Abstract
Loricrin is a major component of the cornified cell envelope, a highly insoluble structure composed of covalently cross-linked proteins. Although loricrin knockout mice only exhibit a mild transient phenotype at birth, they show a marked delay in the formation of an epidermal barrier in utero. We recently discovered that induction of a compensatory response to repair the defective barrier is initiated by amniotic fluid via activation of NF-E2-related factor 2 and identified Sprr2d and Sprr2h as direct transcriptional targets. Proteomic analysis suggested that other proteins were also incorporated into the loricrin knockout cell envelope, in addition to the small proline rich proteins. Here we present evidence suggesting that the late cornified envelope 1 proteins are also compensatory components as determined by their localization within the loricrin knockout cell envelope via immunoelectron microscopy. We also demonstrate that late cornified envelope 1 genes are upregulated at the transcriptional level in loricrin knockout mouse skin and confirm that late cornified envelope 1 genes are transcriptional targets of NRF2. Our present study further highlights the complexity and importance of a compensatory mechanism that evolved in terrestrial animals to ensure the formation of a functional epidermal barrier.
INTRODUCTION
The evolution of terrestrial animals required the development of a barrier between the body and the environment to prevent dehydration and infection. A key element for the mammalian epidermal water barrier is the cornified cell envelope (CE), an insoluble protein/lipid matrix that replaces the plasma membrane of terminally differentiating keratinocytes (Hohl, 1990; Rice and Green, 1977). The assembly of this macromolecular complex is initiated by transglutaminases that catalyze the cross-linking of proteins via γ-glutamyl lysine isopeptide bonds (Rice and Green, 1977). Most of the genes that encode CE components are clustered in the epidermal differentiation complex (EDC) (Mischke et al., 1996) located on mouse chromosome 3 and human chromosome 1q21, respectively. The presence of conserved amino acid sequence motifs in the major CE components suggests that they evolved by duplication of a common ancestral gene (Backendorf and Hohl, 1992). Whole genome sequencing and histochemical analyses revealed that fish and amphibians do not contain CE components, suggesting that the evolution of the EDC coincided with the ability to survive on land (Strasser et al., 2014).
The main protein component of the CE is loricrin (LOR), a glycine (Gly)/serine (Ser)-rich protein that accounts for approximately 70% of the total mass of the CE (Hohl et al., 1991; Mehrel et al., 1990; Yoneda et al., 1992). LOR is considered a reinforcement protein that is incorporated at a late stage of CE formation (Kalinin et al., 2001; Steinert and Marekov, 1995, 1999; Yoneda et al., 1992). Lor knockout (LKO) mice survive to adulthood and develop only a mild, transient phenotype at birth. Nevertheless, examination of LKO embryos revealed a delay in the formation of a permeability barrier in utero (Koch et al., 2000).
The NF-E2-related factor 2 (NRF2)/Kelch-like ECH-associated protein 1 (KEAP1) pathway is a central regulator of the antioxidative response that transactivates a battery of phase 2 detoxifying enzymes (Itoh et al., 2004). However, when Keap1 was ablated from the germline of mice, Keap1-deficient mice died postnatally because of the inability to feed caused by hyperkeratosis of the esophagus/forestomach, suggesting the pathway's prominent role as a regulator of squamous epithelia (Schafer et al., 2012; Wakabayashi et al., 2003). Recently, we found that NRF2 (Moi et al., 1994) and its inhibitor KEAP1 (Itoh et al., 1999) play essential roles in inducing the compensatory response, which leads to the development of functional CEs in LKO mice. Specifically, we demonstrated that exposure of the embryonic LKO skin to amniotic fluid induces upregulation of Sprr2d and Sprr2h gene expression at around embryonic day 16.5 (E16.5). Further, we showed that these two genes are directly regulated by the transcription factor NRF2 (Huebner et al., 2012).
Our previous analysis of the amino acid composition of LKO CE suggested that other components, in addition to SPRR2D and SPRR2H, must be upregulated and incorporated into LKO CE to account for the high Gly and Ser content of LKO CE (Jarnik et al., 2002). Like LOR, the late cornified envelope 1 (LCE1) proteins also contain amino acid sequence motifs enriched in Gly and Ser (Marshall et al., 2001). Here we report that LCE1 proteins are incorporated into LKO CE to compensate the loss of LOR and identify LCE1 family members as NRF2 target genes.
