Abstract
BACKGROUND
Cigarette smoke has been identified as the main cause of oral cavity carcinoma. Recently, the electronic cigarette, a battery-operated device, was developed to help smokers stop their tobacco addiction. This study aimed to evaluate the safety of electronic cigarettes and to establish the possible role of such device in the primary prevention of oral cavity cancer.
SUBJECTS AND METHODS
This study included 65 subjects who were divided into three groups (smokers, e-cigarette smokers, and nonsmokers). All subjects were submitted to cytologic examination by scraping of oral mucosa. The slides were microscopically evaluated through a micronucleus assay test.
RESULTS
The prevalence of micronuclei was significantly decreased in the e-cigarette smoker group. There were no statistically significant differences in micronuclei distribution according to the type of cigarette, gender, and age.
CONCLUSIONS
The use of electronic cigarettes seems to be safe for oral cells and should be suggested as an aid to smoking cessation.
Keywords: electronic cigarettes, e-cigarette, oral cytology, micronuclei, oral squamous cell carcinoma
Introduction
Oral cancer is the eighth leading cause of cancer-related death in the world, with 12.7 million new cases and 7.6 million deaths yearly. Recent epidemiologic data show that there are 73,000 new cases of oral cancer each year in Europe, with an annual mortality rate of 28,200 (38.6%). Approximately 5840 new cases (8%) are diagnosed yearly in Italy, with a male/female ratio of 2:1 and an average age at diagnosis of 50 years.1
Head and neck cancer is the end result of multiple mutations, causing the normal tissue to expand uncontrolled and invade the surrounding tissues.2 Often, many mutations are required, and genetic and epigenetic factors may predispose a specific type of tissue to malignant transformation.3,4
Oral squamous cell carcinoma (OSCC) is the most common histologic type of oral cancer; malignant tumors may originate from all tissues of the oral cavity, which include salivary glands cancers, soft and skeletal tissue sarcomas, melanoma, odontogenic malignant tumors, and lymphoreticular neoplasms. The prognosis for patients with oral cancer is influenced by the disease stage at diagnosis. The five-year survival rate of patients in stages I and II is 80%, with a drastic decrease to 20% for patients in stages III and IV.5 Locoregional recurrences affect survival, reducing the rate to 5.10% at three years from their appearance.6,7 In addition, patients with OSCC have an increased risk of developing more primary tumors of the head, neck, and lungs.8
Tobacco and alcohol play the greatest role in head and neck carcinogenesis. Cigarette smoke has been identified as the main cause of the occurrence of this type of tumors.9 Smokers have a 3.43% risk of oral cancers, which is strictly dose dependent, compared to never smokers.10 Exposure of the upper aerodigestive tract to tobacco and alcohol likely causes premalignant changes in cells and, when paired with inhibition of tumor suppressor genes such as Tp53, enhances the malignant transformation of head and neck mucosa.11,12 Unfortunately, the head and neck mucosa is constantly exposed to unknown substances that may cause premalignant changes.13
Traditional cigarettes were consumed for decades before the carcinogenic effects of tobacco exposure to the head and neck were elucidated, established, and dispersed to the population. Even after the data clearly indicated the harm of tobacco products, many years passed before tobacco use was considered a significant contributor to head and neck cancer.
According to the reported benefits of abstention from smoking, primary prevention campaigns have been developed and specific drugs and devices have been promoted.
The electronic cigarette (e-cigarette) is a recently developed device introduced into the smoking market to help smokers stop their tobacco addiction. This battery-operated device releases nicotine in the form of aerosol along with several substances, including propylene glycol, vegetable glycerin, and flavoring. Its key feature is that it is totally tobacco free and does not generate toxic combustion products.
