ABSTRACT
Hepatitis C virus (HCV) is a major cause of chronic liver disease, infecting approximately 170 million people worldwide. HCV assembly is tightly associated with the lipoprotein pathway. Exchangeable apolipoprotein E (apoE) is incorporated on infectious HCV virions and is important for infectious HCV virion morphogenesis and entry. Moreover, the virion apoE level is positively correlated with its ability to escape E2 antibody neutralization. However, the role of apoE exchange in the HCV life cycle is unclear. In this study, the relationship between apoE expression and cell permissiveness to HCV infection was assessed by infecting apoE knockdown and derived apoE rescue cell lines with HCV. Exchange of apoE between lipoproteins and HCV lipoviral particles (LVPs) was evaluated by immunoprecipitation, infectivity testing, and viral genome quantification. Cell and heparin column binding assays were applied to determine the attachment efficiency of LVPs with different levels of incorporated apoE. The results showed that cell permissiveness for HCV infection was determined by exogenous apoE-associated lipoproteins. Furthermore, apoE exchange did occur between HCV LVPs and lipoproteins, which was important to maintain a high apoE level on LVPs. Lipid-free apoE was capable of enhancing HCV infectivity for apoE knockdown cells but not apoE rescue cells. A higher apoE level on LVPs conferred more efficient LVP attachment to both the cell surface and heparin beads. This study revealed that exogenous apoE-incorporating lipoproteins from uninfected hepatocytes safeguarded the apoE level of LVPs for more efficient attachment during HCV infection.
IMPORTANCE In this study, a neglected but important role of apoE exchange in HCV LVP infectivity after virus assembly and release was identified. The data indicated that apoE expression level in uninfected cells is important for high permissiveness to HCV infection. Secreted apoE-associated lipoprotein specifically enhances infection of HCV LVPs. apoE exchange between HCV LVP and lipoproteins is important to maintain an adequate apoE level on LVPs for their efficient attachment to cell surface. These data defined for the first time an extracellular role of exchangeable apoE in HCV infection and suggested that exchangeable apolipoproteins reach a natural equilibrium between HCV LVPs and lipoprotein particles, which provides a new perspective to the understanding of the heterogeneity of HCV LVPs in composition.
INTRODUCTION
More than 170 million people worldwide are infected with hepatitis C virus (HCV). Up to 80% of infected individuals are unable to clear the virus, and persistent infections lead to a high risk of developing liver cirrhosis and hepatocellular carcinoma (1). Direct-acting antivirals (DAA) significantly improved treatment efficiency, but an effective vaccine for the control of new HCV infection is still needed due to the limited access to anti-HCV treatment. Moreover, the emergence of DAA-resistant viruses calls for alternative strategies to control viral breakthrough (2–4).
A hallmark of HCV infectious particles is their tight connection with very-low-density lipoproteins (VLDL) and low-density lipoproteins (LDL), giving rise to a hybrid form of lipoviral particles (LVPs) with heterogeneous buoyant density (5–12). Nonexchangeable apolipoprotein B (apoB) and several exchangeable apolipoproteins (apoE, apoA-I, and apoC-I) have been found on the LVP surface (13–15). apoB remains associated with triglyceride-rich lipoprotein (TRL) from the beginning of lipoprotein assembly and secretion to the end of remnant particle clearance, whereas exchangeable apolipoproteins are able to dissociate from one lipoprotein and reassociate with another lipoprotein in circulation through an intermediate lipid-free stage (16–18). Among exchangeable apolipoproteins, apoE is dispensable for HCV genome replication but essential for infectious HCV assembly and entry (19–25). Because apoE is a low-density lipoprotein receptor (LDLr) ligand, apoE exchange between lipoproteins is important for cholesterol transport and lipoprotein metabolism (18, 26, 27). However, the role of apoE exchange in HCV infection is not known.
In this study, the role of apoE exchange in HCV infection was examined using an HCV cell culture (HCVcc) system (28–30). We found that the apoE expression level in uninfected hepatic cells is important for their high cell permissiveness to HCV infection. Through apoE exchange, exogenous apoE-incorporating lipoproteins from uninfected hepatocytes safeguard apoE level on LVP for more efficient attachment during HCV infection.
MATERIALS AND METHODS
Cell lines.
The Huh7.5.1 cell lines and its derivatives were cultured in Dulbecco's modified minimal essential medium (DMEM; Invitrogen) supplemented with 2 mM l-glutamine, nonessential amino acids, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum (complete DMEM). Cells were routinely passaged twice a week at a split ratio of 1:7. The 293T cell line is similarly cultured.
Custom DNA fragments encoding transcription activator-like effector nucleases (TALENs) were designed to cleave the human apoE gene. DNA fragments binding to the left and right target sites were cloned into pCMV-SP6-TALEN vector set left and right arms (Sidansai Biotechnology, Shanghai, China), respectively. TALEN plasmids were transfected into Huh7.5.1 cells by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cells were screened with puromycin 24 h after transfection. Single cell clones were isolated afterwards, passaged, and then used for Western blotting. Knockout clones were further verified by sequencing a 500-bp PCR fragment covering the designed cleavage site by using the following primers: 5′-GAGATAAGAGAAGACCAG-3′ and 5′-CTCCTATCTCAAGGATGG-3′.
An apoE stable knockdown cell line and apoE-hemagglutinin (apoE-HA) and apoE-Flag rescue cell lines were generated as previously described. Briefly, lentiviruses encoding short hairpin RNAs (shRNA) were used to establish cell lines sh-NT and sh-apoE. 293T cells were cotransfected with the following vectors: first, transfer vector pAPM, which carries the puromycin resistance gene and an shRNA sequence targeting the 3′ untranslated region of apoE or control shRNA (sh-NT); second, an HIV-1 packaging plasmid (psPAX2); and third, a vesicular stomatitis virus G protein expression vector (pMD2.G). The following shRNA targeting sequences were used: shmirAPOE, 5′-TGCTGTTGACAGTGAGCGCGGACCCTAGTTTAATAAAGATTAGTGAAG CCACAGATGTAATCTTTATTAAACTAGGGTCCATGCCTACTGCCTCGGA-3′, and sh-NT, 5′-TGCTGTTGACAGTGAGCGCTCTCGCTTGGGCGAGAGTAAGTAGTGAAGCCACAGATGTACTTACTCTCGCCCAAGCGAGATAGTGAAGCCACAGATGTA-3′. For the production of lentiviral particles suitable for overexpression, 293T cells were cotransfected with the transfer vector carrying the gene of interest and a blasticidin resistance gene (psPAX2) and pMD2.G. A similar method was applied to produce lentiviral particles encoding green fluorescent protein (GFP).
Plasmids, antibodies, and lipid-free apoE.
Plasmids carrying full-length genomes derived from the HCV chimera Jc1 (pFK_Jc1_dg, pFK_Jc1_FlagE2, and pFK_i389RLuc2a_Core-3′_Jc1; abbreviated as Jc1, Jc1FlagE2, and JcR2a, respectively) have been previously described. The plasmids (pWPI_apoE2-HA, pWPI_apoE3-HA, and pWPI_apoE4-HA) containing the coding sequences of human apoE2, -3, and -4 with C-terminally HA-tagged apoE3 were generated according to a previously described strategy. Mouse anti-NS5A and HCV chimeras were kindly provided by J. Zhong's lab at Institut Pasteur of Shanghai, China. Mouse anti-apoE (monoclonal antibody [MAb] 23) was kindly provided by G. Luo's lab at University of Alabama at Birmingham. Rabbit anti-core was generated in-house. The following commercial antibodies were used: goat anti-human apoE (ab947; Abcam), mouse anti-HA (Sigma), mouse anti-tubulin (Santa Cruz), and mouse anti-CD81 (sc23962; Santa Cruz). Rabbit polyclonal antibodies against occludin (13409), claudin (13050), syndecan1 (10593), SR-BI (21277), epidermal growth factor receptor (EGFR) (22542), and LDLr (10785) were from Proteintech Group. Commercial lipid-free apoE (ALP70) was from Chemicon.
