Abstract
Background
Cesium-131 (Cs-131) brachytherapy is used to reduce local recurrence of resected brain metastases. In order to ensure dose homogeneity and reduce risk of radiation necrosis, inter-seed distance and cavity volume must remain stable during delivery.
Objective
To investigate the efficacy of the “seeds-on-a-string” technique with intracavitary fibrin glue in achieving cavity volume stability.
Methods
We placed intra-operative Cs-131 brachytherapy in 30 cavities post-resection of brain metastases. Seeds-on-a-string were placed like barrel staves within the cavity with fibrin glue. Serial MRI imaging occurred post-operatively. Pre-operative tumor volumes were compared with post-operative cavity volumes to evaluate volume stability. Thirty patients who underwent post-resective stereotactic radiosurgery (SRS) were used as a control group for volumetric comparison.
Results
Cs-131 and SRS patients exhibited consistent cavity shrinkage over the median 110-day follow-up (p<.001), with total median shrinkage of 56.5% (Cs-131) and 84.8% (SRS). During the first month when ~88% of Cs-131 dosage is delivered, however, there was non-significant volume decrease in the Cs-131 group (median 22.0%; p=.063), while SRS patients showed significantly more shrinkage (46.7%; p=.042). No events of radiation necrosis occurred in either group.
Conclusion
Cs-131 patients exhibited significantly less cavity shrinkage than SRS patients during the first critical month with 88% Cs-131 dose delivery. This significant difference in shrinkage suggests that the intracavitary seeds-on-a-string technique facilitates increased cavity stability, promoting more homogenous dose delivery.
Keywords: brachytherapy, brain metastases, cavity volume dynamics, cesium-131 (Cs-131), radiotherapy, resection cavity
Introduction
The incidence of brain metastasis has increased during the last decade, occurring in up to 40% of cancer patients.1-3 Survival without treatment is typically reported as one to two months, but the use of whole brain radiation therapy (WBRT) alone increases outcome to three to six months. The combination of tumor resection with adjuvant WBRT or stereotactic radiosurgery (SRS) is reported to increase survival to 11 months.4-7
WBRT is also associated with reduced quality-of-life measures8,9 and detrimental neurocognitive results.10-13 More localized radiotherapy options such as SRS maintain an equivalent survival benefit with reduced neurocognitive defects.10,14-16 Another option for focal radiation delivery is the intra-operative implantation of radioisotope seeds into the tumor cavity, known as brachytherapy. While brachytherapy achieves similar rates of local control (80%-95%) as post-operative WBRT or SRS,17-25 there have been high rates of reported toxicity, i.e. radiation necrosis, with Iodine-125 (I-125) usage.20,26
High toxicity has been partially attributed to the long half-life (t1/2) of I-125, which allows continued radiation delivery over a long follow-up period during which the cavity is thought to gradually shrink.27-30 Shrinkage increases proximity of the seeds to each other and to normal brain tissue, altering dosage strength and homogeneity. The relationship between shrinkage of the target radiation area and increased necrosis of surrounding tissue was first shown in experiments involving I-125 implantation for inoperable gliomas, and therefore focused on tumor shrinkage rather than cavity shrinkage. In one study, 40% of patients experienced radiation necrosis, which was correlated with faster rates of tumor shrinkage and increased radiation delivery to the brain parenchyma.26 A similar study concluded that tumor shrinkage of over 50% during the first 6 months of I-125 delivery resulted in a 30% increase in radiation delivery to the surrounding tissues and an increased risk for radiation necrosis.31,32
Our institution has attempted to minimize this toxicity by both shortening the duration of radiation delivery and minimizing cavity shrinkage post-resection. In this trial, we instead use Cesium-131 (Cs-131), a novel isotope that has a shorter t1/2 than I-125 (9.7 versus 59.4 days).33,34 Additionally, we use the “seeds-on-a-string” model in an attempt to stabilize cavity volume and minimize shrinkage. With this model, seeds are implanted on strings that line the cavity like barrel staves and are held in place with fibrin glue to prevent inward cavity collapse. To our knowledge, there has not been a long-term analysis of cavity volume dynamics with brachytherapy treatment. Our study investigates whether cavity shrinkage occurs post-implantation of brachytherapy seeds and assesses the efficacy of the seeds-on-a-string technical method in maintaining cavity volume stability.
Methods
Patient selection and surgical technique
Between 2010 and 2014, 30 patients underwent intra-operative Cs-131 brachytherapy for newly resected brain metastases on an IRB-approved prospective trial at New York Presbyterian/Weill Cornell Medical Center. Selection criteria, surgery details, and clinical outcomes are described in a prior study.34 Six more patients are included in this study in comparison to the phase I/II report as the trial is still open and accruing subjects.34 Directly after resection, Cs-131 stranded seeds (IsoRay, Richland, WA) with an activity of 3-5 mCi were implanted with a planned dose of 80 Gy prescribed to a 5 mm depth from the surface of the resection cavity. Seeds were placed roughly 1 cm apart within the cavity. Dose calculations were based on a CT scan done 2 days after surgery. Since cavity shrinkage after placement of the seeds would bring them closer together and create local dose heterogeneity, a strategy was required to try and maintain cavity volume for the first month after seed placement when ~88% of the dose was delivered (based on Cs-131's short t1/2 of 9.7 days). Thus, the seeds were placed within the cavity like “barrel staves” so the tensile strength of the string would keep the cavity open. The seeds were covered with Surgicel (Ethicon) to prevent migration and promote hemostasis. Additionally, fibrin glue was placed within the cavity to keep the seeds along the resection cavity wall, prevent internal migration, and maintain cavity volume until the fibrin glue and strings resorbed. This technique (the seeds-on-a-string model) is demonstrated in Figure 1. Following surgery, all patients were maintained on steroids for 3 weeks on a slow taper.
