP, p300 and CREB binding protein (CBP).
ACKNOWLEDGEMENTS
This work is supported by 5x1000 2013 Lega Italiana per la Lotta contro i Tumori (LILT) and by the Italian Minister of Instruction, University and Research (MIUR), PRIN 2010-11, [20109PLMH2].
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Russell P.J., Khatri A. Novel gene-directed enzyme prodrug therapies against prostate cancer. Expert Opin. Investig. Drugs. 2006;15(8):947–961. doi: 10.1517/13543784.15.8.947. [DOI] [PubMed] [Google Scholar]
- 2.Lichner Z., Fendler A., Saleh C., Nasser A.N., Boles D., Al-Haddad S., Kupchak P., Dharsee M., Nuin P.S., Evans K.R., Jung K., Stephan C., Fleshner N.E., Yousef G.M. MicroRNA signature helps distinguish early from late biochemical failure in prostate cancer. Clin. Chem. 2013;59(11):1595–1603. doi: 10.1373/clinchem.2013.205450. [DOI] [PubMed] [Google Scholar]
- 3.Allegra A., Alonci A., Campo S., Penna G., Petrungaro A., Gerace D., Musolino C. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review). Int. J. Oncol. 2012;41(6):1897–1912. doi: 10.3892/ijo.2012.1647. [review]. [DOI] [PubMed] [Google Scholar]
- 4.Scaggiante B., Dapas B., Farra R., Grassi M., Pozzato G., Giansante C., Fiotti N., Grassi G. Improving siRNA bio-distribution and minimizing side effects. Curr. Drug Metab. 2011;12(1):11–23. doi: 10.2174/138920011794520017. [DOI] [PubMed] [Google Scholar]
- 5.Vasudevan S. Functional validation of microRNA-target RNA interactions. Methods. 2012;58(2):126–134. doi: 10.1016/j.ymeth.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Swierczynski S., Klieser E., Illig R., Alinger-Scharinger B., Kiesslich T., Neureiter D. Histone deacetylation meets miRNA: epigenetics and post-transcriptional regulation in cancer and chronic diseases. Expert Opin. Biol. Ther. 2015;15(5):651–664. doi: 10.1517/14712598.2015.1025047. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Rodero S., Delgado-Álvarez E., Fernández A.F., Fernández-Morera J.L., Menéndez-Torre E., Fraga M.F. Epigenetic alterations in endocrine-related cancer. Endocr. Relat. Cancer. 2014;21(4):R319–R330. doi: 10.1530/ERC-13-0070. [DOI] [PubMed] [Google Scholar]
- 8.Bacalini M.G., Friso S., Olivieri F., Pirazzini C., Giuliani C., Capri M., Santoro A., Franceschi C., Garagnani P. Present and future of anti-ageing epigenetic diets. Mech. Ageing Dev. 2014;136-137(137):101–115. doi: 10.1016/j.mad.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Hizir M.S., Balcioglu M., Rana M., Robertson N.M., Yigit M.V. Simultaneous detection of circulating oncomiRs from body fluids for prostate cancer staging using nanographene oxide. ACS Appl. Mater. Interfaces. 2014;6(17):14772–14778. doi: 10.1021/am504190a. [DOI] [PubMed] [Google Scholar]
- 10.Ellinger J., Müller S.C., Dietrich D. Epigenetic biomarkers in the blood of patients with urological malignancies. Expert Rev. Mol. Diagn. 2015;15(4):505–516. doi: 10.1586/14737159.2015.1019477. [DOI] [PubMed] [Google Scholar]
- 11.Peng X., Guo W., Liu T., Wang X., Tu X., Xiong D., Chen S., Lai Y., Du H., Chen G., Liu G., Tang Y., Huang S., Zou X. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS One. 2011;6(5):e20341–e20354. doi: 10.1371/journal.pone.0020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wach S., Nolte E., Szczyrba J., Stöhr R., Hartmann A., Ørntoft T., Dyrskjøt L., Eltze E., Wieland W., Keck B., Ekici A.B., Grässer F., Wullich B. MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int. J. Cancer. 2012;130(3):611–621. doi: 10.1002/ijc.26064. [DOI] [PubMed] [Google Scholar]
- 13.Patron J.P., Fendler A., Bild M., Jung U., Müller H., Arntzen M.Ø., Piso C., Stephan C., Thiede B., Mollenkopf H.J., Jung K., Kaufmann S.H., Schreiber J. MiR-133b targets antiapoptotic genes and enhances death receptor-induced apoptosis. PLoS One. 2012;7(4):e35345–e35356. doi: 10.1371/journal.pone.0035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suh S.O., Chen Y., Zaman M.S., Hirata H., Yamamura S., Shahryari V., Liu J., Tabatabai Z.L., Kakar S., Deng G., Tanaka Y., Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32(5):772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiyomaru T., Yamamura S., Fukuhara S., Hidaka H., Majid S., Saini S., Arora S., Deng G., Shahryari V., Chang I., Tanaka Y., Tabatabai Z.L., Enokida H., Seki N., Nakagawa M., Dahiya R. Genistein up-regulates tumor suppressor microRNA-5743p in prostate cancer. PLoS One. 2013;8(3):e58929–e58941. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majid S., Dar A.A., Saini S., Chen Y., Shahryari V., Liu J., Zaman M.S., Hirata H., Yamamura S., Ueno K., Tanaka Y., Dahiya R. Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer. Cancer Res. 2010;70(7):2809–2818. doi: 10.1158/0008-5472.CAN-09-4176. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa R., Goto Y., Sakamoto S., Chiyomaru T., Enokida H., Kojima S., Kinoshita T., Yamamoto N., Nakagawa M., Naya Y., Ichikawa T., Seki N. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 2014;105(7):802–811. doi: 10.1111/cas.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima S., Enokida H., Yoshino H., Itesako T., Chiyomaru T., Kinoshita T., Fuse M., Nishikawa R., Goto Y., Naya Y., Nakagawa M., Seki N. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J. Hum. Genet. 2014;59(2):78–87. doi: 10.1038/jhg.2013.121. [DOI] [PubMed] [Google Scholar]