HCVTCP and patient-derived HCV.
HCV transcomplementary particles (HCVTCP) from cell culture were produced as previously described (24). HCV samples from patients with chronic HCV infection were obtained from the First Hospital of Jilin University. All individuals signed an informed-consent form. The study protocol was approved by the Ethics Committee of the First Hospital of Jilin University. Samples 1, 2, and 3 came from patients infected by HCV genotype 1.
In vitro transcription and RNA transfection.
In vitro transcripts generated with pFK-based plasmids were transfected into cells by electroporation as described previously. The concentration of purified RNA was determined by a spectrometer, and the integrity of the transcripts was verified by agarose gel electrophoresis. Transcripts were stored as 10-μg aliquots at −80°C for further use. For RNA transfection, subconfluent Huh7.5 cell monolayers were detached from the culture dish by trypsinization, washed once with phosphate-buffered saline (PBS), and resuspended at a concentration of 1.5 × 107 cells per ml in cytomix containing 2 mM ATP and 5 mM glutathione. Ten micrograms of in vitro transcript was mixed with 400 μl of the cell suspension and transfected by electroporation at 960 mF and 270 V using a GenePulser system (Bio-Rad) and a cuvette with a gap width of 0.4 cm (Bio-Rad). Immediately after electroporation, cells were resuspended in complete DMEM and seeded as required.
Virus purification by using Flag- or HA-specific affinity gels.
Mixtures of virus samples and medium containing exogenous lipoprotein were subjected to either HA- or Flag-specific affinity purification by using HA or Flag affinity gels (Sigma). After four washes with PBS (20-fold bed volume each), immune-captured HCV particles were eluted by using 5 bed volumes of PBS containing 100 μg/ml of HA or Flag peptide (Sigma). Fifty percent tissue culture infective dose (TCID50) assays, Western analysis, and quantitative PCR (qPCR) were applied to measure the infectivity titers, genome copy number, and presence of apoE and HCV proteins.
Iodixanol density gradient analysis for fractionation of HCV LVPs and lipoprotein particles.
Concentrated HCV LVP or lipoprotein samples were analyzed by density gradient centrifugation as described previously. Briefly, samples were collected and concentrated to 0.5 ml by ultrafiltration (Amicon Ultra, Millipore). Concentrated samples were loaded onto a PBS-based 10 to 80% Optiprep (Axis-Shield) gradient and centrifuged at 34,000 rpm for 20 h at 4°C using an SW60 rotor (Beckmann). Fractions were collected from the top of the gradient, and their density was determined by measuring the refractive index of a 5-μl aliquot of each fraction.
Quantification of HCV infectivity.
Infectivity titers were determined by using a limiting-dilution assay. In brief, Huh7.5.1 or derived sh-NT cells, sh-apoE and derived apoE-HA rescued cells, and apoE knockout clone 5 and derived apoE-HA rescued cells were seeded into 96-well plates. The cells were fixed 3 to 4 days after infection. For immunohistochemistry, we used a 1:100 dilution of an antibody reacting specifically with the JFH1 NS5A. Bound antibody was detected with a 1:300 dilution of a peroxidase-conjugated secondary antibody against mouse IgG (Sigma-Aldrich).
Western blot analysis.
Samples for Western blotting were denatured in Laemmli buffer (125 mM Tris-HCl, 2% [wt/vol] SDS, 5% [vol/vol] 2-mercaptoethanol, 10% [vol/vol] glycerol, 0.001% [wt/vol] bromophenol blue, [pH 6.8]) and separated by SDS–12% polyacrylamide gel electrophoresis. Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked overnight in PBS supplemented with 0.5% Tween (PBS-T) and 5% dried milk (PBS-M) at 4°C prior to a 1-h incubation with primary antibody diluted in PBS-M. The membrane was washed 3 times with PBS-T and then incubated for 1 h with horseradish peroxidase-conjugated secondary antibody diluted at 1:10,000 in PBS-M. Bound antibodies were detected after washing 3 times with the ECL reagent (PerkinElmer).
Cell-based and heparin bead-based attachment assays.
For cell-based HCV attachment assay, cold virus samples (4°C) were used to attach prechilled Huh7.5.1 cells (4°C; 10E5 cells in 24-well plates). After incubation for 0.5 or 2 h, inoculated virus samples were removed by washing cells with 1 ml of cold PBS 5 times. To extract HCV genomic RNA for quantitative analysis, cells were subjected to following RNA extraction by using TRIzol reagent (Sigma). For the heparin bead (Sigma)-based attachment assay, cold virus samples were used to attach heparin beads at 4°C for 2 h. Unbound viruses were removed by washing cells with 5 bed volumes of cold PBS 5 times. HCV genomic RNA was similarly extracted by using TRIzol reagent for quantitative analysis. The primer pair 5′-TCTGCGGAACCGGTGAGTA-3′ and 5′-TCAGGCAGTACCACAAGGC-3′ was used for quantitative PCR.
Statistical analysis.
Differences between samples were analyzed using the two-tailed, unpaired Student's t test with the GraphPad Prism 5 software package. P values of less than 0.05 (indicated by asterisks in the figures) were considered statistically significant. The following categories were used: ***, P < 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
RESULTS
High permissiveness of Huh7.5.1 cells to HCV infection is dependent on apoE expression.
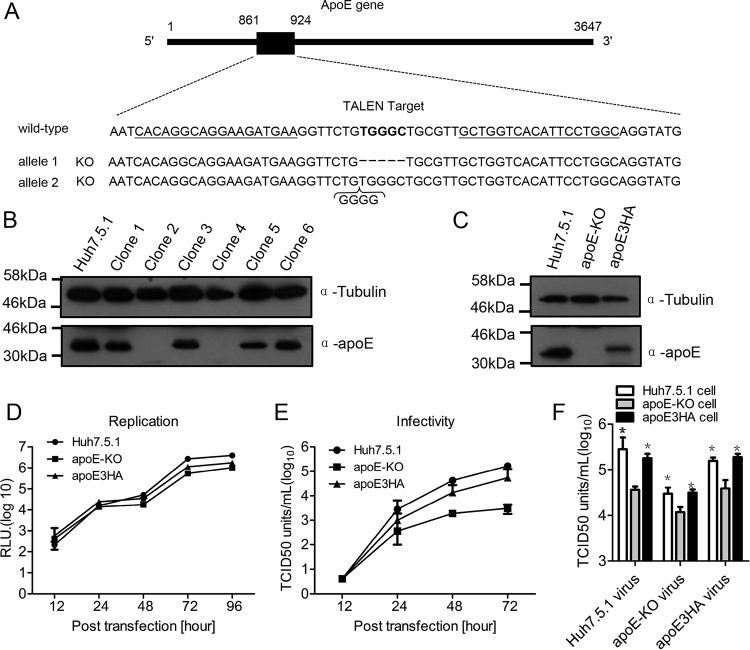
To define the role of apoE exchange in HCV infection, apoE knockout cell clones from Huh7.5.1 cells were generated by using transcription activator-like effector nuclease (TALEN) technology (Fig. 1A). From the apoE knockout experiments, several cell clones were selected and examined for apoE expression. Clones 2 and 4 showed marked reduction of apoE (Fig. 1B). Based on clone 2, the apoE3HA rescue cell line was generated and expression of apoE was detected by Western analysis (Fig. 1C). Clone 2 cells supported efficient HCV (Jc1, a J6/JFH chimera strain) replication (Fig. 1D). In addition, the rescue cell line expressing apoE3HA (24) supported efficient production of infectious HCV particles (Fig. 1E). Using these cell lines, we found that HCV produced from apoE knockout cells (apoE-KO virus) and apoE3-expressing cells (Huh7.5.1 virus and apoE3HA virus) were 3 to 5 times more infectious in apoE-expressing cells than in apoE knockout cells (Fig. 1F). This result suggested that apoE expression in Huh7.5.1 cells is important for new HCV infection.