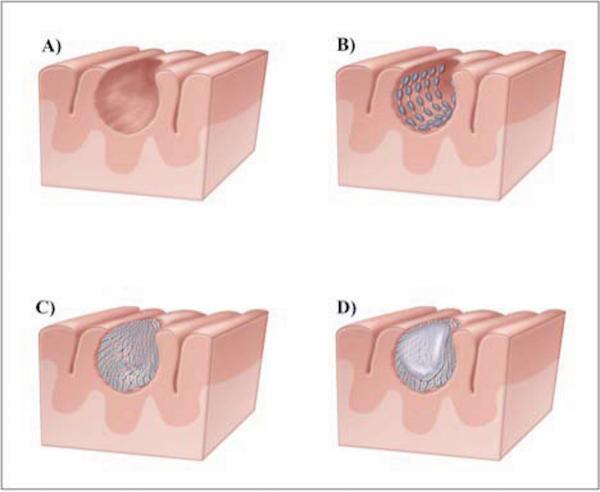
Figure 1.
These images demonstrate the seeds-on-a-string surgical technique utilized to minimize seed migration and cavity shrinkage: A) cavity post-resection; B) seeds are placed within the cavity like barrel staves so the tensile strength of the string keeps the cavity open; C) seeds are covered with Surgicel (Ethicon) to prevent seed movement and promote hemostasis; D) fibrin glue is introduced into the cavity to prevent seed migration and maintain cavity volume.
In order to assess the efficacy of the seeds-on-a-string model in maintaining cavity stability, cavity dynamics in the brachytherapy patients were compared to cavity dynamics in patients who underwent post-resection stereotactic radiosurgery (SRS) for brain metastases. Since there were no patients undergoing brachytherapy seed placement without the seeds-on-a-string method for comparison, SRS patients were selected to serve as the best available controls because SRS is an external radiation delivery modality that lacks intracavitary intervention. Thirty SRS patients who underwent resection of brain metastases and subsequent SRS within a median 50 days ± 19.6 (range 15-94) at New York Presbyterian/ Weill Cornell Medical Center from 2002-2014 were identified for comparison to match the Cs-131 patients. SRS patients received total radiation doses ranging from 15-25 Gy in 1-5 fractions to a 2-mm margin.
Imaging
All patients received a pre-operative and a series of post-operative surveillance MRI scans. The post-operative scans were ideally planned for day one or two, one month, and three months after surgery, though these time points varied slightly. Not all patients received all post-operative scans, and analysis at each time point is limited to the patients with available imaging.
Volumetric analysis
The volumes of the pre-operative tumor and post-operative cavities were calculated for each patient in the Cs-131 and SRS groups. Utilizing iPlan Cranial planning software (Brainlab, Feldkirchen, Germany), the tumor and cavities were contoured on axial slices of 5mm thickness, using the “smartbrush” tool. Smartbrush contouring was based upon a difference in enhancement of the tumor or cavity relative to surrounding parenchyma. The software then calculated three-dimensional volumes through summation of the contoured slices. Pre-operative tumor was contoured on T1+C sequences, while the cavities were contoured using a combination of pre-contrast T1, T1+C, and T2 sequences. Pre- and post-operative edema volumes were similarly calculated on FLAIR or FLAIR+C.
We also measured intra-operative cavity volume as best we could to try to assess how much shrinkage occurred based merely on the removal of the bulk of the tumor. Intraoperative volume was obtained in two ways: 1) fluid was introduced into the cavity and the volume required for filling the cavity was reported. This was only done if the cortical surface was at the most superior part of the resection cavity and there was no communication with the ventricle; 2) dimensions (length, width, and height) of the cavity were measured immediately after resection and volume was calculated using an approximated ellipsoid volume formula typically utilized for hematoma volume measurement 35: (length × width × height) * (1/2).
Descriptive analysis
We calculated the median absolute volume of the cavity at each time point over the follow-up period and the median percent change in volume between different time points for each group. Percent change in volume was calculated as follows, using the example of comparing pre-operative tumor volume to the first post-operative MRI cavity volume [27]: [(cavity volume)-(tumor volume)]/(tumor volume) × 100. Thus, a positive percent change represented cavity expansion, while a negative percent change represented cavity collapse. Percent change in edema volume was calculated in the same way. We analyzed cavity dynamics in comparison to both pre-operative volume and MRI-1 volume baselines, with MRI-1 volume serving as our best available representation for the early, post-debulking volume of the cavity given limitations in intra-operative volume calculation.
Comparison of cavity dynamics and the decay properties of Cs-131 was performed.33 Lastly, rates of radiation necrosis were reported in each group.
Statistical analysis
Statistical analysis was performed with SPSS (version 22, SPSS, Inc), and a 2-sided p-value of <.05 was considered statistically significant. Chi-square, Fisher's exact test, and non-parametric Mann-Whitney tests were used to compare demographic variables between the Cs-131 and SRS groups. Non-parametric Wilcoxon signed-rank tests assessed if changes in cavity volume or edema over different time periods were significant. This analysis was performed within the Cs-131 and SRS groups. Non-parametric Mann-Whitney tests were performed to compare timing of imaging between Cs-131 and SRS patients. The non-parametric Kolmogorov-Smirnov test was used to compare cavity dynamics over the same time period between different groups of patients, with findings confirmed by the Mann-Whitney U test.
Results
In the prospective trial at the time of analysis, there were 30 Cs-131 patients with 31 resected brain metastases. One patient was excluded from volumetric analysis because the resection cavity was continuous with the lateral ventricle and post-operative volumetric analysis could not be performed. One patient had two separate metastases resected 46 days apart that were analyzed separately. Thus, volumetric analysis was limited to a subset of 29 Cs-131 patients with 30 lesions. These patients were compared to a control group with 30 SRS patients.
Demographic and baseline clinical data for the Cs-131 and SRS patient cohorts are included in Table 1. Median age for the Cs-131 and SRS cohorts was 64 years ± 11.9 (range 45-84) and 65 years ± 11.1 (range 32-84), respectively (p=.64). 16 Cs-131 lesions were in females (53.3%), while 14 were in males (46.7%). 14 SRS patients were female (46.7%) and 16 were male (53.3%). There was no statistically significant difference in sex (p=.80) or extent of tumor resection (p=1.00) between the two treatment groups. Statistical analysis could not be performed to compare race, tumor pathology, or tumor localization between the two groups given small sample size. Timing of pre- and post-operative MRI scans in both groups is included in Table 2. There was no statistically significant difference in the imaging timeline between the Cs-131 and SRS patients.