The marketing of electronic cigarettes has expanded considerably in the last 10 years,14,15 whereas there have been few studies on their safety and efficacy in the literature. Thus far, the scientific landscape remains poor in terms of research on screening tests among electronic cigarette smokers. The real effects of electronic cigarette smoke on the oral mucosa have been little evaluated. Most screening tests for secondary prevention are based on the use of biomarkers.16
The micronucleus (MN) assay test is a cytologic method developed by Schmid in 1975 as a screening test for drug toxicity in bone marrow samples from mammals. The technique is based on the identification of micronuclei on smears obtained by oral cavity exfoliation. The presence of MN is assessed as a predictive factor for increased risk of tumoral degeneration, according to the work of Schmid. Schmid used optical microscopy to study erythrocytes from the bone marrow of small mammals exposed to drugs so as to determine chromosomal breakage. The MN assay test was later used in peripheral lymphocytes and in aerodigestive and urinary tract epithelia for identifying cancer risk categories, especially in people with occupational or voluptuary exposure to carcinogens.5,17 In the anaphase, brother chromatids separate and migrate toward the cell poles through the mitotic spindle. Chromosomes and acentric chromosome fragments may remain behind during this stage. In the next telophase, during the reconstitution of the nuclear shells around the genetic kit of new daughter cells, elements that have not previously migrated are excluded from the nucleus and can be incorporated into the cytoplasm, causing the appearance of micronuclei. Therefore, micronuclei are indicative elements of genomic instability and may have clinical application in screening tests for risk categories.
The literature consists of articles about MN tests in order to assess the cytotoxic damage on the oral mucosa in smokers and nonsmokers; in 1987, Sarto et al have applied the technique of the MN research to the exfoliative cytology of oral cavity. The test application to the oral cavity cells has been an important evolution of Schmid theories, in terms of helping to assess the risk of tumor degeneration directly on the target organ.18
In the present study, the MN assay test was used to evaluate the prevalence of cellular changes in the oral mucosa of cigarette and electronic cigarette smokers, in comparison with samples from nonsmokers. The aim of this research was to assess the safety of electronic cigarettes and to establish the possible role of such device in the primary prevention of oral cavity cancer (OSCC).
Subjects and Methods
Between January and June 2015, a sample of previous smokers for at least six months (group A) was selected for the study during routine outpatient consultations. In the same period, a sample of electronic cigarette smokers for at least six months (group B) was obtained during monthly prevention campaigns in collaboration with e-cigarette retailers in Catanzaro, Italy. Finally, a sample of nonsmokers (group C) was selected from among the medical and paramedical staff at the University Hospital of Catanzaro for convenience as a control group. Subjects were adjusted for age and sex, and they were quantitatively uniformed.
All the subjects recruited for the study were submitted to oral cavity examination and assessed according to the following eligibility criteria: age older than 18 years, no dental procedures in the last six months, no oral diseases in the last six months, no occupational exposure to carcinogens, no history of malignancy, no chronic alcoholism, and no concomitant diseases.
This prospective study received approval from the ethics committee of the Magna Graecia University of Catanzaro. The research was conducted in accordance with the principles of the Declaration of Helsinki. All subjects gave a written informed consent indicating their voluntary and anonymous participation in the study. They completed a questionnaire that collected demographic data and information on consumption of cigarettes/e-cigarettes, use of alcohol, exposure to occupational carcinogens, concomitant or previous diseases, and daily dental hygiene routine.
In group A, specific data on cigarette consumption were collected from each subject, including daily and yearly consumption, type of cigarette, possible side effects, and period of consumption or withdrawal. Cigarette consumption was calculated according to the number of cigarettes smoked in 24 hours and the number of packs consumed yearly by using the formula: packages/year = (number of cigarettes smoked per day: 20) × year of consumption. Only subjects who consumed a single type of cigarette were included in the study. Cigarettes were classified according to the average content of nicotine and tar, as shown in Table 1.
Table 1.
Type of cigarette.
| TYPE OF CIGARETTE | CONTENT OF NICOTINE | CONTENT OF TAR |
|---|---|---|
| ULF | <0.5 mg | <5 mg |
| LF | 0.5–0.9 mg | 6–11 mg |
| MF | 1–1.3 mg | 12–17 mg |
| NF | 1.4–1.7 mg | 18–26 mg |
Abbreviations: ULF, ultralight with filter; LF, light with filter; MF, medium with filter; NF, without filter.
Group B included subjects who used different e-cigarette devices and various types of charging liquid. The e-cigarette smokers whom we have considered did not use the traditional cigarette in the last six months. Thus, electronic cigarettes were classified according to the nicotine content of the charging liquid: light (0.4–0.9 mg), medium (0.10–0.12 mg), and heavy (0.13–0.16 mg).
For each consumer, the content (mL) of the reservoir of the device and the number of daily refills were evaluated. The reasons for e-cigarette usage (eg, to stop smoking or to reduce daily cigarette consumption), as well as all oral, cardiovascular, and pulmonary side effects were also investigated.
