ABSTRACT
Unprotected sexual intercourse with HIV-infected men is the major cause of new infections. HIV virions are released into semen by various cells of the male genital tract, as well as by infected monocytes and lymphocytes present in semen. Some of these virions may attach to the surfaces of cells, infected or uninfected. We investigated whether cells carrying attached HIV on their surfaces can transmit infection. We addressed this question in a model system of human tissue exposed ex vivo to monocytes and lymphocytes carrying HIV on their surfaces. We gamma irradiated the cells to prevent their productive infection. In spite of comparable amounts of HIV attached to monocytes and lymphocytes, only monocytes were capable of transmitting infection and triggering productive infection in tissue. This HIV-1 transmission was mediated by cell-cell contacts. Our experiments suggest that in vivo, HIV attached to infected or uninfected monocytes, which far outnumber lymphocytes in HIV-infected semen, may contribute to sexual transmission of HIV from men to their partners.
IMPORTANCE The vast majority of new HIV infections occur through sexual transmission, in which HIV is transferred from the semen of an infected male to an uninfected partner. In semen, HIV-1 particles may exist as free-floating virions; inside infected cells; or attached to the surfaces of cells, whether they are infected or not. Here, we investigated whether HIV attached to the surfaces of monocytes or lymphocytes could transmit infection to human tissue. Incubation of human tissue with monocyte-attached HIV resulted in productive tissue infection. In contrast, there was no infection of tissues when they were incubated with lymphocyte-attached HIV-1. Our results highlight the important role that seminal monocytes may play in HIV transmission in vivo, especially since monocytes far outnumber lymphocytes in the semen of HIV-infected individuals.
INTRODUCTION
Transmission of human immunodeficiency virus (HIV) occurs mainly through unprotected sexual intercourse, in which seminal HIV is deposited on rectal or vaginal mucosa (1–3). The probability of HIV transmission positively correlates with the viral load in semen and varies from 1/200 to 1/2,000 for male-to-female transmission and from 1/10 to 1/1,600 for male-to-male transmission (4). In semen, HIV exists in different forms: (i) free-floating viral particles, (ii) viruses in infected cells (in particular monocytes and lymphocytes [5–8]), and (iii) viral particles that after being released by infected cells reattached to the surfaces of cells, infected or uninfected.
Earlier, it was shown that both cell-free HIV and infected cells are capable of transmitting infection in ex vivo models and in nonhuman primates (9, 10), although the relative contributions of these two pools to HIV transmission remain a matter of debate (reviewed in references 6 and 11). In particular, whether virus attached to the surfaces of seminal cells of HIV-infected individuals can transfer HIV infection is not clear. Since many of the seminal cells express various HIV receptors or express a wide variety of surface molecules involved in HIV attachment (2, 12, 13), there are reasons to consider these cells vehicles for HIV sexual transmission from an infected male to his uninfected partner, as has been suggested with regard to spermatozoa (14).
Here, we address this question by comparing tissue infections ex vivo with cell-free HIV and with cell-attached HIV. In particular, we investigated whether HIV adsorbed at the surfaces of lymphocytes or monocytes, two major types of cells present in the semen of infected individuals, can transmit infection to human tissue ex vivo. We found that in this model, only HIV attached to monocytes, but not that attached to lymphocytes, was able to transmit infection, and the transmitted virus replicated in the recipient tissue similarly to cell-free HIV. Cell-cell contacts between monocytes and target cells were needed for effective HIV transmission.
MATERIALS AND METHODS
Tonsil culture.
Human lymphoid tissues obtained according to an institutional review board (IRB)-approved protocol were surgically removed during routine tonsillectomies at Children's National Medical Center (Washington, DC). They were received within 5 h of excision and were dissected into 2- to 3-mm blocks. The tissue blocks were deposited on a sponge surface at the air-liquid interface and cultured for 15 days with a change of medium every 3 days, as previously described (15).
HIV-1 replication in HIV-inoculated tissue was measured as the production of p24 core antigen released into the medium by means of an HIV-1 p24 Luminex bead assay (16). At each time point postinfection, the amount of p24 produced by the tissues was normalized to the amount of p24 produced in donor-matched tissue infected with cell-free virus. Experiments were repeated n times, each time with tissue from a different donor; the value of n is indicated in the text and figure legends. Graphs were generated and statistical analysis was performed with GraphPad (La Jolla, CA) Prism v5.
Cells and viral adsorption.
Human leukocytes were obtained by leukapheresis from healthy donors, followed by an elutriation procedure to purify monocytes and lymphocytes (all performed at the Department of Transfusion Medicine Clinical Center, National Institutes of Health, Bethesda, MD, USA). Contaminating erythrocytes were removed from elutriated cells (purity, ∼90%, as determined with an automatic blood-counting machine) by centrifugation (400 × g; 30 min) in a Ficoll gradient. Both lymphocytes and monocytes were exposed to 25 Gy of gamma irradiation in a cesium-137 blood irradiator (IBL 437C; CIS Diagnostik). Cell counting was performed in a hemocytometer, and cell viability was evaluated by means of trypan blue (0.1%) exclusion. Our population of lymphocytes was a total population from blood and therefore contained CD4 and CD8 T lymphocytes, as well as B cells.
Trypsin and heparinases were purchased from Thermo-Fisher Scientific (Rockville, MD) and from Sigma-Aldrich (St. Louis, MO), respectively. Anti-CD4 and anti-DC-SIGN were purchased from Beckman-Coulter (Brea, CA) and from Abcam (Cambridge, UK), respectively. Anti-human α4β7 integrin (Act-1) antibody (no. 11718) was obtained from A. A. Ansari through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH.