- 19.Hailer A., Grunewald T.G., Orth M., Reiss C., Kneitz B., Spahn M., Butt E. Loss of tumor suppressor mir-203 mediates overexpression of LIM and SH3 Protein 1 (LASP1) in high-risk prostate cancer thereby increasing cell proliferation and migration. Oncotarget. 2014;5(12):4144–4153. doi: 10.18632/oncotarget.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scaggiante B., Dapas B., Bonin S., Grassi M., Zennaro C., Farra R., Cristiano L., Siracusano S., Zanconati F., Giansante C., Grassi G. Dissecting the expression of EEF1A1/2 genes in human prostate cancer cells: the potential of EEF1A2 as a hallmark for prostate transformation and progression. Br. J. Cancer. 2012;106(1):166–173. doi: 10.1038/bjc.2011.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vislovukh A., Kratassiouk G., Porto E., Gralievska N., Beldiman C., Pinna G., Elskaya A., Harel-Bellan A., Negrutskii B., Groisman I. Proto-oncogenic isoform A2 of eukaryotic translation elongation factor eEF1 is a target of miR-663 and miR-744. Br. J. Cancer. 2013;108(11):2304–2311. doi: 10.1038/bjc.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zang W., Wang Y., Wang T., Du Y., Chen X., Li M., Zhao G. miR-663 attenuates tumor growth and invasiveness by targeting eEF1A2 in pancreatic cancer. Mol. Cancer. 2015;14(1):37–52. doi: 10.1186/s12943-015-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Scaggiante B., Dapas B., Grassi G., Manzini G. Interaction of G-rich GT oligonucleotides with nuclear-associated eEF1A is correlated with their antiproliferative effect in haematopoietic human cancer cell lines. FEBS J. 2006;273(7):1350–1361. doi: 10.1111/j.1742-4658.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- 24.Scaggiante B., Dapas B., Farra R., Tonon F., Abrami M., Grassi M., Musiani F., Zanconati F., Pozzato G., Grassi G. 2014. Translation Elongation. [DOI] [Google Scholar]
- 25.Panasyuk G., Nemazanyy I., Filonenko V., Negrutskii B., Elskaya A.V. A2 isoform of mammalian translation factor eEF1A displays increased tyrosine phosphorylation and ability to interact with different signalling molecules. Int. J. Biochem. Cell Biol. 2008;40(1):63–71. doi: 10.1016/j.biocel.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaggiante B., Dapas B., Pozzato G., Grassi G. The more basic isoform of eEF1A relates to tumour cell phenotype and is modulated by hyper-proliferative/differentiating stimuli in normal lymphocytes and CCRF-CEM T-lymphoblasts. Hematol. Oncol. 2013;31(2):110–116. doi: 10.1002/hon.2022. [DOI] [PubMed] [Google Scholar]
- 27.Zaravinos A. The Regulatory Role of MicroRNAs in EMT and Cancer. J. Oncol. 2015;2015:865816–865829. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren D., Wang M., Guo W., Zhao X., Tu X., Huang S., Zou X., Peng X. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR‑145. Int. J. Oncol. 2013;42(4):1473–1481. doi: 10.3892/ijo.2013.1825. [DOI] [PubMed] [Google Scholar]
- 29.Scaravilli M., Porkka K.P., Brofeldt A., Annala M., Tammela T.L., Jenster G.W., Nykter M., Visakorpi T. MiR-12475p is overexpressed in castration resistant prostate cancer and targets MYCBP2. Prostate. 2015;75(8):798–805. doi: 10.1002/pros.22961. [DOI] [PubMed] [Google Scholar]
- 30.Wan Y., Zeng Z.C., Xi M., Wan S., Hua W., Liu Y.L., Zhou Y.L., Luo H.W., Jiang F.N., Zhong W.D. Dysregulated microRNA-224/apelin axis associated with aggressive progression and poor prognosis in patients with prostate cancer. Hum. Pathol. 2015;46(2):295–303. doi: 10.1016/j.humpath.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Ding M., Lin B., Li T., Liu Y., Li Y., Zhou X., Miao M., Gu J., Pan H., Yang F., Li T., Liu X.Y., Li R. A dual yet opposite growth-regulating function of miR-204 and its target XRN1 in prostate adenocarcinoma cells and neuroendocrine-like prostate cancer cells. Oncotarget. 2015;6(10):7686–7700. doi: 10.18632/oncotarget.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa-Pinheiro P., Ramalho-Carvalho J., Vieira F.Q., Torres-Ferreira J., Oliveira J., Gonçalves C.S., Costa B.M., Henrique R., Jerónimo C. MicroRNA-375 plays a dual role in prostate carcinogenesis. Clin. Epigenetics. 2015;7(1):42–56. doi: 10.1186/s13148-015-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M., Ren D., Guo W., Wang Z., Huang S., Du H., Song L., Peng X. Loss of miR-100 enhances migration, invasion, epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting Argonaute 2. Int. J. Oncol. 2014;45(1):362–372. doi: 10.3892/ijo.2014.2413. [DOI] [PubMed] [Google Scholar]
- 34.Liang J., Li Y., Daniels G., Sfanos K., De Marzo A., Wei J., Li X., Chen W., Wang J., Zhong X., Melamed J., Zhao J., Lee P. LEF1 Targeting EMT in prostate cancer invasion is regulated by miR-34a. Mol. Cancer Res. 2015;13(4):681–688. doi: 10.1158/1541-7786.MCR-14-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rane J.K., Scaravilli M., Ylipää A., Pellacani D., Mann V.M., Simms M.S., Nykter M., Collins A.T., Visakorpi T., Maitland N.J. MicroRNA expression profile of primary prostate cancer stem cells as a source of biomarkers and therapeutic targets. Eur. Urol. 2015;67(1):7–10. doi: 10.1016/j.eururo.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Kao C.J., Martiniez A., Shi X.B., Yang J., Evans C.P., Dobi A., deVere White R.W., Kung H.J. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene. 2014;33(19):2495–2503. doi: 10.1038/onc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ru P., Steele R., Newhall P., Phillips N.J., Toth K., Ray R.B. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol. Cancer Ther. 2012;11(5):1166–1173. doi: 10.1158/1535-7163.MCT-12-0100. [DOI] [PubMed] [Google Scholar]