Sample collection
The study participants rinsed their mouths twice with an isotonic solution of 0.9% NaCl before the sampling. The sampling was carried out from the anterior and middle portions of the oral mucosa of both cheeks in smokers and from one cheek in healthy subjects. This was done by scraping and dragging on the oral mucosa thrice in the posterior–anterior direction with the use of a sterile disposable curette, which was then streaked on a slide. The exfoliative cytology slides were fixed air in dry and closed area for 24 hours.
The slides were subjected to May-Grünwald–Giemsa staining as follows: three minutes in pure May-Grünwald solution, six minutes in 1:1 diluted May-Grünwald solution, one minute in distilled water, and 30 minutes in 1:10 diluted Giemsa solution. Then the stained slides were air dried and assembled with Pertex® solution (Bioptika). These were observed under a light optical microscope with a 100 × oil immersion objective for better visualization of nuclei.
For each sample, at least 1,000 intact epithelial cells were analyzed, eliminating fragmented, anucleated, and binucleated ones. Micronuclei were identified according to Tolbert’s criteria.19 For each subject, the total numbers of micronucleated cells/1000 cells (CMN) and of micronuclei/1000 cells (TMN) were calculated.
Statistic analysis
Statistical analysis was carried out by using the MedCalc software (Mariakerke). Comparison of the CMN and TMN values among the three groups was done by single variance ANOVA. The data were previously treated with Levene’s test to evaluate the variance and log transformation so as to calculate the statistical significance of the ANOVA test. Linear regression was used to control the effect of the CMN and TMN values on the variables, such as gender, age, and type of cigarette/electronic cigarette used. The values lower than 0.05 (P = 0.05) were considered statistically significant. The results are expressed as mean ± standard deviation (SD).
Results
A total of 65 subjects were submitted to oral cavity cytology for micronuclei detection; 23 (35.4%) subjects were included in group A, 22 (33.8%) subjects were included in group B, and 20 (30.8%) subjects were included in group C. Table 2 shows the demographic characteristics of the three groups. There were no statistically significant differences in age or gender between the groups.
Table 2.
Demographic data.
| GROUP | |||
|---|---|---|---|
| SMOKERS n (%) A |
E-CIGARETTES SMOKERS n (%) B |
NONSMOKERS n (%) C |
|
| Sample | 23 (35.4) | 22 (33.8) | 20 (30.8) |
| Sex | |||
| Male | 10 (43.5) | 12 (54.5) | 11 (55) |
| Female | 13 (46.5) | 10 (45.5) | 9 (45) |
| Age (yr) | |||
| Average | 47.6 | 57.8 | 46.7 |
| Range | 23–73 | 27–65 | 23–74 |
Group A
The average consumption of cigarettes in group A was 16 (range, 10–30) cigarettes/day, with a mean number of packs/year of 27.7 (range, 3.5–67.5). Table 3 shows the cigarette consumption data according to the cigarette type smoked. The subgroups were uniformly distributed according to the type and consumption of cigarettes, with consumers of light cigarettes with filter representing 56.6% of all the smokers.
Table 3.
Cigarette type classification.
| TYPE OF CIGARETTE | |||
|---|---|---|---|
| ULF | LF | MF | |
| Smokers (tot. 23) n (%) | 5 (21.7%) | 13 (56.6%) | 5 (21.7%) |
| Cigarettes/day | |||
| Average | 15 | 17.5 | 22.8 |
| Range | 10–20 | 10–40 | 10–40 |
| Packs/year | |||
| Average | 32 | 22.4 | 37 |
| Range | 3.5–60 | 3.5–69 | 7.5–67.5 |
Abbreviations: ULF, ultralight with filter; LF, light with filter; MF, medium with filter.
Group B
The e-cigarette smokers used such device to stop smoking from at least three months; 60% had continued to smoke traditional cigarettes initially and then later abstained from tobacco completely. During the first month of consumption, two subjects developed an episode of gengivostomatitis, consequent to the loss of liquid from the tank; the local irritation resolved without pharmacotherapy in 10 days.
The nicotine content of the charging liquid used by the e-cigarette smokers was 0.9 mg in 50% of cases, 0.12 mg in 12.5% of cases, and 0.16–0.18 mg in 37.5% of cases. The average number of daily refills was 1.8 (range, 1–4), with the device reservoir having an average content of 0.75 mL (range, 0.25–1.2 mL).
