Summary
Quality of life is increasingly recognized as an important secondary endpoint of hematopoietic cell transplantation (HCT). The current study examined the extent to which attrition results in biased estimates of patient quality of life. The study also examined whether patients differ in terms of trajectories of quality of life in the first six months post-transplant. A secondary data analysis was conducted of 701 participants who enrolled in the Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) 0902 trial. Participants completed the SF-36, a measure of quality of life, prior to transplant and 100 and 180 days post-transplant. Results indicated that attrition resulted in slightly biased overestimates of quality of life but the amount of overestimation remained stable over time. Patients could be grouped into three distinct classes based on physical quality of life: 1) low and stable; 2) average and declining, then stable; and 3) average and stable. Four classes of patients emerged for mental quality of life: 1) low and stable; 2) average, improving, then stable; 3) higher than average (by almost 1 SD) and stable; and 4) average and stable. Taken together, these data provide a more comprehensive understanding of quality of life that can be used to educate HCT recipients and their caregivers.
Keywords: hematopoietic stem cell transplant, quality of life
Advances in hematopoietic cell transplant (HCT) have resulted in its increasing use in recent years, giving rise to a growing number of HCT survivors (Majhail, et al 2013, Pasquini and Wang 2011). Nevertheless, HCT remains a physically and emotionally arduous process; patients often suffer from short- and long-term morbidity and deficits in quality of life. Awareness of the importance of quality of life to adult HCT recipients is reflected in a growing body of literature on the topic. Although findings are heterogeneous, in general evidence suggests that patients’ quality of life is impaired relative to population norms before HCT, nadirs within the first 100 days post-transplant, and then demonstrates some recovery in the years thereafter (Grulke, et al 2012, Pidala, et al 2009, Pidala, et al 2010). Nevertheless, long-term quality of life remains impaired relative to population norms (Pidala, et al 2009, Pidala, et al 2010). The domains of quality of life that tend to be most compromised are general health [.7 SD lower than population norms(Andrykowski, et al 2005)], social functioning [.4–.6 SD lower (Andrykowski, et al 2005, Kopp, et al 2005)], role functioning [.4 SD lower (Kopp, et al 2005)], and physical functioning [.3–.5 SD lower (Andrykowski, et al 2005, Kopp, et al 2005)].
While many studies have described quality of life among adult HCT recipients, methodological limitations have prevented widespread use of these findings for clinical education and intervention. For example, study attrition due to morbidity and mortality range from 29–65% in the first year after HCT (Bevans, et al 2006, Broers, et al 2000, Hjermstad, et al 1999b, Lee, et al 2001, McQuellon, et al 1998, Syrjala, et al 2004) and patients with better quality of life are more likely to have complete data (Altmaier, et al 2006, Bevans, et al 2006, Broers, et al 2000, Bush, et al 2000, Syrjala, et al 2004). Attrition has not been statistically addressed in most previous research, leading to potentially biased overestimates of patient quality of life. In addition, quality of life has been almost exclusively described through the use of sample means and standard deviations, which may obscure classes of patients with distinct trajectories of quality of life over time (e.g., patients with early versus late effects).
The current study addressed methodological limitations in existing research by examining change in quality of life in a large sample of HCT patients recruited as part of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902 protocol. The aims of this secondary analysis were threefold. The first aim was to model the mean trajectory of QOL during the first six months after HCT, controlling for attrition. Based on previous research (Pidala, et al 2009, Pidala, et al 2010), we hypothesized that QOL would improve on average over time. The second aim was to determine whether HCT patients can be categorized into distinct classes based on their QOL trajectory. We expected that at least two classes of patients could be identified who differ in their experience of quality of life following HCT (e.g., patients with early versus late effects). The third aim was to examine whether classes differed on the basis of baseline sociodemographic and clinical characteristics. Based on previous literature (Andrykowski, et al 1995, Diez-Campelo, et al 2004, Heinonen, et al 2001, Hjermstad, et al 1999a, Syrjala, et al 1993), we hypothesized that age, gender, race, ethnicity, marital status, education, income, baseline Karnofsky score, pre-HCT comorbidities, transplant type, myeloablative preparative regimen among allogeneic recipients, and length of hospital stay would predict class membership.