- 38.Cai Z.K., Chen Q., Chen Y.B., Gu M., Zheng D.C., Zhou J., Wang Z. microRNA-155 promotes the proliferation of prostate cancer cells by targeting annexin 7. Mol. Med. Rep. 2015;11(1):533–538. doi: 10.3892/mmr.2014.2744. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang Y., Gao P., Zhu B., Chen X., Lin F., Wang X., Wei J., Zhang H. Downregulation of microRNA-429 inhibits cell proliferation by targeting p27Kip1 in human prostate cancer cells. Mol. Med. Rep. 2015;11(2):1435–1441. doi: 10.3892/mmr.2014.2782. [DOI] [PubMed] [Google Scholar]
- 40.Fujii T., Shimada K., Tatsumi Y., Fujimoto K., Konishi N. Syndecan-1 responsive microRNA-126 and 149 regulate cell proliferation in prostate cancer. Biochem. Biophys. Res. Commun. 2015;456(1):183–189. doi: 10.1016/j.bbrc.2014.11.056. [DOI] [PubMed] [Google Scholar]
- 41.Han G., Fan M., Zhang X. microRNA-218 inhibits prostate cancer cell growth and promotes apoptosis by repressing TPD52 expression. Biochem. Biophys. Res. Commun. 2015;456(3):804–809. doi: 10.1016/j.bbrc.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 42.Sohn E.J., Won G., Lee J., Lee S., Kim S.H. Upregulation of miRNA3195 and miRNA374b Mediates the Anti-Angiogenic properties of melatonin in hypoxic PC-3 prostate cancer cells. J. Cancer. 2015;6(1):19–28. doi: 10.7150/jca.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xuan H., Xue W., Pan J., Sha J., Dong B., Huang Y. Downregulation of miR-221, -30d, and -15a contributes to pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry (Mosc.) 2015;80(3):276–283. doi: 10.1134/S0006297915030037. [DOI] [PubMed] [Google Scholar]
- 44.Bian X., Shen Y., Zhang G., Gu C., Cai Y., Wang C., Zhu Y., Zhu Y., Zhang H., Dai B., Ye D. Expression of dicer and its related miRNAs in the progression of prostate cancer. PLoS One. 2015;10(3):e0120159–e0120170. doi: 10.1371/journal.pone.0120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dezhong L., Xiaoyi Z., Xianlian L., Hongyan Z., Guohua Z., Bo S., Shenglei Z., Lian Z. miR-150 is a factor of survival in prostate cancer patients. J. BUON. 2015;20(1):173–179. [PubMed] [Google Scholar]
- 46.Leite K.R., Morais D.R., Florez M.G., Reis S.T., Iscaife A., Viana N., Moura C.M., Silva I.A., Katz B.S., Pontes J. 2015.
- 47.Lu K., Liu C., Tao T., Zhang X., Zhang L., Sun C., Wang Y., Chen S., Xu B., Chen M. MicroRNA-19a regulates proliferation and apoptosis of castration-resistant prostate cancer cells by targeting BTG1. FEBS Lett. 2015;589(13):1485–1490. doi: 10.1016/j.febslet.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 48.Zhang G.M., Bao C.Y., Wan F.N., Cao D.L., Qin X.J., Zhang H.L., Zhu Y., Dai B., Shi G.H., Ye D.W. MicroRNA-302a suppresses tumor cell proliferation by inhibiting AKT in prostate cancer. PLoS One. 2015;10(4):e0124410–e0124422. doi: 10.1371/journal.pone.0124410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuo Z.H., Yu Y.P., Ding Y., Liu S., Martin A., Tseng G., Luo J.H. Oncogenic Activity of miRNA 650 in prostate cancer is mediated by suppression of CSR1 expression. Am. J. Pathol. 2015;185(7):1991–1999. doi: 10.1016/j.ajpath.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casanova-Salas I., Masiá E., Armiñán A., Calatrava A., Mancarella C., Rubio-Briones J., Scotlandi K., Vicent M.J., López-Guerrero J.A. MiR-187 targets the Androgen-Regulated Gene ALDH1A3 in prostate cancer. PLoS One. 2015;10(5):e0125576–e0125590. doi: 10.1371/journal.pone.0125576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang K., Tang Y., He L., Dai Y. MicroRNA-340 inhibits prostate cancer cell proliferation and metastasis by targeting the MDM2-p53 pathway. Oncol. Rep. 2016;35(2):887–895. doi: 10.3892/or.2015.4458. [DOI] [PubMed] [Google Scholar]
- 52.Tian B., Huo N., Li M., Li Y., He Z. let-7a and its target, insulin-like growth factor 1 receptor, are differentially expressed in recurrent prostate cancer. Int. J. Mol. Med. 2015;36(5):1409–1416. doi: 10.3892/ijmm.2015.2357. [DOI] [PubMed] [Google Scholar]
- 53.Fu Q., Liu X., Liu Y., Yang J., Lv G., Dong S. MicroRNA-335 and -543 suppress bone metastasis in prostate cancer via targeting endothelial nitric oxide synthase. Int. J. Mol. Med. 2015;36(5):1417–1425. doi: 10.3892/ijmm.2015.2355. [DOI] [PubMed] [Google Scholar]
- 54.Liao H., Xiao Y., Hu Y., Xiao Y., Yin Z., Liu L. microRNA-32 induces radioresistance by targeting DAB2IP and regulating autophagy in prostate cancer cells. Oncol. Lett. 2015;10(4):2055–2062. doi: 10.3892/ol.2015.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu D., Liu B., Zang L.E., Jiang H. MiR-631/ZAP70: A novel axis in the migration and invasion of prostate cancer cells. Biochem. Biophys. Res. Commun. 2016;469(3):345–351. doi: 10.1016/j.bbrc.2015.11.093. [DOI] [PubMed] [Google Scholar]