RESULTS
LCE1 proteins contain Gly/Ser loop motifs and are good candidates to compensate for the loss of LOR
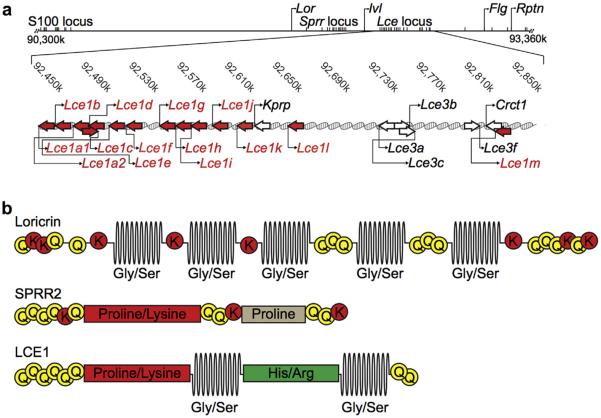
To identify proteins that could account for the unexpected high Gly and Ser content of LKO CEs, we searched the mouse genome for genes encoding Gly- and Ser-rich proteins (Jarnik et al., 2002). We identified the Lce gene family within the EDC as potential candidates (Figure 1a). The predicted LCE1 amino acid sequence shows similarities to LOR, with potential transglutaminase cross-linking sites in both the carboxy and amino termini, and Gly/Ser loops in the center of the protein sequence (Figure 1b). Because of the presence of the Gly/Ser loops, we hypothesize that LCE1 proteins share some of the elastic properties attributed to LOR (Hohl et al., 1991; Steinert et al., 1991). Interestingly, separating the Gly/Ser loop motifs in LCE1 proteins is a histidine-/arginine-rich region that is not present in any other known CE proteins (Figure 1b).
Figure 1. LCE 1 proteins contain Gly/Ser loop motifs and are candidates to compensate for the loss of Lor in LKO mice.
Lce genes are clustered in the EDC and share similarities with both Lor and the Sprr gene family members. (a) Localization of Lce genes in the EDC of chromosome 3 (NCBI build 37.1). The arrows indicate the direction of transcription. The red arrows indicate the Lce1 genes. S100, S100 calcium binding protein gene; Kprp, keratinocyte proline-rich protein gene; Crct1, cysteine-rich C-terminal 1 gene. (b) LOR has multiple unstructured Gly/Ser loops surrounded by conserved glutamine and lysine residues that serve as cross-linking sites for transglutaminases. SPRR2 proteins lack Gly/Ser loops, instead, these proteins are characterized by long stretches of proline and lysine residues. LCE1 proteins resemble both LOR and SPRR2s in their amino acid sequences and the presence of proline and lysine motif as well as two Gly/Ser loop motifs. EDC, epidermal differentiation complex; FLG, filaggrin Gly/Ser, glycine/serine; His/Arg, histidine/arginine; LCE, late cornified envelope; LKO, loricrin knockout; LOR, loricrin.
LCE1 proteins are incorporated into LKO CE
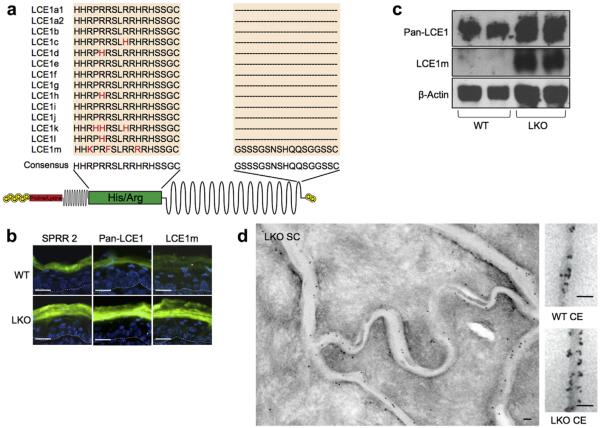
To determine if the LCE1 proteins were incorporated into LKO CE, we generated a pan-LCE1 antibody using a synthetic peptide that corresponds to the specific histidine/arginine region of LCE1 proteins (Figure 2a). We also generated an antibody specific to LCE1m using a synthetic peptide corresponding to its unique carboxy terminus (Figure 2a). Initially, we used these antibodies to perform immunofluorescence and western blot analysis of wild-type (WT) and LKO back skin. Both the pan-LCE1- and LCE1m-specific antibodies showed a marked increase in immunofluorescence staining in LKO epidermis that was initially detected in the uppermost granular layer and persisted throughout the stratum corneum similar to SPRR2 proteins (Figure 2b). Immunoblot analysis of tape-stripped stratum corneum demonstrated a pronounced increase in LCE1 proteins (Figure 2c).
Figure 2. LCE1 proteins are upregulated in LKO epidermis and incorporated into LKO CE.
(a) A pan-LCE1 antibody that recognizes LCE1 family members was generated using a synthetic peptide corresponding to the consensus His/Arg region, and an LCE1m-specific antibody was generated using a synthetic peptide that corresponds to a region in the carboxyl-terminus that is specific for this protein. (b) Immunofluorescent staining of back skin. Increased staining of both SPRR2 and the LCE1 antibodies was detected in the uppermost granular layers and cornified layers of LKO epidermis. Scale bars = 20 μm. (c) Protein from tape-stripped SC was subjected to immunoblotting. Both pan-LCE1 and LCE1m antibodies detected higher expression levels of LCE1 proteins in LKO SC. (d) Immunoelectron microscopic labeling of LCE1 family members in LKO stratum corneum and CEs isolated from WT and LKO mouse skin. Protein A-gold (10 nm) was used to detect bound antibodies on skin cryosections, while CE sections were probed with F(ab′)2 with ultrasmall gold, which was silver-enhanced for greater visibility. Scale bars = 100 nm. CE, cell envelope; His/Arg, histidine/arginine; LCE, late cornified envelope; LKO, loricrin knockout; SC, stratum corneum; WT, wild type.