Materials and Methods
Participants
Participants were recruited as part of a larger, multicenter randomized controlled trial (RCT) of stress management and exercise among HCT patients (BMT CTN 0902) (Jacobsen, et al 2014). Eligibility criteria were that patients be: a) 18 years of age or older, b) able to speak and read English, c) able to exercise at low to moderate intensity as determined by physician judgment and self-reported ability to walk up one flight of stairs without supplementary oxygen, d) willing and able to provide informed consent, e) willing to comply with study procedures and reporting requirements, and f) planning to undergo autologous or allogeneic transplant within six weeks. Patients were excluded if they: a) had orthopedic, neurologic, or other problems which prevented safe ambulation and protocol adherence, b) were participating in another clinical trial with quality of life or functional status as a primary endpoint, c) were planning to receive anti-cytotoxic therapies other than tyrosine kinase inhibitors, d) were planning to receive donor lymphocyte infusion within 100 days of transplant, e) were planning to receive tandem transplant (i.e., planned autologous/autologous or autologous/allogeneic HCT).
Procedure
Eligibility was determined via chart review and in consultation with the attending physician. Eligible patients were recruited and informed consent was obtained prior to the day of graft infusion (i.e., day 0). Following informed consent, participants completed a baseline assessment and were randomized to receive audiovisual materials on exercise, stress management, both, or usual care as part of the larger RCT (Jacobsen, et al 2014). The trial was registered with clinical trials.gov (NCT01278927). In addition to the pre-HCT baseline assessment, participants completed follow-up assessments of quality of life at 100 days and 180 days post-HCT. Participants were recruited between January 2011 and June 2012.
Measures
Demographic and clinical data
Demographic data obtained prior to HCT included age, gender, race, ethnicity, marital status, education, and income. Co-morbid medical conditions were measured using the Hematopoietic Cell Transplant – Comorbidities Index (HCT-CI) (Sorror, et al 2005). Clinical data were collected via BMT CTN reporting and included pre-HCT Karnofsky score, disease type, transplant type, myeloablative preparative regimen among allogeneic recipients, and length of hospital stay.
Quality of life
The acute (one week) version of Medical Outcomes Study Short Form, a 36-item questionnaire, was used to assess health-related quality of life (SF-36) (Ware, et al 1993). The SF-36 is composed of eight subscales: Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health. Scores on these subscales range from 0 to 100 with higher scores reflecting better quality of life. Weighted averages of these eight subscales are used to calculate two summary scores: the Physical Component Summary (PCS) and Mental Component Summary (MCS) (Ware, et al 1994). Component summary scores are expressed as T scores which have been standardized using U.S. population norms.
Statistical Analyses
Change in MCS and PCS, independently, were initially evaluated using mixed models with a focus on linear and quadratic trends among all participants across the pre-transplant, 100-day, and 180-day measures. The final model selected for both PCS and MCS had a linear random effect and a quadratic fixed effect. Attrition was included as a fixed effect in some tested versions (coded as 0 or 1 for participants who completed a Time 3 assessment versus did not, respectively), but was not found to interact with the linear and quadratic trends and was not included in the final model.
To examine trajectories of quality of life, growth mixture modeling (GMM) (Muthén 2004, Ram and Grimm 2009) was used. Adapted from random effects modeling, GMM is able to determine not only whether a group of persons changes on some outcome measure but also whether there are individual differences in the rates of change among group members (Nesselroade and Baltes 1979). Stated differently, GMM evaluates whether there are classes of individuals who differ in terms of their initial starting points and/or rates of change over time. GMM was conducted separately for PCS and MCS. Because change was the outcome of interest, participants were included in GMM analyses if they had completed a baseline assessment and at least one follow-up assessment. Due to the large number of participants required for such analyses, participants were included regardless of transplant type. Beginning with a 1-class model, additional models with incrementally more classes were compared using fit indices and other criteria (e.g., minimal class size of 25) to determine the optimal number of classes for each measure.
Differences in class membership were examined as a function of demographic factors (i.e., age, gender, race, ethnicity, marital status, education, income) and clinical factors (i.e., baseline Karnofsky score, pre-HCT comorbidities, transplant type, myeloablative preparative regimen among allogeneic recipients, length of hospital stay) using correlations and chi-square analyses. Length of hospital stay was conceptualized as a proxy for complications in the acute post-transplant period. PCS and MCS class membership were the outcome measures. GMM was conducted using Mplus v. 7 (Muthén and Muthén 1998–2010), all other analyses were conducted using SAS 9.4 (SAS Institute 2002–2003).