- 56.Kurozumi A., Goto Y., Matsushita R., Fukumoto I., Kato M., Nishikawa R., Sakamoto S., Enokida H., Nakagawa M., Ichikawa T., Seki N. Tumor-suppressive microRNA-223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 2016;107(1):84–94. doi: 10.1111/cas.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L., Song G., Tan W., Qi M., Zhang L., Chan J., Yu J., Han J., Han B. MiR-573 inhibits prostate cancer metastasis by regulating epithelial-mesenchymal transition. Oncotarget. 2015;6(34):35978–35990. doi: 10.18632/oncotarget.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramalinga M., Roy A., Srivastava A., Bhattarai A., Harish V., Suy S., Collins S., Kumar D. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget. 2015;6(33):34446–34457. doi: 10.18632/oncotarget.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong X.J., Duan L.J., Qian X.Q., Xu D., Liu H.L., Zhu Y.J., Qi J. Tumor-suppressive microRNA-497 targets IKKβ to regulate NF-κB signaling pathway in human prostate cancer cells. Am. J. Cancer Res. 2015;5(5):1795–1804. [PMC free article] [PubMed] [Google Scholar]
- 60.Chang Y.L., Zhou P.J., Wei L., Li W., Ji Z., Fang Y.X., Gao W.Q. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6(27):24017–24031. doi: 10.18632/oncotarget.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato M., Goto Y., Matsushita R., Kurozumi A., Fukumoto I., Nishikawa R., Sakamoto S., Enokida H., Nakagawa M., Ichikawa T., Seki N. MicroRNA-26a/b directly regulate La-related protein 1 and inhibit cancer cell invasion in prostate cancer. Int. J. Oncol. 2015;47(2):710–718. doi: 10.3892/ijo.2015.3043. [DOI] [PubMed] [Google Scholar]
- 62.Pasqualini L., Bu H., Puhr M., Narisu N., Rainer J., Schlick B., Schäfer G., Angelova M., Trajanoski Z., Börno S.T., Schweiger M.R., Fuchsberger C., Klocker H. miR-22 and miR-29a Are Members of the Androgen Receptor Cistrome Modulating LAMC1 and Mcl-1 in Prostate Cancer. Mol. Endocrinol. 2015;29(7):1037–1054. doi: 10.1210/me.2014-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang N., Tao L., Zhong H., Zhao S., Yu Y., Yu B., Chen X., Gao J., Wang R. miR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol. Lett. 2016;11(1):543–550. doi: 10.3892/ol.2015.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turchinovich A., Weiz L., Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem. Sci. 2012;37(11):460–465. doi: 10.1016/j.tibs.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Wagner J., Riwanto M., Besler C., Knau A., Fichtlscherer S., Röxe T., Zeiher A.M., Landmesser U., Dimmeler S. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler. Thromb. Vasc. Biol. 2013;33(6):1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 66.Cortez M.A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A.K., Calin G.A. MicroRNAs in body fluidsthe mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., Nitadori-Hoshino A., Hoffman C., Badal K., Garcia B.A., Callahan M.K., Yuan J., Martins V.R., Skog J., Kaplan R.N., Brady M.S., Wolchok J.D., Chapman P.B., Kang Y., Bromberg J., Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Creemers E.E., Tijsen A.J., Pinto Y.M. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110(3):483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 69.Huang X., Yuan T., Liang M., Du M., Xia S., Dittmar R., Wang D., See W., Costello B.A., Quevedo F., Tan W., Nandy D., Bevan G.H., Longenbach S., Sun Z., Lu Y., Wang T., Thibodeau S.N., Boardman L., Kohli M., Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015;67(1):33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mydlarz W.K., Hennessey P.T., Califano J.A. Advances and Perspectives in the molecular diagnosis of head and neck cancer. Expert Opin. Med. Diagn. 2010;4(1):53–65. doi: 10.1517/17530050903338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahn R., Heukamp L.C., Rogenhofer S., von Ruecker A., Müller S.C., Ellinger J. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology. 2011;77(5):1265.e9–1265.e16. doi: 10.1016/j.urology.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Yaman Agaoglu F., Kovancilar M., Dizdar Y., Darendeliler E., Holdenrieder S., Dalay N., Gezer U. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011;32(3):583–588. doi: 10.1007/s13277-011-0154-9. [DOI] [PubMed] [Google Scholar]
- 73.Eckhardt F., Lewin J., Cortese R., Rakyan V.K., Attwood J., Burger M., Burton J., Cox T.V., Davies R., Down T.A., Haefliger C., Horton R., Howe K., Jackson D.K., Kunde J., Koenig C., Liddle J., Niblett D., Otto T., Pettett R., Seemann S., Thompson C., West T., Rogers J., Olek A., Berlin K., Beck S. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat. Genet. 2006;38(12):1378–1385. doi: 10.1038/ng1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gal-Yam E.N., Saito Y., Egger G., Jones P.A. Cancer epigenetics: modifications, screening, and therapy. Annu. Rev. Med. 2008;59:267–280. doi: 10.1146/annurev.med.59.061606.095816. [DOI] [PubMed] [Google Scholar]
- 75.Riggs A.D., Jones P.A. 5-methylcytosine, gene regulation, and cancer. Adv. Cancer Res. 1983;40:1–30. doi: 10.1016/S0065-230X(08)60678-8. [DOI] [PubMed] [Google Scholar]