To confirm that the LCE1 proteins were incorporated into LKO CEs, we performed immunoelectron microscopy on LKO and WT skin. Immunoelectron microscopy using the pan-LCE1 antibody confirmed that LCE1 proteins were localized to the cytosolic side of LKO corneocytes (Figure 2d). Immunoelectron microscopy of isolated CEs showed denser labeling of the LKO CE compared with WT, suggesting increased incorporation of LCE1 proteins into LKO CE (Figure 2d). A similar labeling pattern was detected with the LCE1m antibody (data not shown).
Lce1 genes are transcriptionally upregulated along with other known Nrf2 target genes in postnatal LKO skin
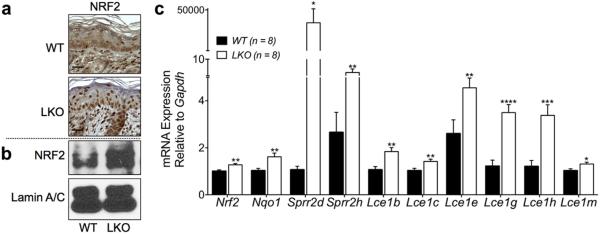
To determine if NRF2 was activated in postnatal LKO skin, we used our previously generated affinity-purified NRF2 antibody (Huebner et al., 2012) and performed immunohistochemistry on back skin obtained from LKO and WT mice and confirmed accumulation of NRF2 in the nuclei of LKO keratinocytes (Figure 3a). Additional evidence documenting NRF2 activation was obtained by immunoblot analysis of nuclear extracts from LKO and WT back skin (Figure 3b).
Figure 3. NRF2 is activated in LKO skin and Lce1 genes are transcriptionally upregulated along with other known NRF2 target genes in LKO skin.
(a) Immunohistochemistry was performed on formalin-fixed paraffin-embedded sections from back skin. Nuclear accumulation of NRF2 was seen in LKO epidermis. Scale bars = 20 μm. (b) Nuclear protein fractions were extracted from back skin and subjected to immunoblotting. LKO nuclear extracts showed a higher level of NRF2 protein. (c) Analysis of mRNA extracted from back skin by qRT-PCR. There was a statistically significant upregulation of Nqo1, Sprr2d, and Sprr2h genes, validated NRF2 downstream targets, in addition to the Nrf2 gene itself, in LKO skin. The transcripts of Lce1b, c, e, g, h, and m were significantly increased in LKO versus WT skin (WT n = 8, LKO n = 8, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001). LCE, late cornified envelope; LKO, loricrin knockout; NRF2, NF-E2-related factor 2; qRT-PCR, quantitative real-time PCR; WT, wild type.
Next, we wanted to determine whether the Lce1 genes were transcriptionally upregulated in postnatal LKO skin. Total RNA was isolated from back skin, and subjected to quantitative real-time PCR analysis using specific primers and probes for each member of the Lce1 family. The transcripts of the Lce1b, Lce1c, Lce1e, Lce1g, Lce1h, and Lce1m genes were significantly increased in LKO skin, along with several known Nrf2 target genes including NAD(P)H dehydrogenase, quinone 1 (Nqo1) (Li and Jaiswal, 1992), Sprr2d and Sprr2h (Huebner et al., 2012), as well as Nrf2 itself (Figure 3c).
Lce1 genes are transcriptionally upregulated after NRF2 activation
To determine whether Lce1 genes were upregulated after NRF2 activation, keratinocytes were isolated from neonatal WT mice, placed in culture, and treated with tert-butylhydroquinone (tBHQ), a well-known activator of the NRF2/KEAP1 pathway (Huang et al., 2000). Immunoblot analysis of nuclear proteins extracted from cultured mouse keratinocytes with tBHQ showed nuclear accumulation of NRF2 (Figure 4a). In addition, immunoblot analysis of the total cellular lysate showed upregulated expression levels of LCE1 proteins, as well as proteins encoded by the known NRF2 target genes, Nqo1 and Sprr2, in a tBHQ dose-dependent manner. Next, we analyzed RNA from cultured keratinocytes isolated from LKO mice and heterozygous littermates (controls) that were also treated with tBHQ. LKO keratinocytes had significantly increased transcripts of Lce1b, Lce1c, Lce1e, Lce1g, Lce1h, and Lce1m, compared with control keratinocytes (Figure 4b).