Results
Participant characteristics are provided in Table 1. The majority of participants were male, non-Hispanic, white, married, had graduated from college, and reported an annual household income of less than $75,000 per year. Participants were on average 54 years of age (range 18–75). The most frequent diagnosis was myeloma (27%) and 50% of the sample received an autologous transplant. A total of 219 (31%) of participants did not complete the Time 3 assessment. Patients not completing the study were more likely to report lower annual household income (p=.006), have more pre-HCT comorbidities (p=.002), and to have received allogeneic HCT (p=.001). No differences were found between patients who did and did not complete the study on gender, age, race/ethnicity, education, marital status, employment status, pre-HCT Karnofsky score, number of days hospitalized in the first 100 days post-HCT, baseline PCS, or baseline MCS (p values >.05). As published previously (Jacobsen, et al 2014), study arms in the larger randomized trial did not demonstrate statistically significant differences on baseline sociodemographic, clinical, or quality of life variables and the intervention did not impact quality of life (Jacobsen, et al 2014); therefore, the study arms were collapsed for the current analyses.
Table 1.
Sociodemographic and Clinical Characteristics of the Sample (n=701).
| Gender: n (%) female | 302 (43) |
| Age: mean (SD) | 54.5 (12.74) |
| Ethnicity: n (%) non-Hispanic | 635 (91) |
| Race: n (%) White | 608 (87) |
| Education: n (%) college graduate | 556 (79) |
| Marital status: n (%) married | 519 (74) |
| Annual household income: n (%) ≥ $75,000 | 200 (41%) |
| Diagnosis | |
| Aplastic anemia | 9 (1) |
| Acute leukemia | 169 (24) |
| Chronic leukemia | 14 (2) |
| Hodgkin’s lymphoma | 48 (7) |
| Myelodysplastic syndrome | 59 (8) |
| Myeloma | 191 (27) |
| Non-Hodgkin’s lymphoma | 177 (25) |
| Solid tumor | 2 (<1) |
| Other | 31 (4) |
| Missing | 1 (<1) |
| Transplant type | |
| Autologous | 354 (50) |
| Allogeneic – myeloablative | 175 (25) |
| Allogeneic – non-myeloablative | 172 (25) |
| Baseline Karnofsky score | |
| 100 | 124 (18) |
| 90 | 283 (40) |
| 80 | 163 (23) |
| ≤70 | 113 (16) |
| Missing | 18 (3) |
| Number of days hospitalized before day 100: mean (SD) | 24.36 (15.73) |
| Attrition: n (%) not completing Time 3 assessment | 219 (31) |
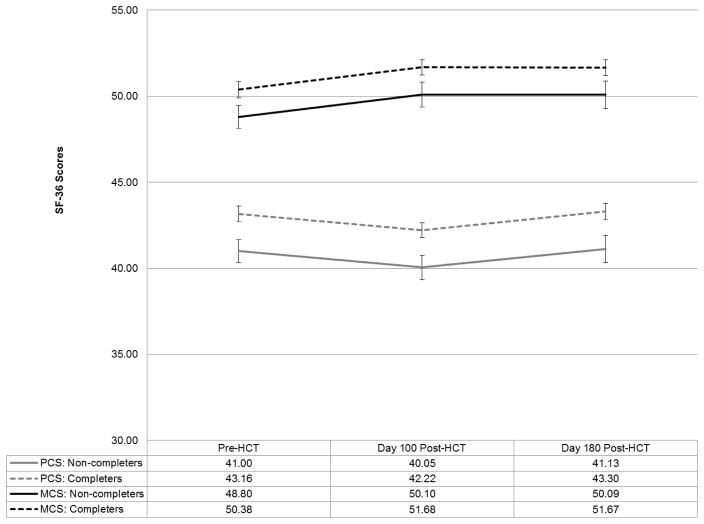
Figure 1 shows mean level changes in study participants over time by study completion status. Time 3 scores for non-completers were estimated based on scores at previous time points and linear and quadratic slopes among completers. Mixed models analysis examining change in PCS indicated significant linear (B=−.67, p=.002) and quadratic (B=.11, p=.001) effects of time controlling for attrition (B=−2.17, p=.005), indicating that PCS scores on average declined slightly from pre-HCT to day 100, then improved from day 100 to day 180 post-HCT. PCS scores were significantly lower at all assessments for patients who did not complete the study but rate of change in PCS was similar between groups. Mixed models analysis examining change in MCS indicated a significant linear effect of time (B=.61, p=.02) but not a quadratic effect (B=−.07, p=.11) controlling for attrition (B=−1.58, p=.04), indicating that MCS scores improved on average over the study period. MCS scores were significantly lower at all assessments for patients who did not complete the study but rate of change in MCS was similar between groups. Mixed models analyses controlling for study arm yielded similar results.