Six months after the sampling, 8 of the 22 subjects (37.5%) stopped smoking and did not use the electronic device, 4 (18.75%) used both traditional and electronic cigarettes, and 10 (43.75%) resumed smoking traditional cigarettes.
Group C
The nonsmokers group included 20 subjects who had never smoked, with no dental procedures in the last six months, no oral diseases in the last six months, no occupational exposure to carcinogens, no history of malignancy, no chronic alcoholism, and no concomitant diseases. This group served as control and was selected from among the medical and paramedical staff at the University Hospital of Catanzaro for convenience.
Distribution of micronuclei
A total of 98 slides were examined under an optical microscope with a 100× oil immersion objective. The micronucleated cells showed the characteristics described by Tolbert. Figures 1 and 2 show the normal oral epithelial cells and the micronucleated cells, respectively.
Figure 1.

Normal oral epithelial cells, absence of micronuclei (May-Grünwald–Giemsa staining, 1000 ×).
Figure 2.
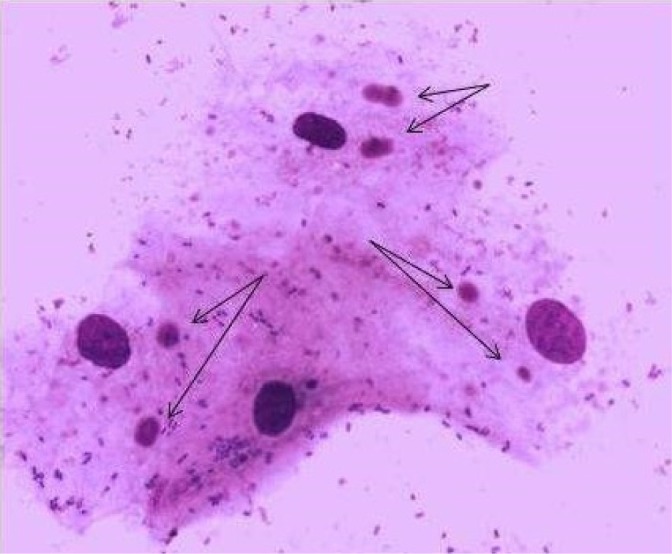
Oral epithelial cells with two micronuclei (May-Grünwald–Giemsa staining, 1000 ×).
The data on the total number of micronucleated cells and on the micronuclei were compared between the three groups through statistical analysis (Table 4). The average total number of micronucleated cells/1000 cells (CMN) was 0.039 (range, 0.01–0.138, SD ± 0.038) in group A, 0.0182 (range, 0.013–0.032, SD ± 0.0064) in group B, and 0.0015 (range, 0.005–0.026, SD ± 0.071) in group C. The average total number of micronuclei/1000 cells (TMN) was 0.088 (range, 0.02–0.362, SD ± 0.0058) in group A, 0.028 (range, 0.016–0.084, SD ± 0.024) in group B, and 0.012 (range, 0.008–0.23, SD ± 0.056) in group C.
Table 4.
Micronuclei distribution.
| GROUP A | GROUP B | GROUP C | |
|---|---|---|---|
| CMN | |||
| Average | 0.039 | 0.0182 | 0.015 |
| Range | 0.02–0.362 | 0.013–0.032 | 0.005–0.026 |
| SD | ± 0.038 | ± 0.006 | ± 0.071 |
| TMN | |||
| Average | 0.088 | 0.028 | 0.012 |
| Range | 0.02–0.362 | 0.016–0.084 | 0.008–0.23 |
| SD | ± 0.0058 | ± 0.024 | ± 0.0056 |
Abbreviations: CMN, micronucleatedcells/1000 cells; TMN, total micronuclei/1000 cells; SD, standard deviation.
The prevalence of micronuclei showed a statistically significant decrease in group B compared to that in group A based on the CMN (P = 0.001) and TMN (P = 0.004) values. There were no statistically significant differences in the distribution of micronuclei according to the type of cigarette, gender, and age.
Discussion
E-cigarettes could represent a smoking cessation device; therefore, studies on their safety are fundamental. The MN assay test has low invasiveness, a rapid execution time, and low cost.20 Thus far, this test has been used to assess OSCC risk in smokers or in subjects exposed to carcinogens.19–24 In the present study, the MN test was used for the first time in e-cigarette smokers.