Figure 4. Lce1 genes are transcriptionally upregulated after NRF2 activation and downregulated following blocking of endogenous NRF2 activity.
(a) Keratinocytes from WT newborn mice were isolated, cultured, and incubated with the indicated concentration of tBHQ. Nuclear protein fractions (upper panels) and whole cell lysates (lower panels) were subjected to immunoblotting. tBHQ induced the accumulation of NRF2 in the nucleus (upper panels). There were increased expression levels of LCE1 proteins in a tBHQ dose-dependent manner, similar to those of validated NRF2 downstream targets; NQO1 and SPRR2 (lower panels). − (DMSO alone), + (tBHQ 10 μM), ++ (tBHQ 30 μM). (b) Keratincytes were isolated from 4-day-old LKO mice and cultured along with littermate controls (heterozygous for Lor). After treatment with tBHQ, LKO keratinocytes exhibited significantly increased transcripts for Lce1b, c, e, g, h, and m, compared with littermate controls. − (DMSO alone), + (tBHQ 4 μM), ++ (tBHQ 10 μM). (CTL (Lor±) n = 3, LKO n = 3, **P < 0.01, ***P < 0.005, ****P < 0.001). (c) The transcripts of Lce1b, c, e, g, h, and m are decreased in the back skin of LKO mice expressing the dnNRF2 transgene, compared with littermate LKO controls (WT n = 5, LKO n = 5, LKOdnNRF2 n = 3, ***P < 0.005, ****P < 0.001, LKOdnNRF2 vs LKO). CTL, control; DMSO, dimethyl sulfoxide; dnNRF2, dominant negative Nrf2 transgene; LCE, late cornified envelope; LKO, loricrin knockout; NQO1, NAD(P)H dehydrogenase, quinone 1; NRF2, NF-E2-related factor 2; tBHQ, tert-butylhydroquinone; WT, wild type.
We previously used a dominant negative Nrf2 transgene to show that blocking endogenous NRF2 activity resulted in suppression of the NRF2 target genes, Sprr2d and Sprr2h, in LKO mice (Huebner et al., 2012). Therefore, we wanted to determine the effect of blocking endogenous NRF2 activity on the induction of Lce1 genes in LKO mice. As previously shown for the Sprr2d and Sprr2h genes (Huebner et al., 2012), LKO mice expressing the dominant negative Nrf2 transgene also exhibited marked suppression of transcription of the following Lce1 genes: Lce1b, Lce1c, Lce1e, Lce1g, Lce1h, and Lce1m (Figure 4c).
Taken together, these results suggest that the Lce1 genes are transcriptionally upregulated after the activation of the NRF2/KEAP1 pathway.
The Lce1e gene is a direct downstream target of NRF2
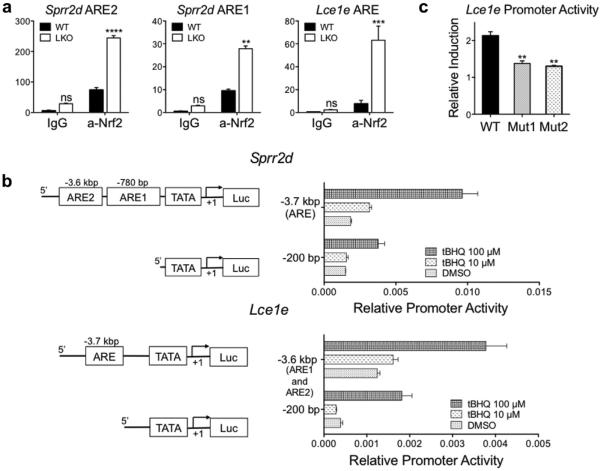
Because NRF2 has a specific target sequence called the antioxidant response element (Rushmore et al., 1991; Wasserman and Fahl, 1997), we searched the putative promoter regions (up to 50 kbp upstream) of all Lce1 genes that were upregulated in LKO skin for the consensus ARE sequence, 5'-TGACnnnGC-3', and found AREs for all of these genes. To confirm the ability of NRF2 to bind to the AREs in Lce1 genes, we focused on the Lce1e gene and performed chromatin immunoprecipitation assays using the NRF2 antibody to determine if NRF2 was bound to the ARE in its promoter region in vivo. Quantitative PCR analysis revealed that there was enrichment of the DNA fragments containing the Sprr2d and Lce1e AREs in the nuclear fraction of LKO skin immunoprecipitated by the NRF2 antibody (Figure 5a).
Figure 5. The Lce1e gene is a direct downstream target of NRF2.