Figure 1.
Mean SF-36 Physical Component Scores (PCS) and Mental Component Scores (MCS) cver Time by Study Completion Status. Error bars indicate standard errors.
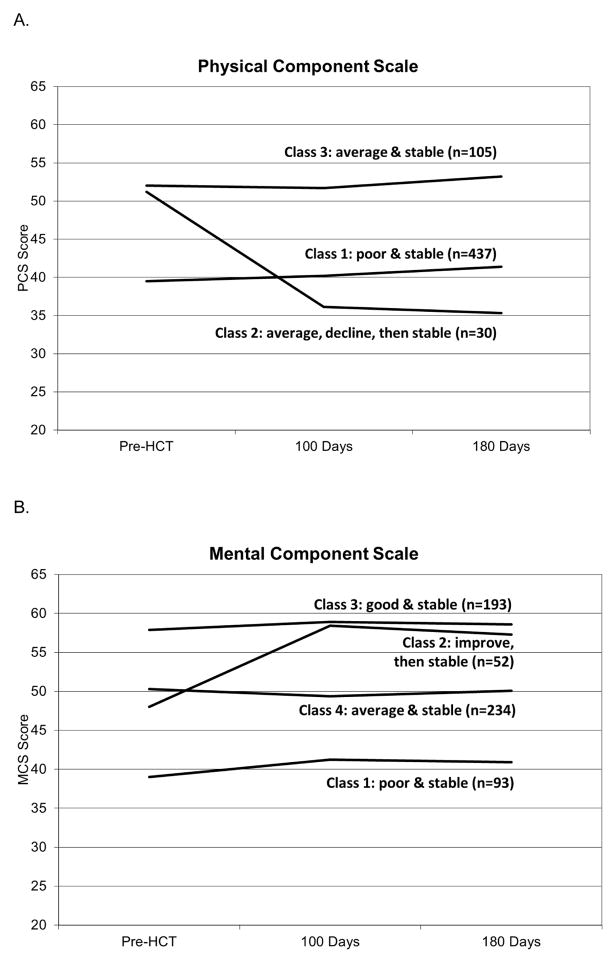
A total of 572 participants completed the baseline assessment and at least one follow-up and were therefore included in GMM analyses. Because mixed models analyses with and without inclusion of attrition as a control variable yielded similar patterns of results, attrition was not controlled in GMM analyses. Based on changes in PCS scores, study participants could be grouped into three distinct classes. As shown in Figure 2A, members of class 1 (n=437) reported poor physical quality of life at baseline (approximately one SD below population norms) and their physical quality of life remained poor across time. Members of class 2 (n=30) reported physical quality of life comparable to population norms at baseline and demonstrated significant declines in quality of life at 90 days post-HCT (i.e., approximately 1.5 SD below population norms) which remained stable at 180 days post-HCT. Members of class 3 (n=105) reported physical quality of life which was comparable to population norms at baseline and across time. Members of class 1 (i.e., poor and stable physical quality of life) tended to be younger than class 3 (p<.05), had greater pre-HCT comorbidities than class 3 (p<.05), and were more likely to have received myeloablative allogeneic HCT than class 3 (p=.01). Members of class 2 (i.e., declining physical quality of life) were more likely to have received allogeneic HCT than classes 1 and 3 (p=.0002), were hospitalized more days in the first 100 days than classes 1 and 3 (p values<.05), and reported lower income than members of classes 1 and 3 (p=.0024). Members of class 3 (i.e., average and stable physical quality of life) were more likely to be working pre-HCT than class 1 (p<.0001) and class 2 (p=.004) and reported greater income than class 1 (p=.0008) and class 2 (p=.02). There were no class differences in physical quality of life by gender, race, ethnicity, education, marital status, or pre-HCT Karnofsky scores (p values>.32)
Figure 2.