Table 1.
Patient Characteristics
| Cs-131 Patients | SRS Patients | p-value * | |
|---|---|---|---|
| Median age (years) at surgery ± SD (range) | 64 ± 11.9 (45-84) | 65 ± 11.1 (32-84) | .64 |
| Sex (%) | .80 | ||
| Male | 14 (46.7%) | 16 (53.3%) | |
| Female | 16 (53.3%) | 14 (46.7%) | |
| Race | n/a† | ||
| White | 21 (70.0%) | 12 (40.0%) | |
| Hispanic | 3 (10.0%) | 1 (3.3%) | |
| Black | 1 (3.3%) | 3 (10.0%) | |
| Asian | 1 (3.3%) | 1 (3.3%) | |
| Other | 0 (0%) | 7 (23.3%) | |
| Not reported or unavailable | 4 (13.3%) | 6 (20.0%) | |
| Tumor pathology | n/a† | ||
| Breast | 3 (10.0%) | 5 (16.7%) | |
| Colorectal | 1 (3.3%) | 2 (6.7%) | |
| Endometrium | 2 (6.7%) | 0 (0%) | |
| Esophagus | 1 (3.3%) | 1 (3.3%) | |
| Lung | 19 (63.3%) | 14 (46.7%) | |
| Melanoma | 2 (6.7%) | 2 (6.7%) | |
| Ovary | 0 (0%) | 1 (3.3%) | |
| Pancreas | 0 (0%) | 1 (3.3%) | |
| Prostate | 0 (0%) | 1 (3.3%) | |
| Renal | 2 (6.7%) | 2 (6.7%) | |
| Salivary gland | 0 (0%) | 1 (3.3%) | |
| Tumor localization | n/a† | ||
| Cerebellum | 6 (20.0%) | 7 (23.3%) | |
| Frontal | 11 (36.7%) | 10 (33.3%) | |
| Parietal | 7 (23.3%) | 4 (13.3%) | |
| Occipital | 2 (6.7%) | 3 (10.0%) | |
| Temporal | 1 (3.3%) | 4 (13.3%) | |
| Fronto-parietal | 1 (3.3%) | 1 (3.3%) | |
| Parieto-occipital | 1 (3.3%) | 1 (3.3%) | |
| Other | 1 (3.3%) | 0 (0%) | |
| Extent of resection | 1.00 | ||
| Gross-total resection | 28 (93.3%) | 27 (90.0%) | |
| Sub-total resection | 2 (6.7%) | 3 (10.0%) |
Statistical comparison of patient characteristics was performed using chi-square analysis, Fisher's exact test, or non-parametric Mann-Whitney U tests, as appropriate.
Statistical analysis could not be performed due to small sample size.
Table 2.
MRI Pre- and Post-Operative Timeline
| Cs-131 Patients | SRS Patients | p-value* | |||
|---|---|---|---|---|---|
| Median timing of scan relative to surgery ± SD (range) | N (cases) | Median timing of scan relative to surgery ± SD (range) | N (cases) | ||
| MRI-pre | 0 days before ± 0.9 (0-3) | 30 | 0 days before ± 1.9 (0-10) | 30 | .424 |
| MRI-1 | 2 days after ± 0.9 (1-5) | 30 | 1 days after ± 1.0 (0-5) | 28 | .094 |
| MRI-2 | 36 days after ± 9.7 (17-57) | 21 | 36 days after ± 10.3 (19-59) | 23 | .466 |
| MRI-3 | 105 days after ± 18.5 (74-136) | 17 | 109 days after ± 17.0 (83-140) | 20 | .383 |
This p-value is the result of a Mann-Whitney test.
Cs-131= Cesium 131; SRS= Stereotactic radiosurgery
Cs-131 cavity dynamics
Volumetric analysis of the Cs-131 patients revealed a decrease in median cavity volume between each successive time point (Table 3). Figure 2 illustrates this trend with MRI scans from a patient who exhibited consistent cavity collapse on T1+C and consistent edema reduction on FLAIR.
Table 3.
Absolute Cavity Volume in Cs-131 vs. SRS Patients
| Cs-131 | SRS | |||
|---|---|---|---|---|
| MRI scan | N (cases) | Median absolute volume (cm3) ± SD (range) | N (cases) | Median absolute volume (cm3) ± SD (range) |
| MRI-pre (tumor) | 30 | 10.7 ± 14.8 (1.9-79.1) | 30 | 13.5 ± 11.6 (1.9-49.0) |
| MRI-1 | 30 | 7.3 ± 6.8 (1.2–28.0) | 28 | 6.9 ± 4.0 (1.1–14.4) |
| MRI-2 | 21 | 5.9 ± 4.4 (1.4–17.1) | 23 | 5.7 ± 4.6 (0.1–14.5) |
| MRI-3 | 17 | 4.1 ± 3.9 (1.2–13.6) | 20 | 2.3 ± 5.1 (0.0–22.8) |
Cs-131= Cesium-131; SRS=Stereotactic radiosurgery
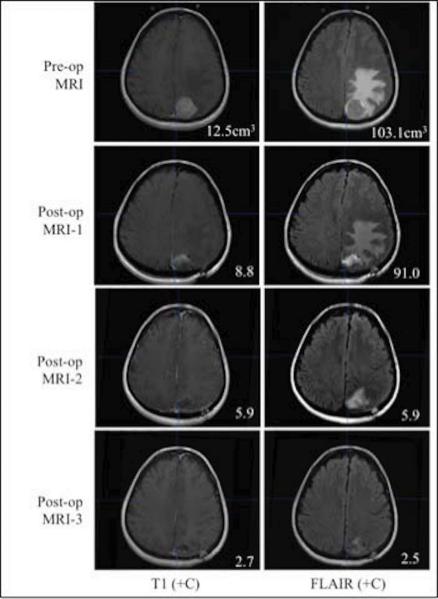
Figure 2.