(a) Chromatin immunoprecipitation assays were performed on nuclear extracts isolated from LKO and WT back skin using the anti-NRF2 antibody. LKO skin showed enrichment of the region flanking AREs, which are upstream of Sprr2d and Lce1e genes (WT n = 3, LKO n = 3, **P < 0.01, ***P < 0.005, ****P < 0.001). (b) Schematic representation of putative promoter regions of the Sprr2d and Lce1e genes (left panel). The Sprr2d gene has an AREs at −780 bp and −3.6 kbp upstream, and Lce1e has an ARE at −3.7 kbp upstream from the transcription start site. Luciferase expression constructs containing the promoter regions of the Sprr2d and Lce1e genes show increased activity in a tBHQ dose-dependent manner (right panel). (c) Mutations in the ARE reduce Lce1e promoter activity. Two different mutations were introduced into the ARE in the promoter region of the Lce1e gene and inserted into the luciferase expression vector. Constructs containing both mutations exhibit reduced activity in response to tBHQ compared with the expression construct containing the WT Lce1e ARE (WT n = 6, Mutants [Muts] n = 6, **P < 0.01). ARE, antioxidant response element; LCE, late cornified envelope; LKO, loricrin knockout; NRF2, NF-E2-related factor 2; tBHQ, tert-butylhydroquinone; WT, wild type.
To confirm that the ARE in the Lce1e gene was functional, we cloned the putative promoter region of this gene, which contains an ARE (5'-TGACATAGC -3') 3.7 kbp upstream from the transcription start site, into a luciferase expression vector, along with vectors containing the promoter region of the Sprr2d gene that contains AREs −780 bp and −3.6 kb upstream (Huebner et al., 2012). Luciferase assays revealed increased transcription activity for both genes in a tBHQ dose-dependent manner (Figure 5b). In addition, vectors containing a mutated ARE, predicted to prevent NRF2 binding, showed reduced transcriptional activity (Figure 5c). These results confirm that the Lce1e gene has a functional ARE site and is thus an NRF2 downstream target.
DISCUSSION
The skin is frequently exposed to environmental stress that can lead to the generation of reactive oxygen species in keratinocytes, a process that is countered by the activation of the NRF2/KEAP1 pathway. Consistent with its role in neutralizing the impact of reactive oxygen species in the outer layers of the epidermis, nuclear localization of NRF2 is particularly obvious in suprabasal keratinocytes (Schafer et al., 2010). Consequently, the expression of proteins encoded by NRF2 target genes, such as the SPRR2 proteins, which function as a protective antioxidant shield (Vermeij et al., 2011; Vermeij and Backendorf, 2010), is initially detected in the suprabasal layers of the epidermis. Similarly, induction of LCE1 proteins has been reported after exposure to stress such as UVB irradiation (Jackson et al., 2005) and mechanical stress (de Cid et al., 2009).
From a phylogenetic perspective, the major CE components (LOR, involucrin, and SPRRs) appear to have emerged by duplication of a common ancestral gene (Backendorf and Hohl, 1992). Furthermore, a recent study revealed that among non-mammalians, EDC homologs were found only in chicken and green anole lizard, not fish or amphibians (Strasser et al., 2014). Thus, the emergence of CE component genes, as well as the entire EDC, coincided with the ability to survive on land. The marked divergence in the chromosomal organization and protein coding sequences of Lce genes between rodents, chimpanzees, and humans (Brown et al., 2007) suggests that CE component genes continued to evolve to adapt to the ever-changing external environment of each mammalian's lifestyle and maintain an optimal epidermal barrier.
We would like to propose LOR as an adapter protein that allows filaggrin (FLG) cross-linking into the CE, which is also rich in histidine-/arginine-like LCE1 proteins. The lack of lysine residues in the FLG monomer suggests the necessity for a lysine donor from elsewhere to make a γ-glutamyl lysine isopeptide bond. Indeed, mass spectrometry analysis revealed a markedly decreased amount of FLG in LKO CE, supporting this hypothesis (unpublished data). Thus, the increased levels of LCE1 proteins, which are rich in potential transglutaminase cross-linking sites, might compensate for this adapter function of LOR in the LKO epidermis, as well as for histidine/arginine that were reduced in LKO CE from the loss of FLG.
Loss-of-function mutations in the Flg gene are bona fide risk factors for developing atopic dermatitis and subsequently, asthma or food allergy (Palmer et al., 2006). Interestingly, deletion of the Lce3b and Lce3c genes has been identified as a susceptibility factor for developing psoriasis (de Cid et al., 2009), which is often accompanied with arthritis (Roberson and Bowcock, 2010). These findings suggest that genetic alterations that impair epidermal barrier function can affect the immune response, which has been documented both clinically (De Benedetto et al., 2012) and experimentally (Fallon et al., 2009; Saunders et al., 2015; Strid et al., 2011). Future studies will determine whether loss-of-function mutations in LOR can affect the immune system and also be a risk factor for developing atopic dermatitis.
MATERIALS AND METHODS
Experimental animal
The LKO mouse (Koch et al., 2000) was maintained on an FvB background. WT mice were purchased from Charles River (Wilmington, MA). All mice were maintained under specific pathogen-free conditions. All animal experiments were approved by the Institutional Animal Use and Care committee and conducted in compliance with all applicable university, state, and federal regulations.