Growth Mixture Models Describing Trajectories of SF-36 Physical Quality of Life (A) and Mental Quality of Life (B).
Participants could be grouped into four distinct classes based on changes in their MCS scores. As shown in Figure 2B, members of class 1 (n=93) reported poor mental quality of life at baseline (i.e., approximately 1 SD below population norms) which remained stable across time. Members of class 2 (n=52) reported mental quality of life at baseline that was comparable to population norms which improved at 90 days post-HCT (i.e., one SD above population norms) and remained stable thereafter. Members of class 3 (n=193) reported good baseline mental quality of life (i.e., approximately 1 SD above population norms), which remained consistently high thereafter. Members of class 4 (n=234) reported baseline mental quality of life comparable to population norms which remained stable over time. Members of class 1 (i.e., poor and stable mental quality of life) were younger on average than the other classes (p<.0001), more likely to be female than classes 3 (p=.03) and 4 (p=.006), reported lower income than classes 2 and 3 (p=.001), had greater pre-HCT comorbidities than classes 3 and 4 (p values<.05), and less likely to be married than the other classes (p values≤.02). Members of class 3 (i.e., good and stable mental quality of life) were more likely to be working pre-HCT compared to class 1 (p=.0009) and class 4 (p=.006). There were no class differences in mental quality of life by race, ethnicity, education, pre-HCT Karnofsky scores, transplant type, myeloablative preparatory regimen among allogeneic recipients, or length of hospital stay (p values>.14).
Conclusions
The current study examined change in physical and mental quality of life over time in a large sample of HCT recipients. The first goal of the study was to examine the effects of attrition on estimates of quality of life. Although patients who did not complete the study demonstrated lower physical and mental quality of life at each assessment than those who did, physical and mental quality of life changed similarly over time for both groups. While group differences were significant, they were not large (i.e., approximately .2 standard deviations). These data suggest that longitudinal quality of life analyses that do not control for attrition in HCT recipients are likely slight overestimates and the amount of overestimation is consistent across time.
The second goal of the study was to examine between-patient variation in quality of life over time. Results indicated significant variation across individuals in both physical and mental quality of life. Regarding physical quality of life, three distinct classes of patients were identified. The majority of patients (i.e., 95%) reported stable physical health over time (classes 1 and 3). The largest class (class 1, 63%) reported quality of life that was significantly below population norms (i.e., approximately 1 SD). This group may reflect advances in transplantation that allow patients with greater comorbidity to be transplanted without subsequent declines in quality of life. A small fraction of patients (class 2) demonstrated average physical quality of life at baseline, then large decrements from baseline to 100 days-HCT, with no change thereafter. These patients were more likely to have had an allogeneic transplant, to have been hospitalized more days in the first 100 days post-HCT, and to report lower pre-HCT income compared to other patients. The only baseline variables that were able to differentiate between these patients and those with average physical quality of life that remained stable (class 3) were income and transplant type. Early identification of patients at risk for poor outcomes remains an elusive research goal. Future research is needed to identify other measurable risk factors. An evolving understanding of the biology of transplant-related toxicity (e.g., GVHD) may point to new risk factors.
Regarding mental quality of life, four classes of patients were identified. The majority of patients (i.e., 91%) reported stable mental health over time. A minority of patients demonstrated change in mental quality of life; these patients reported average mental quality of life at baseline which improved at 100 days post-HCT and remained stable over time. Additionally, 16% of the sample reported poor mental quality of life that remained poor across time. Patients exhibiting low mental quality of life may benefit from early psychosocial intervention, as our data suggest that their mental health will not improve over time. Interestingly, almost half of the sample reported mental quality of life that was better on average than population norms at baseline and across time. Additional research is needed to determine the factors that contribute to their resiliency. For example, qualitative interviews with these individuals might yield valuable information that could be incorporated into future psychosocial interventions.