This is an example of one patient's pre-operative and post-operative MRI series. This specific patient exhibited cavity collapse on T1+C throughout the entire follow-up period as well as a consistent decrease in edema volume visible on FLAIR. The numbers in the left column reflect tumor or cavity volumes (cm3), while the numbers in the right column are edema volumes (cm3) for this patient.
In order to further analyze volume dynamics over time with Cs-131, we assessed cavity volume from the perspective of median percent change in volume between different time points (Table 4).27 We measured percent change in cavity volume from two different starting points: MRI-pre and MRI-1. There were larger decreases in cavity volume when compared to MRI-pre rather than MRI-1. Median percent change in volume compared to MRI-pre was as follows: MRI-pre to MRI-1: −35.1% ± 52.5 (range −78.9 to 129.2); MRI-pre to MRI-2: −46.2% ± 81.3 (−78.4 to 305.9); MRI-pre to MRI-3: −56.5% ± 21.8 (−78.6 to −12.3). The median percent change in volume compared to MRI-1 was as follows: MRI-1 to MRI-2: −22.0% ± 48.3 (−75.8 to 100.0); MRI-1 to MRI-3: −18.4% ± 27.6 (−82.6 to 10.4).
Table 4.
Comparison of Cavity Volume Dynamics between Cs-131 vs. SRS Patients
| Cs-131 | SRS | Cs-131 vs. SRS | |||||
|---|---|---|---|---|---|---|---|
| Time span | N | Median % change in cavity volume ± SD (range) | p* | N | Median % change in cavity volume ± SD (range) | p* | p† |
| MRI-pre to MRI-1 | 30 | −35.1% ± 52.5% (−78.9% – 129.2%) | .002 | 28 | −48.0% ± 44.9% (−85.5% – 76.5%) | <.001 | .17 |
| MRI-pre to MRI-2 | 21 | −46.2% ± 81.3% (−78.4% – 305.9%) | .001 | 23 | −67.8% ± 64.2% (−98.5% – 160.8%) | .001 | .034 |
| MRI-pre to MRI-3 | 17 | −56.5% ± 21.8% (−78.6% – −12.3%) | <.001 | 20 | −84.8% ± 25.5% (−100.0% – −12.5%) | <.001 | .008 |
| MRI-1 to MRI-2 | 21 | −22.0% ± 48.3% (−75.8% – 100.0%) | .063 | 21 | −46.7% ± 49.3% (−94.8% – 76.3%) | .092 | .042 |
| MRI-1 to MRI-3 | 17 | −18.4% ± 27.6% (−82.6% –10.4%) | .001 | 18 | −65.2% ± 63.6% (−100.0% –180.8%) | .004 | .028 |
This p-value is the result of a Wilcoxon signed-rank test comparing the absolute volumes at each endpoint in the time span for the Cs-131 and SRS groups separately.
This p-value is the result of a non-parametric Kolmogorov-Smirnov test comparing the percent change in cavity volume over each time span between the Cs-131 and SRS cohorts.
Cs-131= Cesium-131; SRS=Stereotactic radiosurgery
While there was consistently significant shrinkage between MRI-pre and each postoperative MRI (MRI-1: p=.002; MRI-2: p=.001; MRI-3: p<.001), there was no statistically significant shrinkage between MRI-1 and MRI-2 (p=.063). These findings suggest that the initial collapse of brain tissue into the cavity secondary to surgical debulking between MRI-pre and MRI-1 likely accounts for the majority of cavity shrinkage over the follow-up period. The subsequent period of non-significant shrinkage from MRI-1 to MRI-2 corresponds to the first month after surgery when ~88% of the radiation dose is delivered.
A comparison of intra-operative volume to both pre-operative volume and MRI-1 volume was then performed to investigate shrinkage occurring immediately post-resection for a subset of patients in whom intra-operative volume was available. Using the fluid measurement technique, the median intra-operative volume was 3.13 cm3 ± 4.3 (range 1-17; n=12). The median intra-operative cavity volume using the dimensions formula was 2.4 cm3 ± 5.8 (range 0.2-25; n=21). Both median intra-operative measurements showed significant collapse relative to pre-operative tumor volume (p=.002 for fluid volume and p<.001 for dimension-based volume), providing evidence for immediate significant shrinkage due to tumor debulking. Additional analysis was performed to compare intra-operative volumes with MRI-1 volumes. This analysis demonstrated a statistically significant difference between intra-operative volume and MRI-1 cavity volume (p=.003 for the fluid volume or p=.007 for the dimension-based volume), further supporting early shrinkage.
Comparison of dynamics in Cs-131 and SRS patients
Volumetric analysis of the SRS cavities also revealed a decrease in absolute median cavity volume between each successive time point (Table 3). Further analysis of percent change in cavity volume over time demonstrated consistently significant cavity shrinkage between MRI-pre and all three post-operative MRIs (MRI-1: p<.001; MRI-2: p=.001; MRI-3: p<.001) (Table 4; Figure 3). Median percent change in volume compared to MRI-pre was as follows: MRI-pre to MRI-1: −48.0% ± 44.9 (range: −85.5 to 76.5); MRI-pre to MRI-2: −67.8% ± 64.2 (−98.5 to 160.8); MRI-pre to MRI-3: −84.8% ± 25.5 (−100.0 to −12.5).
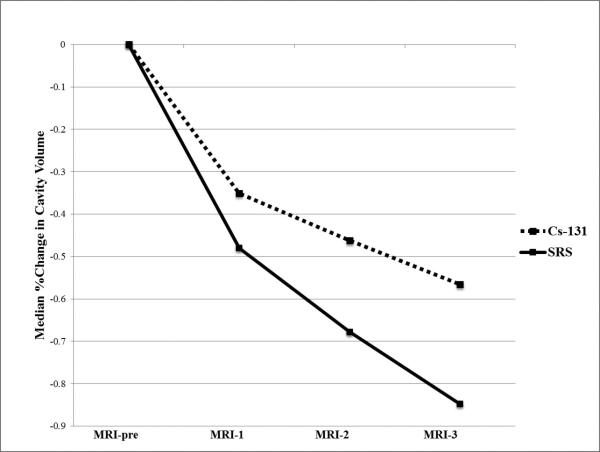
Figure 3.