Generation of antibodies against LCE1 proteins
Polyclonal antibodies were raised in New Zealand white female rabbits. We designed the peptide “HHRPRRSLRRHRHSSGC” to react with several members of the LCE1 family (Figure 2a). We also designed the peptide “GSSSGSNSHQQSGGSSC” to react specifically with LCE1m. Sera were affinity-purified against the synthetic peptides using Affi-Gel 10 (Bio-Rad, Hercules, CA).
Electron microscopy
Immunogold labeling of neonatal WT, LKO skin, and isolated CEs (Jarnik et al., 2002) was performed using a modification of the Tokuyasu technique (Slot and Geuze, 2007). In brief, 1-mm cubes of tissue were fixed in 2% formaldehyde/0.2% glutaraldehyde, infused in 2.3 M sucrose, frozen, and sectioned in a cryo-ultramicrotome (Ultracut UCT; Leica, Solms, Germany). CEs were briefly sonicated and embedded in 10% gelatin, and then subjected to the same procedure as skin tissue. Sections approximately 100 nm thick were labeled with the pan-Lce1 antibody. For skin sections, 10-nm protein A-gold (Aurion, Wageningen, the Netherlands) was used to detect the labeling antibody. With CEs, only low labeling density was obtained with either 10- or 6-nm protein A-gold, so goat-anti-rabbit F(ab′)2 fragments bound to ultrasmall gold (Aurion) were used instead. To make the 0.8-nm ultrasmall gold particles visible, silver enhancement was performed using an Aurion R-Gent SE-EM kit. Antibody to LOR was used as a positive control in WT sections and as negative control in LKO samples. In the latter case, no labeling was observed.
Immunoblotting
Stratum corneum was collected by applying stripping disks (D101; CuDerm, Dallas, TX) twice on the back skin of 4-day-old killed mice. After washing with phosphate buffered saline, the disks were incubated in SDS extraction buffer (Tris-HCL [pH 6.8], 2% SDS, 0.86 M BME and 10% glycerol) for 2 hours at 65 °C with continuous shaking. The nuclear protein fraction from back skin and keratinocyte cultures was extracted using a kit according to the manufacturer's instruction (AY2002; Affymetrix, Santa Clara, CA). Whole cell lysate from keratinocyte cultures was extracted with the SDS extraction buffer. Equal amounts of protein were subjected to Tricine-SDS-PAGE (Schagger and von Jagow, 1987) in a 15% acrylamide gel (stratum corneum and keratinocyte culture) or Tris-SDS-PAGE (Laemmli, 1970) on a 6.5% acrylamide gel and transferred onto a 0.22-μm pore nitrocellulose membrane (1-866-736-1250; GE Osmonics, Minnetonka, MN). Membranes were blocked with nonfat milk and incubated with antibodies against pan-LCE1, LCE1m, β-Actin (A2228; Sigma-Aldrich, St. Louis, MO), NRF2 (Huebner et al., 2012), Lamin A/C (2032S; Cell Signaling, Boston, MA), SPRR2 (a kind gift from Dr Daniel Hohl), and NQO1 (2 μg/ml, GTX100235; GeneTex, Irvine, CA). After incubation with secondary antibodies (Pierce Biotechnology), antibody binding was visualized with a chemiluminescent substrate (34076; Thermo Fisher) and X-ray film (34090; Thermo Fisher, Waltham, MA).
Immunohistochemical and immunofluorescent staining
Paraffin-embedded samples from the back skin of 4-day-old killed mice were sectioned at 6 μm, deparaffinized, and subjected to antigen retrieval. After incubation with 10% goat serum, the sections were incubated with the primary antibody (Huebner et al., 2012). After blocking endogenous peroxidase with phosphate buffered saline containing 0.3% NaN3 and 0.01% H2O2, visualization was performed using an horseradish peroxidase-conjugated secondary antibody (PI-1000; Vector Laboratories, Burlingame, CA), the Vectastain ABC system (PK-7100; Vector Laboratories), and DAB peroxidase substrate (SK-4105; Vector Laboratories).
OCT-embedded frozen 4-day-old skin samples were sectioned at 8 μm. After incubation with 10% goat serum, the sections were incubated with the following primary antibodies: SPRR2, pan-LCE1, and LCE1m. After brief fixation with 10% formalin, antibody binding was visualized with secondary antibodies conjugated with fluorescent dyes (Life Technologies, Carlsbad, CA) and mounting medium (H1200; Vector Laboratories), respectively. All images were taken using a microscopic system (Eclipse 90i and NIS-Elements; Nikon, Tokyo, Japan).