Viral infection.
Viral attachment to cells was performed similarly on both elutriated lymphocytes and monocytes. Briefly, 3 million monocytes or lymphocytes were incubated with 250 μl of R5-tropic HIV-1BaL (which corresponds to 30 ng of p24) at 4°C to avoid virus internalization, as previously described (17). After 2 h of incubation, the cells were washed twice with cold phosphate-buffered saline (PBS) to remove nonattached viruses. To determine the amounts of attached virus on monocytes and lymphocytes, cells were pelleted and resuspended in PBS containing 10% Triton X-100. The HIV-1 p24 content was measured as described above. In each experiment, 3 μl of cells with HIV adsorbed on their surfaces was deposited on top of each tonsil tissue block. Since the amounts of virus attached to the surfaces of monocytes and lymphocytes varied among patients and because we were limited by the number of cells we could add to the tissue blocks, we performed tissue infection using 15 to 20 pg of HIV p24 of cell-associated virus (which corresponds to 4 × 105 to 7 × 105 cells per block). For each donor, a control experiment was performed with an amount of cell-free HIV matching the amount of cell-associated HIV used in the infection experiments.
The TZM-bl cell line, generously donated by John C. Kappes and Xiaoyun Wu, was obtained through the NIH AIDS Reagent Program (18). Cells (2 × 104) in 90 μl of complete medium (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% fetal bovine serum [FBS]) were plated in a 96-well black plate (Sigma-Aldrich, St. Louis, MO) and placed in the incubator for 6 h. We then added 10 μl (corresponding to 15 pg of p24) of either cell-free or cell-associated HIV-1BaL to each well, and the plate was kept in the incubator for 3 days. We added 100 μl of 2× substrate, Bright Glo luciferase buffer (Promega, Madison, WI), to each well. The luminescence was quantified with a Saphire 2 luminometer (Tecan, Switzerland) and shown as relative fluorescence units (RFU). A 2-fold dilution curve of cell-free HIVBaL (in 10 μl) ranging from 2.5 to 320 pg of p24 was built for each experiment. Generally, the limit of HIV detection in the assay was 5 pg of p24 per well. Each condition was tested in six replicates, and the means of luminescence and standard errors of the mean (SEM) were plotted in graphs.
In the transwell experiments, 40 μl of TZM-bl cells (2 × 104 cells) was added to the bottom of a 96-well black plate. Then, 50 μl of lymphocytes or monocytes with attached virus (15 pg of p24) in direct contact with the TZM-bl cells or separated by a virus-permeable membrane (5-μm pores [Corning]) was added. A 2-fold dilution of cell-free HIVBaL was also performed in each transwell assay. After 3 days in culture, the luminescence was quantified as described above. A dose-response curve was plotted for each experiment, and we observed a direct correlation (R2 = 0.99) between the virus concentration and luminescence.
Cytometry.
To assess HIV-infected lymphocytes in human tissues ex vivo, we isolated tonsillar cells by treating them with Liberase DL as previously described (19) and stained them with a LIVE/DEAD dye (Invitrogen) and a combination of the following fluorescence-labeled monoclonal antibodies: anti-human CD3-Pacific Blue (PB), anti-human CD4-QD605, anti-human CD8-QD705, anti-human CD45-allophycocyanin (APC) (Caltag Laboratories, Burlingame, CA), and anti-p24-phycoerythrin (PE) (Beckman Coulter, Brea, CA). Cells were acquired on a BD LSRII flow cytometer equipped with 355-, 407-, 488-, 532-, and 638-nm laser lines (BD Biosciences, San Jose, CA).
To assess cellular proteins incorporated into HIV, virions produced by tonsillar tissue exposed to irradiated monocytes with adsorbed virus or by purified macrophages were captured with anti-2G12 magnetic nanoparticles (MNPs), as previously described (20). Briefly, 15-nm iron oxide MNPs (Ocean NanoTech, Springdale, AR) were coupled with 1 mg of 2G12 antibodies (Polymun Scientific, Austria) according to the manufacturer's protocol. To capture viruses, labeled 2G12-MNPs were incubated with HIV preparations at 37°C for 40 min, and then, complexes with captured virus (HIV-2G12-MNPs) were stained either with anti-CD36-PE antibodies (BioLegend, San Diego, CA) or with the isotype control antibody mouse IgG2a-PE (BioLegend). The resultant complexes were separated from unbound fluorescent antibodies on a magnetic column (Miltenyi Biotech, Germany) in a strong magnetic field generated by an OctoMacs magnet (Miltenyi Biotech) and washed three times with 500 μl of washing buffer (0.5% bovine serum albumin, 2 mM EDTA in PBS). To elute the captured and stained viruses, the column was removed from the magnet and demagnetized, and the complexes were eluted in 400 μl of PBS and fixed with a solution of 1% paraformaldehyde (PFA). The eluted complexes were analyzed on the LSRII cytometer triggering on fluorescence.
RESULTS
HIV-1 attached to monocyte surfaces, but not to lymphocytes, transmitted infection to human tissue ex vivo.
We compared the abilities of HIV attached to lymphocytes and HIV attached to monocytes to be transmitted to human lymphoid tissue ex vivo. We irradiated lymphocytes and monocytes to prevent productive infection of the cells and incubated them with an HIVBaL suspension as described in Materials and Methods. After removing the unattached viral particles by cell centrifugation, we estimated the amount of virus attached at the cell surface. Trypsin treatment removed similar fractions of virus from lymphocytes and monocytes (89.1% ± 1.7% and 88.1% ± 2.5%, respectively; n = 4; P = 0.76), showing that most of the virus was indeed attached at the surfaces of irradiated lymphocytes or monocytes.