FIG 1.
Establishment of apoE knockout cell clone and permissiveness test. (A) Schematic representation of TALEN design and the sequencing of apoE knockout clone 2. A schematic diagram of the apoE gene sequence is shown (nucleotide positions are indicated). The locations of two TALEN arms are underlined, and the TALEN target is in bold. The sequence of apoE knockout clone 2 was aligned with that of the wild-type apoE gene (positive-strand sequence). (B) Western blot verification of apoE knockout in isolated cell clones derived from the Huh7.5.1 cell line. Proteins are specified on the right, and the molecular mass standards are marked on the left. Tubulin was used as a loading control. (C) Western blot verification of apoE3HA rescue cell line based on selected apoE knockout cell clone 2. (D) HCV replication efficiency in Huh7.5.1, apoE-KO, and apoE3HA cell lines was evaluated by electroporating JcR2a in vitro transcripts. (E) HCV production in Huh7.5.1, apoE-KO, and apoEHA cell lines was evaluated by electroporating Jc1 in vitro transcripts. Infectivity of Jc1 virus produced from Huh7.5.1, apoE-KO, and apoE3HA cells was detected by using a limiting-dilution assay in 96-well plates with Huh7.5.1 cells. (F) Detection of infectivity of Jc1 virus produced from Huh7.5.1, apoE-KO, and apoE3HA cell lines by using a limiting-dilution assay in 96-well plates. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
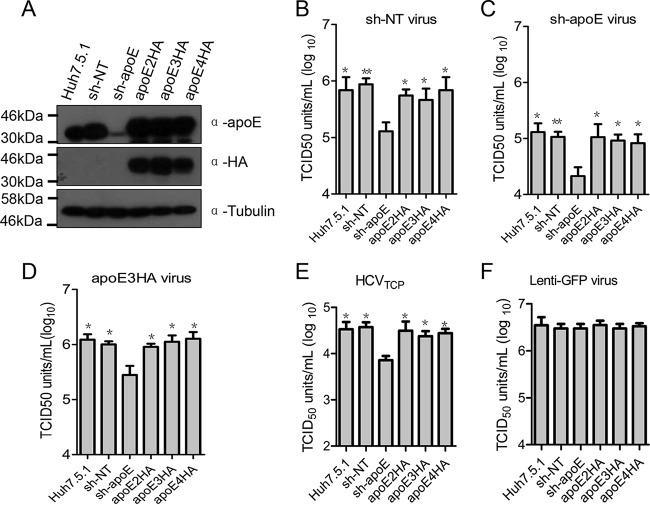
To avoid single clone effects from TALEN selection, we generated apoE stable knockdown cells and derived apoE2HA, apoE3HA, and apoE4HA rescue cell lines (Fig. 2A). We used viruses produced from nontargeting sh-RNA cells (sh-NT virus), apoE stable knockdown cells (sh-apoE virus), and apoE3HA rescue cells (apoE3HA virus) to infect apoE normal (Huh7.5.1 and sh-NT cells), knockdown (sh-apoE cells), and rescued cell lines (apoE2HA-, apoE3HA-, and apoE4HA-expressing cells). We found that regardless of the apoE level of virus-producing cells, virus infectivity in apoE stable knockdown cells was always significantly lower than that in cells with normal apoE levels, including the parental Huh7.5.1 cells, nontargeting sh-NT cell line, and apoE2HA, apoE3HA, and apoE4HA rescue cells (Fig. 2B to D). HCVTCP infectivity was similarly dependent on apoE expression in Huh7.5.1 cells (Fig. 2E). This finding is in agreement with those from apoE knockout and derived rescue cell lines. However, infectivity of lenti-GFP virus was independent of apoE expression in Huh7.5.1 cells (Fig. 2F), suggesting that apoE expression-enhanced viral infectivity is HCV specific. Moreover, infectivity of JFH1 chimeric viruses from other genotypes, including H77(1a), Con1(1b), JFH1(2a), J8(2b), S52(3a), and ED43(4a), all demonstrated a similar dependence on apoE expression in infection targeting cells (Fig. 3). Collectively, our observations indicated that apoE expression, independent of apoE polymorphism, is important for high permissiveness of Huh7.5.1 cells to HCVcc infection.
FIG 2.
Expression of apoE in uninfected cells is important for their permissiveness to HCV infection. (A) Western blot verification of apoE expression in Huh7.5.1 and derived sh-NT, sh-apoE, apoE2HA, apoE3HA, and apoE4HA cell lines; tubulin was detected as a loading control. Proteins are specified on the right. Positions of molecular mass standards are indicated on the left. (B to F) Detection of infectivity of sh-NT virus (B), sh-apoE virus (C), apoE3HA virus (D), HCVTCP (E), and lenti-GFP virus (F) was determined by using a limiting-dilution assay in 96-well plates with Huh7.5.1, sh-NT, sh-apoE, apoE2HA, apoE3HA, and apoE4HA cells, respectively. After 72 h of culture, cells were fixed and probed by anti-NS5A antibody to specify infection-positive wells. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
FIG 3.
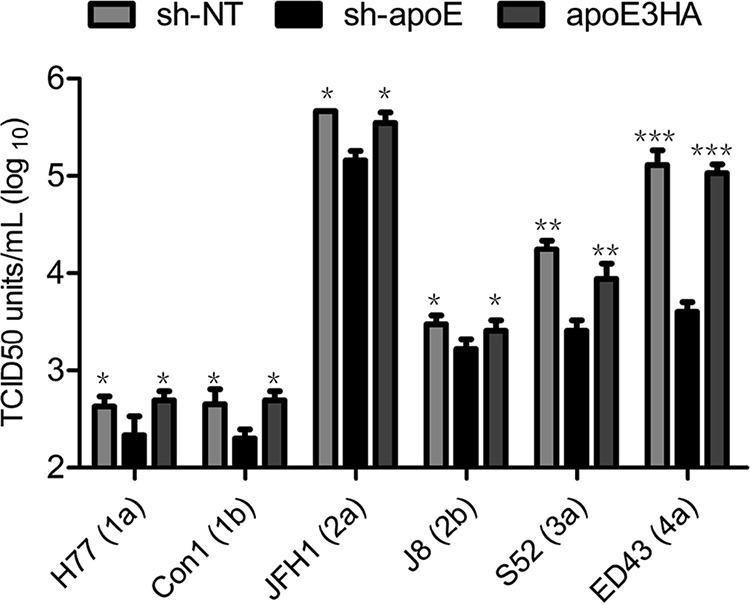
Infectivity of chimeric HCV from other genotypes is dependent on apoE expression in uninfected cells. Detection of infectivity of HCV chimeras including H77(1a), Con1(1b), JFH1(2a), J8(2b), S52(3a), and ED43(4a) was performed by using a limiting-dilution assay in 96-well plates with sh-NT, sh-apoE, and apoE3HA cells. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
Secreted apoE from uninfected cells is responsible for efficient HCV infection.