Quantitative real-time PCR analysis
Back skins from 4-day-old mice were homogenized in TRIzol reagent (15596-026; Life Technologies) and total RNA was isolated. cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (4368814; Life Technologies). Primers specific for each target with double quenched probes were purchased from IDT Primetime Predesigned Library (IDT, Coralville, IA): Nrf2, Mm.PT.47.13038849; Nqo1, Mm.PT.58.10871473; Sprr2d, Mm.PT.47.11936353; Sprr2h, Mm.PT.47.7231243; Lce1b, Mm.PT.42. 13758418; Lce1c, Mm.PT.42.10901888; Lce1e, Mm.PT.42.5128487; Lce1g, Mm.PT.58.42521346.g; Lce1h, Mm.PT.42.10824763; Lce1m, Mm.PT.42.8092836.g. Primers for Gapdh (4352339E, Life Technologies) were used for internal reference. All data were analyzed using an LC480 thermal cycler (Roche, Basel, Switzerland).
Exogenous activation of the NRF2/KEAP1 pathway
Keratinocytes from neonatal ICR (Charles River) or FvB mice were isolated and cultured as described previously (Huebner et al., 2012). Samples were collected 4 hours after incubating in media with indicated concentration of tBHQ (112941; Sigma-Aldrich) dissolved in dimethyl sulfoxide (BP231; Thermo Fisher).
Chromatin immunoprecipitation assay
The back skin of 4-day-old killed mice was collected. Eighty milligrams of skin was used per reaction and incubated with 1 ml of medium (10744-019; Thermo Fisher) supplemented with 4 ng/ml of epidermal growth factor (236-EG; R&D, Minneapolis, MN) and 1% phosphate buffered saline-buffered formalin for 20 minutes, followed by the neutralization with 125 mM of glycine. The nuclear contents were extracted using a Sonicator 3000 (Qsonica, New-town, CT) and immunoprecipitated with 2 μg/reaction of the NRF2 antibody (Huebner et al., 2012), and normal rabbit IgG (sc-2027; Santa Cruz Biotechnology, Santa Cruz, CA) by use of a ChIP kit (P-2003; Epigentek, Brooklyn, NY). The enrichment was determined using SYBR green-based (04707516001; Roche, Basel, Switzerland) quantitative PCR on a real-time PCR system (LC480; Roche) with sequence-specific primers below.
Sprr2d ARE1 (forward: 5'-GCTACATGGGAGATTGGTTAA-3'; reverse: 5'-TTGGAACTCACCAATGATGATAA-3')
Sprr2d ARE2 (forward: 5'-GTGTATGCTGCTTGGTTGATG-3'; reverse: 5'-CCTCCCAATAAGGCCCAATAA-3')
Lce1e ARE (forward: 5'-GGTCTCTGAGCTCATCATGGA-3'; reverse: 5'-CAAGGGGTATGTGGCAGAGT-3')
Luciferase promoter assay
The 5' flanking regions of Sprr2d and Lce1e genes were amplified using a high fidelity polymerase (F-534; Thermo Fisher). The products were cloned into a pGL3 luciferase reporter vector (E1751; Promega, Fitchburg, WI). Mutations in the Lce1e ARE were generated using a site-directed mutagenesis kit (210515; Agilent Technologies, Santa Clara, CA) following the manufacturer's instructions. The expression plasmids and a control beta-galactosidase expression plasmid (E1018; Promega) were introduced into primary mouse keratinocytes at an equal mass ratio using a Nucleofection kit (VCA-1003; Lonza, Basel, Switzerland). The expression levels of luciferase were quantified using a kit (T1003; Life technologies) and a microplate reader (E7031; Promega), 24 hours after adding the indicated concentration of tBHQ to the culture medium that contained 1.2 mM CaCl2.
Statistical analysis
Data are expressed as means ± standard error of the mean. Comparisons among groups were performed with the unpaired t-test with equal SD by use of a software package (Prism 6; GraphPad Software, La Jolla, CA). In all analyses, P < 0.05 was taken to indicate statistical significance.
ACKNOWLEDGMENTS
We thank Michal Jarnik for his contribution to the EM studies. This research was supported in part by the Intramural Research Programs of NIAMS and NIBIB, and a grant from NIAMS (AR47898) to DRR. We also thank the University of Colorado Skin Disease Research Center (P30AR057212) Morphology Phenotyping Core for assistance.
Abbreviations
- CE
cornified cell envelope
- EDC
epidermal differentiation complex
- FLG
filaggrin
- Gly/Ser
glycine/serine
- Keap1
Kelch-like ECH-associated protein 1
- Lce1
late cornified envelope 1
- LKO
Lor knockout
- LOR
loricrin
- Nrf2
NF-E2-related factor 2
- tBHQ
tert-butylhydroquinone
- WT
wild type
Footnotes
ORCIDs Yosuke Ishitsuka: http://orcid.org/0000-0001-9611-3090
Dennis R. Roop: http://orcid.org/0000-0001-5868-5880
CONFLICT OF INTEREST The authors state no conflicts of interest.