The stability of quality of life in most patients raises the possibility of response shift, or changes in patients’ internal standards of quality of life due to changes in their health status. For example, extreme changes in health status during the acute transplant period may affect how patients evaluate their quality of life, such that quality of life that was once considered very poor is now considered to be fair or good. Response shift in cancer patients has been well-documented (Andrykowski, et al 2009, Tierney, et al 2007) and may result in overestimation of quality of life. Future studies of quality of life in HCT should consider evaluating response shift (e.g., using structural equation modeling or the “then test”) (King-Kallimanis, et al 2009, Schwartz and Sprangers 1999) to more accurately model quality of life. The stability of quality of life over time may also be due in part to the measure used; the SF-36 may be less sensitive to change than other measures of quality of life (e.g., the FACT-BMT) (Lee, et al 2006), in part because transplant-related concerns are not represented.
Strengths of the current study include a large, multicenter sample of HCT recipients as well as the use of innovative statistical approaches and validated measures to characterize trajectories of patient quality of life. Study limitations should also be noted, however. Eligible patients were required to be in relatively good health (i.e., able to exercise). Patients who were unwilling to exercise (e.g., due to depression) may have declined study participation. In addition, the sample tended to be relatively homogenous in terms of race and ethnicity. Thus, results of the current study may not be generalizable to the larger population of HCT recipients, particularly those entering transplant with compromised physical function. Due to the sample size requirements of growth mixture modeling, it was not possible to run the models separately for autologous and allogeneic HCT recipients. Future studies with larger samples sizes should examine trajectories separately by transplant type. Of note, quality of life was not collected during the acute transplant period, so short-term changes in quality of life may have been missed. In addition, quality of life was not assessed after 180 days post-HCT, so the impacts of long-term and late effects were not characterized.
Results from the current study have high clinical relevance. First, results suggest that patient attrition only slightly biased estimates of quality of life. These findings provide more confidence in results from existing studies which have not taken attrition into account. In addition, findings suggest that while patients vary widely in terms of their quality of life in the first six months after transplant, large majorities of patients report physical and mental health at 100 days and 180 days post-HCT that is comparable to their baseline levels. This data can be used to better educate patients and caregivers regarding what to expect after HCT. Additional studies should examine trajectories of quality of life over a longer follow-up period to characterize variability due to the impact of late effects among long-term survivors of HCT.
In summary, results from the current study suggest that reports of mean levels of quality of life among HCT recipients may mask significant inter-patient variability. These data are consistent with reports of patient heterogeneity regarding transplant-related morbidities and their effects on quality of life (Pidala, et al 2011). Results point to the importance of developing innovative strategies for analyzing and reporting quality of life data in HCT to provide clinical utility for providers, patients, and caregivers.
Highlights.
This secondary analysis of the BMTCTN 0902 study examined the extent to which attrition results in biased estimates of patient quality of life and whether patients differ in terms of trajectories of quality of life in the first six months post-transplant.
Results indicated that attrition resulted in slightly biased overestimates of quality of life but the amount of overestimation remained stable over time.
Results also indicated that patients could be classified three distinct classes based on physical quality of life and four distinct classes based on mental quality of life.
Taken together, these findings help to better understand the course of recovery after hematopoietic cell transplant.
Acknowledgments
Support for this study was provided by grant U10HL069294 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the above mentioned parties.” The authors wish to thank the participating sites: Baylor College of Medicine/The Methodist Hospital (PI: George Carrum), Blood and Marrow Transplant Program at Northside Hospital (PI: Asad Bashey), City of Hope National Medical Center (PIs: Joseph Alvarnas, Stephen J. Forman), Emory University (PI: Ajay K. Nooka), Fox Chase Cancer Center/Temple University (PI: Mary Ellen Martin), Fred Hutchinson Cancer Research Center (PI: Stephanie J. Lee), Moffitt Cancer Center (PI: Paul B. Jacobsen), Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (PIs: Tracy Douglas, Javier Bolaños-Meade), Karmanos Cancer Institute (PI: Voravit Ratanatharathorn), Medical University of South Carolina (PI: Kylie Perkins Capdevila), Memorial Sloan-Kettering Cancer Center (PIs: Barrie R. Cassileth, Ann A. Jakubowski, Sergio A. Giralt), The Ohio State University Arthur G. James Cancer Hospital (PI: Steven Devine), Oregon Health and Science University (PI: Susan Slater), Rush University Medical Center (PI: John J. Maciejewski), University Hospitals of Cleveland/ Case Western (PI: Hillard Lazarus), University of Florida College of Medicine/Shands Hospital: (PI: John R. Wingard), University of Michigan Medical Center (PI: Carrie Kitko), University of Minnesota (PI: Brian McClune), University of Nebraska Medical Center (PIs: James Armitage, Mojtaba Akhtari, Julie Vose, Philip Bierman), Virginia Commonwealth University/Medical College of Virginia Hospitals (PI: John M. McCarty), Washington University/Barnes Jewish Hospital (PI: Peter Westervelt).