This line graph demonstrates the trend of cavity volume shrinkage over the follow-up period in the Cs-131 versus SRS patient cohorts. Median percent change at each time point (MRI-1, 2, 3) refers to the percent change that occurred over the time period between pre-operative tumor volume (MRI-pre) and those end-points.
There was a statistically significant difference in cavity shrinkage between the Cs-131 and SRS cohorts from MRI-1 to MRI-2 when the majority of Cs-131 radiation is delivered. The median volume shrinkage of 46.7% in SRS patients exceeded that of 22.0% in Cs patients during this first month after surgery (p=.042). Moreover, SRS patients were significantly more likely than Cs-131 patients to experience cavity shrinkage between the following time points: MRI-pre and MRI-2 (p=.034), MRI-pre and MRI-3 (p=.008), and MRI-1 to MRI-3 (p=.028).
Impact of pre-operative tumor size
Volume dynamics were also compared in Cs-131 patients initially presenting with larger tumors versus smaller tumors, in order to investigate a previously reported relationship between larger tumor size and increased post-operative cavity shrinkage.27,29 A volume cut-off of 6 cm3 was used that allowed for sufficiently large subgroups for analysis (Table 5).
Table 5.
Definition and Median Volume of Small versus Large Tumors (Cs-131 Patients Only)
| Volume cut-off | Median volume (cm3) ± SD (range) | Number of lesions | |
|---|---|---|---|
| Small tumors | <6cm3 | 3.9 ± 1.2 (1.9-5.6) | 11 |
| Large tumors | >6cm3 | 15.6 ± 16.1 (6.8-79.1) | 19 |
Median percent change during each time period is reported separately for small and large tumors in Table 6. When comparing post-operative cavity volumes to pre-operative tumor volume, both small and large tumor groups showed consistent median volume decreases, though only large tumors exhibited statistically significant shrinkage at each time point through MRI-3. When comparing cavity dynamics in the small versus large tumor groups, there was a statistically significant difference from MRI-1 to MRI-2. During this critical dose delivery period, small tumors showed a median 33.8% increase in cavity volume, while large tumors showed a median 31.0% decrease in cavity volume (Figure 4). This suggests that cavities resulting from small tumors are more likely to expand while cavities from large tumors are more likely to shrink from MRI-1 to MRI-2.
Table 6.
Comparison of Cavity Volume Dynamics Based upon Initial Tumor Size (Cs-131 Patients Only)
| Small Tumors (<6cm3) | Large Tumors (>6cm3) | Small vs. Large | |||||
|---|---|---|---|---|---|---|---|
| Time span | N | Median % change in cavity volume ± SD (range) | p* | N | Median % change in cavity volume ± SD (range) | p* | p† |
| MRI-pre to MRI-1 | 11 | −45.8% ± 69.8% (−78.9% – 129.2%) | .155 | 19 | −35.0% ± 40.1% (−69.0% – 79.0%) | .008 | .741 |
| MRI-pre to MRI-2 | 8 | −42.0% ± 124.5% (−57.7% – 305.9%) | .208 | 13 | −53.0% ± 26.3% (−78.0% – 5.0%) | .002 | .242 |
| MRI-pre to MRI-3 | 6 | −64.1% ± 21.8% (−76.7% – −16.8%) | .028 | 11 | −47.0% ± 22.3% (−79.0% – −12.0%) | .003 | .359 |
| MRI-1 to MRI-2 | 8 | 33.8% ± 50.3% (−44.0% – 100.0%) | .401 | 13 | −31.0% ± 30.3% (−76.0% – 41.0%) | .019 | .023 |
| MRI-1 to MRI-3 | 6 | −23.0% ± 27.6% (−56.2% – 10.4%) | .116 | 11 | −18.0% ± 28.9% (−83.0% – 1.0%) | .004 | .781 |
This p-value is the result of a Wilcoxon signed-rank test comparing the absolute volumes at each endpoint in the time span for the small and large tumor groups separately.
This p-value is the result of a non-parametric Kolmogorov-Smirnov test comparing the percent change in cavity volume over each time span in small versus large tumors.
Figure 4.
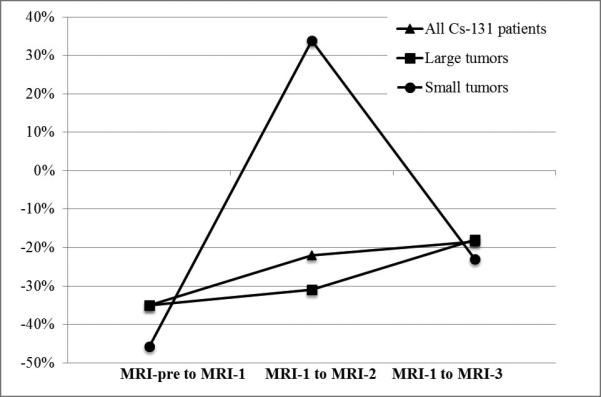
This graph of cavity dynamics compares the median percent change in cavity volume over different time periods. The dynamics of the following groups are illustrated: all Cs-131 patients, Cs-131 patients with large pre-operative tumors, and Cs-131 patients with small pre-operative tumors.
Edema
In the Cs-131 group, median pre-operative edema volume was 42.4 cm3 ± 37.5 (range 6.4-137.6), while the median post-operative edema volume on MRI-3 was 2.5 cm3 ± 8.7 (range 0.04-30.0), representing a median 90.2% reduction. There was a significant decrease in edema volume between MRI-pre and each post-operative scan (MRI-1: p=0.008; MRI-2: p<.001; MRI-3: p<.001), though the largest median decrease occurred between MRI-1 and MRI-2 (91.4%). There was only a median 7.1% decrease between pre-MRI and MRI-1.