REFERENCES
- Backendorf C, Hohl D. A common origin for cornified envelope proteins? Nat Genet. 1992;2:91. doi: 10.1038/ng1092-91. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Tilli CM, Jackson B, Avilion AA, MacLeod MC, Maltais LJ, et al. Rodent Lce gene clusters; new nomenclature, gene organization, and divergence of human and rodent genes. J Invest Dermatol. 2007;127:1782–6. doi: 10.1038/sj.jid.5700792. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(Pt 2):949–63. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet. 2009;41:211–5. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl D. Cornified cell envelope. Dermatologica. 1990;180:201–11. doi: 10.1159/000248031. [DOI] [PubMed] [Google Scholar]
- Hohl D, Mehrel T, Lichti U, Turner ML, Roop DR, Steinert PM. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–36. [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci USA. 2000;97:12475–80. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner AJ, Dai D, Morasso M, Schmidt EE, Schafer M, Werner S, et al. Amniotic fluid activates the nrf2/keap1 pathway to repair an epidermal barrier defect in utero. Dev Cell. 2012;23:1238–46. doi: 10.1016/j.devcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–13. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B, Tilli CM, Hardman MJ, Avilion AA, MacLeod MC, Ashcroft GS, et al. Late cornified envelope family in differentiating epithelia—response to calcium and ultraviolet irradiation. J Invest Dermatol. 2005;124:1062–70. doi: 10.1111/j.0022-202X.2005.23699.x. [DOI] [PubMed] [Google Scholar]
- Jarnik M, de Viragh PA, Scharer E, Bundman D, Simon MN, Roop DR, et al. Quasi-normal cornified cell envelopes in loricrin knockout mice imply the existence of a loricrin backup system. J Invest Dermatol. 2002;118:102–9. doi: 10.1046/j.0022-202x.2001.01661.x. [DOI] [PubMed] [Google Scholar]
- Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114(Pt 17):3069–70. doi: 10.1242/jcs.114.17.3069. [DOI] [PubMed] [Google Scholar]
- Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, Bickenbach J, et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000;151:389–400. doi: 10.1083/jcb.151.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1992;267:15097–104. [PubMed] [Google Scholar]
- Marshall D, Hardman MJ, Nield KM, Byrne C. Differentially expressed late constituents of the epidermal cornified envelope. Proc Natl Acad Sci USA. 2001;98:13031–6. doi: 10.1073/pnas.231489198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrel T, Hohl D, Rothnagel JA, Longley MA, Bundman D, Cheng C, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–12. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- Mischke D, Korge BP, Marenholz I, Volz A, Ziegler A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex (“epidermal differentiation complex”) on human chromosome 1q21. J Invest Dermatol. 1996;106:989–92. doi: 10.1111/1523-1747.ep12338501. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–30. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Rice RH, Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977;11:417–22. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Bowcock AM. Psoriasis genetics: breaking the barrier. Trends Genet. 2010;26:415–23. doi: 10.1016/j.tig.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–9. [PubMed] [Google Scholar]
- Saunders SP, Moran T, Floudas A, Wurlod F, Kaszlikowska A, Salimi M, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J Allergy Clin Immunol. 2015;137:482–96. doi: 10.1016/j.jaci.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Dutsch S, auf dem Keller U, Navid F, Schwarz A, Johnson DA, et al. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010;24:1045–58. doi: 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Farwanah H, Willrodt AH, Huebner AJ, Sandhoff K, Roop D, et al. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol Med. 2012;4:364–79. doi: 10.1002/emmm.201200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. Cryosectioning and immunolabeling. Nat Protoc. 2007;2:2480–91. doi: 10.1038/nprot.2007.365. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Mack JW, Korge BP, Gan SQ, Haynes SR, Steven AC. Glycine loops in proteins: their occurrence in certain intermediate filament chains, loricrins and single-stranded RNA binding proteins. Int J Biol Macromol. 1991;13:130–9. doi: 10.1016/0141-8130(91)90037-u. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–11. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Marekov LN. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell. 1999;10:4247–61. doi: 10.1091/mbc.10.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B, Mlitz V, Hermann M, Rice RH, Eigenheer RA, Alibardi L, et al. Evolutionary origin and diversification of epidermal barrier proteins in amniotes. Mol Biol Evol. 2014;31:3194–205. doi: 10.1093/molbev/msu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–7. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij WP, Alia A, Backendorf C. ROS quenching potential of the epidermal cornified cell envelope. J Invest Dermatol. 2011;131:1435–41. doi: 10.1038/jid.2010.433. [DOI] [PubMed] [Google Scholar]
- Vermeij WP, Backendorf C. Skin cornification proteins provide global link between ROS detoxification and cell migration during wound healing. PLoS One. 2010;5:e11957. doi: 10.1371/journal.pone.0011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–45. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–6. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K, Hohl D, McBride OW, Wang M, Cehrs KU, Idler WW, et al. The human loricrin gene. J Biol Chem. 1992;267:18060–6. [PubMed] [Google Scholar]