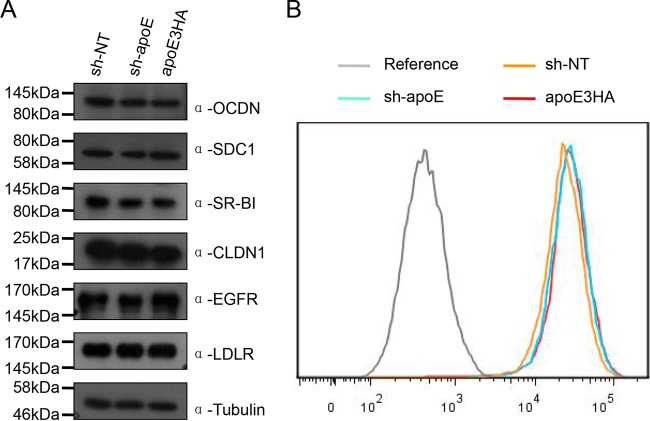
To answer how apoE expression increases permissiveness of Huh7.5.1 cells to HCVcc infection, we tested HCV receptor levels in apoE normal (sh-NT cells), knockdown (sh-apoE cells), and rescued (apoE3HA-expressing cells) cell lines. We found that apoE expression has no detectable effect on HCV receptor expression level (Fig. 4). Therefore, we hypothesized that cell culture medium from apoE-expressing cell lines, compared to that from the apoE knockdown cell line, contains an important component to enhance HCV infectivity titers. To test this hypothesis in sh-apoE cells, we first replaced the culture supernatant with sh-NT medium, sh-apoE medium, and apoE3HA medium and then infected the cells with sh-NT virus, sh-apoE virus, and apoE3HA virus (Fig. 5A). We found that sh-NT medium and apoE3HA medium replacement resulted in a significantly higher infectivity of all three types of viruses than did sh-apoE medium replacement (Fig. 5B). Comparable results were obtained using the sh-NT cell line as an infection target (Fig. 5C). This observation proved that an essential component is present in culture supernatant from apoE-expressing cells.
FIG 4.
Expression level of HCV receptors was not affected by apoE expression. (A) Western blot analysis of expression level of occludin, syndecan 1, SR-BI, claudin 1, EGFR, and LDLr in sh-NT, sh-apoE, and apoE3HA cells. Tubulin was detected as a loading control. Proteins are specified on the right. Positions of molecular mass standards are indicated on the left. (B) Fluorescence-activated cell sorter (FACS) analysis of CD81 expression levels in sh-NT, sh-apoE, and apoE3HA cells.
FIG 5.
apoE-containing medium is important for efficient HCV infection. (A) Schematic representation of experimental design. (B and C) Impact of apoE-containing medium on HCV infectivity. Detection of infectivity of sh-NT virus, sh-apoE virus, and apoE3HA virus by using a limiting-dilution assay was conducted in 96-well plates with sh-apoE (B) and sh-NT (C) cell lines, respectively. Before virus inoculation and TCID50 testing, cell medium from 96-well plates was replaced by sh-NT, sh-apoE, and apoE3HA cell culture medium. After 72 h of culture, cells were fixed and probed by anti-NS5A antibody to identify infection-positive wells. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
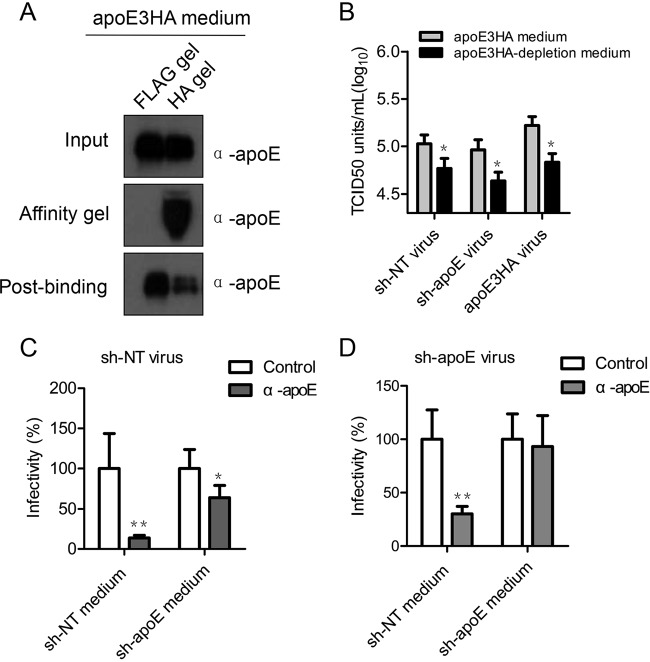
To further answer whether secreted apoE-containing lipoprotein is responsible for this elevated infectivity, we partially depleted apoE3HA-associated lipoproteins from apoE3HA medium by an HA affinity gel (Fig. 6A) and detected a consequential reduction of the apoE3HA medium-mediated infection enhancement (Fig. 6B). Next, we mixed sh-apoE virus with equal volume of either sh-NT medium or sh-apoE medium. This mixture was subjected to an apoE antibody neutralization test. The results showed that infectivity of sh-apoE virus became sensitive to apoE antibody only when the sh-apoE virus was premixed with sh-NT medium and not with sh-apoE medium (Fig. 6D). apoE antibody significantly blocked infection of sh-NT virus mixed both sh-NT medium and sh-apoE medium (Fig. 6C). Collectively, these data strongly indicated that apoE expression in uninfected cells is important for high cell permissiveness to HCV infection, which is due to the exogenous apoE but unlikely caused by the modulation of HCV entry receptor level. Exogenous apoE enhances HCV infection but not that of Japanese encephalitis virus (JEV), herpes simplex virus (HSV), or HIV (data not shown), suggesting that the unique characteristics of HCV LVP might be the major reason. We hypothesized that apoE exchange between apoE-containing lipoproteins secreted from uninfected cells and HCV LVPs might occur for the benefit of HCV infection.
FIG 6.
Exogenous apoE-positive lipoprotein is responsible for efficient HCV infection. (A) Partial depletion of apoE3HA from medium by using a Flag or HA affinity gel. Western blot analysis was used to confirm depletion efficiency. Samples are specified on the right of each panel. Positions of molecular mass standards are indicated on the left. (B) Impact of apoE-containing medium on HCV infectivity. Detection of infectivity of sh-NT virus, sh-apoE virus, and apoE3HA virus by using a limiting-dilution assay was conducted in 96-well plates with the sh-apoE cell line. Before virus inoculation, cell medium from 96-well plates was replaced by apoE3HA medium and apoE3HA partially depleted medium. After 72 h of culture, cells were fixed and probed by anti-NS5A antibody to identify infection-positive wells. (C and D) HCV produced from sh-NT (C) or sh-apoE (D) cells was premixed with sh-NT and sh-apoE medium. After incubation for 4 h at 37°C, the samples were subjected to 1 h of incubation with control or apoE-specific antibody. Infectivity tests were performed afterwards by using a limiting-dilution assay in 96-well plates. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
apoE transfer between LVPs and lipoproteins safeguards high apoE levels of LVPs.
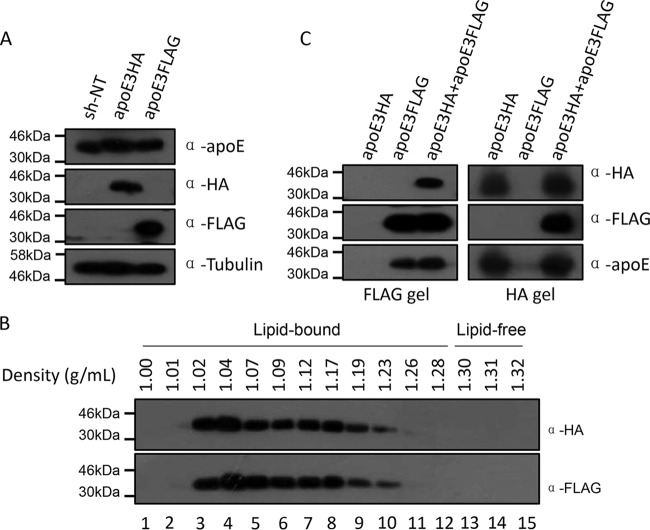
The above-described data led us to hypothesize that apoE exchange between apoE-containing lipoproteins secreted from uninfected cells and HCV LVPs augments HCV infection. Before testing apoE exchange between HCV LVPs and lipoproteins, we first verified apoE exchange between lipoprotein particles using cell lines that express either apoE3Flag or apoE3HA (Fig. 7A). Secreted apoE3HA and apoE3Flag were subjected to density gradient ultracentrifugation to check whether apoE expressed from Huh7.5.1 is fully lipidated like that from other hepatoma cells (31). The profile demonstrated that apoE3HA and apoE3Flag secreted from Huh7.5.1 cells were almost completely associated with lipids, as lipid-free apoE was under detectable levels (Fig. 7B). Subsequently, equal volumes of apoE3HA lipoprotein and apoE3Flag lipoprotein containing culture supernatant were mixed and incubated at 37°C for 4 h. Immunoprecipitation by using either an HA tag or Flag tag affinity gel was performed afterwards (Fig. 7C). The results demonstrated that the Flag tag affinity gel could pull down apoE3HA and the HA tag affinity gel could pull down apoE3Flag. These findings suggest that apoE produced from Huh7.5.1 cells is fully lipidated and that apoE is exchanged spontaneously between lipoprotein particles.