Footnotes
Financial Disclosure: The authors have no conflicts to disclose.
H.S.L.J. performed the research and wrote the manuscript, S.K.S. analyzed and interpreted data, B.J.S. analyzed and interpreted data, P.B.J. designed and conducted the research and wrote the manuscript, W.A.W. performed the research and wrote the manuscript, J.K. performed the research and wrote the manuscript, N.M. designed and conducted the research and wrote the manuscript, K.S. designed and conducted the research and wrote the manuscript, S.J.L. designed and conducted the research and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmaier EM, Ewell M, McQuellon R, Geller N, Carter SL, Henslee-Downey J, Davies S, Papadopoulos E, Yanovich S, Gingrich R. The effect of unrelated donor marrow transplantation on health-related quality of life: a report of the unrelated donor marrow transplantation trial (T-cell depletion trial) Biology of Blood Marrow Transplantation. 2006;12:648–655. doi: 10.1016/j.bbmt.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, Horowitz MM, Sobocinski KA, Rizzo JD, Wingard JR. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. Journal of Clinical Oncology. 2005;23:599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Andrykowski MA, Donovan KA, Jacobsen PB. Magnitude and correlates of response shift in fatigue ratings in women undergoing adjuvant therapy for breast cancer. Journal of Pain and Symptom Management. 2009;37:341–351. doi: 10.1016/j.jpainsymman.2008.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrykowski MA, Greiner CB, Altmaier EM, Burish TG, Antin JH, Gingrich R, McGarigle C, Henslee-Downey PJ. Quality of life following bone marrow transplantation: findings from a multicentre study. British Journal of Cancer. 1995;71:1322–1329. doi: 10.1038/bjc.1995.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans MF, Marden S, Leidy NK, Soeken K, Cusack G, Rivera P, Mayberry H, Bishop MR, Childs R, Barrett AJ. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2006;38:101–109. doi: 10.1038/sj.bmt.1705406. [DOI] [PubMed] [Google Scholar]
- Broers S, Kaptein AA, Le Cessie S, Fibbe W, Hengeveld MW. Psychological functioning and quality of life following bone marrow transplantation: a 3-year follow-up study. Journal of Psychosomatic Research. 2000;48:11–21. doi: 10.1016/s0022-3999(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Bush NE, Donaldson GW, Haberman MH, Dacanay R, Sullivan KM. Conditional and unconditional estimation of multidimensional quality of life after hematopoietic stem cell transplantation: a longitudinal follow-up of 415 patients. Biology of Blood Marrow Transplantation. 2000;6:576–591. doi: 10.1016/s1083-8791(00)70067-x. [DOI] [PubMed] [Google Scholar]
- Diez-Campelo M, Perez-Simon JA, Gonzalez-Porras JR, Garcia-Cecilia JM, Salinero M, Caballero MD, Canizo MC, Ocio EM, Miguel JF. Quality of life assessment in patients undergoing reduced intensity conditioning allogeneic as compared to autologous transplantation: results of a prospective study. Bone Marrow Transplantation. 2004;34:729–738. doi: 10.1038/sj.bmt.1704646. [DOI] [PubMed] [Google Scholar]
- Grulke N, Albani C, Bailer H. Quality of life in patients before and after haematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 2012;47:473–482. doi: 10.1038/bmt.2011.107. [DOI] [PubMed] [Google Scholar]
- Heinonen H, Volin L, Uutela A, Zevon M, Barrick C, Ruutu T. Gender-associated differences in the quality of life after allogeneic BMT. Bone Marrow Transplantation. 2001;28:503–509. doi: 10.1038/sj.bmt.1703158. [DOI] [PubMed] [Google Scholar]
- Hjermstad M, Holte H, Evensen S, Fayers P, Kaasa S. Do patients who are treated with stem cell transplantation have a health-related quality of life comparable to the general population after 1 year? Bone Marrow Transplantation. 1999a;24:911–918. doi: 10.1038/sj.bmt.1701998. [DOI] [PubMed] [Google Scholar]