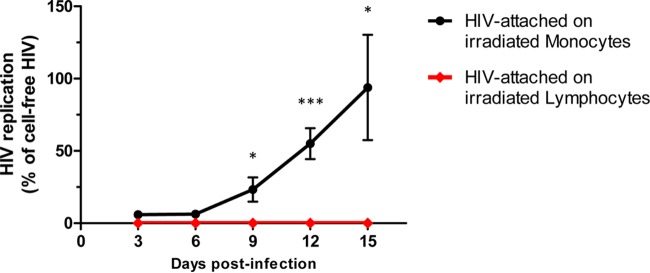
Similar amounts of p24 of cell-attached viruses (as well as cell-free viruses as a control) were then applied on top of blocks of human tissue. When the tissues were exposed to free HIVBaL or HIVBaL attached to monocytes, lymphoid tissues became productively infected (Fig. 1). The cumulative HIV p24 at day 15 postinfection in tissues from different donors varied from 2 to 13 ng/ml in tissues exposed to cell-free HIVBaL and from 3 to 11 ng/ml in tissues exposed to monocyte-attached HIVBaL. There was no replication observed in tissue inoculated with lymphocyte-attached HIVBaL. The replication in tissue inoculated with monocyte-attached HIV was statistically significantly different from that in tissue inoculated with lymphocyte-attached HIV (Fig. 1).
FIG 1.
HIV-1 production by tissues exposed to either lymphocyte- or monocyte-associated HIV-1. Three million monocytes or lymphocytes were incubated with 250 μl of R5-tropic HIV-1BaL (corresponding to 30 ng of p24) at 4°C for 2 h. Donor-matched human lymphoid tissues were then exposed to HIV attached to irradiated lymphocytes or monocytes. Control matched tissues were inoculated with a similar amount of cell-free HIV. The culture medium was changed every 3 days. HIV replication was evaluated from the amount of p24 released into the medium. Shown is HIV replication in tissues inoculated with cell-associated HIV normalized to those inoculated with cell-free HIV (cell-free HIV was set as 100% at each depicted point). Means ± SEM of data obtained from six donors are presented. The asterisks denote statistical differences in HIV replication in tissues inoculated with monocyte- and lymphocyte-attached HIV (*, P < 0.05; ***, P < 0.001).
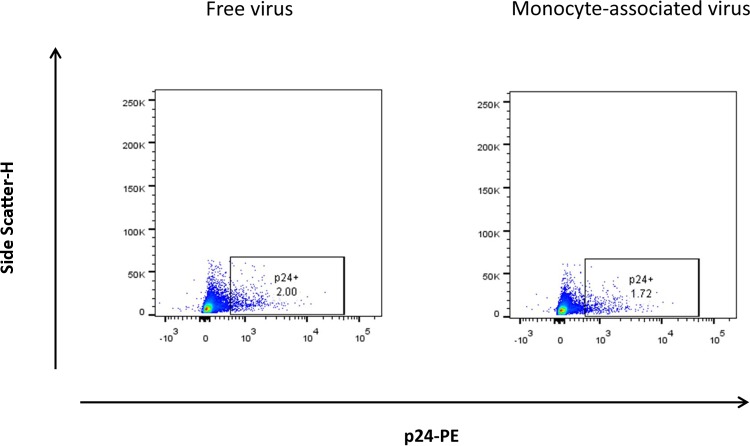
In contrast to monocytes, HIVBaL attached at the surfaces of lymphocytes did not transmit infection (Fig. 1). In early days postexposure, tissues exposed to cell-free HIVBaL produced more HIV than tissues exposed to HIVBaL attached to monocytes, as evaluated by measurement of p24 in the culture medium. After 15 days of infection, however, HIV production levels were similar (Fig. 1). These results were confirmed with flow-cytometric analysis of the tissue infection at day 15 postinfection, as the numbers of T lymphocytes (CD3+) positive for intracellular p24 were similar in tissues exposed to cell-free and to monocyte-associated virus (Fig. 2).
FIG 2.
Infection of T cells in human lymphoid tissue exposed to monocyte-attached HIV. Fractions of HIV-infected T cells in tissue exposed to cell-free or to monocyte-associated virus were determined. Blocks of donor-matched tissue were inoculated with 15 pg p24 of monocyte-associated or cell-free HIV-1. On day 15 postinoculation, cells were isolated from the tissue and stained with a LIVE/DEAD kit for cell viability, as well as for surface CD45 and CD3 and for intracellular p24. Shown is the tissue analysis of the results of a representative experiment with two different donors.
Lymphocytes and monocytes attached similar amounts of HIV-1 on their surfaces.
To investigate whether the difference between the abilities of monocytes and lymphocytes to transmit HIV infection could be the result of different abilities of these cells to attach virions, we evaluated the numbers of virions attached to the cells. As shown in Fig. 3, on average, 51.8 pg of p24 per million lymphocytes was attached to the surfaces of the cells under our protocol. The number of virions attached to lymphocytes was not statistically different from that attached to monocytes: 46.3 pg of p24 per million monocytes (P = 0.66; n = 7). Thus, lymphocytes and monocytes bound similar amounts of HIVBaL at their surfaces.
FIG 3.