- 76.Singh P.K., Campbell M.J. The Interactions of microRNA and epigenetic modifications in prostate cancer. Cancers (Basel) 2013;5(3):998–1019. doi: 10.3390/cancers5030998. [Basel]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paone A., Galli R., Fabbri M. MicroRNAs as new characters in the plot between epigenetics and prostate cancer. Front. Genet. 2011;2(2):62. doi: 10.3389/fgene.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bishop K.S., Ferguson L.R. The interaction between epigenetics, nutrition and the development of cancer. Nutrients. 2015;7(2):922–947. doi: 10.3390/nu7020922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhatnagar N., Li X., Padi S.K., Zhang Q., Tang M.S., Guo B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1(12):e105–e113. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fabbri M., Garzon R., Cimmino A., Liu Z., Zanesi N., Callegari E., Liu S., Alder H., Costinean S., Fernandez-Cymering C., Volinia S., Guler G., Morrison C.D., Chan K.K., Marcucci G., Calin G.A., Huebner K., Croce C.M. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA. 2007;104(40):15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morita S., Horii T., Kimura M., Ochiya T., Tajima S., Hatada I. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci. 2013;14(7):14647–14658. doi: 10.3390/ijms140714647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishikawa R., Goto Y., Kojima S., Enokida H., Chiyomaru T., Kinoshita T., Sakamoto S., Fuse M., Nakagawa M., Naya Y., Ichikawa T., Seki N. Tumor-suppressive microRNA-29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int. J. Oncol. 2014;45(1):401–410. doi: 10.3892/ijo.2014.2437. [DOI] [PubMed] [Google Scholar]
- 83.Steele R., Mott J.L., Ray R.B. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1(4):381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galli R., Paone A., Fabbri M., Zanesi N., Calore F., Cascione L., Acunzo M., Stoppacciaro A., Tubaro A., Lovat F., Gasparini P., Fadda P., Alder H., Volinia S., Filippini A., Ziparo E., Riccioli A., Croce C.M. Toll-like receptor 3 (TLR3) activation induces microRNA-dependent reexpression of functional RARβ and tumor regression. Proc. Natl. Acad. Sci. USA. 2013;110(24):9812–9817. doi: 10.1073/pnas.1304610110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theodore S.C., Davis M., Zhao F., Wang H., Chen D., Rhim J., Dean-Colomb W., Turner T., Ji W., Zeng G., Grizzle W., Yates C. MicroRNA profiling of novel African American and Caucasian Prostate Cancer cell lines reveals a reciprocal regulatory relationship of miR-152 and DNA methyltranferase 1. Oncotarget. 2014;5(11):3512–3525. doi: 10.18632/oncotarget.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y.L., Wu S., Jiang B., Yin F.F., Zheng S.S., Hou S.C. Role of MicroRNAs in prostate cancer pathogenesis. Clin. Genitourin. Cancer. 2015;13(4):261–270. doi: 10.1016/j.clgc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Xue G., Ren Z., Chen Y., Zhu J., Du Y., Pan D., Li X., Hu B. A feedback regulation between miR-145 and DNA methyltransferase 3b in prostate cancer cell and their responses to irradiation. Cancer Lett. 2015;361(1):121–127. doi: 10.1016/j.canlet.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 88.Soni A., Bansal A., Singh L.C., Mishra A.K., Majumdar M., Regina T., Mohanty N.K., Saxena S. Gene expression profile and mutational analysis of DNA mismatch repair genes in carcinoma prostate in Indian population. OMICS. 2011;15(5):319–324. doi: 10.1089/omi.2010.0110. [DOI] [PubMed] [Google Scholar]
- 89.Basu S., Majumder S., Bhowal A., Ghosh A., Naskar S., Nandy S., Mukherjee S., Sinha R.K., Basu K., Karmakar D., Banerjee S., Sengupta S. A study of molecular signals deregulating mismatch repair genes in prostate cancer compared to benign prostatic hyperplasia. PLoS One. 2015;10(5):e0125560–e0125582. doi: 10.1371/journal.pone.0125560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noonan E.J., Place R.F., Pookot D., Basak S., Whitson J.M., Hirata H., Giardina C., Dahiya R. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28(14):1714–1724. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- 91.Wilting R.H., Yanover E., Heideman M.R., Jacobs H., Horner J., van der Torre J., DePinho R.A., Dannenberg J.H. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J. 2010;29(15):2586–2597. doi: 10.1038/emboj.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ocker M., Schneider-Stock R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int. J. Biochem. Cell Biol. 2007;39(7-8):1367–1374. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 93.Vashishta A., Hetman M. Inhibitors of histone deacetylases enhance neurotoxicity of DNA damage. Neuromolecular Med. 2014;16(4):727–741. doi: 10.1007/s12017-014-8322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellis L., Pili R. Histone deacetylase inhibitors: Advancing therapeutic strategies in hematological and solid malignancies. Pharmaceuticals (Basel) 2010;3(8):2411–2469. doi: 10.3390/ph3082441. [Basel]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halkidou K., Cook S., Leung H.Y., Neal D.E., Robson C.N. Nuclear accumulation of histone deacetylase 4 (HDAC4) coincides with the loss of androgen sensitivity in hormone refractory cancer of the prostate. Eur. Urol. 2004;45(3):382–389. doi: 10.1016/j.eururo.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Weichert W., Röske A., Gekeler V., Beckers T., Stephan C., Jung K., Fritzsche F.R., Niesporek S., Denkert C., Dietel M., Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer. 2008;98(3):604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Majid S., Dar A.A., Saini S., Shahryari V., Arora S., Zaman M.S., Chang I., Yamamura S., Tanaka Y., Chiyomaru T., Deng G., Dahiya R. miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin. Cancer Res. 2013;19(1):73–84. doi: 10.1158/1078-0432.CCR-12-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verma M. Cancer control and prevention by nutrition and epigenetic approaches. Antioxid. Redox Signal. 2012;17(2):355–364. doi: 10.1089/ars.2011.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phuah N.H., Nagoor N.H. Regulation of microRNAs by natural agents: new strategies in cancer therapies. 2014. [DOI] [PMC free article] [PubMed]