In the SRS control group, median pre-operative edema volume was 41.0 cm3 ± 44.6 (range 2.4-166.3), while the median post-operative edema volume on MRI-3 was 3.4 cm3 ± 4.4 (range 0.3-19.1), representing a median 92.2% reduction. There was a significant decrease in edema volume between MRI-pre and both MRI-2 (p=.002) and MRI-3 (p<.001), though there was no significant decrease between MRI-pre and MRI-1 (p=.133). Similarly to the Cs-131 group, the largest median decrease in edema in the SRS group occurred between MRI-1 and MRI-2 (78.6%). There was only a median 3.9% decrease between pre-MRI and MRI-1.
Radiation Necrosis
Ultimately, the proof of principal for the efficacy of this technique will be the reduction of radiation necrosis rates. In both the Cs-131 and SRS group, there were 0 cases of radiation necrosis.
Discussion
The concept of post-resection intracavitary brachytherapy for brain metastases is appealing. Without additional radiation therapy, the local recurrence rate is high and residual tumor cells are found at the margin of the cavity. Brachytherapy targets this exact area and allows the delivery of therapy at the ideal time when the population of multiplying cells is at its lowest. Early studies of brachytherapy, however, reported high rates of radiation necrosis, leading to strong criticisms of brachytherapy-related toxicity. Brachytherapy traditionally involved the usage of high-dose permanent implants20 and was performed either concurrently with WBRT25 or post-WBRT.17,24 In more recent trials attempting to minimize toxicity, brachytherapy has been performed without WBRT and with lowered doses. Studies using low-dose implants showed that reduction in radiation necrosis could be achieved at the cost of sacrificing local control, and vice versa with high-dose implants.18,20 Together, these findings point to the need to optimize brachytherapy radiation dose and seed activity to minimize radiation necrosis and maximize local control.
Our center has attempted to address toxicity concerns while maintaining local control with the usage of the novel Cs-131 isotope and its limited time of dose delivery. The permanent Cs-131 seeds used in this study, with an activity of 3-5 mCi, were implanted with a planned dose of 80 Gy to a 5 mm depth from the surface of the resection cavity. Compared to the historically-utilized I-125 isotope, Cs-131 has more beneficial physical and radiobiological properties. Cs-131 has a higher dose rate of .342 Gy/h compared to I-125's dose rate of .069Gy/h; Cs-131 therefore has a shorter t1/2 (9.7 days compared to 59.4 days for I-125), resulting in reduced long-term seed activity.33 Additionally, Cs-131 has a high mean energy of 29 keV, which facilitates the implantation of fewer seeds per unit volume. Finally, studies of brachytherapy usage for prostate cancer show that Cs-131 is clinically superior to I-125 and Palladium-103 isotopes.30 The previously reported clinical outcomes from our Cs-131 trial are encouraging, with no reported toxicity (radiation necrosis) or local recurrence, only one regional recurrence event (6.2%), and one-year survival of 50.0%.34 The consistent and drastic decrease in edema over the follow-up period also suggests that there is decreased injury of the surrounding tissues. The results in this study support our prior conclusions with dramatically lower rates of radiation necrosis compared with prior trials of brachytherapy.
One variable affecting radiation delivery and the risk of subsequent toxicity to the surrounding parenchyma is the stability of tumor cavity volume post-resection.26,28,31,32 Early studies of I-125 implantation for primary gliomas showed that tumor shrinkage during active radiation delivery is correlated with increased radiation of surrounding parenchyma and increased risk of radiation necrosis. Looking at cavity dynamics from a different perspective, two recent SRS studies have analyzed post-resection cavity dynamics to explore if there is an optimal time to perform SRS after surgery. Jarvis et al. demonstrated the potential for cavity volume change in the mean 30 days between tumor resection and SRS, with 23.3% of cavities shrinking more than 2 cm3 and 30.2% of cavities increasing in size more than 2 cm3.29 Analysis of mean volumes of the entire patient population showed shrinkage from the mean pre-operative tumor volume of 14.2 cm3 to the immediately post-operative cavity volume of 8.5 cm3. Relatively minimal change was then reported at the second post-operative MRI, with a mean volume of 8.77 cm3. Atalar et al. similarly reported significant cavity shrinkage occurring immediately after surgery in a different SRS population, without further significant change over the remainder of the 33-day follow-up period.27
In order to avoid this early cavity volume fluctuation, we used the “seeds-on-a-string” method. We stabilized the cavity by implanting seeds on strands that exert an outward force on the cavity and introduced fibrin glue into the cavity. After a dramatic decrease in volume that results from tumor removal and successfully alleviates any symptoms of mass effect, our results show a minimal change in cavity size in the first post-operative month when most of the Cs-131 dose is delivered. Furthermore, compared to patients who underwent SRS, an external radiation technique, the Cs-131 patients exhibited significantly less cavity shrinkage over the entire follow-up period and specifically during this critical period of maximal dose delivery. This difference in cavity volume dynamics suggests that the physical qualities of the brachytherapy seeds and the usage of fibrin glue during implantation are successful at minimizing the shrinkage that occurs in the absence of this intracavitary intervention. However, given that both techniques (i.e., the seeds and the fibrin glue) were used together, we cannot differentiate which is more important. Moreover, both groups exhibited significant and substantial edema resolution from MRI-1 to MRI-2, likely promoting a relief of inward-directed pressure on the resection cavity. Despite this relief, the SRS group continued to exhibit significant shrinkage during this period while the Cs-131 group did not, suggesting that the seeds-on-a-string plus fibrin glue exert an outward force that helps maintain cavity stability.