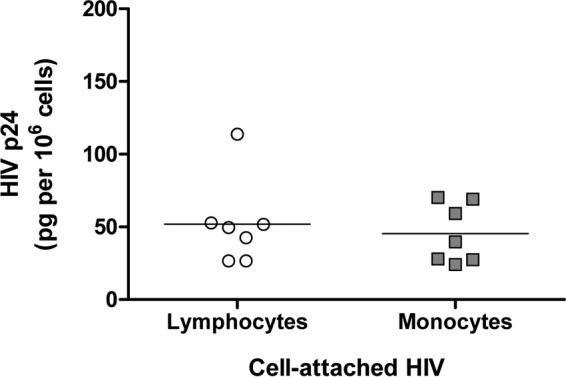
Attachment of HIV-1 to the surfaces of lymphocytes and monocytes. Three million irradiated human lymphocytes or monocytes were exposed to HIVBaL (250 μl, corresponding to 30 ng of p24) for 2 h at 4°C. Unattached virus was washed out by cell centrifugation, and the cell pellets were lysed with 10% Triton X-100. The concentrations of HIV p24 in the cell lysates were evaluated with a specific Luminex bead assay. Shown are the amounts of p24 for both lymphocytes and monocytes for seven donors. Horizontal bars represent the means.
To address the nature of virus-cell association, we investigated the roles of CD4, α4β7, DC-SIGN, and heparan sulfate (HS) in virus attachment to monocytes and lymphocytes. To achieve this goal, we preincubated irradiated monocytes and lymphocytes with blocking antibodies at 10 μg/ml or pretreated the irradiated cells with a mixture of heparinases I, II, and III (10 U/ml) (14, 21).
Following the treatment with either blocking antibodies or heparinases, we incubated cells with HIVBaL and compared HIV binding to treated cells with binding to untreated controls.
On average, anti-CD4, anti-α4β7 integrin, and anti-DC-SIGN reduced HIVBaL binding to lymphocytes by 1.5% ± 1%, 4.3% ± 2%, and 1.8% ± 0.9% (P > 0.05; n = 6), respectively. Similarly, anti-CD4 and anti-α4β7 integrin did not reduce HIVBaL binding to monocytes, and anti-DC-SIGN reduced HIVBaL binding to monocytes by only 3.4% ± 2% (P > 0.05; n = 6). In contrast, heparinase treatment reduced HIVBaL binding to lymphocytes and monocytes, respectively, by 63.1% ± 10.3% and 72.6% ± 8.1% (P = 0.02 and P = 0.01; n = 6).
Thus, irradiated lymphocytes and monocytes attached equal amounts of virus to their surfaces predominantly through HS rather than specific HIV receptors.
Viruses attached to monocytes rather than released viruses transmitted infection.
In principle, the virus that monocytes transmit to tissue may not be the one constantly attached to the cell surface but rather some virus released during incubation with the tissue. The source of this virus that essentially became free may be the pool that detached from the cell surface or may be a potential residual productive infection persisting in spite of gamma irradiation of monocytes.
To evaluate the first possibility, we determined the numbers of attached virions that detached and become free during cell incubation with the tissue. We cultured irradiated lymphocytes or monocytes with attached HIVBaL for 3 days without tissue and quantified the amounts of virus (measured as p24) released in the medium in 3 days at 37°C. As shown in Fig. 4A, there was no statistically significant difference (P = 0.35; n = 6) between the amounts of p24 released by lymphocytes and monocytes (18% ± 5% and 28% ± 9%, respectively). Next, we assessed whether this released virus was infectious for human tissue ex vivo and for TZM-bl cells. We found no productive infection of tonsils (n = 3) (data not shown) when lymphoid tissues were incubated with the amount of HIVBaL released after 3 days in culture, despite the fact that the viruses were infectious to TZM-bl cells (Fig. 4B). Thus, virions that spontaneously detached from monocytes were infectious but were not able to transmit tissue infection.
FIG 4.
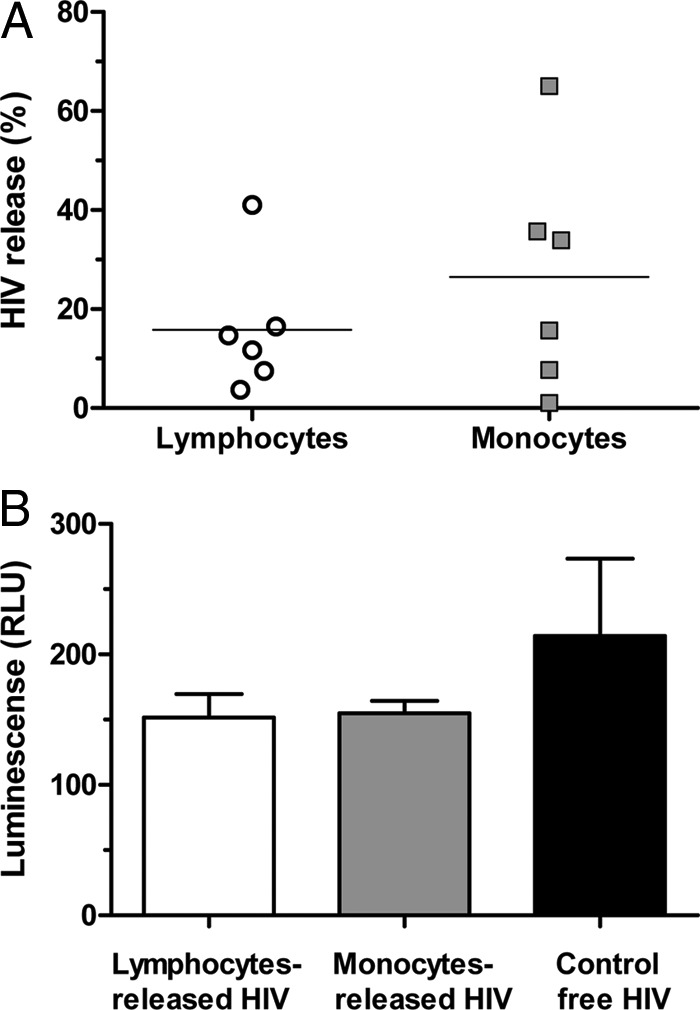
(A) Infectivity of HIV released from the surfaces of lymphocytes and monocytes. Three million monocytes or lymphocytes were incubated with 250 μl of R5-tropic HIV-1BaL (corresponding to 30 ng of p24) at 4°C for 2 h. Irradiated lymphocytes or monocytes with attached HIVBaL on their surfaces were cultured for 3 days. Shown are the fractions of HIV p24 released by lymphocytes and by monocytes relative to the amount of initially attached HIV p24 from six different donors. (B) On day 3, the cells were centrifuged, and the supernatants were analyzed for HIV p24. The released virus and free virus at the same concentration (control) were applied to TZM-bl cells. Infection was revealed in TZM-bl cells by luminescence after substrate interaction with cell-expressed luciferase. The plotted values are presented as means of luminescence substracted from background (noninfected controls) plus SEM (n = 3).