- 100.Smoliga J.M., Blanchard O. Enhancing the delivery of resveratrol in humans: if low bioavailability is the problem, what is the solution? Molecules. 2014;19(11):17154–17172. doi: 10.3390/molecules191117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang C.S., Landau J.M., Huang M.T., Newmark H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 102.Yang M.H., Kim J., Khan I.A., Walker L.A., Khan S.I. Nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) modulators from natural products as anti-cancer agents. Life Sci. 2014;100(2):75–84. doi: 10.1016/j.lfs.2014.01.075. [DOI] [PubMed] [Google Scholar]
- 103.Cimino S., Sortino G., Favilla V., Castelli T., Madonia M., Sansalone S., Russo G. I., Morgia G. 2012.
- 104.Li Y., Sarkar F.H. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002;8(7):2369–2377. [PubMed] [Google Scholar]
- 105.Lazarevic B., Boezelijn G., Diep L.M., Kvernrod K., Ogren O., Ramberg H., Moen A., Wessel N., Berg R.E., Egge-Jacobsen W., Hammarstrom C., Svindland A., Kucuk O., Saatcioglu F., Taskèn K.A., Karlsen S.J. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer. 2011;63(6):889–898. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 106.Fritz W.A., Eltoum I.E., Cotroneo M.S., Lamartiniere C.A. Genistein alters growth but is not toxic to the rat prostate. J. Nutr. 2002;132(10):3007–3011. doi: 10.1093/jn/131.10.3007. [DOI] [PubMed] [Google Scholar]
- 107.Mentor-Marcel R., Lamartiniere C.A., Eltoum I.E., Greenberg N.M., Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. 2001;61(18):6777–6782. [PubMed] [Google Scholar]
- 108.Adjakly M., Ngollo M., Dagdemir A., Judes G., Pajon A., Karsli-Ceppioglu S., Penault-Llorca F., Boiteux J.P., Bignon Y.J., Guy L., Bernard-Gallon D. Prostate cancer: The main risk and protective factors-Epigenetic modifications. Ann. Endocrinol. (Paris) 2015;76(1):25–41. doi: 10.1016/j.ando.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Vardi A., Bosviel R., Rabiau N., Adjakly M., Satih S., Dechelotte P., Boiteux J.P., Fontana L., Bignon Y.J., Guy L., Bernard-Gallon D.J. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo. 2010;24(4):393–400. [PubMed] [Google Scholar]
- 110.Phillip C.J., Giardina C.K., Bilir B., Cutler D.J., Lai Y.H., Kucuk O., Moreno C.S. Genistein cooperates with the histone deacetylase inhibitor vorinostat to induce cell death in prostate cancer cells. BMC Cancer. 2012;12:145–156. doi: 10.1186/1471-2407-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirata H., Hinoda Y., Shahryari V., Deng G., Tanaka Y., Tabatabai Z.L., Dahiya R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer. 2014;110(6):1645–1654. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaman M.S., Chen Y., Deng G., Shahryari V., Suh S.O., Saini S., Majid S., Liu J., Khatri G., Tanaka Y., Dahiya R. The functional significance of microRNA-145 in prostate cancer. Br. J. Cancer. 2010;103(2):256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y., Kong D., Ahmad A., Bao B., Dyson G., Sarkar F.H. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics. 2012;7(8):940–949. doi: 10.4161/epi.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y., Zaman M.S., Deng G., Majid S., Saini S., Liu J., Tanaka Y., Dahiya R. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev. Res. (Phila.) 2011;4(1):76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiyomaru T., Yamamura S., Zaman M.S., Majid S., Deng G., Shahryari V., Saini S., Hirata H., Ueno K., Chang I., Tanaka Y., Tabatabai Z.L., Enokida H., Nakagawa M., Dahiya R. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS One. 2012;7(8):e43812–e43821. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Q., Wang B., Zang W., Wang X., Liu Z., Li W., Jia J. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS One. 2013;8(11):e70627–e70636. doi: 10.1371/journal.pone.0070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bosutti A., Degens H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci. Rep. 2015;5:12592–12619. doi: 10.1038/srep08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheth S., Jajoo S., Kaur T., Mukherjea D., Sheehan K., Rybak L.P., Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7(12):e51655–e51669. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhar S., Hicks C., Levenson A.S. Resveratrol and prostate cancer: promising role for microRNAs. Mol. Nutr. Food Res. 2011;55(8):1219–1229. doi: 10.1002/mnfr.201100141. [DOI] [PubMed] [Google Scholar]
- 120.Ullah M.F., Bhat S.H., Husain E., Abu-Duhier F., Hadi S.M., Sarkar F.H., Ahmad A. Pharmacological Intervention through dietary nutraceuticals in gastrointestinal neoplasia. Crit. Rev. Food Sci. Nutr. 2016;56(9):1501–1518. doi: 10.1080/10408398.2013.772091. [DOI] [PubMed] [Google Scholar]