E-cigarettes are currently used to stop smoking or reduce the consumption of traditional cigarettes. However, the Food and Drug Administration has yet to fully regulate the composition of the liquid refill of electronic cigarettes and to approve their use, although it has provided information about the currently known side effects of such usage (eg, congestive heart failure, cough, hypotension, and mental confusion) and defined the common components of the liquid refill.25
The cytotoxic effects of cigarette smoking have been widely evaluated in the literature; however, studies about e-cigarettes are often related to their use for smoking cessation or to case series of their side effects.26–28 Some components of the refill liquid, such as propylene glycol and glycerin, may have irritating effects on the mucous membrane of the airways. A recent study evaluated the cytotoxic effects in embryonic cell and adult lung fibroblast cultures exposed to refill liquids and their vapors; the effects were found to be greater in stem and progenitor cells than in adult ones.29 Yu et al30 evaluated the cytotoxicity and genotoxicity of short- and long-term e-cigarette vapor exposure in a panel of normal epithelial and head and neck squamous cell carcinoma cell lines. They found that cells exposed to e-cigarettes had significantly reduced cell viability and clonogenic survival, along with increased rates of apoptosis and necrosis, regardless of the e-cigarette vapor nicotine content. The cells also showed a significantly increased comet tail length and accumulation of γ-H2AX foci, indicating increased DNA strand breaks. However, the important limitations of this study were its non-reproducibility in vivo and the use of head and neck cancer cell lines, which are genotypically damaged and already directed toward cancer development.
There have been some in vitro studies comparing exposure to e-cigarettes with exposure to traditional cigarettes. One preliminary study demonstrated that high nicotine e-cigarette conditioned media induced a gene expression pattern similar to that seen when the same cell cultures were exposed to traditional cigarette conditioned media.31 Another study demonstrated an equivalent or greater toxicity to immune regulatory cells, such as dendritic cells, when exposed to extracts from presumed “reduced harm” cigarettes. These immune regulatory cells are identified in the pathogenesis of smoking-induced disease, such as chronic obstructive pulmonary disease.32
In contrast to these in vitro studies, in our in vivo study, the oral cavity cells of e-cigarette smokers showed CMN and TMN values similar to those of healthy controls, indicating the safety of e-cigarettes. The CMN and TMN values of e-cigarette smokers were similar to those of healthy controls.
The nicotine content of e-cigarettes varies from 14.8 to 87.2 mg/mL according to the composition of the refill liquid; thus, e-cigarette smokers may develop cardiovascular side effects. However, the levels of tobacco-specific nitrosamines in the refill liquid are similar to those in nicotine patches.28 This explains the importance of establishing the refill liquid composition and e-cigarette safety.
In the present study, 30% of e-cigarette smokers stopped smoking six months after the sampling. This finding is similar to that reported by other authors;33,34 however, another study found that the use of e-cigarettes did not play a significant role in smoking cessation in patients with head and neck carcinomas.35
Although there are much discordant data in the literature, our results show that e-cigarettes cause no harm in the oral cavity and, therefore, should be suggested as a reliable aid to smoking cessation compared to alternative methods.
Footnotes
ACADEMIC EDITOR: Brenda Anne Wilson, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 948 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Involved in the study design, literature search, clinical studies, data acquisition, and article preparation: TF. Involved in data and statistical analysis and article editing: ST. Involved in the experimental study: LP. Involved in the study concept, definition of intellectual content, and manuscript review: EA. All authors read and approved the final article.