- Hjermstad MJ, Evensen SA, Kvaloy SO, Fayers PM, Kaasa S. Health-related quality of life 1 year after allogeneic or autologous stem-cell transplantation: a prospective study. Journal of Clinical Oncology. 1999b;17:706–718. doi: 10.1200/JCO.1999.17.2.706. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, Wu J, Majhail NS, Wood W, Rizzo JD, Geller NL, Kitko C, Faber E, Abidi MH, Slater S, Horowitz MM, Lee SJ. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20:1530–1536. doi: 10.1016/j.bbmt.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Kallimanis BL, Oort FJ, Visser MR, Sprangers MA. Structural equation modeling of health-related quality-of-life data illustrates the measurement and conceptual perspectives on response shift. Journal of Clinical Epidemiology. 2009;62:1157–1164. doi: 10.1016/j.jclinepi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Kopp M, Holzner B, Meraner V, Sperner-Unterweger B, Kemmler G, Nguyen-Van-Tam DP, Nachbaur D. Quality of life in adult hematopoietic cell transplant patients at least 5 yr after treatment: a comparison with healthy controls. European Journal of Haematology. 2005;74:304–308. doi: 10.1111/j.1600-0609.2004.00402.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Fairclough D, Parsons SK, Soiffer RJ, Fisher DC, Schlossman RL, Antin JH, Weeks JC. Recovery after stem-cell transplantation for hematologic diseases. Journal of Clinical Oncology. 2001;19:242–252. doi: 10.1200/JCO.2001.19.1.242. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kim HT, Ho VT, Cutler C, Alyea EP, Soiffer RJ, Antin JH. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplantation. 2006;38:305–310. doi: 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]
- Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, Hahn T, Jakubowski A, Joffe S, Lazarus HM, Parsons SK, Robien K, Lee SJ, Kuntz KM. Prevalence of hematopoietic cell transplant survivors in the United States. Biology of Blood and Marrow Transplantation. 2013;19:1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuellon RP, Russell GB, Rambo TD, Craven BL, Radford J, Perry JJ, Cruz J, Hurd DD. Quality of life and psychological distress of bone marrow transplant recipients: the ‘time trajectory’ to recovery over the first year. Bone Marrow Transplantation. 1998;21:477–486. doi: 10.1038/sj.bmt.1701115. [DOI] [PubMed] [Google Scholar]
- Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The Sage handbook of quantitative methodology for the social sciences. Sage; Thousand Oaks CA: 2004. pp. 345–368. [Google Scholar]
- Muthén LK, Muthén B. Mplus User’s Guide. Muthén & Muthén; Los Angeles, CA: 1998–2010. [Google Scholar]
- Nesselroade JR, Baltes PB. Longitudinal research in the study of behavior and development. Academic Press; New York: 1979. [Google Scholar]
- Pasquini M, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides 2011 [Google Scholar]
- Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. British Journal of Haematology. 2010;148:373–385. doi: 10.1111/j.1365-2141.2009.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, Cutler C, Jacobsohn D, Palmer J, Arai S, Jagasia M, Lee SJ. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Grimm KJ. Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development. 2009;33:565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS 9.1.3. SAS Institute, Inc; Cary, NC: 2002–2003. [Google Scholar]
- Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Social Science and Medicine. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplantation. 1993;11:319–327. [PubMed] [Google Scholar]
- Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, Martin PJ. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- Tierney DK, Facione N, Padilla G, Dodd M. Response shift: a theoretical exploration of quality of life following hematopoietic cell transplantation. Cancer Nursing. 2007;30:125–138. doi: 10.1097/01.NCC.0000265002.79687.af. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. The Health Institute; Boston: 1994. [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute; Boston: 1993. [Google Scholar]