- 121.Wargovich M.J. Experimental evidence for cancer preventive elements in foods. Cancer Lett. 1997;114(1-2):11–17. doi: 10.1016/S0304-3835(97)04616-8. [DOI] [PubMed] [Google Scholar]
- 122.Gupta S.C., Kim J.H., Prasad S., Aggarwal B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29(3):405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karunagaran D., Rashmi R., Kumar T.R. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets. 2005;5(2):117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 124.Liu H.L., Chen Y., Cui G.H., Zhou J.F. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol. Sin. 2005;26(5):603–609. doi: 10.1111/j.1745-7254.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 125.Khor T.O., Huang Y., Wu T.Y., Shu L., Lee J., Kong A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82(9):1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 126.Meja K.K., Rajendrasozhan S., Adenuga D., Biswas S.K., Sundar I.K., Spooner G., Marwick J.A., Chakravarty P., Fletcher D., Whittaker P., Megson I.L., Kirkham P.A., Rahman I. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 2008;39(3):312–323. doi: 10.1165/rcmb.2008-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teiten M.H., Dicato M., Diederich M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013;57(9):1619–1629. doi: 10.1002/mnfr.201300201. [DOI] [PubMed] [Google Scholar]
- 128.Li Y., Ahmad A., Kong D., Bao B., Sarkar F.H. Recent progress on nutraceutical research in prostate cancer. Cancer Metastasis Rev. 2014;33(2-3):629–640. doi: 10.1007/s10555-013-9478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bao B., Ahmad A., Kong D., Ali S., Azmi A.S., Li Y., Banerjee S., Padhye S., Sarkar F.H. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One. 2012;7(8):e43726–e43737. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang C.H., Yue J., Sims M., Pfeffer L.M. The curcumin analog EF24 targets NF-κB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS One. 2013;8(8):e71130–e71142. doi: 10.1371/journal.pone.0071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yallapu M.M., Khan S., Maher D.M., Ebeling M.C., Sundram V., Chauhan N., Ganju A., Balakrishna S., Gupta B.K., Zafar N., Jaggi M., Chauhan S.C. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35(30):8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siddiqui I.A., Asim M., Hafeez B.B., Adhami V.M., Tarapore R.S., Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25(4):1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee Y.H., Kwak J., Choi H.K., Choi K.C., Kim S., Lee J., Jun W., Park H.J., Yoon H.G. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012;30(1):69–74. doi: 10.3892/ijmm.2012.966. [DOI] [PubMed] [Google Scholar]
- 134.Narayanan R., Jiang J., Gusev Y., Jones A., Kearbey J.D., Miller D.D., Schmittgen T.D., Dalton J.T. MicroRNAs are mediators of androgen action in prostate and muscle. PLoS One. 2010;5(10):e13637–e13650. doi: 10.1371/journal.pone.0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Waltering K.K., Porkka K.P., Jalava S.E., Urbanucci A., Kohonen P.J., Latonen L.M., Kallioniemi O.P., Jenster G., Visakorpi T. Androgen regulation of micro-RNAs in prostate cancer. Prostate. 2011;71(6):604–614. doi: 10.1002/pros.21276. [DOI] [PubMed] [Google Scholar]
- 136.Sun T., Wang Q., Balk S., Brown M., Lee G.S., Kantoff P. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69(8):3356–3363. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chmelar R., Buchanan G., Need E.F., Tilley W., Greenberg N.M. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int. J. Cancer. 2007;120(4):719–733. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 138.Xiao J., Gong A.Y., Eischeid A.N., Chen D., Deng C., Young C.Y., Chen X.M. miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate. 2012;72(14):1514–1522. doi: 10.1002/pros.22501. [DOI] [PubMed] [Google Scholar]
- 139.Gong A.Y., Eischeid A.N., Xiao J., Zhao J., Chen D., Wang Z.Y., Young C.Y., Chen X.M. miR-175p targets the p300/CBP-associated factor and modulates androgen receptor transcriptional activity in cultured prostate cancer cells. BMC Cancer. 2012;12:492–502. doi: 10.1186/1471-2407-12-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma S., Chan Y.P., Kwan P.S., Lee T.K., Yan M., Tang K.H., Ling M.T., Vielkind J.R., Guan X.Y., Chan K.W. MicroRNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI-2. Cancer Res. 2011;71(2):583–592. doi: 10.1158/0008-5472.CAN-10-2587. [DOI] [PubMed] [Google Scholar]
- 141.Ribas J., Ni X., Haffner M., Wentzel E.A., Salmasi A.H., Chowdhury W.H., Kudrolli T.A., Yegnasubramanian S., Luo J., Rodriguez R., Mendell J.T., Lupold S.E. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009;69(18):7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi X.B., Xue L., Yang J., Ma A.H., Zhao J., Xu M., Tepper C.G., Evans C.P., Kung H.J., deVere White R.W. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc. Natl. Acad. Sci. USA. 2007;104(50):19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chakrabarti M., Ai W., Banik N.L., Ray S.K. Overexpression of miR-71 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem. Res. 2013;38(2):420–432. doi: 10.1007/s11064-012-0936-5. [DOI] [PubMed] [Google Scholar]