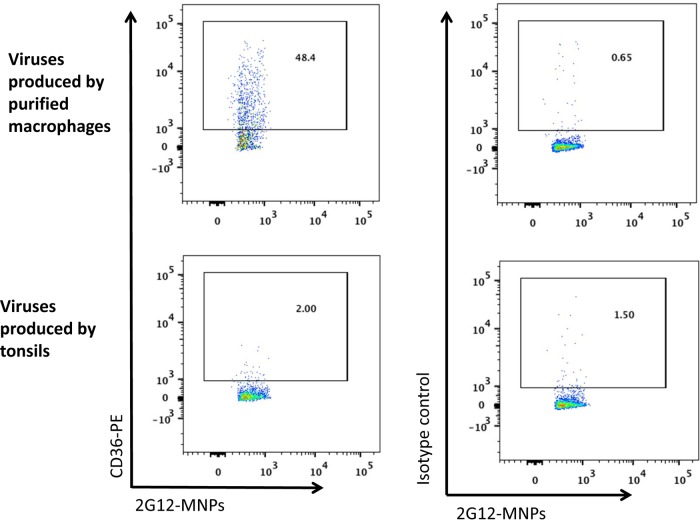
To evaluate whether virions that may be released in the course of potential productive infection of monocytes can be responsible for the transmission of infection, we performed the following control experiments. (i) Instead of R5 HIVBaL, we attached X4 HIVLAI.04, which does not replicate in monocytes (22, 23). We incubated monocyte-attached X4 HIVLAI.04 with lymphoid tissue blocks and found that these monocytes transmitted infection to the tissue, with tissue cells being productively infected (data not shown). (ii) We evaluated the phenotype of the virus produced by the tissue exposed to HIVBaL attached to monocytes. Virions produced by various cells carry some of the cell membrane proteins (24, 25). In particular, viruses produced by monocytes carry CD36, a protein expressed by these cells but not by T lymphocytes (25, 26). Using the technique of flow virometry (20), we found that HIVBaL produced by the tissues exposed to HIVBaL attached to the surfaces of monocytes was CD36 negative (Fig. 5).
FIG 5.
Phenotypic analysis of virions produced by human lymphoid tissue exposed to monocyte-attached HIV. Virions produced by human lymphoid tissue exposed to monocyte-attached HIV were collected on day 15 postinoculation, captured with MNPs coupled with HIV anti-gp120 antibody (2G12), and analyzed for the presence of CD36 by means of flow virometry. (Bottom) Viruses produced by exposed tissue were stained with fluorescent anti-CD36 antibody (left) or with isotype control antibody (right). (Top) Virions from purified macrophages were stained with anti-CD36 antibody (left) or with isotype control antibody (right). Shown are histograms of the results of one of two similar experiments.
Cell contact between monocyte-attached HIV and cell targets was required to efficiently transfer infection to tissues.
We used a transwell system to check whether cell contact was needed for viral transfer from monocytes to target cells. For this purpose, HIVBaL attached to lymphocytes or monocytes was cocultured with TZM-bl cells in a system separated by a virus-permeable membrane (5-μm pore size). TZM-bl cells became infected from contact with monocytes carrying HIVBaL but not from contact with lymphocytes carrying HIVBaL (lymphocytes in contact with TZM-bl cells, 335 ± 3.5 RFU; background noninfected controls, 311 ± 10.3 RFU; P = 0.17), similar to what we observed in lymphoid tissue. Conversely, impeding contact between transmitters (monocytes) and target cells (TZM-bl cells) by means of the transwell chamber abolished TZM-bl cell infection (Fig. 6). This result cannot be attributed to an inability of the virus to cross the 5-μm-pore-size membrane, as cell-free virus added to the upper chamber was able to cross the membrane and infect TZM-bl cells in a dose-dependent manner (data not shown).
FIG 6.