REFERENCES
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Allegra E, Baudi F, La Boria A, Fagiani F, Garozzo A, Costanzo FS. Multiple head and neck tumours and their genetic relationship. Acta Otorhinolaryngol Ital. 2009;29(5):237. [PMC free article] [PubMed] [Google Scholar]
- 3.Renan MJ. How many mutations are required for tumorigenesis? Implications from human cancer data. Mol Carcinog. 1993;7(3):139–146. doi: 10.1002/mc.2940070303. [DOI] [PubMed] [Google Scholar]
- 4.Allegra E, Trapasso S. Cancer stem cells in head and neck cancer. Onco Targets Ther. 2012;5:375–383. doi: 10.2147/OTT.S38694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Bloebaum M, Poort L, Böckman R, Kessler P. Survival after curative surgical treatment for primary oral squamous cell carcinoma. J Craniomaxillofac Surg. 2014;42:1572–1576. doi: 10.1016/j.jcms.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Sklenicka S, Gardiner S, Dierks EK, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome? J Oral Maxillofac Surg. 2010;68:1270–1275. doi: 10.1016/j.joms.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Chuang SC, Scelo G, Tonita JM, et al. Risk of second primary cancer among patients with head and neck cancers: a pooled analysis of 13 cancer registries. Int J Cancer. 2008;123:2390–2396. doi: 10.1002/ijc.23798. [DOI] [PubMed] [Google Scholar]
- 9.Warnakulasuriya S, Sutherland G, Scully C. Tobacco, oral cancer, treatment of dependence. Oral Oncol. 2005;41:244–269. doi: 10.1016/j.oraloncology.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 13.Allegra E, Lombardo N, Puzzo L, Garozzo A. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngol Ital. 2009;29(4):187. [PMC free article] [PubMed] [Google Scholar]
- 14.Lazuras L, Muzi M, Grano C, Lucidi F. E-cigarettesas smoking cessation aids: a survey among practitioners in Italy. Int J Public Health. 2016;61:243–248. doi: 10.1007/s00038-015-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17:219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloching M, Hofmann A, Lautenschläger C, Berghaus A, Grummt T. Exfoliative cytology of normal buccal mucosa to predict the relative risk of cancer in the upper aerodigestive tract using the MN-assay. Oral Oncol. 2000;36:550–555. doi: 10.1016/s1368-8375(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 17.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 18.Sarto F, Finotto S, Giacomelli L, Mazzotti D, Tomanin R, Levis AG. The micronucleus assay in exfoliated cells of the human buccal mucosa. Mutagenesis. 1987;2:11–17. doi: 10.1093/mutage/2.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: a field test in snuff users. Am J Epidemiol. 1991;134:840–850. doi: 10.1093/oxfordjournals.aje.a116159. [DOI] [PubMed] [Google Scholar]
- 20.Palaskar S, Jindal C. Evaluation of micronuclei using Papanicolau and May Grunwald Giemsa stain in individuals with different tobacco habits—a comparative study. J Clin Diagn Res. 2010;4:3607–3613. [Google Scholar]
- 21.Grover S, Mujib A, Jahagirdar A, Telagi N, Kulkarni P. A comparative study for selectivity of micronuclei in oral exfoliated epithelial cells. J Cytol. 2012;29:230–235. doi: 10.4103/0970-9371.103940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casartelli G, Bonatti S, De Ferrari M, et al. Micronuclei frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol. 2000;22:486–492. [PubMed] [Google Scholar]
- 23.Jadhav K, Gupta N, Ahmed MB. Micronuclei: an essential biomarker in oral exfoliated cells for grading of oral squamous cell carcinoma. J Cytol. 2001;28:7–12. doi: 10.4103/0970-9371.76941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bansal H, Sandhu VS, Bhandari R, Sharma D. Evaluation of micronuclei in tobacco users: a study in Punjabi population. Contemp Clin Dent. 2012;3:184–187. doi: 10.4103/0976-237X.96825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food And Drug Administration, Public Health Service, US Department Of Health And Human Services Shared risk evaluation mitigation strategy for all immediate-release transmucosal fentanyl dosage forms. J Pain Palliat Care Pharmacother. 2012;26:123–126. [Google Scholar]
- 26.Westenberger BJ. Evaluation of E-Cigarettes. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis; St. Louis, MO: 2009. [Google Scholar]
- 27.Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23:36–40. doi: 10.1136/tobaccocontrol-2013-051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahn Z, Seigel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- 29.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Yu V, Rahimy M, Korrapati A, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. doi: 10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassallo R, Wang L, Hirano Y, Walters P, Grill D. Extracts from presumed “reduced harm” cigarettes induce equivalent or greater toxicity in antigen-presenting cells. Toxicology. 2015;335:46–54. doi: 10.1016/j.tox.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomized controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 34.Born H, Persky M, Kraus DH, Peng R, Amin MR, Branski RC. Electronic cigarettes: a primer for clinicians. Otolaryngol Head Neck Surg. 2015;153:5–14. doi: 10.1177/0194599815585752. [DOI] [PubMed] [Google Scholar]
- 35.Mcqueen N, Partington EJ, Harrington KF, Rosenthal EL, Carroll WR, Schmalbach CE. Smoking cessation and electronic cigarette use among head and neck cancer patients. Otolaryngol Head Neck Surg. 2016;154:73–79. doi: 10.1177/0194599815613279. [DOI] [PubMed] [Google Scholar]