Another method of brachytherapy that similarly attempts to maintain cavity volume over the course of radiation delivery with intracavitary intervention is the GliaSite system. GliaSite is a temporary high-dose rapid-delivery radiation modality in which an intracavitary balloon catheter is implanted to fill and stabilize the cavity before seeds are introduced. In a prospective Gliasite trial, Rogers et al. implanted brachytherapy seeds (with a total dosage of 60 Gy) into the cavity 21 days after resection of brain metastases, resulting in radiation necrosis in 15.5% of patients.23 While GliaSite effectively controls cavity dynamics, we prefer the seeds-on-a-string model that starts radiation delivery without delay after resection, uses a lower dosage, and results in fewer cases of radiation necrosis.34
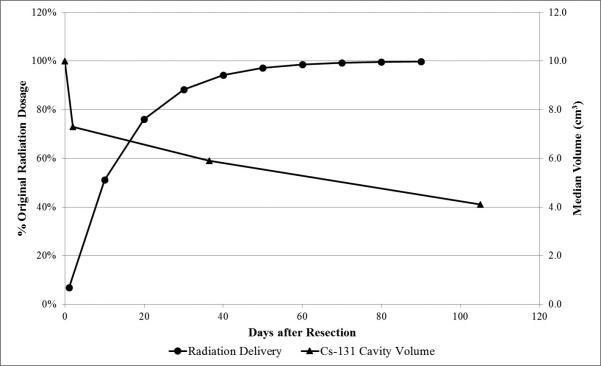
Figure 5 superimposes the trajectory of Cs-131 radiation delivery with the changing cavity volume over the follow-up. The majority of radiation is delivered in the first month following surgery after the initial significant decrease in cavity volume from surgical bulking has already occurred. Since dosimetry is adjusted real-time based upon intra-operative volume (ie. the volume of the cavity dictates the spacing of seeds, normally spaced at 1-cm intervals, which then dictates later dose calculation), the immediate collapse due to tumor debulking does not affect dose delivery. We therefore emphasize the comparison of radiation delivery dynamics to volume dynamics during the period following MRI-1, with MRI-1 volume serving as our best available representation for the early, post-debulking volume of the cavity given limitations in intra-operative volume calculation. During this period, there is non-significant shrinkage in the Cs-131 patients (from MRI-1 to MRI-2) and significantly less shrinkage relative to the SRS patients (from MRI-1 to MRI-2 and MRI-3). Only 23.9% and 11.7% of radiation remains at day 20 and day 30 post-implantation, respectively. By MRI-2 (at median day 36), more than 88.3% of the radiation has already been delivered. Thus, the majority of radiation is delivered when the Cs-131 cavity does not exhibit significant shrinkage, which is corroborated by the previously reported absence of radiation necrosis in this patient population, though these patients were only followed over a median 19.3-month period.34 In contrast to Cs-131, I-125 has a longer t1/2 (59.4 days), which may increase the clinical repercussions of cavity shrinkage. There continues to be significant I-125 radiation delivery as the cavity volume shrinks post-operatively, possibly accounting for its higher reported rates of radiation necrosis (0-40%).20,26,31
Figure 5.
This composite graph shows the calculated radiation delivery curve of Cesium-131 superimposed upon the changing median cavity volume over the follow-up period.
Based upon previous findings,27,29 we also compared cavity dynamics in patients presenting with large versus small tumors. In a post-SRS setting, it has been reported that cavities resulting from larger tumors tended to shrink more than cavities resulting from smaller tumors.27,29 Our results were slightly different and a bit more complicated. In our Cs-131 patient population, smaller tumors actually shrunk more after initial debulking than larger tumors, although the difference between the two groups was minimal. Interestingly, smaller tumor cavities then showed an increase in median volume during the first month after surgery, in significant contrast to the larger tumors. This is likely caused by the dramatic decrease in edema combined with the slight outward pressure provided by the tensile strength of the Cs-131 seeds and the fibrin glue. In the long run, however, both large and small tumor subgroups showed significant shrinkage between MRI-pre and MRI-3. While this analysis is limited due to our small patient population, these results may suggest that cavities from small tumors are more likely to fluctuate in volume while cavities from large tumors exhibit more consistent shrinkage. Future studies should further address this relationship with larger patient populations.
Limitations
Our usage of SRS patients as controls for the Cs-131 patients introduces a limitation into the study because SRS is a delayed therapy after surgery, while Cs-131 implantation occurs intra-operatively. The median timing of SRS was 50 days following surgery, which falls in the time period from MRI-2 to MRI-3. While this radiosurgical intervention is a potential confounding variable that could promote additional cavity shrinkage during this later time period, most SRS patients did not undergo radiosurgical intervention until after the critical period from MRI-1 to MRI-2 that we emphasize as most important for comparison. Another possible control group would be patients who had brachytherapy seeds placed that were not on a string and without fibrin glue in the cavity. However, such a control group was not available to us.
Due to scheduling and other logistical constraints, only a subset of patients had a full series of three post-operative scans. The resulting small patient populations hindered some statistical analysis, especially when comparing patient subgroups. Additionally, the majority of patients did not have intra-operative cavity volume measured due to logistical difficulties. In the future, more accurate and reliable intra-operative cavity volume calculation should be performed using intra-operative imaging to create a more clinically relevant baseline for cavity dynamics analysis.
Conclusion
Our data show that after an initial rapid decrease in cavity volume due to surgical debulking, the strategy of lining the cavity with Cs-131 seeds on a string and using fibrin glue successfully maintains cavity volume in the first 30 days after the seeds are placed. Maintenance of volume during this period likely promotes homogenous radiation during the time of Cs-131 maximal dose delivery given the lack of radiation necrosis in this cohort.34 Compared to the Cs-131 cohort, SRS patients lacked intracavitary intervention and exhibited significantly more cavity volume shrinkage during this critical period of dose delivery and the entire follow-up period. Intracavitary Cs-131 brachytherapy should therefore be paired with a volume-maintenance technique such as the seeds-on-a-string method with fibrin glue to promote dose homogeneity and minimize risk of radiation-induced injury to surrounding parenchyma.
Acknowledgments
Financial support: Dr. A. Gabriella Wernicke was supported by the NIH KL2 grant 3KL2RR024997. Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-TR000457-06).
Abbreviations
- Cs-131
Cesium-131
- I-125
Iodine-125
- MRI
magnetic resonance imaging
- SRS
stereotactic radiosurgery
- t1/2
half-life
- WBRT
whole brain radiotherapy
Footnotes
Disclosure: Theodore Schwartz: Visionsense (Consultant), Karl Storz (Consultant). The other authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. This work was presented at ASTRO's 55th Annual Meeting, Atlanta, GA, 2013.