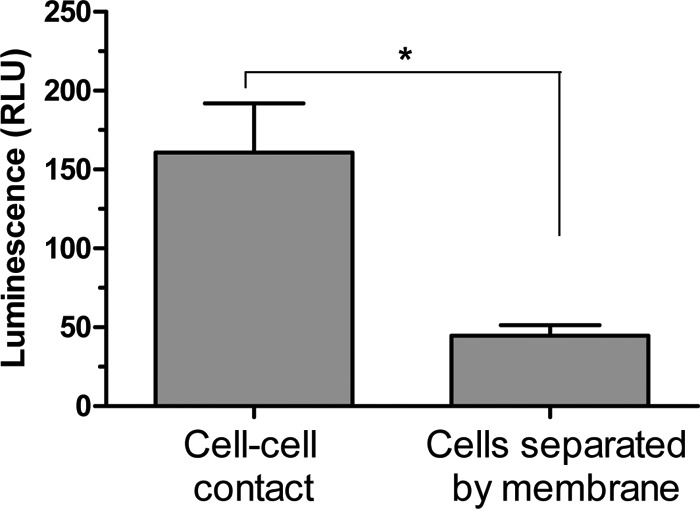
Transmission of monocyte-attached HIV through cell-cell contacts. Irradiated monocytes with 15 pg of HIVBaL p24 attached to their surfaces were cultured with TZM-bl cells either in direct contact or in different chambers separated by a virus-permeable membrane (transwell system). Infection was evaluated as luminescence, as described in the legend to Fig. 4. Shown are means and SEM of six replicates.
DISCUSSION
Sexual HIV transmission from men to their uninfected partners is mediated by HIV present in semen, where HIV exists in cell-free and in cell-associated forms, predominantly in infected lymphocytes and monocytes (6). However, it is not entirely clear whether these cells actually transfer HIV directly to target cells through cell-cell contacts or whether the transmission occurs through the free virus released by infected cells. Also, virus released from HIV-infected cells can attach to uninfected cells, which may serve as vehicles for HIV transmission.
In the present work, we examined HIV transmission to human tissue exposed ex vivo either to cell-free HIV suspension or to HIV attached to the surfaces of monocytes or lymphocytes, the two major types of immune cells found in semen (6, 27). We irradiated the cells to prevent the release of HIV, which would act as cell-free virus in our system. Trypsin treatment of the cells to which HIV was attached removed over 90% of the viruses, indicating that virions were attached to proteins on the cell surface. The majority of virions seemed to attach through cell surface HS, as cell pretreatment with heparinases prevented HIV adsorption. HSs are glycosaminoglycans found on cell surfaces that have been identified as a major source of HIV binding in epithelial cells (21), spermatozoa (14), and leukocytes, including monocytes and lymphocytes (28–30; reviewed in references 31 and 32).
In most of our experiments, we used HIVBaL, a prototypical HIV of CCR5 phenotype that is predominantly transmitted through sexual intercourse (33). Importantly, the amount of virus in the inoculum used in our experiments (15 to 20 pg) corresponds to the amount of HIV found in the semen of some HIV-infected men (considering that 1 pg of HIV p24 is equivalent to 12,500 copies of HIV RNA) (34–36). Moreover, we used human lymphoid tissue rather than single-cell cultures to assess the ability of cell-attached HIV to transmit and establish infection. These ex vivo tissues preserve their in vivo cytoarchitecture and do not require artificial activation/stimulation to become productively infected (15), thus simulating in vivo transmission more adequately.
First, we showed that HIV attached to proteins on the cell membranes of both primary human lymphocytes and monocytes. However, upon incubation with lymphoid tissue ex vivo, only HIV attached to monocytes, but not that attached to lymphocytes, readily induced productive infection in the recipient tissues. Transmission of monocyte-attached HIV in donor-matched ex vivo tissues seemed to be less efficient than that of control free virus in the first days, as evaluated from virus replication in the recipient tissue. However, the levels of replication became similar after 15 days of culture.
In the next series of experiments, we attempted to explain the dramatic difference between the abilities of lymphocytes and monocytes to transmit infection to lymphoid tissue. First, we excluded some of the trivial reasons that might explain this difference. We showed that (i) the numbers of virions attached to monocytes and to lymphocytes were similar; (ii) the numbers of virions that might spontaneously detach from monocytes and lymphocytes over the 3 days of incubation were also comparable; and (iii) in any event, the numbers of detached virions were not sufficient to infect tissue. Thus, the differential transmission abilities of the two cell types were not the result of different amounts of attached or detached virus.
Although we irradiated monocytes to prevent them from producing virus after differentiation into macrophages, we nevertheless confirmed that HIV detected in tissue culture came from the tissue cells rather than being produced by monocytes/macrophages. We demonstrated that (i) virions collected from infected tissue did not carry CD36, a marker of macrophage-produced HIV (25, 26), and (ii) when HIVLAI.04, a CXCR4-tropic HIV strain not able to replicate in monocytes/macrophages, was attached to monocytes, HIV infection was nonetheless transferred to human tissues ex vivo and virus replicated similarly to what was observed with CCR5-tropic HIVBaL.
Finally, we investigated whether cell-cell contacts between monocytes and recipient tissue cells were needed to effectively transmit cell surface-attached HIV. To achieve this goal, we compared the transmission of lymphocyte- and monocyte-attached HIV to TZM-bl cells directly or through a 5-μm transwell membrane, which was permeable to HIV but not to cells. We found that lymphocyte-attached HIV was not transmitted to TZM-bl cells regardless of whether lymphocytes and TZM-bl cells were in direct contact or separated by the membrane. However, TZM-bl cells became infected only when they were in direct contact with monocyte-attached HIV. When cells were physically separated by the membrane, no transmission was observed. Thus, cell-cell contacts are required for HIV-attached monocytes to be transmitted.