FIG 7.
apoE exchange between lipoprotein particles produced from Huh7.5.1 cells. (A) Western blot verification of apoE3HA and apoE3Flag expression in Huh7.5.1 cells. Tubulin was detected as a loading control. Proteins are specified on the right. (B) Density profile of secreted apoE3HA and apoE3Flag. Cell culture supernatants containing apoE3HA and apoE3Flag were collected, concentrated by ultrafiltration, and fractionated by density gradient centrifugation. Fifteen fractions were recovered from each gradient and analyzed by Western blotting using antibodies against HA tag and Flag tag. (C) Equal volumes of apoE3HA medium and apoE3Flag medium were mixed and incubated for 4 h at 37°C. HA or Flag affinity purification of lipoprotein particles and Western analysis were conducted to test apoE exchange between lipoprotein particles from apoE3HA- and apoE3Flag-expressing cells. Proteins are specified on the right. Positions of molecular mass standards are indicated on the left.
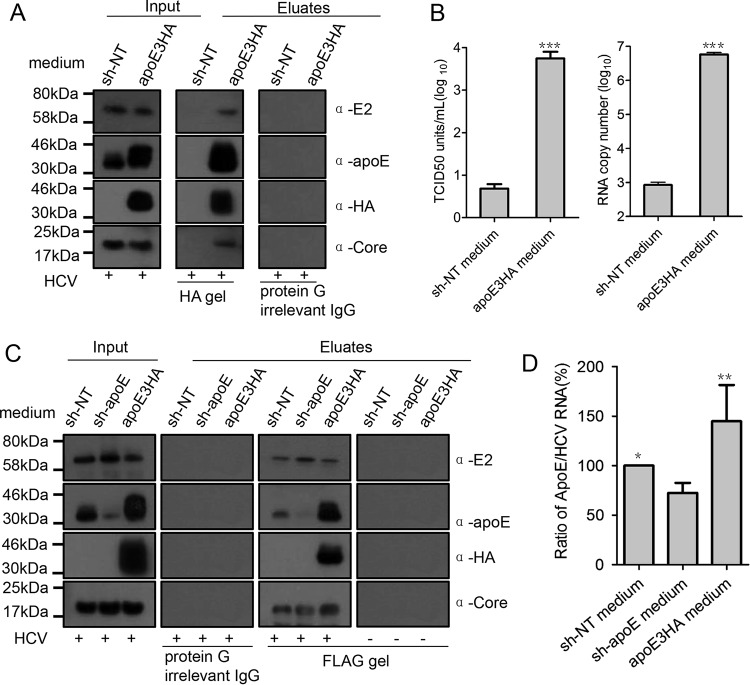
To investigate whether exogenous apoE produced from HCV infection targeting cells could be mobilized to HCV LVPs, a two-direction immunoprecipitation experiment was designed. We first mixed sh-NT/apoE3HA culture medium with an equal volume of Jc1 virus generated from Huh7.5.1 cells. This mixture was subjected to HA tag affinity purification after incubation at 37°C for 4 h. From the HA tag affinity gel, HCV infectious virus particles, genome, and core protein were specifically recovered (Fig. 8A and B). Subsequently, we mixed equal volumes of sh-NT/sh-apoE/apoE3HA cell line culture medium and Huh7.5.1-derived Jc1-FlagE2 virus (envelope protein E2-tagged Jc1) (32). This mixture was subjected to Flag tag affinity purification after a similar prior incubation. From the Flag tag affinity gel, HCV core protein and apoE3HA were specifically obtained (Fig. 8C). More importantly, mixing apoE-containing medium (sh-NT/apoE3HA) resulted in a more abundant apoE level on Flag tag-purified virus than did mixing sh-apoE medium (Fig. 8D). These two-direction immunoprecipitation assays demonstrated exogenous apoE transfer to HCV infectious LVPs. In addition, a high exogenous apoE level from HCV infection targeting cells contributes significantly to viral infectivity by providing an optimal apoE equilibrium to maintain a sufficient apoE level on LVPs.
FIG 8.
apoE is exchanged to HCV LVPs for their high apoE levels. (A) Flag-tagged HCV produced from Huh7.5.1 cells was mixed with equal volumes of sh-NT medium and apoE3HA medium and incubated for 4 h at 37°C. Afterwards, HA affinity purification of HCV LVPs was conducted to evaluate mobilization of apoE3HA to HCV LVPs. After HA affinity gel purification, HCV E2, apoE3HA, and core protein in eluates were detected by Western blot analysis. (B) Infectivity and genome copy number of eluates were detected by limiting-dilution assay and quantitative reverse transcriptase PCR (qRT-PCR), respectively. Input and eluted proteins are shown. Molecular sizes are indicated on the left, and proteins are specified on the right. (C) Flag-tagged HCV produced from Huh7.5.1 cells was mixed with equal volumes of sh-NT medium, sh-apoE, and apoE3HA medium and incubated for 4 h at 37°C. Afterwards, Flag affinity purification of HCV LVPs was conducted to reevaluate mobilization of apoE3HA to HCV LVPs. After Flag affinity gel purification, HCV E2, apoE3HA, and core protein in eluates were detected by Western blot analysis. (D) ApoE abundance on Flag affinity-purified LVP was evaluated by calculating the ratio of apoE amount to genome copy number. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
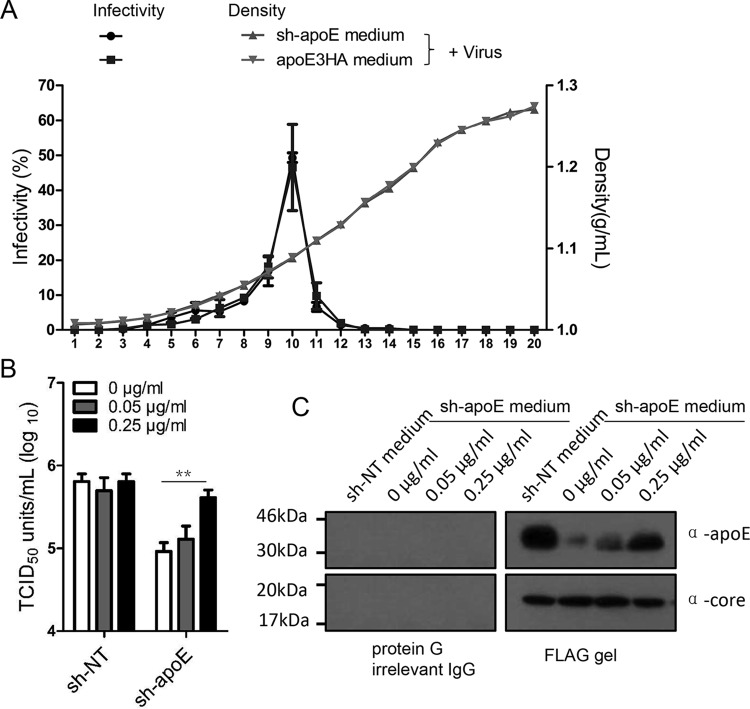
Lipid-free apoE enhances HCV infection in apoE knockdown cells.