- 144.McCann M.J., Rowland I.R., Roy N.C. The anti-proliferative effects of enterolactone in prostate cancer cells: evidence for the role of DNA licencing genes, mi-R106b cluster expression, and PTEN dosage. Nutrients. 2014;6(11):4839–4855. doi: 10.3390/nu6114839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarkar F.H., Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat. Res. 2004;555(1-2):53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 146.Verhoeven D.T., Goldbohm R.A., van Poppel G., Verhagen H., van den Brandt P.A. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomarkers Prev. 1996;5(9):733–748. [PubMed] [Google Scholar]
- 147.Le H.T., Schaldach C.M., Firestone G.L., Bjeldanes L.F. Plant-derived 3,3-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003;278(23):21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 148.Zhang W.W., Feng Z., Narod S.A. Multiple therapeutic and preventive effects of 3,3-diindolylmethane on cancers including prostate cancer and high grade prostatic intraepithelial neoplasia. J. Biomed. Res. 2014;28(5):339–348. doi: 10.7555/JBR.28.20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kong D., Heath E., Chen W., Cher M.L., Powell I., Heilbrun L., Li Y., Ali S., Sethi S., Hassan O., Hwang C., Gupta N., Chitale D., Sakr W.A., Menon M., Sarkar F.H. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One. 2012;7(3):e33729–e33740. doi: 10.1371/journal.pone.0033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Varambally S., Cao Q., Mani R.S., Shankar S., Wang X., Ateeq B., Laxman B., Cao X., Jing X., Ramnarayanan K., Brenner J.C., Yu J., Kim J.H., Han B., Tan P., Kumar-Sinha C., Lonigro R.J., Palanisamy N., Maher C.A., Chinnaiyan A.M. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322(5908):1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin J.M., Amin S., Trushin N., Hecht S.S. Effects of isothiocyanates on tumorigenesis by benzo[a]pyrene in murine tumor models. Cancer Lett. 1993;74(3):151–159. doi: 10.1016/0304-3835(93)90237-4. [DOI] [PubMed] [Google Scholar]
- 152.Jiao D., Eklind K.I., Choi C.I., Desai D.H., Amin S.G., Chung F.L. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994;54(16):4327–4333. [PubMed] [Google Scholar]
- 153.Yu C., Gong A.Y., Chen D., Solelo Leon D., Young C.Y., Chen X.M. Phenethyl isothiocyanate inhibits androgen receptor-regulated transcriptional activity in prostate cancer cells through suppressing PCAF. Mol. Nutr. Food Res. 2013;57(10):1825–1833. doi: 10.1002/mnfr.201200810. [DOI] [PubMed] [Google Scholar]
- 154.Hsu A., Wong C.P., Yu Z., Williams D.E., Dashwood R.H., Ho E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenetics. 2011;3:3–12. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cao P., Deng Z., Wan M., Huang W., Cramer S.D., Xu J., Lei M., Sui G. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol. Cancer. 2010;9:108–120. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carter L.G., DOrazio J.A., Pearson K.J. Resveratrol and cancer: focus on in vivo evidence. Endocr. Relat. Cancer. 2014;21(3):R209–R225. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gerhardt T., Jones R., Park J., Lu R., Chan H.W., Fang Q., Singh N., Lai H. Effects of antioxidants and pro-oxidants on cytotoxicity of dihydroartemisinin to Molt-4 human leukemia cells. Anticancer Res. 2015;35(4):1867–1871. [PubMed] [Google Scholar]
- 158.Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6(221):221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 159.Gontero P., Marra G., Soria F., Oderda M., Zitella A., Baratta F., Chiorino G., Gregnanin I., Daniele L., Cattel L., Frea B., Brusa P. A randomized double-blind placebo controlled phase I-II study on clinical and molecular effects of dietary supplements in men with precancerous prostatic lesions. Chemoprevention or chemopromotion? Prostate. 2015;75(11):1177–1186. doi: 10.1002/pros.22999. [DOI] [PubMed] [Google Scholar]
- 160.Wang P., Aronson W.J., Huang M., Zhang Y., Lee R.P., Heber D., Henning S.M. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev. Res. (Phila.) 2010;3(8):985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Banerjee S., Li Y., Wang Z., Sarkar F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Biswas S.K., McClure D., Jimenez L.A., Megson I.L., Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005;7(1-2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 163.Li G., Rivas P., Bedolla R., Thapa D., Reddick R.L., Ghosh R., Kumar A.P. Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplastic lesions: involvement of SIRT1/S6K axis. Cancer Prev. Res. (Phila.) 2013;6(1):27–39. doi: 10.1158/1940-6207.CAPR-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Powell M.J., Casimiro M.C., Cordon-Cardo C., He X., Yeow W.S., Wang C., McCue P.A., McBurney M.W., Pestell R.G. Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation. Cancer Res. 2011;71(3):964–975. doi: 10.1158/0008-5472.CAN-10-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gupta S., Hastak K., Ahmad N., Lewin J.S., Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. USA. 2001;98(18):10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Adhami V.M., Siddiqui I.A., Ahmad N., Gupta S., Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64(23):8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 167.Adhami V.M., Ahmad N., Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J. Nutr. 2003;133(7) Suppl.:2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]