How can our results be extrapolated to in vivo conditions? Our ex vivo experiments suggest that cells do not have to be productively infected to contribute to HIV infection, and therefore, in vivo, HIV attached to infected or uninfected cells may contribute to HIV spread. Different cells may differ in this capability, as we found that monocytes, but not lymphocytes, were able to efficiently transfer infection to lymphoid tissues in our experimental model. Similar findings were reported for granulocytes. In a recent study, Jiang et al. showed that only viruses harbored on the surfaces of basophils, but not eosinophils or neutrophils, were transferred to T cells (37). Monocytes and basophils are not the only cell types that capture HIV at their surfaces and transfer it to target cells, as it was shown that both human genital epithelial cells and spermatozoa capture HIV-1 through HS and efficiently transmit the virus (14, 21). Whether HIV is transmitted to dendritic cells (DCs) or T cells (14, 21) or to TZM-bl cells, as in our study, cell-cell contact is required for an efficient transfer of HIV from donor to target cells.
Here, we were not able to determine why lymphocytes did not transmit HIV infection. One of the possible explanations why, in contrast to monocytes, lymphocytes did not transmit HIV infection may be related to the ability of monocytes to establish close contact with target cells through lamellipodia, increasing the probability of transmission of surface-attached HIV (38, 39). In particular, Groot et al. showed that HIV-infected macrophages can transiently interact with noninfected CD4+ T cells and efficiently transfer virus after being cocultured for as little as 1 h (38). On the basis of the numbers of macrophages and T cells interacting with each other over time, it was estimated that each productively infected macrophage is able to infect at least one T cell every 6 h (38). In contrast to monocytes, lymphocytes are less able to establish adhesion contact (40, 41), suggesting that they are less likely to transmit HIV to recipient cells.
The strategy used in the present work has several advantages: (i) we assessed the efficiency of HIV transmission in the tissue that permits viral replication, rather than mere transfer of the virus to the recipient cells; (ii) we focused exclusively on the role of HIV attached to cells, thus excluding possible transmission by the cell-free virus; and (iii) in contrast to many published experiments, the amount of virus used for infection in this study was close to the physiological amount of HIV in semen. On the other hand, our work has significant limitations. (i) We were limited to the use of lymphoid rather than cervicovaginal tissue, which would be even more relevant to transmission, because the latter has considerably fewer target cells for HIV infection (one of the possible reasons for the relatively low rate of HIV transmission in vivo) and therefore requires a much higher inoculum. (ii) We do not know whether virions, which bud from the infected cells and remain on their surfaces, behave in the same way as the virus attached to cell surfaces investigated in our experiments. (iii) Although our results demonstrated a dramatic difference between the abilities of monocytes and lymphocytes to transmit infection, this difference should be confirmed in vivo with simian immunodeficiency virus (SIV) in nonhuman primates.
In conclusion, we compared cell-free and cell-attached viruses in regard to their abilities to infect lymphoid tissues and showed that monocyte-attached HIV is a form of cell-associated virus that is directly involved in HIV spread. Specifically, the pattern of HIV transmission described here may be important in vivo, since in the semen of HIV-infected individuals, monocytes far outnumber lymphocytes (6).
ACKNOWLEDGMENTS
V.B.-D.-S. was partially supported by fellowships from the Brazilian Ministry of Education/CAPES and from the Brazilian Ministry of Science and Technology/CNPq. A.A., S.Z., L.M., and C.V. were supported by the NICHD Intramural Program.
Funding Statement
This work, including the efforts of Victor Barreto-de-Souza, Anush Arakelyan, Sonia Zicari, Leonid Margolis, and Christophe Vanpouille, was funded by the NICHD Intramural Program. This work, including the efforts of Victor Barreto-de-Souza, was funded by the Brazilian Ministry of Education and the Brazilian Ministry of Science and Technology.
REFERENCES
- 1.Royce RA, Sena A, Cates W Jr, Cohen MS. 1997. Sexual transmission of HIV. N Engl J Med 336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 2.Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat Rev Immunol 8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L, Tang Y, Hobbs M, Hoffman I, Kazembe P, Jones CD, Borrow P, Fiscus S, Cohen MS, Swanstrom R, Center for HIV/AIDS Vaccine Immunology. 2010. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog 6:e1001053. doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvin SR, Cohen MS. 2004. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 5.Quayle AJ, Xu C, Mayer KH, Anderson DJ. 1997. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis 176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. 2010. Targeting Trojan Horse leukocytes for HIV prevention. AIDS 24:163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poli G. 2013. Cell-to-cell vs. cell-free HIV-1 transmission from macrophages to CD4+ T lymphocytes: lessons from the virology textbook. AIDS 27:2307–2308. doi: 10.1097/QAD.0b013e328363619a. [DOI] [PubMed] [Google Scholar]