A remarkable feature of HCV LVPs is their low buoyant density, ranging between 1.03 and 1.15 g/ml (9, 33). In theory, HCV LVPs might exist either as single hybrid particles with a continuous membrane or as complex particles consisting of conventional virions decorated with HDL or LDL particles through transient apoE and E1E2 interaction. Therefore, apoE could be transferred from lipoprotein particles to HCV LVPs by either apoE direct exchange to hybrid particles or a transient association between apoE-containing lipoprotein and the complex particles. To clarify the role of these two possible modes of apoE exchange, we first performed density gradient analysis of a mixture of HCV LVPs produced from Huh7.5.1 cells with culture supernatant from either apoE knockdown cell or apoE3HA rescue cells (Fig. 8A). Dilution of apoE-positive lipoprotein transient association by mixing LVPs with apoE-negative medium could, in theory, shift the density of HCV LVP infectivity to a higher density range. Density gradient analysis showed no significant shift of HCV infectivity between HCV LVPs mixed with apoE-containing medium and those mixed with apoE knockdown medium, which suggested that the apoE-positive lipoprotein transient association with HCV virions might play only a minor role. To further examine whether apoE is exchanged through an intermediate lipid-free stage, lipid-free apoE at different concentrations was added to different infection target cells (Huh7.5.1 and sh-apoE cells) and then HCV infectivity was measured. The addition of lipid-free apoE did not change HCV infectivity for Huh7.5.1 cells but enhanced HCV infectivity for apoE knockdown cells 5-fold (Fig. 9B). Moreover, the addition of lipid-free apoE could similarly increase the apoE level of Flag-tagged purified HCV LVPs (Fig. 9C). Collectively, these observations demonstrated that the major mechanism responsible for exogenous apoE-enhanced HCV infection is apoE exchange between HCV LVPs and lipoproteins.
FIG 9.
Lipid-free apoE enhances HCV infection in apoE knockdown cells. (A) Biophysical property of HCV LVPs conditioned with sh-apoE medium and apoE3HA medium. One milliliter of HCV produced from Huh7.5.1 cells was mixed with 30 ml of either sh-apoE medium or apoE3HA medium. The mixture was incubated for 4 h at 37°C, followed by concentration by ultrafiltration and fractionation by density gradient centrifugation. The virus infectivity of each fraction was determined as described above. Shown are the means of 3 independent experiments; error bars indicate standard deviations from the means. (B) Different amounts of lipid-free apoE were added into 96-well plates containing either Huh7.5.1 or sh-apoE cells. The final concentrations of lipid-free apoE were 0, 0.05, and 0.25 μg/ml. Infectivity of HCV was tested in these plates afterwards. Shown are the means of 3 independent experiments; error bars indicate standard deviations from the means. (C) Flag-tagged HCV produced from Huh7.5.1 cells was mixed with equal volumes of sh-NT medium with different amounts of lipid-free apoE. The mixture was incubated for 4 h at 37°C. Flag affinity purification of HCV LVPs was conducted to evaluate the apoE amounts on HCV LVPs by Western analysis. Molecular sizes are indicated on the left, and proteins are specified on the right.
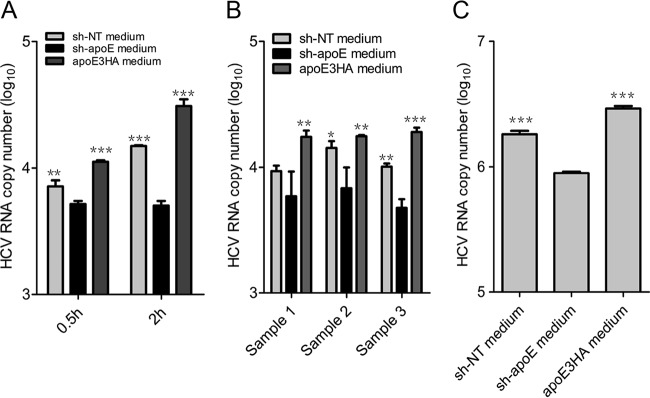
Higher apoE levels of LVPs confer more efficient HCV attachment.
apoE exchange between lipoproteins is critical for lipoprotein clearance through apoE interaction with low-density lipoprotein receptor (LDLr), heparan sulfate polyglycans (HSPG), and other receptors (16, 18). Before HCV engagement with cellular receptors (15), cell surface HSPG was reported to be the major HCV attachment factor (21, 22). We hypothesize that higher apoE levels of LVPs facilitate the initial attachment step of HCV entry. Therefore, assays of HCV infection attachment on the Huh7.5.1 cell surface and heparin column were performed. We showed that higher apoE levels on HCV particles confer more efficient attachment of HCV particles to both culture heparin column and Huh7.5.1 cells (Fig. 10A and C). The more efficient attachment to the cell surface was also observed for patient-derived virus (Fig. 10B). These findings confirmed that apoE exchange plays an important role in HCV infectivity. Additionally, secreted apoE from uninfected hepatocytes helps to maintain high apoE levels on LVPs for efficient attachment to the cell surface and HSPG during HCV infection.
FIG 10.
Cell attachment assay of HCVcc- and patient-derived HCV samples. (A) Cell attachment of sh-NT medium, sh-apoE medium, and apoE3HA medium-mixed HCV. Huh7.5.1 cells and viruses were precooled to 4°C. Attachment was performed on ice for 0.5 h and 2 h. After attachment, cells were washed 5 times with cold PBS, followed by TRIzol treatment and RNA extraction for qRT-PCR. (B) Huh7.5.1 cells and patient-derived HCV (samples 1, 2, and 3) were precooled to 4°C. Attachment was performed on ice for 2 h. After attachment, RNA quantification was conducted as described above. (C) HCV was mixed with sh-NT medium, sh-apoE medium, and apoE3HA medium, followed by incubation at 37°C for 4 h. The resulting samples were used to perform a heparin column attachment assay. After 1 h of attachment, the heparin column was washed 4 times with cold PBS and subjected to TRIzol treatment and RNA extraction for qRT-PCR. Shown are the means from 3 independent experiments; error bars indicate standard deviations from the means.
DISCUSSION
In this study, a neglected but important role of apoE exchange in HCV LVP infectivity after virus assembly and release was identified. We found that the apoE expression level in uninfected cells is important for high HCV infection permissiveness. In addition, exogenous apoE-associated lipoprotein specifically enhances HCV infection, which is independent of apoE genetically determined polymorphism. apoE3 and apoE4 bind with similar affinities to the low-density lipoprotein receptor (LDLr), whereas apoE2 has 2% or less of this binding capability. This difference suggests that LDLr might not be the major HCVcc attachment receptor during the initial step of viral entry. apoE secreted from Huh7.5.1 cells is fully lapidated, and apoE exchange between lipoproteins takes place spontaneously. apoE exchange between HCV LVPs and lipoproteins occurs too, which is important for maintaining a sufficient apoE level on LVPs. apoE exchange is further corroborated by lipid-free apoE-increased HCV LVP infectivity for apoE knockdown cells. Finally, higher apoE levels on HCV LVPs were found to be associated with more efficient HCV attachment, most likely through interaction between apoE and HSPG. SR-BI has been shown to serve as a rate-limiting factor which mediates the initial capture of HCV (H77/JFH1 chimera) particles of intermediate density (34). However, SR-BI knockdown did not change the overall attachment efficiency of Jc1 (unpublished data), suggesting that the role of SR-BI in HCV entry might be density and virus strain specific. Detailed research involving more HCV strains and spatio-temporal analysis of HCV engagement to multiple entry factors from the initial attachment to functional internalization will be required for a precise understanding of HCV entry.