References
- 1.Bradley KA, Mehta MP. Management of brain metastases. Semin Oncol. 2004;31:693–701. doi: 10.1053/j.seminoncol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Weil RJ. Does trastuzumab increase the risk of isolated central nervous system metastases in patients with breast cancer? Nat Clin Pract Oncol. 2006;3:236–237. doi: 10.1038/ncponc0487. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 6.Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:33–43. doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 8.Chow E, Davis L, Holden L, Tsao M, Danjoux C. Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage. 2005;30:18–23. doi: 10.1016/j.jpainsymman.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients' perspective regarding complications. Am J Clin Oncol. 2005;28:173–179. doi: 10.1097/01.coc.0000143016.15783.5b. [DOI] [PubMed] [Google Scholar]
- 10.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 11.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 13.Nieder C, Schwerdtfeger K, Steudel WI, Schnabel K. Patterns of relapse and late toxicity after resection and whole-brain radiotherapy for solitary brain metastases. Strahlenther Onkol. 1998;174:275–278. doi: 10.1007/BF03038721. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 15.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 16.Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519–526. doi: 10.1016/s0360-3016(02)02770-0. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein M, Cabantog A, Laperriere N, Leung P, Thomason C. Brachytherapy for recurrent single brain metastasis. Can J Neurol Sci. 1995;22:13–16. doi: 10.1017/s0317167100040439. [DOI] [PubMed] [Google Scholar]
- 18.Bogart JA, Ungureanu C, Shihadeh E, et al. Resection and permanent I-125 brachytherapy without whole brain irradiation for solitary brain metastasis from non-small cell lung carcinoma. J Neurooncol. 1999;44:53–57. doi: 10.1023/a:1006285304892. [DOI] [PubMed] [Google Scholar]
- 19.Dagnew E, Kanski J, McDermott MW, et al. Management of newly diagnosed single brain metastasis using resection and permanent iodine-125 seeds without initial whole-brain radiotherapy: a two institution experience. Neurosurg Focus. 2007;22:E3. doi: 10.3171/foc.2007.22.3.4. [DOI] [PubMed] [Google Scholar]
- 20.Huang K, Sneed PK, Kunwar S, et al. Surgical resection and permanent iodine-125 brachytherapy for brain metastases. J Neurooncol. 2009;91:83–93. doi: 10.1007/s11060-008-9686-2. [DOI] [PubMed] [Google Scholar]
- 21.McDermott MW, Cosgrove GR, Larson DA, Sneed PK, Gutin PH. Interstitial brachytherapy for intracranial metastases. Neurosurg Clin N Am. 1996;7:485–495. [PubMed] [Google Scholar]
- 22.Ostertag CB, Kreth FW. Interstitial iodine-125 radiosurgery for cerebral metastases. Br J Neurosurg. 1995;9:593–603. doi: 10.1080/02688699550040873. [DOI] [PubMed] [Google Scholar]
- 23.Rogers LR, Rock JP, Sills AK, et al. Results of a phase II trial of the GliaSite radiation therapy system for the treatment of newly diagnosed, resected single brain metastases. J Neurosurg. 2006;105:375–384. doi: 10.3171/jns.2006.105.3.375. [DOI] [PubMed] [Google Scholar]
- 24.Schulder M, Black PM, Shrieve DC, et al. Permanent low-activity iodine-125 implants for cerebral metastases. J Neurooncol. 1997;33:213–221. doi: 10.1023/a:1005798027813. [DOI] [PubMed] [Google Scholar]
- 25.Zamorano L, Yakar D, Dujovny M, et al. Permanent iodine-125 implant and external beam radiation therapy for the treatment of malignant brain tumors. Stereotact Funct Neurosurg. 1992;59:183–192. doi: 10.1159/000098940. [DOI] [PubMed] [Google Scholar]
- 26.Wowra B, Schmitt HP, Sturm V. Incidence of late radiation necrosis with transient mass effect after interstitial low dose rate radiotherapy for cerebral gliomas. Acta Neurochir (Wien) 1989;99(3-4):104–8. doi: 10.1007/BF01402316. [DOI] [PubMed] [Google Scholar]
- 27.Atalar B, Choi CY, Harsh GR, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72:180–185. doi: 10.1227/NEU.0b013e31827b99f3. [DOI] [PubMed] [Google Scholar]
- 28.Dale RG, Jones B, Coles IP. Effect of tumour shrinkage on the biological effectiveness of permanent brachytherapy implants. Br J Radiol. 1994;67:639–645. doi: 10.1259/0007-1285-67-799-639. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis LA, Simmons NE, Bellerive M, et al. Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys. 2012;84:943–948. doi: 10.1016/j.ijrobp.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 30.Yang R, Wang J, Zhang H. Dosimetric study of CS-131, I-125 and Pd-103 seeds for permanent prostate brachytherapy. Cancer Biother Radiopharm. 2009;24:701–705. doi: 10.1089/cbr.2009.0648. [DOI] [PubMed] [Google Scholar]
- 31.Dale RG, Jones B. Enhanced normal tissue doses caused by tumor shrinkage during brachytherapy. Br J Radiol. 1999;72(857):499–501. doi: 10.1259/bjr.72.857.10505017. [DOI] [PubMed] [Google Scholar]
- 32.Kreth FW, Faist M, Rossner R, et al. The risk of interstitial radiotherapy of low-grade gliomas. Radiother Oncol. 1997;43:253–60. doi: 10.1016/s0167-8140(97)01948-8. [DOI] [PubMed] [Google Scholar]
- 33.Henschke UK, Lawrence DC. Cesium-131 Seeds for Permanent Implants. Radiology. 1965;85:1117–1119. doi: 10.1148/85.6.1117. [DOI] [PubMed] [Google Scholar]
- 34.Wernicke AG, Yondorf MZ, Peng L, et al. Phase I/II study of neurosurgical resection and intra-operative cesium-131 radio-isotope brachytherapy in patients with newly diagnosed brain metastases. J Neurosurg. 2014;121(2):338–348. doi: 10.3171/2014.3.JNS131140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]