- 8.Politch JA, Marathe J, Anderson DJ. 2014. Characteristics and quantities of HIV host cells in human genital tract secretions. J Infect Dis 210(Suppl 3):S609–S615. doi: 10.1093/infdis/jiu390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, Ho DD. 1996. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol 70:3098–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. 2010. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis 202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 11.Barreto-de-Souza V, Arakelyan A, Margolis L, Vanpouille C. 2014. HIV-1 vaginal transmission: cell-free or cell-associated virus? Am J Reprod Immunol 71:589–599. doi: 10.1111/aji.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brogi A, Presentini R, Moretti E, Strazza M, Piomboni P, Costantino-Ceccarini E. 1998. New insights into the interaction between the gp120 and the HIV receptor in human sperm (human.sperm/gp120/galactoglycerolipid/antigalactosylceramide/seminolip id/spermatogonia). J Reprod Immunol 41:213–231. doi: 10.1016/S0165-0378(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, KewalRamani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceballos A, Remes Lenicov F, Sabatte J, Rodriguez Rodrigues C, Cabrini M, Jancic C, Raiden S, Donaldson M, Agustin Pasqualini R Jr, Marin-Briggiler C, Vazquez-Levin M, Capani F, Amigorena S, Geffner J. 2009. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. J Exp Med 206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grivel JC, Margolis L. 2009. Use of human tissue explants to study human infectious agents. Nat Protoc 4:256–269. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biancotto A, Brichacek B, Chen SS, Fitzgerald W, Lisco A, Vanpouille C, Margolis L, Grivel JC. 2009. A highly sensitive and dynamic immunofluorescent cytometric bead assay for the detection of HIV-1 p24. J Virol Methods 157:98–101. doi: 10.1016/j.jviromet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marechal V, Clavel F, Heard JM, Schwartz O. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J Virol 72:2208–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Introini A, Vanpouille C, Lisco A, Grivel JC, Margolis L. 2013. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog 9:e1003148. doi: 10.1371/journal.ppat.1003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakelyan A, Fitzgerald W, Margolis L, Grivel JC. 2013. Nanoparticle-based flow virometry for the analysis of individual virions. J Clin Invest 123:3716–3727. doi: 10.1172/JCI67042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Chen Z, Phillips DM. 2003. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ cells: implications for mechanisms of sexual transmission. J Infect Dis 188:1473–1482. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 22.Collman R, Hassan NF, Walker R, Godfrey B, Cutilli J, Hastings JC, Friedman H, Douglas SD, Nathanson N. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med 170:1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collman RG, Yi Y, Liu QH, Freedman BD. 2000. Chemokine signaling and HIV-1 fusion mediated by macrophage CXCR4: implications for target cell tropism. J Leukoc Biol 68:318–323. [PubMed] [Google Scholar]
- 24.Tremblay MJ, Fortin JF, Cantin R. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today 19:346–351. doi: 10.1016/S0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Roberts BD, Griffin GE, Folks TM, Butera ST. 2000. Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J Virol 74:139–145. doi: 10.1128/JVI.74.1.139-145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moniuszko M, Kowal K, Rusak M, Pietruczuk M, Dabrowska M, Bodzenta-Lukaszyk A. 2006. Monocyte CD163 and CD36 expression in human whole blood and isolated mononuclear cell samples: influence of different anticoagulants. Clin Vaccine Immunol 13:704–707. doi: 10.1128/CVI.00417-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, Cosma A, Dereuddre-Bosquet N, Le Grand R. 2013. Semen CD4+ T cells and macrophages are productively infected at all stages of SIV infection in macaques. PLoS Pathog 9:e1003810. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses 9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 29.Ohshiro Y, Murakami T, Matsuda K, Nishioka K, Yoshida K, Yamamoto N. 1996. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol Immunol 40:827–835. doi: 10.1111/j.1348-0421.1996.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 30.Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol 75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallay P. 2004. Syndecans and HIV-1 pathogenesis. Microbes Infect 6:617–622. doi: 10.1016/j.micinf.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Connell BJ, Lortat-Jacob H. 2013. Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition. Front Immunol 4:385. doi: 10.3389/fimmu.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph SB, Swanstrom R, Kashuba AD, Cohen MS. 2015. Bottlenecks in HIV-1 transmission: insights from the study of founder viruses. Nat Rev Microbiol 13:414–425. doi: 10.1038/nrmicro3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, Cronin M, Rinaldo CR. 1997. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol 71:6271–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Politch JA, Tucker L, Mayer KH, Seage GR III, Anderson DJ. 1997. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis 176:941–947. doi: 10.1086/516539. [DOI] [PubMed] [Google Scholar]
- 36.Jennings C, Fiscus SA, Crowe SM, Danilovic AD, Morack RJ, Scianna S, Cachafeiro A, Brambilla DJ, Schupbach J, Stevens W, Respess R, Varnier OE, Corrigan GE, Gronowitz JS, Ussery MA, Bremer JW. 2005. Comparison of two human immunodeficiency virus (HIV) RNA surrogate assays to the standard HIV RNA assay. J Clin Microbiol 43:5950–5956. doi: 10.1128/JCM.43.12.5950-5956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang AP, Jiang JF, Guo MG, Jin YM, Li YY, Wang JH. 2015. Human blood-circulating basophils capture HIV-1 and mediate viral trans-infection of CD4+ T cells. J Virol 89:8050–8062. doi: 10.1128/JVI.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groot F, Welsch S, Sattentau QJ. 2008. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 39.Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, Bess JW Jr, Bavari S, Lowekamp BC, Bliss D, Lifson JD, Subramaniam S. 2010. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A 107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lidington EA, McCormack AM, Yacoub MH, Rose ML. 1998. The effects of monocytes on the transendothelial migration of T lymphocytes. Immunology 94:221–227. doi: 10.1046/j.1365-2567.1998.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CN, Chang SF, Lee PL, Chang K, Chen LJ, Usami S, Chien S, Chiu JJ. 2006. Neutrophils, lymphocytes, and monocytes exhibit diverse behaviors in transendothelial and subendothelial migrations under coculture with smooth muscle cells in disturbed flow. Blood 107:1933–1942. doi: 10.1182/blood-2005-08-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]