HCV virion morphogenesis has been shown to take place at the connection site between the endoplasmic reticulum (ER) membrane and the surface of lipid droplets (LD) (7–9, 12, 35). With assistance from microsomal triglyceride transfer protein (MTTP) and its close link to the very-low-density-lipoprotein pathway, HCV virions are assembled as LVPs possessing characteristics similar to those of lipoproteins for their association with apolipoproteins and neutral lipids (8, 10, 11, 32, 35). Many apolipoproteins, including apoB, apoE, apoC, apoA-I, and apoJ, were demonstrated to play an important intracellular functional role in HCV LVP assembly and secretion (7, 17, 35–37). Unlike apoB, which remains associated with triglyceride-rich lipoproteins from the beginning of assembly to the end of remnant clearance, exchangeable apolipoproteins (apoE, apoA-I, and apoC) are able to dissociate from and reassociate with lipoprotein particles in circulation (18). In this study, we demonstrated apoE exchange between lipoprotein particles and further defined, for the first time, an extracellular role of exchangeable apoE in HCV infection. These data suggest that a natural exchange equilibrium of exchangeable apolipoproteins is reached between HCV LVPs and lipoprotein particles. These findings provide a new perspective to understand the heterogeneity of HCV LVPs in composition.
On the surface of HCV LVPs, both HCV envelope proteins and apolipoproteins (including apoB, apoE, and apoA-I) are present. However, a major unresolved issue is whether LVPs represent either hybrid particles that share an envelope or conventional virus particles that spontaneously interact with separable serum lipoprotein particles (9, 12). On E2-specific antibody-coated electron microscopy (EM) grids, affinity-purified HCV LVPs are generally spherical and well separated under electron microscopy (14, 32), indicating that most of the LVPs are in a hybrid particle form which resembles lipoprotein particles and support exchangeable apolipoprotein transfer. The diameters of HCV LVPs range from 40 nm to 100 nm, suggesting various extents of neutral lipid incorporation. Addition of lipid-free apoE secured apoE levels on HCV LVP and increased HCV LVP infectivity for apoE knockdown cells 5-fold, indicating that apoE exchange is indeed the major reason.
E1E2 protein on HCV LVPs might interact spontaneously with apoE (13, 38, 39), forming larger complexes in which conventional virions are transiently decorated with apoE-coated HDL/LDL particles. Hypothetically, for this complex type of HCV LVPs, apoE-positive lipoprotein could also assist HCV LVP infection through virion and lipoprotein particle association. However, the possibility of this mode of assistance is relatively low for the following reasons. (i) The diameters of HDL and LDL decorating virions range from 7 nm to 60 nm, but virion particles with a mean diameter of 50 nm associated with one or more HDL or LDL particles were not frequently observed on E1E2 affinity antibody-coated grids (14). (ii) Less decoration of virions by lipoprotein particles could theoretically shift HCV LVP infectivity from a lower to a higher density range. In our study, however, the addition of apoE-negative medium to HCV LVPs did not bring significant change to the biophysical property of LVP. (iii) The addition of lipid-free apoE increased HCV LVP infectivity for apoE knockdown cells 5-fold (comparable to the effect from addition of medium containing apoE-positive lipoproteins), suggesting that exogenous apoE-enhanced LVP infectivity is mainly due to apoE exchange. Based on this rationale, we propose that either larger complex forms of HCV LVPs represented only a minor fraction or apoE-positive lipoprotein association with or dissociation from virions was inefficient. Nevertheless, apoE exchange may still happen between lipoproteins from infection targeting cells to high-order complex HCV LVP virion-associated lipoproteins from virus-producing cells. More in-depth analysis involving protease digestion, gel filtration, and phospholipid labeling will be required to measure the precise ratio between the hybrid model and complex model of HCV LVPs.
In previous reports, it was shown that exchangeable apolipoproteins redundantly participated in the formation of HCV infectious particles (40). And surprisingly, short segments of amphipathic α-helices from apoE are equivalent to full-length apoE in supporting infectious HCV assembly and release (33, 40, 41). Lacking receptor bind domain from intact apoE did not significantly reduce HCV infectivity. These HCV particles could use other apolipoproteins, such as apoA, I and apoC, to gain efficient virus entry through interacting with LDLr and HSPG, or they could obtain intact apoE or other exchangeable apolipoproteins through exchange from permissive cells for efficient attachment. HCV-infected cells produce both infectious HCV LVPs and a large excess of apoE-positive lipoproteins to protect viral infectivity. During new infection, apoE produced by uninfected cells is essential to maintain a high apoE level on HCV LVPs, and the infectivity of small HCV inocula is then secured by apoE exchange equilibrium provided by uninfected cells. Neutralizing antibody evasion of HCV LVPs through apoE masking of E1E2 glycoprotein (42) could be dependent on both HCV LVP-producing cells and infection targeting cells.
Similar to the initiation of HCVcc infection in uninfected Huh7.5.1 cells, in infection of a new human host by HCV or repopulation in an infected human host by DAA-resistant HCV, the high apoE circulation level in blood might be a previously unappreciated factor. Interestingly, Younossi et al. found that successful ledipasvir (LDV)/sofosbuvir (SOF) with ribavirin (RBV) treatment of HCV is associated with decreased apoE and apoA-II levels (43), suggesting that besides the direct antivirus effect, LDV/SOF/RBV treatment may help HCV eradication in patients by reducing the contribution of exchangeable apoE to limit the repopulation of resistant viruses. It was reported that HCV infection of hepatocarcinoma cells was increased by addition of HDL (44). This increased infection could be attributed to the major exchangeable apolipoproteins (apoA-I and apoE) incorporated in HDL.
In summary, this study demonstrated an important role for apoE exchange in HCV infection. Exogenous apoE-incorporating lipoproteins from uninfected hepatocytes safeguarded the apoE level on LVPs for efficient attachment during HCV infection. Our data highlighted a previously underappreciated dynamic dimension in heterogeneous composition of infectious HCV LVPs and suggested high circulating exchangeable apoE as a neglected factor for HCV infection.
ACKNOWLEDGMENTS
We thank F. Chisari for the gift of Huh7.5.1 cells, G. Luo for the gift of MAb 23 against apoE, and T. Wakita, C. M. Rice, J. Bukh, and R. Bartenschlager for providing HCV strains. We are grateful to Z. Ma, X. Liang, and J. Wang for assistance with JEV, HSV, and HIV infections. We apologize to many respected colleagues whom we could not cite because of space limitations.
We have no conflicts of interest to disclose.
Z.Y., X.P., and G.L. designed the experiments. Z.Y., X.W., X.C., F.Z., J.G., and P.M. performed the experiments. Z.Y., J.Z., J.N., X.P., and G.L. analyzed the data. Z.Y., X.P., and G.L. wrote the manuscript.
REFERENCES
- 1.Yamane D, McGivern DR, Masaki T, Lemon SM. 2013. Liver injury and disease pathogenesis in chronic hepatitis C. Curr Top Microbiol Immunol 369:263–288. [DOI] [PubMed] [Google Scholar]
- 2.Larrat S, Vallet S, David-Tchouda S, Caporossi A, Margier J, Ramiere C, Scholtes C, Haim-Boukobza S, Roque-Afonso AM, Besse B, Andre-Garnier E, Mohamed S, Halfon P, Pivert A, LeGuillou-Guillemette H, Abravanel F, Guivarch M, Mackiewicz V, Lada O, Mourez T, Plantier JC, Baazia Y, Alain S, Hantz S, Thibault V, Gaudy-Graffin C, Bouvet D, Mirand A, Henquell C, Gozlan J, Lagathu G, Pronier C, Velay A, Schvoerer E, Trimoulet P, Fleury H, Bouvier-Alias M, Brochot E, Duverlie G, Maylin S, Gouriou S, Pawlotsky JM, Morand P. 2015. Naturally occurring resistance-associated variants of hepatitis C virus protease inhibitors in poor responders to pegylated interferon-ribavirin. J Clin Microbiol 53:2195–2202. doi: 10.1128/JCM.03633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlotsky JM. 2014. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. 2015. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol 62:S87–S99. doi: 10.1016/j.jhep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 5.André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Brechot C, Paranhos-Baccala G, Lotteau V. 2002. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol 76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calattini S, Fusil F, Mancip J, Dao Thi VL, Granier C, Gadot N, Scoazec JY, Zeisel MB, Baumert TF, Lavillette D, Dreux M, Cosset FL. 2015. Functional and biochemical characterization of hepatitis C virus (HCV) particles produced in a humanized liver mouse model. J Biol Chem 290:23173–23187. doi: 10.1074/jbc.M115.662999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuhara T, Ono C, Puig-Basagoiti F, Matsuura Y. 2015. Roles of lipoproteins and apolipoproteins in particle formation of hepatitis C virus. Trends Microbiol 23:618–629. doi: 10.1016/j.tim.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr, Ye J. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci U S A 104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenbach BD, Rice CM. 2013. The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol 11:688–700. doi: 10.1038/nrmicro3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. 2006. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol 80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen SU, Bassendine MF, Martin C, Lowther D, Purcell PJ, King BJ, Neely D, Toms GL. 2008. Characterization of hepatitis C RNA-containing particles from human liver by density and size. J Gen Virol 89:2507–2517. doi: 10.1099/vir.0.2008/000083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul D, Madan V, Bartenschlager R. 2014. Hepatitis C virus RNA replication and assembly: living on the fat of the land. Cell Host Microbe 16:569–579. doi: 10.1016/j.chom.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer A, Dumans A, Beaumont E, Etienne L, Roingeard P, Meunier JC. 2014. The association of hepatitis C virus glycoproteins with apolipoproteins E and B early in assembly is conserved in lipoviral particles. J Biol Chem 289:18904–18913. doi: 10.1074/jbc.M113.538256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catanese MT, Uryu K, Kopp M, Edwards TJ, Andrus L, Rice WJ, Silvestry M, Kuhn RJ, Rice CM. 2013. Ultrastructural analysis of hepatitis C virus particles. Proc Natl Acad Sci U S A 110:9505–9510. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuisson J, Cosset FL. 2014. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol 61:S3–S13. doi: 10.1016/j.jhep.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Phillips MC. 2014. Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life 66:616–623. doi: 10.1002/iub.1314. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu Y, Hishiki T, Ujino S, Sugiyama K, Funami K, Shimotohno K. 2011. Lipoprotein component associated with hepatitis C virus is essential for virus infectivity. Curr Opin Virol 1:19–26. doi: 10.1016/j.coviro.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Sundaram M, Yao Z. 2012. Intrahepatic role of exchangeable apolipoproteins in lipoprotein assembly and secretion. Arterioscler Thromb Vasc Biol 32:1073–1078. doi: 10.1161/ATVBAHA.111.241455. [DOI] [PubMed] [Google Scholar]
- 19.Albecka A, Belouzard S, Op de Beeck A, Descamps V, Goueslain L, Bertrand-Michel J, Terce F, Duverlie G, Rouille Y, Dubuisson J. 2012. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology 55:998–1007. doi: 10.1002/hep.25501. [DOI] [PubMed] [Google Scholar]
- 20.Chang KS, Jiang J, Cai Z, Luo G. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J Virol 81:13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. 2012. Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol 86:7256–7267. doi: 10.1128/JVI.07222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, Wu X, Tang H, Luo G. 2013. Apolipoprotein E mediates attachment of clinical hepatitis C virus to hepatocytes by binding to cell surface heparan sulfate proteoglycan receptors. PLoS One 8:e67982. doi: 10.1371/journal.pone.0067982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, McCormick KD, Zhao W, Zhao T, Fan D, Wang T. 2012. Human apolipoprotein E peptides inhibit hepatitis C virus entry by blocking virus binding. Hepatology 56:484–491. doi: 10.1002/hep.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long G, Hiet MS, Windisch MP, Lee JY, Lohmann V, Bartenschlager R. 2011. Mouse hepatic cells support assembly of infectious hepatitis C virus particles. Gastroenterology 141:1057–1066. doi: 10.1053/j.gastro.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Owen DM, Huang H, Ye J, Gale M Jr. 2009. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology 394:99–108. doi: 10.1016/j.virol.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Mahley RW. 2014. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis 72(Part A):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen D, Dhanasekaran P, Phillips MC, Lund-Katz S. 2009. Molecular mechanism of apolipoprotein E binding to lipoprotein particles. Biochemistry 48:3025–3032. doi: 10.1021/bi9000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 29.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. 2005. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillard BK, Lin HY, Massey JB, Pownall HJ. 2009. Apolipoproteins A-I, A-II and E are independently distributed among intracellular and newly secreted HDL of human hepatoma cells. Biochim Biophys Acta 1791:1125–1132. doi: 10.1016/j.bbalip.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merz A, Long G, Hiet MS, Brugger B, Chlanda P, Andre P, Wieland F, Krijnse-Locker J, Bartenschlager R. 2011. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem 286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin C, Nielsen SU, Ibrahim S, Bassendine MF, Toms GL. 2008. Binding of liver derived, low density hepatitis C virus to human hepatoma cells. J Med Virol 80:816–823. doi: 10.1002/jmv.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dao Thi VL, Granier C, Zeisel MB, Guerin M, Mancip J, Granio O, Penin F, Lavillette D, Bartenschlager R, Baumert TF, Cosset FL, Dreux M. 2012. Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps. J Biol Chem 287:31242–31257. doi: 10.1074/jbc.M112.365924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol 82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Luo G. 2009. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol 83:12680–12691. doi: 10.1128/JVI.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CC, Tsai P, Sun HY, Hsu MC, Lee JC, Wu IC, Tsao CW, Chang TT, Young KC. 2014. Apolipoprotein J, a glucose-upregulated molecular chaperone, stabilizes core and NS5A to promote infectious hepatitis C virus virion production. J Hepatol 61:984–993. doi: 10.1016/j.jhep.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Acosta EG, Stoeck IK, Long G, Hiet MS, Mueller B, Fackler OT, Kallis S, Bartenschlager R. 2014. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J Virol 88:12422–12437. doi: 10.1128/JVI.01660-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazumdar B, Banerjee A, Meyer K, Ray R. 2011. Hepatitis C virus E1 envelope glycoprotein interacts with apolipoproteins in facilitating entry into hepatocytes. Hepatology 54:1149–1156. doi: 10.1002/hep.24523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuhara T, Wada M, Nakamura S, Ono C, Shiokawa M, Yamamoto S, Motomura T, Okamoto T, Okuzaki D, Yamamoto M, Saito I, Wakita T, Koike K, Matsuura Y. 2014. Amphipathic alpha-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles. PLoS Pathog 10:e1004534. doi: 10.1371/journal.ppat.1004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cun W, Jiang J, Luo G. 2010. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol 84:11532–11541. doi: 10.1128/JVI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fauvelle C, Felmlee DJ, Crouchet E, Lee J, Heydmann L, Lefevre M, Magri A, Hiet MS, Fofana I, Habersetzer F, Foung SK, Milne R, Patel AH, Vercauteren K, Meuleman P, Zeisel MB, Bartenschlager R, Schuster C, Baumert TF. 2016. Apolipoprotein E mediates evasion from hepatitis C virus neutralizing antibodies. Gastroenterology 150:206–217 e4. doi: 10.1053/j.gastro.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Younossi ZM, Elsheikh E, Stepanova M, Gerber L, Nader F, Stamm LM, Brainard DM, McHutchinson JG. 2015. Ledipasvir/sofosbuvir treatment of hepatitis C virus is associated with reduction in serum apolipoprotein levels. J Viral Hepat 22:977–982. doi: 10.1111/jvh.12448. [DOI] [PubMed] [Google Scholar]
- 44.Bartosch B, Vermey G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. 2005. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol 79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]